Perspectives on the Parathyroid–Thymus Interconnection—A Literature Review
Abstract
1. Introduction
2. Material and Methods
- The inclusion criteria for the development of the glands were based on keywords such as molecular research on thymic organogenesis and molecular research on parathyroid gland organogenesis.
- Regarding the functional relationship between these, we used the following keywords: thymus function, parathyroid and thymus function, T lymphocytes and Ca.
- To highlight the etiopathogenic relationship, we used the following keywords: thymic cause of hypoparathyroidism, parathyroid and thymic dysfunctions, causes of myasthenia gravis.
3. Results
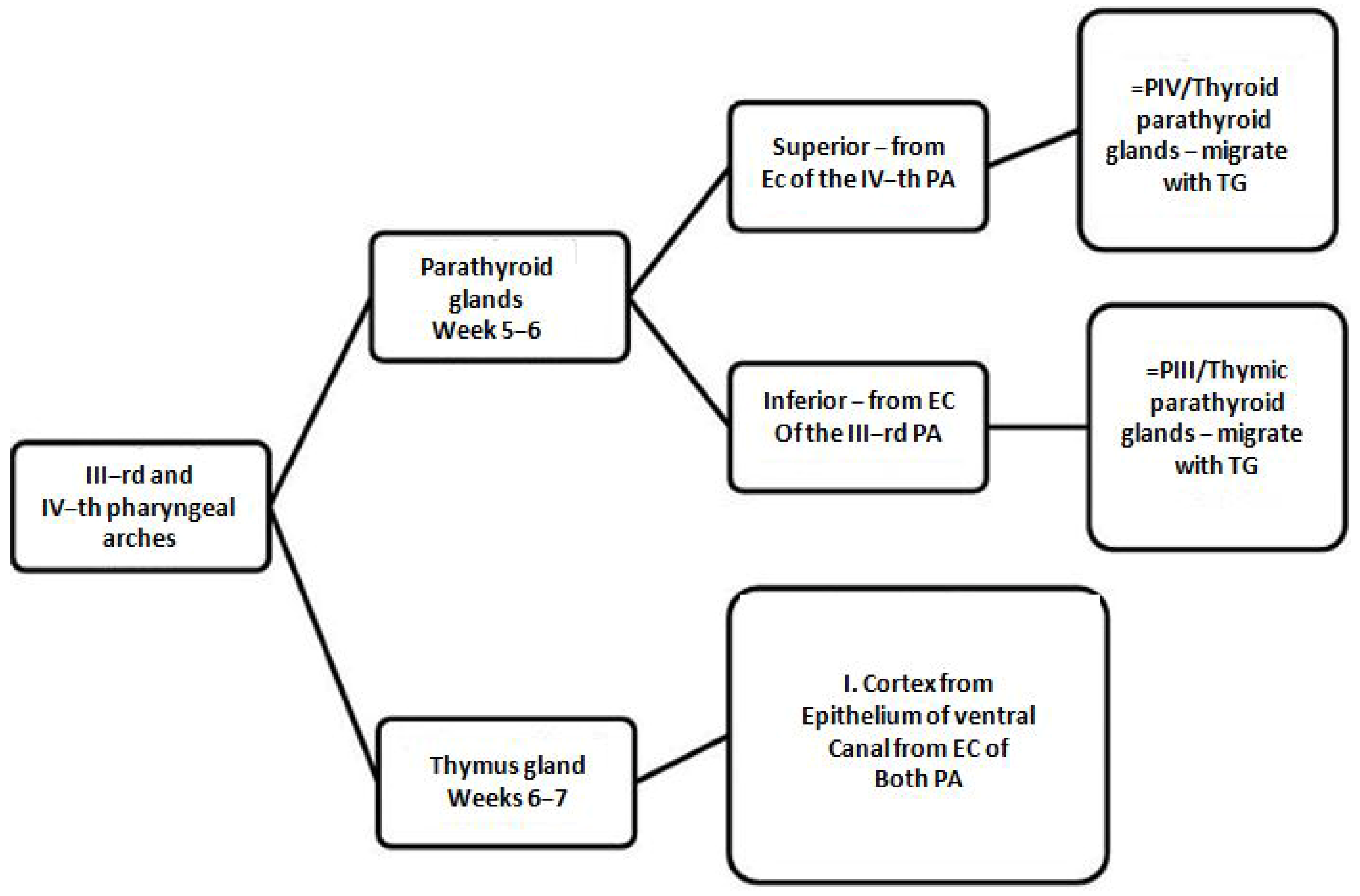
3.1. Common Development Molecular Research on Thymic and Parathyroid Glands
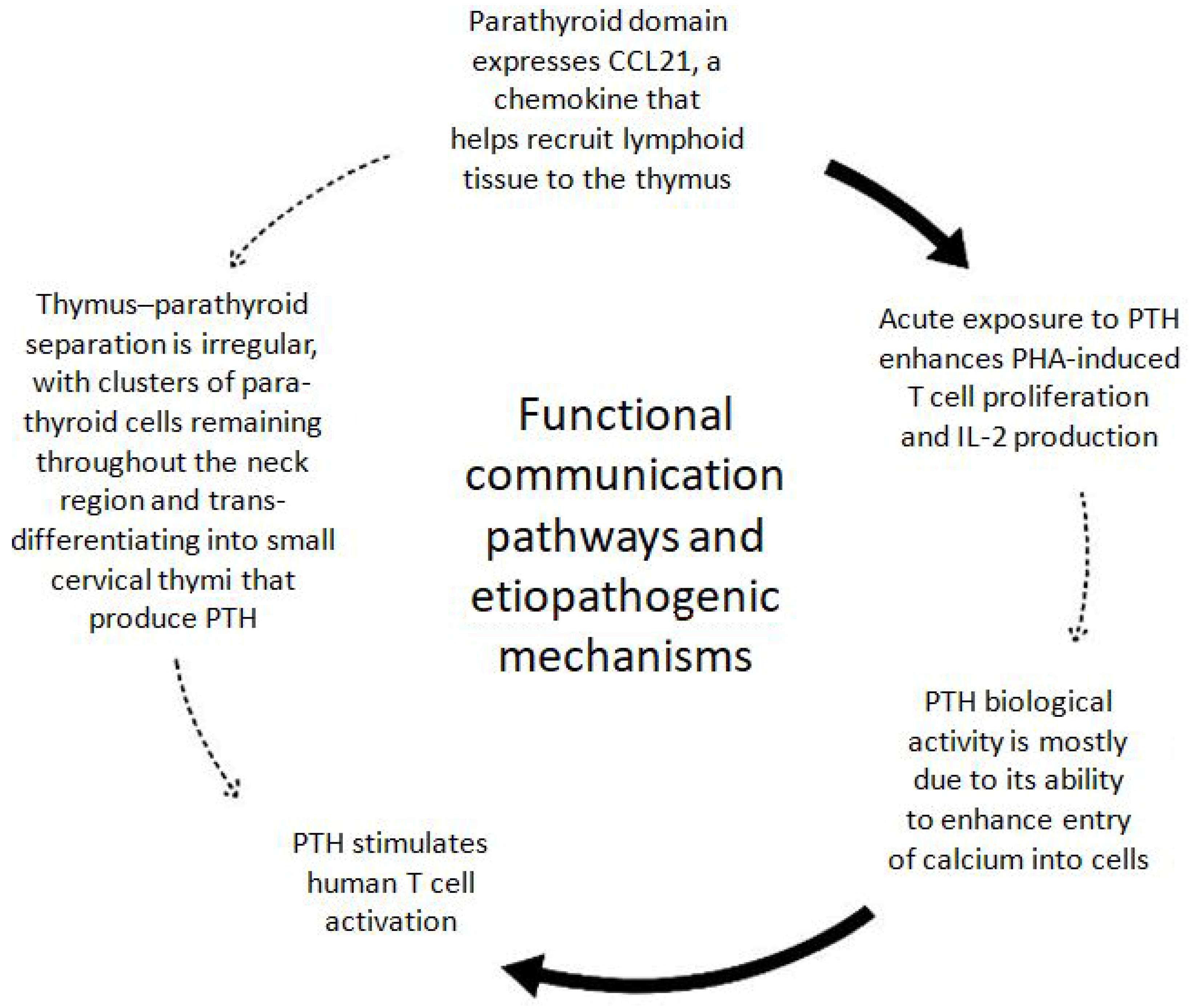
3.2. Functional Relationship Molecular Research on Thymic and Parathyroid Glands
- The role of the thymus in the immune system:
- ⮚
- oncologic immunology;
- ⮚
- systemic viral infections;
- ⮚
- organ transplantation;
- ⮚
- maternal–fetal tolerance.
