Abstract
Helicobacter pylori (H. pylori) infection is a leading cause of gastritis, peptic ulcers, and gastric cancer, affecting more than half of the global population. Its persistence in the acidic gastric environment and its ability to evade host immunity present major treatment challenges. Although antibiotics remain the standard therapy, rising antimicrobial resistance has reduced treatment efficacy, prompting the search for alternative and adjunct approaches. Emerging therapies include probiotics, antimicrobial peptides (AMPs), and plant-derived compounds, which target H. pylori through membrane disruption, immunomodulation, or direct antimicrobial activity. Novel drug delivery systems and microbiota-sparing interventions are also being investigated. Additionally, vaccine development offers a promising strategy for long-term protection, though challenges related to antigenic variability and host-specific responses remain. Despite these advances, treatment variability and the limited clinical validation of alternatives hinder progress. A multifaceted approach integrating microbiome research, host–pathogen interactions, and new therapeutic agents is essential for future success.
1. Helicobacter pylori Infection
Helicobacter pylori (H. pylori) is a common, chronic bacterial infection that significantly contributes to various gastrointestinal disorders. The infection can range from asymptomatic gastritis to severe conditions such as peptic ulcers, gastric mucosa-associated lymphoid tissue (MALT) lymphoma, and gastric cancer [1]. The transmission routes are predominantly via direct contact, oral–oral routes, and contaminated water or food sources. One study mentioned that approximately 50% of the world’s population is estimated to be infected with H. pylori [2]. Specifically, H. pylori infection is significantly more prevalent, often reaching 80–90%, in developing countries like Africa, South Asia, and Latin America. Factors such as poor sanitation, contaminated water, crowded living conditions, co-infections (soil-transmitted helminths), and limited healthcare access contribute to this high prevalence [3,4]. In contrast, developed countries, including those in Western Europe and North America, exhibit much lower prevalence rates, ranging from 20% to 40% due to improved sanitation, better hygiene practices, and greater healthcare access [5]. Additionally, a decreasing trend in H. pylori prevalence is observed in these regions due to improved hygiene, healthcare access, and awareness [6].
In general, H. pylori infection is acquired during childhood, with infection rates increasing with age. Studies have shown that the highest incidence of seroconversion occurs in early childhood [7]. As children grow older, the rate of new infections decreases, but the overall prevalence increases with age due to the cumulative effect of acquiring the infection over time [1]. Conversely, higher prevalence has been noted in populations with a family history of gastric diseases, such as gastric cancer, peptic ulcer disease, and relapses in previously treated individuals. This is likely due to genetic factors that may make some individuals more susceptible to infection and its complications [8]. The infection is also linked to other gastrointestinal issues like duodenal ulcers and vitamin B12 deficiency [6]. Diagnosis involves both invasive and non-invasive methods, including stool antigen tests, PCR stool tests, urea breath tests, and upper gastrointestinal procedures such as endoscopy or X-rays [9]. Currently, urease testing and histological analysis are widely regarded as the gold standard in diagnosing H. pylori infection in many clinical situations. However, no single method can be universally defined as the gold standard for diagnosis. Non-invasive tests such as the urea breath test and stool antigen test are highly recommended and commonly used [10]. Further evidence is needed before any diagnostic method can be established as the definitive gold standard for diagnosing H. pylori infection across different clinical scenarios. The infection is typically treated with a combination of antibiotics and acid suppressants to eradicate the bacteria and promote stomach healing [11].
2. Current Therapeutic Approaches for H. pylori Infection
Common antibiotics used include amoxicillin (AMX), clarithromycin (CLR), metronidazole (MTZ), rifabutin, rifaximin, and sitafloxacin, with the latter three being considered for third-line treatment [12]. Proton pump inhibitors (PPIs), such as omeprazole and esomeprazole, help reduce stomach acid, while bismuth subsalicylate (Pep-to-Bismol) protects ulcers from acid. Histamine (H-2) blockers, such as cimetidine, block acid production [13]. On the other hand, potassium-competitive acid blockers (P-CABs), such as Vonoprazan, play a crucial role in contemporary H. pylori eradication therapies [12]. When combined with antibiotics such as AMX and CLR, it often outperforms traditional PPIs, contributing to higher eradication rates and improved treatment efficacy. Among all these, rifabutin-based and CLR-based triple therapies are the most common [14]. However, there is no treatment that guarantees a 100% cure rate, and antibiotic resistance must be considered when selecting a therapeutic approach. Figure 1 illustrates a comprehensive overview of H. pylori infection and global prevalence, showing high infection rates in developing countries (80–90% in Africa, South Asia, and Latin America) and lower rates in developed countries (20–40% in Western Europe and North America); transmission routes, detailing direct contact, oral–oral transmission, and contamination through water and food; risk factors, including poor sanitation, overcrowded living conditions, limited healthcare access, family history, and age-related factors (higher rates in children, cumulative increase with age); diagnostic methods, distinguishing between non-invasive approaches (stool antigen test, PCR stool test, urea breath test) and invasive procedures (endoscopy, urease testing, histological analysis); and treatment approaches, categorizing options into antibiotics (amoxicillin, clarithromycin, metronidazole, rifabutin, rifaximin, sitafloxacin), acid suppressants (PPIs and H-2 blockers), and protective agents (bismuth subsalicylate), providing a complete picture of therapeutic strategies.

Figure 1.
The overview of H. pylori infection.
3. Antibiotic Resistance in H. pylori
The prevalence of antibiotic resistance in H. pylori varies significantly worldwide, often exceeding 95% in certain regions, particularly in low- and middle-income countries where the misuse and overuse of antibiotics are prevalent [15]. This widespread resistance compromises the efficacy of standard eradication therapies and heightens the risk of treatment failure. However, the emergence of resistance to commonly used antibiotics, such as MTZ, CLR, and levofloxacin (LVX), has drastically reduced treatment success rates worldwide. AMX is widely used as a first-line treatment for H. pylori eradication owing to its affordability and safety, and resistance remains low (0–10%) in most countries, with low resistance observed in Asia (~3%), China (2.8%), Europe (~0.4%), and Algeria (0%), but high rates reported in Vietnam (25.7%) and Africa (up to 100%) [16]. Resistance arises through three primary mechanisms, which are mutations in penicillin-binding proteins (PBPs), particularly PBP1A, which reduce AMX binding affinity via amino acid substitutions (S402G, T555S); followed by β-Lactamase production such as the blaTEM-1 gene, which degrades AMX and may spread via horizontal gene transfer; and lastly, porin and efflux pump modifications, where mutations in proteins like helicobacter outer membrane protein B (HopB), helicobacter efflux protein A (HefA), and outer membrane protein (omp25) decrease AMX permeability and intracellular accumulation. Long-term low-dose exposure further alters porin expression, complicating treatment outcomes [17,18].
MTZ, a nitroimidazole antibiotic, shows mixed trends globally, particularly in East and Southeast Asia, with rates reaching 87.8% in China, 94.6% in Bangladesh, and 81.6% in India. In Europe, resistance averages 32%, peaking in France (58.6%) and falling to 10.2% in Austria. Oceania reports ~50% resistance, while the Americas average 23% but decreases in Chile and Spain [19]. Resistance is particularly prevalent in developing countries compared to Western nations, possibly due to the widespread and frequent use of MTZ for other infections, which increases selective pressure on H. pylori [20]. Patients who have previously undergone treatment for H. pylori are more likely to develop resistance, as are those diagnosed with nonulcerated duodenal ulcers (NUD). MTZ resistance primarily results from genetic mutations in the rdxA, frxA, or fdxB genes, which result in a reduction of nitroreductase activity, leading to the decreased activation of the drug and rendering it ineffective [21]. The high expression of efflux pump genes like hp1165 and hefA also contributes to resistance and is associated with multidrug resistance [22].
CLR resistance has risen in many regions, particularly in China and treated patients in France and Taiwan [23]; also, some significant regional variations were observed, including about 18% in Europe, 33% in the Eastern Mediterranean, 34% in the Western Pacific, less than 10% in the Americas and Southeast Asia, and less than 29.2% in Africa, though specific countries like Congo reported lower rates (1.7%) [24]. Resistance primarily arises from mutations in the 23S rRNA gene (e.g., A2142G, A2143G), which hinder CLR binding, along with synergistic mutations in genes like rpl22 and infB [25]. Efflux pump systems, particularly the RND family, actively expel CLR, reducing its efficacy, with inhibitors like PaβN and CCCP showing the potential to reverse this effect. OMP alterations, such as changes in HopT and OMP31 expression, further contribute to resistance by decreasing membrane permeability [26]. These mechanisms collectively undermine CLR’s effectiveness, posing challenges for H. pylori treatment.
Tetracycline (TET) resistance remains low globally, except for notable increases in Iran and China, ranging from 0.6% to 8%, and in Europe (below 1%). The United States and Australia also report low resistance, making it a preferred option in quadruple therapy, achieving over 90% eradication success even in areas with high resistance to other antibiotics like CLR and MTZ [27]. TET’s mechanism of action involves inhibiting protein synthesis by binding bacterial ribosomes, but resistance occurs mainly through mutations in the 16S rRNA gene that prevent TET binding [28]. High-level resistance results from triple mutations at the TET binding site, while lower resistance is linked to double or single mutations. Additionally, TET resistance can be enhanced by proton motive force-dependent efflux pump systems, with the Hp1165 gene playing a key role in some strains [19,29]. However, other efflux pump determinants may also contribute to resistance, suggesting a complex mechanism of TET resistance in H. pylori.
LVX, a fluoroquinolone antibiotic resistance, has escalated in Europe, Asia, and the Western Pacific, exceeding 30% in countries like China (30.29%), India (54.9%), the U.S. (37.6%), and Iran (18%), while lower rates are observed in Pakistan, Thailand, and Brazil [23]. Due to high resistance, LVX is mainly used in remedial treatments after initial failure or based on drug susceptibility tests. Resistance arises primarily from mutations in the quinolone resistance determination region (QRDR) of the gyrA gene, particularly at amino acids 87 and 91 (N87K, D91G), and sometimes in the gyrB gene [30]. These mutations prevent LVX from binding to DNA gyrase, rendering the antibiotic ineffective [31]. Other mutations in gyrA and gyrB also contribute to LVX resistance, though their impact requires further investigation [32].
Multidrug resistance (MDR) is rising, with double and triple resistance patterns becoming more prevalent, especially in regions like Chile and Peru [33]. Several studies have highlighted significant antibiotic resistance in H. pylori strains across various regions. In Isfahan, Iran, resistance rates for CLR, MTZ, and AMX were 15.3%, 55.1%, and 6.4%, respectively, with one multidrug-resistant isolate identified [34]. A study in Myanmar revealed high MDR, particularly to MTZ and LVX, with 84.2% showing dual-drug resistance [35]. In Saudi Arabia, 8.8% of isolates were MDR, with high resistance to MTZ (48.2%) and CLR (27.7%) [36]. Italy demonstrated an increase in resistance over time, particularly for CLR (35.9%) and MTZ (40.2%) [37]. Meanwhile, in Malaysia, a study reported high resistance to MTZ (82.4%) and CLR (72.5%), with 82.4% of isolates exhibiting multidrug resistance [38]. Resistance rates were 65.2% for MTZ and 34.8% for LVX, with no resistance observed for AMX in a study conducted in Shanghai [39]. These trends highlight the urgent need for tailored treatment strategies and enhanced antibiotic stewardship to combat H. pylori resistance. The challenges associated with broad-spectrum antibiotic therapy are particularly pronounced in pediatric patients, where the loss of gut flora during extended treatments poses significant health risks. Moreover, the increasing failure of eradication strategies and the desire to prevent gastric cancer have spurred interest in more targeted therapeutic approaches, such as local drug delivery methods. Table 1 provides antibiotic resistance patterns and mechanisms against H. pylori.

Table 1.
The global resistance patterns, mechanisms, and regional variations of antibiotics used against H. pylori.
4. Biofilms of H. pylori
H. pylori biofilms are now recognized as one of the most important causes of antibiotic resistance and relapse infection [40]. Biofilm growth is a survival strategy that enables H. pylori to colonize and persist in the hostile gastric environment [41]. It also acts as a shield against host immunity and acid stress and eventually inhibits drug penetration, enhances metabolic dormancy, and enhances phenotypic heterogeneity that eventually decreases therapeutic effectiveness [42]. The structures are also involved in transmission and reinfection and have been observed not only on gastric mucosa but also abiotically on water systems, serving as reservoirs in the environment [43,44]. Mature biofilm dispersal allows for intra-host transmission as well as inter-host transmission [45,46]. H. pylori biofilm development is a multi-step process initiated by bacterial adhesion to surfaces, stimulated by outer membrane adhesins such as BabA, SabA, and AlpA/B [47]. Motility and chemotaxis via chemoreceptors such as TlpA–D also favor niche localization [48]. The matrix of the biofilm consists of exopolysaccharides (EPS), extracellular DNA (eDNA), proteins, and OMVs, along with other substances [49,50]. OMVs, formed through outer membrane blebbing, represent the predominant type of bacterial extracellular vesicles (bEVs) produced by Gram-negative bacteria such as H. pylori [51]. OMVs are lipidated with lipopolysaccharides (LPS), outer membrane proteins (OMPs), and virulence factors such as CagA, VacA, and urease, playing a multifunctional role in biofilm integrity, bacterial survival, and modulation of the host immune system [52]. EPS and eDNA are involved in matrix cohesion and horizontal gene transfer, enabling adaptability and resistance [53,54,55]. Quorum sensing through the AI-2 signaling system, provided by luxS, controls biofilm architecture and virulence. TlpB, in particular, perceives AI-2 as a chemorepellent and affects dispersal dynamics; luxS mutation enhances biomass and cluster formation, highlighting its regulatory function [56,57]. Biofilms, when mature, form 3D architectures with microcolony heterogeneity, reduced antibiotic permeability, and enhanced expression of efflux pumps [58,59]. Dispersal, generally induced by stress or starvation, is regulated by ECM-degrading enzymes like proteases and DNases, releasing coccoid cells in a resting state with enhanced resistance and persistence potential [60,61,62]. Figure 2 illustrates the complete biofilm formation life cycle from adhesion to dispersal through ECM maturation.
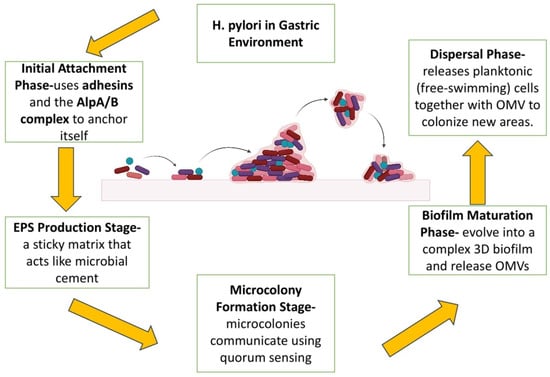
Figure 2.
H. pylori biofilm formation. The sequential stages of H. pylori biofilm formation within the gastric environment, beginning with initial attachment where the bacterium uses adhesins such as BabA, SabA, and the AlpA/B complex to anchor itself to biotic or abiotic surfaces, aided by flagellar motility and chemotaxis. This is followed by the EPS production stage, where H. pylori secrete extracellular polymeric substances (EPS) including eDNA, proteins, and polysaccharides, forming a sticky matrix that acts like microbial cement. In the microcolony formation stage, bacterial cells aggregate into clusters and communicate via quorum sensing through autoinducer-2 (AI-2) and the LuxS gene product. These microcolonies mature into complex 3D biofilm structures in the biofilm maturation phase, characterized by increased antibiotic resistance and the release of outer membrane vesicles (OMVs) carrying virulence factors such as CagA, VacA, LPS, and outer membrane proteins (OMPs). Finally, during the dispersal phase, nutrient depletion or stress triggers the release of single, planktonic cells along with OMVs, enabling the colonization of new niches and perpetuating the infection cycle.
Factors Influencing Formation of Biofilms
The development of H. pylori biofilms is driven by environmental stresses such as gastric acidity, nutrient starvation, and intercellular communication, which together enhance bacterial survival and promote chronic infection [63,64,65,66].
H. pylori’s metabolic flexibility and its ability to form biofilms on both biotic and abiotic surfaces, including medical devices, expand its ecological niches and enhance its potential for persistence [67]. The virulence factors VacA and CagA play synergistic roles in promoting biofilm formation and gastric pathogenesis [68,69,70]. CagA, delivered into gastric epithelial cells via the type IV secretion system, disrupts cellular polarity, tight junctions, and cytoskeletal structures. It also controls NF-κB-mediated inflammatory responses, which can induce carcinogenesis [68,69,70,71].
VacA induces vacuolation, disrupts endolysosomal trafficking, and suppresses autophagy, leading to the intracellular accumulation of CagA and increased cytotoxicity [72,73,74]. In combination, these factors augment mucosal damage and are associated with more severe gastroduodenal disease [75]. Notably, sub-therapeutic antibiotic exposure can paradoxically induce biofilm formation by triggering stress responses and enhancing matrix production [76].
Under such stress, H. pylori may adopt a viable but non-culturable (VBNC) coccoid form, in which it is metabolically inactive but capable of reactivation. VBNC cells contribute matrix material to biofilms through lysis, releasing DNA and proteins and thereby strengthening biofilm integrity [77,78]. This mutual relationship stabilizes biofilms and promotes immune evasion. VBNC cells are highly antibiotic-resistant and evade detection by standard culture methods, often leading to treatment failures, relapses, and delayed diagnoses [79,80,81,82,83].
Together, H. pylori biofilms, facilitated by the coordination of virulence factors, environmental stress responses, and the VBNC state, form a chronic reservoir that is resistant to eradication and contributes to long-term gastric disease [84,85].
5. The Influence of Bacterial Extracellular Vesicles During H. pylori Infection
bEVs are nanosized, membrane-bound particles secreted by Gram-negative and Gram-positive bacteria. They harbor diverse biomolecular cargo, including lipids, proteins, toxins, nucleic acids, and signaling molecules [86,87]. Initially believed to be unique to Gram-negative bacteria due to their outer membrane, bEVs are now recognized as universal structures across bacterial species, with Gram-positive bacteria using autolysins and endolysins to traverse their thick peptidoglycan walls [88]. These vesicles are central mediators of bacterial physiology, facilitating intercellular communication both among microbial communities and at host–microbe interfaces [89]. In H. pylori, OMVs, a subcategory of bEVs, are the major secreted vesicle type and play a critical role in the delivery of virulence, immune evasion, and biofilm formation.
