Association of Tissue Expression of LAG-3 and TIM-3 with Clinical Features in Ovarian Cancer
Abstract
1. Introduction
2. Results
2.1. Group Characteristics and Evaluation of Tissue Expression
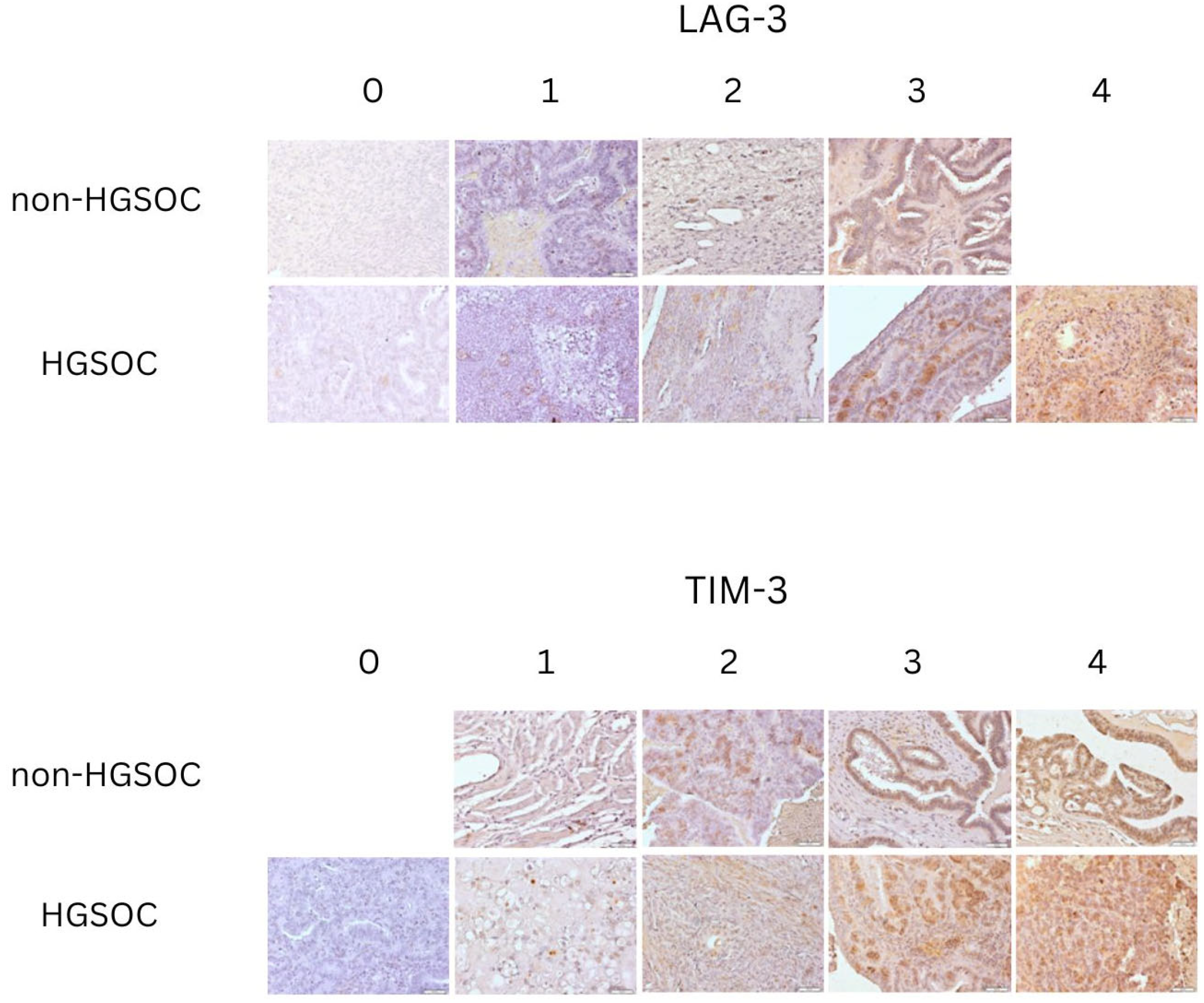
2.2. Comparison of Tissue Expression of LAG-3 Between Non-HGSOC and HGSOC
2.3. Comparison of Tissue Expression of TIM-3 Between Non-HGSOC and HGSOC
2.4. Association of LAG-3 and TIM-3 Protein Expression with Clinical Features
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Immunohistochemical (IHC) Expression of LAG-3 and TIM-3 in Ovarian Cancer Tissue Samples
4.3. Statistical Calculations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ovarian Cancer Statistics. Available online: https://www.wcrf.org/cancer-trends/ovarian-cancer-statistics/ (accessed on 14 December 2024).
