Repurposing Caffeine, Metformin, and Furosemide to Target Schizophrenia-Related Impairments in a Triple-Hit Rat Model
Abstract
1. Introduction
2. Results
2.1. General Observations
2.2. Behavioral Differences Between the Water-Drinking LE and Lisket Animals
2.3. Behavioral Results in Pharmacological Treatments in Lisket Animals
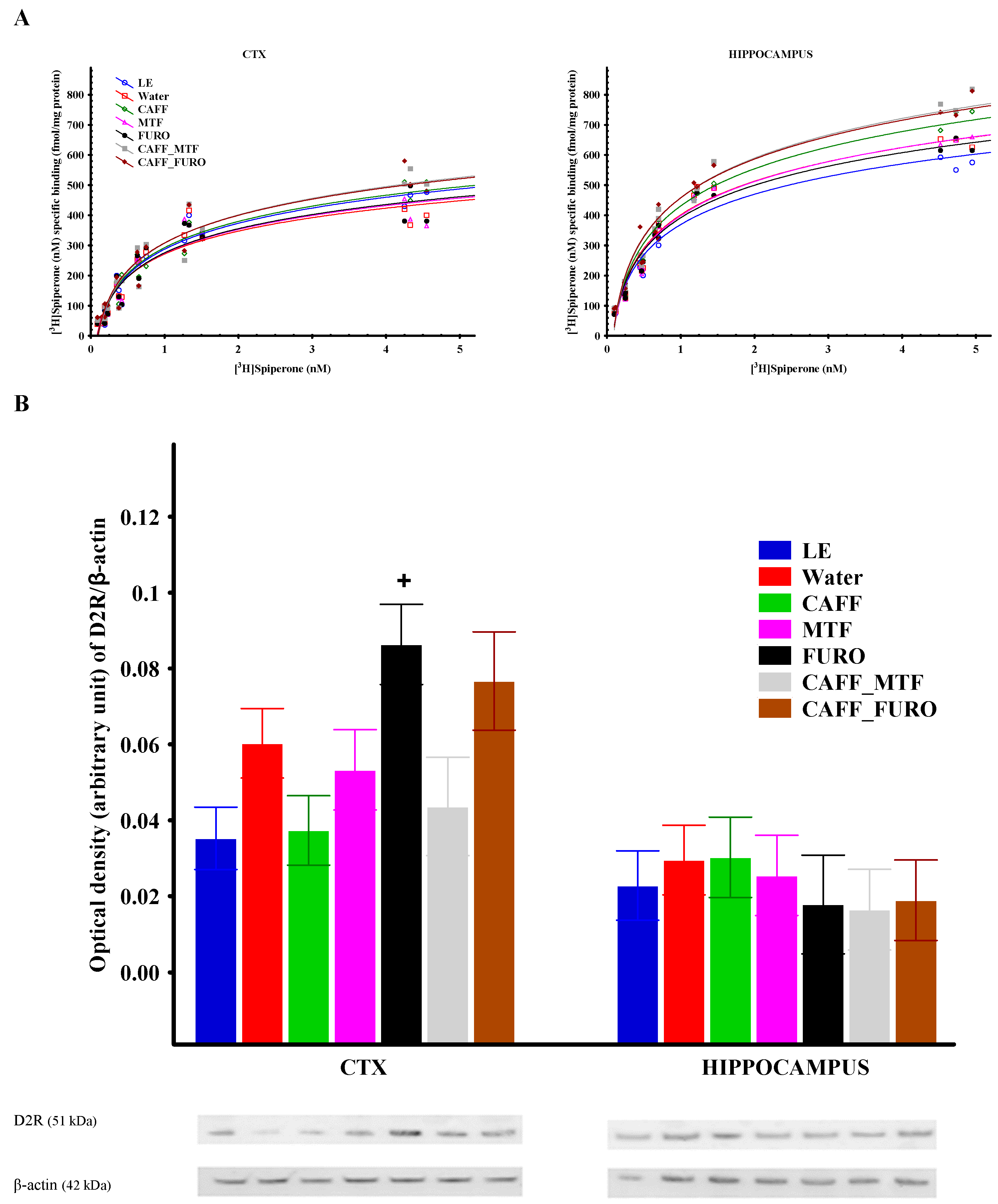
2.4. In Vitro Results
Receptor Binding Assay
2.5. Receptor Protein Expression
3. Discussion
Limitations
4. Methods
4.1. Animals
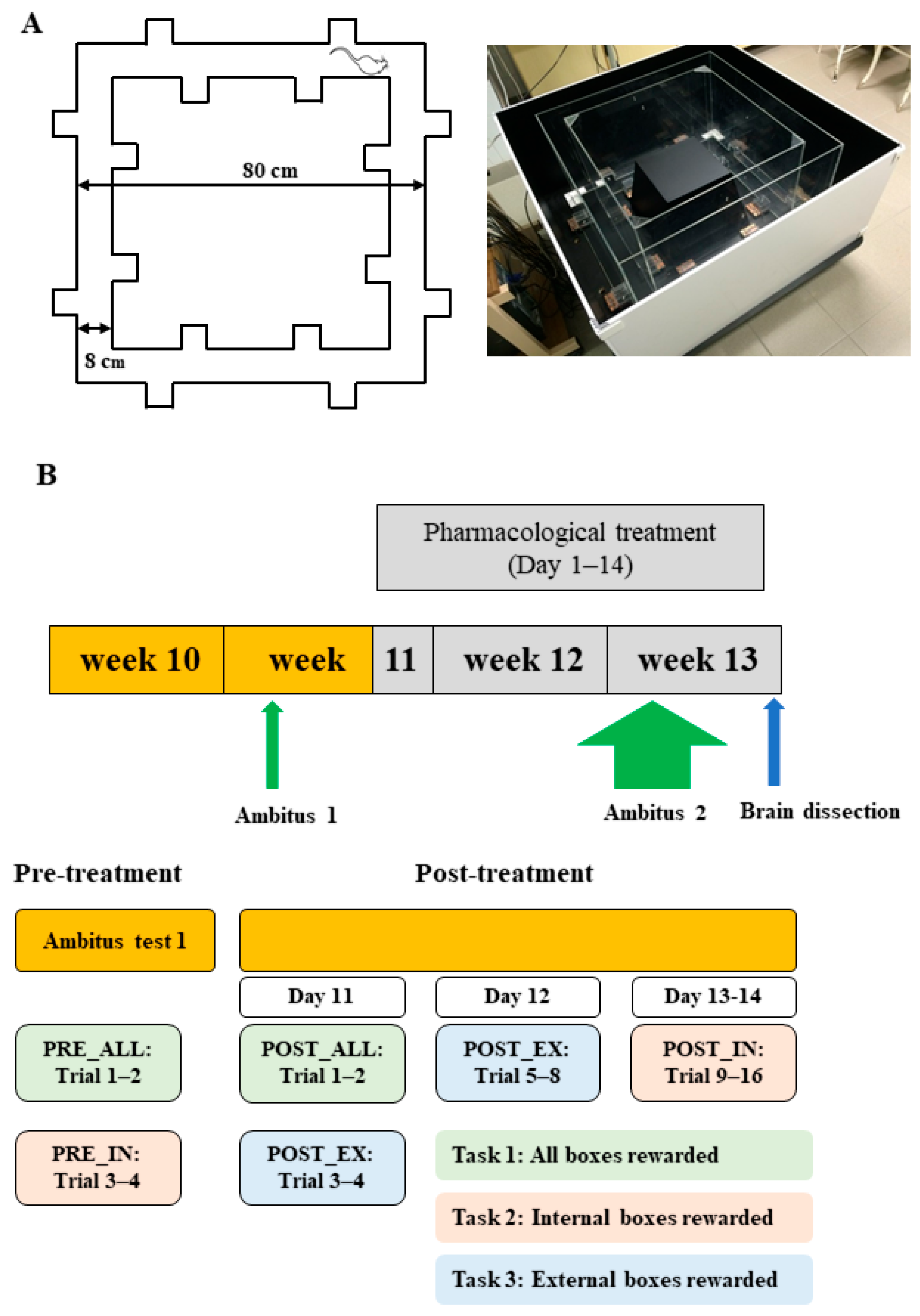
4.2. Experimental Paradigm
4.3. Ambitus Test
4.4. In Vitro Experiments
4.4.1. Preparation of Brain Samples for Receptor Binding Assays
4.4.2. Receptor Binding Experiment
4.4.3. Western Blot Analysis
4.5. Measurements and Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| D2R | Dopamine2 receptor |
| LE | Long Evans |
| LE_W | Water-drinking LE animals |
| Lisket_W | Water-drinking Lisket animals |
| CTX | Cerebral cortex |
| CAFF | Caffeine |
| MTF | Metformin |
| FURO | Furosemide |
| CAFF_MTF | Caffeine and metformin double treatment |
| CAFF_FURO | Caffeine and furosemide double treatment |
| MTF_FURO | Metformin and furosemide double treatment, |
| TRIPLE | Caffeine and metformin and furosemide triple treatment |
| Bmax | Maximal binding capacity |
| Kd | Dissociation constant |
| PFC | Prefrontal cortex |
| NKCC1 | Sodium–potassium–chloride cotransporter 1 |
| KCC2 | Potassium–chloride cotransporter 2 |
| AMPK | Adenosine monophosphate protein kinase |
References
- Gray, J.A.; Roth, B.L. Molecular Targets for Treating Cognitive Dysfunction in Schizophrenia. Schizophr. Bull. 2007, 33, 1100–1119. [Google Scholar] [CrossRef] [PubMed]
- Lesh, T.A.; Niendam, T.A.; Minzenberg, M.J.; Carter, C.S. Cognitive Control Deficits in Schizophrenia: Mechanisms and Meaning. Neuropsychopharmacology 2011, 36, 316–338. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Chant, D.; Welham, J.; McGrath, J. A Systematic Review of the Prevalence of Schizophrenia. PLoS Med. 2005, 2, 0413–0433. [Google Scholar] [CrossRef] [PubMed]
- McCleery, A.; Nuechterlein, K.H. Cognitive Impairment in Psychotic Illness: Prevalence, Profile of Impairment, Developmental Course, and Treatment Considerations. Dialogues Clin. Neurosci. 2019, 21, 239–248. [Google Scholar] [CrossRef]
- Hede, V.; Devillé, C. Treating Psychiatric Symptoms and Disorders with Non-Psychotropic Medications. Dialogues Clin. Neurosci. 2019, 21, 193–201. [Google Scholar] [CrossRef]
- Jiang, W.L.; Cai, D.B.; Yin, F.; Zhang, L.; Zhao, X.W.; He, J.; Ng, C.H.; Ungvari, G.S.; Sim, K.; Hu, M.L.; et al. Adjunctive Metformin for Antipsychotic-Induced Dyslipidemia: A Meta-Analysis of Randomized, Double-Blind, Placebo-Controlled Trials. Transl. Psychiatry 2020, 10, 117. [Google Scholar] [CrossRef]
- Subramaniam, K.; Luks, T.L.; Garrett, C.; Chung, C.; Fisher, M.; Nagarajan, S.; Vinogradov, S. Intensive Cognitive Training in Schizophrenia Enhances Working Memory and Associated Prefrontal Cortical Efficiency in a Manner That Drives Long-Term Functional Gains. NeuroImage 2014, 99, 281–292. [Google Scholar] [CrossRef]
- Strittmatter, S.M. Overcoming Drug Development Bottlenecks with Repurposing: Old Drugs Learn New Tricks. Nat. Med. 2014, 20, 590–591. [Google Scholar] [CrossRef]
- Strassnig, M.; Brar, J.S.; Ganguli, R. Increased Caffeine and Nicotine Consumption in Community-Dwelling Patients with Schizophrenia. Schizophr. Res. 2006, 86, 269–275. [Google Scholar] [CrossRef]
- Dall’Igna, O.P.; Da Silva, A.L.; Dietrich, M.O.; Hoffmann, A.; De Oliveira, R.V.; Souza, D.O.; Lara, D.R. Chronic Treatment with Caffeine Blunts the Hyperlocomotor but Not Cognitive Effects of the N-Methyl-D-Aspartate Receptor Antagonist MK-801 in Mice. Psychopharmacology 2003, 166, 258–263. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Bättig, K.; Holmén, J.; Nehlig, A.; Zvartau, E.E. Actions of Caffeine in the Brain with Special Reference to Factors That Contribute to Its Widespread Use. Pharmacol. Rev. 1999, 51, 83–133. [Google Scholar] [CrossRef] [PubMed]