- The role of the thymus in maintaining the homeostasis of lipid and carbohydrate metabolism.
- The evaluation of the possibilities of regaining thymic functions.
- The role of the thymus in wound repair.
- The role of Ca+ channels in thymic function.
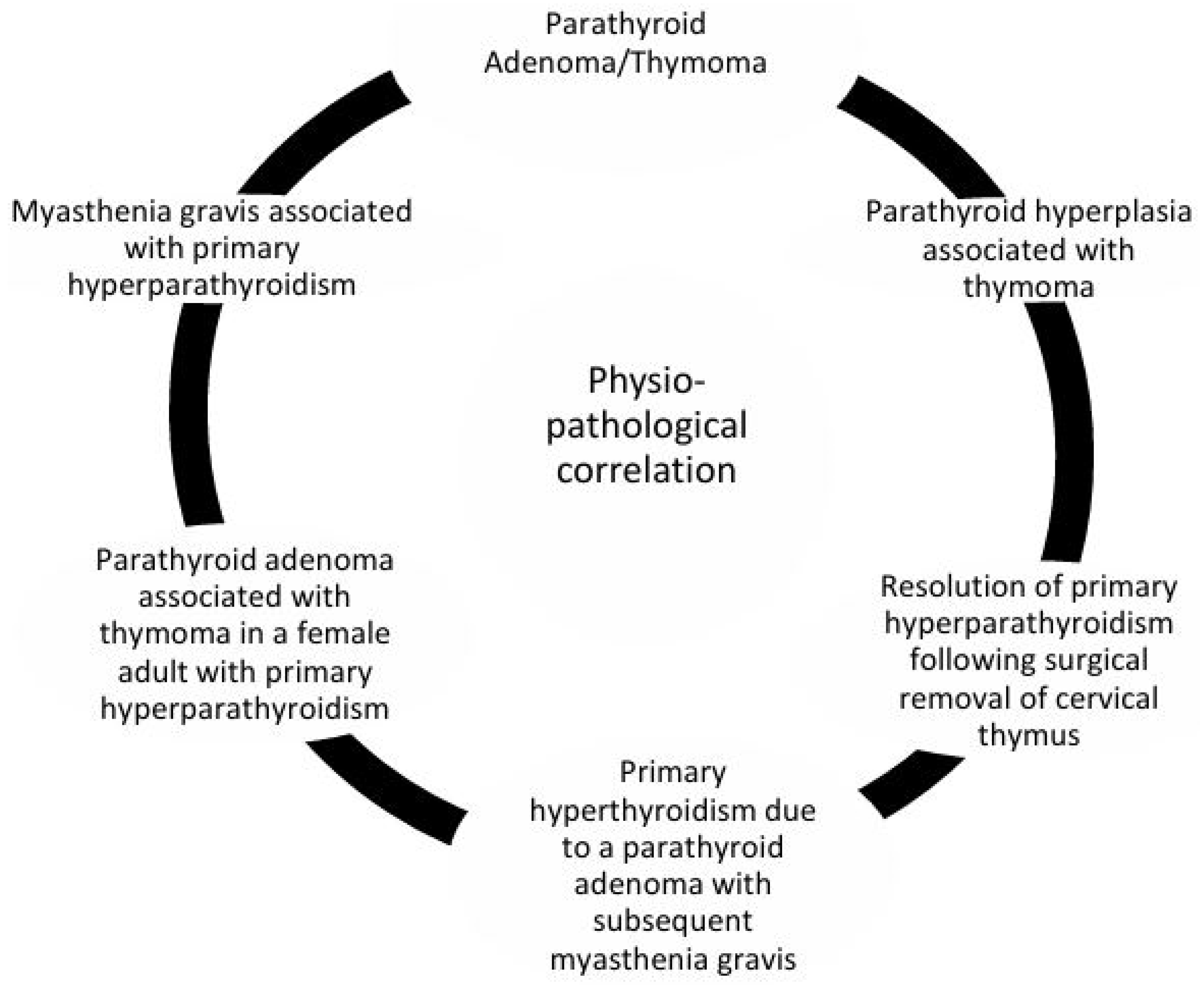
3.3. Etiopathogenic Relationship Molecular Research on Thymic and Parathyroid Glands
4. Discussions
4.1. Convergent Molecular Pathways in and Parathyroid Glands Organogenesis
4.2. Functional Interactions and Molecular Crosstalk Between the Thymus and Parathyroid Glands
4.3. Etiopathogenic Interactions and Molecular Insights Linking the Thymus and Parathyroid Glands
5. Conclusions and Future Directions
- A parathyroid adenoma/thymoma association was observed; this coexistence of parathyroid adenomas and thymomas suggests a developmental or functional link between these glands.
- Instances of MG coexisting with primary hyperparathyroidism (PHPT) imply shared immune or endocrine regulatory pathways.
- A review of parathyroid hyperplasia in patients with thymoma highlights the potential for thymic abnormalities to influence parathyroid function.
- In adult females, cases of parathyroid adenomas co-occurring with thymomas have been documented in the context of PHPT, further linking the two organs.
- Rare cases describe PHPT caused by parathyroid adenomas that later lead to the development of MG, possibly through immune dysregulation.
- The resolution of PHPT after thymus surgical excision of the cervical thymus has, in some cases, resulted in the resolution of primary hyperparathyroidism, indicating the presence of ectopic or residual parathyroid tissue within the thymus [71].
