Solvent Performance Evaluation of Heavy Oil in Coal–Oil Co-Liquefaction
Abstract
1. Introduction
2. Results
2.1. Column Chromatography and Co-Liquefaction Experiments of Heavy Oil
2.1.1. Analysis of Group Component Contents in Heavy Oil
2.1.2. Co-Liquefaction Performance
2.2. Characteristic and Structural Analyses of Heavy Oil
2.2.1. FT-IR Analysis of Heavy Oil
2.2.2. 13C-NMR Analysis of Heavy Oil
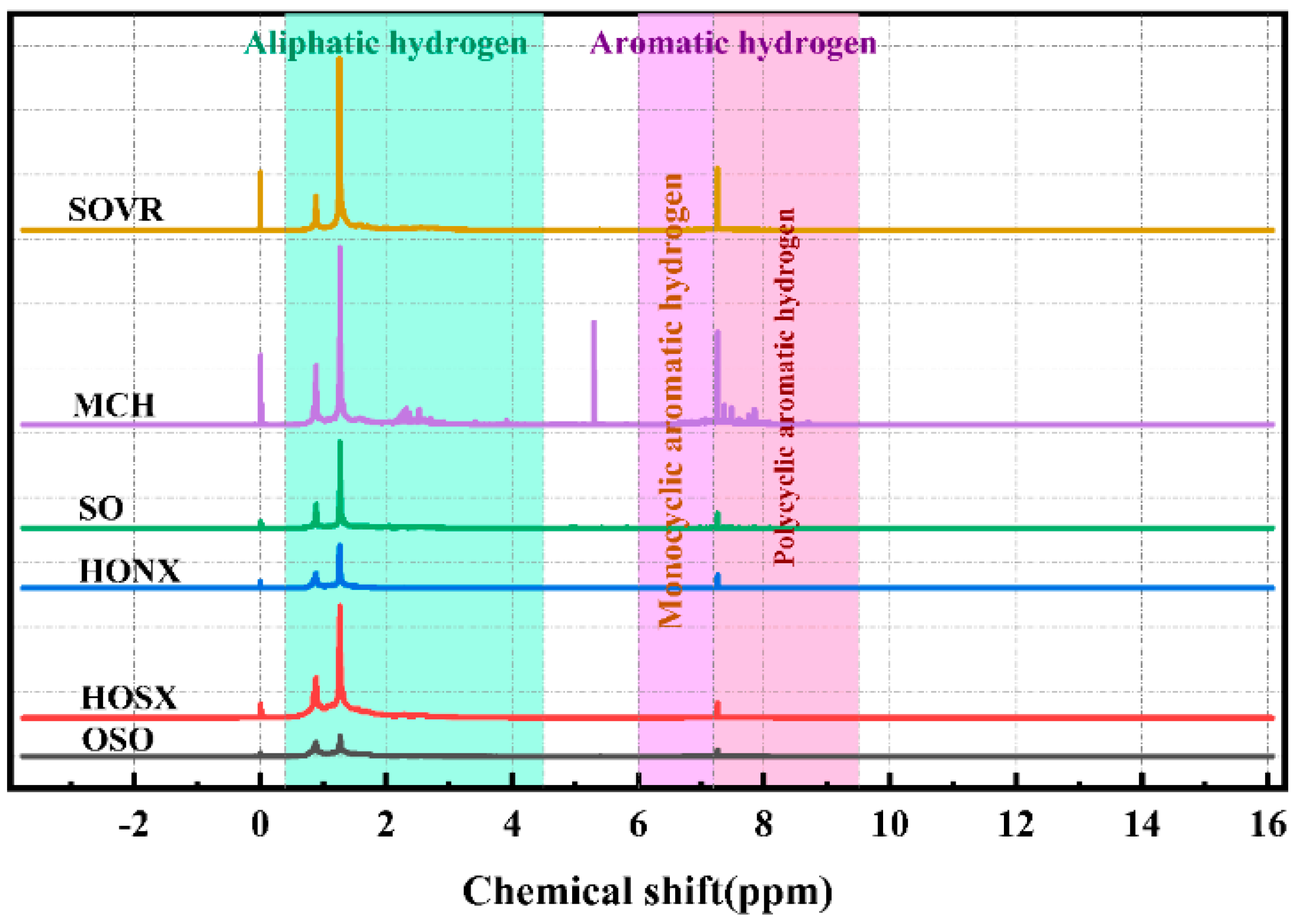
2.2.3. 1H-NMR Analysis of Heavy Oil
2.3. Evaluation and Analysis of Heavy Oil Co-Liquefaction Performance
3. Discussion
4. Materials and Methods
4.1. Material
4.2. Experimental Process and Analytical Method
4.2.1. COCL Experiment
4.2.2. Characterization of Heavy Oil
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Song, W.; Liu, P.G.; Chen, X.Y.; Wang, T.; He, C.R.; Hao, R.; Liu, K.Y. Research progress of catalysts for direct coal liquefaction. J. Energy Chem. 2025, 100, 481–497. [Google Scholar] [CrossRef]
- Zhang, X.B.; Wang, A.Q.; Wang, X.B.; Li, W.Y. Theoretical analysis of hydrogen solubility in direct coal liquefaction solvents. Int. J. Coal Sci. Technol. 2024, 11, 28. [Google Scholar] [CrossRef]
- Kong, H.; Sun, Y.Q.; Li, Z.; Zheng, H.F.; Wang, J.; Wang, H.S. The development path of direct coal liquefaction system under carbon neutrality target: Coupling green hydrogen or CCUS technology. Appl. Energy 2023, 347, 121451. [Google Scholar] [CrossRef]
- JIA, M.T.; Gao, S.S.; Zhang, Y.J.; Zhang, D.X. Co-hydrogenation behavior of Hami coal with Tahe residue. J. Fuel Chem. Technol. 2021, 49, 902–908. [Google Scholar] [CrossRef]
- Zhou, X.D.; Wu, H.; Liu, J.-M.; Huang, X.L.; Fan, X.; Jin, L.J.; Zhu, Y.F.; Ma, F.Y.; Zhong, M. Study on oxygen species in the products of co-liquefaction of coal and petroleum residues. Energy 2022, 260, 124945. [Google Scholar] [CrossRef]
- Wang, G.Y.; Zhang, X.J.; Chen, G.F.; Li, P.L.; Yan, B.F. Influence of raw oil structure on hydrogen donation capacity during coal oil co-processing. Clean Coal Technol. 2015, 21, 83–87. [Google Scholar]
- Painter, P.C.; Graf, J.; Coleman, M.M. Coal Solubility and Swelling. 1. Solubility Parameters for Coal and the Flory χ Parameter. Energy Fuels 1990, 4, 379–384. [Google Scholar] [CrossRef]
- Guin, J.; Tarrer, A.; Taylor, L.; Prather, J.; Green, S. Mechanisms of Coal Particle Dissolution. Ind. Eng. Chem. Process Des. Dev. 1976, 15, 490–494. [Google Scholar] [CrossRef]
