Echocardiographic Assessment of Cardiac Function in Mouse Models of Heart Disease
Abstract
1. Introduction
2. Echocardiographic Imaging Techniques in Murine Models
2.1. Preparation, Anesthesia, and Imaging Techniques
2.2. Standard Views and Functional Modalities
2.3. Assessment of Left Ventricular Systolic Function
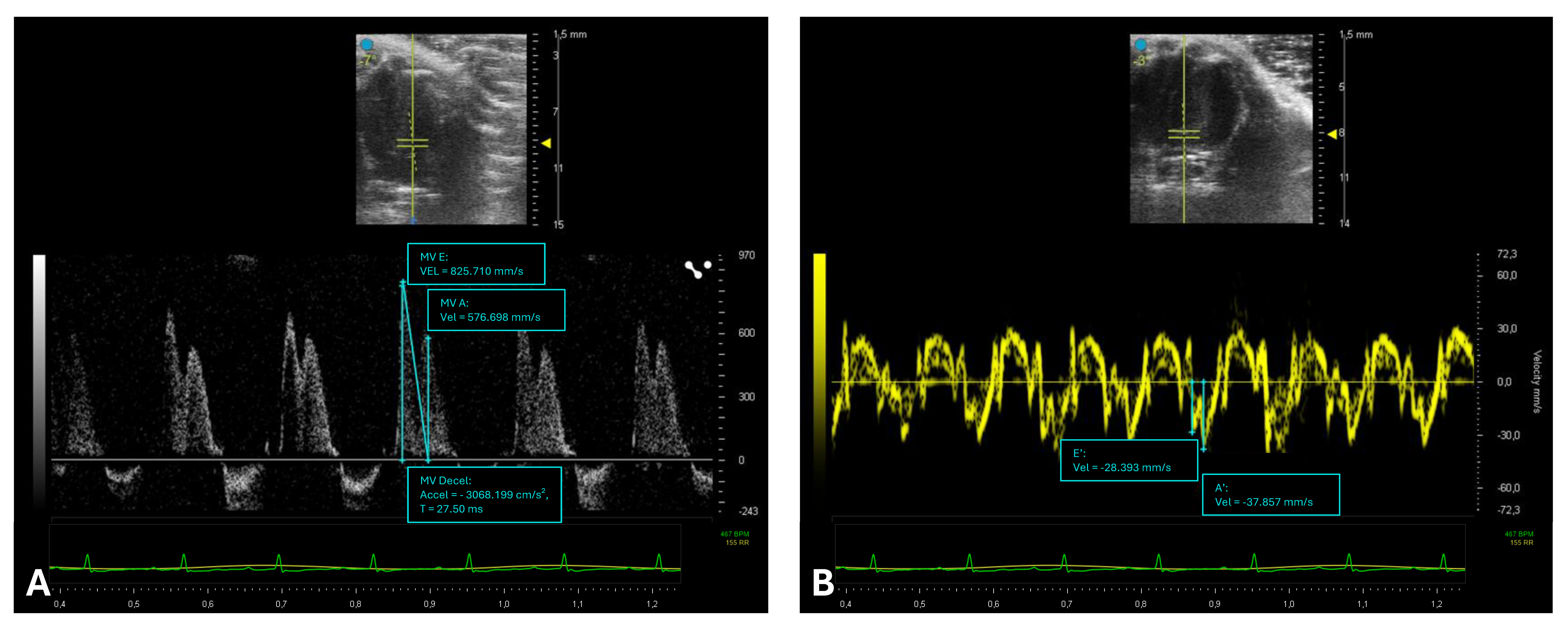
2.4. Assessment of Left Ventricular Diastolic Function
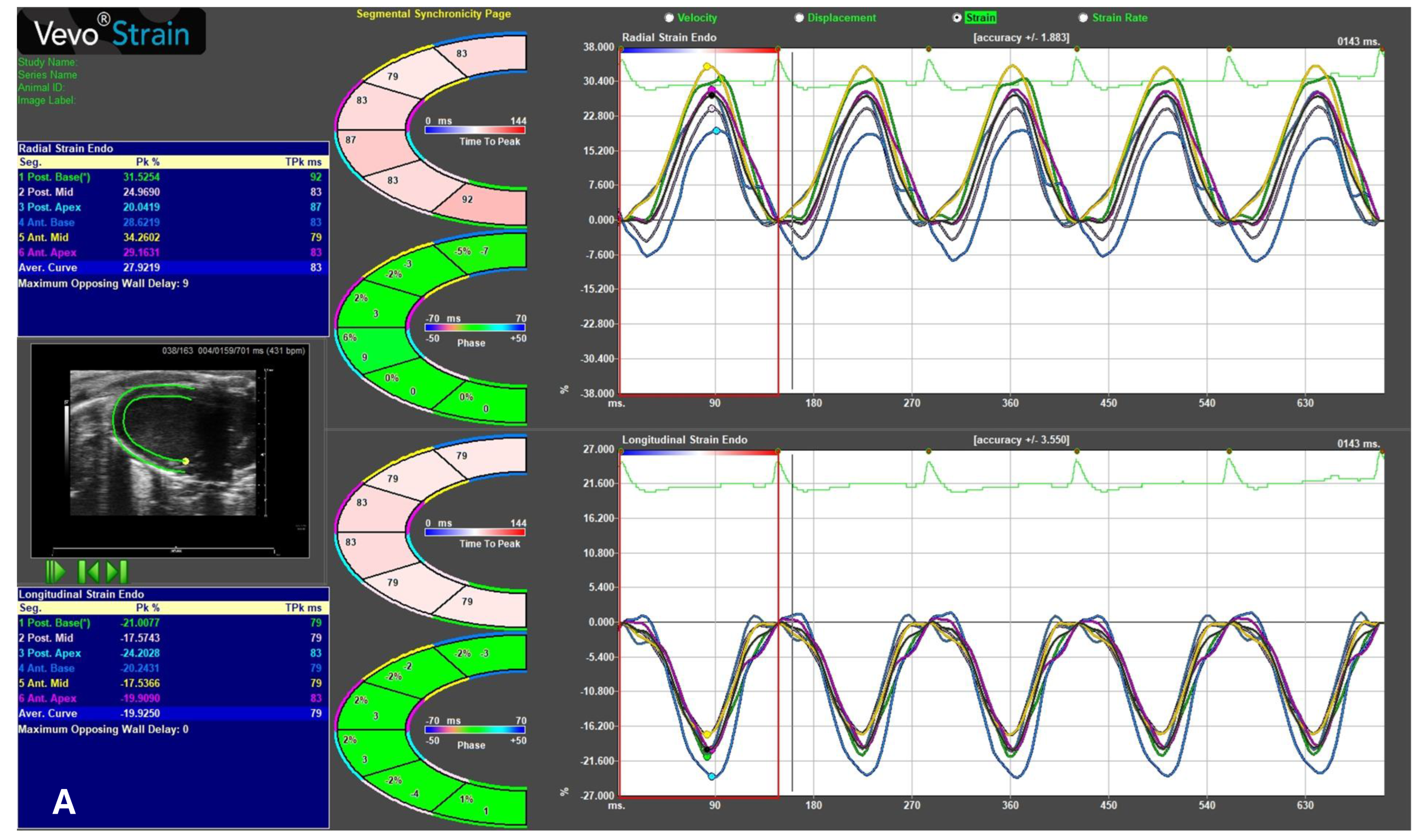
2.5. Speckle-Tracking Echocardiography
- Global longitudinal strain (GLS, Figure 5A) measures contraction along the long axis of the left ventricle (LV), typically from the parasternal long-axis (PLAX) view, with normal values around −22%;
- Global radial strain (GRS, Figure 5B) reflects myocardial thickening and thinning perpendicular to the wall, assessed from the parasternal short-axis (PSAX) view, with normal values near +35%;
- Global circumferential strain (GCS, Figure 5C) represents circumferential shortening around the LV and from PSAX, with normal values around −30%.