Functionally, bEVs participate in nutrient acquisition, stress responses, biofilm development, and horizontal gene transfer. They also serve as delivery vehicles for virulence factors, toxins, and immunomodulatory molecules, thereby affecting bacterial pathogenicity and the host immune response [90,91]. bEVs also contain pathogen-associated molecular patterns (PAMPs) that interact with host pattern recognition receptors, inducing innate and adaptive immunity, thereby a dual function in both pathogenesis and immunoregulation [92].
In Gram-negative bacteria like H. pylori, bEVs are primarily OMVs, which are generated through outward blebbing of the outer membrane. The outer membrane is an asymmetric bilayer composed of phospholipids in the inner leaflet and lipopolysaccharides (LPS) in the outer leaflet, together with OMPs involved in adhesion, nutrient uptake, and environmental sensing [93,94]. OMVs from these membranes include a mixture of bioactive molecules, including LPS, OMPs, phospholipids, peptidoglycan fragments, enzymes, and toxins such as VacA and CagA, which are key players in H. pylori pathogenesis [95]. OMV biogenesis is a regulated process of vesicle formation via membrane curvature and pinching that enables the bacterium to release toxic or immunomodulatory effectors in a controlled manner [95].
Recent studies have extended the understanding of vesicle heterogeneity in Gram-negative bacteria. Among them is the outer–inner membrane vesicle (OIMV), containing outer and inner membrane material, likely produced by explosive lysis propelled by phage-encoded endolysins. This vesicle contains abundant cytoplasmic cargo, including DNA, cytosolic proteins, and enzymes [96,97]. One of these new subtypes is the explosive outer membrane vesicle (EOMV), which also derives from cell lysis events, yet differs from OMVs in its disordered, unstructured assembly. EOMVs encapsulate large, random quantities of cellular contents and can be agents of horizontal gene transfer or bactericidal activity towards competing microbes [98,99,100].
Although Gram-positive bacteria lack an outer membrane, they also shed vesicles, which are commonly referred to as cytoplasmic membrane vesicles (CMVs). These vesicles are discharged through processes of “bubbling cell death,” a form of autolysis in which bulging of the cytoplasmic membrane leads to vesicle release, mediated by autolysins that degrade the peptidoglycan layer [101,102,103]. Interestingly, vesicle formation in Gram-positive bacteria is not solely associated with cell death; viable cells can also release CMVs as part of regulated secretion processes involved in immune modulation, biofilm formation, and intercellular communication [104,105].
While the therapeutic promise of bEVs, particularly H. pylori-derived OMVs, is becoming more apparent in drug delivery, vaccine engineering, and biomarker identification, there are several challenges that must be surmounted. These include the heterogeneity of vesicle populations, scalability of production and purification, guarantee of safety and specificity, and information on their in vivo kinetics and mode of action [106]. There is a need for the standardization of bEV isolation protocols and for understanding their roles in health and disease through more studies. Nonetheless, bEVs represent a promising avenue for future antimicrobial and immunotherapeutic strategies to H. pylori and other persistent infections. Table 2 explains the multifaceted roles of bEVs in the context of H. pylori infection, from their composition and function to their potential in diagnostics and treatment.

Table 2.
The differences in bEVs from Gram-negative versus Gram-positive bacteria.
Role of OMVs in H. pylori Pathogenesis, Biofilm Formation, and Antibiotic Resistance
H. pylori have evolved sophisticated mechanisms to survive the gastric acidic environment, and OMVs play a key role in host–pathogen interactions. OMVs are nanometer-sized (20–300 nm) vesicles that are formed by the outward blebbing of the bacterial outer membrane, and they contain a heterogeneous cargo of virulence factors such as CagA, VacA, urease subunits, γ-glutamyltransferase (GGT), and adhesins such as BabA and SabA that enhance bacterial adhesion to host epithelial cells [107,108]. In addition to presenting virulence factors, OMVs also transport immunomodulatory molecules that can induce inflammation for colonization and also allow immune evasion [109].
OMVs are carriers of PAMPs such as LPS and peptidoglycan that activate host pattern recognition receptors (PRRs) such as TLRs and NOD1 [110]. This triggers inflammatory cascades mediated by NF-κB, caspases, and cytokines such as IL-8, IL-6, IL-1β, and TNF-α, facilitating immune cell recruitment and inflammation [110,111]. Conversely, OMVs allow for immune suppression by activating heme oxygenase-1 (HO-1) via Akt-Nrf2 and mTOR–IKK–NF-κB signaling pathways in dendritic cells and macrophages [112]. Moreover, OMV-associated factors like GGT and VacA induce T cell apoptosis, cell cycle arrest, and disordered proliferation, while small non-coding RNAs and nucleic acids modulate host gene expression, inhibit autophagy, and interfere with antigen presentation by the interaction of, for instance, VacA with CD18 on T cells [113,114,115].
The antioxidant load in OMVs, including catalase (KatA), which is seven times more abundant on OMVs than in the outer membrane, is crucial to neutralize host-generated reactive oxygen species (ROS), particularly hydrogen peroxidase (H2O2), from neutrophils and polymorphonuclear leukocytes [116]. Meanwhile, OMVs can also cause oxidative stress by Helicobacter pylori neutrophil-activating protein (HP-NAP)-induced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activation, which produces a paradoxical pro-inflammatory milieu conducive to bacterial persistence [117,118].
Interestingly, OMVs cause the M2 polarization of macrophages, a tissue repair and immune tolerance-related phenotype through CD206 upregulation and CD86 downregulation, the increased production of IL-10 and IL-4, and the decreased production of IL-12 and IFN-γ [119,120]. All these reinforce an immunosuppressive microenvironment favorable for chronic infection. OMVs also modulate T cell survival through the induction of apoptosis in CD4+ and CD8+ subsets by cyclooxygenase-2 (COX-2) induction and prostaglandin E2 (PGE2) synthesis pathways suppressing T cell growth and IL-2 production [121,122]. VacA-enriched OMVs can also disrupt the immunological synapse and cause cytoskeletal degradation, nuclear factor of activated T-cells (NFAT) suppression, and glutamine starvation through gamma-glutamyltransferase (GGT), disrupting T cell function [123]. These vesicles also participate in extragastric diseases like liver fibrosis, cardiovascular disease, and neurodegeneration by modulating immune responses or transporting bacterial products to target tissues [124].
OMVs also play a significant role in H. pylori biofilm formation. For example, OMVs of the TK1402 isolate induce biofilm formation in a dose-dependent manner by acting as a structural backbone that provides orienting signals to bacterial cells and extracellular matrix components [49]. The coating of biofilm surfaces by OMV has been demonstrated using transmission electron microscopy, and proteomic profiling defined OMPs such as AlpB are required for adhesion and stability. Defective biofilm formation in AlpB mutants is corrected upon AlpB reintroduction [125]. Adhesin-enriched OMVs bearing adhesins such as BabA also promote early surface attachment, a critical event during the initiation of the biofilm [58].
Biofilm OMVs ensnare antibiotics like AMX and TET, forming diffusion barriers and protecting dormant bacterial populations [126]. Bacterial OMVs contain higher levels of extracellular DNA (eDNA) than planktonic OMVs and contribute to matrix stability and nuclease resistance degradation [127]. OMVs also facilitate horizontal gene transfer by delivering eDNA for natural transformation in biofilms, disseminating resistance and virulence genes [128]. Notably, EVs from infected host cells sometimes harbor hybrid bacterial host signatures, reflecting a complex intercellular cargo exchange [129]. Size heterogeneity in OMVs is also linked to functional heterogeneity, influencing adhesion, matrix support, and signaling functions in the biofilm microenvironment [129].
OMVs convey antibiotic resistance through a variety of mechanisms. They facilitate horizontal gene transfer by delivering resistance genes and enzymes like β-lactamases that degrade antibiotics [130]. OMVs also serve as decoys, trapping antibiotics before they reach their destination [131]. Certain OMVs cause active drug extrusion, such as OMV-mediated bismuth extrusion, which enables H. pylori to tolerate sublethal levels of bismuth [131,132]. Interestingly, OMVs selectively protect against clarithromycin and levofloxacin but not against amoxicillin or metronidazole [133], indicating their targeted role in modulating drug susceptibility.
Overall, H. pylori OMVs are multifunctional nanoscale vesicles that are attributed to orchestrating biofilm formation, immune evasion, and antibiotic resistance. Their roles in the modulation of infection niche, chronic colonization, and interaction with the host highlight their importance as potential therapeutic targets. An improved understanding of OMV biogenesis and the mechanism of action could lead to identifying OMV-disrupting compounds or vaccines for the eradication of H. pylori infections. Table 3 summarizes the various roles of H. pylori OMVs in immunomodulation, biofilm formation, and antibiotic resistance.

Table 3.
The key immunomodulatory, biofilm-related, and antibiotic resistance functions of H. pylori OMVs.
6. The Influence of H. pylori on Gut Microbiota
The human gastrointestinal (GI) tract contains a remarkably diverse and dense microbial community, with bacterial cell estimates in the colon ranging from 1011 to 1012 per milliliter, ranking it among the most densely inhabited environments on the planet [134,135]. The gut microbiome comprises more than 3.3 million genes, 150 times more genes than humans’ own genome, generating thousands of metabolites, greatly exceeding the human genome’s 23,000 genes [136]; the bacterial diversity analysis showed that about 1000 bacterial species live in our gut, and a majority of them belongs to the divisions of Bacillota (formerly Firmicutes), which includes genera like Lactobacillus, Clostridium, and Faecalibacterium, playing a role in short-chain fatty acids (SCFAs) production, energy metabolism, and gut health. Bacteroidota (formerly Bacteroidetes) [137], which includes Bacteroides and Prevotella, is involved in carbohydrate fermentation and SCFA production. Actinomycetota (formerly Actinobacteria), notably including Bifidobacterium, has beneficial effects on gut health, enhancing the mucosal barrier and inhibiting pathogens. Pseudomonadota (formerly Proteobacteria), including Escherichia and Enterobacter, is often associated with dysbiosis when in high abundance. Verrucomicrobiota (formerly Verrucomicrobia), with Akkermansia muciniphila as a key member, is linked to maintaining mucosal integrity and anti-inflammatory effects [138]. The human gut microbiota also includes a small but important component of fungal and yeast species, such as Saccharomyces cerevisiae, which can influence gut health and immune responses [139]. Additionally, archaea, like Methanobrevibacter smithii, are involved in methane production, contributing to the overall balance of the gut microbiota [140]. The virome, which consists of bacteriophages (viruses that infect bacteria) plays a significant role in regulating bacterial populations and maintaining the balance of the microbiota [141]. These diverse microbial entities, although smaller in number, collectively contribute to the complexity and functionality of the gut ecosystem, influencing digestion, immunity, and overall health. This has uncovered the microbiota’s variety and richness, which enhance its function as a “superorganism” with metabolic and immune roles, interacting symbiotically with the host [142].
H. pylori infection significantly alters the composition of the gut microbiota, leading to dysbiosis that may create an environment conducive to gastric atrophy and intestinal metaplasia [143]. H. pylori produce urease, which increases gastric pH by breaking down urea into ammonia. This altered pH can make the stomach less hospitable for acid-tolerant beneficial microbes, leading to colonization by opportunistic pathogens [144]. Early research using in situ hybridization and bacterial culturing found increased growth of Lactobacillus acidophilus in H. pylori-positive patients, indicating an imbalance in the gut microbiota, as these bacteria might proliferate in response to the changes in gut pH and the disruption caused by H. pylori [145], while a reduction in Clostridia and anaerobes was observed in comparison to H. pylori-negative subjects, which may impact processes such as fermentation and immune system modulation, potentially leading to gastrointestinal symptoms or an increased risk of other conditions like colorectal cancer [146]. Subsequent studies using fecal 16S rRNA analysis confirmed these findings and revealed that H. pylori infection leads to a decrease in microbial diversity, including an increased abundance of genera like Haemophilus and Ralstonia, while genera such as Parasutterella and Pseudoflavonifractor are found in lower levels [147]. The decreased levels of Pseudoflavonifractor, an SCFA producer, could negatively impact intestinal health, as SCFAs like butyrate are crucial for immune modulation and gut health [148]. Moreover, the infection is linked to a shift towards a Prevotella-dominated microbiome, regardless of dietary influences [149]. Other studies have reported that H. pylori infection could also enrich the gut microbiota with specific genera, such as Succinivibrio, Coriobacteriaceae, Enterococcaceae, and Rikenellaceae [150]. Notably, a large population study demonstrated that H. pylori-positive individuals exhibited a higher microbial diversity, along with increased abundance of genera such as Actinomyces, Gemella, Streptococcus, and Haemophilus [151]. However, in individuals with H. pylori colonization, the microbiota shifts towards an enrichment of Spirochetes and Proteobacteria. Carcinogenic metabolites, such as N-nitroso compounds produced by Staphylococcus, Lactobacillus, and Escherichia coli were observed, are implicated in cellular mechanisms that promote inflammation and tumor angiogenesis [152]. A study found that H. pylori infection increases the abundance of bacterial families like Succinivibrionaceae, Coriobacteriaceae, Enterococcaceae, and Rikenellaceae, as well as fungal species such as Candida glabrata. These microbial shifts may disrupt the intestinal mucosal barrier, potentially contributing to early-stage colorectal carcinoma [153]. In elderly individuals, reduced beneficial bacteria like butyrate-producing Clostridium clusters contribute to gastric carcinogenesis. Gastric cancer microbiota commonly includes strains of Streptococcus, Lactobacillus, Veillonella, and Prevotella, with specific Streptococcus species prevalent in gastric carcinoma [154]. Altered microbial communities in gastric cancer are linked to increased Proteobacteria, Lactobacillus, Clostridium, and Rhodococcus, highlighting the microbiota’s role in disease progression [155]. Persistent inflammation or atrophy in some patients was associated with shifts in microbial clusters, including increased Acinetobacter and Streptococcus anginosus [156]. Studies have also suggested that factors such as age, BMI, sex, and diet influence gut microbiota composition, highlighting the importance of controlling for these variables when evaluating the impact of H. pylori [157]. Overall, H. pylori infection alters gut microbiota diversity and composition, while eradication therapy involving broad-spectrum antibiotics significantly impacts microbial richness and specific populations. These shifts due to therapy affect metabolic health, microbial balance, and antibiotic resistance [158]. Although these microbial alterations are usually temporary, with the microbiota often returning to baseline within eight to ten weeks post-treatment, the recovery process can vary among individuals. The change in gut population includes an increased abundance of Enterobacteriaceae, Clostridiaceae, and certain Proteobacteria, alongside decreased levels of beneficial genera like Bifidobacterium, Faecalibacterium, Actinobacteria, and Lactobacillus, leading to temporary or prolonged dysbiosis, affecting gut barrier function and immunity [159]. Specifically, triple therapy moderately reduces diversity with notable increases in Enterobacteriaceae, whereas quadruple therapy causes broader microbial disruption. MTZ-containing regimens promote facultative anaerobes, and bismuth-based treatments are associated with prolonged dysbiosis, leading to B vitamins and folate, leading to deficiencies, and underscoring the variable impacts of different regimens [160]. Megamonas and the Rikenellaceae family, known for SCFA (butyrate and propionate) production, demonstrate alterations linked to improved glucose homeostasis after eradication therapy [161], while the use of antibiotics in eradication therapies has been associated with an increase in antibiotic resistance genes. For example, genes like ermB (macrolide resistance) and tetQ (TET resistance) have been identified; however, in some cases, the tetO and tetW were found to be decreased [162]. Eradication regimens also promote transient increases in genes like ermB, CFX, and tetQ, particularly after LVX-based therapies [163]. Another study found increases in opportunistic pathogens, such as Enterococcus faecium and Clostridium difficile, and a notable rise in antibiotic resistance genes [164]. This rise poses challenges for future treatment options and highlights the need for judicious antibiotic use. Table 4 provides the microbial shifts in response to H. pylori infection, indicating changes in bacterial and fungal genera and their implications on gut health, microbial diversity, and potential links to gastric and colorectal cancer.

Table 4.
Microbial changes induced by H. pylori infection.
7. Potential Treatment for Microbiota Recovery After H. pylori Infection
Current clinical practice guidelines recommend the use of combination regimens for the eradication of H. pylori, of which PPIs together with antibiotics such as clarithromycin, amoxicillin, or metronidazole form the basis of triple therapy. Quadruple therapy with bismuth and TET is applied in cases of antibiotic resistance or treatment failure [165]. The efficacy of these regimens is declining globally due to increasing antibiotic resistance, necessitating the pursuit of alternative or adjunct therapies [166]. Furthermore, conventional eradication therapies tend to induce drastic alterations in gut microbiota composition, resulting in dysbiosis, compromised gut barrier function, and increased susceptibility to secondary infection [167]. In response to these challenges, microbiota-sparing strategies have been proposed. Emerging evidence suggests that incorporating adjunctive therapies such as probiotics, prebiotics, or faecal microbiota transplantation (FMT) can accelerate the restoration of microbial balance and improve clinical outcomes [168,169].
Probiotics produce antimicrobial substances that directly inhibit the growth of H. pylori, such as acetic acid and lactic acid, H2O2, and bacteriocins. These products lower gastric pH, annihilate H. pylori cells, and suppress bacterial growth [170]. Probiotics, particularly Lactobacillus strains such as Lactobacillus plantarum, Lactobacillus reuteri, and Lactobacillus casei, also compete with H. pylori for adherence sites on the gastric mucosa and nutrients, suppressing colonization through the formation of protective barriers and the exclusion of pathogenic bacteria [171,172,173]. Saccharomyces boulardii is also particularly effective against the growth inhibition of H. pylori by modulating the immune response to H. pylori, restoring the gut microbiota balance disrupted by infection and antibiotics, and reducing the abundance of antibiotic-resistance genes [174]. In addition to their direct antimicrobial activity, certain Lactobacillus strains can stimulate the production of mucin, a protective coating in the stomach that prevents H. pylori from binding to gastric mucosa by modulating the immune response to better control infection [175,176]. A study found that Lactobacillus plantarum strains Q21, Q25, and QA85 were found to reduce H. pylori load, improve gastrointestinal symptoms, and modulate inflammation, but they cannot fully eradicate H. pylori when used as monotherapy [177]. Thus, the application of probiotics in conjunction with standard eradication therapies, such as triple therapy or PPIs, has been shown to improve eradication rates and reduce adverse effects [178]. Combinations like Bifidobacterium–Lactobacillus and Bifidobacterium–Lactobacillus–Saccharomyces have had the best overall performance in improving both eradication and side effect outcomes, whereas Lactobacillus–Propionibacterium was efficacious in eradication but with increased side effects [179]. Furthermore, multi-strain probiotic combinations, particularly those with Saccharomyces boulardii and combinations like Clostridium butyricum with Bacillus coagulans, have shown increased efficacy compared to single strains in the treatment of gastrointestinal disorders and H. pylori eradication [180]. The combinations have also shown promise with minimal side effects. Strain variability in efficacy and the need for further research into the dosing and duration of treatment are, nevertheless, still hindrances to their clinical application [181].
