Multi-Target Protective Effects of β-Caryophyllene (BCP) at the Intersection of Neuroinflammation and Neurodegeneration
Abstract
1. Introduction
2. Results
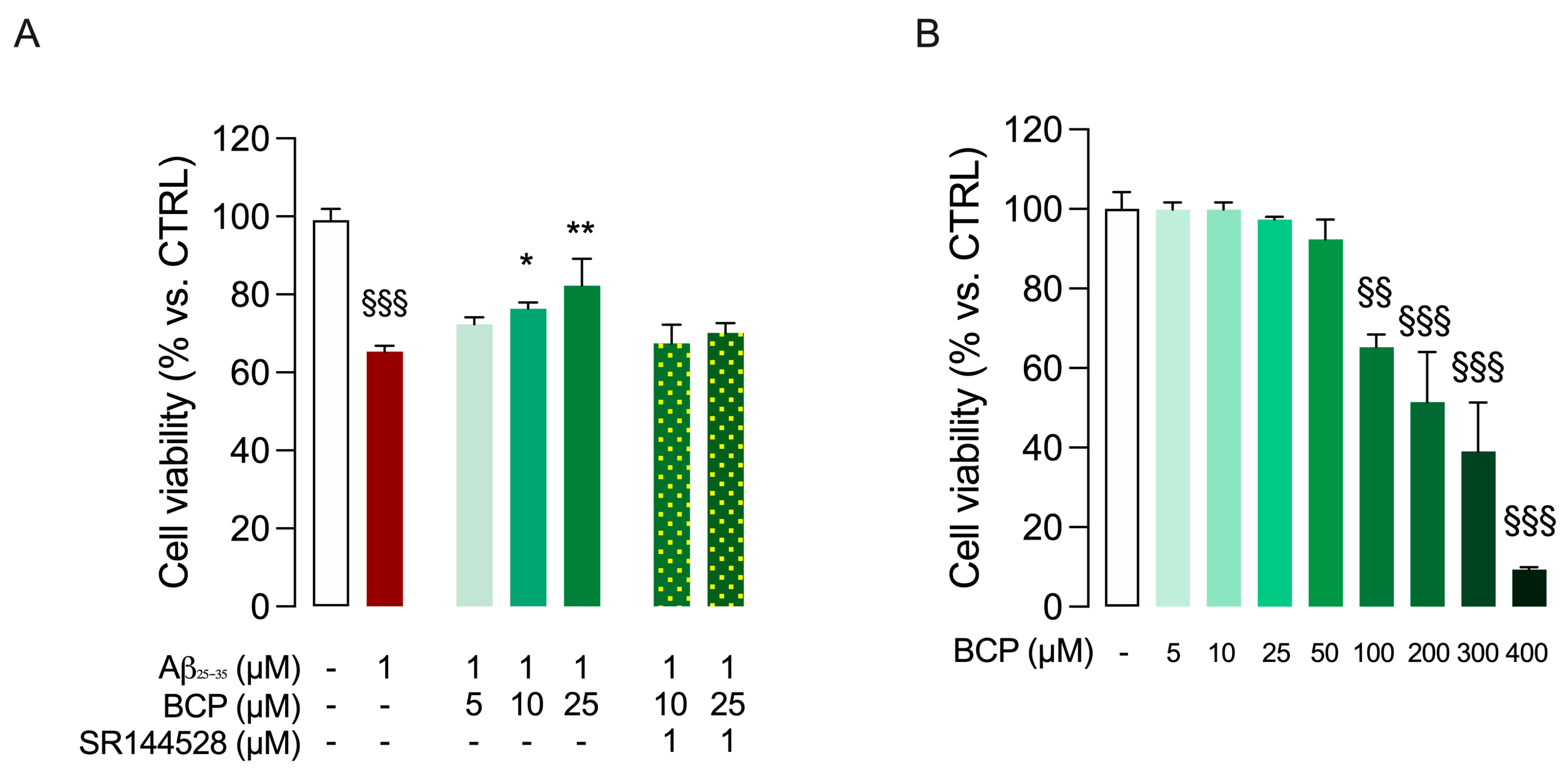
2.1. BCP-CB2R System Protects HMC3 Cells from Aβ25-35-Induced Cytotoxic Effect
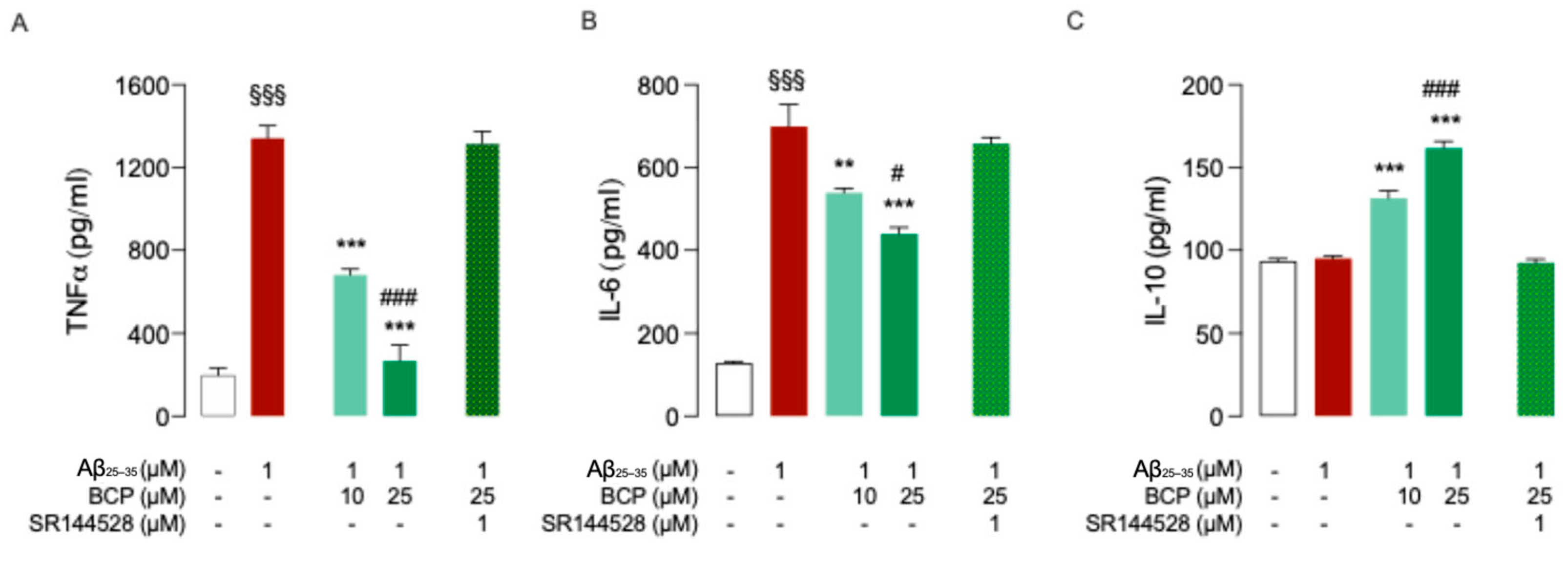
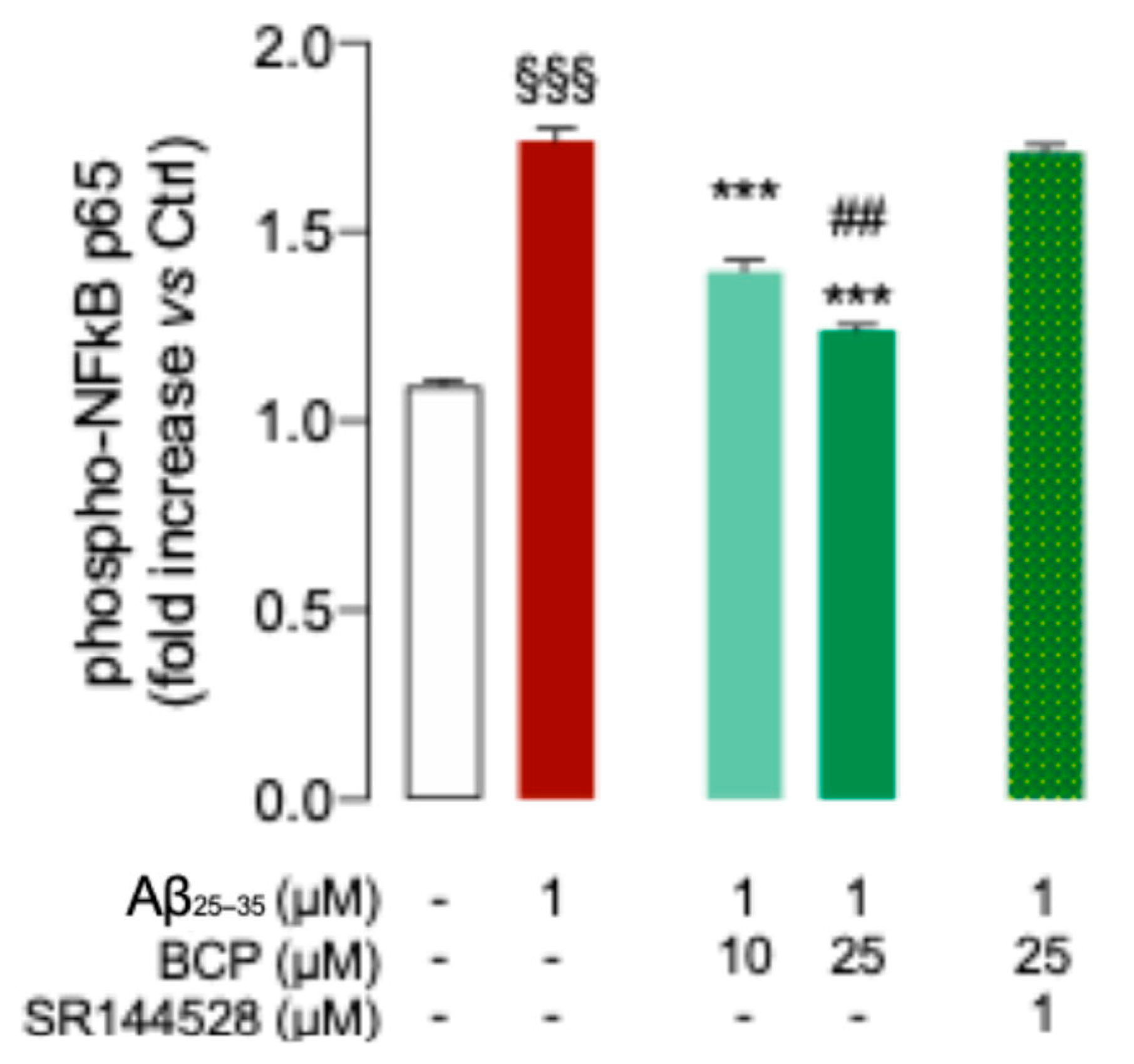
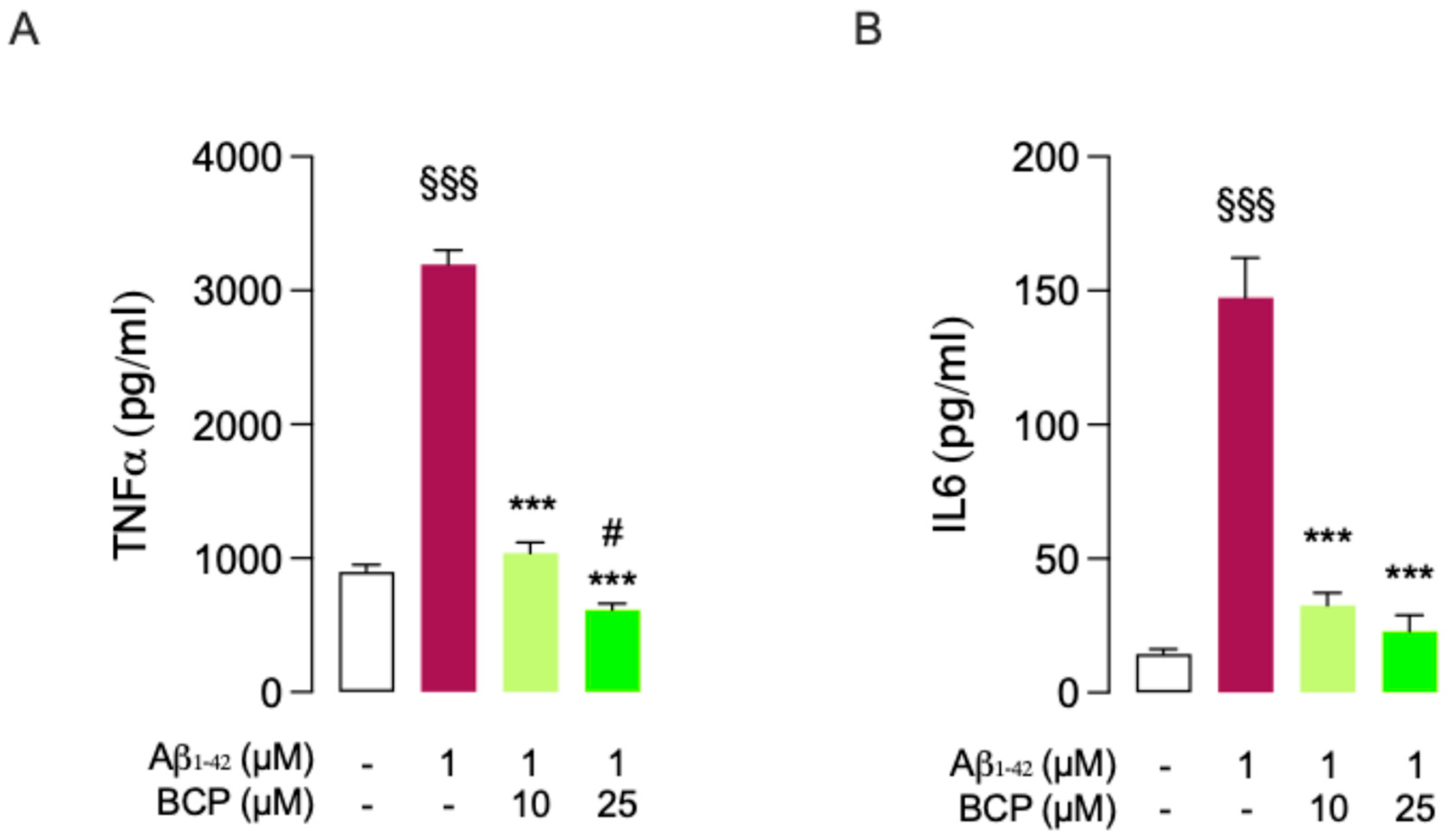
2.2. BCP-CB2R System Protects HMC3 Cells from Aβ25-35-Induced Pro-Inflammatory Response
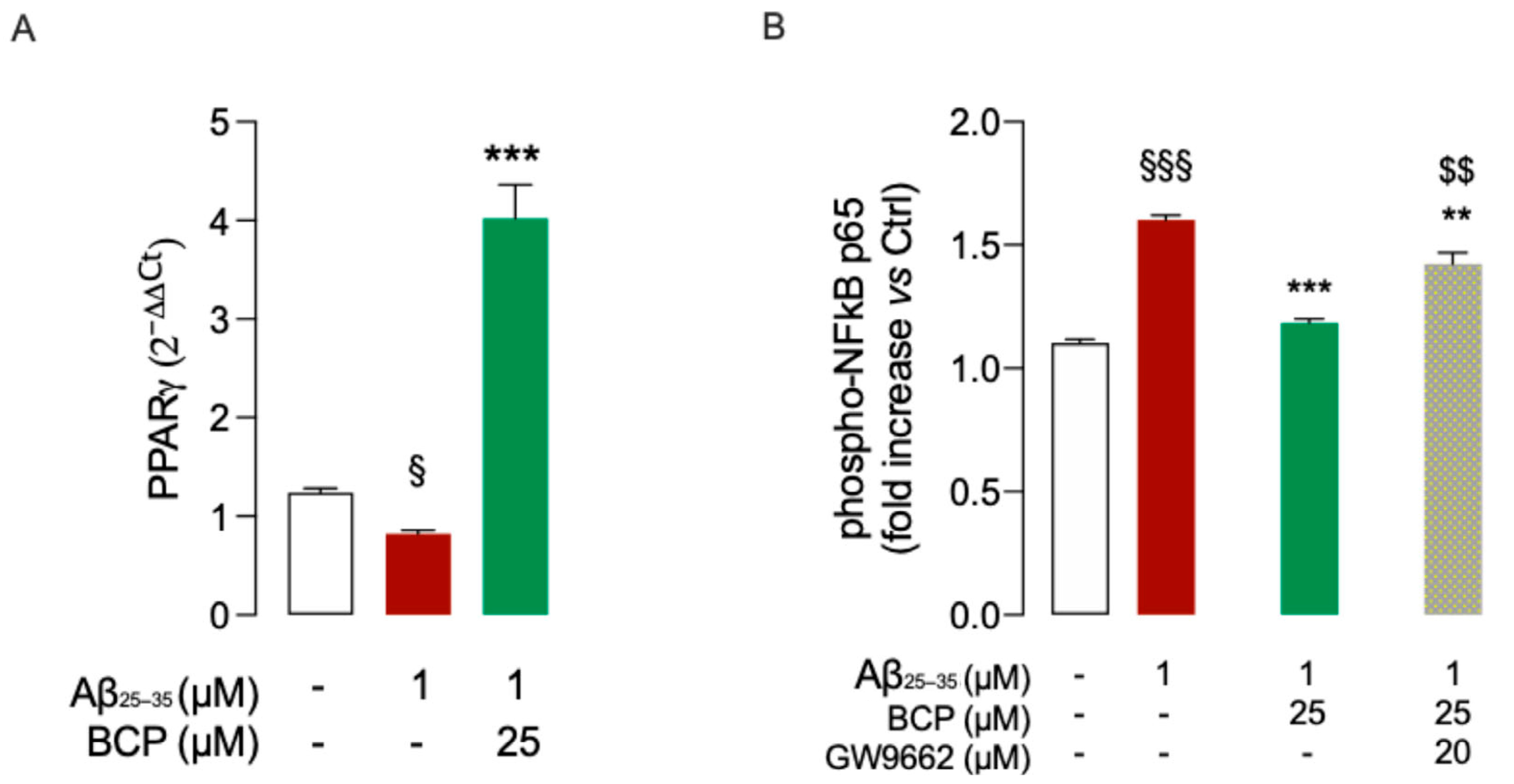
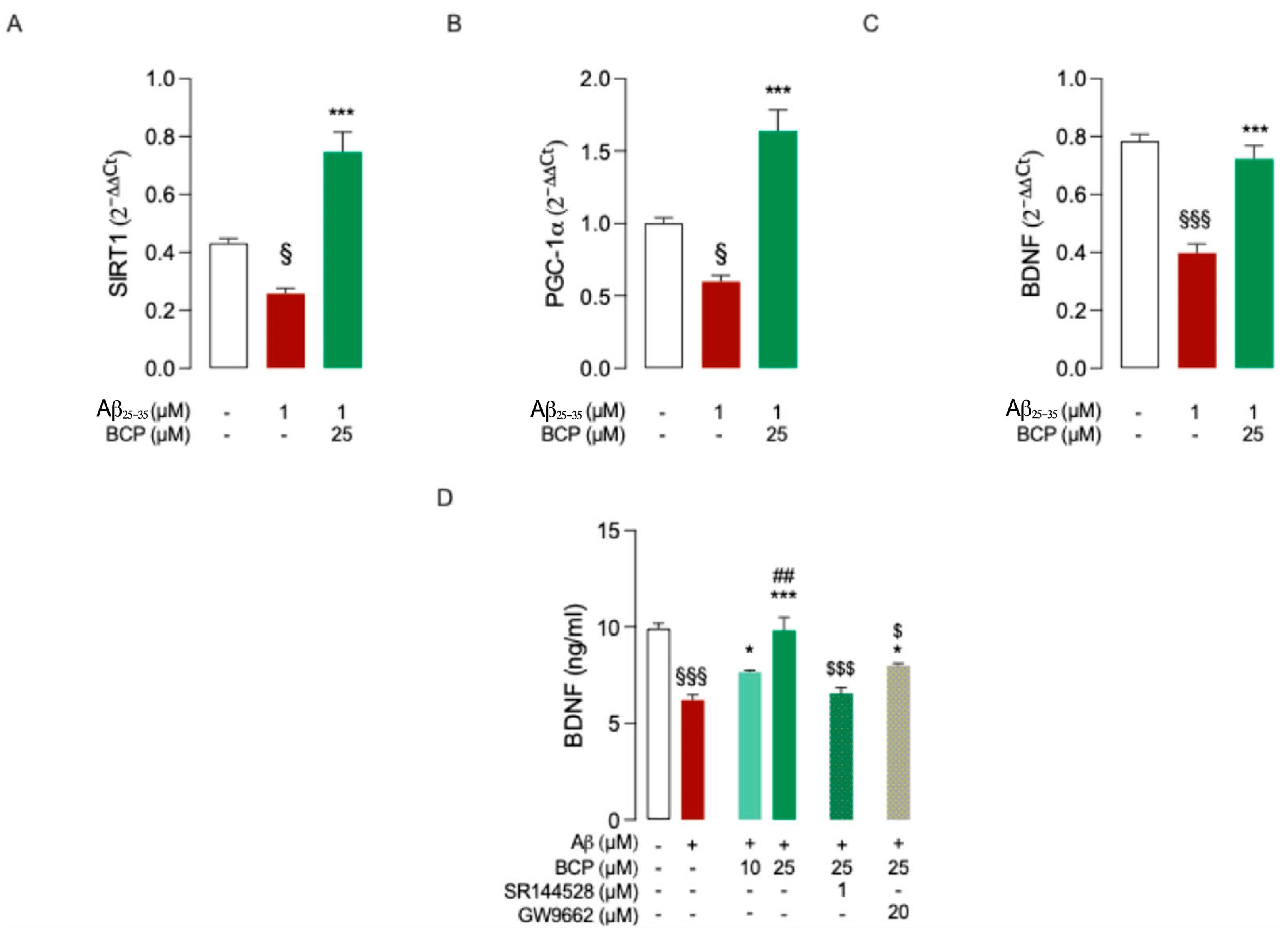
2.3. BCP Modulates BDNF’s Activity upon Activation of the PGC-1α and SIRT1 Pathways
2.4. BCP Prevents Aβ1-42-Mediated Release of Pro-Inflammatory Factors in Mouse Organotypic Brain Slice Cultures (BSCs)
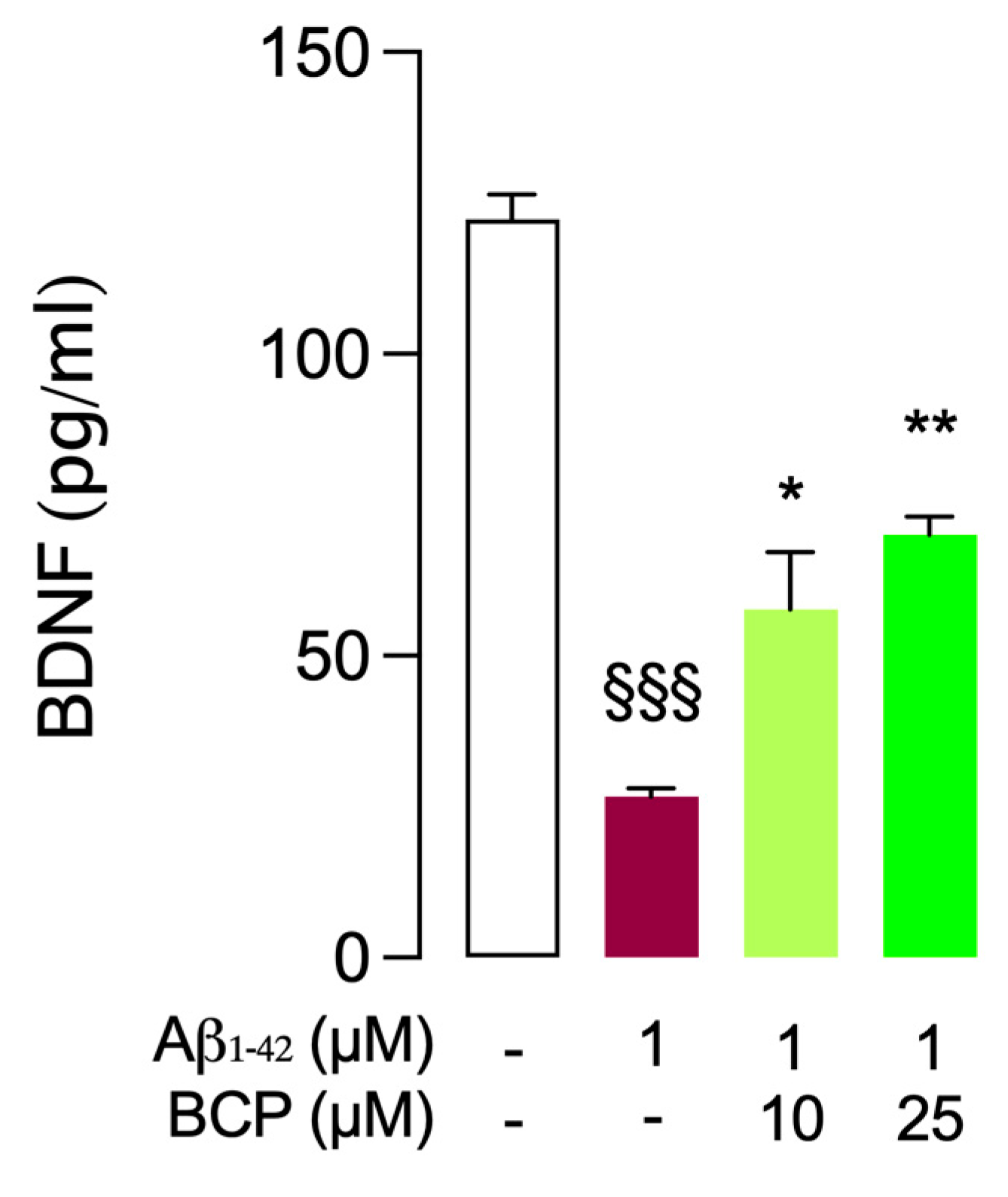
2.5. Protective Effect of BCP Against Aβ1-42-Induced Neurotoxicity: Modulation of BDNF Activity in Mouse BSCs
2.6. BCP Acute Perfusion Counteracts Aβ1-42-Induced Inhibition of Long-Term Potentiation (LTP) in Mouse Brain Slices
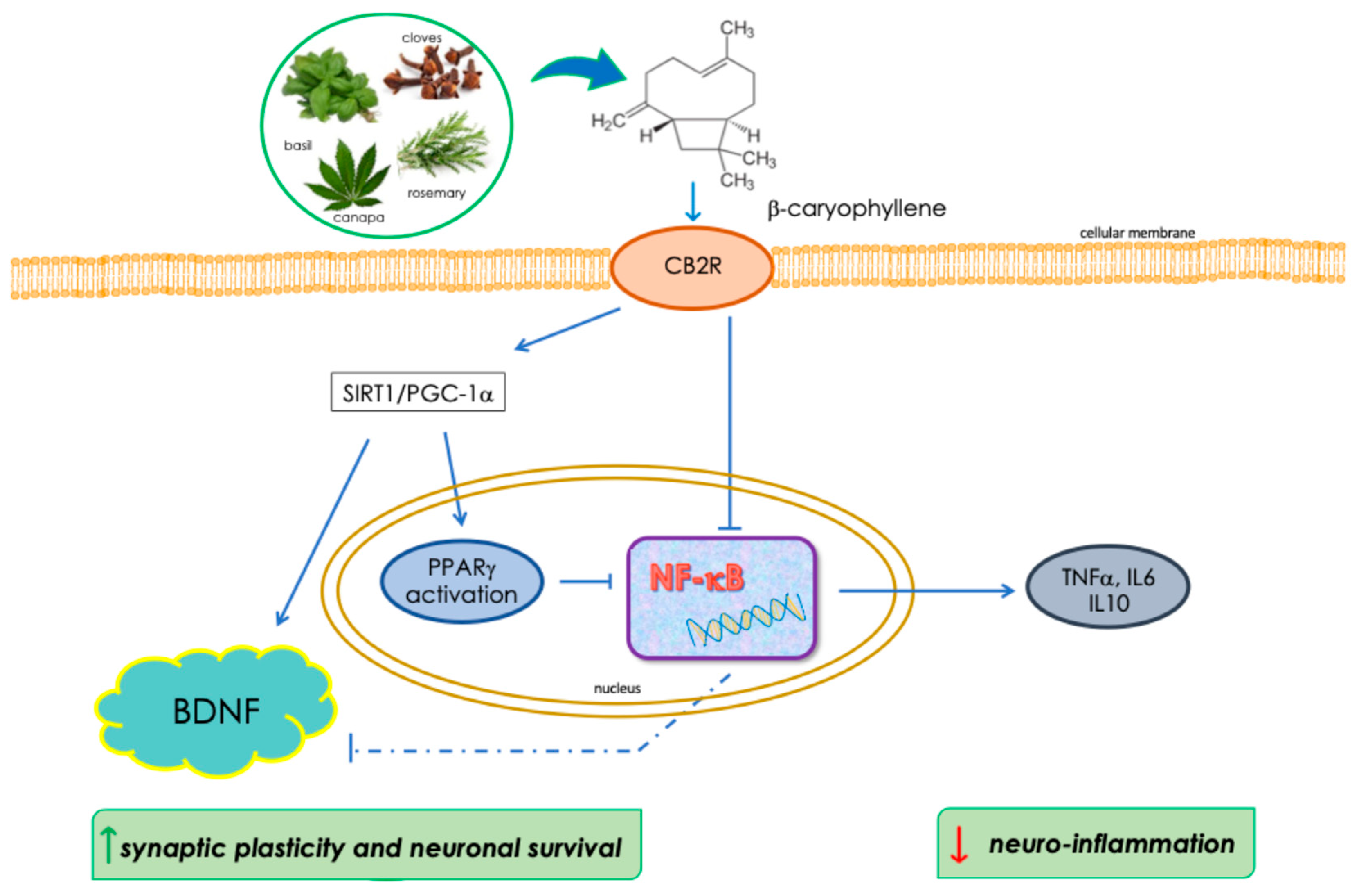
3. Discussion
4. Materials and Methods
4.1. Test Compounds
4.2. Cell Cultures and Reagents
4.3. MTT Assay (Cell Viability Assay)
4.4. Gene Expression Analysis
4.5. Animals
4.6. Brain Horizontal EC Slices Preparation
4.7. Organotypic Cultures
4.8. In Vitro Electrophysiology
4.9. ELISA Assays
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grabon, W.; Rheims, S.; Smith, J.; Bodennec, J.; Belmeguenai, A.; Bezin, L. CB2 Receptor in the CNS: From Immune and Neuronal Modulation to Behavior. Neurosci. Biobehav. Rev. 2023, 150, 105226. [Google Scholar] [CrossRef]
- Rakotoarivelo, V.; Mayer, T.Z.; Simard, M.; Flamand, N.; Di Marzo, V. The Impact of the CB2 Cannabinoid Receptor in Inflammatory Diseases: An Update. Molecules 2024, 29, 3381. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Cao, Z.; Wang, W.; Zhou, N. New Insights in Cannabinoid Receptor Structure and Signaling. Curr. Mol. Pharmacol. 2019, 12, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Cassano, T.; Calcagnini, S.; Pace, L.; De Marco, F.; Romano, A.; Gaetani, S. Cannabinoid Receptor 2 Signaling in Neurodegenerative Disorders: From Pathogenesis to a Promising Therapeutic Target. Front. Neurosci. 2017, 11. [Google Scholar] [CrossRef]
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A Review of the Common Neurodegenerative Disorders: Current Therapeutic Approaches and the Potential Role of Nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef]