3. Echocardiography in Murine Models of Heart Diseases
3.1. Echocardiography in Murine Non-Ischemic Dilated Cardiomyopathy
3.2. Echocardiography in Murine Diabetic Cardiomyopathy
3.3. Echocardiographic Assessment in Pressure-Overload Heart Disease Murine Models
| Mouse Model | Mechanism | Model | Main Features | Echocardiographic Assessment |
|---|---|---|---|---|
| Pressure Overload/Hypertensive heart disease | Surgical method | Ascending aortic constriction (AAC) [105] | LV pressure overload induced by aortic constriction | Eccentric cardiac hypertrophy + reduced sistolic function + dyastolic disfunction:
|
| Transverse aortic constriction (TAC) [106] | ||||
| Suprarenal abdominal aortic banding (AAB) [112] | ||||
| Aortocaval fistula (shunt) [119] | LV volume overload induced by the creation of a shunt between the aorta and vena cava inferior | Eccentric cardiac hypertrophy + hypercontractile stage with increased sistolic function + dyastolic disfunction:
| ||
| Chemical induction | DOCA-salt + unilateral nephrectomy + 1% NaCl drinking water solution [120] | Renal imbalance with increased reabsorption of sodium and water resulting in hypervolemia | Concentric cardiac hypertrophy + preserved sistolic function + early dyastolic disfunction:
| |
| Angiotensin II infusion (1,4 mg/kg/die) [122] | Increase blood pressure via vasoconstriction | Eccentric cardiac hypertrophy + hypercontractile stage with increased sistolic function + dyastolic disfunction:
| ||
| Isoproterenol (30 mg/kg/die) [127] | Hypertrophic response to adrenergic stimulation:
| Concentric cardiac hypertrophy + hypercontractile stage with increased sistolic function + dyastolic disfunction:
| ||
| Genetically induced | Mybpc3−/−mice [131] | Mutation in endogenous cardiac (c) MyBP-C gene and absence of cMyBP-C results in familial hypertrophic cardiomyopathy | Eccentric cardiac hypertrophy + hypercontractile stage with increased sistolic function + dyastolic disfunction:
|
3.4. Echocardiographic Assessment of Ischemic Heart Disease Murine Models
| Mouse Model | Mechanism | Model | Main Features | Echocardiographic Assessment |
|---|---|---|---|---|
| Ischemic cardiomyopathy | Irreversible surgical methods | Permanent left anterior descending artery ligation [143] | Suture of the left anterior descending coronary artery with a needle; confirm the color change of the left ventricle within 10 s and ST elevation with an EKG monitor:
| Ventricular dilation + reduced sistolic function + dyastolic disfunction:
|
| Reversible surgical methods | Ischemia/reperfusion (I/R) model [151] | Temporary ischemia followed by restoration of blood flow:
| Ventricular dilation + reduced sistolic function + dyastolic disfunction:
|
4. Conclusions
- -
- Imaging Settings: 30–40 MHz probe; ≥200 fps for 2D; ≥1 kHz sampling for PW Doppler; narrow sector width; focus at mid-myocardium.
- -
- Anesthesia and Monitoring: Isoflurane 1–1.5% with a 10 min stabilization period; maintain heart rate at 400–600 bpm; keep body temperature at 37 ± 0.5 °C.
- -
- Gating and Acquisition: ECG and respiratory gating; optimize gain for clear endocardial borders; acquire ≥ 3 stable cine-loops per view.