Autoprobiotics represent a novel approach to probiotic therapy, which are strains of indigenous microbiota recovered from the individual’s own body. The personalized strain formulation is tailored to the individual’s microbial milieu, which renders autoprobiotics more potent and capable of persisting in the gut for longer periods of time [182]. This reduces the requirement for prolonged treatments in comparison with conventional probiotics. Experimental evidence suggests that autoprobiotics, such as Lactobacillus, Bifidobacteria, or Enterococci, can accelerate the recovery of microbiota in dysbiosis induced by antibiotics in rats. In H. pylori chronic gastritis, autoprobiotics using native non-pathogenic Enterococci achieved 80% eradication and 100% symptom elimination in 20 days [183].
In addition, EVs derived from probiotics are also emerging as important mediators of bacteria–host interactions, offering potential therapeutic applications [184]. EVs carry bioactive molecules like proteins, lipids, and RNA that can shape immune responses, affect inflammation, and restore gut microbial homeostasis [185,186,187]. Further studies are needed to optimize their utilization in the clinic. EVs, particularly from Lactobacillus crispatus strains, are shown to modulate immune responses by regulating pro-inflammatory and anti-inflammatory cytokines [188]. For instance, EVs from L. crispatus significantly reduce pro-inflammatory cytokines IL-1β, IL-6, IL-8, and TNF-α while elevating the levels of anti-inflammatory cytokines IL-10 and TGF-β. Immunomodulation by this action averts excessive inflammatory reaction and tissue damage caused by H. pylori and, thereby, reduces gastritis and potentially improves healing in gastric epithelial cells [189]. Probiotic EVs are also involved in the maintenance of gastric and intestinal barrier function, an important determinant of gastrointestinal mucosa safety against pathogen invasion. EVs from Lacticaseibacillus species, such as L. casei and L. rhamnosus, contain proteins like p40 and p75 that prevent epithelial cell apoptosis and maintain tight junctions [190]. These proteins are responsible for keeping the mucosal surface intact and preventing pathogen-caused damage, thereby developing a protective barrier against H. pylori colonization [191]. Probiotic EVs can carry bacteriocins that target and inhibit pathogenic bacteria. While direct evidence of bacteriocins from probiotic EVs inhibiting H. pylori is still being explored, the antimicrobial properties of these molecules suggest a potential role in reducing bacterial load or preventing colonization [192]. The delivery of these antimicrobial agents via EVs enhances the targeted and sustained delivery to infected areas, such as the gastric mucosa, potentially improving therapeutic outcomes. Probiotic EVs also interact with immune cells, including macrophages. These interactions have the potential to reprogram macrophage polarization into an anti-inflammatory phenotype, enhancing their activity in phagocytosis and killing bacterial pathogens [193]. Such immunomodulation is particularly beneficial in controlling H. pylori infection and reducing the consequential tissue damage, further indicating the potential of probiotic EVs to control the infection and promote healing.
Prebiotics are non-digestible food ingredients that selectively stimulate the growth and activity of beneficial gut bacteria, particularly Bifidobacterium and Lactobacillus species [194] that can produce antimicrobial substances (bacteriocins, lactic acid) that inhibit H. pylori growth. These compounds, primarily oligosaccharides and non-starch polysaccharides, resist digestion in the small intestine and reach the large intestine intact, where they are fermented by beneficial microorganisms [195]. Common prebiotics include fructo-oligosaccharides (FOS), inulin, and galacto-oligosaccharides, which are found in various fruits and vegetables [194]. Prebiotics offer several advantages over probiotics, including lower cost, reduced risk, and easier incorporation into the diet [195]. They are also heat-resistant, allowing them to be used in baked goods and other processed foods [196]. By promoting the growth of beneficial bacteria, prebiotics can improve gut health, enhance resistance to pathogens, and confer various health benefits to the host [197]. Prebiotics stimulate beneficial gut bacteria, leading to increased SCFAs, such as butyrate, which lowers gastric pH and inhibits H. pylori growth. This effect is often associated with the increased population of beneficial bacteria like Lactobacillus and Bifidobacterium, which are stimulated by prebiotics [198], leading to the promotion of balanced gut microbiota. Prebiotics strengthen the mucosal barrier and reduce gastric inflammation [197]. While prebiotics alone are not sufficient enough to eradicate H. pylori, combining probiotics and prebiotics (synbiotics) improves gut microbiota and immune response, making it more effective than probiotics alone for supporting eradication therapy, but more robust studies are needed for definitive confirmation [199]. For an example, the combination of probiotics and prebiotics like butyric acid and inulin has also shown promise in reducing adverse events during H. pylori eradication treatment [197,198,199,200].
Similarly, FMT has shown promise in restoring microbiota composition and function by replenishing lost microbial diversity [201]. However, its role as a prophylactic measure to prevent bacterial infections in high-risk individuals remains under investigation. A pilot study on washed microbiota transplantation (WMT), another type of FMT for H. pylori eradication, showed a 40.6% eradication rate in patients. The study found that a higher pre-WMT PGR (probiotic growth ratio) was associated with a greater likelihood of successful eradication [202]. In a randomized controlled trial, patients receiving standard bismuth quadruple therapy (BQT) followed by a single FMT dose showed alleviation of gastrointestinal symptoms such as diarrhea compared to the placebo, although FMT did not significantly accelerate gut microbiota restoration. Post-antibiotic FMT can improve patient comfort and potentially reduce adverse effects associated with antibiotic regimens for H. pylori eradication [203]. Safety is a critical aspect of FMT, with potential risks including pathogen transmission from donors. Rigorous screening processes and large randomized controlled trials are requisite to confirm efficacy, refine protocols, and clarify mechanisms through which FMT cures H. pylori infection [204]. Adding FMT to conventional eradication therapy could enhance overall treatment success and counteract antibiotic-associated dysbiosis and resistance. Post-eradication management should not be overlooked; it should include non-invasive confirmatory testing (urea breath test or stool antigen test) to verify successful eradication, along with short-term microbiome restoration strategies such as targeted probiotic supplementation to mitigate lingering dysbiosis and reduce recurrence risk.
Probiotics, particularly Lactobacillus, Bifidobacterium, and Saccharomyces boulardii, have been extensively studied for adjunctive therapy in H. pylori eradication treatment. A meta-analysis of 45 randomized controlled trials in about 7000 patients showed that probiotic supplementation increased eradication rates by approximately 13% and significantly reduced common gastrointestinal side effects like diarrhea, nausea, and bloating (relative risk ~0.59) [205]. A further meta-analysis of networks encompassing 5792 patients reinforced these findings, confirming a statistically significant increase in eradication outcomes with the incorporation of probiotics. S. boulardii, in specific, has been shown to be quite effective in reducing treatment side effects and to modestly improve eradication when supplemented with routine triple therapy [206]. Preclinical studies further corroborate these findings because in vitro and animal models have proven that probiotic strains have efficacy in enhancing gut barrier function, influencing mucosal immunity, producing antimicrobial substances such as lactic acid and bacteriocins, and competing with H. pylori adhesion to gastric mucosa, which results in reduced bacterial burden and inflammation [207]. Autoprobiotics represent a new personalized treatment, with early clinical trials showing promising results. For instance, a pilot study of indigenous Enterococcus faecium achieved an 80% eradication rate and symptom improvement in H. pylori-associated chronic gastritis patients, although these results need to be confirmed by large-scale, controlled clinical trials [208]. Autoprobiotics, using animal models, have been found to have the ability to rapidly reconstitute the microbiota community following antibiotic-induced dysbiosis and confer anti-inflammatory activity. Another new approach is employing probiotic-derived EVs, but their clinical application is yet in its early stages. To date, no human clinical trials have assessed the efficacy of these EVs on H. pylori therapy. Preclinical evidence shows that EVs from species like Lactobacillus crispatus can modulate the inflammatory response, enhance the integrity of epithelial barriers by p40/p75 proteins, and regulate cytokine production, IL-1β, IL-6, IL-8, and TNF-α suppression, and stimulation of IL-10 and TGF-β, highlighting their immune-modulating nanocarrier properties. Prebiotics such as non-digestible fibers such as fructo-oligosaccharides (FOS), inulin, and galacto-oligosaccharides have limited clinical evidence on their own but are well established in preclinical studies for their ability to selectively stimulate the growth of beneficial microbes such as Lactobacillus and Bifidobacterium, enhance short-chain fatty acid production (butyrate), and promote mucosal and immune health [209]. Synbiotics, the combination of prebiotics and probiotics, are synergistically useful and have been shown to be clinically associated with reduced inflammation, enhanced mucosal immunity, and augmentation in numbers of health-promoting gut bacteria [210]. These are, however, formulation-dependent, and, more importantly against H. pylori, very few such trials have been conducted. FMT is established for recurrent Clostridioides difficile infection but remains under investigation in its application in the eradication of H. pylori. A pilot study using washed microbiota transplantation (WMT) reported a 40.6% rate of eradication and remission of symptoms but did not significantly accelerate the reconstitution of the microbiome compared to controls [211]. Preclinical models in animals have shown that FMT can reproducibly reconstitute microbial diversity and alleviate dysbiosis, holding promise for its future use as a microbiota-sparing intervention. All these microbiome therapies represent an intriguing future prospect for H. pylori management, with a progressively defined spectrum of clinical maturity, from well-established probiotics to new probiotic-derived EVs, and they emphasize the need for further randomized controlled trials to refine their roles and optimize incorporation into current treatment regimens. Table 5 shows the potential treatments for microbiota recovery after H. pylori infection.

Table 5.
Summary of microbiota recovery strategies after H. pylori infection.
8. Emerging Non-Antibiotic Therapies
Phage therapy has emerged as a promising alternative treatment for H. pylori infections, especially given the increasing antibiotic resistance of the bacterium, which affects over half of the global population and is associated with several gastric diseases [212]. The use of bacteriophages, particularly lytic phages, offers several advantages over traditional antibiotic treatments, including high specificity, safety, and the ability to rapidly adapt to bacterial resistance [213]. A study demonstrated that phages could resist simulated gastric juice when carried by H. pylori, making them a feasible treatment option and able to retain their ability to infect and destroy H. pylori cells [214]. Combining H. pylori phages with protective carriers like lactoferrin adsorbed on hydroxyapatite nanoparticles has been shown to enhance phage antibacterial activity and protect phages from gastric acidity [215]. Despite these promising findings, there is a lack of comprehensive phage collections, and phage resistance is a concern. Additionally, further research is needed to identify and characterize more lytic phages for use in therapy, as many studies have focused on the potential of prophages, which do not have the same therapeutic applications as bacteriophages of H. pylori.
Photodynamic therapy (PDT) has emerged as a promising alternative treatment for H. pylori infections, addressing the growing concern of antibiotic resistance, which utilizes the photosensitizing agents and light to generate reactive oxygen species that inactivate bacteria [216]. In vitro studies demonstrated that violet light (405 nm) alone was particularly effective in eradicating H. pylori, even without the use of added photosensitizers. This suggests that light-induced PDT can be a non-invasive, powerful treatment option [217]. Clinical trials have shown that PDT can reduce H. pylori load in the stomach, with the greatest effects observed in the antrum, the part of the stomach most commonly affected by H. pylori [218]. New developments, such as ingestible PDT capsules, have significantly improved the delivery of PDT to treat H. pylori infections, with some studies reporting up to 96% bacterial killing efficiency [219]. However, further research is required to optimize PDT protocols, refine safety, and establish its long-term efficacy as a mainstream treatment option for H. pylori eradication [220].
Despite decades of research, a widely available vaccine for H. pylori remains elusive, although significant progress has been made. The most impressive achievement was the development of an oral recombinant vaccine in China, which was approximately 72% effective against natural H. pylori infection in children in a Phase 3 clinical trial [221]. The vaccine employed the urease subunit B (UreB) with the heat-labile enterotoxin B subunit (LTB) as a mucosal adjuvant. Despite being successful, its development was ceased, showing the complex path from clinical usefulness to commercial success [222]. Many other contenders have emerged in preclinical phases. Multi-antigenic and multi-epitope vaccines such as CTB-multiHp have a strong immune reactivity and are being considered further for their protective function [223]. A whole-cell H. pylori inactivated vaccine combined with a non-toxic mucosal adjuvant (mmCT) significantly reduced bacterial colonization in mouse models [224]. Similarly, a multivalent epitope-based vaccine (CWAE) targeting heterogeneous H. pylori antigens exhibited therapeutic effects in Mongolian gerbils via the inhibition of colonization and robust immune responses [225]. Another promising candidate, Epivac, a protein CD4+ T cell epitope-based vaccine containing H. pylori protein, induced Th1-skewed immune responses and provided protection in mice [226]. In addition to classic antigens such as urease, VacA, and CagA, newer strategies are examining immune evasion targets such as γ-glutamyltranspeptidase (GGT) to make vaccines more effective. Advances in immunoinformatics and molecular docking have enabled the design of non-allergenic, stable multi-epitope constructs that can stimulate immune receptors like Toll-like receptors (TLRs) 2 and 4 [227]. The majority of these candidates, though, are effective in animal models but pose a difficult task to translate preclinical success into an effective human vaccine. The absence of natural sterilizing immunity, early age of acquisition, and H. pylori’s strategies for evading the host’s immune response all add to the challenge. However, with novel adjuvants, nucleic acid platforms (DNA and mRNA vaccines), and artificial intelligence-informed design tools, it is now more feasible to construct a safe, effective, and universally accessible H. pylori vaccine. According to experts’ estimates, the vaccine will be available within 8–10 years, only if research is funded regularly and it is kept on the top of the world’s health agenda. Ongoing research focuses on identifying protective immune mechanisms and optimizing vaccine formulations for broad protection. Figure 3 shows the overall available and potential treatments to treat the infection.
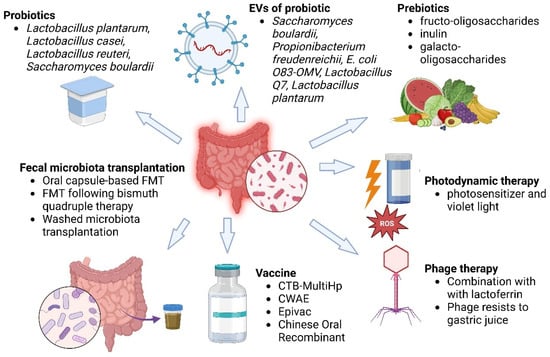
Figure 3.
The various treatments against H. pylori. This figure shows some of the new strategies against H. pylori by microbiota modulation and adjunct therapies. The key strategies are listed as follows: Probiotics (Lactobacillus plantarum, L. casei, L. reuteri, Saccharomyces boulardii) are used to inhibit H. pylori colonization and gut health. EVs of probiotics derived from strains like S. boulardii, Propionibacterium freudenreichii, and L. plantarum can have antimicrobial and immunomodulatory activities. Prebiotics (fructo-oligosaccharides, inulin, galacto-oligosaccharides) enhance the growth of favourable flora. FMT via oral capsules or post-bismuth treatment restores gut microbial homeostasis. Vaccines (CTB-MultiHp, CWAE, Epivac, Chinese oral recombinant vaccines) are under development for H. pylori prevention. Photodynamic treatment with a photosensitizer and violet light can generate ROS and eliminate H. pylori. Phage therapy, often in combination with lactoferrin, can be used for H. pylori with phages that are resistant to gastric acidity. All these strategies reflect a multidisciplinary approach to combat antibiotic resistance and promote effective H. pylori eradication.
9. Natural Products as Promising Alternative Agents for H. pylori Eradication
Natural products have emerged as a promising multi-targeted strategy to combat H. pylori, especially in the context of rising antibiotic resistance, biofilm-associated persistence, and virulence-driven inflammation. By targeting many pathways, several phytochemicals including phenols, terpenoids, flavonoids, alkaloids, and bee-derived molecules have shown strong anti-H. pylori action [228,229,230].
Particularly, biofilms shield H. pylori from host immune clearance and antibiotics, and natural products have demonstrated significant potential in disrupting such structures. Several natural molecules have shown promising antibiofilm activity through different mechanisms. Eugenol, a phenolic component of clove oil, destabilizes bacterial membranes, quenches quorum sensing, and downregulates key virulence genes such as cagA and vacA [228]. Terpenoids thymol and carvacrol, present in Thymus kotschyanus and Origanum vulgare, respectively, destabilize membranes, inhibit ATP synthesis, and inhibit biofilm maturation [229]. Myricetin and curcumin are polyphenols with potent antibiofilm activities that compromise urease activity, enhance immune recognition, and modulate NF-κB and MAPK inflammatory pathways [230]. Cumin, rosemary, cinnamon, and ylang-ylang essential oils (EQs) are a good number of oils from plants that interfere with H. pylori biofilms by disrupting adhesion and matrix stability [231]. Ginger extract at low concentrations inhibits biofilm formation by over 90% and possesses antibacterial and anti-inflammatory activities [231]. Aloe-emodin targets outer membrane proteins that are essential for the architecture of biofilms, induces oxidative stress, and destabilizes established biofilms [232]. Green tea coumarins (Camellia sinensis) suppress bacterial adhesion, DNA gyrase, and quorum sensing signals [233]. These compounds target by multiple mechanisms that encompass membrane disruption, quorum sensing inhibition, urease inhibition, silencing virulence genes, and immunomodulation, with the additional advantage of synergizing with conventional antibiotics [234,235]. Together, natural products represent a multimodal approach to the therapy of H. pylori infection, including biofilm-related resistance, and a compelling platform for adjunctive or alternative treatment.