- Khan, S.; Barve, K.H.; Kumar, M.S. Recent Advancements in Pathogenesis, Diagnostics and Treatment of Alzheimer’s Disease. Curr. Neuropharmacol. 2020, 18, 1106–1125. [Google Scholar] [CrossRef]
- Scipioni, L.; Ciaramellano, F.; Carnicelli, V.; Leuti, A.; Lizzi, A.R.; De Dominicis, N.; Oddi, S.; Maccarrone, M. Microglial Endocannabinoid Signalling in AD. Cells 2022, 11, 1237. [Google Scholar] [CrossRef]
- Aso, E.; Ferrer, I. Cannabinoids for Treatment of Alzheimer’s Disease: Moving toward the Clinic. Front. Pharmacol. 2014, 5, 37. [Google Scholar] [CrossRef]
- Hashiesh, H.M.; Sharma, C.; Goyal, S.N.; Sadek, B.; Jha, N.K.; Kaabi, J.A.; Ojha, S. A Focused Review on CB2 Receptor-Selective Pharmacological Properties and Therapeutic Potential of β-Caryophyllene, a Dietary Cannabinoid. Biomed. Pharmacother. 2021, 140, 111639. [Google Scholar] [CrossRef]
- Ullah, H.; Di Minno, A.; Santarcangelo, C.; Khan, H.; Daglia, M. Improvement of Oxidative Stress and Mitochondrial Dysfunction by β-Caryophyllene: A Focus on the Nervous System. Antioxidants 2021, 10, 546. [Google Scholar] [CrossRef]
- Solas, M.; Francis, P.T.; Franco, R.; Ramirez, M.J. CB2 Receptor and Amyloid Pathology in Frontal Cortex of Alzheimer’s Disease Patients. Neurobiol. Aging 2013, 34, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Klauke, A.-L.; Racz, I.; Pradier, B.; Markert, A.; Zimmer, A.M.; Gertsch, J.; Zimmer, A. The Cannabinoid CB2 Receptor-Selective Phytocannabinoid Beta-Caryophyllene Exerts Analgesic Effects in Mouse Models of Inflammatory and Neuropathic Pain. Eur. Neuropsychopharmacol. 2014, 24, 608–620. [Google Scholar] [CrossRef]
- Scandiffio, R.; Geddo, F.; Cottone, E.; Querio, G.; Antoniotti, S.; Gallo, M.P.; Maffei, M.E.; Bovolin, P. Protective Effects of (E)-β-Caryophyllene (BCP) in Chronic Inflammation. Nutrients 2020, 12, 3273. [Google Scholar] [CrossRef]
- Youssef, D.A.; El-Fayoumi, H.M.; Mahmoud, M.F. Beta-Caryophyllene Alleviates Diet-Induced Neurobehavioral Changes in Rats: The Role of CB2 and PPAR-γ Receptors. Biomed. Pharmacother. 2019, 110, 145–154. [Google Scholar] [CrossRef]
- O’Sullivan, S.E. An Update on PPAR Activation by Cannabinoids. Br. J. Pharmacol. 2016, 173, 1899–1910. [Google Scholar] [CrossRef]
- Esposito, E.; Cuzzocrea, S. Targeting the Peroxisome Proliferator-Activated Receptors (PPARs) in Spinal Cord Injury. Expert Opin. Ther. Targets 2011, 15, 943–959. [Google Scholar] [CrossRef]