- -
- Quality Control and Reporting: Operator training with blinded re-reads (CV < 10%); annual equipment calibration; report imaging view, probe settings, heart rate, anesthetic protocol, gating method, and refer to the international consensus for reference values.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full Term. |
| 2-D echo | Two-Dimensional Mode. |
| 3-D echo | Three-Dimensional Echocardiography. |
| 4-D echo | Four-Dimensional Echocardiography. |
| 5-FU | 5-Flurouracil. |
| A | A Wave. |
| A4C | Apical Four-Chamber. |
| AAB | Aortic Arch Banding. |
| AI | Artificial Intelligence. |
| ANG II | Angiotensin II. |
| AO | Aorta. |
| AV | Aortic Valve. |
| B-mode | Two-Dimensional Mode. |
| BW | Body Weight. |
| BPM | Beats Per Minute. |
| CFM | Color Flow Mapping. |
| CMR | Cardiac Magnetic Resonance. |
| CO | Cardiac Output. |
| CWD | Continuous-Wave Doppler. |
| DCM | Dilated Cardiomyopathy. |
| DOCA | Deoxycorticosterone Acetate. |
| DT | Deceleration Time. |
| E | E Wave. |
| ECG | Electrocardiographic. |
| EF | Ejection Fraction. |
| FS | Fractional Shortening. |
| GCS | Global Circumferential Strain. |
| GLS | Global Longitudinal Strain. |
| GRS | Global Radial Strain. |
| HF | Heart Failure. |
| HFD | High-Fat Diet. |
| HFpEF | Heart Failure With Preserved Ejection Fraction. |
| HFrEF | Heart Failure With Reduced Ejection Fraction. |
| I/R | Ischemia/Reperfusion. |
| ISO | Isoproterenol. |
| IVRT | Isovolumic Relaxation Time. |
| LA | Left Atrium. |
| LAD | Left Anterior Descending. |
| LV | Left Ventricle. |
| LVEDV | Left Ventricular End-Diastolic Volume. |
| LVESV | Left Ventricular End-Systolic Volume. |
| LVIDd | Ventricular End-Diastolic Internal Diameter. |
| LVIDs | Left Ventricular End-Systolic Internal Diameter. |
| LVM | Left Ventricular Mass. |
| MI | Myocardial Infarction. |
| M-mode | Motion Mode. |
| MyBP-c | Myosin-Binding Protein C. |
| NOD | Non-Obese Diabetic. |
| PLAX | Parasternal Long-Axis. |
| PM | Papillary Muscle. |
| PSAX | Parasternal Short-Axis. |
| PWD | Pulsed-Wave Doppler. |
| ROI | Region Of Interest. |
| ROS | Reactive Oxygen Species. |
| RV | Right Ventricle. |
| SIC | Stress-Induced Cardiomyopathy. |
| STE | Speckle-Tracking Echocardiography. |
| STZ | Streptozotocin. |
| SV | Stroke Volume. |
| T1D | Type 1 Diabetes Mellitus. |
| T2D | Type 2 Diabetes Mellitus. |
| TAC | Transverse Aortic Constriction. |
| TDI | Tissue Doppler Imaging. |
| TTE | Transthoracic Echocardiography. |
| TTP | Time To Peak. |
| WMSI | Wall Motility Score Index. |
References
- Picano, E.; Pierard, L.; Peteiro, J.; Djordjevic-Dikic, A.; Sade, L.E.; Cortigiani, L.; Van De Heyning, C.M.; Celutkiene, J.; Gaibazzi, N.; Ciampi, Q.; et al. The clinical use of stress echocardiography in chronic coronary syndromes and beyond coronary artery disease: A clinical consensus statement from the European Association of Cardiovascular Imaging of the ESC. Eur. Heart J.-Cardiovasc. Imaging 2024, 25, e65–e90. [Google Scholar] [CrossRef] [PubMed]
- Phoon, C.K.L.; Turnbull, D.H. Cardiovascular Imaging in Mice. CP Mouse Biol. 2016, 6, 15–38. [Google Scholar] [CrossRef] [PubMed]
