Serum IgE and IgA Levels in Pediatric Henoch–Schönlein Purpura: Clinical Characteristics and Immunological Correlations in the Context of Infectious Diseases—A Five-Year Retrospective Analysis
Abstract
1. Introduction
2. Results
2.1. Descriptive Statistics
2.2. Immunologic Panel Results
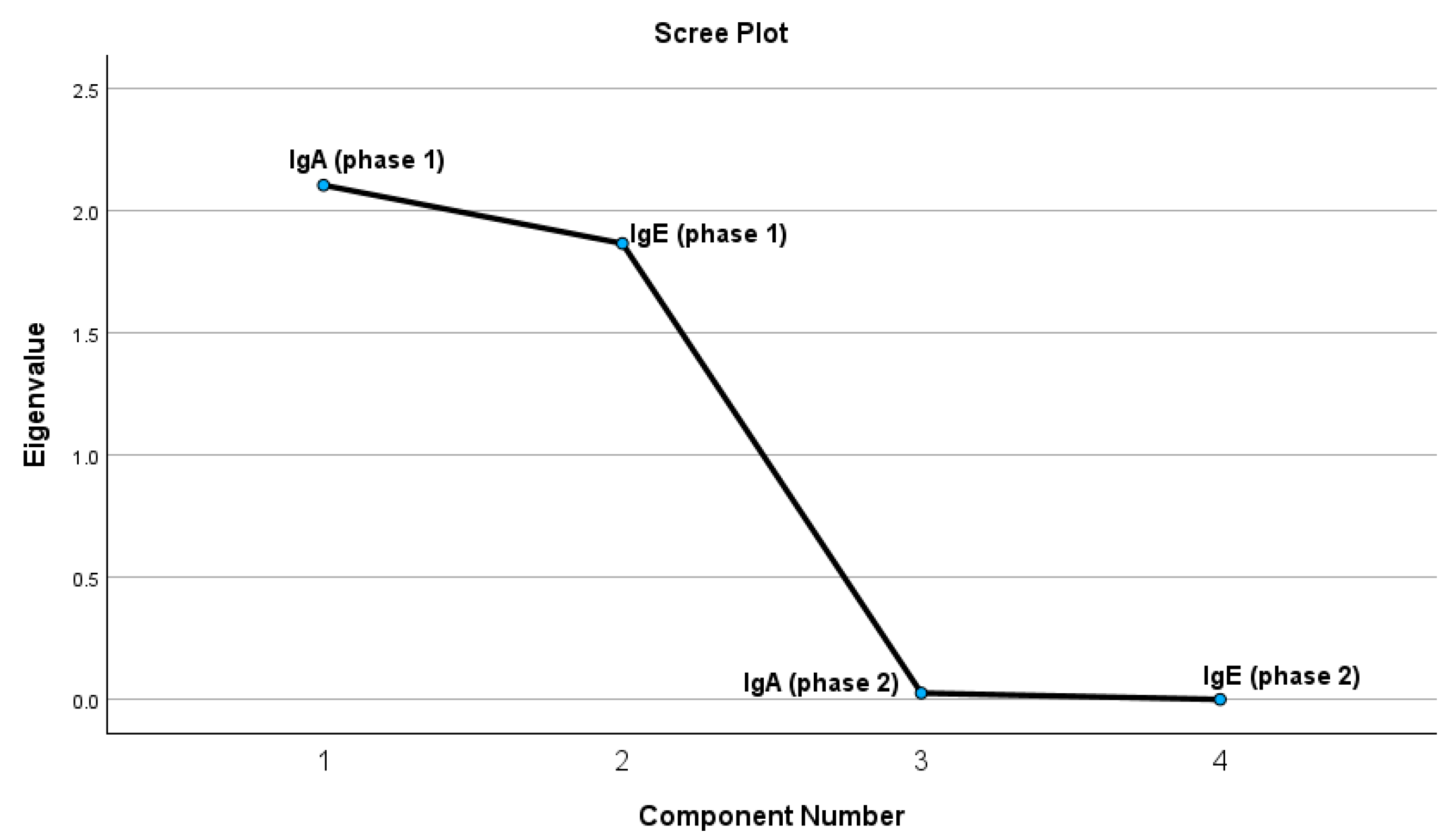
2.2.1. Principal Component Analysis Results
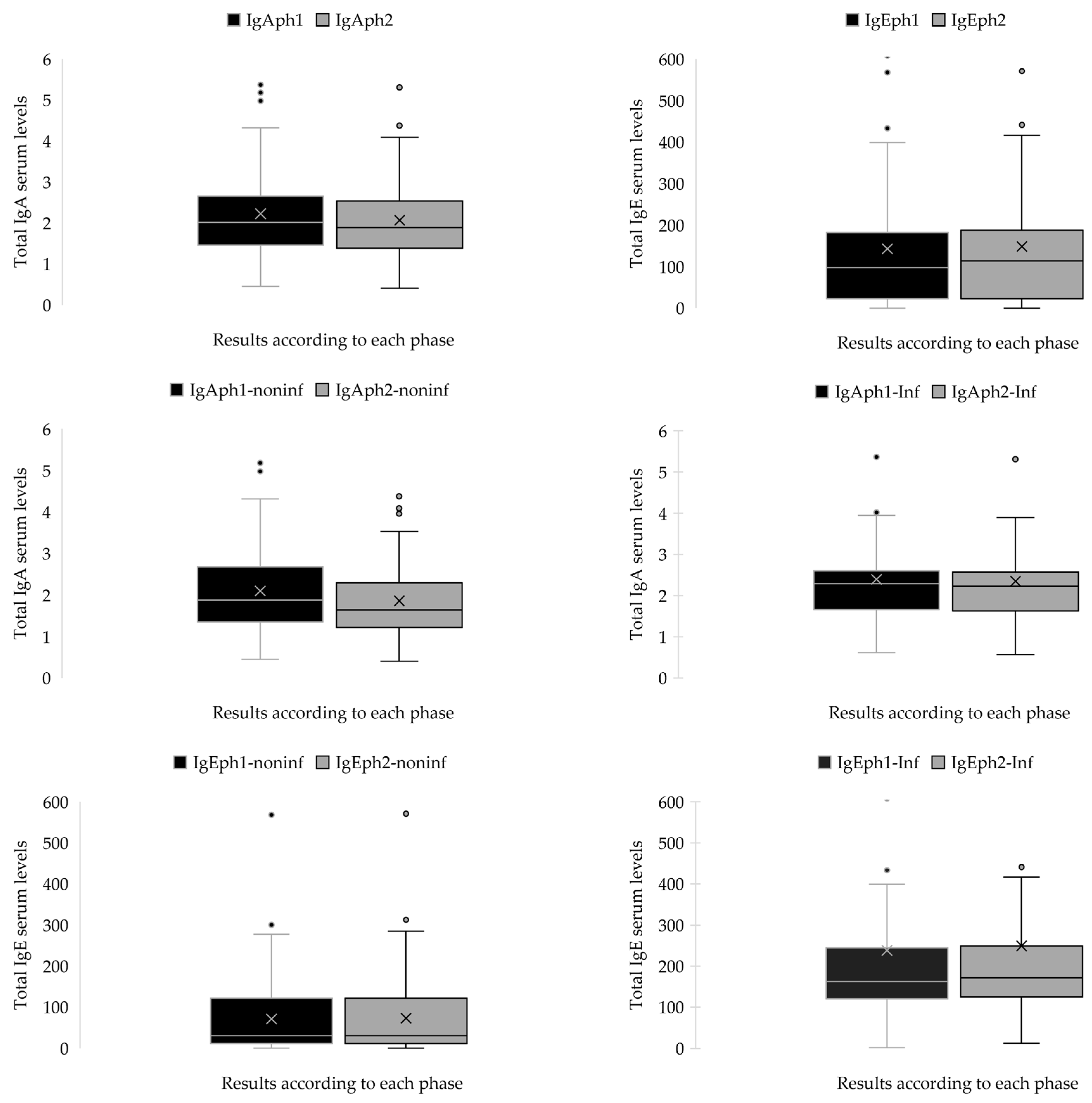
2.2.2. The Statistical Analyses of IgA and IgE Serum Concentrations (g/L) for Both Patient Cohorts During Both Phases
2.3. Patients with Infectious Diseases as Secondary Diagnoses
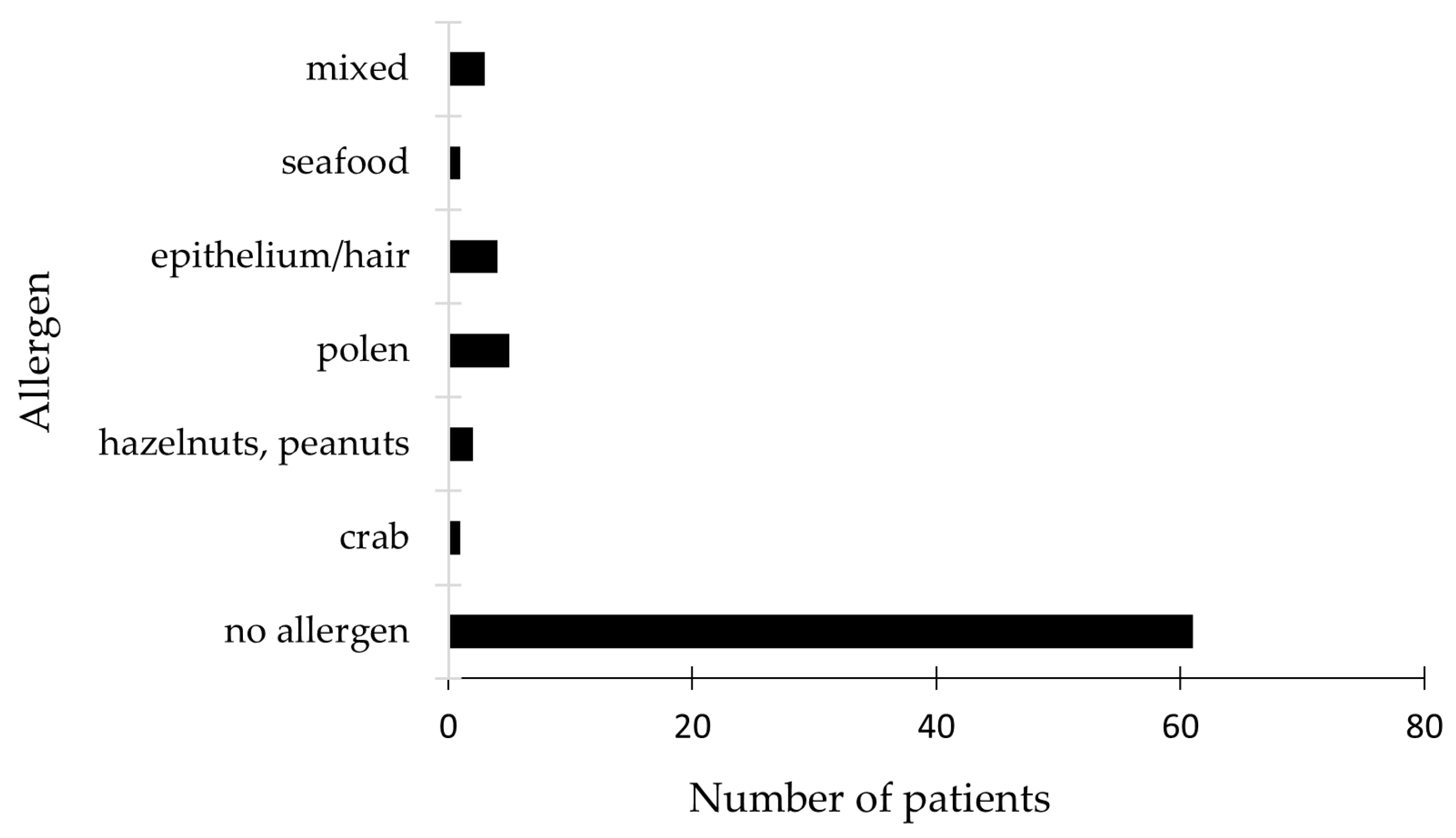
2.4. Allergen Panel Results and Treatment Approaches
3. Discussion
3.1. Demographic Characteristics Correlated with HSP Manifestations
3.2. Immune Response Profiles in Children with Henoch–Schönlein Purpura and Associated Infections
3.3. High IgE Levels Without Allergen Panel Positivity
3.4. Therapeutic Approaches in Managing Pediatric HSP
4. Materials and Methods
4.1. Study Design
4.2. Descriptive Analysis of the Patients’ Series
4.2.1. Inclusion Criteria
4.2.2. Exclusion Criteria
4.2.3. Statistical Analysis of Data
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IgE | Immunoglobulin E |
| IgA | Immunoglobulin A |
| HSP | Henoch–Schönlein purpura |
| IgAV | Immunoglobulin A vasculitis |
Appendix A
| IgE Levels | |
|---|---|
| Age | Values (UI/mL) |
| <1 | <15 |
| 1 to 5 | <60 |
| 6 to 9 | <90 |
| 10 to 15 | <200 |
| >15 | <100 |
| Allergen Lists | |
|---|---|
| Respiratory | Food |
| Pollen Phleum pratense | Peanuts |
| Pollen graminee mix (g × 6) | Hazelnut |
| Pollen Artemisia vulgaris | Carrot |
| Pollen Ambrosia elatior | Potato |
| Parietaria officinalis | Wheat |
| Pollen Betulla verrucosa | Gluten |
| Pollen Alnus incana | Rice |
| Dermatophagoides pteronyssinus | Soya |
| Dermatophagoides farinae | Egg white |
| Fur and epithelium (cat) | Yolk |
| Fur and epithelium (dog) | Cow’s milk |
| Blatella germanica | Alpha-lactalbumin |
| Penicillium chrysogenum, Cladosporium herbarum, Aspergillus fumigatus, Alternaria tenuis | Beta-lactoglobulin |
| Casein | |
| Crab | |
| Seafood | |
| Fish (code) | |
| Shrimps | |
| Mean | Std. Deviation | Std. Error Mean | 95% Confidence Interval of the Difference | t | df | One-Sided p | Two-Sided p | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||||
| Pair 1 | IgAph1Ninf-IgAph2NInf | 0.23983 | 0.35063 | 0.04565 | 0.14846 | 0.33121 | 5.254 | 58 | <0.001 | <0.001 |
| Pair 2 | IgAph1Inf-IgAph2Inf | 0.04593 | 0.15397 | 0.02005 | 0.00581 | 0.08606 | 2.291 | 58 | 0.013 | 0.026 |
| Pair 3 | IgEph1NInf-IgEph2NInf | −1.59322 | 3.16292 | 0.41178 | −2.41748 | −0.76896 | −3.869 | 58 | <0.001 | <0.001 |