- McMillen, D.F.; Malhotra, R.; Tse, D.S. Interactive effects between solvent components: Possible chemical origin of synergy in liquefaction and coprocessing. Energy Fuels 1991, 5, 179–187. [Google Scholar] [CrossRef]
- Doust, A.M.; Rahimi, M.; Feyzi, M. Effects of solvent addition and ultrasound waves on viscosity reduction of residue fuel oil. Chem. Eng. Process. 2015, 95, 353–361. [Google Scholar] [CrossRef]
- Shen, T.; Hu, Z.W.; Liu, Q.Y.; Yan, Y.X.; Liu, Z.Y. Evaluation of hydrogen donation ability of real hydrogen storage solvents by radical precursors. Fuel 2022, 327, 125070. [Google Scholar] [CrossRef]
- Niu, B.; Jin, L.J.; Li, Y.; Shi, Z.W.; Hu, H.Q. Isotope analysis for understanding the hydrogen transfer mechanism in direct liquefaction of Bulianta coal. Fuel 2017, 203, 82–89. [Google Scholar] [CrossRef]
- Li, H.J.; Zhang, X.B.; Wang, X.B.; Li, W.Y. Research and prospects of coal direct liquefaction solvents. J. China Coal Soc. 2022, 47, 3791–3804. [Google Scholar]
- Flynn, T.; Kemp, W.; Steedman, W.; Bartle, K.D.; Burke, M.; Taylor, N.; Wallace, S. Thermal extraction of bituminous coal with petroleum-derived solvents. Fuel Process. Technol. 1989, 23, 197–204. [Google Scholar] [CrossRef]
- Grint, A.; Jackson, W.R.; Larkins, F.P.; Louey, M.B. Effect of aromatic solvents in coal liquefaction: Residence-time studies. Fuel 1994, 73, 381–386. [Google Scholar] [CrossRef]
- Li, W.; Li, W.Y.; Wang, X.B.; Feng, J. Regulation of radicals by hydrogen-donor solvent in direct coal liquefaction. Front. Chem. Sci. Eng. 2022, 16, 1689–1699. [Google Scholar] [CrossRef]
- Wiehe, I.A. A phase-separation kinetic model for coke formation. Ind. Eng. Chem. Res. 1993, 32, 2447–2454. [Google Scholar] [CrossRef]
- Quan, H.P.; Chen, L.; Huang, Z.Y.; Zheng, C.C. The effect of a kind of hyperbranched polyester with different carbon length on flowability for crude oil. Fuel 2018, 214, 356–362. [Google Scholar] [CrossRef]
- Tian, B.; Qiao, Y.Y.; Tian, Y.Y.; Xie, K.C.; Liu, Q.; Zhou, H.F. FTIR study on structural changes of different–rank coals caused by single/multiple extraction with cyclohexanone and NMP/CS2 mixed solvent. Fuel Process. Technol. 2016, 154, 210–218. [Google Scholar] [CrossRef]
- Li, S.K.; Zhu, Y.M.; He, R. Mechanistic analysis of chemical structure evolution for coals under igneous intrusion. Fuel 2024, 357, 130055. [Google Scholar] [CrossRef]