- Donner, D.G.; Kiriazis, H.; Du, X.-J.; Marwick, T.H.; McMullen, J.R. Improving the quality of preclinical research echocardiography: Observations, training, and guidelines for measurement. Am. J. Physiol.-Heart Circ. Physiol. 2018, 315, H58–H70. [Google Scholar] [CrossRef] [PubMed]
- Roth, D.M.; Swaney, J.S.; Dalton, N.D.; Gilpin, E.A.; Ross, J. Impact of anesthesia on cardiac function during echocardiography in mice. Am. J. Physiol.-Heart Circ. Physiol. 2002, 282, H2134–H2140. [Google Scholar] [CrossRef]
- Cheng, Z.; Ito, S.; Nishio, N.; Thanasegaran, S.; Fang, H.; Isobe, K. Characteristics of cardiac aging in C57BL/6 mice. Exp. Gerontol. 2013, 48, 341–348. [Google Scholar] [CrossRef]
- Zacchigna, S.; Paldino, A.; Falcão-Pires, I.; Daskalopoulos, E.P.; Ferro, M.D.; Vodret, S.; Lesizza, P.; Cannatà, A.; Miranda-Silva, D.; Lourenço, A.P.; et al. Towards standardization of echocardiography for the evaluation of left ventricular function in adult rodents: A position paper of the ESC Working Group on Myocardial Function. Cardiovasc. Res. 2021, 117, 43–59. [Google Scholar] [CrossRef]
- Hoffman, M.; Kyriazis, I.D.; Lucchese, A.M.; de Lucia, C.; Piedepalumbo, M.; Bauer, M.; Schulze, P.C.; Bonios, M.J.; Koch, W.J.; Drosatos, K. Myocardial Strain and Cardiac Output are Preferable Measurements for Cardiac Dysfunction and Can Predict Mortality in Septic Mice. JAHA 2019, 8, e012260. [Google Scholar] [CrossRef]
- Tarin, D.; Sturdee, A. Surgical anaesthesia of mice: Evaluation of tribromo-ethanol, ether, halothane and methoxyflurane and development of a reliable technique. Lab Anim. 1972, 6, 79–84. [Google Scholar] [CrossRef]
- Takuma, S.; Suehiro, K.; Cardinale, C.; Hozumi, T.; Yano, H.; Shimizu, J.; Mullis-Jansson, S.; Sciacca, R.; Wang, J.; Burkhoff, D.; et al. Anesthetic inhibition in ischemic and nonischemic murine heart: Comparison with conscious echocardiographic approach. Am. J. Physiol.-Heart Circ. Physiol. 2001, 280, H2364–H2370. [Google Scholar] [CrossRef]
- Pachon, R.E.; Scharf, B.A.; Vatner, D.E.; Vatner, S.F. Best anesthetics for assessing left ventricular systolic function by echocardiography in mice. Am. J. Physiol.-Heart Circ. Physiol. 2015, 308, H1525–H1529. [Google Scholar] [CrossRef]
- Navarro, K.L.; Huss, M.; Smith, J.C.; Sharp, P.; Marx, J.O.; Pacharinsak, C. Mouse Anesthesia: The Art and Science. ILAR J. 2021, 2, 238–273. [Google Scholar] [CrossRef] [PubMed]
- Gardin, J.M.; Siri, F.M.; Kitsis, R.N.; Edwards, J.G.; Leinwand, L.A. Echocardiographic Assessment of Left Ventricular Mass and Systolic Function in Mice. Circ. Res. 1995, 76, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Scherrer-Crosbie, M.; Thibault, H.B. Echocardiography in Translational Research: Of Mice and Men. J. Am. Soc. Echocardiogr. 2008, 21, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Pistner, A.; Belmonte, S.; Coulthard, T.; Blaxall, B. Murine echocardiography and ultrasound imaging. JoVE 2010, 42, 2100. [Google Scholar] [CrossRef]
- Ram, R.; Mickelsen, D.M.; Theodoropoulos, C.; Blaxall, B.C. New approaches in small animal echocardiography: Imaging the sounds of silence. Am. J. Physiol.-Heart Circ. Physiol. 2011, 301, H1765–H1780. [Google Scholar] [CrossRef]