| Pair 4 | IgEph1Inf-IgEph2Inf | −10.99458 | 9.45152 | 1.23048 | −13.45766 | −8.53150 | −8.935 | 58 | <0.001 | <0.001 |
| F | Sig. | t | df | Significance | Mean Difference | Std. Error Difference | 95% Confidence Interval of the Difference | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| One-Sided p | Two-Sided p | Lower | Upper | ||||||||
| IgAph1 | Equal variances assumed | 0.029 | 0.866 | 1.413 | 136 | 0.080 | 0.160 | 0.29733 | 0.21037 | −0.11868 | 0.71334 |
| Equal variances not assumed | 1.353 | 101.803 | 0.090 | 0.179 | 0.29733 | 0.21979 | −0.13863 | 0.73329 | |||
| IgAph2 | Equal variances assumed | 0.068 | 0.795 | 2.635 | 136 | 0.005 | 0.009 | 0.49254 | 0.18695 | 0.12282 | 0.86225 |
| Equal variances not assumed | 2.474 | 91.638 | 0.008 | 0.015 | 0.49254 | 0.19909 | 0.09711 | 0.88796 | |||
| IgEph1 | Equal variances assumed | 10.094 | 0.002 | 4.801 | 136 | <0.001 | <0.001 | 166.75904 | 34.73431 | 98.06984 | 235.44824 |
| Equal variances not assumed | 4.256 | 66.547 | <0.001 | <0.001 | 166.75904 | 39.18586 | 88.53393 | 244.98414 | |||
| IgEph2 | Equal variances assumed | 10.039 | 0.002 | 5.031 | 136 | <0.001 | <0.001 | 176.01944 | 34.98582 | 106.83286 | 245.20602 |
| Equal variances not assumed | 4.463 | 66.846 | <0.001 | <0.001 | 176.01944 | 39.43635 | 97.30082 | 254.73805 | |||
References
- Gardner-Medwin, J.M.; Dolezalova, P.; Cummins, C.; Southwood, T.R. Incidence of Henoch-Schönlein purpura, Kawasaki disease, and rare vasculitides in children of different ethnic origins. Lancet 2002, 360, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.C.; Barankin, B.; Leong, K.F. Henoch-Schönlein Purpura in Children: An Updated Review. Curr. Pediatr. Rev. 2020, 16, 265–276. [Google Scholar] [CrossRef]
- Goldstein, A.R.; White, R.H.; Akuse, R.; Chantler, C. Long-term follow-up of childhood Henoch-Schönlein nephritis. Lancet 1992, 339, 280–282. [Google Scholar] [CrossRef] [PubMed]
- Ozen, S.; Pistorio, A.; Iusan, S.M.; Bakkaloglu, A.; Herlin, T.; Brik, R.; Buoncompagni, A.; Lazar, C.; Bilge, I.; Uziel, Y.; et al. EULAR/PRINTO/PRES criteria for Henoch-Schönlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part II: Final classification criteria. Ann. Rheum. Dis. 2010, 69, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Piram, M.; Maldini, C.; Biscardi, S.; De Suremain, N.; Orzechowski, C.; Georget, E.; Regnard, D.; Koné-Paut, I.; Mahr, A. Incidence of IgA vasculitis in children estimated by four-source capture–recapture analysis: A population-based study. Rheumatology 2017, 56, 1358–1366. [Google Scholar] [CrossRef]