- Poole, R.L.; Braak, D.; Gould, T.J. Concentration- and Age-Dependent Effects of Chronic Caffeine on Contextual Fear Conditioning in C57BL/6J Mice. Behav. Brain Res. 2016, 298, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, K.K.; Williams, J.M.; Menza, M.; Galazyn, M.; Benowitz, N.L. Higher Serum Caffeine in Smokers with Schizophrenia Compared to Smoking Controls. Drug Alcohol. Depend. 2010, 110, 151–155. [Google Scholar] [CrossRef][Green Version]
- Hughes, J.R.; McHugh, P.; Holtzman, S.; Frances, R.J. Caffeine and Schizophrenia. Psychiatr. Serv. 1998, 49, 1415–1417. [Google Scholar] [CrossRef]
- Núñez, C.; Stephan-Otto, C.; Cuevas-Esteban, J.; Maria Haro, J.; Huerta-Ramos, E.; Ochoa, S.; Usall, J.; Brébion, G. Effects of Caffeine Intake and Smoking on Neurocognition in Schizophrenia. Psychiatry Res. 2015, 230, 924–931. [Google Scholar] [CrossRef]
- Alzoubi, K.H.; Abdul-Razzak, K.K.; Khabour, O.F.; Al-Tuweiq, G.M.; Alzubi, M.A.; Alkadhi, K.A. Caffeine Prevents Cognitive Impairment Induced by Chronic Psychosocial Stress and/or High Fat-High Carbohydrate Diet. Behav. Brain Res. 2013, 237, 7–14. [Google Scholar] [CrossRef]
- Angelucci, M.E.M.; Vital, M.A.B.F.; Cesário, C.; Zadusky, C.R.; Rosalen, P.L.; Da Cunha, C. The Effect of Caffeine in Animal Models of Learning and Memory. Eur. J. Pharmacol. 1999, 373, 135–140. [Google Scholar] [CrossRef]
- Horvath, G.; Adam, G.; Tuboly, G.; Kekesi, G.; Büki, A.; Ducza, E.; Szűcs, E.; Benyhe, S.; Benedek, G. Caffeine—Treat or Trigger? Disparate Behavioral and Long-Term Dopaminergic Changes in Control and Schizophrenia-like Wisket Rats. Physiol. Behav. 2021, 236, 113410. [Google Scholar] [CrossRef]
- Horvath, G.; Kertész, I.; Nagy, T.; Adlan, L.G.; Kekesi, G.; Büki, A.; Tuboly, G.; Trencsényi, G. Caffeine-Induced Acute and Delayed Responses in Cerebral Metabolism of Control and Schizophrenia-Like Wisket Rats. Int. J. Mol. Sci. 2022, 23, 8186. [Google Scholar] [CrossRef]
- Sandner, G.; Angst, M.J.; Guiberteau, T.; Guignard, B.; Nehlig, A. Effects of Caffeine or RX821002 in Rats with a Neonatal Ventral Hippocampal Lesion. Front. Behav. Neurosci. 2014, 8, 15. [Google Scholar] [CrossRef][Green Version]
- Sanday, L.; Zanin, K.A.; Patti, C.L.; Fernandes-Sandos, L.; Oliveira, L.C.; Longo, B.M.; Andersen, M.L.; Tufik, S.; Frussa-Filho, R. Role of State-Dependent Learning in the Cognitive Effects of Caffeine in Mice. Int. J. Neuropsychopharmacol. 2013, 16, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Pedraza, L.K.; Sierra, R.O.; Lotz, F.N.; Alvares, L.D.O. Periodical Reactivation under the Effect of Caffeine Attenuates Fear Memory Expression in Rats. Sci. Rep. 2018, 8, 7260. [Google Scholar] [CrossRef] [PubMed]
- Dubroqua, S.; Low, S.R.L.; Yee, B.K.; Singer, P. Caffeine Impairs the Acquisition and Retention, but Not the Consolidation of Pavlovian Conditioned Freezing in Mice. Psychopharmacology 2015, 232, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, K. Drug Effects on Cognitive Function in Mice Determined by the Non-Matching to Sample Task Using a 4-Arm Maze. Jpn. J. Pharmacol. 1991, 56, 483–493. [Google Scholar] [CrossRef]
- Spinetta, M.J.; Woodlee, M.T.; Feinberg, L.M.; Stroud, C.; Schallert, K.; Cormack, L.K.; Schallert, T. Alcohol-Induced Retrograde Memory Impairment in Rats: Prevention by Caffeine. Psychopharmacology 2008, 201, 361–371. [Google Scholar] [CrossRef]
- Borota, D.; Murray, E.; Keceli, G.; Chang, A.; Watabe, J.M.; Ly, M.; Toscano, J.P.; Yassa, M.A. Post-Study Caffeine Administration Enhances Memory Consolidation in Humans. Nat. Neurosci. 2014, 17, 201–203. [Google Scholar] [CrossRef]
- Penninx, B.W.J.H.; Lange, S.M.M. Metabolic Syndrome in Psychiatric Patients: Overview, Mechanisms, and Implications. Dialogues Clin. Neurosci. 2018, 20, 63–73. [Google Scholar] [CrossRef]