- Lindsey, M.L.; Kassiri, Z.; Virag, J.A.I.; De Castro Brás, L.E.; Scherrer-Crosbie, M. Guidelines for measuring cardiac physiology in mice. Am. J. Physiol.-Heart Circ. Physiol. 2018, 314, H733–H752. [Google Scholar] [CrossRef]
- de Lucia, C.; Wallner, M.; Eaton, D.M.; Zhao, H.; Houser, S.R.; Koch, W.J. Echocardiographic Strain Analysis for the Early Detection of Left Ventricular Systolic/Diastolic Dysfunction and Dyssynchrony in a Mouse Model of Physiological Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 455–461. [Google Scholar] [CrossRef]
- Franchi, F.; E Knudsen, B.; Oehler, E.; Textor, S.C.; O Lerman, L.; Grande, J.P.; Rodriguez-Porcel, M. Non-invasive assessment of cardiac function in a mouse model of renovascular hypertension. Hypertens. Res. 2013, 36, 770–775. [Google Scholar] [CrossRef]
- Villalba-Orero, M.; Garcia-Pavia, P.; Lara-Pezzi, E. Non-invasive assessment of HFpEF in mouse models: Current gaps and future directions. BMC Med. 2022, 20, 349. [Google Scholar] [CrossRef]
- Schaefer, A. Evaluation of left ventricular diastolic function by pulsed Doppler tissue imaging in mice. J. Am. Soc. Echocardiogr. 2003, 16, 1144–1149. [Google Scholar] [CrossRef]
- Moran, C.M.; Thomson, A.J.W.; Rog-Zielinska, E.; Gray, G.A. High-resolution echocardiography in the assessment of cardiac physiology and disease in preclinical models. Exp. Physiol. 2013, 98, 629–644. [Google Scholar] [CrossRef] [PubMed]
- Rottman, J.N.; Ni, G.; Brown, M. Echocardiographic Evaluation of Ventricular Function in Mice. Echocardiography 2007, 24, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Stypmann, J.; Engelen, M.A.; Troatz, C.; Rothenburger, M.; Eckardt, L.; Tiemann, K. Echocardiographic assessment of global left ventricular function in mice. Lab Anim. 2009, 43, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Polimeni, A.; Albanese, M.; Salerno, N.; Aquila, I.; Sabatino, J.; Sorrentino, S.; Leo, I.; Cacia, M.; Signorile, V.; Mongiardo, A.; et al. Predictors of outcomes in patients with mitral regurgitation undergoing percutaneous valve repair. Sci. Rep. 2020, 10, 17144. [Google Scholar] [CrossRef]
- Scherrer-Crosbie, M.; Kurtz, B. Ventricular remodeling and function: Insights using murine echocardiography. J. Mol. Cell. Cardiol. 2010, 48, 512–517. [Google Scholar] [CrossRef]
- O’Riordan, C.E.; Trochet, P.; Steiner, M.; Fuchs, D. Standardisation and future of preclinical echocardiography. Mamm. Genome 2023, 34, 123–155. [Google Scholar] [CrossRef]
- Dawson, D.; Lygate, C.A.; Saunders, J.; Schneider, J.E.; Ye, X.; Hulbert, K.; Noble, J.A.; Neubauer, S. Quantitative 3-Dimensional Echocardiography for Accurate and Rapid Cardiac Phenotype Characterization in Mice. Circulation 2004, 110, 1632–1637. [Google Scholar] [CrossRef]
- Scherrer-Crosbie, M.; Steudel, W.; Hunziker, P.R.; Liel-Cohen, N.; Ullrich, R.; Zapol, W.M.; Picard, M.H. Three-Dimensional Echocardiographic Assessment of Left Ventricular Wall Motion Abnormalities in Mouse Myocardial Infarction. J. Am. Soc. Echocardiogr. 1999, 12, 834–840. [Google Scholar] [CrossRef]