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| TEC | thymic epithelial cell |
| non-TEC stromal cells | Non-Epithelial Thymic Stromal Cells |
| TCR | T cell receptor |
| ETPs | Early T-cell progenitors |
| Treg | regulatory T cells |
| TRAs | peripheral tissue-restricted antigens |
| FRCs | fibroblastic reticular cells |
| ETPs | early T-cell progenitors |
| RANKL | Receptor activator of nuclear factor kappa B ligand |
| PAX8 | Paired-Box Gene 8 |
| TEPC | thymic epithelial progenitor cell |
| IHC | Immunohistochemistry |
| FTOC | fetal thymic organ culture |
| 3PP | third pharyngeal pouch |
| hESCs | human embryonic stem cells |
| GSEA | Gene set enrichment analysis |
| ChIP Assay Kit | Chromatin Immunoprecipitation (ChIP) Assay Kit |
| NF-κB | nuclear factor-kappaB |
| Tregs | Regulatory CD4 T cells |
| PTGs | parathyroid glands |
| PSCs | pluripotent stem cells |
| mESCs | mouse embryonic stem cells |
| BC | blastocyst complementation |
| GCM2 | Glial cells missing homolog 2 |
| TF5 | Thymosin Fraction V |
| Tα1 | Thymosin alpha 1 |
| Tβ4 | Thymosin beta 4 |
| TP | Thymopoietin |
| TH | Thymulin |
| TFX® | Thymus factor X/Thymostimulinum |
| tTreg | thymus-derived Treg |
| pTreg | peripherally derived Treg |
| iTreg | in vitro induced Treg |
| TNFR | novel tumor necrosis factor receptor |
| S1P | HIV-1 on Sphingosine-1-phosphate |
| S1PR1 | S1P receptor 1 |
| PLWH | people living with HIV |
| RT-qRT-PCR | Real-Time Quantitative Reverse-Transcription PCR |
| PBMC | peripheral blood mononuclear cells |
| BMP4 | Bone Morphogenic Protein 4 |
| KGF/FGF-7 | Keratinocyte growth factor |
| AIRE | Autoimmune regulatory protein |
| CCL2 | C-C Motif Chemokine Ligand 2 |
| FoxN1 | Thymus transcription factors forkhead box N1 |
| T1DM/T2DM | Type 1 and 2 diabetes mellitus |
| adipokines | cytokines produced by adipose tissue |
| sj-TREC | T cell receptor excision circles |
| ApoB | apolipoprotein A |
| UCP1 | mitochondrial uncoupling protein |
| TBX1 | T-box transcription factor |
| NKT | Natural killer T cells |
| KCa3.1 and Kv1.3 | potassium (K+) channel |
| PBMCs | Peripheral blood mononuclear cells |
| VDCCs | voltage-dependent Ca2+ channels |
| MLPA | multiplex ligation-dependent probe amplification |
| CCHD | Conotruncal Congenital Heart Disease |
| APS-1 | Autoimmune polyglandular syndrome type 1 |
| HP | hypoparathyroidism |
| AC-Abs | Antibodies to adrenal cortex |
| STC-Abs | antibodies to steroid-producing cells |
References
- Marra, F.; Lunetti, P.; Curcio, R.; Lasorsa, F.M.; Capobianco, L.; Porcelli, V.; Dolce, V.; Fiermonte, G.; Scarcia, P. An Overview of Mitochondrial Protein Defects in Neuromuscular Diseases. Biomolecules 2021, 11, 1633. [Google Scholar] [CrossRef]
- Nicolle, M.W. Myasthenia Gravis and Lambert-Eaton Myasthenic Syndrome. Muscle Neuromuscul. Junction Disord. 2016, 22, 1978–2005. [Google Scholar] [CrossRef]
- Gilmour, J. The embryology of the parathyroid glands, the thymus and certain associated rudiments. J. Pathol. Bacteriol. 2005, 45, 507–522. [Google Scholar] [CrossRef]
- Sakr, M. Embryology of the Parathyroid Glands; StatPearls Publishing: Treasure Island, FL, USA, 2022; pp. 11–21. [Google Scholar]
- Corbin, H.; Yip, L.; Carty, S.E.; Reyes-Múgica, M.; Seethala, R.R. Characterisation of Kürsteiner canals of parathyroid: Imparting relevance to a one-and-a-quarter-century-old concept. Histopathology 2025, 86, 341–351. [Google Scholar] [CrossRef]
- Liu, Z.; Farley, A.; Chen, L.; Kirby, B.J.; Kovacs, C.S.; Blackburn, C.C.; Manley, N.R. Thymus-associated parathyroid hormone has two cellular origins with distinct endocrine and immunological functions. PLoS Genet. 2010, 6, e1001251. [Google Scholar] [CrossRef]
- Zangari, M.; Yoo, H.; Shin, I.; Kim, B.; Edmondson, R.; Morgan, G.J.; Suva, L.J.; Yoon, D. Thymic PTH Increases After Thyroparathyroidectomy in C57BL/KaLwRij Mice. Endocrinology 2018, 159, 1561–1569. [Google Scholar] [CrossRef]
- Neves, H.; Dupin, E.; Parreira, L.; Le Douarin, N.M. Modulation of Bmp4 signalling in the epithelial-mesenchymal interactions that take place in early thymus and parathyroid development in avian embryos. Dev. Biol. 2012, 361, 208–219. [Google Scholar] [CrossRef]
- Kearns, N.A.; Lobo, M.; Genga, R.M.J.; Abramowitz, R.G.; Parsi, K.M.; Min, J.; Kernfeld, E.M.; Huey, J.D.; Kady, J.; Hennessy, E.; et al. Generation and molecular characterization of human pluripotent stem cell-derived pharyngeal foregut endoderm. Dev. Cell 2023, 58, 1801–1818.e1815. [Google Scholar] [CrossRef]
- Gordon, J.; Manley, N.R. Mechanisms of thymus organogenesis and morphogenesis. Development 2011, 138, 3865–3878. [Google Scholar] [CrossRef]
- Gardiner, J.R.; Jackson, A.L.; Gordon, J.; Lickert, H.; Manley, N.R.; Basson, M.A. Localised inhibition of FGF signalling in the third pharyngeal pouch is required for normal thymus and parathyroid organogenesis. Development 2012, 139, 3456–3466. [Google Scholar] [CrossRef]
- Gordon, J.; Wilson, V.A.; Blair, N.F.; Sheridan, J.; Farley, A.; Wilson, L.; Manley, N.R.; Blackburn, C.C. Functional evidence for a single endodermal origin for the thymic epithelium. Nat. Immunol. 2004, 5, 546–553. [Google Scholar] [CrossRef]
- Gordon, J.; Patel, S.R.; Mishina, Y.; Manley, N.R. Evidence for an early role for BMP4 signaling in thymus and parathyroid morphogenesis. Dev. Biol. 2010, 339, 141–154. [Google Scholar] [CrossRef]
- Terszowski, G.; Müller, S.M.; Bleul, C.C.; Blum, C.; Schirmbeck, R.; Reimann, J.; Pasquier, L.D.; Amagai, T.; Boehm, T.; Rodewald, H.R. Evidence for a functional second thymus in mice. Science 2006, 312, 284–287. [Google Scholar] [CrossRef]
- Liu, C.; Saito, F.; Liu, Z.; Lei, Y.; Uehara, S.; Love, P.; Lipp, M.; Kondo, S.; Manley, N.; Takahama, Y. Coordination between CCR7- and CCR9-mediated chemokine signals in prevascular fetal thymus colonization. Blood 2006, 108, 2531–2539. [Google Scholar] [CrossRef]
- Liu, C.; Ueno, T.; Kuse, S.; Saito, F.; Nitta, T.; Piali, L.; Nakano, H.; Kakiuchi, T.; Lipp, M.; Hollander, G.A.; et al. The role of CCL21 in recruitment of T-precursor cells to fetal thymi. Blood 2005, 105, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Peacock, M. Calcium metabolism in health and disease. Clin. J. Am. Soc. Nephrol. 2010, 5 (Suppl. 1), S23–S30. [Google Scholar] [CrossRef]