- Key Statistics for Ovarian Cancer. Available online: https://www.cancer.org/cancer/types/ovarian-cancer/about/key-statistics.html (accessed on 14 December 2024).
- Colombo, N.; Sessa, C.; Du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO–ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef] [PubMed]
- Margoni, A.; Gargalionis, A.N.; Papavassiliou, A.G. CA-125:CA72-4 ratio—Towards a promising cost-effective tool in ovarian cancer diagnosis and monitoring of post-menopausal women under hormone treatment. J. Ovarian Res. 2024, 17, 164. [Google Scholar] [CrossRef]
- Zhang, R.; Siu, M.K.Y.; Ngan, H.Y.S.; Chan, K.K.L. Molecular Biomarkers for the Early Detection of Ovarian Cancer. Int. J. Mol. Sci. 2022, 23, 12041. [Google Scholar] [CrossRef]
- Menon, U.; Gentry-Maharaj, A.; Burnell, M.; Singh, N.; Ryan, A.; Karpinskyj, C.; Carlino, G.; Taylor, J.; Massingham, S.K.; Raikou, M.; et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): A randomised controlled trial. Lancet 2021, 397, 2182–2193. [Google Scholar] [CrossRef] [PubMed]
- Buys, S.S. Effect of Screening on Ovarian Cancer Mortality. JAMA 2011, 305, 2295. [Google Scholar] [CrossRef]
- Passaro, A.; Al Bakir, M.; Hamilton, E.G.; Diehn, M.; André, F.; Roy-Chowdhuri, S.; Mountzios, G.; Wistuba, I.I.; Swanton, C.; Peters, S. Cancer biomarkers: Emerging trends and clinical implications for personalized treatment. Cell 2024, 187, 1617–1635. [Google Scholar] [CrossRef]
- Morand, S.; Devanaboyina, M.; Staats, H.; Stanbery, L.; Nemunaitis, J. Ovarian Cancer Immunotherapy and Personalized Medicine. Int. J. Mol. Sci. 2021, 22, 6532. [Google Scholar] [CrossRef]
- Siminiak, N.; Czepczyński, R.; Zaborowski, M.P.; Iżycki, D. Immunotherapy in Ovarian Cancer. Arch. Immunol. Ther. Exp. 2022, 70, 19. [Google Scholar] [CrossRef] [PubMed]
- Kozłowski, M.; Borzyszkowska, D.; Cymbaluk-Płoska, A. The Role of TIM-3 and LAG-3 in the Microenvironment and Immunotherapy of Ovarian Cancer. Biomedicines 2022, 10, 2826. [Google Scholar] [CrossRef]
- Tyrinova, T.V.; Chernykh, E.R. Inhibitory Checkpoint Receptor TIM-3 as a Regulator of the Functional Activity of Dendritic Cells. Bull. Exp. Biol. Med. 2024, 177, 287–292. [Google Scholar] [CrossRef]
- Acharya, N.; Sabatos-Peyton, C.; Anderson, A.C. Tim-3 finds its place in the cancer immunotherapy landscape. J. Immunother. Cancer 2020, 8, e000911. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, L.; Yang, R.; Wang, Z.; Li, Q.; Li, J.; Liu, Y. Diagnostic and prognostic value of CD44v9 and TIM3 expression in CK− and CK+ regions in gastric cancer tissues. Oncol. Lett. 2024, 28, 479. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, F.; Peng, J.; Zhao, D.; Xu, L.; Li, H.; Ma, S.; Peng, X.; Sheng, X.; Sun, Y.; et al. Soluble Tim-3 serves as a tumor prognostic marker and therapeutic target for CD8+ T cell exhaustion and anti-PD-1 resistance. Cell Rep. Med. 2024, 5, 101686. [Google Scholar] [CrossRef]
- Chen, Y.; Shao, Z.; Hao, Z.; Xin, Z.; Chen, X.; Huang, L.; Chen, D.; Lin, M.; Liu, Q.; Xu, X.; et al. Epithelium/imcDC2 axis facilitates the resistance of neoadjuvant anti-PD-1 in human NSCLC. J. Immunother. Cancer 2024, 12, e007854. [Google Scholar] [CrossRef]