- Fakhfouri, G.; Ahmadiani, A.; Rahimian, R.; Grolla, A.A.; Moradi, F.; Haeri, A. WIN55212-2 Attenuates Amyloid-Beta-Induced Neuroinflammation in Rats through Activation of Cannabinoid Receptors and PPAR-γ Pathway. Neuropharmacology 2012, 63, 653–666. [Google Scholar] [CrossRef]
- Gonzalez-Mateo, G.T.; Aroeira, L.S.; Lopez-Cabrera, M.; Ruiz-Ortega, M.; Ortiz, A.; Selgas, R. Pharmacological Modulation of Peritoneal Injury Induced by Dialysis Fluids: Is It an Option? Nephrol. Dial. Transplant. 2012, 27, 478–481. [Google Scholar] [CrossRef][Green Version]
- Scuderi, C.; Steardo, L.; Esposito, G. Cannabidiol Promotes Amyloid Precursor Protein Ubiquitination and Reduction of Beta Amyloid Expression in SHSY5Y APP+ Cells Through PPARγ Involvement. Phytother. Res. 2014, 28, 1007–1013. [Google Scholar] [CrossRef]
- Youssef, D.A.; El-Fayoumi, H.M.; Mahmoud, M.F. Beta-Caryophyllene Protects against Diet-Induced Dyslipidemia and Vascular Inflammation in Rats: Involvement of CB2 and PPAR-γ Receptors. Chem.-Biol. Interact. 2019, 297, 16–24. [Google Scholar] [CrossRef]
- Russo, E.B. Taming THC: Potential Cannabis Synergy and Phytocannabinoid-terpenoid Entourage Effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Liu, T.; Mao, Y.; Peng, B. Novel Microglia-Based Therapeutic Approaches to Neurodegenerative Disorders. Neurosci. Bull. 2023, 39, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Barger, S.; Barnum, S.; Bradt, B.; Bauer, J.; Cole, G.M.; Cooper, N.R.; Eikelenboom, P.; Emmerling, M.; Fiebich, B.L.; et al. Inflammation and Alzheimer’s Disease. Neurobiol. Aging 2000, 21, 383–421. [Google Scholar] [CrossRef]
- Shi, S.; Liang, D.; Chen, Y.; Xie, Y.; Wang, Y.; Wang, L.; Wang, Z.; Qiao, Z. Gx-50 Reduces β-Amyloid-Induced TNF-α, IL-1β, NO, and PGE2 Expression and Inhibits NF-κB Signaling in a Mouse Model of Alzheimer’s Disease. Eur. J. Immunol. 2016, 46, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Cisbani, G.; Rivest, S. Targeting Innate Immunity to Protect and Cure Alzheimer’s Disease: Opportunities and Pitfalls. Mol. Psychiatry 2021, 26, 5504–5515. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Bian, J.-S. Hydrogen Sulfide Protects Amyloid-β Induced Cell Toxicity in Microglia. J. Alzheimers Dis. 2010, 22, 1189–1200. [Google Scholar] [CrossRef]