- Shi, Q.L.; Qin, B.T.; Bi, Q.; Qu, B. An experimental study on the effect of igneous intrusions on chemical structure and combustion characteristics of coal in Daxing Mine. China Fuel 2018, 226, 307–315. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, D.; Wang, P.; Bai, J.R.; Wang, Z.C.; Liu, B. A comparison of the structures of >300 °C fractions in six Chinese shale oils obtained from different locations using 1H NMR, 13C NMR and FT-IR analyses. Fuel 2018, 211, 341–352. [Google Scholar] [CrossRef]
- Axelson, D.E. Solid state 13C nuclear magnetic resonance of heavy oil/bitumen-derived solids. Fuel 1987, 66, 40–43. [Google Scholar] [CrossRef]
- Jameel, A.G.A.; Elbaz, A.M.; Emwas, A.H.; Roberts, W.L.; Sarathy, S.M. Calculation of Average Molecular Parameters, Functional Groups, and a Surrogate Molecule for Heavy Fuel Oils Using 1H and 13C Nuclear Magnetic Resonance Spectroscopy. Energy Fuels 2016, 30, 3894–3905. [Google Scholar] [CrossRef]
- Jones, M.; Taylor, S.E. NMR relaxometry and diffusometry in characterizing structural, interfacial and colloidal properties of heavy oils and oil sands. Adv. Colloid Interface Sci. 2015, 224, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.C.; Ge, Y.; Shui, H.F.; Ren, S.B.; Pan, C.X.; Kang, S.G.; Lei, Z.P.; Zhao, Z.J.; Hu, J.C. Molecular structure and size of asphaltene and preasphaltene from direct coal liquefaction. Fuel Process. Technol. 2015, 137, 305–311. [Google Scholar] [CrossRef]
- Derbyshire, F.J.; Varghese, P.; Whitehurst, D.D. Synergistic effects between light and heavy solvent components during coal liquefaction. Fuel 1982, 61, 859–864. [Google Scholar] [CrossRef]
- Liu, H.Y.; Zhang, R.; Zheng, X.; Xie, J.; Tang, B.W.; Li, Z. Hydrogen-donating behavior and hydrogen-shuttling mechanism of liquefaction solvents under Fe- and Ni-based catalysts. Fuel 2024, 368, 131586. [Google Scholar] [CrossRef]
- Zhou, X.D.; Yin, X.L.; Liu, L.; Huang, X.L.; Liu, J.M.; Liu, T.; Lin, H.; Ma, F.Y. Using isotope-tracer method with deuterium for quantitative study of hydrogen activation mechanism in direct coal liquefaction. Fuel 2025, 382, 133646. [Google Scholar] [CrossRef]
- GB/T 33690-2017; Test Method of Coal Liquefaction Activity by Autoclave. National Standards of People’s Republic of China: Beijing, China, 2017.