Natural products also act as inhibitors of urease for H. pylori. The urease enzyme is central to the pathogenicity and survival of H. pylori by hydrolyzing urea into ammonia and carbon dioxide, which in turn neutralizes stomach acid and supports the colonization of the acidic stomach lining [144]. Urease is also involved in mucosal inflammation, immune evasion, and epithelial injury. The inhibition of urease is hence a powerful strategy against H. pylori infection, that is, one which is specific to antibiotic resistance [236]. Natural compounds with diverse structures, for example, flavonoids, polyphenols, coumarins, and phytochemicals, have been found to possess remarkable urease inhibitory activity with diverse mechanisms. The most promising inhibitors of urease are flavonoids and polyphenols, which are well known to exhibit antioxidative and antimicrobial activity, and they also possess in vitro urease inhibitory effects, with IC50 values ranging from approximately 11 to 100 µM [237]. Genistein, an isoflavone, a member of the Fabaceae plant, exhibits the 50% inhibition of urease at 430 µg/mL, whereas baicalin and scutellarin, flavone glucuronides of Scutellaria baicalensis and Erigeron breviscapus, have been found to chelate directly to the active sites of the enzyme [238]. Particularly notable are methyl gallate and penta-O-galloyl-β-D-glucoside (PGG) from Paeonia lactiflora roots, whose activity is almost as effective as the established synthetic urease inhibitor acetohydroxamic acid [239]. Certain plant extracts have also been examined for anti-urease activity. Methanolic extracts of Camellia sinensis (green tea), rich in epigallocatechin gallate (EGCG) and other related catechins, have very high urease inhibitory activity in vitro, with an IC50 of around 13 µM [240]. Other phenolic-rich plant extracts of Punica granatum (pomegranate), Origanum vulgare (oregano), Vaccinium macrocarpon (cranberry), Matricaria recutita (chamomile), and Nasturtium officinale (watercress) have also exhibited significant urease inhibition, largely as a result of their presence of flavonoids and tannins [241]. Ginkgo biloba extract showed very good efficacy with an IC50 of 36.17 µg/mL, which was better than the positive control hydroxyurea and Rhus coriaria (sumac) extract inhibited at 80.29 µg/mL, consistent with its ancient antimicrobial use in gastrointestinal disease [242]. Zerumbone, a sesquiterpene derived from Zingiber zerumbet (wild ginger), has also inhibited H. pylori urease activity effectively, but its precise IC50 is not given [243]. Mechanistically, naturally occurring urease inhibitors function primarily by binding the nickel-containing catalytic site of the enzyme or with sulfhydryl (-SH) groups of cysteine residues, obstructing substrate access and enzymatic activity. Flavonoids and polyphenols predominantly are metal-chelating agents that sequester the essential metal ions in the active site. Other compounds suppress urease gene expression or disrupt the quaternary structure of the enzyme, contributing to its dysfunction. Through this, these agents debilitate the ability of the bacterium to resist gastric acid, reduce colonization competence, and enhance vulnerability to immune clearance and antibiotics [244]. The large diversity of natural compounds with urease inhibitory activity emphasizes their therapeutic potential. They can also be applied as adjuvants to conventional triple or quadruple therapy, potentially lowering the doses of antibiotics needed and reducing resistance [245]. Moreover, their immunomodulatory as well as antioxidant activities may help in mucosal healing and resolving inflammation.
In terms of membrane-targeting activity, natural compounds such as coptisine (from Rhizoma coptidis) compromise H. pylori viability by exteriorizing phosphatidylserine, inducing DNA fragmentation, and damaging virulence factor expression [246]. Similarly, sanguinarine (from Zanthoxylum nitidum) causes cell lysis and urease inhibition, while squalamine, a steroidal alkaloid, compromises membrane integrity and increases antibiotic permeability [247]. Piperine interferes with motility and adhesion, impairing the colonizing capacity of the bacterium. Terpenoid-containing EQs such as thymol, carvacrol, and eugenol interact with lipid bilayers, permeabilize, and interfere with membrane-bound processes such as efflux and virulence [248]. Phytochemicals such as kaempferol, tellimagrandin I, curcumin, and propolis-derived flavonoids also destabilize membrane integrity. Aloe-emodin and ginger phenolics also play a role in membrane dysfunction, proving to be valuable adjuvants in the eradication of drug-resistant H. pylori strains [249].
Several plant extracts and purified molecules have been found to demonstrate quorum-sensing inhibitory activity against H. pylori. MeMe of Chelidonium majus and Corydalis cheilanthifolia has shown significant in vitro QS inhibition against a number of H. pylori strains [250]. Likewise, Atractylodes lancea volatile oils and Pistacia vera oleoresins have shown in vitro and in vivo anti-QS activity and are thus potential systemic anti-virulence agents [251]. In silico docking studies have identified a series of bioactive phytochemicals that include β-carotene, β-amyrin, taraxasterol, bauerenol, taraxacin, and benzoyl peroxide as highly potent binders to the H. pylori chemoreceptor TlpB, the central sensor of the AI-2-mediated QS system. The binding of these molecules to TlpB effectively inhibits the perception of AI-2, which in turn affects signal transduction, gene regulation, and ensuing pathogenic processes [252,253]. These compounds also bear promising pharmacological attributes like low toxicity and good bioavailability in model systems. EQs and their major constituents are also another vital category of natural QS inhibitors. The EOs of essential herbs of the Lamiaceae family, including Origanum vulgare (oregano), Thymus vulgaris (thyme), and Rosmarinus officinalis (rosemary), have exhibited significant anti-QS and antibiofilm activities against H. pylori and related pathogens [254]. Key EO constituents such as carvacrol, thymol, eugenol, linalool, limonene, γ-terpinene, and 1,8-cineole disrupt QS-regulated behavior by inhibiting AI-2 synthesis, transport, or binding to receptors. Lipophilic molecules such as these can diffuse through bacterial membranes, interfere with signaling proteins, and rewire gene expression patterns governing adhesion, motility, and toxin production [255].
Flavonoids and furanones have also been found to have strong QS inhibitory activity. While certain flavonoids such as H. pylori QS are likewise yet to be well understood, their broad-spectrum QS inhibition in other models of bacteria suggests similar modes of action. These compounds typically act by competing with AI-2 at the receptor level, blocking signal perception, or inhibiting LuxS-dependent AI-2 biosynthesis [256]. Synthetic furanones that mimicked natural halogenated furanones of marine algae were well documented to inhibit QS in Gram-negative bacteria by facilitating the degradation of signal receptors or the simulation of signal molecules to competitively inhibit binding [257].
The inhibition of virulence gene expression in H. pylori is an excellent method used to combat its pathogenicity, limit tissue damage, and increase eradication rates. Virulence factors such as CagA, VacA, urease, outer membrane adhesins, and biofilm-related genes play a key role in H. pylori’s ability to colonize the gastric mucosa, evade immune detection, and induce chronic inflammation. A wide range of natural products, particularly alkaloids, polyphenols, flavonoids, terpenoids, and bee-derived compounds, have demonstrated the capacity to suppress these virulence-related genes and pathways through multiple mechanisms [258]. Eugenol not only exhibits strong antibiofilm and membrane-disruptive properties but also downregulates the expression of key virulence genes, attenuating the ability of H. pylori to establish persistent infection [259]. Similarly, terpenoids such as thymol and carvacrol, respectively, interfere with membrane integrity and pH homeostasis, thus indirectly inhibiting the expression of virulence genes related to biofilm and adhesion. Alkaloids such as coptisin (from Rhizoma coptidis) have also been found to suppress the expression of the Cag pathogenicity island. Sanguinarine, which is another Zanthoxylum nitidum alkaloid, exhibits urease inhibition and cell lysis, contributing to diminished virulence expression and bacterial burden [260]. Among the most active polyphenols and flavonoids, kaempferol inhibits bacterial membrane function, which inhibits virulence factor functionality. Chalcone derivatives, from plant families like Leguminosae, Asteraceae, and Moraceae, inhibit H. pylori motility, adhesion, and urease activity, thereby downregulating virulence at structural and enzymatic levels [261]. Myricetin, from Myrica species, improves the immune recognition of H. pylori, sensitizes to antibiotics, and reduces biofilm formation, all of which are linked with suppressed virulence gene expression. Curcumin is also well known for urease inhibition and NF-κB inhibition, diminishing inflammation and indirectly suppressing the synthesis of virulence factors. Apigenin, a flavonoid in celery and parsley, also disrupts NF-κB pathways, thereby inhibiting the inflammatory damage caused by H. pylori virulence action [262]. Another important class includes bee-derived products, specifically propolis, which is rich in polyphenolic compounds like caffeic acid phenethyl ester (CAPE). CAPE has been shown to inhibit the bacterial peptide deformylase enzyme required for protein maturation, thus impacting H. pylori protein expression, including virulence-related proteins [263]. Moreover, extracts of Acacia nilotica, Calotropis procera, and Geranium wilfordii plants have been reported to display anti-urease and anti-adhesive activity, with evidence pointing towards their potential wider role of modulating virulence-associated gene networks [264]. In summary, natural products exert strong anti-H. pylori properties via a constellation of mechanisms, including biofilm disruption, urease inhibition, membrane weakening, virulence gene silencing, and quorum sensing blocking. Their multitarget activity, low resistance potential, and synergy with antibiotics underscore their value as adjunctive or alternative therapeutics. This integrated phytochemical approach, supported by both experimental research and mechanistic insight, paves the way for new strategies to cure H. pylori infection, especially those complicated by resistance or recurrence.
10. Conclusions and Future Perspective
The successful eradication of H. pylori in the era of rising antimicrobial resistance requires a multifaceted, stepwise strategy. Clinicians should first consider region-specific antibiotic resistance patterns, prioritizing bismuth quadruple therapy in high clarithromycin resistance settings (>15%) and using tailored triple therapy where resistance remains low. The adjunctive use of probiotics, particularly strains like Lactobacillus plantarum, Saccharomyces boulardii, or multi-strain formulations, can enhance eradication rates and reduce gastrointestinal side effects, thereby improving adherence. In refractory or recurrent infections, combining antibiotics with biofilm-disrupting agents such as N-acetylcysteine or natural compounds (curcumin, berberine) may help overcome biofilm-associated persistence. For multidrug-resistant cases, emerging alternatives like AMPs, nanoparticle-mediated drug delivery systems, or phage therapy offer promising avenues, although further clinical validation is required. Long-term success will depend on the development of vaccines and microbiota-sparing interventions such as autoprobiotics, probiotic-derived EVs, and FMT to support microbiome recovery and reduce antibiotic-induced side effects. This also warrants further exploration through rigorously designed randomized trials. Furthermore, real-world clinical data reinforce the utility of microbiota-targeted adjuncts. For example, probiotic–antibiotic co-administration has been shown in multiple trials to reduce adverse events such as diarrhea, nausea, and bloating by 30–40%, thereby improving patient tolerance and adherence. Pretreatment resistance testing, especially in high-burden regions, remains crucial for guiding optimal therapy selection. Patient education should also be emphasized to improve compliance with complex multidrug regimens. In resource-limited settings, low-cost adjuncts such as standardized garlic or ginger extracts may serve as practical and accessible options to support eradication efforts. Lastly, integrating multi-omics approaches (metagenomics, transcriptomics, proteomics) may help to identify predictive biomarkers of treatment response and enable precision-guided interventions tailored to the individual patient’s microbiota and immune landscape. In addition, nanoparticle-based drug delivery systems and vaccines targeting H. pylori antigens hold even more promise in the fight against this devious pathogen. The future of H. pylori therapy is a part of an interdisciplinary approach involving alternative therapies, precision medicine, and enhanced antibiotic stewardship in addressing the challenge of resistance. Research into the role of microbiota in the dynamics of infection and the optimization of non-antibiotic treatment will be vital in overcoming the limitations of current treatment modalities. Finally, a more complete and individualized therapy regimen will be essential in controlling H. pylori infections and in diminishing the threat of gastric cancers and other such illnesses. As the resistance to antibiotics continues to climb worldwide, novel and adjunct treatments will increasingly be needed. Developing personalized, microbiota-tolerant, and sustainable treatment choices will be the key to the future of H. pylori treatment and worldwide efforts to counter antibiotic resistance. Figure 4 explains a stepwise algorithm integrating diagnosis, resistance-guided treatment, adjunctive support, and long-term microbiome recovery.
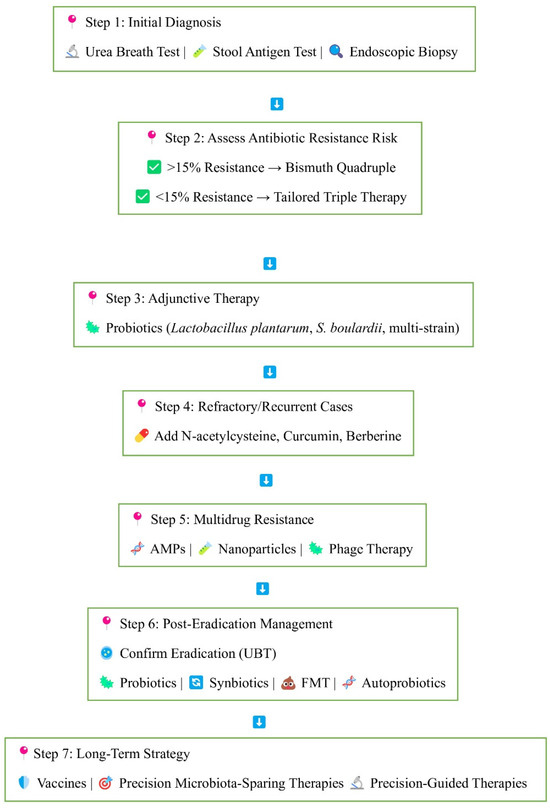
Figure 4.
Stepwise approach to H. pylori eradication and microbiota recovery. The flowchart illustrates a comprehensive approach to H. pylori management. Step 1: Initial non-invasive (urea breath test, stool antigen test) or invasive (endoscopic biopsy) diagnosis. Step 2: Treatment is selected on the basis of local antibiotic resistance prevalence: Bismuth quadruple therapy for resistance >15%, tailored triple therapy for resistance <15%. Step 3: Probiotic adjunctive therapy (Lactobacillus plantarum, S. boulardii) may enhance efficacy and reduce side effects. Step 4: For treatment-resistant or recurrent infection, additional agents such as N-acetylcysteine, curcumin, and berberine are used. Step 5: For multidrug resistance, newer strategies include antimicrobial peptides (AMPs), nano-particles, and phage therapy. Step 6: Post-eradication confirmation by UBT is important, followed by the restoration of gut microbiota by probiotics, synbiotics, fecal microbiota transplantation (FMT), or autoprobiotics. Step 7: Long-term strategies focus on preventive and precision strategies such as vaccines, micro-biota-sparing therapy, and precision-guided therapies.