- Russo, I.; Micotti, E.; Fumagalli, F.; Magnoli, M.; Ristagno, G.; Latini, R.; Staszewsky, L. A novel echocardiographic method closely agrees with cardiac magnetic resonance in the assessment of left ventricular function in infarcted mice. Sci. Rep. 2019, 9, 3580. [Google Scholar] [CrossRef]
- Damen, F.W.; Berman, A.G.; Soepriatna, A.H.; Ellis, J.M.; Buttars, S.D.; Aasa, K.L.; Goergen, C.J. High-Frequency 4-Dimensional Ultrasound (4DUS): A Reliable Method for Assessing Murine Cardiac Function. Tomography 2017, 3, 180–187. [Google Scholar] [CrossRef]
- Dann, M.M.; Clark, S.Q.; Trzaskalski, N.A.; Earl, C.C.; Schepers, L.E.; Pulente, S.M.; Lennord, E.N.; Annamalai, K.; Gruber, J.M.; Cox, A.D.; et al. Quantification of murine myocardial infarct size using 2-D and 4-D high-frequency ultrasound. Am. J. Physiol.-Heart Circ. Physiol. 2022, 322, H359–H372. [Google Scholar] [CrossRef] [PubMed]
- Haesen, S.; Steegen, L.; Deluyker, D.; Bito, V. Comprehensive transthoracic echocardiographic evaluation of doxorubicin-induced cardiotoxicity: A multimodal imaging approach in an animal model. Eur. Heart J.-Imaging Methods Pract. 2025, 3, qyaf006. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Ho, D.; Vatner, D.E.; Vatner, S.F. Echocardiography in Mice. CP Mouse Biol. 2011, 1, 71–83. [Google Scholar] [CrossRef]
- Schnelle, M.; Catibog, N.; Zhang, M.; Nabeebaccus, A.A.; Anderson, G.; Richards, D.A.; Sawyer, G.; Zhang, X.; Toischer, K.; Hasenfuss, G.; et al. Echocardiographic evaluation of diastolic function in mouse models of heart disease. J. Mol. Cell. Cardiol. 2018, 114, 20–28. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, T.; Liu, F.; Cohen, E.D.; Patel, V.V. An Evaluation of Transmitral and Pulmonary Venous Doppler Indices for Assessing Murine Left Ventricular Diastolic Function. J. Am. Soc. Echocardiogr. 2010, 23, 887–897. [Google Scholar] [CrossRef]
- Erkens, R.; Kramer, C.M.; Lückstädt, W.; Panknin, C.; Krause, L.; Weidenbach, M.; Dirzka, J.; Krenz, T.; Mergia, E.; Suvorava, T.; et al. Left ventricular diastolic dysfunction in Nrf2 knock out mice is associated with cardiac hypertrophy, decreased expression of SERCA2a, and preserved endothelial function. Free Radic. Biol. Med. 2015, 89, 906–917. [Google Scholar] [CrossRef]
- Schmidt, A.G.; Gerst, M.; Zhai, J.; Carr, A.N.; Pater, L.; Kranias, E.G.; Hoit, B.D. Evaluation of left ventricular diastolic function from spectral and color M-mode Doppler in genetically altered mice. J. Am. Soc. Echocardiogr. 2002, 15, 1065–1073. [Google Scholar] [CrossRef]
- Tsujita, Y.; Kato, T.; Sussman, M.A. Evaluation of Left Ventricular Function in Cardiomyopathic Mice by Tissue Doppler and Color M-Mode Doppler Echocardiography. Echocardiography 2005, 22, 245–253. [Google Scholar] [CrossRef]
- Du, J.; Liu, J.; Feng, H.-Z.; Hossain, M.M.; Gobara, N.; Zhang, C.; Li, Y.; Jean-Charles, P.-Y.; Jin, J.-P.; Huang, X.-P.; et al. Impaired relaxation is the main manifestation in transgenic mice expressing a restrictive cardiomyopathy mutation, R193H, in cardiac TnI. Am. J. Physiol.-Heart Circ. Physiol. 2008, 294, H2604–H2613. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Sun, H.; Kopelen, H.A.; Middleton, K.J.; Khoury, D.S. Hemodynamic determinants of the mitral annulus diastolic velocities by tissue Doppler. J. Am. Coll. Cardiol. 2001, 37, 278–285. [Google Scholar] [CrossRef]