- Kanome, Y.; Gao, J.; Hashimoto, A.; Ogawa, Y.; Nakatsu, M.; Kohno, M.; Fukui, K. Effects of cerium oxide nanoparticles on adenine-induced chronic kidney disease model rats. Geriatr. Gerontol. Int. 2024, 24 (Suppl. 1), 88–95. [Google Scholar] [CrossRef]
- Nagakubo, D.; Hirakawa, M.; Iwanami, N.; Boehm, T. Limits to in vivo fate changes of epithelia in thymus and parathyroid by ectopic expression of transcription factors Gcm2 and Foxn1. Sci. Rep. 2022, 12, 13554. [Google Scholar] [CrossRef]
- Geng, C.; Liu, J.; Guo, B.; Liu, K.; Gong, P.; Wang, B.; Wan, Q.; Sun, L.; Zhao, J.; Song, Y. High lymphocyte signature genes expression in parathyroid endocrine cells and its downregulation linked to tumorigenesis. EBioMedicine 2024, 102, 105053. [Google Scholar] [CrossRef] [PubMed]
- Patrick, M.S.; Cheng, N.-L.; Kim, J.; An, J.; Dong, F.; Yang, Q.; Zou, I.; Weng, N.-P. Human T Cell Differentiation Negatively Regulates Telomerase Expression Resulting in Reduced Activation-Induced Proliferation and Survival. Front. Immunol. 2019, 10, 1993. [Google Scholar] [CrossRef]
- Diogenes, L.; Dellavance, A.; Baldo, D.; Gozzi-Silva, S.; Gomes, K.; Prado, M.; Andrade, L.; Keppeke, G. Detection of Autoantibodies Against the Acetylcholine Receptor, Evaluation of Commercially Available Methodologies: Fixed Cell-Based Assay, Radioimmunoprecipitation Assay and Enzyme-Linked Immunosorbent Assay1. J. Neuromuscul. Dis. 2024, 11, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Han, L.; Zhu, D.; Peng, J.; Li, J.; Ding, J.; Luo, J.; Hong, R.; Wang, K.; Wan, W.; et al. A Stable Cell Line Expressing Clustered AChR: A Novel Cell-Based Assay for Anti-AChR Antibody Detection in Myasthenia Gravis. Front. Immunol. 2021, 12, 666046. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Bajoghli, B.; Dick, A.M.; Claasen, A.; Doll, L.; Aghaallaei, N. Zebrafish and Medaka: Two Teleost Models of T-Cell and Thymic Development. Int. J. Mol. Sci. 2019, 20, 4179. [Google Scholar] [CrossRef]
- Newton, H.S.; Gawali, V.S.; Chimote, A.A.; Lehn, M.A.; Palackdharry, S.M.; Hinrichs, B.H.; Jandarov, R.; Hildeman, D.; Janssen, E.M.; Wise-Draper, T.M.; et al. PD1 blockade enhances K+ channel activity, Ca2+ signaling, and migratory ability in cytotoxic T lymphocytes of patients with head and neck cancer. J. Immunother. Cancer 2020, 8, e000844. [Google Scholar] [CrossRef]
- Fenninger, F.; Han, J.; Stanwood, S.R.; Nohara, L.L.; Arora, H.; Choi, K.B.; Munro, L.; Pfeifer, C.G.; Shanina, I.; Horwitz, M.S.; et al. Mutation of an L-Type Calcium Channel Gene Leads to T Lymphocyte Dysfunction. Front. Immunol. 2019, 10, 2473. [Google Scholar] [CrossRef]
- Cirillo, A.; Lioncino, M.; Maratea, A.; Passariello, A.; Fusco, A.; Fratta, F.; Monda, E.; Caiazza, M.; Signore, G.; Esposito, A.; et al. Clinical Manifestations of 22q11.2 Deletion Syndrome. Heart Fail. Clin. 2022, 18, 155–164. [Google Scholar] [CrossRef]
- Campos-Garcia, F.-J.; Castillo-Espinola, A.-M.; Medina-Escobedo, C.-E.; Zenteno, J.C.; Lara-Riegos, J.-C.; Rubio-Zapata, H.; Cruz-Robles, D.; Velazquez-Ibarra, A.-I. Screening Method for 22q11 Deletion Syndrome Involving the Use of TaqMan qPCR for TBX1 in Patients with Conotruncal Congenital Heart Disease. Cardiogenetics 2022, 12, 253–260. [Google Scholar] [CrossRef]
- Szczawińska-Popłonyk, A.; Schwartzmann, E.; Chmara, Z.; Głukowska, A.; Krysa, T.; Majchrzycki, M.; Olejnicki, M.; Ostrowska, P.; Babik, J. Chromosome 22q11.2 Deletion Syndrome: A Comprehensive Review of Molecular Genetics in the Context of Multidisciplinary Clinical Approach. Int. J. Mol. Sci. 2023, 24, 8317. [Google Scholar] [CrossRef]
- Blackburn, C.C.; Manley, N.R. Developing a new paradigm for thymus organogenesis. Nat. Rev. Immunol. 2004, 4, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.A.; Wilkerson, W.M.; Shaffer, D.W.; Armstrong, E.G. “Practicing” medicine without risk: Students’ and educators’ responses to high-fidelity patient simulation. Acad. Med. 2001, 76, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhang, X.; Wu, H.; Zhang, P.; Liu, S.; Guo, T.; Shan, H.; Liang, Y.; Chen, H.; Xie, J.; et al. HOXA3 functions as the on-off switch to regulate the development of hESC-derived third pharyngeal pouch endoderm through EPHB2-mediated Wnt pathway. Front. Immunol. 2023, 14, 1258074. [Google Scholar] [CrossRef] [PubMed]
- Mulder, G.B.; Manley, N.; Maggio-Price, L. Retinoic acid-induced thymic abnormalities in the mouse are associated with altered pharyngeal morphology, thymocyte maturation defects, and altered expression of Hoxa3 and Pax1. Teratology 1998, 58, 263–275. [Google Scholar] [CrossRef]
- Handra-Luca, A. Adult perithyroid and cervical thymus-parathyroid unit: Case reports of a rare entity. Medicine 2014, 93, e201. [Google Scholar] [CrossRef]
- O’Connor, K.; Alzahrani, H.; Murad, F.; Daroca, P.; Kandil, E. An ectopic intrathyroidal thymic tissue and intrathymic parathyroid tissue in a patient with Graves disease. Gland. Surg. 2017, 6, 726–728. [Google Scholar] [CrossRef]
- Wu, S.; Gupta, D.; Connelly, J. Adult ectopic thymus adjacent to thyroid and parathyroid. Arch. Pathol. Lab. Med. 2001, 125, 842–843. [Google Scholar] [CrossRef]
- Perniola, R. Twenty Years of AIRE. Front. Immunol. 2018, 9, 98. [Google Scholar] [CrossRef]
- Gao, S.; Li, X.; Amendt, B.A. Understanding the role of Tbx1 as a candidate gene for 22q11.2 deletion syndrome. Curr. Allergy Asthma Rep. 2013, 13, 613–621. [Google Scholar] [CrossRef]
- Prapa, M.; Ho, S.Y. Human Genetics of Semilunar Valve and Aortic Arch Anomalies. Adv. Exp. Med. Biol. 2024, 1441, 761–775. [Google Scholar] [CrossRef]
- Zeng, Y.; Liu, C.; Gong, Y.; Bai, Z.; Hou, S.; He, J.; Bian, Z.; Li, Z.; Ni, Y.; Yan, J.; et al. Single-Cell RNA Sequencing Resolves Spatiotemporal Development of Pre-thymic Lymphoid Progenitors and Thymus Organogenesis in Human Embryos. Immunity 2019, 51, 930–948.e936. [Google Scholar] [CrossRef]
- Farley, A.; Chengrui, A.; Palmer, S.; Liu, D.; Kousa, A.; Rouse, P.; Major, V.; Sweetman, J.; Morys, J.; Corsinotti, A.; et al. Thymic epithelial cell fate and potency in early organogenesis assessed by single cell transcriptional and functional analysis. Front. Immunol. 2023, 14, 1202163. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Kousa, A.I.; O’Neill, K.E.; Rouse, P.; Popis, M.; Farley, A.M.; Tomlinson, S.R.; Ulyanchenko, S.; Guillemot, F.; Seymour, P.A.; et al. Canonical Notch signaling controls the early thymic epithelial progenitor cell state and emergence of the medullary epithelial lineage in fetal thymus development. Development 2020, 147, dev178582. [Google Scholar] [CrossRef] [PubMed]