Author Contributions
B.M., W.W., A.S. and A.H.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing. A.H.: conception and design, assembly of data, data analysis, final approval of manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by research grants from the Centre for Research and Instrumentation Management (CRIM), Universiti Kebangsaan Malaysia (Grant ID: DIP-2024-005). The funder had no role in the manuscript submission and publication.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Elbehiry, A.; Marzouk, E.; Aldubaib, M.; Abalkhail, A.; Anagreyyah, S.; Anajirih, N.; Almuzaini, A.M.; Rawway, M.; Alfadhel, A.; Draz, A.; et al. Helicobacter pylori Infection: Current Status and Future Prospects on Diagnostic, Therapeutic and Control Challenges. Antibiotics 2023, 12, 191. [Google Scholar] [CrossRef] [PubMed]
- Salih, B.A. Helicobacter pylori infection in developing countries: The burden for how long? Saudi J. Gastroenterol. 2009, 15, 201–207. [Google Scholar] [CrossRef]
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Shirani, M.; Pakzad, R.; Haddadi, M.H.; Akrami, S.; Asadi, A.; Kazemian, H.; Moradi, M.; Kaviar, V.H.; Zomorodi, A.R.; Khoshnood, S.; et al. The global prevalence of gastric cancer in Helicobacter pylori-infected individuals: A systematic review and meta-analysis. BMC Infect. Dis. 2023, 23, 543. [Google Scholar] [CrossRef]
- Chen, Y.C.; Malfertheiner, P.; Yu, H.T.; Kuo, C.L.; Chang, Y.Y.; Meng, F.T.; Wu, Y.X.; Hsiao, J.L.; Chen, M.J.; Lin, K.P.; et al. Global Prevalence of Helicobacter pylori Infection and Incidence of Gastric Cancer Between 1980 and 2022. Gastroenterology 2024, 166, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Camargo, M.C.; El-Omar, E.; Liou, J.M.; Peek, R.; Schulz, C.; Smith, S.I.; Suerbaum, S. Helicobacter pylori infection. Nat. Rev. Dis. Primers 2023, 9, 19. [Google Scholar] [CrossRef]
- Nguyen, J.; Kotilea, K.; Bontems, P.; Miendje Deyi, V.Y. Helicobacter pylori Infections in Children. Antibiotics 2023, 12, 1440. [Google Scholar] [CrossRef]
- Reyes, V.E. Helicobacter pylori and Its Role in Gastric Cancer. Microorganisms 2023, 11, 1312. [Google Scholar] [CrossRef]
- Patel, S.K.; Pratap, C.B.; Jain, A.K.; Gulati, A.K.; Nath, G. Diagnosis of Helicobacter pylori: What should be the gold standard? World J. Gastroenterol. 2014, 20, 12847–12859. [Google Scholar] [CrossRef]
- Talebi Bezmin Abadi, A. Diagnosis of Helicobacter pylori Using Invasive and Noninvasive Approaches. J. Pathog. 2018, 2018, 9064952. [Google Scholar] [CrossRef]
- Godavarthy, P.K.; Puli, C. From Antibiotic Resistance to Antibiotic Renaissance: A New Era in Helicobacter pylori Treatment. Cureus 2023, 15, e36041. [Google Scholar] [CrossRef] [PubMed]
- Gugnani, J.S.; Abhishek, F.; Agarwal, Y.; Damera, A.R.; Kaur, H.; Taleb, B.; Mane, R.; Soni, U.; Nayar, K.D. Effectiveness of Rifabutin-Based Regimens in Treating Helicobacter pylori Infections. Cureus 2023, 15, e50541. [Google Scholar] [CrossRef] [PubMed]
- Begg, M.; Tarhuni, M.; N Fotso, M.; Gonzalez, N.A.; Sanivarapu, R.R.; Osman, U.; Latha Kumar, A.; Sadagopan, A.; Mahmoud, A.; Khan, S. Comparing the Safety and Efficacy of Proton Pump Inhibitors and Histamine-2 Receptor Antagonists in the Management of Patients with Peptic Ulcer Disease: A Systematic Review. Cureus 2023, 15, e44341. [Google Scholar] [CrossRef]
- Gisbert, J.P. Rifabutin for the Treatment of Helicobacter pylori Infection: A Review. Pathogens 2020, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xue, J.; Lin, F.; Liu, D.; Zhang, W.; Ru, S.; Jiang, F. Global Primary Antibiotic Resistance Rate of Helicobacter pylori in Recent 10 years: A Systematic Review and Meta-Analysis. Helicobacter 2024, 29, e13103. [Google Scholar] [CrossRef]
- Dascălu, R.I.; Bolocan, A.; Păduaru, D.N.; Constantinescu, A.; Mitache, M.M.; Stoica, A.D.; Andronic, O. Multidrug resistance in Helicobacter pylori infection. Front. Microbiol. 2023, 14, 1128497. [Google Scholar] [CrossRef]
- Attaran, B.; Salehi, N.; Ghadiri, B.; Esmaeili, M.; Kalateh, S.; Tashakoripour, M.; Eshagh Hosseini, M.; Mohammadi, M. The penicillin binding protein 1A of Helicobacter pylori, its amoxicillin binding site and access routes. Gut Pathog. 2021, 13, 43. [Google Scholar] [CrossRef]
- Boyanova, L.; Hadzhiyski, P.; Gergova, R.; Markovska, R. Evolution of Helicobacter pylori Resistance to Antibiotics: A Topic of Increasing Concern. Antibiotics 2023, 12, 332. [Google Scholar] [CrossRef]
- Lin, Y.; Shao, Y.; Yan, J.; Ye, G. Antibiotic resistance in Helicobacter pylori: From potential biomolecular mechanisms to clinical practice. J. Clin. Lab. Anal. 2023, 37, e24885. [Google Scholar] [CrossRef]
- Thung, I.; Aramin, H.; Vavinskaya, V.; Gupta, S.; Park, J.Y.; Crowe, S.E.; Valasek, M.A. Review article: The global emergence of Helicobacter pylori antibiotic resistance. Aliment. Pharmacol. Ther. 2016, 43, 514–533. [Google Scholar] [CrossRef]
- Jenks, P.J.; Edwards, D.I. Metronidazole resistance in Helicobacter pylori. Int. J. Antimicrob. Agents 2002, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Krzyżek, P. Helicobacter pylori Efflux Pumps: A Double-Edged Sword in Antibiotic Resistance and Biofilm Formation. Int. J. Mol. Sci. 2024, 25, 12222. [Google Scholar] [CrossRef]
- Savoldi, A.; Carrara, E.; Graham, D.Y.; Conti, M.; Tacconelli, E. Prevalence of Antibiotic Resistance in Helicobacter pylori: A Systematic Review and Meta-analysis in World Health Organization Regions. Gastroenterology 2018, 155, 1372–1382. [Google Scholar] [CrossRef]
- Kocsmár, É.; Buzás, G.M.; Szirtes, I.; Kocsmár, I.; Kramer, Z.; Szijártó, A.; Fadgyas-Freyler, P.; Szénás, K.; Rugge, M.; Fassan, M.; et al. Primary and secondary clarithromycin resistance in Helicobacter pylori and mathematical modeling of the role of macrolides. Nat. Commun. 2021, 12, 2255. [Google Scholar] [CrossRef]
- Albasha, A.M.; Elnosh, M.M.; Osman, E.H.; Zeinalabdin, D.M.; Fadl, A.A.; Ali, M.A.; Altayb, H.N. Helicobacter pylori 23S rRNA gene A2142G 2021, A2143G, T2182C, and C2195T mutations associated with clarithromycin resistance detected in Sudanese patients. BMC Microbiol. 2021, 21, 38. [Google Scholar] [CrossRef]
- Gaurav, A.; Bakht, P.; Saini, M.; Pandey, S.; Pathania, R. Role of bacterial efflux pumps in antibiotic resistance, virulence, and strategies to discover novel efflux pump inhibitors. Microbiology 2023, 169, 001333. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.R.; Wang, X.J.; Zhu, M.J.; Chen, A.L.; Zhang, D.; Du, Q.; Kim, J.J.; Hu, W.L. Efficacy and safety of low-dose tetracycline, amoxicillin quadruple therapy in Helicobacter pylori infection: A retrospective single center study. World J. Gastroenterol. 2024, 30, 4295–4304. [Google Scholar] [CrossRef] [PubMed]
- Dailidiene, D.; Bertoli, M.T.; Miciuleviciene, J.; Mukhopadhyay, A.K.; Dailide, G.; Pascasio, M.A.; Kupcinskas, L.; Berg, D.E. Emergence of tetracycline resistance in Helicobacter pylori: Multiple mutational changes in 16S ribosomal DNA and other genetic loci. Antimicrob. Agents Chemother. 2002, 46, 3940–3946. [Google Scholar] [CrossRef]
- Ribeiro, M.L.; Gerrits, M.M.; Benvengo, Y.H.; Berning, M.; Godoy, A.P.; Kuipers, E.J.; Mendonça, S.; van Vliet, A.H.; Pedrazzoli, J., Jr.; Kusters, J.G. Detection of high-level tetracycline resistance in clinical isolates of Helicobacter pylori using PCR-RFLP. FEMS Immunol. Med. Microbiol. 2004, 40, 57–61. [Google Scholar] [CrossRef]
- Minarini, L.A.; Darini, A.L. Mutations in the quinolone resistance-determining regions of gyrA and parC in Enterobacteriaceae isolates from Brazil. Braz. J. Microbiol. 2012, 43, 1309–1314. [Google Scholar] [CrossRef]
- Miftahussurur, M.; Shrestha, P.K.; Subsomwong, P.; Sharma, R.P.; Yamaoka, Y. Emerging Helicobacter pylori levofloxacin resistance and novel genetic mutation in Nepal. BMC Microbiol. 2016, 16, 256. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.; Cheng, H.; Hu, F.L.; Li, J. Distribution of gyrA mutations in fluoroquinolone-resistant Helicobacter pylori strains. World J. Gastroenterol. 2010, 16, 2272–2277. [Google Scholar] [CrossRef] [PubMed]
- Parra-Sepúlveda, C.; Merino, J.S.; Sáez-Carrillo, K.; González, C.; García-Cancino, A. Antibiotic resistance surveillance of Helicobacter pylori at the Biobío region (Chile) in a decade. Arq. Gastroenterol. 2019, 56, 361–366. [Google Scholar] [CrossRef]
- Farzi, N.; Yadegar, A.; Sadeghi, A.; Asadzadeh Aghdaei, H.; Marian Smith, S.; Raymond, J.; Suzuki, H.; Zali, M.R. High Prevalence of Antibiotic Resistance in Iranian Helicobacter pylori Isolates: Importance of Functional and Mutational Analysis of Resistance Genes and Virulence Genotyping. J. Clin. Med. 2019, 8, 2004. [Google Scholar] [CrossRef]
- Subsomwong, P.; Doohan, D.; Fauzia, K.A.; Akada, J.; Matsumoto, T.; Yee, T.T.; Htet, K.; Waskito, L.A.; Tuan, V.P.; Uchida, T.; et al. Next-Generation Sequencing-Based Study of Helicobacter pylori Isolates from Myanmar and Their Susceptibility to Antibiotics. Microorganisms 2022, 10, 196. [Google Scholar] [CrossRef] [PubMed]
- Alsohaibani, F.; Al Ashgar, H.; Al Kahtani, K.; Kagevi, I.; Peedikayil, M.; Alfadda, A.; Khan, M. Prospective trial in Saudi Arabia comparing the 14-day standard triple therapy with the 10-day sequential therapy for treatment of Helicobacter pylori infection. Saudi J. Gastroenterol. 2015, 21, 220–225. [Google Scholar] [CrossRef]
- Nista, E.C.; Pellegrino, A.; Giuli, L.; Candelli, M.; Schepis, T.; De Lucia, S.S.; Ojetti, V.; Franceschi, F.; Gasbarrini, A. Clinical Implications of Helicobacter pylori Antibiotic Resistance in Italy: A Review of the Literature. Antibiotics 2022, 11, 1452. [Google Scholar] [CrossRef]
- Sukri, A.; Hanafiah, A.; Yusoff, H.; Shamsul Nizam, N.A.; Nameyrra, Z.; Wong, Z.; Raja Ali, R.A. Multidrug-Resistant Helicobacter pylori Strains: A Five-Year Surveillance Study and Its Genome Characteristics. Antibiotics 2022, 11, 1391. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Yang, F.; Chi, W.; Ding, L.; Liu, T.; Zhu, F.; Ji, D.; Zhou, J.; Fang, Y.; et al. Antimicrobial resistance patterns and genetic elements associated with the antibiotic resistance of Helicobacter pylori strains from Shanghai. Gut Pathog. 2022, 14, 14. [Google Scholar] [CrossRef]
- Hou, C.; Yin, F.; Wang, S.; Zhao, A.; Li, Y.; Liu, Y. Helicobacter pylori Biofilm-Related Drug Resistance and New Developments in Its Anti-Biofilm Agents. Infect. Drug Resist. 2022, 15, 1561–1571. [Google Scholar] [CrossRef]
- Elbehiry, A.; Abalkhail, A.; Anajirih, N.; Alkhamisi, F.; Aldamegh, M.; Alramzi, A.; AlShaqi, R.; Alotaibi, N.; Aljuaid, A.; Alzahrani, H.; et al. Helicobacter pylori: Routes of Infection, Antimicrobial Resistance, and Alternative Therapies as a Means to Develop Infection Control. Diseases 2024, 12, 311. [Google Scholar] [CrossRef] [PubMed]
- Fauzia, K.A.; Effendi, W.I.; Alfaray, R.I.; Malaty, H.M.; Yamaoka, Y.; Mifthussurur, M. Molecular Mechanisms of Biofilm Formation in Helicobacter pylori. Antibiotics 2024, 13, 976. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Xin, S.; Liu, X.; He, C.; Yu, X.; Pan, L.; Zhang, S.; Gao, H.; Xu, J. Multiple biological characteristics and functions of intestinal biofilm extracellular polymers: Friend or foe? Front. Microbiol. 2024, 15, 1445630. [Google Scholar] [CrossRef]
- Hathroubi, S.; Zerebinski, J.; Clarke, A.; Ottemann, K.M. Helicobacter pylori Biofilm Confers Antibiotic Tolerance in Part via A Protein-Dependent Mechanism. Antibiotics 2020, 9, 355. [Google Scholar] [CrossRef]
- Hathroubi, S.; Servetas, S.L.; Windham, I.; Merrell, D.S.; Ottemann, K.M. Helicobacter pylori Biofilm Formation and Its Potential Role in Pathogenesis. Microbiol. Mol. Biol. Rev. 2018, 82, e00001-18. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Suleman, L. Biofilms and Helicobacter pylori: Dissemination and persistence within the environment and host. World J. Gastrointest. Pathophysiol. 2014, 5, 122–132. [Google Scholar] [CrossRef]
- Matos, R.; Amorim, I.; Magalhães, A.; Haesebrouck, F.; Gärtner, F.; Reis, C.A. Adhesion of Helicobacter Species to the Human Gastric Mucosa: A Deep Look into Glycans Role. Front. Mol. Biosci. 2021, 8, 656439. [Google Scholar] [CrossRef]
- Colin, R.; Ni, B.; Laganenka, L.; Sourjik, V. Multiple functions of flagellar motility and chemotaxis in bacterial physiology. FEMS Microbiol. Rev. 2021, 45, fuab038. [Google Scholar] [CrossRef]
- Jarzab, M.; Posselt, G.; Meisner-Kober, N.; Wessler, S. Helicobacter pylori-Derived Outer Membrane Vesicles (OMVs): Role in Bacterial Pathogenesis? Microorganisms 2020, 8, 1328. [Google Scholar] [CrossRef]
- Muiznieks, L.D.; Keeley, F.W. Molecular assembly and mechanical properties of the extracellular matrix: A fibrous protein perspective. Biochim. Biophys. Acta 2013, 1832, 866–875. [Google Scholar] [CrossRef]
- Liu, B.D.; Akbar, R.; Oliverio, A.; Thapa, K.; Wang, X.; Fan, G.C. Bacterial extracellular vesicles in the regulation of inflammatory response and host-microbe interactions. Shock 2024, 61, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, S.; Zhao, J. The role and mechanisms of Helicobacter pylori outer membrane vesicles in the pathogenesis of extra-gastrointestinal diseases. Microb. Pathog. 2025, 200, 107312. [Google Scholar] [CrossRef]
- Gunn, J.S.; Bakaletz, L.O.; Wozniak, D.J. What’s on the Outside Matters: The Role of the Extracellular Polymeric Substance of Gram-negative Biofilms in Evading Host Immunity and as a Target for Therapeutic Intervention. J. Biol. Chem. 2016, 291, 12538–12546. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; You, J.; Yin, S.; Yang, H.; He, S.; Feng, L.; Li, J.; Zhao, Q.; Wei, L. Extracellular polymeric substances-antibiotics interaction in activated sludge: A review. Environ. Sci. Ecotechnol. 2022, 13, 100212. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.K.; Rajpurohit, Y.S. Multitasking functions of bacterial extracellular DNA in biofilms. J. Bacteriol. 2024, 206, e0000624. [Google Scholar] [CrossRef]
- Rader, B.A.; Campagna, S.R.; Semmelhack, M.F.; Bassler, B.L.; Guillemin, K. The quorum-sensing molecule autoinducer 2 regulates motility and flagellar morphogenesis in Helicobacter pylori. J. Bacteriol. 2007, 189, 6109–6117. [Google Scholar] [CrossRef]
- Clyne, M.; Ó Cróinín, T. Pathogenicity and virulence of Helicobacter pylori: A paradigm of chronic infection. Virulence 2024, 16, 2438735. [Google Scholar] [CrossRef]
- Yonezawa, H.; Osaki, T.; Kamiya, S. Biofilm Formation by Helicobacter pylori and Its Involvement for Antibiotic Resistance. BioMed Res. Int. 2015, 2015, 914791. [Google Scholar] [CrossRef]
- Elshenawi, Y.; Hu, S.; Hathroubi, S. Biofilm of Helicobacter pylori: Life Cycle, Features, and Treatment Options. Antibiotics 2023, 12, 1260. [Google Scholar] [CrossRef]
- Guzmán-Soto, I.; McTiernan, C.; Gonzalez-Gomez, M.; Ross, A.; Gupta, K.; Suuronen, E.J.; Mah, T.F.; Griffith, M.; Alarcon, E.I. Mimicking biofilm formation and development: Recent progress in in vitro and in vivo biofilm models. iScience 2021, 24, 102443. [Google Scholar] [CrossRef]
- Hathroubi, S.; Mekni, M.A.; Domenico, P.; Nguyen, D.; Jacques, M. Biofilms: Microbial Shelters Against Antibiotics. Microb. Drug Resist. 2017, 23, 147–156. [Google Scholar] [CrossRef]
- Pai, L.; Patil, S.; Liu, S.; Wen, F. A growing battlefield in the war against biofilm-induced antimicrobial resistance: Insights from reviews on antibiotic resistance. Front. Cell. Infect. Microbiol. 2023, 13, 1327069. [Google Scholar] [CrossRef]
- Zhao, A.; Sun, J.; Liu, Y. Understanding bacterial biofilms: From definition to treatment strategies. Front. Cell. Infect. Microbiol. 2023, 13, 1137947. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Maurya, S.; Verma, H.; Verma, V.K. Unraveling the factors and mechanism involved in persistence: Host-pathogen interactions in Helicobacter pylori. J. Cell. Biochem. 2019, 120, 18572–18587. [Google Scholar] [CrossRef] [PubMed]
