Bitter Taste Receptor TAS2R43 Co-Regulates Mechanisms of Gastric Acid Secretion and Zinc Homeostasis
Abstract
1. Introduction
2. Results
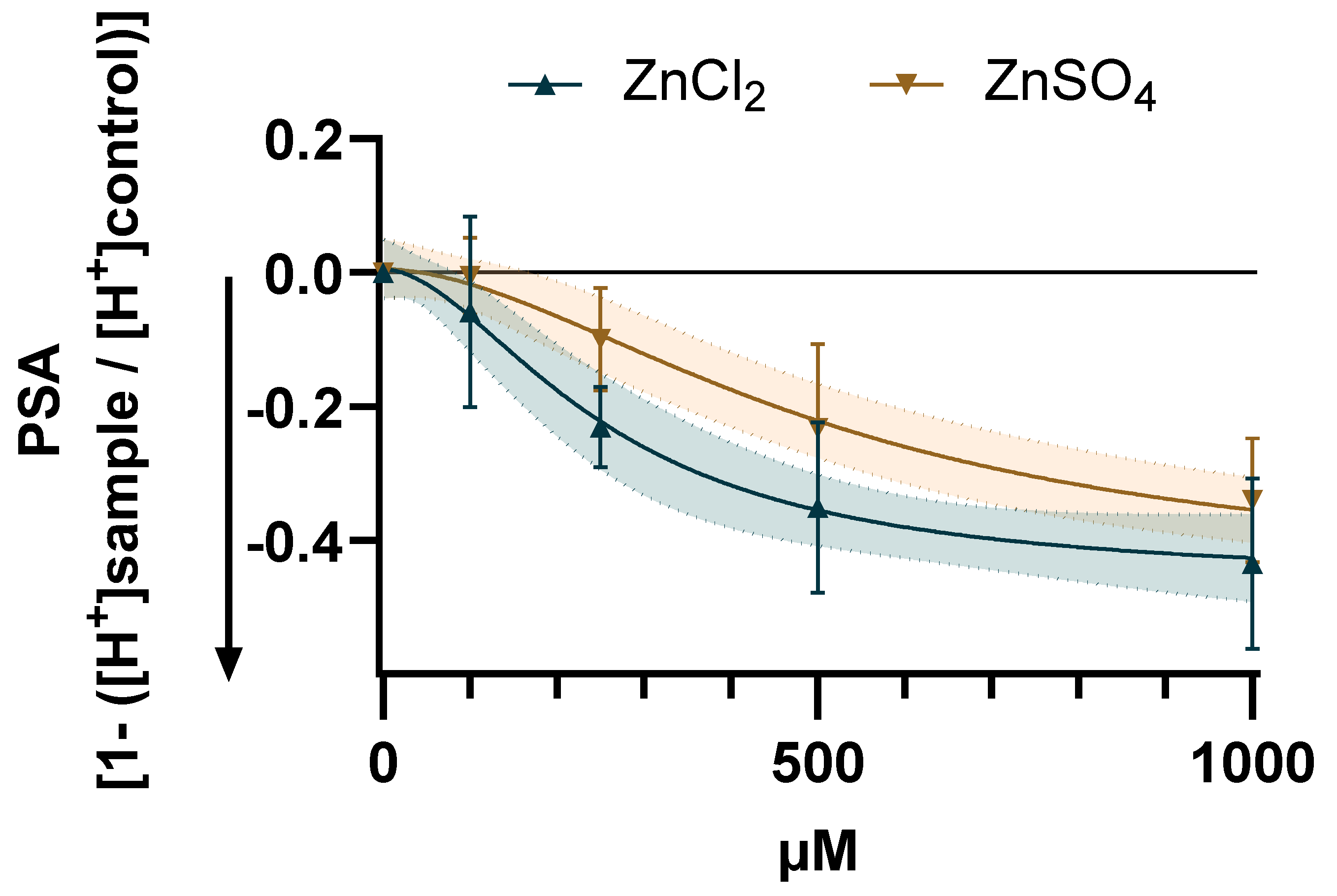
2.1. Zinc-Induced Reduction of Proton Secretory Activity (PSA) in HGT-1 Cells Is Mediated by TAS2Rs
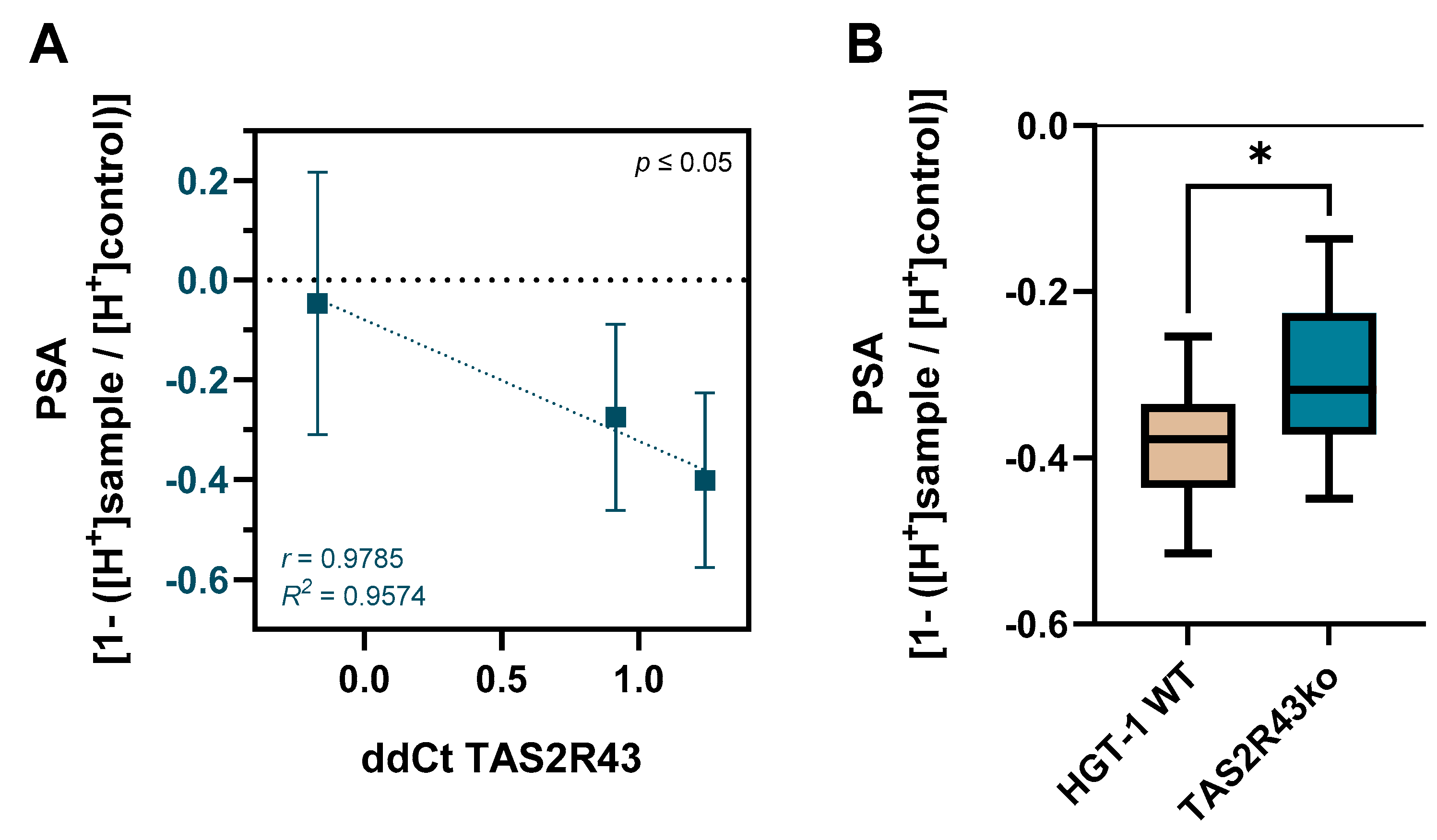
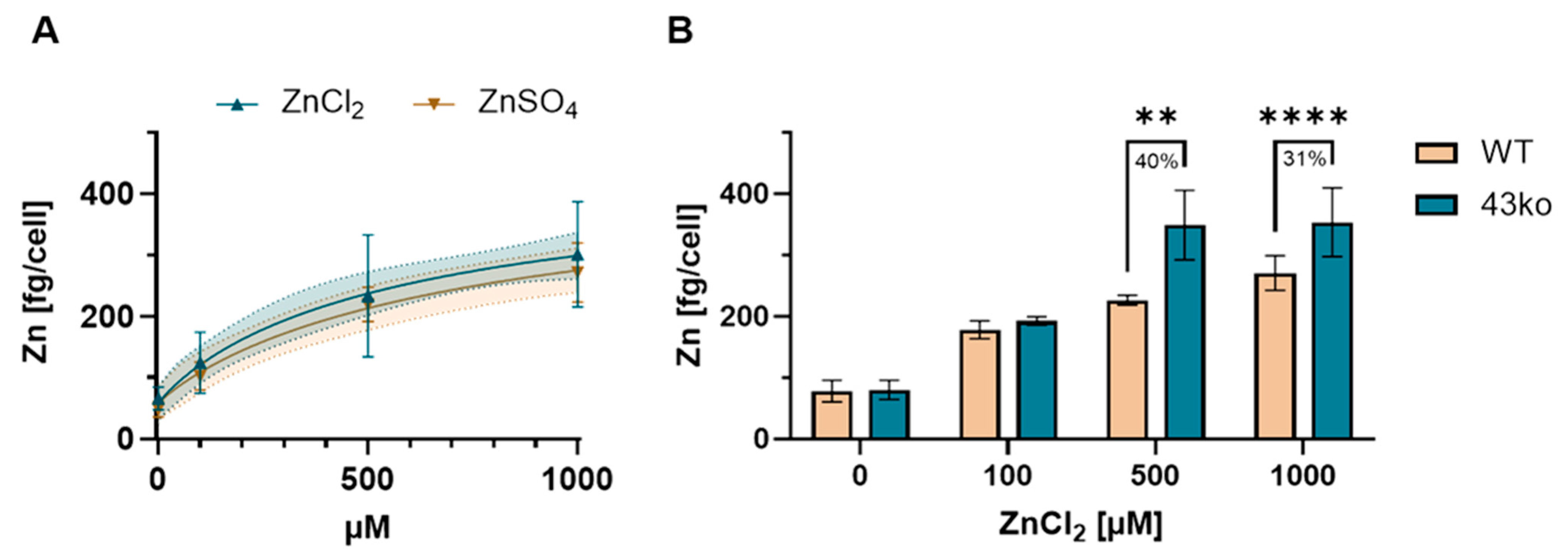
2.2. Protective Role of TAS2R43 Against Excessive Cellular Zn2+ Uptake
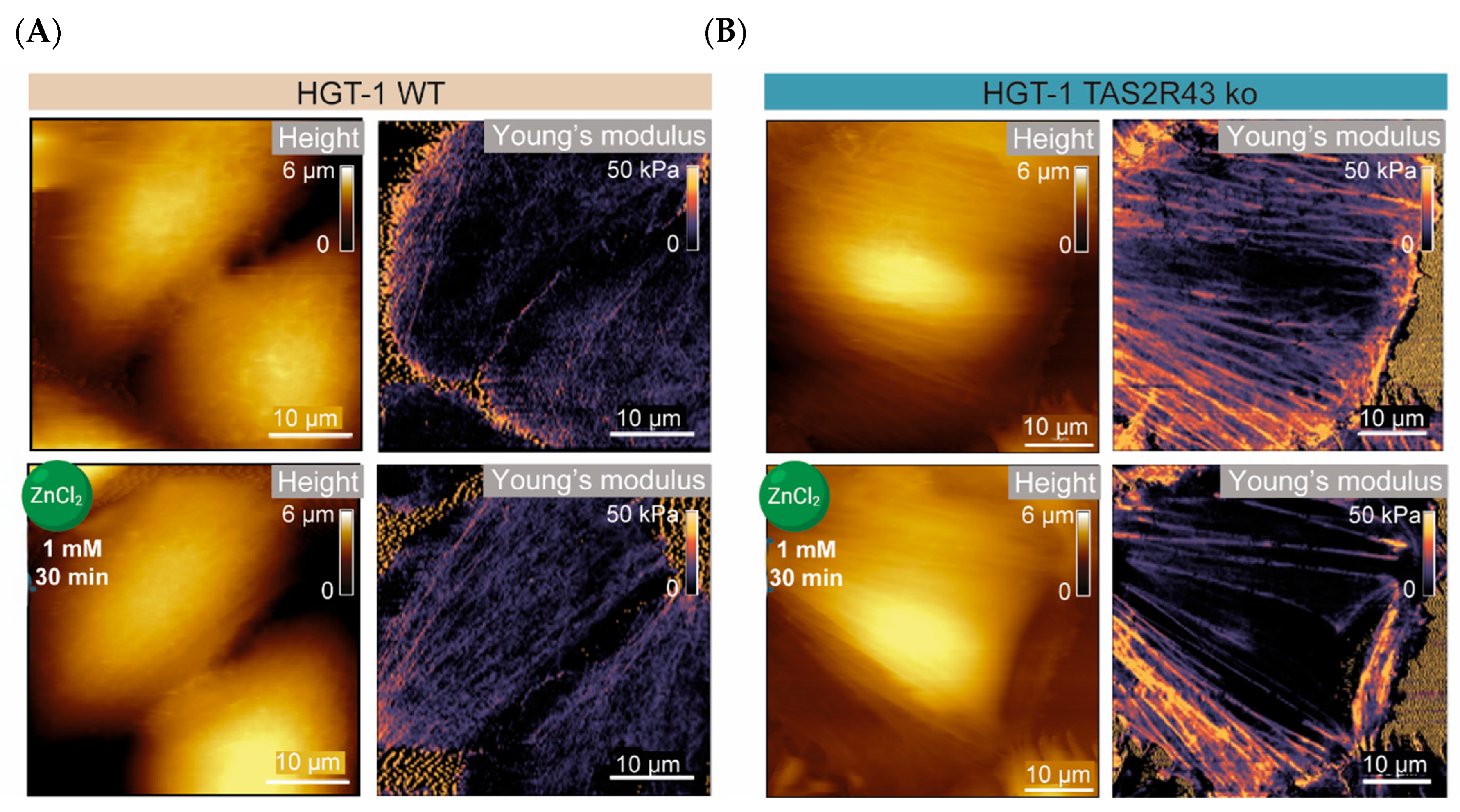
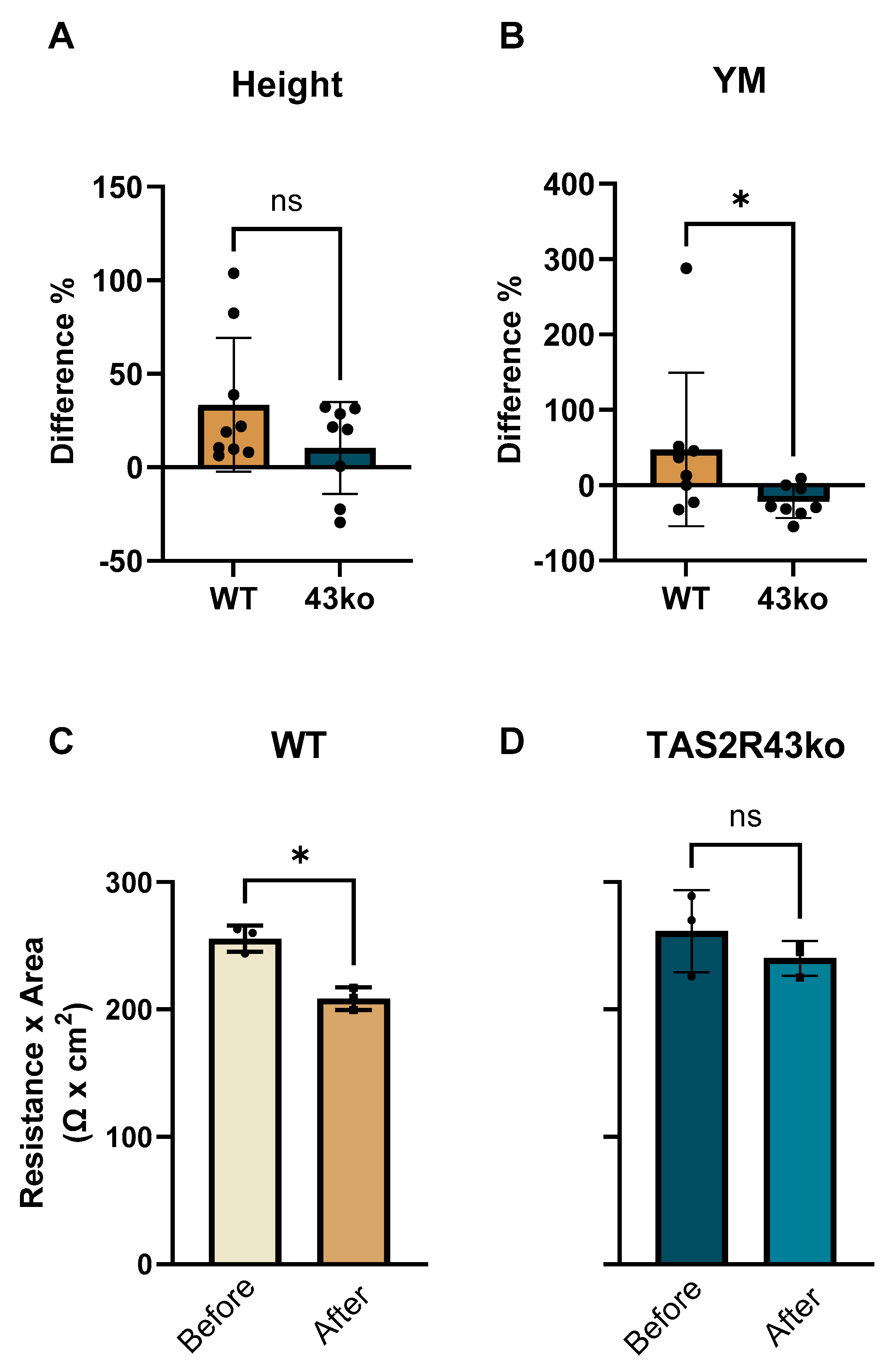
2.3. Impaired Cell Membrane Morphology of TAS2R43ko-Cells by Atomic Force Microscopy and TEER Analysis
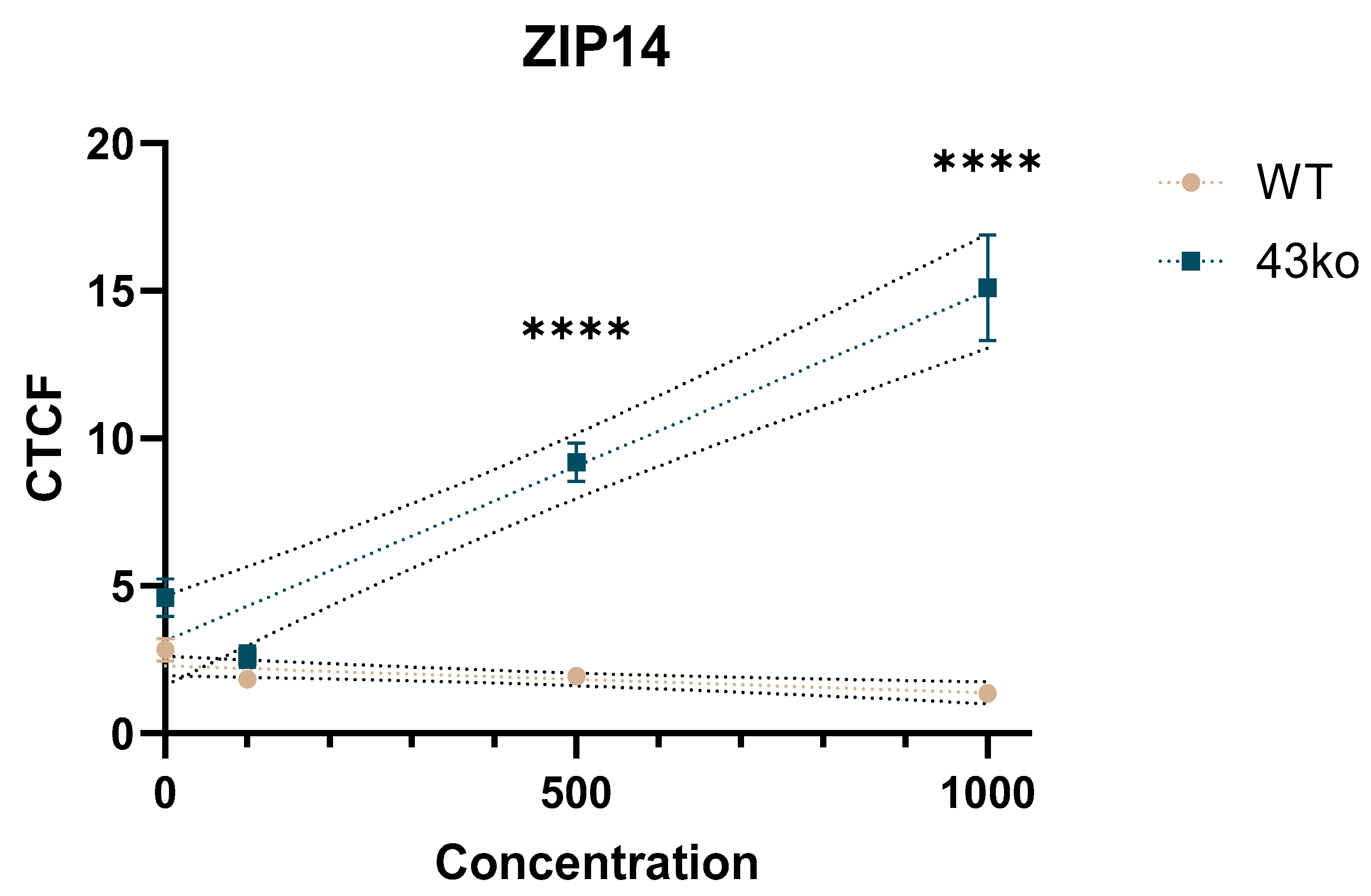
2.4. Increased Protein Expression of Zinc Transporter ZIP14 in HGT-1 TAS2R43ko vs. WT-Cells
3. Discussion
4. Material & Methods
4.1. Chemicals
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Proton Secretory Activity (PSA)
4.5. TAS2R Gene Expression
4.6. Calcium Assay Using Cal-520
4.7. Intracellular Zinc Concentration
4.8. cDNA Microarrays
4.9. Cell Membrane Height and Stiffness by Atomic Force Microscopy (AFM) as Well as Membrane Permeability by Trans-Epithelial Electrical Resistance (TEER)
4.10. Immunocytochemistry Staining
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lang, T.; Di Pizio, A.; Risso, D.; Drayna, D.; Behrens, M. Activation Profile of TAS2R2, the 26th Human Bitter Taste Receptor. Mol. Nutr. Food Res. 2023, 67, 2200775. [Google Scholar] [CrossRef] [PubMed]
- Liszt, K.I.; Ley, J.P.; Lieder, B.; Behrens, M.; Stöger, V.; Reiner, A.; Hochkogler, C.M.; Köck, E.; Marchiori, A.; Hans, J.; et al. Caffeine Induces Gastric Acid Secretion via Bitter Taste Signaling in Gastric Parietal Cells. Proc. Natl. Acad. Sci. USA 2017, 114, E6260–E6269. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, J.; Mueller, K.L.; Hoon, M.A.; Adler, E.; Feng, L.; Guo, W.; Zuker, C.S.; Ryba, N.J.P. T2Rs Function as Bitter Taste Receptors. Cell 2000, 100, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Adler, E.; Hoon, M.A.; Mueller, K.L.; Chandrashekar, J.; Ryba, N.J.P.; Zuker, C.S. A Novel Family of Mammalian Taste Receptors. Cell 2000, 100, 693–702. [Google Scholar] [CrossRef]
- Richter, P.; Sebald, K.; Fischer, K.; Behrens, M.; Schnieke, A.; Somoza, V. Bitter Peptides YFYPEL, VAPFPEVF, and YQEPVLGPVRGPFPIIV, Released during Gastric Digestion of Casein, Stimulate Mechanisms of Gastric Acid Secretion via Bitter Taste Receptors TAS2R16 and TAS2R38. J. Agric. Food Chem. 2022, 70, 11591–11602. [Google Scholar] [CrossRef]