- Ruperto, N.; Ozen, S.; Pistorio, A.; Dolezalova, P.; Brogan, P.; Cabral, D.A.; Cuttica, R.; Khubchandani, R.; Lovell, D.J.; O’Neil, K.M.; et al. EULAR/PRINTO/PRES criteria for Henoch-Schönlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part I: Overall methodology and clinical characterisation. Ann. Rheum. Dis. 2010, 69, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Piram, M.; Mahr, A. Epidemiology of immunoglobulin A vasculitis (Henoch-Schönlein): Current state of knowledge. Curr. Opin. Rheumatol. 2013, 25, 171–178. [Google Scholar] [CrossRef]
- Rigante, D.; Castellazzi, L.; Bosco, A.; Esposito, S. Is there a crossroad between infections, genetics, and Henoch-Schönlein purpura? Autoimmun. Rev. 2013, 12, 1016–1021. [Google Scholar] [CrossRef]
- Pillebout, E.; Sunderkötter, C. IgA vasculitis. Semin. Immunopathol. 2021, 43, 729–738. [Google Scholar] [CrossRef]
- Oruc, Z.; Oblet, C.; Boumediene, A.; Druilhe, A.; Pascal, V.; Le Rumeur, E.; Cuvillier, A.; El Hamel, C.; Lecardeur, S.; Leanderson, T.; et al. IgA Structure Variations Associate with Immune Stimulations and IgA Mesangial Deposition. J. Am. Soc. Nephrol. 2016, 27, 2748–2761. [Google Scholar] [CrossRef]
- Oprițescu, S.; Nițescu, G.V.; Golumbeanu, M.; Boghițoiu, D.; Ioniță, E.I.; Ușurelu, D.-A.; Lucaci, C.; Negoiță, A.; Moroșan, E. The Impact of Infectious Diseases on Clinical Characteristics and Immunological Correlations in Pediatric Henoch–Schönlein Purpura: A Five-Year Retrospective Study. Biomedicines 2025, 13, 113. [Google Scholar] [CrossRef]
- Popescu, I.-M.; Margan, M.-M.; Anghel, M.; Mocanu, A.; Laitin, S.M.D.; Margan, R.; Capraru, I.D.; Tene, A.-A.; Gal-Nadasan, E.-G.; Cirnatu, D.; et al. Developing Prediction Models for COVID-19 Outcomes: A Valuable Tool for Resource-Limited Hospitals. Int. J. Gen. Med. 2023, 16, 3053–3065. [Google Scholar] [CrossRef]
- Ruan, J.W.; Fan, G.Z.; Niu, M.M.; Jiang, Q.; Li, R.X.; Qiu, Z.; Hu, P. Serum immunoglobulin profiles in Chinese children with Henoch-Schönlein purpura. Scand. J. Immunol. 2022, 96, e13191. [Google Scholar] [CrossRef] [PubMed]
- Horgos, M.S.; Pop, O.L.; Sandor, M.; Borza, I.L.; Negrean, R.A.; Cote, A.; Neamtu, A.-A.; Grierosu, C.; Sachelarie, L.; Huniadi, A. Platelets Rich Plasma (PRP) Procedure in the Healing of Atonic Wounds. J. Clin. Med. 2023, 12, 3890. [Google Scholar] [CrossRef]
- Bardana, E.J., Jr. Immunoglobulin E- (IgE) and non-IgE-mediated reactions in the pathogenesis of atopic eczema/dermatitis syndrome (AEDS). Allergy 2004, 59 (Suppl. S78), 25–29. [Google Scholar] [CrossRef] [PubMed]