- Kolopaking, M.S. Urease, Gastric Bacteria and Gastritis. Acta Medica Indones. 2022, 54, 1–2. [Google Scholar]
- Rumbaugh, K.P.; Sauer, K. Biofilm dispersion. Nat. Rev. Microbiol. 2020, 18, 571–586. [Google Scholar] [CrossRef]
- Coppola, F.; Fratianni, F.; Bianco, V.; Wang, Z.; Pellegrini, M.; Coppola, R.; Nazzaro, F. New Methodologies as Opportunities in the Study of Bacterial Biofilms, Including Food-Related Applications. Microorganisms 2025, 13, 1062. [Google Scholar] [CrossRef]
- Imoto, I.; Oka, S.; Katsurahara, M.; Nakamura, M.; Yasuma, T.; Akada, J.; D’Alessandro-Gabazza, C.N.; Toda, M.; Horiki, N.; Gabazza, E.C.; et al. Helicobacter pylori infection: Is there circulating vacuolating cytotoxin A or cytotoxin-associated gene A protein? Gut Pathog. 2022, 14, 43. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Q.L.; Cheng, D.D.; Xu, W.T.; Lu, N.H. Adhesion and Invasion of Gastric Mucosa Epithelial Cells by Helicobacter pylori. Front. Cell. Infect. Microbiol. 2016, 6, 159. [Google Scholar] [CrossRef]
- Hatakeyama, M. Structure and function of Helicobacter pylori CagA, the first-identified bacterial protein involved in human cancer. Proc. Jpn. Academy. Ser. B Phys. Biol. Sci. 2017, 93, 196–219. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, C.; Zhang, Z.; Zhang, H.; Hu, H. NF-κB signaling in inflammation and cancer. MedComm 2021, 2, 618–653. [Google Scholar] [CrossRef] [PubMed]
- Jarzab, M.; Skorko-Glonek, J. There Are No Insurmountable Barriers: Passage of the Helicobacter pylori VacA Toxin from Bacterial Cytoplasm to Eukaryotic Cell Organelle. Membranes 2024, 14, 11. [Google Scholar] [CrossRef]
- Ricci, V. Relationship Between VacA Toxin and Host Cell Autophagy in Helicobacter pylori Infection of the Human Stomach: A Few Answers, Many Questions. Toxins 2016, 8, 203. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.; Greenfield, L.K.; Bronte-Tinkew, D.; Capurro, M.I.; Rizzuti, D.; Jones, N.L. VacA promotes CagA accumulation in gastric epithelial cells during Helicobacter pylori infection. Sci. Rep. 2019, 9, 38. [Google Scholar] [CrossRef]
- Takahashi-Kanemitsu, A.; Knight, C.T.; Hatakeyama, M. Molecular anatomy and pathogenic actions of Helicobacter pylori CagA that underpin gastric carcinogenesis. Cell. Mol. Immunol. 2020, 17, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Krzyżek, P.; Migdał, P.; Tusiewicz, K.; Zawadzki, M.; Szpot, P. Subinhibitory concentrations of antibiotics affect development and parameters of Helicobacter pylori biofilm. Front. Pharmacol. 2024, 15, 14773. [Google Scholar] [CrossRef]
- Ierardi, E.; Losurdo, G.; Mileti, A.; Paolillo, R.; Giorgio, F.; Principi, M.; Di Leo, A. The Puzzle of Coccoid Forms of Helicobacter pylori: Beyond Basic Science. Antibiotics 2020, 9, 293. [Google Scholar] [CrossRef]
- Abadi, A.T.B. Strategies used by Helicobacter pylori to establish persistent infection. World J. Gastroenterol. 2017, 23, 2870–2882. [Google Scholar] [CrossRef]
- Zhao, X.; Zhong, J.; Wei, C.; Lin, C.W.; Ding, T. Current Perspectives on Viable but Non-culturable State in Foodborne Pathogens. Front. Microbiol. 2017, 8, 580. [Google Scholar] [CrossRef]
- Li, J.; Zheng, T.; Shen, D.; Chen, J.; Pei, X. Research progress in the Helicobacter pylori with viable non-culturable state. J. Cent. South Univ. Med. Sci. 2021, 46, 1423–1429. [Google Scholar] [CrossRef]
- Orta de Velásquez, M.T.; Yáñez Noguez, I.; Casasola Rodríguez, B.; Román Román, P.I. Effects of ozone and chlorine disinfection on VBNC Helicobacter pylori by molecular techniques and FESEM images. Environ. Technol. 2017, 38, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Mahdizade Ari, M.; Scholz, K.J.; Cieplik, F.; Al-Ahmad, A. Viable but non-cultivable state in oral microbiota: A critical review of an underexplored microbial survival strategy. Front. Cell. Infect. Microbiol. 2025, 15, 1533768. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Mendis, N.; Trigui, H.; Oliver, J.D.; Faucher, S.P. The importance of the viable but non-culturable state in human bacterial pathogens. Front. Microbiol. 2014, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Manna, O.M.; Caruso Bavisotto, C.; Gratie, M.I.; Damiani, P.; Tomasello, G.; Cappello, F. The Role of Helicobacter pylori Heat Shock Proteins in Gastric Diseases’ Pathogenesis. Int. J. Mol. Sci. 2025, 26, 5065. [Google Scholar] [CrossRef]
- Moghadam, M.T.; Chegini, Z.; Khoshbayan, A.; Farahani, I.; Shariati, A. Helicobacter pylori Biofilm and New Strategies to Combat it. Curr. Mol. Med. 2021, 21, 549–561. [Google Scholar] [CrossRef]
- Ciobanasu, C. Bacterial Extracellular Vesicles and Antimicrobial Peptides: A Synergistic Approach to Overcome Antimicrobial Resistance. Antibiotics 2025, 14, 414. [Google Scholar] [CrossRef]
- Barathan, M.; Ng, S.-L.; Lokanathan, Y.; Ng, M.H.; Law, J.X. Unseen Weapons: Bacterial Extracellular Vesicles and the Spread of Antibiotic Resistance in Aquatic Environments. Int. J. Mol. Sci. 2024, 25, 3080. [Google Scholar] [CrossRef]
- Margutti, P.; D’Ambrosio, A.; Zamboni, S. Microbiota-Derived Extracellular Vesicle as Emerging Actors in Host Interactions. Int. J. Mol. Sci. 2024, 25, 8722. [Google Scholar] [CrossRef]
- Xiu, L.; Wu, Y.; Lin, G.; Zhang, Y.; Huang, L. Bacterial membrane vesicles: Orchestrators of interkingdom interactions in microbial communities for environmental adaptation and pathogenic dynamics. Front. Immunol. 2024, 15, 1371317. [Google Scholar] [CrossRef]
- Rima, M.; Dakramanji, M.; El Hayek, E.; El Khoury, T.; Fajloun, Z.; Rima, M. Unveiling the wonders of bacteria-derived extracellular vesicles: From fundamental functions to beneficial applications. Heliyon 2025, 11, e42509. [Google Scholar] [CrossRef]
- Pawar, R.R.; Bardeskar, P.; Bhor, V.M. Opportunities and challenges in harnessing the immunomodulatory potential of bacterial extracellular vesicles as vaccine candidates. Discov. Immun. 2025, 2, 2. [Google Scholar] [CrossRef]
- Amabebe, E.; Kumar, A.; Tatiparthy, M.; Kammala, A.K.; Taylor, B.D.; Menon, R. Cargo exchange between human and bacterial extracellular vesicles in gestational tissues: A new paradigm in communication and immune development. Extracell. Vesicles Circ. Nucleic Acids 2024, 5, 297–328. [Google Scholar] [CrossRef] [PubMed]
- De Langhe, N.; Van Dorpe, S.; Guilbert, N.; Vander Cruyssen, A.; Roux, Q.; Deville, S.; Dedeyne, S.; Tummers, P.; Denys, H.; Vandekerckhove, L.; et al. Mapping bacterial extracellular vesicle research: Insights, best practices and knowledge gaps. Nat. Commun. 2024, 15, 9410. [Google Scholar] [CrossRef]
- Sun, J.; Rutherford, S.T.; Silhavy, T.J.; Huang, K.C. Physical properties of the bacterial outer membrane. Nat. Rev. Microbiol. 2022, 20, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Sartorio, M.G.; Pardue, E.J.; Feldman, M.F.; Haurat, M.F. Bacterial Outer Membrane Vesicles: From Discovery to Applications. Annu. Rev. Microbiol. 2021, 75, 609–630. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Suh, J.W.; Kang, J.S.; Kim, S.B.; Yoon, Y.K.; Sohn, J.W. Gram-Negative Bacteria’s Outer Membrane Vesicles. Infect. Chemother. 2023, 55, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Toyofuku, M.; Schild, S.; Kaparakis-Liaskos, M.; Eberl, L. Composition and functions of bacterial membrane vesicles. Nat. Rev. Microbiol. 2023, 21, 415–430. [Google Scholar] [CrossRef]
- Juodeikis, R.; Carding, S.R. Outer Membrane Vesicles: Biogenesis, Functions, and Issues. Microbiol. Mol. Biol. Rev. 2022, 86, e0003222. [Google Scholar] [CrossRef]
- Magaña, G.; Harvey, C.; Taggart, C.C.; Rodgers, A.M. Bacterial Outer Membrane Vesicles: Role in Pathogenesis and Host-Cell Interactions. Antibiotics 2024, 13, 32. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, X.; Xiang, X.; Hao, C.; Ma, D. Roles of bacterial extracellular vesicles in systemic diseases. Front. Microbiol. 2023, 14, 1258860. [Google Scholar] [CrossRef]
- Hosseini-Giv, N.; Basas, A.; Hicks, C.; El-Omar, E.; El-Assaad, F.; Hosseini-Beheshti, E. Bacterial extracellular vesicles and their novel therapeutic applications in health and cancer. Front. Cell. Infect. Microbiol. 2022, 12, 962216. [Google Scholar] [CrossRef]
- Gan, Y.; Zhao, G.; Wang, Z.; Zhang, X.; Wu, M.X.; Lu, M. Bacterial Membrane Vesicles: Physiological Roles, Infection Immunology, and Applications. Adv. Sci. 2023, 10, e2301357. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Meng, L.; Chen, Y.; Dong, Z.; Peng, Q. Bacterial outer membrane vesicles as potential biological nanomaterials for antibacterial therapy. Acta Biomater. 2022, 140, 102–115. [Google Scholar] [CrossRef]
- Villageliu, D.N.; Samuelson, D.R. The Role of Bacterial Membrane Vesicles in Human Health and Disease. Front. Microbiol. 2022, 13, 828704. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Aggarwal, S.; Singh, D.V.; Acharya, N. Extracellular vesicles: An emerging platform in gram-positive bacteria. Microbiol. Cell 2020, 7, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Dai, K.; Liao, B.; Huang, X.; Liu, Q. Consistency in bacterial extracellular vesicle production: Key to their application in human health. Extracell. Vesicles Circ. Nucleic Acids 2025, 6, 1–20. [Google Scholar] [CrossRef]
- Sharndama, H.C.; Mba, I.E. Helicobacter pylori: An up-to-date overview on the virulence and pathogenesis mechanisms. Braz. J. Microbiol. 2022, 53, 33–50. [Google Scholar] [CrossRef]
- González, M.F.; Díaz, P.; Sandoval-Bórquez, A.; Herrera, D.; Quest, A.F.G. Helicobacter pylori Outer Membrane Vesicles and Extracellular Vesicles from Helicobacter pylori-Infected Cells in Gastric Disease Development. Int. J. Mol. Sci. 2021, 22, 4823. [Google Scholar] [CrossRef]
- Chmiela, M.; Walczak, N.; Rudnicka, K. Helicobacter pylori outer membrane vesicles involvement in the infection development and Helicobacter pylori-related diseases. J. Biomed. Sci. 2018, 25, 78. [Google Scholar] [CrossRef] [PubMed]
- Resta, S.C.; Guerra, F.; Talà, A.; Bucci, C.; Alifano, P. Beyond Inflammation: Role of Pyroptosis Pathway Activation by Gram-Negative Bacteria and Their Outer Membrane Vesicles (OMVs) in the Interaction with the Host Cell. Cells 2024, 13, 1758. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, Z.; Jiao, Y.; Feng, Y.; Zhang, S.; Yang, A. Innate Immunity in Helicobacter pylori Infection and Gastric Oncogenesis. Helicobacter 2025, 30, e70015. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.H.; Rho, D.J.; Jeon, J.I.; Kim, Y.J.; Woo, H.A.; Kim, N.; Kim, J.M. Crude Preparations of Helicobacter pylori Outer Membrane Vesicles Induce Upregulation of Heme Oxygenase-1 via Activating Akt-Nrf2 and mTOR-IκB Kinase-NF-κB Pathways in Dendritic Cells. Infect. Immun. 2016, 84, 2162–2174. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Mao, L.; Yao, Y. Helicobacter pylori outer membrane vesicles and infected cell exosomes: New players in host immune modulation and pathogenesis. Front. Immunol. 2024, 15, 1512935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Chen, C.; Hu, J.; Su, R.; Zhang, J.; Han, Z.; Chen, H.; Li, Y. Molecular mechanism of Helicobacter pylori-induced autophagy in gastric cancer. Oncol. Lett. 2019, 18, 6221–6227. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Zhu, J.; Xu, H. Strategies of Helicobacter pylori in evading host innate and adaptive immunity: Insights and prospects for therapeutic targeting. Front. Cell. Infect. Microbiol. 2024, 14, 1342913. [Google Scholar] [CrossRef]
- Lekmeechai, S.; Su, Y.C.; Brant, M.; Alvarado-Kristensson, M.; Vallström, A.; Obi, I.; Arnqvist, A.; Riesbeck, K. Helicobacter pylori Outer Membrane Vesicles Protect the Pathogen from Reactive Oxygen Species of the Respiratory Burst. Front. Microbiol. 2018, 9, 1837. [Google Scholar] [CrossRef]
- Sah, D.K.; Arjunan, A.; Lee, B.; Jung, Y.D. Reactive Oxygen Species and H. pylori Infection: A Comprehensive Review of Their Roles in Gastric Cancer Development. Antioxidants 2023, 12, 1712. [Google Scholar] [CrossRef]
- Fu, H.-W.; Lai, Y.-C. The Role of Helicobacter pylori Neutrophil-Activating Protein in the Pathogenesis of H. pylori and Beyond: From a Virulence Factor to Therapeutic Targets and Therapeutic Agents. Int. J. Mol. Sci. 2023, 24, 91. [Google Scholar] [CrossRef]
- Fehlings, M.; Drobbe, L.; Moos, V.; Renner Viveros, P.; Hagen, J.; Beigier-Bompadre, M.; Pang, E.; Belogolova, E.; Churin, Y.; Schneider, T.; et al. Comparative analysis of the interaction of Helicobacter pylori with human dendritic cells, macrophages, and monocytes. Infect. Immun. 2012, 80, 2724–2734. [Google Scholar] [CrossRef]
- Fei, X.; Chen, S.; Li, L.; Xu, X.; Wang, H.; Ke, H.; He, C.; Xie, C.; Wu, X.; Liu, J.; et al. Helicobacter pylori infection promotes M1 macrophage polarization and gastric inflammation by activation of NLRP3 inflammasome via TNF/TNFR1 axis. Cell Commun. Signal. 2025, 23, 6. [Google Scholar] [CrossRef]
- Lamb, A.; Chen, L.F. Role of the Helicobacter pylori-induced inflammatory response in the development of gastric cancer. J. Cell. Biochem. 2013, 114, 491–497. [Google Scholar] [CrossRef]
- Dai, Y.K.; Zhang, Y.Z.; Li, D.Y.; Chen, X.; Gong, L.; Luo, Q.; Lan, S.Y.; Chen, B.; Wu, J.Y.; Zhang, Z.J.; et al. Interaction of Cyclooxygenase-2 with Helicobacter pylori Induces Gastric Chronic Nonresolving Inflammation and the Formation of Syndrome of Internal Block of Static Blood in Helicobacter pylori-Related Gastric Diseases. Evid.-Based Complement. Altern. Med. Ecam 2020, 2020, 7340814. [Google Scholar] [CrossRef] [PubMed]
- Schmees, C.; Prinz, C.; Treptau, T.; Rad, R.; Hengst, L.; Voland, P.; Bauer, S.; Brenner, L.; Schmid, R.M.; Gerhard, M. Inhibition of T-cell proliferation by Helicobacter pylori gamma-glutamyl transpeptidase. Gastroenterology 2007, 132, 1820–1833. [Google Scholar] [CrossRef]
- Ahmed, A.A.Q.; Besio, R.; Xiao, L.; Forlino, A. Outer Membrane Vesicles (OMVs) as Biomedical Tools and Their Relevance as Immune-Modulating Agents Against H. pylori Infections: Current Status and Future Prospects. Int. J. Mol. Sci. 2023, 24, 8542. [Google Scholar] [CrossRef]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef]
- Bano, S.; Hassan, N.; Rafiq, M.; Hassan, F.; Rehman, M.; Iqbal, N.; Ali, H.; Hasan, F.; Kang, Y.-Q. Biofilms as Battlefield Armor for Bacteria against Antibiotics: Challenges and Combating Strategies. Microorganisms 2023, 11, 2595. [Google Scholar] [CrossRef] [PubMed]
- Lander, S.M.; Fisher, G.; Everett, B.A.; Tran, P.; Prindle, A. Secreted nucleases reclaim extracellular DNA during biofilm development. NPJ Biofilms Microbiomes 2024, 10, 103. [Google Scholar] [CrossRef]
- Marinacci, B.; Krzyżek, P.; Pellegrini, B.; Turacchio, G.; Grande, R. Latest Update on Outer Membrane Vesicles and Their Role in Horizontal Gene Transfer: A Mini-Review. Membranes 2023, 13, 860. [Google Scholar] [CrossRef]
- Schwab, A.; Meyering, S.S.; Lepene, B.; Iordanskiy, S.; van Hoek, M.L.; Hakami, R.M.; Kashanchi, F. Extracellular vesicles from infected cells: Potential for direct pathogenesis. Front. Microbiol. 2015, 6, 1132. [Google Scholar] [CrossRef]
- Furuyama, N.; Sircili, M.P. Outer Membrane Vesicles (OMVs) Produced by Gram-Negative Bacteria: Structure, Functions, Biogenesis, and Vaccine Application. BioMed Res. Int. 2021, 2021, 1490732. [Google Scholar] [CrossRef]
- Kumar, S.; Schmitt, C.; Gorgette, O.; Marbouty, M.; Duchateau, M.; Giai Gianetto, Q.; Matondo, M.; Guigner, J.M.; De Reuse, H. Bacterial Membrane Vesicles as a Novel Strategy for Extrusion of Antimicrobial Bismuth Drug in Helicobacter pylori. mBio 2022, 13, e0163322. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, D.; Cui, G.; Lee, K.H.; Yang, T.; Zhang, Z.; Liu, Q.; Zhang, J.; Chua, E.G.; Chen, Z. Association Between Biofilm Formation and Structure and Antibiotic Resistance in H. pylori. Infect. Drug Resist. 2024, 17, 2501–2512. [Google Scholar] [CrossRef]