- Sterneder, S.; Stoeger, V.; Dugulin, C.A.; Liszt, K.I.; Di Pizio, A.; Korntheuer, K.; Dunkel, A.; Eder, R.; Ley, J.P.; Somoza, V. Astringent Gallic Acid in Red Wine Regulates Mechanisms of Gastric Acid Secretion via Activation of Bitter Taste Sensing Receptor TAS2R4. J. Agric. Food Chem. 2021, 69, 10550–10561. [Google Scholar] [CrossRef]
- Stoeger, V.; Holik, A.K.; Hölz, K.; Dingjan, T.; Hans, J.; Ley, J.P.; Krammer, G.E.; Niv, M.Y.; Somoza, M.M.; Somoza, V. Bitter-Tasting Amino Acids l-Arginine and l-Isoleucine Differentially Regulate Proton Secretion via T2R1 Signaling in Human Parietal Cells in Culture. J. Agric. Food Chem. 2020, 68, 3434–3444. [Google Scholar] [CrossRef]
- Duarte, A.C.; Rosado, T.; Costa, A.R.; Santos, J.; Gallardo, E.; Quintela, T.; Ishikawa, H.; Schwerk, C.; Schroten, H.; Gonçalves, I.; et al. The Bitter Taste Receptor TAS2R14 Regulates Resveratrol Transport across the Human Blood-Cerebrospinal Fluid Barrier. Biochem. Pharmacol. 2020, 177, 113953. [Google Scholar] [CrossRef]
- Behrens, M.; Redel, U.; Blank, K.; Meyerhof, W. The Human Bitter Taste Receptor TAS2R7 Facilitates the Detection of Bitter Salts. Biochem. Biophys. Res. Commun. 2019, 512, 877–881. [Google Scholar] [CrossRef]
- Wang, Y.; Zajac, A.L.; Lei, W.; Christensen, C.M.; Margolskee, R.F.; Bouysset, C.; Golebiowski, J.; Zhao, H.; Fiorucci, S.; Jiang, P. Metal Ions Activate the Human Taste Receptor TAS2R7. Chem. Senses 2019, 44, 339–347. [Google Scholar] [CrossRef]
- Mariani, E.; Mangialasche, F.; Feliziani, F.T.; Cecchetti, R.; Malavolta, M.; Bastiani, P.; Baglioni, M.; Dedoussis, G.; Fulop, T.; Herbein, G.; et al. Effects of Zinc Supplementation on Antioxidant Enzyme Activities in Healthy Old Subjects. Exp. Gerontol. 2008, 43, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Foster, M.; Samman, S. Zinc and Regulation of Inflammatory Cytokines: Implications for Cardiometabolic Disease. Nutrients 2012, 4, 676–694. [Google Scholar] [CrossRef] [PubMed]
- Sunderman, F.W.J. The Influence of Zinc on Apoptosis. Ann. Clin. Lab. Sci. 1995, 25, 134–142. [Google Scholar] [PubMed]