- Praharaj, S.K.; Jana, A.K.; Goyal, N.; Sinha, V.K. Metformin for Olanzapine-Induced Weight Gain: A Systematic Review and Meta-Analysis. Br. J. Clin. Pharmacol. 2011, 71, 377–382. [Google Scholar] [CrossRef]
- Markowicz-Piasecka, M.; Sikora, J.; Szydłowska, A.; Skupień, A.; Mikiciuk-Olasik, E.; Huttunen, K.M. Metformin—A Future Therapy for Neurodegenerative Diseases: Theme: Drug Discovery, Development and Delivery in Alzheimer’s Disease Guest Editor: Davide Brambilla. Pharm. Res. 2017, 34, 2614–2627. [Google Scholar] [CrossRef]
- Ryu, Y.K.; Park, H.Y.; Go, J.; Choi, D.H.; Kim, Y.H.; Hwang, J.H.; Noh, J.R.; Lee, T.G.; Lee, C.H.; Kim, K.S. Metformin Inhibits the Development of L-DOPA-Induced Dyskinesia in a Murine Model of Parkinson’s Disease. Mol. Neurobiol. 2018, 55, 5715–5726. [Google Scholar] [CrossRef]
- Horvath, G.; Kis, G.; Kekesi, G.; Büki, A.; Adlan, L.G.; Szűcs, E.; Heni, H.E.; Benyhe, S. Interaction of Clozapine with Metformin in a Schizophrenia Rat Model. Sci. Rep. 2021, 11, 16862. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Luo, C.; Mao, X.Y.; Li, X.; Yin, J.Y.; Zhang, W.; Zhou, H.H.; Liu, Z.Q. Metformin Reverses the Schizophrenia-like Behaviors Induced by MK-801 in Rats. Brain Res. 2019, 1719, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Kong, D.; Xue, R.; Chen, M.; Li, G.; Xu, Y.; Liu, S.; Tian, H.; Zhuo, C. Metformin Enhances Antidepressant/Antipsychotic Combination Therapy of Schizophrenia With Comorbid Depression in a Murine Model. Front. Neurosci. 2020, 14, 517. [Google Scholar] [CrossRef] [PubMed]
- Hannaert, P.; Alvarez-Guerra, M.; Pirot, D.; Nazaret, C.; Garay, R.P. Rat NKCC2/NKCC1 Cotransporter Selectivity for Loop Diuretic Drugs. Naunyn Schmiedebergs Arch. Pharmacol. 2002, 365, 193–199. [Google Scholar] [CrossRef]
- Kaur, A.; Bali, A.; Singh, N.; Jaggi, A.S. Investigating the Stress Attenuating Potential of Furosemide in Immobilization and Electric Foot-Shock Stress Models in Mice. Naunyn Schmiedebergs Arch. Pharmacol. 2015, 388, 497–507. [Google Scholar] [CrossRef]
- Kim, H.R.; Rajagopal, L.; Meltzer, H.Y.; Martina, M. Depolarizing GABAA Current in the Prefrontal Cortex Is Linked with Cognitive Impairment in a Mouse Model Relevant for Schizophrenia. Sci. Adv. 2021, 7, eaba5032. [Google Scholar] [CrossRef]
- Krystal, A.D.; Sutherland, J.; Hochman, D.W. Loop Diuretics Have Anxiolytic Effects in Rat Models of Conditioned Anxiety. PLoS ONE 2012, 7, e35417. [Google Scholar] [CrossRef][Green Version]
- Lam, P.; Newland, J.; Faull, R.L.M.; Kwakowsky, A. Cation-Chloride Cotransporters KCC2 and NKCC1 as Therapeutic Targets in Neurological and Neuropsychiatric Disorders. Molecules 2023, 28, 1344. [Google Scholar] [CrossRef]
- Löscher, W.; Puskarjov, M.; Kaila, K. Cation-Chloride Cotransporters NKCC1 and KCC2 as Potential Targets for Novel Antiepileptic and Antiepileptogenic Treatments. Neuropharmacology 2013, 69, 62–74. [Google Scholar] [CrossRef]
- Shaker, E.; El Agami, O.; Salamah, A. Bumetanide, a Diuretic That Can Help Children with Autism Spectrum Disorder. CNS Neurol. Disord. Drug Targets 2024, 23, 536–542. [Google Scholar] [CrossRef]
- Lemonnier, E.; Lazartigues, A.; Ben-Ari, Y. Treating Schizophrenia With the Diuretic Bumetanide: A Case Report. Clin. Neuropharmacol. 2016, 39, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Rahmanzadeh, R.; Eftekhari, S.; Shahbazi, A.; Khodaei Ardakani, M.-R.; Rahmanzade, R.; Mehrabi, S.; Barati, M.; Joghataei, M.T. Effect of Bumetanide, a Selective NKCC1 Inhibitor, on Hallucinations of Schizophrenic Patients; a Double-Blind Randomized Clinical Trial. Schizophr. Res. 2017, 184, 145–146. [Google Scholar] [CrossRef] [PubMed]
- Kharod, S.C.; Kang, S.K.; Kadam, S.D. Off-Label Use of Bumetanide for Brain Disorders: An Overview. Front. Neurosci. 2019, 13, 310. [Google Scholar] [CrossRef] [PubMed]