- Fucikova, J.; Rakova, J.; Hensler, M.; Kasikova, L.; Belicova, L.; Hladikova, K.; Truxova, I.; Skapa, P.; Laco, J.; Pecen, L.; et al. TIM-3 Dictates Functional Orientation of the Immune Infiltrate in Ovarian Cancer. Clin. Cancer Res. 2019, 25, 4820–4831. [Google Scholar] [CrossRef]
- Wu, J.-L.; Zhao, J.; Zhang, H.-B.; Zuo, W.-W.; Li, Y.; Kang, S. Genetic variants and expression of the TIM-3 gene are associated with clinical prognosis in patients with epithelial ovarian cancer. Gynecol. Oncol. 2020, 159, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Workman, C.J.; Vignali, D.A.A. Negative Regulation of T Cell Homeostasis by Lymphocyte Activation Gene-3 (CD223). J. Immunol. 2005, 174, 688–695. [Google Scholar] [CrossRef]
- Blackburn, S.D.; Shin, H.; Haining, W.N.; Zou, T.; Workman, C.J.; Polley, A.; Betts, M.R.; Freeman, G.J.; Vignali, D.A.A.; Wherry, E.J. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 2009, 10, 29–37. [Google Scholar] [CrossRef]
- Woo, S.-R.; Turnis, M.E.; Goldberg, M.V.; Bankoti, J.; Selby, M.; Nirschl, C.J.; Bettini, M.L.; Gravano, D.M.; Vogel, P.; Liu, C.L.; et al. Immune Inhibitory Molecules LAG-3 and PD-1 Synergistically Regulate T-cell Function to Promote Tumoral Immune Escape. Cancer Res. 2012, 72, 917–927. [Google Scholar] [CrossRef]
- Hong, J.H.; Cho, H.W.; Ouh, Y.-T.; Lee, J.K.; Chun, Y. Lymphocyte activation gene (LAG)-3 is a potential immunotherapeutic target for microsatellite stable, programmed death-ligand 1 (PD-L1)-positive endometrioid endometrial cancer. J. Gynecol. Oncol. 2023, 34, e18. [Google Scholar] [CrossRef]
- Eurich, K.; De La Cruz, P.; Laguna, A.; Woodman, M.; McAdams, J.; Lips, E.; Ebott, J.; DiSilvestro, J.; Ribeiro, J.; James, N. Multiplex serum immune profiling reveals circulating LAG-3 is associated with improved patient survival in high grade serous ovarian cancer. Gynecol. Oncol. 2023, 174, 200–207. [Google Scholar] [CrossRef]
- Zaitsu, S.; Yano, M.; Adachi, S.; Miwa, M.; Katoh, T.; Kawano, Y.; Yasuda, M. Lymphocyte-activation gene 3 protein expression in tumor-infiltrating lymphocytes is associated with a poor prognosis of ovarian clear cell carcinoma. J. Ovarian Res. 2023, 16, 93. [Google Scholar] [CrossRef] [PubMed]
- Rådestad, E.; Klynning, C.; Stikvoort, A.; Mogensen, O.; Nava, S.; Magalhaes, I.; Uhlin, M. Immune profiling and identification of prognostic immune-related risk factors in human ovarian cancer. Oncoimmunology 2019, 8, e1535730. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, C.; Qian, S.; Hou, H. The Expression of Tim-3 in Peripheral Blood of Ovarian Cancer. DNA Cell Biol. 2013, 32, 648–653. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, H.; Huang, Y.; Rui, X.; Zheng, F. Role of TIM-3 in ovarian cancer. Clin. Transl. Oncol. 2017, 19, 1079–1083. [Google Scholar] [CrossRef]