- Hövelmeyer, N.; Schmidt-Supprian, M.; Ohnmacht, C. NF-κB in control of regulatory T cell development, identity, and function. J. Mol. Med. 2022, 100, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Dhar, A.; Chawla, M.; Chattopadhyay, S.; Oswal, N.; Umar, D.; Gupta, S.; Bal, V.; Rath, S.; George, A.; Arimbasseri, G.A.; et al. Role of NF-kappaB2-p100 in regulatory T cell homeostasis and activation. Sci. Rep. 2019, 9, 13867. [Google Scholar] [CrossRef]
- Kano, M.; Mizuno, N.; Sato, H.; Kimura, T.; Hirochika, R.; Iwasaki, Y.; Inoshita, N.; Nagano, H.; Kasai, M.; Yamamoto, H.; et al. Functional calcium-responsive parathyroid glands generated using single-step blastocyst complementation. Proc. Natl. Acad. Sci. USA 2023, 120, e2216564120. [Google Scholar] [CrossRef]
- Kano, M. Parathyroid Gland Generation from Pluripotent Stem Cells. Endocrinol. Metab. 2024, 39, 552–558. [Google Scholar] [CrossRef]
- Yamada, T.; Tatsumi, N.; Anraku, A.; Suzuki, H.; Kamejima, S.; Uchiyama, T.; Ohkido, I.; Yokoo, T.; Okabe, M. Gcm2 regulates the maintenance of parathyroid cells in adult mice. PLoS ONE 2019, 14, e0210662. [Google Scholar] [CrossRef]
- Georg, B.; Jørgensen, H.L.; Hannibal, J. PER1 Oscillation in Rat Parathyroid Hormone and Calcitonin Producing Cells. Int. J. Mol. Sci. 2024, 25, 13006. [Google Scholar] [CrossRef]
- Artym, J.; Zimecki, M. An overview on immunological activity of calf thymus extract (TFX®) and its therapeutic benefits. J. Biomed. Res. Ther. 2022, 1, 1–22. [Google Scholar] [CrossRef]
- Besman, M.; Zambrowicz, A.; Matwiejczyk, M. Review of Thymic Peptides and Hormones: From Their Properties to Clinical Application. Int. J. Pept. Res. Ther. 2024, 31, 10. [Google Scholar] [CrossRef]
- Li, W.; Wang, B.; Jiang, Z.G.; Feng, Y.J.; Zhang, W.; Qiu, M. The role of thymus preservation in parathyroid gland function and surgical completeness after bilateral central lymph node dissection for papillary thyroid cancer: A randomized controlled study. Int. J. Surg. 2019, 65, 1–6. [Google Scholar] [CrossRef]
- Miller, J. How the thymus shaped immunology and beyond. Immunol. Cell Biol. 2019, 97, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Savino, W.; Lepletier, A. Thymus-derived hormonal and cellular control of cancer. Front. Endocrinol. 2023, 14, 1168186. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.; Aguilera, A.R.; Sundarrajan, A.; Corvino, D.; Stannard, K.; Krumeich, S.; Das, I.; Lima, L.G.; Meza Guzman, L.G.; Li, K.; et al. CD155 on Tumor Cells Drives Resistance to Immunotherapy by Inducing the Degradation of the Activating Receptor CD226 in CD8+ T Cells. Immunity 2020, 53, 805–823.e815. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Rosichini, M.; Bordoni, V.; Silvestris, D.A.; Mariotti, D.; Matusali, G.; Cardinale, A.; Zambruno, G.; Condorelli, A.G.; Flamini, S.; Genah, S.; et al. SARS-CoV-2 infection of thymus induces loss of function that correlates with disease severity. J. Allergy Clin. Immunol. 2023, 151, 911–921. [Google Scholar] [CrossRef]
- Li, Z.; Liang, X.; Chen, X.; Chen, Y.; Wang, F.; Wang, S.; Liao, Y.; Li, L. The role of thymus- and extrathymus-derived regulatory T cells in maternal-fetal tolerance. Front. Immunol. 2023, 14, 1109352. [Google Scholar] [CrossRef]
- Dai, X.; Hua, L.; Chen, H.; Li, Q.; Chen, W.; Liang, C. What’s the role of thymus in diabetes mellitus? Int. Immunopharmacol. 2023, 116, 109765. [Google Scholar] [CrossRef]
- Ruiz Pérez, M.; Vandenabeele, P.; Tougaard, P. The thymus road to a T cell: Migration, selection, and atrophy. Front. Immunol. 2024, 15, 1443910. [Google Scholar] [CrossRef]
- Kinsella, S.; Dudakov, J.A. When the Damage Is Done: Injury and Repair in Thymus Function. Front. Immunol. 2020, 11, 1745. [Google Scholar] [CrossRef] [PubMed]
- Firoozbahr, M.; Kingshott, P.; Palombo, E.A.; Zaferanloo, B. Recent Advances in Using Natural Antibacterial Additives in Bioactive Wound Dressings. Pharmaceutics 2023, 15, 644. [Google Scholar] [CrossRef]
- Dresser, L.; Wlodarski, R.; Rezania, K.; Soliven, B. Myasthenia Gravis: Epidemiology, Pathophysiology and Clinical Manifestations. J. Clin. Med. 2021, 10, 2235. [Google Scholar] [CrossRef] [PubMed]
- Watad, A.; De Marco, G.; Mahajna, H.; Druyan, A.; Eltity, M.; Hijazi, N.; Haddad, A.; Elias, M.; Zisman, D.; Naffaa, M.E.; et al. Immune-Mediated Disease Flares or New-Onset Disease in 27 Subjects Following mRNA/DNA SARS-CoV-2 Vaccination. Vaccines 2021, 9, 435. [Google Scholar] [CrossRef]
- Lazaridis, K.; Tzartos, S.J. Autoantibody Specificities in Myasthenia Gravis; Implications for Improved Diagnostics and Therapeutics. Front. Immunol. 2020, 11, 212. [Google Scholar] [CrossRef]
- Kameda, Y. Cellular and molecular mechanisms of the organogenesis and development, and function of the mammalian parathyroid gland. Cell Tissue Res. 2023, 393, 425–442. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.J.; Duarte, M.J.; Machado, M.C.V.; Mascarenhas, R.S.; Palma, P.V.B.; García, H.D.M.; Nakaya, H.I.; Cunha, T.M.; Donadi, E.A.; Passos, G.A. The single-cell transcriptome of mTECs and CD4(+) thymocytes under adhesion revealed heterogeneity of mTECs and a network controlled by Aire and lncRNAs. Front. Immunol. 2024, 15, 1376655. [Google Scholar] [CrossRef]
- Kim, H.Y.; Seok, J.M.; Jung, S.Y.; Lee, M.J.; Tran, A.N.-T.; Yeo, S.J.; Park, S.A.; Kim, H.S. Development of Surgically Transplantable Parathyroid Hormone-Releasing Microbeads. Biomedicines 2022, 10, 440. [Google Scholar] [CrossRef]
- Santos, M.F.; Rappa, G.; Karbanová, J.; Diana, P.; Cirrincione, G.; Carbone, D.; Manna, D.; Aalam, F.; Wang, D.; Vanier, C.; et al. HIV-1-induced nuclear invaginations mediated by VAP-A, ORP3, and Rab7 complex explain infection of activated T cells. Nat. Commun. 2023, 14, 4588. [Google Scholar] [CrossRef]
- Kaminski, H.J.; Sikorski, P.; Coronel, S.I.; Kusner, L.L. Myasthenia gravis: The future is here. J. Clin. Investig. 2024, 134, e179742. [Google Scholar] [CrossRef]
- Geng, Y.; Zhang, H.; Wang, Y. Risk factors of myasthenia crisis after thymectomy among myasthenia gravis patients: A meta-analysis. Medicine 2020, 99, e18622. [Google Scholar] [CrossRef] [PubMed]