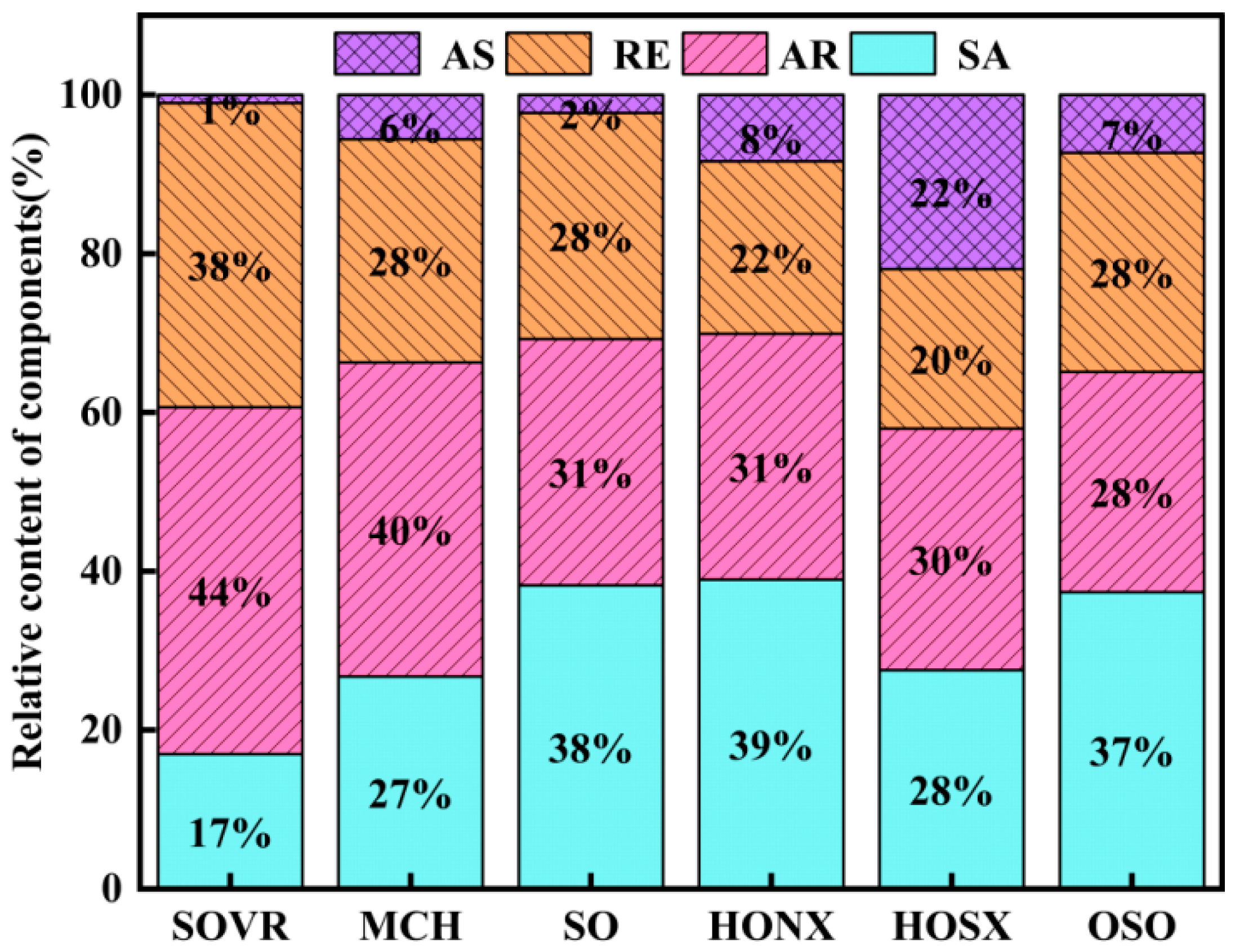
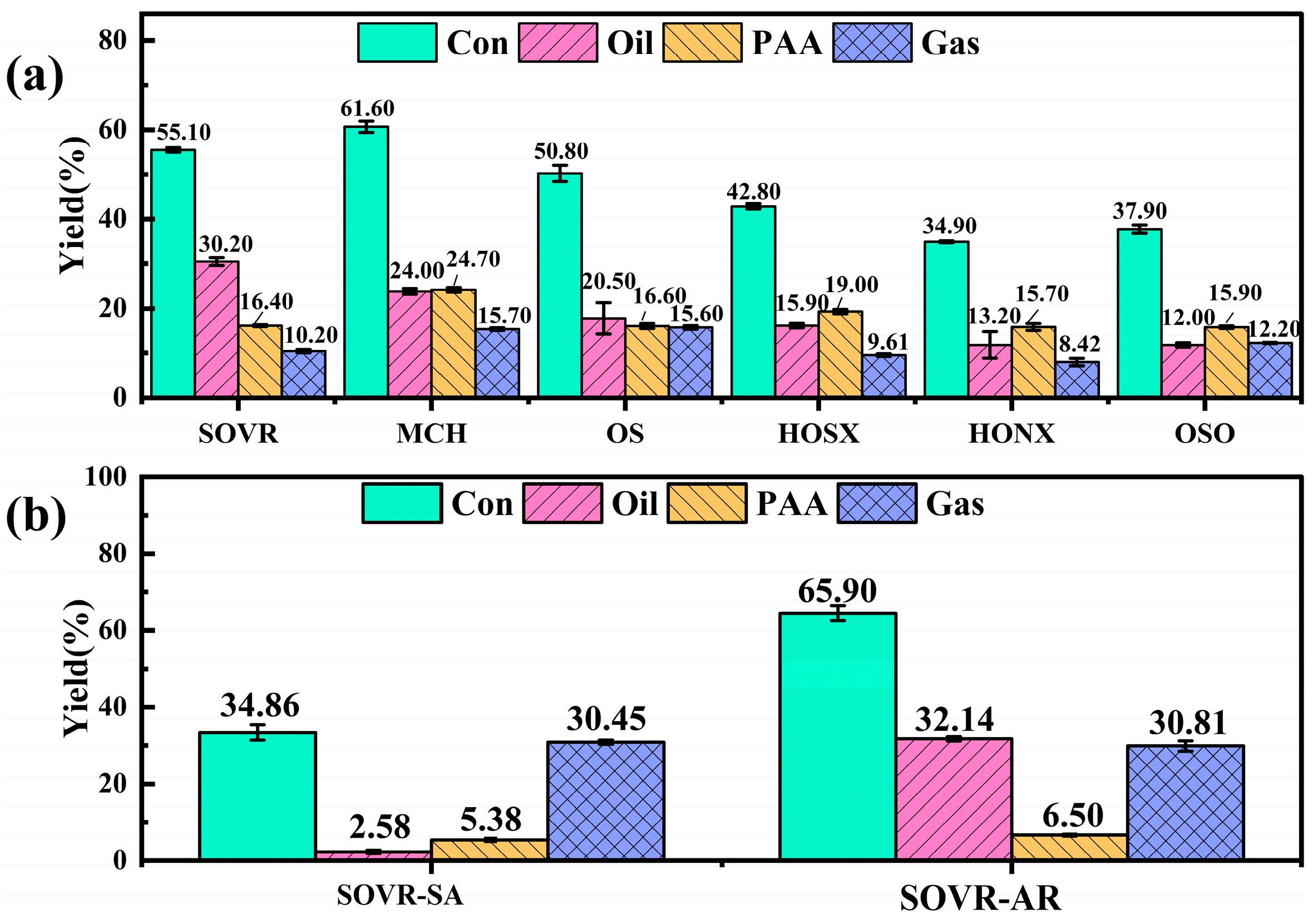
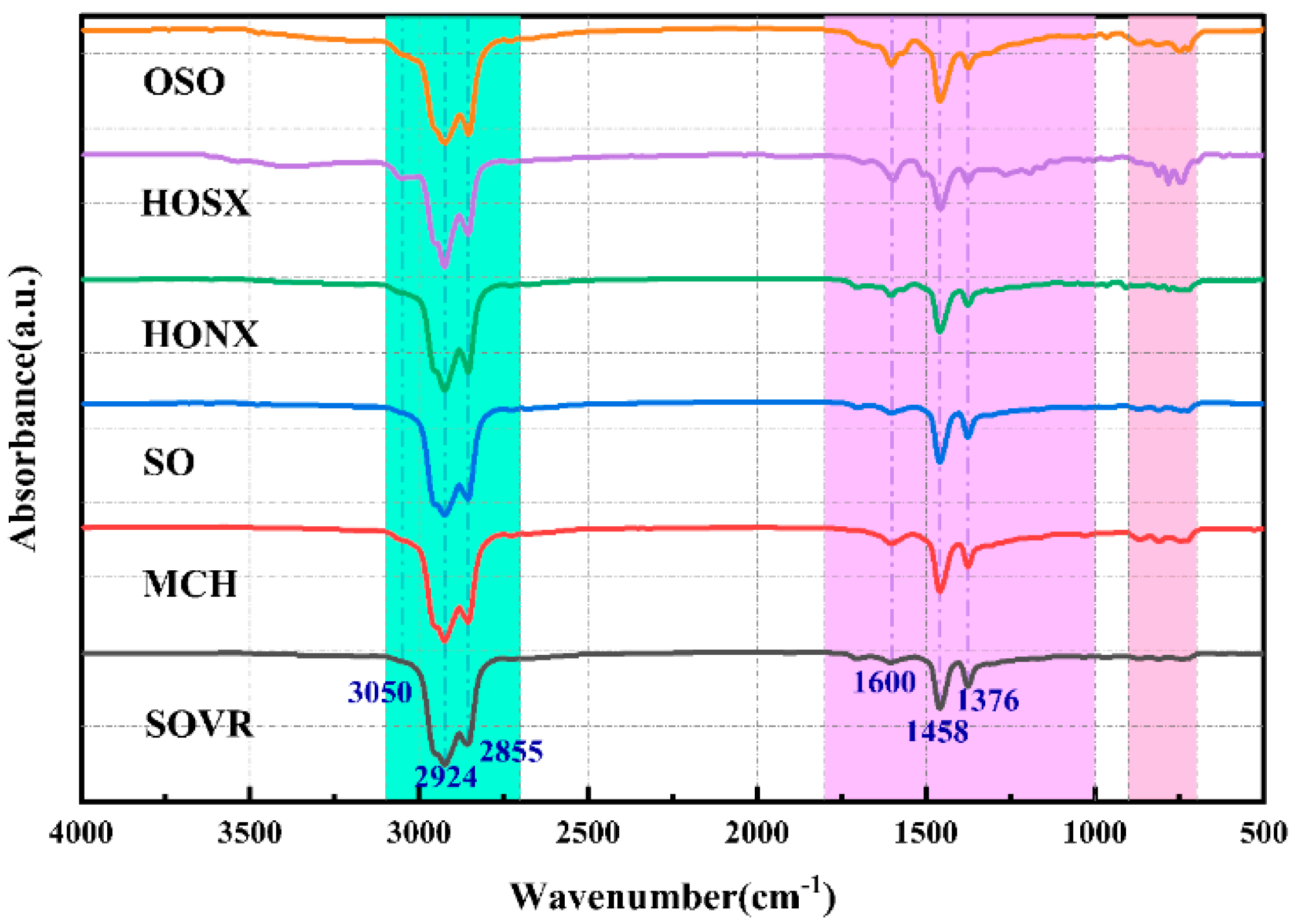
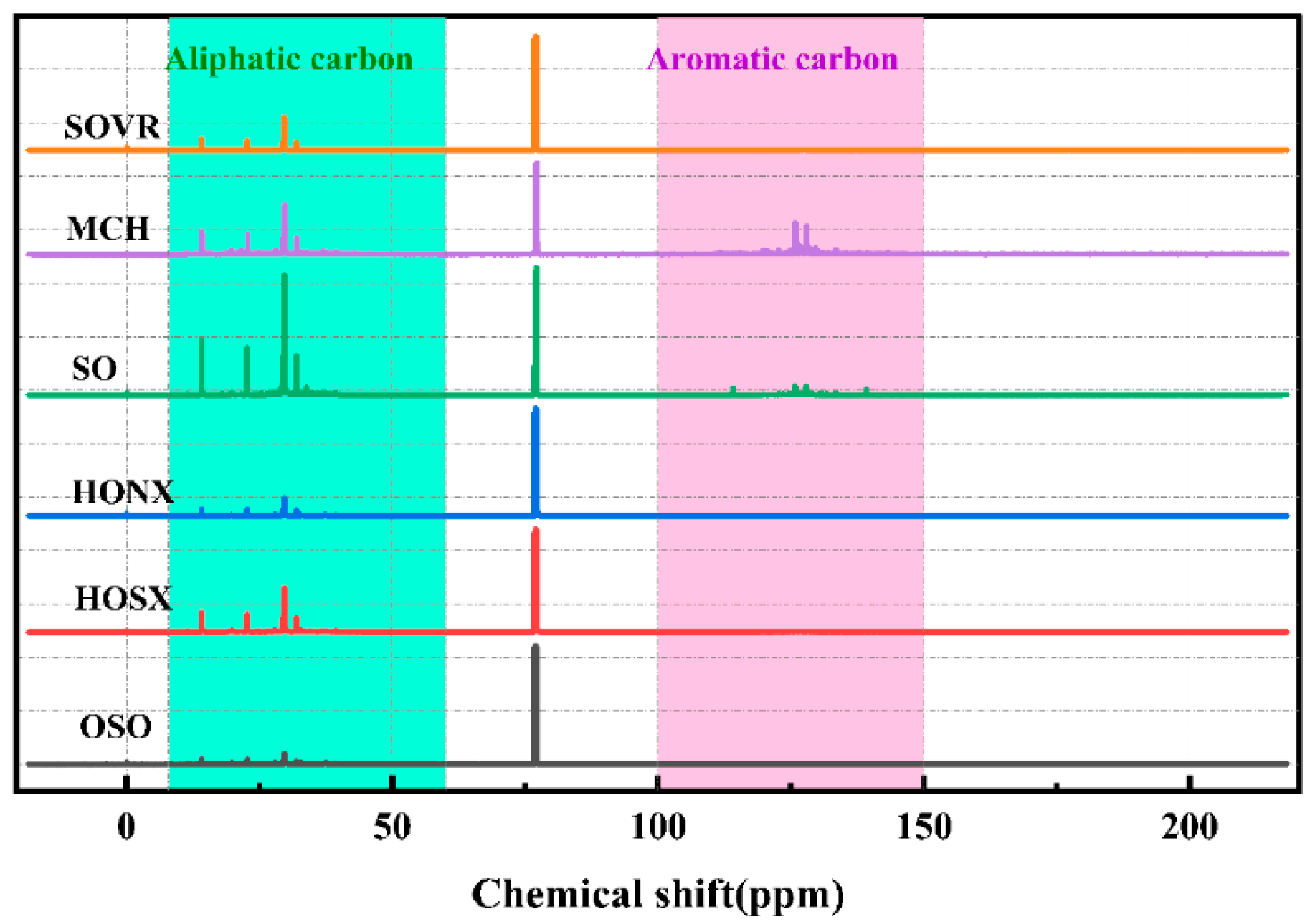

| Band Position/cm−1 | Functional Group | Area Percentage/% | |||||
|---|---|---|---|---|---|---|---|
| SOVR | MCH | SO | HONX | HOSX | OSO | ||
| 2950–2930 | Aliphatic -CH3 | 57.61 | 54.64 | 57.90 | 55.15 | 37.44 | 60.84 |
| 2870–2850 | Symmetric aliphatic -CH2 | 22.05 | 14.30 | 19.66 | 13.38 | 14.36 | 17.53 |
| 1600 | Aromatic C=C | 2.33 | 4.32 | 2.76 | 2.75 | 7.02 | 1.22 |
| 1480–1400 | Asymmetric CH3, CH2 | 9.66 | 13.19 | 11.27 | 9.28 | 9.50 | 11.93 |
| 1400–1240 | Symmetric deformation -CH3 | 1.95 | 4.79 | 5.20 | 16.29 | 25.54 | 5.62 |
| 900–860 | Five adjacent H deformations | 0.55 | 0.00 | 0.27 | 0.69 | 1.07 | 0.67 |