- Ralay Ranaivo, H.; Craft, J.M.; Hu, W.; Guo, L.; Wing, L.K.; Van Eldik, L.J.; Watterson, D.M. Glia as a Therapeutic Target: Selective Suppression of Human Amyloid-Beta-Induced Upregulation of Brain Proinflammatory Cytokine Production Attenuates Neurodegeneration. J. Neurosci. 2006, 26, 662–670. [Google Scholar] [CrossRef]
- Wang, C.; Fan, L.; Khawaja, R.R.; Liu, B.; Zhan, L.; Kodama, L.; Chin, M.; Li, Y.; Le, D.; Zhou, Y.; et al. Microglial NF-κB Drives Tau Spreading and Toxicity in a Mouse Model of Tauopathy. Nat. Commun. 2022, 13, 1969. [Google Scholar] [CrossRef]
- Irrera, N.; D’Ascola, A.; Pallio, G.; Bitto, A.; Mazzon, E.; Mannino, F.; Squadrito, V.; Arcoraci, V.; Minutoli, L.; Campo, G.M.; et al. β-Caryophyllene Mitigates Collagen Antibody Induced Arthritis (CAIA) in Mice Through a Cross-Talk between CB2 and PPAR-γ Receptors. Biomolecules 2019, 9, 326. [Google Scholar] [CrossRef]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol Improves Health and Survival of Mice on a High-Calorie Diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef]
- Iannotti, F.A.; Vitale, R.M. The Endocannabinoid System and PPARs: Focus on Their Signalling Crosstalk, Action and Transcriptional Regulation. Cells 2021, 10, 586. [Google Scholar] [CrossRef]
- Zheng, X.; Sun, T.; Wang, X. Activation of Type 2 Cannabinoid Receptors (CB2R) Promotes Fatty Acid Oxidation through the SIRT1/PGC-1α Pathway. Biochem. Biophys. Res. Commun. 2013, 436, 377–381. [Google Scholar] [CrossRef]
- Jeong, H.; Cohen, D.E.; Cui, L.; Supinski, A.; Savas, J.N.; Mazzulli, J.R.; Yates, J.R.; Bordone, L.; Guarente, L.; Krainc, D. Sirt1 Mediates Neuroprotection from Mutant Huntingtin by Activation of the TORC1 and CREB Transcriptional Pathway. Nat. Med. 2012, 18, 159–165. [Google Scholar] [CrossRef]
- Noraberg, J.; Poulsen, F.R.; Blaabjerg, M.; Kristensen, B.W.; Bonde, C.; Montero, M.; Meyer, M.; Gramsbergen, J.B.; Zimmer, J. Organotypic Hippocampal Slice Cultures for Studies of Brain Damage, Neuroprotection and Neurorepair. Curr. Drug Targets CNS Neurol. Disord. 2005, 4, 435–452. [Google Scholar] [CrossRef]
- Accorroni, A.; Rutigliano, G.; Sabatini, M.; Frascarelli, S.; Borsò, M.; Novelli, E.; Bandini, L.; Ghelardoni, S.; Saba, A.; Zucchi, R.; et al. Exogenous 3-Iodothyronamine Rescues the Entorhinal Cortex from β-Amyloid Toxicity. Thyroid 2020, 30, 147–160. [Google Scholar] [CrossRef]
- Origlia, N.; Righi, M.; Capsoni, S.; Cattaneo, A.; Fang, F.; Stern, D.M.; Chen, J.X.; Schmidt, A.M.; Arancio, O.; Yan, S.D.; et al. Receptor for Advanced Glycation End Product-Dependent Activation of P38 Mitogen-Activated Protein Kinase Contributes to Amyloid-Beta-Mediated Cortical Synaptic Dysfunction. J. Neurosci. 2008, 28, 3521–3530. [Google Scholar] [CrossRef]