- He, J.S.; Narayanan, S.; Subramaniam, S.; Ho, W.Q.; Lafaille, J.J.; Curotto de Lafaille, M.A. Biology of IgE production: IgE cell differentiation and the memory of IgE responses. Curr. Top. Microbiol. Immunol. 2015, 388, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Devereux, G. The increase in the prevalence of asthma and allergy: Food for thought. Nat. Rev. Immunol. 2006, 6, 869–874. [Google Scholar] [CrossRef]
- Belmesk, L.; Muntyanu, A.; Cantin, E.; AlHalees, Z.; Jack, C.S.; Le, M.; Sasseville, D.; Iannattone, L.; Ben-Shoshan, M.; Litvinov, I.V.; et al. Prominent Role of Type 2 Immunity in Skin Diseases: Beyond Atopic Dermatitis. J. Cutan. Med. Surg. 2022, 26, 33–49. [Google Scholar] [CrossRef]
- Devilliers, H.; Turcu, A.; Vernier, N.; Muller, G.; Bielefeld, P.; Bonniaud, P.; Besancenot, J.F. Hyper-IgE in internal medicine. Rev. Med. Interne 2018, 39, 332–338. [Google Scholar] [CrossRef]
- Freeman, A.F.; Kleiner, D.E.; Nadiminti, H.; Davis, J.; Quezado, M.; Anderson, V.; Puck, J.M.; Holland, S.M. Causes of death in hyper-IgE syndrome. J. Allergy Clin. Immunol. 2007, 119, 1234–1240. [Google Scholar] [CrossRef]
- Freeman, A.F.; Milner, J.D. The Child with Elevated IgE and Infection Susceptibility. Curr. Allergy Asthma Rep. 2020, 20, 65. [Google Scholar] [CrossRef] [PubMed]
- Duréault, A.; Tcherakian, C.; Poiree, S.; Catherinot, E.; Danion, F.; Jouvion, G.; Bougnoux, M.E.; Mahlaoui, N.; Givel, C.; Castelle, M.; et al. Spectrum of Pulmonary Aspergillosis in Hyper-IgE Syndrome with Autosomal-Dominant STAT3 Deficiency. J. Allergy Clin. Immunol. Pract. 2019, 7, 1986–1995.e1983. [Google Scholar] [CrossRef]
- Sherrill, D.L.; Halonen, M.; Burrows, B. Relationships between total serum IgE, atopy, and smoking: A twenty-year follow-up analysis. J. Allergy Clin. Immunol. 1994, 94, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Zetterström, O.; Osterman, K.; Machado, L.; Johansson, S.G. Another smoking hazard: Raised serum IgE concentration and increased risk of occupational allergy. Br. Med. J. (Clin. Res. Ed.) 1981, 283, 1215–1217. [Google Scholar] [CrossRef] [PubMed]
- Burrows, B.; Halonen, M.; Lebowitz, M.D.; Knudson, R.J.; Barbee, R.A. The relationship of serum immunoglobulin E, allergy skin tests, and smoking to respiratory disorders. J. Allergy Clin. Immunol. 1982, 70, 199–204. [Google Scholar] [CrossRef]
- Omenaas, E.; Bakke, P.; Elsayed, S.; Hanoa, R.; Gulsvik, A. Total and specific serum IgE levels in adults: Relationship to sex, age and environmental factors. Clin. Exp. Allergy 1994, 24, 530–539. [Google Scholar] [CrossRef]
- Bergerson, J.R.E.; Freeman, A.F. An Update on Syndromes with a Hyper-IgE Phenotype. Immunol. Allergy Clin. N. Am. 2019, 39, 49–61. [Google Scholar] [CrossRef]