- Yamasaki, S.; Sakata-Sogawa, K.; Hasegawa, A.; Suzuki, T.; Kabu, K.; Sato, E.; Kurosaki, T.; Yamashita, S.; Tokunaga, M.; Nishida, K.; et al. Zinc is a Novel Intracellular Second Messenger. J. Cell Biol. 2007, 177, 637–645. [Google Scholar] [CrossRef]
- Wijesekara, N.; Chimienti, F.; Wheeler, M.B. Zinc, a Regulator of Islet Function and Glucose Homeostasis. Diabetes, Obes. Metab. 2009, 11, 202–214. [Google Scholar] [CrossRef]
- Guthrie, G.J.; Aydemir, T.B.; Troche, C.; Martin, A.B.; Chang, S.M.; Cousins, R.J. Influence of ZIP14 (Slc39A14) on Intestinal Zinc Processing and Barrier Function. Am. J. Physiol.—Gastrointest. Liver Physiol. 2015, 308, G171–G178. [Google Scholar] [CrossRef]
- Hu, H.; Bandell, M.; Petrus, M.J.; Zhu, M.X.; Patapoutian, A. Zinc Activates Damage-Sensing TRPA1 Ion Channels. Nat. Chem. Biol. 2009, 5, 183–190. [Google Scholar] [CrossRef]
- Lee, H.H.; Prasad, A.S.; Brewer, G.J.; Owyang, C. Zinc Absorption in Human Small Intestine. Am. J. Physiol.—Gastrointest. Liver Physiol. 1989, 256, G87–G91. [Google Scholar] [CrossRef]
- Yasuno, T.; Okamoto, H.; Nagai, M.; Kimura, S.; Yamamoto, T.; Nagano, K.; Furubayashi, T.; Yoshikawa, Y.; Yasui, H.; Katsumi, H.; et al. In Vitro Study on the Transport of Zinc across Intestinal Epithelial Cells Using Caco-2 Monolayers and Isolated Rat Intestinal Membranes. Biol. Pharm. Bull. 2012, 35, 588–593. [Google Scholar] [CrossRef]
- Lodemann, U.; Einspanier, R.; Scharfen, F.; Martens, H.; Bondzio, A. Effects of Zinc on Epithelial Barrier Properties and Viability in a Human and a Porcine Intestinal Cell Culture Model. Toxicol. Vitr. 2013, 27, 834–843. [Google Scholar] [CrossRef]
- Noël, L.; Huynh-Delerme, C.; Guérin, T.; Huet, H.; Frémy, J.M.; Kolf-Clauw, M. Cadmium Accumulation and Interactions with Zinc, Copper, and Manganese, Analysed by ICP-MS in a Long-Term Caco-2 TC7 Cell Model. BioMetals 2006, 19, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Naik, H.B.; Beshire, M.; Walsh, B.M.; Liu, J.; Soybel, D.I.; Hb, N.; Beshire, M.; Bm, W.; Liu, J.; Secretory, S.D.I. Secretory State Regulates Zn2+ Transport in Gastric Parietal Cell of the Rabbit. Am. J. Physiol. Physiol. 2009, 297, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Office of Dietary Supplements Zinc—Fact Sheet. Available online: https://ods.od.nih.gov/factsheets/Zinc-HealthProfessional/ (accessed on 2 June 2025).