| 860–810 | Four adjacent H deformations | 0.76 | 1.80 | 0.58 | 0.79 | 1.95 | 0.65 |
| 810–750 | Three adjacent H deformations | 3.86 | 2.36 | 0.47 | 0.00 | 2.37 | 0.91 |
| 750–720 | Two adjacent H deformations | 1.23 | 4.60 | 1.89 | 1.66 | 0.74 | 0.63 |
| Symbol | δC | Relative Content (%) | |||||
|---|---|---|---|---|---|---|---|
| SOVR | MCH | SO | HONX | HOSX | OSO | ||
| faliC | 8.0–60.0 | 44.25 | 50.77 | 53.77 | 56.77 | 59.77 | 62.77 |
| fC1 | 8.0–15.0 | 3.57 | 4.57 | 5.57 | 6.57 | 7.57 | 8.57 |
| fC2 | 15.0–22.5 | 3.93 | 4.93 | 5.93 | 6.93 | 7.93 | 8.93 |
| fC3 | 22.5–60.0 | 36.75 | 37.75 | 38.75 | 39.75 | 40.75 | 41.75 |
| farC | 100.0–150.0 | 55.75 | 49.24 | 33.25 | 44.32 | 38.46 | 45.50 |
| fC1 + fC2 | - | 7.50 | 9.50 | 11.50 | 13.50 | 15.50 | 17.50 |
| Symbol | δH | Relative Content (%) | |||||
|---|---|---|---|---|---|---|---|
| SOVR | MCH | SO | HONX | HOSX | OSO | ||
| Hγ | 0.4–1.0 | 10.99 | 17.94 | 17.80 | 26.51 | 23.54 | 31.33 |
| Hβ | 1.0–1.9 | 57.49 | 44.03 | 59.41 | 57.33 | 53.49 | 54.61 |
| Hα | 1.9–4.5 | 18.99 | 24.80 | 14.75 | 9.996 | 16.24 | 9.58 |
| HA | 6.0–9.5 | 12.54 | 13.23 | 8.03 | 6.20 | 6.73 | 4.47 |
| HA1 | 6.0–7.2 | 4.39 | 5.83 | 3.28 | 2.00 | 2.59 | 1.90 |
| HA2 | 7.2–7.7 | 2.31 | 3.16 | 2.09 | 1.45 | 1.01 | 1.07 |
| HA3 | 7.7–9.5 | 5.84 | 4.24 | 2.66 | 2.75 | 3.13 | 1.50 |
| HA2 + HA3 | - | 8.15 | 7.4 | 4.75 | 4.2 | 4.14 | 2.57 |
| Industrial Analysis w/% | Elemental Analysis wdaf/% | H/C (mol Ratio) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mad | Ad | Vdaf | FCdaf | C | H | N | S | Oa | |