- Nabavi, S.; Fox, R.; Proulx, C.D.; Lin, J.Y.; Tsien, R.Y.; Malinow, R. Engineering a Memory with LTD and LTP. Nature 2014, 511, 348–352. [Google Scholar] [CrossRef]
- Tozzi, F.; Rutigliano, G.; Borsò, M.; Falcicchia, C.; Zucchi, R.; Origlia, N. T1AM-TAAR1 Signalling Protects against OGD-Induced Synaptic Dysfunction in the Entorhinal Cortex. Neurobiol. Dis. 2021, 151, 105271. [Google Scholar] [CrossRef]
- Ferrisi, R.; Gado, F.; Ricardi, C.; Polini, B.; Manera, C.; Chiellini, G. The Interplay between Cannabinoid Receptors and Microglia in the Pathophysiology of Alzheimer’s Disease. J. Clin. Med. 2023, 12, 7201. [Google Scholar] [CrossRef]
- Askari, V.R.; Shafiee-Nick, R. The Protective Effects of β-Caryophyllene on LPS-Induced Primary Microglia M1/M2 Imbalance: A Mechanistic Evaluation. Life Sci. 2019, 219, 40–73. [Google Scholar] [CrossRef]
- Clunas, H.; Walpole, S.; Babic, I.; Nair, M.; May, N.; Huang, X.-F.; Solowij, N.; Newell, K.A.; Weston-Green, K. Improved Recognition Memory and Reduced Inflammation Following β-Caryophyllene Treatment in the Wistar-Kyoto Rodent Model of Treatment-Resistant Depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2025, 138, 111312. [Google Scholar] [CrossRef] [PubMed]
- Sudeep, H.V.; Venkatakrishna, K.; Amritharaj; Gouthamchandra, K.; Reethi, B.; Naveen, P.; Lingaraju, H.B.; Shyamprasad, K. A Standardized Black Pepper Seed Extract Containing β-Caryophyllene Improves Cognitive Function in Scopolamine-Induced Amnesia Model Mice via Regulation of Brain-Derived Neurotrophic Factor and MAPK Proteins. J. Food Biochem. 2021, 45, e13994. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Oliveira, G.L.; Da Silva, J.C.C.L.; Dos Santos C. L Da Silva, A.P.; Feitosa, C.M.; De Castro Almeida, F.R. Anticonvulsant, Anxiolytic and Antidepressant Properties of the β-Caryophyllene in Swiss Mice: Involvement of Benzodiazepine-GABAAergic, Serotonergic and Nitrergic Systems. CMP 2020, 14, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Mecha, M.; Feliú, A.; Iñigo, P.M.; Mestre, L.; Carrillo-Salinas, F.J.; Guaza, C. Cannabidiol Provides Long-Lasting Protection against the Deleterious Effects of Inflammation in a Viral Model of Multiple Sclerosis: A Role for A2A Receptors. Neurobiol. Dis. 2013, 59, 141–150. [Google Scholar] [CrossRef]