- Lee, J.-M.; Botesteanu, D.-A.; Tomita, Y.; Yuno, A.; Lee, M.-J.; Kohn, E.C.; Annunziata, C.M.; Matulonis, U.; Macdonald, L.A.; Nair, J.R.; et al. Patients with BRCA mutated ovarian cancer may have fewer circulating MDSC and more peripheral CD8+ T cells compared with women with BRCA wild type disease during the early disease course. Oncol. Lett. 2019, 18, 3914–3924. [Google Scholar] [CrossRef]
- Tu, L.; Guan, R.; Yang, H.; Zhou, Y.; Hong, W.; Ma, L.; Zhao, G.; Yu, M. Assessment of the expression of the immune checkpoint molecules PD-1, CTLA4, TIM-3 and LAG-3 across different cancers in relation to treatment response, tumor-infiltrating immune cells and survival. Int. J. Cancer 2020, 147, 423–439. [Google Scholar] [CrossRef]
- Blanc-Durand, F.; Genestie, C.; Galende, E.Y.; Gouy, S.; Morice, P.; Pautier, P.; Maulard, A.; Mesnage, S.; Le Formal, A.; Brizais, C.; et al. Distribution of novel immune-checkpoint targets in ovarian cancer tumor microenvironment: A dynamic landscape. Gynecol. Oncol. 2021, 160, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Weimer, P.; Wellbrock, J.; Sturmheit, T.; Oliveira-Ferrer, L.; Ding, Y.; Menzel, S.; Witt, M.; Hell, L.; Schmalfeldt, B.; Bokemeyer, C.; et al. Tissue-Specific Expression of TIGIT, PD-1, TIM-3, and CD39 by γδ T Cells in Ovarian Cancer. Cells 2022, 11, 964. [Google Scholar] [CrossRef]
- Jafarzadeh, L.; Masoumi, E.; Mirzaei, H.R.; Alishah, K.; Fallah-Mehrjardi, K.; Khakpoor-Koosheh, M.; Rostamian, H.; Noorbakhsh, F.; Hadjati, J. Targeted knockdown of Tim3 by short hairpin RNAs improves the function of anti-mesothelin CAR T cells. Mol. Immunol. 2021, 139, 1–9. [Google Scholar] [CrossRef]
- Akbari, B.; Soltantoyeh, T.; Shahosseini, Z.; Yarandi, F.; Hadjati, J.; Mirzaei, H.R. The inhibitory receptors PD1, Tim3, and A2aR are highly expressed during mesoCAR T cell manufacturing in advanced human epithelial ovarian cancer. Cancer Cell Int. 2023, 23, 104. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.-Y.; Francois, A.; McGray, A.R.; Miliotto, A.; Odunsi, K. Compensatory upregulation of PD-1, LAG-3, and CTLA-4 limits the efficacy of single-agent checkpoint blockade in metastatic ovarian cancer. Oncoimmunology 2017, 6, e1249561. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.-Y.; Eppolito, C.; Lele, S.; Shrikant, P.; Matsuzaki, J.; Odunsi, K. LAG3 and PD1 co-inhibitory molecules collaborate to limit CD8+ T cell signaling and dampen antitumor immunity in a murine ovarian cancer model. Oncotarget 2015, 6, 27359–27377. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, J.; Gnjatic, S.; Mhawech-Fauceglia, P.; Beck, A.; Miller, A.; Tsuji, T.; Eppolito, C.; Qian, F.; Lele, S.; Shrikant, P.; et al. Tumor-infiltrating NY-ESO-1–specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc. Natl. Acad. Sci. USA 2010, 107, 7875–7880. [Google Scholar] [CrossRef]
- James, N.E.; Miller, K.; LaFranzo, N.; Lips, E.; Woodman, M.; Ou, J.; Ribeiro, J.R. Immune Modeling Analysis Reveals Immunologic Signatures Associated With Improved Outcomes in High Grade Serous Ovarian Cancer. Front. Oncol. 2021, 11, 622182. [Google Scholar] [CrossRef]
- Ma, S.; Zheng, Y.; Fei, C. Identification of Key Factors Associated with Early- and Late-Onset Ovarian Serous Cystadenocarcinoma. Future Oncol. 2020, 16, 2821–2833. [Google Scholar] [CrossRef]