- Ryu, M.-S.; Aydemir, T.B. Zinc. In Present Knowledge in Nutrition; Volume 1: Basic Nutrition and Metabolism; Marriott, B.P., Birt, D.F., Stalling, V.A., Yates, A.A., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 393–408. ISBN 9780323661621. [Google Scholar]
- Hamano, H.; Yoshinaga, K.; Eta, R.; Emori, Y.; Kawasaki, D.; Iino, Y.; Sawada, M.; Kuroda, H.; Takei, M. Effect of Polaprezinc on Taste Disorders in Zinc-Deficient Rats. BioFactors 2006, 28, 185–193. [Google Scholar] [CrossRef]
- Keast, R.S.J.; Canty, T.M.; Breslin, P.A.S. Oral Zinc Sulfate Solutions Inhibit Sweet Taste Perception. Chem. Senses 2004, 29, 513–521. [Google Scholar] [CrossRef]
- Keast, R.S.J. The Effect of Zinc on Human Taste Perception. J. Food Sci. 2003, 68, 1871–1877. [Google Scholar] [CrossRef]
- Kirchhoff, P.; Socrates, T.; Sidani, S.; Duffy, A.; Breidthardt, T.; Grob, C.; Viehl, C.T.; Beglinger, C.; Oertli, D.; Geibel, J.P. Zinc Salts Provide a Novel, Prolonged and Rapid Inhibition of Gastric Acid Secretion. Am. J. Gastroenterol. 2011, 106, 62–70. [Google Scholar] [CrossRef]
- Puscas, I.; Sturzu, L.; Buzas, G. Effect of ZnSO4 upon Gastric Acid Secretion and Carbonic Anhydrase. Int. J. Clin. Pharmacol. Ther. Toxicol. 1985, 23, 609–612. [Google Scholar]
- Yamaguchi, M.; Yoshino, T.; Okada, S. Effect of Zinc on the Acidity of Gastric Secretion in Rats. Toxicol. Appl. Pharmacol. 1980, 54, 526–530. [Google Scholar] [CrossRef]
- Gradl, K.; Richter, P.; Somoza, V. Bitter Peptides Formed During In-Vitro Gastric Digestion Induce Mechanisms of Gastric Acid Secretion and Release Satiating Serotonin via Bitter Taste Receptors TAS2R4 and TAS2R43 in Human Parietal Cells in Culture. Food Chem. 2025, 482, 144174. [Google Scholar] [CrossRef]
- Dufner, M.M.; Kirchhoff, P.; Remy, C.; Hafner, P.; Müller, M.K.; Cheng, S.X.; Tang, L.Q.; Hebert, S.C.; Geibel, J.P.; Wagner, C.A. The Calcium-Sensing Receptor Acts as a Modulator of Gastric Acid Secretion in Freshly Isolated Human Gastric Glands. Am. J. Physiol.—Gastrointest. Liver Physiol. 2005, 289, 1084–1090. [Google Scholar] [CrossRef]