| Search Query | Publication Years | Filters Applied | Date Run | Results |
|---|---|---|---|---|
| Parathyroid gland organogenesis | 2024, 2023, 2022, 2020, 2019 | Article or Review Article, All Open Access | 24 January 2025, 15:23:07 GMT+0200 | 3 |
| Thymic organogenesis | 2024, 2023, 2022, 2021, 2020, 2019 | Article or Review Article, All Open Access, English | 24 January 2025, 15:13:37 GMT+0200 | 51 |
| Thymic organogenesis | 2024, 2023, 2022, 2021, 2020, 2019 | Article or Review Article, English | 24 January 2025, 15:13:25 GMT+0200 | 57 |
| Thymic organogenesis | 2024, 2023, 2022, 2021, 2020, 2019 | Article or Review Article | 24 January 2025, 15:13:19 GMT+0200 | 58 |
| Records Removed Before Screening | Duplicate Records Removed | 0 |
|---|---|---|
| Automatically removed records (ineligible) | 0 | |
| Records removed for other reasons | 0 | |
| Screening | Records subject to screening | 64 |
| Excluded records | 1 | |
| Obtaining reports | Reports requested for evaluation (after record exclusion) | 63 |
| Reports that could not be obtained | 10 | |
| Eligibility | Reports assessed for eligibility | 53 |
| Excluded reports | 0 | |
| Included | Studies included in the analysis (review) | 53 |
| Reports of the included studies | 53 |
| Criteria | Thymus | Parathyroid | |
|---|---|---|---|
| Biomolecule(s) | Material and Methods | Results | |
| TEC | Mouse single-cell transcriptional landscape of non-hematopoietic cell | characterization of cell lineage differentiation, maturation, and temporal dynamics | |
| non-TEC stromal cells, TCR, ETPs, Treg, TRAs, FRCs | Review | better understanding of the cellular and molecular basis of the entire set of thymic stromal cells insights toward the in vivo reconstitution of the thymus | |
| TEC, ETPs, transcription factors Foxn1 and Bcl11b, chemokine Ccl25a | Review on Zebrafish and Medaka in vivo imaging and loss-of-function analysis transcription factors Foxn1 and Bcl11b chemokine Ccl25a | insight into the general principles of vertebrate T-cell development and thymopoiesis | |
| Thymic Crosstalk, Regenerative Pathways, RANKL | Review | Strategies to promote thymic function | |
| PAX8, DLX Genes | Review | Cancer therapy, | |
| Single-cell RNA-seq | droplet-based (10× Genomics Chromium System) Transcription RNA Sequencing [STRT-seq]) | transcriptomic profiling of thymic organogenesis | |
| TEC, TEPC, | Mice single cell (sc) RNAseq lineage-tracing Tamoxifen administration Microdissection of fetal thymic lobes Cell process by Flow cytometry IHC Statistical models | fully defined medium able to support different TEC differentiation states, and of cTEPC and mTEPC-specific differentiation conditions may produce functional TEC from pluripotent stem cells (PSC) or by reprogramming | |
| TEC, Lin28, FOXC genes family | transgenic mice Flow cytometry analysis and antibodies Immunofluorescence BrdU incorporation and Annexin V staining RT−PCR and Q-PCR Sex steroid ablation Statistical analysis | Lin28 is regulating the development and differentiation of TECs by modulating MHCII expression and TEC proliferation throughout thymic ontogeny and involution | |
| NOTCH signaling, Foxn1Cre; Rbpjfl/fl and Foxa2Cre; dnMAML | Mice FTOC in the presence of a NOTCH inhibitor | NOTCH as a potent regulator of TEPC and mTEC fate during fetal thymus development | |
| Endoderm of 3PP transcription factor HOXA3, hESCs | Human stem cells mimicking developmental queues with Activin A, WNT3A, retinoic acid and BMP4 qRT-PCR Immunofluorescence assays Flow cytometry analysis Western blotting analysis Knockdown assay by siRNA Cell proliferation cell cycle and apoptosis analysis kit RNA-seq DESeq2 software 138.3.to identify differentially expressed genes GSEA ChIP Assay Kit Statistical analysis | HOXA3 functioned as the on-off switch to regulate the development of hESC-derived 3PP endoderm | |
| NF-κB, Tregs | Mice Ex-vivo cell isolation Flow cytometry Bi-parental bone marrow chimera generation Ex-vivo cell sorting Treg suppression In vitro iTreg generation In vivo parking experiment RNA-seq analysis Statistical analyses | p100 normally restrains RelB-mediated Treg activation, and in the absence of p100, p50-RelB dimers can contribute to Treg activation | |
| In vivo generating PTGs from the PSCs of the patient Genes Related to PTG Development In vivo generation of PTGs from rodent PSCs | Review | current achievements and challenges in present and future PTG regenerative medicine | |
| Single-step blastocyst complementation | mESCs CRISPR-Cas9-mediated zygote genome editing BC | BC can produce functional endocrine organs and constitute a concept in treatment of hypoparathyroidism | |
| GCM2 | Knockout mouse assay using Tamoxifen solution Genotyping by genomic DNA PCR SPOTCHEM D-02 for total Ca and P analysis PTH measured by Mouse PTH 1–84 ELISA Kit Paraffin section in situ hybridization Rabbit Ki-67 and PCNA Apoptotic signals detected by TUNEL assay Statistical analyses | Gcm2 plays a prominent role in adult parathyroid cell proliferation and maintenance | |
| Phase | Description | Number (No.) |
|---|---|---|
| Identification | Records identified from databases | 3703 |
| Records identified from registers | 0 | |
| Total records identified | 3703 | |
| Removed before screening | Duplicate records removed | 0 |
| Records removed by automation tools | 0 | |
| Records removed for other reasons | 0 | |
| Screening | Records screened | 3703 |
| Records excluded | 4048 | |
| Reports sought for retrieval | (Not explicitly shown, inferred total) | 2664 |
| Reports not retrieved | 57 | |
| Eligibility | Reports assessed for eligibility | 2607 |
| Reports excluded: not in English | 42 | |
| Reports excluded: no open access | 9 | |
| Total reports excluded | 51 | |
| Included | Studies included in review | 2558 |
| Reports of included studies | 2558 |
| Criteria | Thymic Function | Parathyroid Function | |
|---|---|---|---|
| Biomolecule(s) | Material and Methods | Results | |
| prothymosin alpha, thymosin alpha 1, thymosin beta 4 and thymosin beta 10, thymulin and thymopoietin | Review | activate the immune system through several mechanisms and signaling pathways, including stimulation of T-cell differentiation and maturation, activation NK and DC cells, and induction of the release of proinflammatory cytokines | |