- Horvath, G.; Liszli, P.; Kekesi, G.; Büki, A.; Benedek, G. Characterization of Exploratory Activity and Learning Ability of Healthy and “Schizophrenia-like” Rats in a Square Corridor System (AMBITUS). Physiol. Behav. 2017, 169, 155–164. [Google Scholar] [CrossRef]
- Horvath, G.; Liszli, P.; Kekesi, G.; Büki, A.; Benedek, G. Cognitive Training Improves the Disturbed Behavioral Architecture of Schizophrenia-like Rats, “Wisket. ” Physiol. Behav. 2019, 201, 70–82. [Google Scholar] [CrossRef]
- Kekesi, G.; Petrovszki, Z.; Benedek, G.; Horvath, G. Sex-Specific Alterations in Behavioral and Cognitive Functions in a “Three Hit” Animal Model of Schizophrenia. Behav. Brain Res. 2015, 284, 85–93. [Google Scholar] [CrossRef]
- Holahan, M.R.; Rekart, J.L.; Sandoval, J.; Routtenberg, A. Spatial Learning Induces Presynaptic Structural Remodeling in the Hippocampal Mossy Fiber System of Two Rat Strains. Hippocampus 2006, 16, 560–570. [Google Scholar] [CrossRef]
- Kumar, G.; Talpos, J.; Steckler, T. Strain-Dependent Effects on Acquisition and Reversal of Visual and Spatial Tasks in a Rat Touchscreen Battery of Cognition. Physiol. Behav. 2015, 144, 26–36. [Google Scholar] [CrossRef]
- Peinado, A.; Abrams, C.K. Patterns of Spontaneous Local Network Activity in Developing Cerebral Cortex: Relationship to Adult Cognitive Function. PLoS ONE 2015, 10, e0131259. [Google Scholar] [CrossRef][Green Version]
- Gogos, A.; Sbisa, A.; Witkamp, D.; van den Buuse, M. Sex Differences in the Effect of Maternal Immune Activation on Cognitive and Psychosis-like Behaviour in Long Evans Rats. Eur. J. Neurosci. 2020, 52, 2614–2626. [Google Scholar] [CrossRef]
- Uttl, L.; Petrasek, T.; Sengul, H.; Svojanovska, M.; Lobellova, V.; Vales, K.; Radostova, D.; Tsenov, G.; Kubova, H.; Mikulecka, A.; et al. Chronic MK-801 Application in Adolescence and Early Adulthood: A Spatial Working Memory Deficit in Adult Long-Evans Rats but No Changes in the Hippocampal NMDA Receptor Subunits. Front. Pharmacol. 2018, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Hernádi, Z.; Adlan, L.; Kekesi, G.; Büki, A.; Tuboly, G.; Horvath, G. PERSONALIZED BEHAVIORAL ANALYSIS OF RODENTS IN HOME-CAGE CONDITION. IBRO Neurosci. Rep. 2023, 15, S579. [Google Scholar] [CrossRef]
- Büki, A.; Bohár, Z.; Kekesi, G.; Vécsei, L.; Horvath, G. Wisket Rat Model of Schizophrenia: Impaired Motivation and, Altered Brain Structure, but No Anhedonia. Physiol. Behav. 2022, 244, 113651. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, M.; Carlsson, A. Schizophrenia: A Subcortical Neurotransmitter Imbalance Syndrome? Schizophr. Bull. 1990, 16, 425–432. [Google Scholar] [CrossRef]
- Howes, O.D.; Kapur, S. The Dopamine Hypothesis of Schizophrenia: Version III–The Final Common Pathway. Schizophr. Bull. 2009, 35, 549–562. [Google Scholar] [CrossRef]
- Lodge, D.J.; Grace, A.A. Hippocampal Dysregulation of Dopamine System Function and the Pathophysiology of Schizophrenia. Trends Pharmacol. Sci. 2011, 32, 507–513. [Google Scholar] [CrossRef]
- Perez-Costas, E.; Melendez-Ferro, M.; Roberts, R.C. Basal Ganglia Pathology in Schizophrenia: Dopamine Connections and Anomalies. J. Neurochem. 2010, 113, 287–302. [Google Scholar] [CrossRef]
- Yoon, J.H.; Minzenberg, M.J.; Raouf, S.; D’Esposito, M.; Carter, C.S. Impaired Prefrontal-Basal Ganglia Functional Connectivity and Substantia Nigra Hyperactivity in Schizophrenia. Biol. Psychiatry 2013, 74, 122–129. [Google Scholar] [CrossRef]
- Szűcs, E.; Ducza, E.; Büki, A.; Kekesi, G.; Benyhe, S.; Horvath, G. Characterization of Dopamine D2 Receptor Binding, Expression and Signaling in Different Brain Regions of Control and Schizophrenia-Model Wisket Rats. Brain Res. 2020, 1748, 147074. [Google Scholar] [CrossRef]
- Luck, S.J.; Gold, J.M. The Construct of Attention in Schizophrenia. Biol. Psychiatry 2008, 64, 34–39. [Google Scholar] [CrossRef]