- Turcotte, C.; Blanchet, M.-R.; Laviolette, M.; Flamand, N. The CB2 Receptor and Its Role as a Regulator of Inflammation. Cell. Mol. Life Sci. 2016, 73, 4449–4470. [Google Scholar] [CrossRef]
- Cheng, Y.; Dong, Z.; Liu, S. β-Caryophyllene Ameliorates the Alzheimer-Like Phenotype in APP/PS1 Mice through CB2 Receptor Activation and the PPARγ; Pathway. Pharmacology 2014, 94, 1–12. [Google Scholar] [CrossRef]
- Bento, A.F.; Marcon, R.; Dutra, R.C.; Claudino, R.F.; Cola, M.; Leite, D.F.P.; Calixto, J.B. β-Caryophyllene Inhibits Dextran Sulfate Sodium-Induced Colitis in Mice through CB2 Receptor Activation and PPARγ Pathway. Am. J. Pathol. 2011, 178, 1153–1166. [Google Scholar] [CrossRef]
- Irrera, N.; D’Ascola, A.; Pallio, G.; Bitto, A.; Mannino, F.; Arcoraci, V.; Rottura, M.; Ieni, A.; Minutoli, L.; Metro, D.; et al. β-Caryophyllene Inhibits Cell Proliferation through a Direct Modulation of CB2 Receptors in Glioblastoma Cells. Cancers 2020, 12, 1038. [Google Scholar] [CrossRef]
- Agnes, J.P.; Dos Santos, B.; Das Neves, R.N.; Luciano, V.M.M.; Benvenutti, L.; Goldoni, F.C.; Schran, R.G.; Santin, J.R.; Quintão, N.L.M.; Zanotto-Filho, A. β-Caryophyllene Inhibits Oxaliplatin-Induced Peripheral Neuropathy in Mice: Role of Cannabinoid Type 2 Receptors, Oxidative Stress and Neuroinflammation. Antioxidants 2023, 12, 1893. [Google Scholar] [CrossRef]
- Shapira-Lichter, I.; Beilin, B.; Ofek, K.; Bessler, H.; Gruberger, M.; Shavit, Y.; Seror, D.; Grinevich, G.; Posner, E.; Reichenberg, A.; et al. Cytokines and Cholinergic Signals Co-Modulate Surgical Stress-Induced Changes in Mood and Memory. Brain Behav. Immun. 2008, 22, 388–398. [Google Scholar] [CrossRef]
- Khosropoor, S.; Alavi, M.S.; Etemad, L.; Roohbakhsh, A. Cannabidiol Goes Nuclear: The Role of PPARγ. Phytomedicine 2023, 114, 154771. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.D.S.; Newton, W.R.; Tassinari, I.D.; Da Cunha Xavier, F.H.; Marx, A.; De Fraga, L.S.; Wright, K.; Guedes, R.P.; Bambini-Jr, V. Cannabidiol Prevents LPS-Induced Inflammation by Inhibiting the NLRP3 Inflammasome and iNOS Activity in BV2 Microglia Cells via CB2 Receptors and PPARγ. Neurochem. Int. 2024, 177, 105769. [Google Scholar] [CrossRef]
- Bouhadfane, M.; Tazerart, S.; Moqrich, A.; Vinay, L.; Brocard, F. Sodium-Mediated Plateau Potentials in Lumbar Motoneurons of Neonatal Rats. J. Neurosci. 2013, 33, 15626–15641. [Google Scholar] [CrossRef]