| Clinical–Demographical Characteristics | Total Cohort (n = 58) | Non-HGSOC (n = 26) | HGSOC (n = 32) | p-Value * | OR (95%CI) |
|---|---|---|---|---|---|
| Median (IQR) | |||||
| Age (years old) | 63.0 (20) | 51.0 (23) | 63.5 (12.5) | 0.005 | |
| BMI (kg/m2) | 24.7 (6.9) | 25.4 (6.6) | 23.4 (7.1) | NS | |
| Number (%) | |||||
| LAG-3 protein expression intensity | 0.7 (0.3–2.1) | ||||
| 0-1 | 27 (46.6%) | 11 (42.3%) | 16 (50%) | NS | |
| 2-4 | 31 (53.4%) | 15 (57.7%) | 16 (50%) | ||
| TIM-3 protein expression intensity | 0.5 (0.2–1.6) | ||||
| 0-1 | 18 (31.0%) | 6 (23.1%) | 12 (37.5%) | NS | |
| 2-4 | 40 (69.0%) | 20 (76.9%) | 20 (62.5%) | ||
| Age (years old) | |||||
| <65 | 33 (56.9%) | 17 (65.4%) | 16 (50%) | NS | |
| ≥65 | 25 (43.1%) | 9 (34.6%) | 16 (50%) | ||
| BMI | |||||
| <25 | 30 (51.7%) | 11 (42.3%) | 19 (59.4%) | NS | |
| ≥25 | 28 (48.3%) | 15 (57.7%) | 13 (40.6%) | ||
| Menopausal status | |||||
| Premenopausal | 20 (34.5%) | 14 (53.8%) | 6 (18.7%) | 0.006 | |
| Postmenopausal | 38 (65.5%) | 12 (46.2%) | 26 (81.3%) | ||
| Grade | |||||
| High (G2 + G3) | 40 (69.0%) | 8 (30.8%) | 32 (100%) | 0.0001 | |
| Low (G1) | 18 (31.0%) | 18 (69.2%) | 0 (0%) | ||
| Histological type | |||||
| Serous | 44 (75.9%) | 12 (46.2%) | 32 (100%) | 0.0001 | |
| Non-serous | 14 (24.1%) | 14 (53.8%) | 0 (0%) | ||
| FIGO | |||||
| I–II | 19 (32.8%) | 15 (57.7%) | 4 (12.5%) | 0.0002 | |
| II–IV | 39 (67.2%) | 11 (42.3%) | 28 (87.5%) | ||
| Non-HGSOC (n = 11) | HGSOC (n = 16) | p-Value * | |
|---|---|---|---|
| LAG-3 (0-1) | |||
| Grade | |||
| High | 4 (36.4%) | 16 (100%) | 0.0002 |
| Low | 7 (63.6%) | 0 (0%) | |
| Histological type | |||
| serous | 5 (45.5%) | 16 (100%) | 0.0008 |
| Non-serous | 6 (54.5%) | 0 (0%) | |
| Menopausal status | |||
| Premenopausal | 5 (45.5%) | 4 (25%) | NS |
| Postmenopausal | 6 (54.5%) | 12 (75%) | |
| FIGO | |||
| I-II | 6 (54.5%) | 3 (18.8%) | NS |
| III-IV | 5 (45.5%) | 13 (81.2%) | |
| BMI | |||
| <25 | 7 (63.6%) | 9 (56.3%) | NS |
| >25 | 4 (36.4%) | 7 (43.7%) | |
| Age | |||
| <65 | 7 (63.6%) | 9 (56.3%) | NS |
| >65 | 4 (36.4%) | 7 (43.7%) | |
| Non-HGSOC (n = 15) | HGSOC (n = 16) | p-Value * | |
|---|---|---|---|
| LAG-3 (2-4) | |||
| Grade | |||
| High | 4 (26.7%) | 16 (100%) | 0.0001 |
| Low | 11 (73.3%) | 0 (0%) | |
| Histological type | |||
| Serous | 7 (46.7%) | 16 (100%) | 0.0007 |
| Non-serous | 8 (53.3%) | 0 (0%) | |
| Menopausal status | |||
| Premenopausal | 9 (60%) | 2 (12.5%) | 0.005 |
| Postmenopausal | 6 (40%) | 14 (87.5%) | |
| FIGO | |||
| I–II | 9 (60%) | 1 (6.3%) | 0.001 |
| III–IV | 6 (40%) | 15 (93.7%) | |
| BMI | |||
| <25 | 4 (26.7%) | 10 (62.5%) | 0.04 |
| >25 | 11 (73.3%) | 6 (37.5%) | |
| Age | |||
| <65 | 10 (66.7%) | 7 (43.8%) | NS |
| >65 | 5 (33.3%) | 9 (56.2%) | |
| Non-HGSOC (n = 6) | HGSOC (n = 12) | p-Value * | |
|---|---|---|---|
| TIM-3 (0–1) | |||
| Grade | |||
| High | 3 (50%) | 12 (100%) | 0.007 |
| Low | 3 (50%) | 0 (0%) | |
| Histological type | |||
| Serous | 3 (50%) | 12 (100%) | 0.007 |