- Morrens, M.; Hulstijn, W.; Sabbe, B. Psychomotor Slowing in Schizophrenia. Schizophr. Bull. 2007, 33, 1038–1053. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, A.A.; Garami, J.K.; Mahlberg, J.; Golembieski, J.; Keri, S.; Frydecka, D. Cognitive Function in Schizophrenia: Conflicting Findings and Future Directions. Rev. Neurosci. 2016, 27, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Sumiyoshi, T.; Matsumoto, M.; Murayama, K.; Ikezawa, S.; Matsumoto, K.; Nakagome, K. Neural Correlates for Intrinsic Motivational Deficits of Schizophrenia; Implications for Therapeutics of Cognitive Impairment. Front. Psychiatry 2018, 9, 178. [Google Scholar] [CrossRef] [PubMed]
- Tandon, R.; Nasrallah, H.A.; Keshavan, M.S. Schizophrenia, “Just the Facts” 4. Clinical Features and Conceptualization. Schizophr. Res. 2009, 110, 1–23. [Google Scholar] [CrossRef]
- Lau, C.I.; Wang, H.C.; Hsu, J.L.; Liu, M.E. Does the Dopamine Hypothesis Explain Schizophrenia? Rev. Neurosci. 2013, 24, 389–400. [Google Scholar] [CrossRef]
- Owen, M.J.; Sawa, A.; Mortensen, P.B. Schizophrenia. Lancet 2016, 388, 86–97. [Google Scholar] [CrossRef]
- Okano, H.; Hirano, T.; Balaban, E. Learning and Memory. Proc. Natl. Acad. Sci. USA 2000, 97, 12403–12404. [Google Scholar] [CrossRef]
- Takahashi, H.; Higuchi, M.; Suhara, T. The Role of Extrastriatal Dopamine D2 Receptors in Schizophrenia. Biol. Psychiatry 2006, 59, 919–928. [Google Scholar] [CrossRef]
- Seeman, M.V.; Seeman, P. Is Schizophrenia a Dopamine Supersensitivity Psychotic Reaction? Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 48, 155–160. [Google Scholar] [CrossRef]
- Deslauriers, J.; Larouche, A.; Sarret, P.; Grignon, S. Combination of Prenatal Immune Challenge and Restraint Stress Affects Prepulse Inhibition and Dopaminergic/GABAergic Markers. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 45, 156–164. [Google Scholar] [CrossRef]
- De Oliveira, R.V.; Dall’Igna, O.P.; Tort, A.B.L.; Schuh, J.F.; Neto, P.F.; Gomes, M.W.S.; Souza, D.O.; Lara, D.R. Effect of Subchronic Caffeine Treatment on MK-801-Induced Changes in Locomotion, Cognition and Ataxia in Mice. Behav. Pharmacol. 2005, 16, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Boison, D.; Singer, P.; Shen, H.Y.; Feldon, J.; Yee, B.K. Adenosine Hypothesis of Schizophrenia—Opportunities for Pharmacotherapy. Neuropharmacology 2012, 62, 1527–1543. [Google Scholar] [CrossRef] [PubMed]
- Prediger, R.D.S.; Pamplona, F.A.; Fernandes, D.; Takahashi, R.N. Caffeine Improves Spatial Learning Deficits in an Animal Model of Attention Deficit Hyperactivity Disorder (ADHD) -- the Spontaneously Hypertensive Rat (SHR). Int. J. Neuropsychopharmacol. 2005, 8, 583–594. [Google Scholar] [CrossRef]
- Pires, V.A.; Pamplona, F.A.; Pandolfo, P.; Prediger, R.D.S. Chronic Caffeine Treatment during Prepubertal Period Confers Long-Term Cognitive Benefits in Adult Spontaneously Hypertensive Rats (SHR), an Animal Model of Attention Deficit Hyperactivity Disorder (ADHD). Behav. Brain Res. 2010, 215, 39–44. [Google Scholar] [CrossRef]
- Prediger, R.D.S.; Batista, L.C.; Takahashi, R.N. Caffeine Reverses Age-Related Deficits in Olfactory Discrimination and Social Recognition Memory in Rats. Involvement of Adenosine A1 and A2A Receptors. Neurobiol. Aging 2005, 26, 957–964. [Google Scholar] [CrossRef]
- Molinengo, L.; Scordo, I.; Pastorello, B. Action of Caffeine, L-PIA and Their Combination on Memory Retention in the Rat. Life Sci. 1994, 54, 1247–1250. [Google Scholar] [CrossRef]
- SanMiguel, N.; Pardo, M.; Carratalá-Ros, C.; López-Cruz, L.; Salamone, J.D.; Correa, M. Individual Differences in the Energizing Effects of Caffeine on Effort-Based Decision-Making Tests in Rats. Pharmacol. Biochem. Behav. 2018, 169, 27–34. [Google Scholar] [CrossRef]