- Eckl-Dorna, J.; Villazala-Merino, S.; Campion, N.J.; Byazrova, M.; Filatov, A.; Kudlay, D.; Karsonova, A.; Riabova, K.; Khaitov, M.; Karaulov, A.; et al. Tracing IgE-Producing Cells in Allergic Patients. Cells 2019, 8, 994. [Google Scholar] [CrossRef] [PubMed]
- Audemard-Verger, A.; Pillebout, E.; Guillevin, L.; Thervet, E.; Terrier, B. IgA vasculitis (Henoch-Shönlein purpura) in adults: Diagnostic and therapeutic aspects. Autoimmun. Rev. 2015, 14, 579–585. [Google Scholar] [CrossRef]
- Hamdan, J.M.; Barqawi, M.A. Henoch-Schonlein purpura in children. Influence of age on the incidence of nephritis and arthritis. Saudi Med. J. 2008, 29, 549–552. [Google Scholar]
- He, X.; Yu, C.; Zhao, P.; Ding, Y.; Liang, X.; Zhao, Y.; Yue, X.; Wu, Y.; Yin, W. The genetics of Henoch-Schönlein purpura: A systematic review and meta-analysis. Rheumatol. Int. 2013, 33, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Jauhola, O.; Ronkainen, J.; Koskimies, O.; Ala-Houhala, M.; Arikoski, P.; Hölttä, T.; Jahnukainen, T.; Rajantie, J.; Ormälä, T.; Nuutinen, M. Clinical course of extrarenal symptoms in Henoch-Schonlein purpura: A 6-month prospective study. Arch. Dis. Child. 2010, 95, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Berthoux, F.; Suzuki, H.; Thibaudin, L.; Yanagawa, H.; Maillard, N.; Mariat, C.; Tomino, Y.; Julian, B.A.; Novak, J. Autoantibodies targeting galactose-deficient IgA1 associate with progression of IgA nephropathy. J. Am. Soc. Nephrol. 2012, 23, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Yu, G.; Zhang, X.; Xie, X.; Wang, J.; Shi, S.; Liu, L.; Lv, J.; Zhang, H. Plasma Galactose-Deficient IgA1 and C3 and CKD Progression in IgA Nephropathy. Clin. J. Am. Soc. Nephrol. 2019, 14, 1458–1465. [Google Scholar] [CrossRef]
- Calviño, M.C.; Llorca, J.; García-Porrúa, C.; Fernández-Iglesias, J.L.; Rodriguez-Ledo, P.; González-Gay, M.A. Henoch-Schönlein purpura in children from northwestern Spain: A 20-year epidemiologic and clinical study. Medicine 2001, 80, 279–290. [Google Scholar] [CrossRef]
- Chen, O.; Zhu, X.B.; Ren, P.; Wang, Y.B.; Sun, R.P.; Wei, D.E. Henoch Schonlein Purpura in children: Clinical analysis of 120 cases. Afr. Health Sci. 2013, 13, 94–99. [Google Scholar] [CrossRef]
- Nikolaishvili, M.; Pazhava, A.; Di Lernia, V. Viral Infections May Be Associated with Henoch-Schönlein Purpura. J. Clin. Med. 2023, 12, 697. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Luo, L.; Fu, M.; Li, Z.; Liu, J. Analysis of children with Henoch–Schonlein purpura secondary to infection. Clin. Rheumatol. 2022, 41, 803–810. [Google Scholar] [CrossRef]
- Farooq, H.; Aemaz Ur Rehman, M.; Asmar, A.; Asif, S.; Mushtaq, A.; Qureshi, M.A. The pathogenesis of COVID-19-induced IgA nephropathy and IgA vasculitis: A systematic review. J. Taibah Univ. Med. Sci. 2022, 17, 1–13. [Google Scholar] [CrossRef]