| TF5 | mass spectrometry | Increase T-cell numbers and functions | |
| Tα1 | Review | Role as immune-enhancing, immune-modulating, and immune-restoring agent Activation of TLR-2 and 9 Stimulation of IL-2,-10, 12, IFN-α and -γ Activation of DC, NK cells and macrophages Inhibitation of viral replication Protection agains oxidative stress Inhibition of IL-1β and TNF-α | |
| Tβ4 | Review | Actin polymerization Cell migration and crosstalk signaling Collagen deposition Tissue repair Supressing expression of TNF-α Ferroptosis modulation | |
| TP | Review | TEC differentiation | |
| TH | Review | TEC differentiation Anti-proinflammatory Activation of NK cells Thymus–Pituitary axis transmitter between the neuroendocrine system and the immune system increase the production of GH, PRL, TSH | |
| TFX® | Review | inhibition of herpes simplex virus type 1 replication inhibition of proinflammatory cytokine production by LPS-stimulated macrophages | |
| Treg, tTreg, pTreg, iTreg | Transgenic mice Induction of pTreg cells in vivo Treg cell depletion and adoptive transfer of Treg cells Isolation of thymus, spleen or LN mononuclear cells by mashing through a 40 μm cell strainer Centrifugation of blood samples on a Ficoll-Hypaque Premium Filtering decidua mononuclear cells Magnetically depleted Non-CD4+ T Treg cells were sorted by a FACSAria cell sorter Flow cytometry RT-PCR measuring of mRNA levels of chemokines Western blot Chemotaxis experiments Statistical analysis | In vitro generated iTreg cells may also play a role in maternal-fetal tolerance | |
| intestinal microbiota | Review | molecules and metabolites derived from the intestinal microbiota impact T-cell ontogeny | |
| RANKL, TNFR | Review | RANKL signaling via RANK is the master factor for osteoclastogenesis mTECs act as mediators of the central tolerance process by which self-reactive T cells are eliminated while regulatory T cells are generated | |
| S1P, S1PR1 | in vitro primary human thymocytes and in vivo and ex vivo humanized mice Thymocite line culture Mice renal implantation of human fetal thymus/liver Flow Cytometry and S1P Exposure RT-qRT-PCR Statistical Analysis | Circulating thymocytes that are not functionally mature from the thymus to peripheral blood and lymphoid organs may have implications for the immune function of PLWH | |
| NOTCH 1 signaling | Medaka In vivo reverse genetic approaches and whole-thymus live imaging | Notch1 controls the migratory behavior of thymocytes through controlling the chemokine receptor Ccr9b and thereby influence the T-cell receptor (TCR) activation | |
| CD31-positive naive T-cell differentiation status of the memory of TEC | Human PBMC isolation Expression on either CD4 or CD8, CCR7 and the absence of CD45RO | severe reduction in circulating naïve T cells because of a decrease in recent thymic emigrants is highly associated with all-cause mortality after renal transplantation | |
| Interleukin-22 BMP4, BMP4R antagonist Noggin KGF/FGF-7 RANKL | Review | Molecular mechanisms for endogenous thymic regeneration Strategies of Thymic Regeneration by Cell Therapies and Bioengineering, Modulation of Hormones and Metabolism, using Thymic T Cell Precursors and Bone Marrow Progenitors, Targeting Non-Hematopoeitic Cells | |
| inflammatory-immune cells inflammatory cytokines insulin resistance Th1 master transcription factor Th17 cells AIRE insulin-related peptides in the thymus Ins1 and Ins2 genes NADPH oxidase IL-10, TNF-α CCL2, FoxN1, | Review | changes of TEC could induce autoimmune diabetes T1DM is pa complex TEC autoimmune disease in which the pancreatic insulin-producing β-cells are selectively destroyed by the immune system thymus function have potential impact on insulin resistance T2DM is induced by chronic inflamatory diseases and high cytokine levels | |
| adipokines leptin and ghrelin sj-TREC ApoB | Review | Thymus has a significant role in the development of atherosclerosis and metabolic health | |
| C57BL/6J UCP1 TBX1 | Stromal Cell Isolation DC Isolation Measurement of Mitochondrial H2O2 In Vitro Stimulation of Splenic T CellsImmunofluorescence Microscopy from Cytospin Autophagy Analysis by Flow Cytometry T Cell Clonal Deletion Chemokine Expression qRT-PCR Reciprocal BMT Antinuclear Antibody | Thymus role in diminished expression of tissue restricted antigens in thymus (ApoB antigen), decreased frequency of cells undergoing clonal deletion in thymus, appearance of ApoB-specific T lymphocytes in circulation Tregs Lymphocytes Control Atherogenesis | |
| CD4(+)CD25(+)CD127(low) CD45RO(−) CD45RA(+)CD31(+) TNF concentration IL-10/TNF ratio IL-2, IL-6, IL-8, IFN-γ, TNF-α and IL-1β CD4+Foxp3+Helios+ Tregs HDL-C | ApoE−/− mice depleted of Tregs LDLR−/− mice depeleted of Tregs CAD patients | Thymus generates atheroprotective natural T regulatory cells in wound healing | |
| thymic microenvironment | Review | TECs migrate to the and exert specialized effector functions and orchestrate the immune responses against tumor cells, pathogens and self-antigens Tα1 accelerates the replenishment and maturation of macrophages; block the intratumoral accumulation of myeloid suppressor cells; restrain tumor growth by its proapoptotic and anti-proliferative properties Tβ4 decreases proliferative and migratory capacities of tumor cells Tβ10 inhibites tumor cell invasion and metastasis type I NKT cells promote anti-tumor immunity and type II NKT cells suppress it | |
| Ca2+ fluxing abilities KCa3.1 and Kv1.3 | Isolation of PBMCs Tumor single-cell suspension and TIL isolation Electrophysiology Ca2+ measured by a TCR-independent/ion channel-dependent method Flow cytometry Ion channel antibody specificity Statistical analysis | Cancer therapy pembrolizumab elicits an ion channel and functional phenotype in responder patients’ cytotoxic T cells that is conducive to a heightened ability of these cells to chemotax and kill cancer cells | |
| VDCCs, pore-forming CaV (α1)-, the β regulatory- | infection mice models Splenocyte Isolation Flow Cytometry Surface Staining Ca2+ Flux Assay Statistical Tests | diminished Ca2+ flux suggests the exhaustion of T lymphocytes in CaV1.4-deficient mice, provided by assessing T lymphocyte functions, such as cytotoxicity L-type Ca2+ channel deficiencies often lead to a phenotype that includes impaired TCR signaling, resulting in diminished T cell effector functions and reduced T-cell survival | |