- Angelucci, M.E.M.; Cesário, C.; Hiroi, R.H.; Rosalen, P.L.; Da Cunha, C. Effects of Caffeine on Learning and Memory in Rats Tested in the Morris Water Maze. Braz. J. Med. Biol. Res. 2002, 35, 1201–1208. [Google Scholar] [CrossRef]
- Pandolfo, P.; Machado, N.J.; Kofalvi, A.; Takahashia, R.N.; Cunhab, R.A. Caffeine Regulates Frontocorticostriatal Dopamine Transporter Density and Improves Attention and Cognitive Deficits in an Animal Model of Attention Deficit Hyperactivity Disorder. Eur. Neuropsychopharmacol. 2013, 23, 317–328. [Google Scholar] [CrossRef]
- Nikodijević, O.; Jacobson, K.A.; Daly, J.W. Locomotor Activity in Mice during Chronic Treatment with Caffeine and Withdrawal. Pharmacol. Biochem. Behav. 1993, 44, 199–216. [Google Scholar] [CrossRef]
- McLellan, T.M.; Caldwell, J.A.; Lieberman, H.R. A Review of Caffeine’s Effects on Cognitive, Physical and Occupational Performance. Neurosci. Biobehav. Rev. 2016, 71, 294–312. [Google Scholar] [CrossRef] [PubMed]
- Ongini, E.; Fredholm, B.B. Pharmacology of Adenosine A2A Receptors. Trends Pharmacol. Sci. 1996, 17, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Quarta, D.; Ferre, S.; Solinas, M.; You, Z.B.; Hockemeyer, J.; Popoli, P.; Goldberg, S.R. Opposite Modulatory Roles for Adenosine A1 and A2A Receptors on Glutamate and Dopamine Release in the Shell of the Nucleus Accumbens. Effects of Chronic Caffeine Exposure. J. Neurochem. 2004, 88, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.J. Metformin: Therapeutic Profile in the Treatment of Type 2 Diabetes. Diabetes Obes. Metab. 2024, 26 (Suppl. 3), 3–19. [Google Scholar] [CrossRef]
- Birajdar, S.V.; Mazahir, F.; Alam, M.I.; Kumar, A.; Yadav, A.K. Repurposing and Clinical Attributes of Antidiabetic Drugs for the Treatment of Neurodegenerative Disorders. Eur. J. Pharmacol. 2023, 961, 176117. [Google Scholar] [CrossRef]
- Łabuzek, K.; Suchy, D.; Gabryel, B.; Bielecka, A.; Liber, S.; Okopień, B. Quantification of Metformin by the HPLC Method in Brain Regions, Cerebrospinal Fluid and Plasma of Rats Treated with Lipopolysaccharide. Pharmacol. Rep. 2010, 62, 956–965. [Google Scholar] [CrossRef]
- Zhang, Y.; Zong, H.; Qi, Y.; Chang, L.; Gao, Y.; Zhou, T.; Yin, T.; Liu, M.; Pan, K.; Chen, W.; et al. Anxiolytic Effect of Antidiabetic Metformin Is Mediated by AMPK Activation in mPFC Inhibitory Neurons. Mol. Psychiatry 2023, 28, 3955–3965. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Viollet, B. Metformin: Update on Mechanisms of Action and Repurposing Potential. Nat. Rev. Endocrinol. 2023, 19, 460–476. [Google Scholar] [CrossRef]
- Kang, H.; Khang, R.; Ham, S.; Jeong, G.R.; Kim, H.; Jo, M.; Lee, B.D.; Lee, Y.I.; Jo, A.; Park, C.; et al. Activation of the ATF2/CREB-PGC-1α Pathway by Metformin Leads to Dopaminergic Neuroprotection. Oncotarget 2017, 8, 48603–48618. [Google Scholar] [CrossRef]
- Nagy, S.; Maurer, G.W.; Hentze, J.L.; Rose, M.; Werge, T.M.; Rewitz, K. AMPK Signaling Linked to the Schizophrenia-Associated 1q21.1 Deletion Is Required for Neuronal and Sleep Maintenance. PLoS Genet. 2018, 14, e1007623. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Y.; Han, G.; Deng, K.; Liu, X.; Bao, X.; Feng, M.; Yao, Y.; Lian, W.; Xing, B.; et al. Metformin Inhibits Growth and Prolactin Secretion of Pituitary Prolactinoma Cells and Xenografts. J. Cell Mol. Med. 2018, 22, 6368–6379. [Google Scholar] [CrossRef] [PubMed]
- Al Za’abi, M.; Ali, B.H.; Al Suleimani, Y.; Al-Zakwani, I.; Al-Fulaiti, B.; Manoj, P.; Nemmar, A. The Effects of Furosemide on Behavioral and Hormonal Parameters in Male and Female Mice Subjected to Immobilization and Cold-Water Stress. J. Exp. Pharmacol. 2021, 13, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Dargaei, Z.; Bang, J.Y.; Mahadevan, V.; Khademullah, C.S.; Bedard, S.; Parfitt, G.M.; Kim, J.C.; Woodin, M.A. Restoring GABAergic Inhibition Rescues Memory Deficits in a Huntington’s Disease Mouse Model. Proc. Natl. Acad. Sci. USA 2018, 115, E1618–E1626. [Google Scholar] [CrossRef] [PubMed]