- Murray, B.O.; Dawson, R.A.; Alsharaf, L.M.; Anne Winter, J. Protective effects of Helicobacter pylori membrane vesicles against stress and antimicrobial agents. Microbiology 2020, 166, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem Across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V.; et al. Human gut microbiota in health and disease: Unveiling the relationship. Front. Microbiol. 2022, 13, 999001. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Kerstens, R.; Joyce, P. The Gut Microbiome as a Catalyst and Emerging Therapeutic Target for Parkinson’s Disease: A Comprehensive Update. Biomedicines 2024, 12, 1738. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Q.; Yang, Y.; Zhong, W.; He, F.; Li, J. The mycobiome as integral part of the gut microbiome: Crucial role of symbiotic fungi in health and disease. Gut Microbes 2024, 16, 2440111. [Google Scholar] [CrossRef]
- Volmer, J.G.; McRae, H.; Morrison, M. The evolving role of methanogenic archaea in mammalian microbiomes. Front. Microbiol. 2023, 14, 1268451. [Google Scholar] [CrossRef]
- Emencheta, S.C.; Olovo, C.V.; Eze, O.C.; Kalu, C.F.; Berebon, D.P.; Onuigbo, E.B.; Vila, M.M.D.C.; Balcão, V.M.; Attama, A.A. The Role of Bacteriophages in the Gut Microbiota: Implications for Human Health. Pharmaceutics 2023, 15, 2416. [Google Scholar] [CrossRef]
- Matijašić, M.; Meštrović, T.; Paljetak, H.Č.; Perić, M.; Barešić, A.; Verbanac, D. Gut Microbiota beyond Bacteria-Mycobiome, Virome, Archaeome, and Eukaryotic Parasites in IBD. Int. J. Mol. Sci. 2020, 21, 2668. [Google Scholar] [CrossRef]
- Xu, W.; Xu, L.; Xu, C. Relationship between Helicobacter pylori infection and gastrointestinal microecology. Front. Cell. Infect. Microbiol. 2022, 12, 938608. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.Y.; Sheu, B.S.; Wu, J.J. Helicobacter pylori infection: An overview of bacterial virulence factors and pathogenesis. Biomed. J. 2016, 39, 14–23. [Google Scholar] [CrossRef]
- Fakharian, F.; Asgari, B.; Nabavi-Rad, A.; Sadeghi, A.; Soleimani, N.; Yadegar, A.; Zali, M.R. The interplay between Helicobacter pylori and the gut microbiota: An emerging driver influencing the immune system homeostasis and gastric carcinogenesis. Front. Cell. Infect. Microbiol. 2022, 12, 953718. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, W.; Lee, A.; He, J.; Huang, B.; Zheng, W.; Su, T.; Lai, S.; Long, Y.; Chu, H.; et al. The impact of Helicobacter pylori infection, eradication therapy and probiotic supplementation on gut microenvironment homeostasis: An open-label, randomized clinical trial. EBioMedicine 2018, 35, 87–96. [Google Scholar] [CrossRef]
- Serrano, C.; Harris, P.R.; Smith, P.D.; Bimczok, D. Interactions between H. pylori and the Gastric Microbiome: Impact on Gastric Homeostasis and Disease. Curr. Opin. Physiol. 2021, 21, 57–64. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Wei, K.; He, J.; Ding, N.; Hua, J.; Zhou, T.; Niu, F.; Zhou, G.; Shi, T.; et al. Review: Effect of Gut Microbiota and Its Metabolite SCFAs on Radiation-Induced Intestinal Injury. Front. Cell. Infect. Microbiol. 2021, 11, 577236. [Google Scholar] [CrossRef] [PubMed]
- Frost, F.; Kacprowski, T.; Rühlemann, M.; Bang, C.; Franke, A.; Zimmermann, K.; Nauck, M.; Völker, U.; Völzke, H.; Biffar, R.; et al. Helicobacter pylori infection associates with fecal microbiota composition and diversity. Sci. Rep. 2019, 9, 20100. [Google Scholar] [CrossRef]
- Francisco, A.J. Helicobacter pylori Infection Induces Intestinal Dysbiosis That Could Be Related to the Onset of Atherosclerosis. BioMed Res. Int. 2022, 2022, 9943158. [Google Scholar] [CrossRef]
- Iino, C.; Shimoyama, T. Impact of Helicobacter pylori infection on gut microbiota. World J. Gastroenterol. 2021, 27, 6224–6230. [Google Scholar] [CrossRef]
- Sgamato, C.; Rocco, A.; Compare, D.; Priadko, K.; Romano, M.; Nardone, G. Exploring the Link between Helicobacter pylori, Gastric Microbiota and Gastric Cancer. Antibiotics 2024, 13, 484. [Google Scholar] [CrossRef] [PubMed]
- Tohumcu, E.; Kaitsas, F.; Bricca, L.; Ruggeri, A.; Gasbarrini, A.; Cammarota, G.; Ianiro, G. Helicobacter pylori and the Human Gastrointestinal Microbiota: A Multifaceted Relationship. Antibiotics 2024, 13, 584. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Ağagündüz, D.; Cocozza, E.; Cemali, Ö.; Bayazıt, A.D.; Nanì, M.F.; Cerqua, I.; Morgillo, F.; Saygılı, S.K.; Berni Canani, R.; Amero, P.; et al. Understanding the role of the gut microbiome in gastrointestinal cancer: A review. Front. Pharmacol. 2023, 14, 1130562. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.J.Y.; Coker, O.O.; Chu, E.; Szeto, C.H.; Luk, S.T.Y.; Lau, H.C.H.; Yu, J. Gastric microbes associated with gastric inflammation, atrophy and intestinal metaplasia 1 year after Helicobacter pylori eradication. Gut 2020, 69, 1572–1580. [Google Scholar] [CrossRef] [PubMed]
- Rueda-Robles, A.; Rubio-Tomás, T.; Plaza-Diaz, J.; Álvarez-Mercado, A.I. Impact of Dietary Patterns on H. pylori Infection and the Modulation of Microbiota to Counteract Its Effect. A Narrative Review. Pathogens 2021, 10, 875. [Google Scholar] [CrossRef]
- Martín-Núñez, G.M.; Cornejo-Pareja, I.; Coin-Aragüez, L.; Roca-Rodríguez, M.D.M.; Muñoz-Garach, A.; Clemente-Postigo, M.; Cardona, F.; Moreno-Indias, I.; Tinahones, F.J.H. Pylori eradication with antibiotic treatment causes changes in glucose homeostasis related to modifications in the gut microbiota. PLoS ONE 2019, 14, e0213548. [Google Scholar] [CrossRef]
- Pilla, R.; Suchodolski, J.S. The Role of the Canine Gut Microbiome and Metabolome in Health and Gastrointestinal Disease. Front. Vet. Sci. 2020, 6, 498. [Google Scholar] [CrossRef]
- Chen, J.; Guo, Y.; Huang, Y.; Ding, Z.; Wang, J.; Liang, X.; Xu, P.; Han, Y.; Lu, H. Rifabutin-Containing Triple Therapy Versus Bismuth Quadruple Therapy for Helicobacter pylori Rescue Treatment: A Multicenter, Randomized Controlled Trial. J. Infect. Dis. 2023, 228, 511–518. [Google Scholar] [CrossRef]
- Zhang, D.; Jian, Y.P.; Zhang, Y.N.; Li, Y.; Gu, L.T.; Sun, H.H.; Liu, M.D.; Zhou, H.L.; Wang, Y.S.; Xu, Z.X. Short-chain fatty acids in diseases. Cell Commun. Signal. 2023, 21, 212. [Google Scholar] [CrossRef]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of Antibiotics and Antibiotic Resistance, and Their Impacts on Drug Development: A Narrative Review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Chung, J.W. Best Helicobacter pylori Eradication Strategy in the Era of Antibiotic Resistance. Antibiotics 2020, 9, 436. [Google Scholar] [CrossRef]
- Dongre, D.S.; Saha, U.B.; Saroj, S.D. Exploring the role of gut microbiota in antibiotic resistance and prevention. Ann. Med. 2025, 57, 2478317. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Baik, G.H.; Kim, Y.S.; Suk, K.T.; Shin, W.G.; Kim, K.H.; Kim, K.O.; Park, C.H.; Baik, I.H.; Jang, H.J.; et al. Comparison of the Eradication Rate Between 1- and 2-Week Bismuth-Containing Quadruple Rescue Therapies for Helicobacter pylori Eradication. Gut Liver 2012, 6, 434–439. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Becattini, S.; Taur, Y.; Pamer, E.G. Antibiotic-Induced Changes in the Intestinal Microbiota and Disease. Trends Mol. Med. 2016, 22, 458–478. [Google Scholar] [CrossRef]
- Saleem, N.; Howden, C.W. Update on the Management of Helicobacter pylori Infection. Curr. Treat. Option. Gastroenterol. 2020, 18, 476–487. [Google Scholar] [CrossRef]
- Hrncir, T. Gut Microbiota Dysbiosis: Triggers, Consequences, Diagnostic and Therapeutic Options. Microorganisms 2022, 10, 578. [Google Scholar] [CrossRef]
- Khoruts, A. Targeting the microbiome: From probiotics to fecal microbiota transplantation. Genome Med. 2018, 10, 80. [Google Scholar] [CrossRef]
- Rabetafika, H.N.; Razafindralambo, A.; Ebenso, B.; Razafindralambo, H.L. Probiotics as Antibiotic Alternatives for Human and Animal Applications. Encyclopedia 2023, 3, 561–581. [Google Scholar] [CrossRef]
- Keikha, M.; Karbalaei, M. Probiotics as the live microscopic fighters against Helicobacter pylori gastric infections. BMC Gastroenterol. 2021, 21, 388. [Google Scholar] [CrossRef] [PubMed]
- Homan, M.; Orel, R. Are probiotics useful in Helicobacter pylori eradication? World J. Gastroenterol. 2015, 21, 10644–10653. [Google Scholar] [CrossRef]
- Mestre, A.; Sathiya Narayanan, R.; Rivas, D.; John, J.; Abdulqader, M.A.; Khanna, T.; Chakinala, R.C.; Gupta, S. Role of Probiotics in the Management of Helicobacter pylori. Cureus 2022, 14, e26463. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, P.A.; Garcés, D.; Prado-Vivar, B.; Flores, N.; Fornasini, M.; Cohen, H.; Salvador, I.; Cargua, O.; Baldeón, M.E. Effect of Saccharomyces boulardii CNCM I-745 as complementary treatment of Helicobacter pylori infection on gut microbiome. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1365–1372. [Google Scholar] [CrossRef]
- Liévin-Le Moal, V.; Servin, A.L. Anti-infective activities of lactobacillus strains in the human intestinal microbiota: From probiotics to gastrointestinal anti-infectious biotherapeutic agents. Clin. Microbiol. Rev. 2014, 27, 167–199. [Google Scholar] [CrossRef] [PubMed]
- Baryshnikova, N.V.; Ilina, A.S.; Ermolenko, E.I.; Uspenskiy, Y.P.; Suvorov, A.N. Probiotics and autoprobiotics for treatment of Helicobacter pylori infection. World J. Clin. Cases 2023, 11, 4740–4751. [Google Scholar] [CrossRef]
- Yang, H.; Lin, Y.; Ma, Y.; Li, J.; Li, J.; Huo, Z.; Yang, P.; Zhang, C. Screening Probiotics for Anti-Helicobacter pylori and Investigating the Effect of Probiotics on Patients with Helicobacter pylori Infection. Foods 2024, 13, 1851. [Google Scholar] [CrossRef]
- Mukai, R.; Handa, O.; Suyama, Y.; Majima, A.; Naito, Y. Effectiveness of including probiotics to Helicobacter pylori eradication therapies. J. Clin. Biochem. Nutr. 2020, 67, 102–104. [Google Scholar] [CrossRef]
- Vlasova, A.N.; Kandasamy, S.; Chattha, K.S.; Rajashekara, G.; Saif, L.J. Comparison of probiotic lactobacilli and bifidobacteria effects, immune responses and rotavirus vaccines and infection in different host species. Vet. Immunol. Immunopathol. 2016, 172, 72–84. [Google Scholar] [CrossRef]
- Rau, S.; Gregg, A.; Yaceczko, S.; Limketkai, B. Prebiotics and Probiotics for Gastrointestinal Disorders. Nutrients 2024, 16, 778. [Google Scholar] [CrossRef]
- Zhu, M.; Tse, M.W.; Weller, J.; Chen, J.; Blainey, P.C. The future of antibiotics begins with discovering new combinations. Ann. N. Y. Acad. Sci. 2021, 1496, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Abouelela, M.E.; Helmy, Y.A. Next-Generation Probiotics as Novel Therapeutics for Improving Human Health: Current Trends and Future Perspectives. Microorganisms 2024, 12, 430. [Google Scholar] [CrossRef] [PubMed]
- Suvorov, A.; Karaseva, A.; Kotyleva, M.; Kondratenko, Y.; Lavrenova, N.; Korobeynikov, A.; Kozyrev, P.; Kramskaya, T.; Leontieva, G.; Kudryavtsev, I.; et al. Autoprobiotics as an Approach for Restoration of Personalised Microbiota. Front. Microbiol. 2018, 9, 1869. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, Q.; Xie, J.; Nie, S. Immunomodulatory Effects of Probiotic-Derived Extracellular Vesicles: Opportunities and Challenges. J. Agric. Food Chem. 2024, 72, 19259–19273. [Google Scholar] [CrossRef]
- Krzyżek, P.; Marinacci, B.; Vitale, I.; Grande, R. Extracellular Vesicles of Probiotics: Shedding Light on the Biological Activity and Future Applications. Pharmaceutics 2023, 15, 522. [Google Scholar] [CrossRef]
- Ma, B.; Barathan, M.; Ng, M.H.; Law, J.X. Oxidative Stress, Gut Microbiota, and Extracellular Vesicles: Interconnected Pathways and Therapeutic Potentials. Int. J. Mol. Sci. 2025, 26, 3148. [Google Scholar] [CrossRef]
- Barathan, M.; Zulpa, A.K.; Ng, S.L.; Lokanathan, Y.; Ng, M.H.; Law, J.X. Innovative Strategies to Combat 5-Fluorouracil Resistance in Colorectal Cancer: The Role of Phytochemicals and Extracellular Vesicles. Int. J. Mol. Sci. 2024, 25, 7470. [Google Scholar] [CrossRef]
- Manna, O.M.; Caruso Bavisotto, C.; Gratie, M.I.; Damiani, P.; Bonaventura, G.; Cappello, F.; Tomasello, G.; D’Andrea, V. Targeting Helicobacter pylori Through the “Muco-Microbiotic Layer” Lens: The Challenge of Probiotics and Microbiota Nanovesicles. Nutrients 2025, 17, 569. [Google Scholar] [CrossRef]
- Fakharian, F.; Sadeghi, A.; Pouresmaeili, F.; Soleimani, N.; Yadegar, A. Anti-inflammatory effects of extracellular vesicles and cell-free supernatant derived from Lactobacillus crispatus strain RIGLD-1 on Helicobacter pylori-induced inflammatory response in gastric epithelial cells in vitro. Folia Microbiol. 2024, 69, 927–939. [Google Scholar] [CrossRef]
- Domínguez Rubio, A.P.; D’Antoni, C.L.; Piuri, M.; Pérez, O.E. Probiotics, Their Extracellular Vesicles and Infectious Diseases. Front. Microbiol. 2022, 13, 864720. [Google Scholar] [CrossRef]
- Sanwlani, R.; Bramich, K.; Mathivanan, S. Role of probiotic extracellular vesicles in inter-kingdom communication and current technical limitations in advancing their therapeutic utility. Extracell. Vesicles Circ. Nucleic Acids 2024, 5, 509–526. [Google Scholar] [CrossRef]
- Ji, J.; Yang, H. Using Probiotics as Supplementation for Helicobacter pylori Antibiotic Therapy. Int. J. Mol. Sci. 2020, 21, 1136. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Luo, H.; Xie, Y.; Cao, H.; Mao, L.; Liu, T.; Yue, Y.; Qian, H. Extracellular vesicles in Helicobacter pylori-mediated diseases: Mechanisms and therapeutic potential. Cell. Commun. Signal 2025, 23, 79. [Google Scholar] [CrossRef]
- Smolinska, S.; Popescu, F.-D.; Zemelka-Wiacek, M. A Review of the Influence of Prebiotics, Probiotics, Synbiotics, and Postbiotics on the Human Gut Microbiome and Intestinal Integrity. J. Clin. Med. 2025, 14, 3673. [Google Scholar] [CrossRef]
- Kaur, A.P.; Bhardwaj, S.; Dhanjal, D.S.; Nepovimova, E.; Cruz-Martins, N.; Kuča, K.; Chopra, C.; Singh, R.; Kumar, H.; Șen, F.; et al. Plant Prebiotics and Their Role in the Amelioration of Diseases. Biomolecules 2021, 11, 440. [Google Scholar] [CrossRef]
- Bamigbade, G.B.; Subhash, A.J.; Kamal-Eldin, A.; Nyström, L.; Ayyash, M. An Updated Review on Prebiotics: Insights on Potentials of Food Seeds Waste as Source of Potential Prebiotics. Molecules 2022, 27, 5947. [Google Scholar] [CrossRef] [PubMed]
- Maftei, N.-M.; Raileanu, C.R.; Balta, A.A.; Ambrose, L.; Boev, M.; Marin, D.B.; Lisa, E.L. The Potential Impact of Probiotics on Human Health: An Update on Their Health-Promoting Properties. Microorganisms 2024, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- Leser, T.; Baker, A. Molecular Mechanisms of Lacticaseibacillus rhamnosus, LGG® Probiotic Function. Microorganisms 2024, 12, 794. [Google Scholar] [CrossRef] [PubMed]
- Shafaghi, A.; Pourkazemi, A.; Khosravani, M.; Fakhrie Asl, S.; Amir Maafi, A.; Atrkar Roshan, Z.; Abaspour Rahimabad, J. The Effect of Probiotic Plus Prebiotic Supplementation on the Tolerance and Efficacy of Helicobacter pylori Eradication Quadruple Therapy: A Randomized Prospective Double Blind Controlled Trial. Middle East J. Dig. Dis. 2016, 8, 179–188. [Google Scholar] [CrossRef]
- Ji, J.; Jin, W.; Liu, S.J.; Jiao, Z.; Li, X. Probiotics, prebiotics, and postbiotics in health and disease. MedComm 2023, 4, e420. [Google Scholar] [CrossRef]