- Garzoni, L.; Vanoni, F.; Rizzi, M.; Simonetti, G.D.; Goeggel Simonetti, B.; Ramelli, G.P.; Bianchetti, M.G. Nervous system dysfunction in Henoch-Schonlein syndrome: Systematic review of the literature. Rheumatology 2009, 48, 1524–1529. [Google Scholar] [CrossRef]
- Aleyd, E.; Heineke, M.H.; van Egmond, M. The era of the immunoglobulin A Fc receptor FcαRI; its function and potential as target in disease. Immunol. Rev. 2015, 268, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Oprițescu, S.; Nițescu, G.V.; Cîrnațu, D.; Trifunschi, S.; Munteanu, M.; Golumbeanu, M.; Boghițoiu, D.; Dărăban, A.M.; Ilie, E.I.; Moroșan, E. Elevated Immunoglobulin E Serum Levels: Possible Underlying Factors That Can Cause an Inborn Error of Immunity in the Pediatric Population with Recurrent Infections. Antibodies 2024, 13, 47. [Google Scholar] [CrossRef]
- Peru, H.; Soylemezoglu, O.; Bakkaloglu, S.A.; Elmas, S.; Bozkaya, D.; Elmaci, A.M.; Kara, F.; Buyan, N.; Hasanoglu, E. Henoch Schonlein purpura in childhood: Clinical analysis of 254 cases over a 3-year period. Clin. Rheumatol. 2008, 27, 1087–1092. [Google Scholar] [CrossRef]
- Mao, Y.; Yin, L.; Huang, H.; Zhou, Z.; Chen, T.; Zhou, W. Henoch-Schönlein purpura in 535 Chinese children: Clinical features and risk factors for renal involvement. J. Int. Med. Res. 2014, 42, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Lawee, D. Atypical clinical course of Henoch-Schonlein purpura. Can. Fam. Physician 2008, 54, 1117–1120. [Google Scholar]
- Davin, J.C.; Pierard, G.; Dechenne, C.; Grossman, D.; Nagy, J.; Quacoe, M.; Malaise, M.; Hall, M.; Jansen, F.; Chantraine, J.M.; et al. Possible pathogenic role of IgE in Henoch-Schönlein purpura. Pediatr. Nephrol. 1994, 8, 169–171. [Google Scholar] [CrossRef]
- AlYafie, R.; Velayutham, D.; van Panhuys, N.; Jithesh, P.V. The genetics of hyper IgE syndromes. Front. Immunol. 2025, 16, 1516068. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, H.; Mohebbi, M.; Mehravaran, S.; Mazloumi, M.; Jahanbani-Ardakani, H.; Abtahi, S.H. Hyperimmunoglobulin E syndrome: Genetics, immunopathogenesis, clinical findings, and treatment modalities. J. Res. Med. Sci. 2017, 22, 53. [Google Scholar] [CrossRef]
- Boyd, J.K.; Barratt, J. Inherited IgA glycosylation pattern in IgA nephropathy and HSP nephritis: Where do we go next? Kidney Int. 2011, 80, 8–10. [Google Scholar] [CrossRef]
- Chandesris, M.O.; Melki, I.; Natividad, A.; Puel, A.; Fieschi, C.; Yun, L.; Thumerelle, C.; Oksenhendler, E.; Boutboul, D.; Thomas, C.; et al. Autosomal dominant STAT3 deficiency and hyper-IgE syndrome: Molecular, cellular, and clinical features from a French national survey. Medicine 2012, 91, e1–e19. [Google Scholar] [CrossRef]