| gene AIRE (21q) | Observational study on type 1 autoimmune polyendocrinopathies of the patients | If transcription of autoantibodies are inhibited, specially at the thymus level, parathyroid agenesis appears | |
| Stage | Description | Number (No.) |
|---|---|---|
| Identification | Records identified from databases | 2805 |
| Records identified from registers | 0 | |
| Total records identified | 2805 | |
| Exclusions before screening | Duplicate records removed | 0 |
| Records removed by automated tools | 0 | |
| Records removed for other reasons | 0 | |
| Screening | Records subject to screening | 2805 |
| Records excluded | 328 | |
| Obtaining reports | Reports requested for evaluation | 715 |
| Reports that could not be obtained | 20 | |
| Eligibility | Reports assessed for eligibility | 502 |
| Reports excluded (not in English) | 7 | |
| Reports excluded (no open access) | 3 | |
| Total reports excluded | 10 | |
| Included | Studies included in the review | 492 |
| Reports of included studies | 492 |
| Criteria | Thymic Function | Parathyroid Function | |
|---|---|---|---|
| Biomolecule(s) | Material and Methods | Results | |
| 22q11.2 deletion | TBX1 qPCR probe qPCR TaqMan technique MLPA R Studio 2024.12.1+563 for statistical analysis | The use of the same sample for qPCR and MLPA is a possible newborn screening method for 22q11.2 deletion syndrome in CCHD patients | parathyroid and thyroid gland hormonal dysfunctions |
| 22q11.2 deletion | Review | genetic modifiers and environmental factors, as well as the impact of hemizygosity on the remaining allele, contribute to the intricate genotype–phenotype relationships | parathyroid and thyroid gland hormonal dysfunctions |
| Oxidative Stress 22q11.2 deletion | Review | autoimmune disorders due to immunodeficiency and immune dysregulation caused by thymic dysfunction and hypoparathyroidism/neonatal hypocalcemia related to parathyroid abnormal development consistently contribute to the clinical phenotype | thymic hypoplasia or aplasia with consequent immune deficiency, cardiac malformations, hypoparathyroidism |
| CDNA, RNA, | Transgenic Mice Immunofluorescence RNA Extraction and Quantitative PCR Statistical Analysis NanoDrop | APS-1 is an inherited autosomal disorder caused by mutations in the autoimmune regulator (AIRE) gene, leading to adrenocortical failure, hypoparathyroidism, and chronic mucocutaneous candidiasis due to impaired central tolerance in the thymus. | HP |
| 21q22.3 gene, AIRE, AC-Abs, STC-Abs, Antibodies to germline cells, Antibodies to CYP Enzymes, Epitope Targeting, Adrenal antibody, | Review | APS1 is based on the classic triad idiopathic hypoparathyroidism (HPT)—chronic mucocutaneous candidiasis—autoimmune Addison’s disease non-dependence on AIRE of the main adrenal self-antigens in the human thymus raises questions about the intrinsic mechanisms that trigger autoimmunity in APS1 | HP |
| 22q11.2 deletion syndrome, TBX, FOXN1, scRNA-Seq | Review | mesenchymal cells were causal to the small embryonic thymuses in the 22q11.2DS mouse models | Hypoplastic embryonic thymuses from 22q11.2DS mouse models maintain normal thymopoiesis. 22q11.2DS causes congenital malformations affecting the thymus, heart, and parathyroids |
| Nicotinic AChR, AChR antibodies IgG1 and IgG3, membrane attack complex, MuSK, Lrp4 Antibody, B Cell Tolerance, TECs, Cytokines, Tregs | Review | autoantibodies disrupt cholinergic transmission between nerve terminals and muscle fibers by causing downregulation, destruction, functional blocking of AChRs, or disrupting the clustering of AChRs in the postsynaptic membrane | |
| Author(s) | Year | Study Model | Experimental Evidence | Relevance to Thymus–Parathyroid Axis |
|---|---|---|---|---|
| Blackburn and Manley [32] | 2004 | Mouse model | Conditional deletion of Foxn1 and Gcm2 disrupted thymus and parathyroid development | Shows shared embryologic origin and gene dependency |
| Gordon et al. [33] | 2001 | Mouse mutant (nude/SCID) | Lack of Foxn1 impairs thymic epithelium; adjacent parathyroid displacement observed | Functional and spatial link during development |
| Fu et al. [34,35] | 2003 | Human fetal tissue | HOXA3 and TBX1 co-expression in pharyngeal endoderm | Identifies key genes guiding organ separation |
| Liu et al. [6] | 2010 | Zebrafish model | Dual expression of GCM genes in 3rd pouch derivatives | Evolutionary conservation of thymus–parathyroid ontogeny |
| Wu et al. [36,37,38] | 2001 | Human adults | Persistent thymic -parathyroid remnants near thyroid | Suggests incomplete organ separation clinically |
| Neves et al. [8] | 2012 | In vitro co-culture | PTH modulates thymocyte activation in stromal microenvironments | Possible endocrine–immune interaction |
| Perniola et al. [39] | 2018 | APS-1 patient data | AIRE mutation disrupts thymic tolerance; leads to hypoparathyroidism | Pathogenic link via shared central tolerance |
| Gao et al. [31,40,41] | 2013, 2024, 2023 | Mouse knockout (Tbx1−/−) | Thymus and parathyroid aplasia with cardiac outflow defects | Reinforces TBX1 as shared morphogenetic factor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Comănescu, M.-P.; Boișteanu, O.; Hînganu, D.; Hînganu, M.V.; Grigorovici, R.; Grigorovici, A. Perspectives on the Parathyroid–Thymus Interconnection—A Literature Review. Int. J. Mol. Sci. 2025, 26, 6000. https://doi.org/10.3390/ijms26136000
Comănescu M-P, Boișteanu O, Hînganu D, Hînganu MV, Grigorovici R, Grigorovici A. Perspectives on the Parathyroid–Thymus Interconnection—A Literature Review. International Journal of Molecular Sciences. 2025; 26(13):6000. https://doi.org/10.3390/ijms26136000
Chicago/Turabian StyleComănescu, Maria-Paula, Otilia Boișteanu, Delia Hînganu, Marius Valeriu Hînganu, Roxana Grigorovici, and Alexandru Grigorovici. 2025. "Perspectives on the Parathyroid–Thymus Interconnection—A Literature Review" International Journal of Molecular Sciences 26, no. 13: 6000. https://doi.org/10.3390/ijms26136000
APA StyleComănescu, M.-P., Boișteanu, O., Hînganu, D., Hînganu, M. V., Grigorovici, R., & Grigorovici, A. (2025). Perspectives on the Parathyroid–Thymus Interconnection—A Literature Review. International Journal of Molecular Sciences, 26(13), 6000. https://doi.org/10.3390/ijms26136000