| Non-serous | 3 (50%) | 0 (0%) | |
| Menopausal status | |||
| Premenopausal | 2 (33.3%) | 1 (8.3%) | NS |
| Postmenopausal | 4 (66.7%) | 11 (91.7%) | |
| FIGO | |||
| I–II | 3 (50%) | 1 (8.3%) | 0.04 |
| III-IV | 3 (50%) | 11 (91.7%) | |
| BMI | |||
| <25 | 4 (66.7%) | 8 (66.7%) | NS |
| >25 | 2 (33.3%) | 4 (33.3%) | |
| Age | |||
| <65 | 4 (66.7%) | 6 (50%) | NS |
| >65 | 2 (33.3%) | 6 (50%) | |
| Non-HGSOC (n = 20) | HGSOC (n = 20) | p-Value * | |
|---|---|---|---|
| TIM-3 (2-4) | |||
| Grade | |||
| High | 5 (25%) | 20 (100%) | 0.0001 |
| Low | 15 (75%) | 0 (0%) | |
| Histological type | |||
| Serous | 9 (45%) | 20 (100%) | 0.0001 |
| Non-serous | 11 (55%) | 0 (0%) | |
| Menopausal status | |||
| Premenopausal | 12 (60%) | 15 (75%) | NS |
| Postmenopausal | 8 (40%) | 5 (25%) | |
| FIGO | |||
| I–II | 12 (60%) | 3 (15%) | 0.003 |
| III–IV | 8 (40%) | 17 (85%) | |
| BMI | |||
| <25 | 7 (35%) | 11 (55%) | NS |
| >25 | 13 (65%) | 9 (45%) | |
| Age | |||
| <65 | 13 (65%) | 10 (50%) | NS |
| >65 | 7 (35%) | 10 (50%) | |
| Rho | p-Value | |
|---|---|---|
| LAG-3, TIM-3 | 0.609 | <0.0001 |
| LAG-3, age | −0.068 | 0.6129 |
| LAG-3, BMI | 0.143 | 0.2864 |
| TIM-3, age | −0.291 | 0.0262 |
| TIM-3, BMI | 0.063 | 0.6387 |
| Rho | p-Value | |
|---|---|---|
| LAG-3, TIM-3 | 0.535 | 0.0013 |
| LAG-3, age | 0.125 | 0.4995 |
| LAG-3, BMI | −0.084 | 0.6515 |
| TIM-3, age | −0.161 | 0.3828 |
| TIM-3, BMI | −0.114 | 0.5387 |
| Rho | p-Value | |
|---|---|---|
| LAG-3, TIM-3 | 0.710 | <0.0001 |
| LAG-3, age | −0.164 | 0.4270 |
| LAG-3, BMI | 0.405 | 0.0394 |
| TIM-3, age | −0.223 | 0.2766 |
| TIM-3, BMI | 0.239 | 0.2433 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borzyszkowska, D.; Kozłowski, M.; Golara, A.; Piotrowska, K.; Brodowska, A.; Brodowski, J.; Bojar, I.; Kempinska-Podhorodecka, A.; Cymbaluk-Płoska, A. Association of Tissue Expression of LAG-3 and TIM-3 with Clinical Features in Ovarian Cancer. Int. J. Mol. Sci. 2025, 26, 5996. https://doi.org/10.3390/ijms26135996
Borzyszkowska D, Kozłowski M, Golara A, Piotrowska K, Brodowska A, Brodowski J, Bojar I, Kempinska-Podhorodecka A, Cymbaluk-Płoska A. Association of Tissue Expression of LAG-3 and TIM-3 with Clinical Features in Ovarian Cancer. International Journal of Molecular Sciences. 2025; 26(13):5996. https://doi.org/10.3390/ijms26135996
Chicago/Turabian StyleBorzyszkowska, Dominika, Mateusz Kozłowski, Anna Golara, Katarzyna Piotrowska, Agnieszka Brodowska, Jacek Brodowski, Iwona Bojar, Agnieszka Kempinska-Podhorodecka, and Aneta Cymbaluk-Płoska. 2025. "Association of Tissue Expression of LAG-3 and TIM-3 with Clinical Features in Ovarian Cancer" International Journal of Molecular Sciences 26, no. 13: 5996. https://doi.org/10.3390/ijms26135996
APA StyleBorzyszkowska, D., Kozłowski, M., Golara, A., Piotrowska, K., Brodowska, A., Brodowski, J., Bojar, I., Kempinska-Podhorodecka, A., & Cymbaluk-Płoska, A. (2025). Association of Tissue Expression of LAG-3 and TIM-3 with Clinical Features in Ovarian Cancer. International Journal of Molecular Sciences, 26(13), 5996. https://doi.org/10.3390/ijms26135996