- Tsilifis, C.; Freeman, A.F.; Gennery, A.R. STAT3 Hyper-IgE Syndrome-an Update and Unanswered Questions. J. Clin. Immunol. 2021, 41, 864–880. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Hong, L.; Chen, T.X. Clinical Manifestation of Hyper IgE Syndrome Including Otitis Media. Curr. Allergy Asthma Rep. 2018, 18, 51. [Google Scholar] [CrossRef]
- Kostova, P.; Papochieva, V.; Miteva, D.; Georgieva, B.; Mileva, S.; Shahid, M.; Lukanov, T.; Petrova, G. Elevated IgE Levels-An Allergy or an Underlying Inborn Error of Immunity in Children with Recurrent Infections? Antibodies 2023, 12, 70. [Google Scholar] [CrossRef]
- Lin, I.H.; Tsai, M.-C.; Chen, J.-P.; Fu, L.-S. Allergic children with extremely high total IgE but no allergen identified in the initial screening panel. J. Microbiol. Immunol. Infect. 2021, 54, 474–481. [Google Scholar] [CrossRef]
- Crago, S.S.; Kutteh, W.H.; Moro, I.; Allansmith, M.R.; Radl, J.; Haaijman, J.J.; Mestecky, J. Distribution of IgA1-, IgA2-, and J chain-containing cells in human tissues. J. Immunol. 1984, 132, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Davin, J.C.; Ten Berge, I.J.; Weening, J.J. What is the difference between IgA nephropathy and Henoch-Schönlein purpura nephritis? Kidney Int. 2001, 59, 823–834. [Google Scholar] [CrossRef]
- de Sousa-Pereira, P.; Woof, J.M. IgA: Structure, Function, and Developability. Antibodies 2019, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Donadio, J.V.; Grande, J.P. IgA nephropathy. N. Engl. J. Med. 2002, 347, 738–748. [Google Scholar] [CrossRef]
- Invitrogen. Human IgE ELISA Kit. Available online: https://www.thermofisher.com/elisa/product/Human-IgE-ELISA-Kit/BMS2097 (accessed on 10 November 2024).
- Invitrogen. Human IgA ELISA Kit. Available online: https://www.thermofisher.com/elisa/product/Human-IgA-ELISA-Kit/BMS2096 (accessed on 10 November 2024).
- Invitrogen. Human Complement C3 ELISA Kit. Available online: https://www.thermofisher.com/elisa/product/Human-Complement-C3a-ELISA-Kit/BMS2089 (accessed on 10 November 2024).
- Invitrogen. Human Complement C4 ELISA Kit. Available online: https://www.thermofisher.com/elisa/product/Human-Complement-C4-ELISA-Kit/EEL075 (accessed on 10 November 2024).
- Merrett, J.; Merrett, T.G. Phadiatop—A novel IgE antibody screening test. Clin. Exp. Allergy 1987, 17, 409–416. [Google Scholar] [CrossRef]
- Scientific, T. ImmunoCAP Phadiatop. Available online: https://www.thermofisher.com/phadia/us/en/product-catalog.html?articleNumber=14-4405-35®ion=RO (accessed on 7 April 2025).