- Hediyal, T.A.; Vichitra, C.; Anand, N.; Bhaskaran, M.; Essa, S.M.; Kumar, P.; Qoronfleh, M.W.; Akbar, M.; Kaul-Ghanekar, R.; Mahalakshmi, A.M.; et al. Protective effects of fecal microbiota transplantation against ischemic stroke and other neurological disorders: An update. Front. Immunol. 2024, 15, 1324018. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.N.; Xia, H.H.; Zhang, R.; Li, L.; Wu, L.H.; Liu, X.J.; Xie, W.R.; He, X.X. The Efficacy of Washed Microbiota Transplantation on Helicobacter pylori Eradication: A Pilot Study. Gastroenterol. Res. Pract. 2020, 2020, 8825189. [Google Scholar] [CrossRef]
- Zhao, J.T.; Zhang, Y.; Wang, X.W.; Zou, P.Y.; Zhao, Z.; Mei, H.; Liu, Y.X.; Su, N.Y.; Zhu, Y.J.; Wang, B.; et al. Long-term effects of fecal microbiota transplantation on gut microbiota after Helicobacter pylori eradication with bismuth quadruple therapy: A randomized controlled trial. Helicobacter 2024, 29, e13079. [Google Scholar] [CrossRef]
- Merrick, B.; Allen, L.; Masirah M Zain, N.; Forbes, B.; Shawcross, D.L.; Goldenberg, S.D. Regulation, risk and safety of Faecal Microbiota Transplant. Infect. Prev. Pract. 2020, 2, 100069. [Google Scholar] [CrossRef] [PubMed]
- Lü, M.; Yu, S.; Deng, J.; Yan, Q.; Yang, C.; Xia, G.; Zhou, X. Efficacy of Probiotic Supplementation Therapy for Helicobacter pylori Eradication: A Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2016, 11, e0163743. [Google Scholar] [CrossRef]
- Li, M.; Xie, Y. Efficacy and safety of Saccharomyces boulardii as an adjuvant therapy for the eradication of Helicobacter pylori: A meta-analysis. Front. Cell. Infect. Microbiol. 2025, 15, 1441185. [Google Scholar] [CrossRef] [PubMed]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. Cells 2023, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Xiao, S.; Li, S.; Suo, B.; Wang, Y.; Meng, L.; Liu, Z.; Yin, Z.; Xue, Y.; Zhou, L. The impact of Helicobacter pylori infection, eradication therapy, and probiotics intervention on gastric microbiota in young adults. Helicobacter 2021, 26, e12848. [Google Scholar] [CrossRef]
- Li, M.; Mao, B.; Tang, X.; Zhang, Q.; Zhao, J.; Chen, W.; Cui, S. Lactic acid bacteria derived extracellular vesicles: Emerging bioactive nanoparticles in modulating host health. Gut Microbes 2024, 16, 2427311. [Google Scholar] [CrossRef]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, prebiotics and synbiotics—A review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef]
- Obaid, N.A. Alternative treatment of recurrent Clostridioides difficile infection in adults by fecal transplantation: An overview of phase I–IV studies from Clinicaltrials.gov. Front. Microbiol. 2024, 15, 1374774. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Mukhopadhyay, A.K. Progress of bacteriophage as therapeutic agent against Helicobacter pylori, especially in India. Discov. Appl. Sci. 2025, 7, 399. [Google Scholar] [CrossRef]
- Lin, D.M.; Koskella, B.; Lin, H.C. Phage therapy: An alternative to antibiotics in the age of multi-drug resistance. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.; Sousa, C.; Gonçalves, R.F.S.; Pinheiro, A.C.; Oleastro, M.; Wagemans, J.; Lavigne, R.; Figueiredo, C.; Azeredo, J.; Melo, L.D.R. Characterization and Genomic Analysis of a New Phage Infecting Helicobacter pylori. Int. J. Mol. Sci. 2022, 23, 7885. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, P.; Papaianni, M.; Fulgione, A.; Guerra, F.; Capparelli, R.; Medaglia, C. An Innovative Approach to Control H. pylori-Induced Persistent Inflammation and Colonization. Microorganisms 2020, 8, 1214. [Google Scholar] [CrossRef]
- Badran, Z.; Rahman, B.; De Bonfils, P.; Nun, P.; Coeffard, V.; Verron, E. Antibacterial nanophotosensitizers in photodynamic therapy: An update. Drug Discov. Today 2023, 28, 103493. [Google Scholar] [CrossRef]
- Hu, J.; Shi, X.; Cao, S.; Dong, X.; Dai, J.; Yin, H. Exploring the phototherapy modalities and dosages for an ingestible light-emitting diode capsule to eliminate Helicobacter pylori infection. J. Photochem. Photobiol. B Biol. 2025, 267, 113155. [Google Scholar] [CrossRef]
- Ganz, R.A.; Viveiros, J.; Ahmad, A.; Ahmadi, A.; Khalil, A.; Tolkoff, M.J.; Nishioka, N.S.; Hamblin, M.R. Helicobacter pylori in patients can be killed by visible light. Lasers Surg. Med. 2005, 36, 260–265. [Google Scholar] [CrossRef]
- Luo, Q.; Liu, C.; Zhang, A.; Zhang, D. Research progress in photodynamic therapy for Helicobacter pylori infection. Helicobacter 2024, 29, e13068. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, X.; Zhou, S.; Wang, L.; Fang, Y.; Xing, L.; Zhao, Y.; Zhang, L.P.; Qiu, H.; Zeng, J.; et al. Selective photodynamic inactivation of Helicobacter pylori by a cationic benzylidene cyclopentanone photosensitizer—An in vitro and ex vivo study. J. Photochem. Photobiol. B. 2021, 223, 112287. [Google Scholar] [CrossRef]
- Zeng, M.; Mao, X.H.; Li, J.X.; Tong, W.D.; Wang, B.; Zhang, Y.J.; Guo, G.; Zhao, Z.J.; Li, L.; Wu, D.L.; et al. Efficacy, safety, and immunogenicity of an oral recombinant Helicobacter pylori vaccine in children in China: A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015, 386, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhao, W.; Xia, L.; Kong, L.; Yang, L. How Long Will It Take to Launch an Effective Helicobacter pylori Vaccine for Humans? Infect. Drug Resist. 2023, 16, 3787–3805. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, J.; Nordqvist, S.; Blomquist, M.; Jeverstam, F.; Lebens, M.; Raghavan, S. Preclinical immunogenicity and protective efficacy of an oral Helicobacter pylori inactivated whole cell vaccine and multiple mutant cholera toxin: A novel and non-toxic mucosal adjuvant. Vaccine 2018, 36, 6223–6230. [Google Scholar] [CrossRef]
- Hasanzadeh Haghighi, F.; Menbari, S.; Mohammadzadeh, R.; Pishdadian, A.; Farsiani, H. Developing a potent vaccine against Helicobacter pylori: Critical considerations and challenges. Expert Rev. Mol. Med. 2024, 27, e12. [Google Scholar] [CrossRef]
- Guo, L.; Hong, D.; Wang, S.; Zhang, F.; Tang, F.; Wu, T.; Chu, Y.; Liu, H.; He, M.; Yang, H.; et al. Therapeutic Protection Against H. pylori Infection in Mongolian Gerbils by Oral Immunization with a Tetravalent Epitope-Based Vaccine with Polysaccharide Adjuvant. Front. Immunol. 2019, 10, 1185. [Google Scholar] [CrossRef]
- Li, H.B.; Zhang, J.Y.; He, Y.F.; Chen, L.; Li, B.; Liu, K.Y.; Yang, W.C.; Zhao, Z.; Zou, Q.M.; Wu, C. Systemic immunization with an epitope-based vaccine elicits a Th1-biased response and provides protection against Helicobacter pylori in mice. Vaccine 2012, 31, 120–126. [Google Scholar] [CrossRef]
- Sarabi, P.A.; Rismani, E.; Shabanpouremam, M.; Talehahmad, S.; Vosough, M. Developing a multi-epitope vaccine against Helicobacter pylori. Hum. Immunol. 2025, 86, 111212. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.A.N.; Dos Santos, G.A.; Dos Santos, C.T.; Soares, D.C.F.; Saraiva, M.F.; Leal, D.H.S.; Sachs, D. Eugenol as a promising antibiofilm and anti-quorum sensing agent: A systematic review. Microb. Pathog. 2024, 196, 106937. [Google Scholar] [CrossRef]
- Asadi, S.; Rahimi, E.; Shakerian, A. Anti-Helicobacter pylori Effects of Thymus caramanicus Jalas Essential Oils: A New Antimicrobial Approach. Evid.-Based Complement. Altern. Med. 2024, 2024, 3627074. [Google Scholar] [CrossRef]
- Frenț, O.D.; Stefan, L.; Morgovan, C.M.; Duteanu, N.; Dejeu, I.L.; Marian, E.; Vicaș, L.; Manole, F. A Systematic Review: Quercetin-Secondary Metabolite of the Flavonol Class, with Multiple Health Benefits and Low Bioavailability. Int. J. Mol. Sci. 2024, 25, 12091. [Google Scholar] [CrossRef]
- Elbestawy, M.K.M.; El-Sherbiny, G.M.; Moghannem, S.A.; Farghal, E.E. Antibacterial, Antibiofilm, and Anti-Inflammatory Activities of Ginger Extract against Helicobacter pylori. Microbiol. Res. 2023, 14, 1124–1138. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Z.; Cai, Y.; Xue, J.; Zhang, L.; Wang, L.; Zhao, M.; Zheng, Y.; Xia, T.; Yu, H.; et al. Aloe-emodin destroys the biofilm of Helicobacter pylori by targeting the outer membrane protein 6. Microbiol. Res. 2024, 278, 127539. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, W.C. Green Tea Catechins: Their Use in Treating and Preventing Infectious Diseases. BioMed Res. Int. 2018, 2018, 9105261. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.T.; Berchová, K.; Majerová, M.; Pokorná, M.; Švajdlenka, E. In vitro synergistic effect of Hibiscus sabdariffa aqueous extract in combination with standard antibiotics against Helicobacter pylori clinical isolates. Pharm. Biol. 2016, 54, 1736–1740. [Google Scholar] [CrossRef] [PubMed]
- Touati, A.; Mairi, A.; Ibrahim, N.A.; Idres, T. Essential Oils for Biofilm Control: Mechanisms, Synergies, and Translational Challenges in the Era of Antimicrobial Resistance. Antibiotics 2025, 14, 503. [Google Scholar] [CrossRef]
- Hassan, S.T.; Žemlička, M. Plant-Derived Urease Inhibitors as Alternative Chemotherapeutic Agents. Arch. Der Pharm. 2016, 349, 507–522. [Google Scholar] [CrossRef]
- Šudomová, M.; Hassan, S.T.S. Gossypol from Gossypium spp. Inhibits Helicobacter pylori Clinical Strains and Urease Enzyme Activity: Bioactivity and Safety Assessments. Sci. Pharm. 2022, 90, 29. [Google Scholar] [CrossRef]
- Yu, X.D.; Zheng, R.B.; Xie, J.H.; Su, J.Y.; Huang, X.Q.; Wang, Y.H.; Zheng, Y.F.; Mo, Z.Z.; Wu, X.L.; Wu, D.W.; et al. Biological evaluation and molecular docking of baicalin and scutellarin as Helicobacter pylori urease inhibitors. J. Ethnopharmacol. 2015, 162, 69–78. [Google Scholar] [CrossRef]
- Kim, K.H.; Shim, J.S.; Kim, H.J.; Son, E.D. Penta-O-galloyl-β-D-glucose from Paeonia lactiflora Pall. root extract enhances the expression of skin barrier genes via EGR3. J. Ethnopharmacol. 2020, 248, 112337. [Google Scholar] [CrossRef]
- Nagle, D.G.; Ferreira, D.; Zhou, Y.D. Epigallocatechin-3-gallate (EGCG): Chemical and biomedical perspectives. Phytochemistry 2006, 67, 1849–1855. [Google Scholar] [CrossRef]
- Lin, Y.T.; Kwon, Y.I.; Labbe, R.G.; Shetty, K. Inhibition of Helicobacter pylori and associated urease by oregano and cranberry phytochemical synergies. Appl. Environ. Microbiol. 2005, 71, 8558–8564. [Google Scholar] [CrossRef]
- Mahernia, S.; Bagherzadeh, K.; Mojab, F.; Amanlou, M. Urease Inhibitory Activities of some Commonly Consumed Herbal Medicines. Iran. J. Pharm. Res. 2015, 14, 943–947. [Google Scholar]
- Woo, H.J.; Yang, J.Y.; Lee, P.; Kim, J.B.; Kim, S.H. Zerumbone Inhibits Helicobacter pylori Urease Activity. Molecules 2021, 26, 2663. [Google Scholar] [CrossRef] [PubMed]
- Svane, S.; Sigurdarson, J.J.; Finkenwirth, F.; Eitinger, T.; Karring, H. Inhibition of urease activity by different compounds provides insight into the modulation and association of bacterial nickel import and ureolysis. Sci. Rep. 2020, 10, 8503. [Google Scholar] [CrossRef] [PubMed]
- Almarmouri, C.; El-Gamal, M.I.; Haider, M.; Hamad, M.; Qumar, S.; Sebastian, M.; Ghemrawi, R.; Muhammad, J.S.; Burucoa, C.; Khoder, G. Anti-urease therapy: A targeted approach to mitigating antibiotic resistance in Helicobacter pylori while preserving the gut microflora. Gut Pathog. 2025, 17, 37. [Google Scholar] [CrossRef]
- Li, C.; Huang, P.; Wong, K.; Xu, Y.; Tan, L.; Chen, H.; Lu, Q.; Luo, C.; Tam, C.; Zhu, L.; et al. Coptisine-induced inhibition of Helicobacter pylori: Elucidation of specific mechanisms by probing urease active site and its maturation process. J. Enzym. Inhib. Med. Chem. 2018, 33, 1362–1375. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Zhang, Z.; Xu, Y.; Chen, Y.; Li, C. Sanguinarine, a major alkaloid from Zanthoxylum nitidum (Roxb.) DC.; inhibits urease of Helicobacter pylori and jack bean: Susceptibility and mechanism. J. Ethnopharmacol. 2022, 295, 115388. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Mohammed, D.M.; Korma, S.A.; Alshahrani, M.Y.; Ahmed, A.E.; Ibrahim, E.H.; Salem, H.M.; Alkafaas, S.S.; Saif, A.M.; et al. Medicinal plants: Bioactive compounds, biological activities, combating multidrug-resistant microorganisms, and human health benefits—A comprehensive review. Front. Immunol. 2025, 16, 1491777. [Google Scholar] [CrossRef]
- Tandoro, Y.; Chen, B.K.; Ali, A.; Wang, C.K. Review of Phytochemical Potency as a Natural Anti-Helicobacter pylori and Neuroprotective Agent. Molecules 2023, 28, 7150. [Google Scholar] [CrossRef]
- Krzyżek, P.; Junka, A.; Słupski, W.; Dołowacka-Jóźwiak, A.; Płachno, B.J.; Sobiecka, A.; Matkowski, A.; Chodaczek, G.; Płusa, T.; Gościniak, G.; et al. Antibiofilm and Antimicrobial-Enhancing Activity of Chelidonium majus and Corydalis cheilanthifolia Extracts against Multidrug-Resistant Helicobacter pylori. Pathogens 2021, 10, 1033. [Google Scholar] [CrossRef]
- Savitri, C.M.A.; Fauzia, K.A.; Alfaray, R.I.; Aftab, H.; Syam, A.F.; Lubis, M.; Yamaoka, Y.; Miftahussurur, M. Opportunities for Helicobacter pylori Eradication beyond Conventional Antibiotics. Microorganisms 2024, 12, 1986. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Ashok, A.K.; Bhat, T.; Ballamoole, K.; Gollapalli, P. Computational-driven discovery of AI-2 quorum sensing inhibitor targeting the 5′- methylthioadenosine/S-adenosylhomocysteine nucleosidase (MTAN) to combat drug-resistant Helicobacter pylori. Comput. Biol. Med. 2025, 184, 109409. [Google Scholar] [CrossRef]
- Tekpinar, M.; Yildirim, A.; Wassenaar, T.A. Molecular dynamics study of the effect of active site protonation on Helicobacter pylori 5′-methylthioadenosine/S-adenosylhomocysteine nucleosidase. Eur. Biophys. J. 2015, 44, 685–696. [Google Scholar] [CrossRef]
- Khwaza, V.; Aderibigbe, B.A. Antibacterial Activity of Selected Essential Oil Components and Their Derivatives: A Review. Antibiotics 2025, 14, 68. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Casado, J.; Lanas, Á. Fighting the Antibiotic Crisis: Flavonoids as Promising Antibacterial Drugs Against Helicobacter pylori Infection. Front. Cell. Infect. Microbiol. 2021, 11, 709749. [Google Scholar] [CrossRef]
- Tang, K.; Zhang, X.H. Quorum quenching agents: Resources for antivirulence therapy. Mar. Drugs 2014, 12, 3245–3282. [Google Scholar] [CrossRef] [PubMed]
- Sedarat, Z.; Taylor-Robinson, A.W. Helicobacter pylori Outer Membrane Proteins and Virulence Factors: Potential Targets for Novel Therapies and Vaccines. Pathogens 2024, 13, 392. [Google Scholar] [CrossRef]
- Elbestawy, M.K.M.; El-Sherbiny, G.M.; Moghannem, S.A. Antibacterial, Antibiofilm and Anti-Inflammatory Activities of Eugenol Clove Essential Oil against Resistant Helicobacter pylori. Molecules 2023, 28, 2448. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Tian, W.; Cui, X.; Tu, P.; Li, J.; Shi, S.; Liu, X. Biosynthesis Investigations of Terpenoid, Alkaloid, and Flavonoid Antimicrobial Agents Derived from Medicinal Plants. Antibiotics 2022, 11, 1380. [Google Scholar] [CrossRef]
- Choi, H.R.; Lim, H.; Lee, J.H.; Park, H.; Kim, H.P. Interruption of Helicobacter pylori-Induced NLRP3 Inflammasome Activation by Chalcone Derivatives. Biomol. Ther. 2021, 29, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Krzyżek, P.; Migdał, P.; Paluch, E.; Karwańska, M.; Wieliczko, A.; Gościniak, G. Myricetin as an Antivirulence Compound Interfering with a Morphological Transformation into Coccoid Forms and Potentiating Activity of Antibiotics against Helicobacter pylori. Int. J. Mol. Sci. 2021, 22, 2695. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; Lu, W.; Zhu, L.; Shen, X.; Huang, J. Caffeic acid phenethyl ester (CAPE), an active component of propolis, inhibits Helicobacter pylori peptide deformylase activity. Biochem. Biophys. Res. Commun. 2013, 435, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Al-Rajhi, A.M.H.; Qanash, H.; Bazaid, A.S.; Binsaleh, N.K.; Abdelghany, T.M. Pharmacological Evaluation of Acacia nilotica Flower Extract against Helicobacter pylori and Human Hepatocellular Carcinoma In Vitro and In Silico. J. Funct. Biomater. 2023, 14, 237. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).