- Pérez, C.A.; Huang, L.; Rong, M.; Kozak, J.A.; Preuss, A.K.; Zhang, H.; Max, M.; Margolskee, R.F. A Transient Receptor Potential Channel Expressed in Taste Receptor cells. Nat. Neurosci. 2002, 5, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hoon, M.A.; Chandrashekar, J.; Mueller, K.L.; Cook, B.; Wu, D.; Zuker, C.S.; Ryba, N.J.P. Coding of Sweet, Bitter, and Umami Tastes: Different Receptor Cells Sharing Similar Signaling Pathways. Cell 2003, 112, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Navratil, J.; Kratochvilova, M.; Raudenska, M.; Balvan, J.; Vicar, T.; Petrlakova, K.; Suzuki, K.; Jadrna, L.; Bursa, J.; Kräter, M.; et al. Long-Term Zinc Treatment Alters the Mechanical Properties and Metabolism of Prostate Cancer Cells. Cancer Cell Int. 2024, 24, 313. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.K.; Quist, A.P.; Lal, R. Amyloid β Protein-(1-42) Forms Calcium-Permeable, Zn2+-Sensitive Channel. J. Biol. Chem. 1998, 273, 13379–13382. [Google Scholar] [CrossRef]
- Radmacher, M. Measuring the Elastic Properties of Living Cells. In Methods in Cell Biology; Bhanu, P., Jena, J.K., Hörber, H., Eds.; Academic Press: Cambridge, MA, USA, 2002; Volume 68, pp. 67–90. ISBN 9780125441711. [Google Scholar]
- Cragg, R.A.; Christie, G.R.; Phillips, S.R.; Russi, R.M.; Küry, S.; Mathers, J.C.; Taylor, P.M.; Ford, D. A Novel Zinc-Regulated Human Zinc Transporter, hZTL1, is Localized to the Enterocyte Apical Membrane. J. Biol. Chem. 2002, 277, 22789–22797. [Google Scholar] [CrossRef]
- Thornton, J.K.; Taylor, K.M.; Ford, D.; Valentine, R.A. Differential Subcellular Localization of the Splice Variants of the Zinc Transporter ZnT5 is Dictated by the Different C-Terminal Regions. PLoS ONE 2011, 6, e23878. [Google Scholar] [CrossRef]
- Valentine, R.A.; Jackson, K.A.; Christie, G.R.; Mathers, J.C.; Taylor, P.M.; Ford, D. ZnT5 Variant B is a Bidirectional Zinc Transporter and Mediates Zinc Uptake in Human Intestinal Caco-2 Cells. J. Biol. Chem. 2007, 282, 14389–14393. [Google Scholar] [CrossRef]
- Wilkes, J.M.; Scott, D.R.; Hersey, S.J.; Sachs, G. Second Messengers in the Gastric Gland: A Focus on Calcium. Scand. J. Gastroenterol. 1991, 26, 70–84. [Google Scholar] [CrossRef]
- Hershfinkel, M.; Moran, A.; Grossman, N.; Sekler, I. A Zinc-Sensing Receptor Triggers the Release of Intracellular Ca2+ and Regulates Ion Transport. Proc. Natl. Acad. Sci. USA 2001, 98, 11749–11754. [Google Scholar] [CrossRef]
- Richter, P.; Andersen, G.; Kahlenberg, K.; Mueller, A.U.; Pirkwieser, P.; Boger, V.; Somoza, V. Sodium-Permeable Ion Channels TRPM4 and TRPM5 Are Functional in Human Gastric Parietal Cells in Culture and Modulate the Cellular Response to Bitter-Tasting Food Constituents. J. Agric. Food Chem. 2024, 72, 4906–4917. [Google Scholar] [CrossRef]