- McGinnis, M.M.; Parrish, B.C.; McCool, B.A. Withdrawal from Chronic Ethanol Exposure Increases Postsynaptic Glutamate Function of Insular Cortex Projections to the Rat Basolateral Amygdala. Neuropharmacology 2020, 172, 108129. [Google Scholar] [CrossRef]
- Serra, M.P.; Boi, M.; Carta, A.; Murru, E.; Carta, G.; Banni, S.; Quartu, M. Anti-Inflammatory Effect of Beta-Caryophyllene Mediated by the Involvement of TRPV1, BDNF and trkB in the Rat Cerebral Cortex after Hypoperfusion/Reperfusion. Int. J. Mol. Sci. 2022, 23, 3633. [Google Scholar] [CrossRef]
- Rosa, É.V.F.; Da Silveira, A.R.; Sari, M.H.M.; Sampaio, T.B.; Dos Santos, J.T.; Müller, S.G.; Fighera, M.R.; Royes, L.F.F.; Nogueira, C.W.; Oliveira, M.S.; et al. Beta-Caryophyllene Mitigates the Cognitive Impairment Caused by Repeated Exposure to Aspartame in Rats: Putative Role of BDNF-TrKB Signaling Pathway and Acetylcholinesterase Activity. Behav. Brain Res. 2023, 453, 114615. [Google Scholar] [CrossRef]
- Laske, C.; Stellos, K.; Hoffmann, N.; Stransky, E.; Straten, G.; Eschweiler, G.W.; Leyhe, T. Higher BDNF Serum Levels Predict Slower Cognitive Decline in Alzheimer’s Disease Patients. Int. J. Neuropsychopharmacol. 2011, 14, 399–404. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, Y.; Sterling, K.; Song, W. Brain-Derived Neurotrophic Factor in Alzheimer’s Disease and Its Pharmaceutical Potential. Transl. Neurodegener. 2022, 11, 4. [Google Scholar] [CrossRef]
- Criscuolo, C.; Fabiani, C.; Bonadonna, C.; Origlia, N.; Domenici, L. BDNF Prevents Amyloid-Dependent Impairment of LTP in the Entorhinal Cortex by Attenuating P38 MAPK Phosphorylation. Neurobiol. Aging 2015, 36, 1303–1309. [Google Scholar] [CrossRef]
- Sharma, C.; M. Al Kaabi, J.; M. Nurulain, S.; N. Goyal, S.; Amjad Kamal, M.; Ojha, S. Polypharmacological Properties and Therapeutic Potential of β-Caryophyllene: A Dietary Phytocannabinoid of Pharmaceutical Promise. CPD 2016, 22, 3237–3264. [Google Scholar] [CrossRef]
- Ahmed, E.A. The Potential Therapeutic Role of Beta-Caryophyllene as a Chemoensitizer and an Inhibitor of Angiogenesis in Cancer. Molecules 2025, 30, 1751. [Google Scholar] [CrossRef] [PubMed]
- Polini, B.; Ricardi, C.; Bertolini, A.; Carnicelli, V.; Rutigliano, G.; Saponaro, F.; Zucchi, R.; Chiellini, G. T1AM/TAAR1 System Reduces Inflammatory Response and β-Amyloid Toxicity in Human Microglial HMC3 Cell Line. Int. J. Mol. Sci. 2023, 24, 11569. [Google Scholar] [CrossRef] [PubMed]