- Deidda, G.; Parrini, M.; Naskar, S.; Bozarth, I.F.; Contestabile, A.; Cancedda, L. Reversing Excitatory GABAAR Signaling Restores Synaptic Plasticity and Memory in a Mouse Model of Down Syndrome. Nat. Med. 2015, 21, 318–326. [Google Scholar] [CrossRef]
- Santhakumar, V.; Hanchar, H.J.; Wallner, M.; Olsen, R.W.; Otis, T.S. Contributions of the GABAA Receptor Alpha6 Subunit to Phasic and Tonic Inhibition Revealed by a Naturally Occurring Polymorphism in the Alpha6 Gene. J. Neurosci. 2006, 26, 3357–3364. [Google Scholar] [CrossRef]
- Lundy, R.F.; Caloiero, V.; Bradley, C.; Liang, N.-C.; Norgren, R. Furosemide-Induced Food Avoidance: Evidence for a Conditioned Response. Physiol. Behav. 2004, 81, 397–408. [Google Scholar] [CrossRef]
- Delpire, E.; Staley, K.J. Novel Determinants of the Neuronal Cl− Concentration. J. Physiol. 2014, 592, 4099–4114. [Google Scholar] [CrossRef]
- Arion, D.; Lewis, D.A. Altered Expression of Regulators of the Cortical Chloride Transporters NKCC1 and KCC2 in Schizophrenia. Arch. Gen. Psychiatry 2011, 68, 21–31. [Google Scholar] [CrossRef]
- Gharaylou, Z.; Shafaghi, L.; Oghabian, M.A.; Yoonessi, A.; Tafakhori, A.; Shahsavand Ananloo, E.; Hadjighassem, M. Longitudinal Effects of Bumetanide on Neuro-Cognitive Functioning in Drug-Resistant Epilepsy. Front. Neurol. 2019, 10, 483. [Google Scholar] [CrossRef]
- Morita, Y.; Callicott, J.H.; Testa, L.R.; Mighdoll, M.I.; Dickinson, D.; Chen, Q.; Tao, R.; Lipska, B.K.; Kolachana, B.; Law, A.J.; et al. Characteristics of the Cation Cotransporter NKCC1 in Human Brain: Alternate Transcripts, Expression in Development, and Potential Relationships to Brain Function and Schizophrenia. J. Neurosci. 2014, 34, 4929–4940. [Google Scholar] [CrossRef]
- Marx, B.; Scuvée, É.; Scuvée-Moreau, J.; Seutin, V.; Jouret, F. Mechanisms of caffeine-induced diuresis. Med. Sci. 2016, 32, 485–490. [Google Scholar] [CrossRef]
- Acquas, E.; Tanda, G.; Di Chiara, G. Differential Effects of Caffeine on Dopamine and Acetylcholine Transmission in Brain Areas of Drug-Naive and Caffeine-Pretreated Rats. Neuropsychopharmacology 2002, 27, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Petrovszki, Z.; Adam, G.; Tuboly, G.; Kekesi, G.; Benedek, G.; Keri, S.; Horvath, G. Characterization of Gene-Environment Interactions by Behavioral Profiling of Selectively Bred Rats: The Effect of NMDA Receptor Inhibition and Social Isolation. Behav. Brain Res. 2013, 240, 134–145. [Google Scholar] [CrossRef]
- Ardais, A.P.; Rocha, A.S.; Borges, M.F.; Fioreze, G.T.; Sallaberry, C.; Mioranzza, S.; Nunes, F.; Pagnussat, N.; Botton, P.H.S.; Cunha, R.A.; et al. Caffeine Exposure during Rat Brain Development Causes Memory Impairment in a Sex Selective Manner That Is Offset by Caffeine Consumption throughout Life. Behav. Brain Res. 2016, 303, 76–84. [Google Scholar] [CrossRef]
- Wang, B.; Wang-France, J.; Li, H.; Sansom, S.C. Furosemide Reduces BK-Aβ4-Mediated K+ Secretion in Mice on an Alkaline High-K+ Diet. Am. J. Physiol. Ren. Physiol. 2019, 316, F341–F350. [Google Scholar] [CrossRef]
- Benyhe, S.; Farkas, J.; Tóth, G.; Wollemann, M.; Toth, G.; Wollemann, M. Met5-Enkephalin-Arg6-Phe7, an Endogenous Neuropeptide, Binds to Multiple Opioid and Nonopioid Sites in Rat Brain. J. Neurosci. Res. 1997, 48, 249–258. [Google Scholar] [CrossRef]
- Szucs, E.; Büki, A.; Kékesi, G.; Horváth, G.; Benyhe, S. Mu-Opioid (MOP) Receptor Mediated G-Protein Signaling Is Impaired in Specific Brain Regions in a Rat Model of Schizophrenia. Neurosci. Lett. 2016, 619, 29–33. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for Quantitation of Microgram Quantities of Protein Utilizing Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Janowsky, A.; Neve, K.A.; Kinzie, J.M.; Taylor, B.; de Paulis, T.; Belknap, J.K. Extrastriatal Dopamine D2 Receptors: Distribution, Pharmacological Characterization and Region-Specific Regulation by Clozapine. J. Pharmacol. Exp. Ther. 1992, 261, 1282–1290. [Google Scholar] [CrossRef]