- Mueller, A.U.; Andersen, G.; Richter, P.; Somoza, V. Activation of the TRPML1 Ion Channel Induces Proton Secretion in the Human Gastric Parietal Cell Line HGT-1. Int. J. Mol. Sci. 2024, 25, 8829. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.L.; Stork, C.J.; Li, Y.V. Determining Zinc with Commonly Used Calcium and Zinc Fluorescent Indicators, a Question on Calcium Signals. Cell Calcium 2006, 40, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Stork, C.J.; Li, Y.V. Intracellular Zinc Elevation Measured with a “Calcium-Specific” Indicator During Ischemia and Reperfusion in Rat Hippocampus: A Question on Calcium Overload. J. Neurosci. 2006, 26, 10430–10437. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, L.R.; Sterneder, S.; Hasural, A.; Paetz, S.; Hans, J.; Ley, J.P.; Somoza, V. Reducing the Bitter Taste of Pharmaceuticals Using Cell-Based Identification of Bitter-Masking Compounds. Pharmaceuticals 2022, 15, 317. [Google Scholar] [CrossRef]
- Hershfinkel, M. The Zinc Sensing Receptor, ZnR/GPR39, in Health and Disease. Int. J. Mol. Sci. 2018, 19, 439. [Google Scholar] [CrossRef]
- Liu, J.J.; Kohler, J.E.; Blass, A.L.; Moncaster, J.A.; Mocofanescu, A.; Marcus, M.A.; Blakely, E.A.; Bjornstad, K.A.; Amarasiriwardena, C.; Casey, N.; et al. Demand for Zn2+ in Acid-Secreting Gastric Mucosa and Its Requirement for Intracellular Ca2+. PLoS ONE 2011, 6, e19638. [Google Scholar] [CrossRef]
- Luo, Q.; Kuang, D.; Zhang, B.; Song, G. Cell Stiffness Determined by Atomic Force Microscopy and Its Correlation with Cell Motility. Biochim. Biophys. Acta—Gen. Subj. 2016, 1860, 1953–1960. [Google Scholar] [CrossRef]
- Permyakov, S.E.; Yundina, E.N.; Kazakov, A.S.; Permyakova, M.E.; Uversky, V.N.; Permyakov, E.A. Mouse S100G Protein Exhibits Properties Characteristic of a Calcium Sensor. Cell Calcium 2020, 87, 102185. [Google Scholar] [CrossRef]
- Baudier, J.; Deloulme, J.C.; Shaw, G.S. The Zn2+ and Ca2+-Binding S100B and S100A1 Proteins: Beyond the Myths. Biol. Rev. 2020, 95, 738–758. [Google Scholar] [CrossRef]
- Steimle, B.L.; Bailey, D.K.; Smith, F.M.; Rosenblum, S.L.; Kosman, D.J. Calcium and the Ca-ATPase SPCA1 Modulate Plasma Membrane Abundance of ZIP8 and ZIP14 to Regulate Mn(II) Uptake in Brain Microvascular Endothelial Cells. J. Biol. Chem. 2022, 298, 102211. [Google Scholar] [CrossRef]