- Gerace, E.; Landucci, E.; Scartabelli, T.; Moroni, F.; Pellegrini-Giampietro, D.E. Rat Hippocampal Slice Culture Models for the Evaluation of Neuroprotective Agents. Methods Mol. Biol. 2012, 846, 343–354. [Google Scholar] [CrossRef]
- Origlia, N.; Capsoni, S.; Cattaneo, A.; Fang, F.; Arancio, O.; Yan, S.D.; Domenici, L. Aβ-Dependent Inhibition of LTP in Different Intracortical Circuits of the Visual Cortex: The Role of RAGE. JAD 2009, 17, 59–68. [Google Scholar] [CrossRef]
- Morcuende, A.; García-Gutiérrez, M.S.; Tambaro, S.; Nieto, E.; Manzanares, J.; Femenia, T. Immunomodulatory Role of CB2 Receptors in Emotional and Cognitive Disorders. Front. Psychiatry 2022, 13, 866052. [Google Scholar] [CrossRef]

| Reference Sequence RNA | Gene Symbol | Forward Primer | Reverse Primer |
|---|---|---|---|
| NM_001354667.3 | PPARg | AGCCTGCGAAAGCCTTTTGGTG | GGCTTCACATTCAGCAAACCTGG |
| NM_001142498.2 | SIRT1 | TAGACACGCTGGAACAGGTTGC | CTCCTCGTACAGCTTCACAGTC |
| NM_013261 | PGC-1a | CCAAAGGATGCGCTCTCGTTCA | CGGTGTCTGTAGTGGCTTGACT |
| NM_170734 | BDNF | CATCCGAGGACAAGGTGGCTTG | GCCGAACTTTCTGGTCCTCATC |
| NM_002046 | GAPDH | GTCTCCTCTGACTTCAACAGCG | ACCACCCTGTTGCTGTAGCCAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricardi, C.; Mazzierli, A.; Guglielmo, S.; Origlia, N.; Gado, F.; Manera, C.; Chiellini, G.; Polini, B. Multi-Target Protective Effects of β-Caryophyllene (BCP) at the Intersection of Neuroinflammation and Neurodegeneration. Int. J. Mol. Sci. 2025, 26, 6027. https://doi.org/10.3390/ijms26136027
Ricardi C, Mazzierli A, Guglielmo S, Origlia N, Gado F, Manera C, Chiellini G, Polini B. Multi-Target Protective Effects of β-Caryophyllene (BCP) at the Intersection of Neuroinflammation and Neurodegeneration. International Journal of Molecular Sciences. 2025; 26(13):6027. https://doi.org/10.3390/ijms26136027
Chicago/Turabian StyleRicardi, Caterina, Anna Mazzierli, Stefano Guglielmo, Nicola Origlia, Francesca Gado, Clementina Manera, Grazia Chiellini, and Beatrice Polini. 2025. "Multi-Target Protective Effects of β-Caryophyllene (BCP) at the Intersection of Neuroinflammation and Neurodegeneration" International Journal of Molecular Sciences 26, no. 13: 6027. https://doi.org/10.3390/ijms26136027
APA StyleRicardi, C., Mazzierli, A., Guglielmo, S., Origlia, N., Gado, F., Manera, C., Chiellini, G., & Polini, B. (2025). Multi-Target Protective Effects of β-Caryophyllene (BCP) at the Intersection of Neuroinflammation and Neurodegeneration. International Journal of Molecular Sciences, 26(13), 6027. https://doi.org/10.3390/ijms26136027