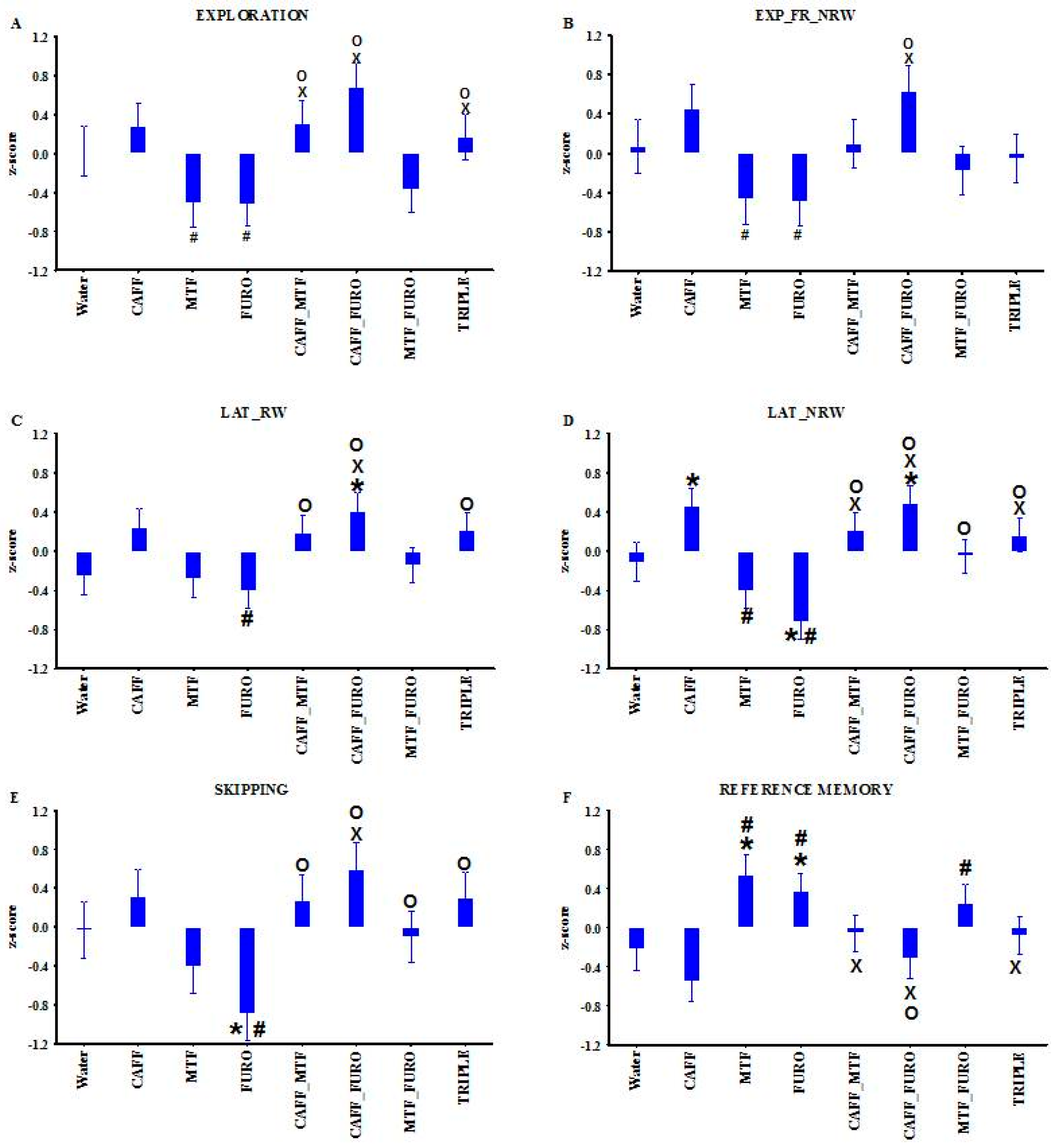
| Parameters Definition: Calculation | Group | Group and Phase Interaction | Treatment |
|---|---|---|---|
| 1. Locomotion (Loco, n): Number of entries into the corridors up to 5 min | 5.30; (1, 52) < 0.05 | ||
| 2. Exploration (Expl, n): Overall number of box visits up to 5 min | 8.26; (4, 52) < 0.0001 | 3.32; (7, 75) < 0.01 | |
| 3. Expl_FR_RW: Exploration frequency into the rewarded boxes up to the collection of all rewards: (number of RW box visits) × 300/(Eat_T) | 17.06; (1, 52) < 0.005 | ||
| 4. Expl_FR_NRW: Exploration frequency into the non-rewarded boxes up to the collection of all rewards: (number of NRW box visits) × 300/(Eat_T) | 3.97; (2, 26) < 0.05 | 2.43; (7, 75) < 0.05 | |
| 5. LAT_RW: Time up to the first exploration into a rewarded box | 33.25; (1, 52) < 0.0001 | 2.69; (7, 75) < 0.05 | |
| 6. LAT_NRW: Time up to the first exploration into a non-rewarded box | 5.28; (7, 75) < 0.0001 | ||
| 7. Skipping: Number of entries into the corridors before the first exploration | 27.33; (1, 52) < 0.0005 | 3.16; (7, 75) < 0.01 | |
| 8. Adequate exploration (A_E, %): (Eat_N) × 100/(number of explorations up to Eat_T) | 6.59; (1, 52) < 0.05 | ||
| 9. Learning capacity (L_C, %): [(Eat_N) × 300 × 100]/[(number of rewards) × (Eat_T)] | 23.46; (1, 52) < 0.0005 | ||
| 10. Working memory (W_M, %): (Eat_N) × 100/(number of exploration into the RW boxes up to collection of rewards) | 4.92; (1, 26) < 0.05 | ||
| 11. Reference memory (R_M, %): (number of exploration into the RW boxes up to collection of rewards) × 100/(number of exploration into all boxes up to collection of rewards) | 3.43; (7, 75) < 0.005 |
| Region | Treatment | Bmax + S.E.M. (fmol/mg Protein) | Kd + S.E.M. (nM) |
|---|---|---|---|
| Cortex | LE | 578.6 + 38.0 | 1.01 + 0.16 |
| Water | 495.2 + 36.7 | 0.77 + 0.15 | |
| CAFF | 619.2 + 37.5 | 1.16 + 0.17 | |
| MTF | 509.0 + 41.4 | 0.80 + 0.17 | |
| FURO | 527.4 + 45.5 | 0.88 + 0.19 | |
| CAFF_MTF | 671.2 + 57.5 | 1.25 + 0.25 | |
| CAFF_FURO | 661.7 + 55.7 | 1.20 + 0.24 | |
| Hippocampus | LE | 701.6 + 31.8 | 0.86 + 0.10 |
| Water | 788.0 + 29.2 | 0.94 + 0.09 | |
| CAFF | 897.5 + 26.7 **; p = 0.05 # | 1.10 + 0.08 | |
| MTF | 800.9 + 28.8 | 1.00 + 0.09 | |
| FURO | 763.0 + 23.8+ | 0.92 + 0.07 | |
| CAFF_MTF | 971.5 + 30.2 ***; $; # | 1.16 + 0.09 | |
| CAFF_FURO | 938.6 + 39.1 **; o; # | 1.06 + 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horvath, G.; Plesz, S.B.; Ducza, E.; Varga, D.; Szucs, E.; Benyhe, S.; Adlan, L.G.; Braunitzer, G.; Kekesi, G. Repurposing Caffeine, Metformin, and Furosemide to Target Schizophrenia-Related Impairments in a Triple-Hit Rat Model. Int. J. Mol. Sci. 2025, 26, 6019. https://doi.org/10.3390/ijms26136019
Horvath G, Plesz SB, Ducza E, Varga D, Szucs E, Benyhe S, Adlan LG, Braunitzer G, Kekesi G. Repurposing Caffeine, Metformin, and Furosemide to Target Schizophrenia-Related Impairments in a Triple-Hit Rat Model. International Journal of Molecular Sciences. 2025; 26(13):6019. https://doi.org/10.3390/ijms26136019
Chicago/Turabian StyleHorvath, Gyongyi, Szonja Bianka Plesz, Eszter Ducza, Dorottya Varga, Edina Szucs, Sándor Benyhe, Leatitia Gabriella Adlan, Gabor Braunitzer, and Gabriella Kekesi. 2025. "Repurposing Caffeine, Metformin, and Furosemide to Target Schizophrenia-Related Impairments in a Triple-Hit Rat Model" International Journal of Molecular Sciences 26, no. 13: 6019. https://doi.org/10.3390/ijms26136019
APA StyleHorvath, G., Plesz, S. B., Ducza, E., Varga, D., Szucs, E., Benyhe, S., Adlan, L. G., Braunitzer, G., & Kekesi, G. (2025). Repurposing Caffeine, Metformin, and Furosemide to Target Schizophrenia-Related Impairments in a Triple-Hit Rat Model. International Journal of Molecular Sciences, 26(13), 6019. https://doi.org/10.3390/ijms26136019