| 10.58 | 6.51 | 48.97 | 42.66 | 73.76 | 6.16 | 0.82 | 0.48 | 18.78 | 1.00 |
| Sample | Elemental Analysis wdaf/% | H/C (mol Ratio) | ||||
|---|---|---|---|---|---|---|
| C | H | O | N | S | ||
| SOVR | 85.87 | 12.09 | <0.5 | 2.07 | <0.5 | 1.69 |
| MCH | 85.12 | 13.68 | <0.5 | 1.25 | <0.5 | 1.93 |
| SO | 85.05 | 13.68 | <0.5 | 1.25 | <0.5 | 1.93 |
| HONX | 85.36 | 13.48 | <0.5 | 0.68 | 0.99 | 1.89 |
| HOSX | 85.16 | 12.85 | <0.5 | 0.43 | 2.29 | 1.81 |
| OSO | 86.33 | 13.08 | <0.5 | 1.08 | <0.5 | 1.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Ma, J.; Chen, C.; Cui, T.; He, Y.; Liu, T. Solvent Performance Evaluation of Heavy Oil in Coal–Oil Co-Liquefaction. Int. J. Mol. Sci. 2025, 26, 6048. https://doi.org/10.3390/ijms26136048
Yang G, Ma J, Chen C, Cui T, He Y, Liu T. Solvent Performance Evaluation of Heavy Oil in Coal–Oil Co-Liquefaction. International Journal of Molecular Sciences. 2025; 26(13):6048. https://doi.org/10.3390/ijms26136048
Chicago/Turabian StyleYang, Guanghua, Juan Ma, Caitao Chen, Tingting Cui, Yingluo He, and Ting Liu. 2025. "Solvent Performance Evaluation of Heavy Oil in Coal–Oil Co-Liquefaction" International Journal of Molecular Sciences 26, no. 13: 6048. https://doi.org/10.3390/ijms26136048
APA StyleYang, G., Ma, J., Chen, C., Cui, T., He, Y., & Liu, T. (2025). Solvent Performance Evaluation of Heavy Oil in Coal–Oil Co-Liquefaction. International Journal of Molecular Sciences, 26(13), 6048. https://doi.org/10.3390/ijms26136048