- Seier Poulsen, S.; Kirkegaard, P.; Skov Olsen, P.; Kürstein Jensen, K.; Christiansen, J. Role of Delayed Gastric Emptying in the Pathogenesis of Cysteamine-Induced Duodenal Ulcer in the Rat. Scand. J. Gastroenterol. 1982, 17, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Behrens, M.; Reichling, C.; Batram, C.; Brockhoff, A.; Meyerhof, W. Bitter Taste Receptors and Their Cells. Ann. N. Y. Acad. Sci. 2009, 1170, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Poverini, R.; Di Lullo, G.; Modesti, A.; Modica, A.; Scarino, M.L. Heavy Metal Toxicity Following Apical and Basolateral Exposure in the Human Intestinal Cell Line Caco-2. Toxicol. Vitr. 1996, 10, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Weiss, C.; Rubach, M.; Lang, R.; Seebach, E.; Blumberg, S.; Frank, O.; Hofmann, T.; Somoza, V. Measurement of the Intracellular Ph in Human Stomach Cells: A Novel Approach to Evaluate the Gastric Acid Secretory Potential of Coffee Beverages. J. Agric. Food Chem. 2010, 58, 1976–1985. [Google Scholar] [CrossRef]
- Roper, S.D. Signal Transduction and Information Processing in Mammalian Taste Buds. Pflüg. Arch.—Eur. J. Physiol. 2007, 454, 759–776. [Google Scholar] [CrossRef]
- Meyer, S.; López-Serrano, A.; Mitze, H.; Jakubowski, N.; Schwerdtle, T. Single-Cell Analysis by ICP-MS/MS as a Fast Tool for Cellular Bioavailability Studies of Arsenite. Metallomics 2018, 10, 73–76. [Google Scholar] [CrossRef]
- Holik, A.K.; Schweiger, K.; Stoeger, V.; Lieder, B.; Reiner, A.; Zopun, M.; Hoi, J.K.; Kretschy, N.; Somoza, M.M.; Kriwanek, S.; et al. Gastric Serotonin Biosynthesis and its Functional Role in l-Arginine-Induced Gastric Proton Secretion. Int. J. Mol. Sci. 2021, 22, 5881. [Google Scholar] [CrossRef]
- Behr, J.; Michel, T.; Giridhar, M.; Santhosh, S.; Das, A.; Sabzalipoor, H.; Keki, T.; Parlar, E.; Ilić, I.; Ibáñez-Redín, G.; et al. An Open—Source Advanced Maskless Synthesizer for Light—Directed Chemical Synthesis of Large Nucleic Acid Libraries and Microarrays. ChemRxiv 2024, 1–20. [Google Scholar] [CrossRef]
- Hölz, K.; Hoi, J.K.; Schaudy, E.; Somoza, V.; Lietard, J.; Somoza, M.M. High-Efficiency Reverse (5′ → 3′) Synthesis of Complex DNA Microarrays. Sci. Rep. 2018, 8, 15099. [Google Scholar] [CrossRef]