- Ozen, S.; Ruperto, N.; Dillon, M.J.; Bagga, A.; Barron, K.; Davin, J.C.; Kawasaki, T.; Lindsley, C.; Petty, R.E.; Prieur, A.M.; et al. EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann. Rheum. Dis. 2006, 65, 936–941. [Google Scholar] [CrossRef]


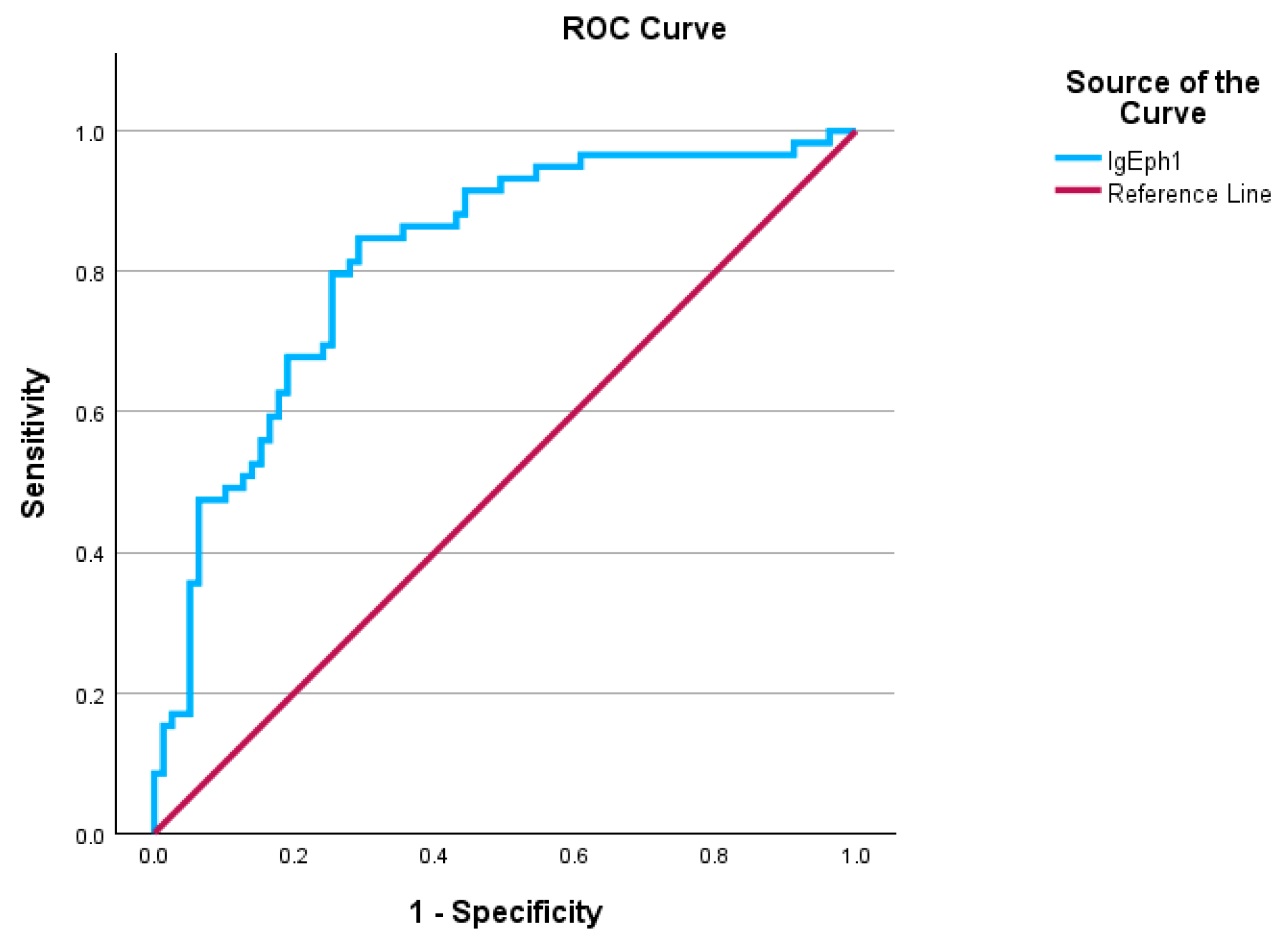
| Area | Std. Error a | Asymptotic Sig. b | Asymptotic 95% Confidence Interval | |
|---|---|---|---|---|
| Lower Bound | Upper Bound | |||
| 0.817 | 0.031 | 0.000 | 0.778 | 0.907 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oprițescu, S.; Nițescu, G.V.; Golumbeanu, M.; Boghițoiu, D.; Ioniță, E.I.; Licu, M.; Chirigiu, L.-M.-E.; Popovici, V.; Marin, L.-M.; Moroșan, E. Serum IgE and IgA Levels in Pediatric Henoch–Schönlein Purpura: Clinical Characteristics and Immunological Correlations in the Context of Infectious Diseases—A Five-Year Retrospective Analysis. Int. J. Mol. Sci. 2025, 26, 6053. https://doi.org/10.3390/ijms26136053
Oprițescu S, Nițescu GV, Golumbeanu M, Boghițoiu D, Ioniță EI, Licu M, Chirigiu L-M-E, Popovici V, Marin L-M, Moroșan E. Serum IgE and IgA Levels in Pediatric Henoch–Schönlein Purpura: Clinical Characteristics and Immunological Correlations in the Context of Infectious Diseases—A Five-Year Retrospective Analysis. International Journal of Molecular Sciences. 2025; 26(13):6053. https://doi.org/10.3390/ijms26136053
Chicago/Turabian StyleOprițescu, Sînziana, Gabriela Viorela Nițescu, Mihaela Golumbeanu, Dora Boghițoiu, Elena Iuliana Ioniță, Monica Licu, Larisa-Marina-Elisabeth Chirigiu, Violeta Popovici, Loredana-Maria Marin, and Elena Moroșan. 2025. "Serum IgE and IgA Levels in Pediatric Henoch–Schönlein Purpura: Clinical Characteristics and Immunological Correlations in the Context of Infectious Diseases—A Five-Year Retrospective Analysis" International Journal of Molecular Sciences 26, no. 13: 6053. https://doi.org/10.3390/ijms26136053
APA StyleOprițescu, S., Nițescu, G. V., Golumbeanu, M., Boghițoiu, D., Ioniță, E. I., Licu, M., Chirigiu, L.-M.-E., Popovici, V., Marin, L.-M., & Moroșan, E. (2025). Serum IgE and IgA Levels in Pediatric Henoch–Schönlein Purpura: Clinical Characteristics and Immunological Correlations in the Context of Infectious Diseases—A Five-Year Retrospective Analysis. International Journal of Molecular Sciences, 26(13), 6053. https://doi.org/10.3390/ijms26136053