- Hutter, J.L.; Bechhoefer, J. Calibration of Atomic-Force Microscope tips. Rev. Sci. Instrum. 1993, 64, 1868–1873. [Google Scholar] [CrossRef]
- Hertz, H. On the Fixed Elastic Body Contact. J. für die reine und Angew. Math. 1882, 1882, 156–171. [Google Scholar] [CrossRef]
- Schillers, H.; Rianna, C.; Schäpe, J.; Luque, T.; Doschke, H.; Wälte, M.; Uriarte, J.J.; Campillo, N.; Michanetzis, G.P.A.; Bobrowska, J.; et al. Standardized Nanomechanical Atomic Force Microscopy Procedure (SNAP) for Measuring Soft and Biological Samples. Sci. Rep. 2017, 7, 5117. [Google Scholar] [CrossRef] [PubMed]
- Danzer, B.; Jukic, M.; Dunkel, A.; Andersen, G.; Lieder, B.; Schaudy, E.; Stadlmayr, S.; Lietard, J.; Michel, T.; Krautwurst, D.; et al. Impaired Metal Perception and Regulation of Associated Human Foliate Papillae Tongue Transcriptome in Long-COVID-19. Sci. Rep. 2024, 14, 15408. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orth, H.N.; Pirkwieser, P.; Benthin, J.; Koehler, M.; Sterneder, S.; Parlar, E.; Schaudy, E.; Lietard, J.; Michel, T.; Boger, V.; et al. Bitter Taste Receptor TAS2R43 Co-Regulates Mechanisms of Gastric Acid Secretion and Zinc Homeostasis. Int. J. Mol. Sci. 2025, 26, 6017. https://doi.org/10.3390/ijms26136017
Orth HN, Pirkwieser P, Benthin J, Koehler M, Sterneder S, Parlar E, Schaudy E, Lietard J, Michel T, Boger V, et al. Bitter Taste Receptor TAS2R43 Co-Regulates Mechanisms of Gastric Acid Secretion and Zinc Homeostasis. International Journal of Molecular Sciences. 2025; 26(13):6017. https://doi.org/10.3390/ijms26136017
Chicago/Turabian StyleOrth, H. Noreen, Philip Pirkwieser, Julia Benthin, Melanie Koehler, Sonja Sterneder, Etkin Parlar, Erika Schaudy, Jory Lietard, Timm Michel, Valerie Boger, and et al. 2025. "Bitter Taste Receptor TAS2R43 Co-Regulates Mechanisms of Gastric Acid Secretion and Zinc Homeostasis" International Journal of Molecular Sciences 26, no. 13: 6017. https://doi.org/10.3390/ijms26136017
APA StyleOrth, H. N., Pirkwieser, P., Benthin, J., Koehler, M., Sterneder, S., Parlar, E., Schaudy, E., Lietard, J., Michel, T., Boger, V., Dunkel, A., Somoza, M. M., & Somoza, V. (2025). Bitter Taste Receptor TAS2R43 Co-Regulates Mechanisms of Gastric Acid Secretion and Zinc Homeostasis. International Journal of Molecular Sciences, 26(13), 6017. https://doi.org/10.3390/ijms26136017