- Daniels, L.J.; Macindoe, C.; Koutsifeli, P.; Annandale, M.; James, S.L.; Watson, L.E.; Coffey, S.; Raaijmakers, A.J.A.; Weeks, K.L.; Bell, J.R.; et al. Myocardial deformation imaging by 2D speckle tracking echocardiography for assessment of diastolic dysfunction in murine cardiopathology. Sci. Rep. 2023, 13, 12344. [Google Scholar] [CrossRef] [PubMed]
- Methawasin, M.; Strom, J.; Borkowski, T.; Hourani, Z.; Runyan, R.; Smith, J.E.; Granzier, H. Phosphodiesterase 9a Inhibition in Mouse Models of Diastolic Dysfunction. Circ: Heart Fail. 2020, 13, e006609. [Google Scholar] [CrossRef] [PubMed]
- Long, V.; Motok, B.; Leblanc, É.; Tannous, G.; Pyle, W.G.; Fiset, C. Arrhythmogenic atrial remodeling during pregnancy in mice. Heart Rhythm 2024, S1547527124035276. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-B.; Chen, K.-K.; Li, S.; Cai, M.-Q.; Yuan, M.-J.; Wang, Y.-P.; Zhang, X.; Wei, M.; Yan, M.-L.; Ma, X.-X.; et al. Impaired Left Atrial Performance Resulting From Age-Related Arial Fibrillation Is Associated With Increased Fibrosis Burden: Insights From a Clinical Study Combining With an in vivo Experiment. Front. Cardiovasc. Med. 2021, 7, 615065. [Google Scholar] [CrossRef]
- Colazzo, F.; Castiglioni, L.; Sironi, L.; Fontana, L.; Nobili, E.; Franzosi, M.; Guerrini, U.; Meloni, M. Murine Left Atrium and Left Atrial Appendage Structure and Function: Echocardiographic and Morphologic Evaluation. PLoS ONE 2015, 10, e0125541. [Google Scholar] [CrossRef]
- Zhang, M.J.; Gyberg, D.J.; Healy, C.L.; Zhang, N.; Liu, H.; Dudley, S.C.; O’cOnnell, T.D. Atrial Myopathy Quantified by Speckle-tracking Echocardiography in Mice. Circ. Cardiovasc. Imaging 2023, 16, e015735. [Google Scholar] [CrossRef]
- Schultheiss, H.-P.; Fairweather, D.; Caforio, A.L.P.; Escher, F.; Hershberger, R.E.; Lipshultz, S.E.; Liu, P.P.; Matsumori, A.; Mazzanti, A.; McMurray, J.; et al. Dilated cardiomyopathy. Nat. Rev. Dis. Primers 2019, 5, 32. [Google Scholar] [CrossRef]
- Codd, M.B.; Sugrue, D.D.; Gersh, B.J.; Melton, L.J. Epidemiology of idiopathic dilated and hypertrophic cardiomyopathy. A population-based study in Olmsted County, Minnesota, 1975–1984. Circulation 1989, 80, 564–572. [Google Scholar] [CrossRef]
- Hershberger, R.E.; Hedges, D.J.; Morales, A. Dilated cardiomyopathy: The complexity of a diverse genetic architecture. Nat. Rev. Cardiol. 2013, 10, 531–547. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Bawa-Khalfe, T.; Lu, L.-S.; Lyu, Y.L.; Liu, L.F.; Yeh, E.T.H. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 2012, 18, 1639–1642. [Google Scholar] [CrossRef]
- Sabatino, J.; De Rosa, S.; Tammè, L.; Iaconetti, C.; Sorrentino, S.; Polimeni, A.; Mignogna, C.; Amorosi, A.; Spaccarotella, C.; Yasuda, M.; et al. Empagliflozin prevents doxorubicin-induced myocardial dysfunction. Cardiovasc. Diabetol. 2020, 19, 66. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Redfors, B.; Ståhlman, M.; Täng, M.S.; Miljanovic, A.; Möllmann, H.; Troidl, C.; Szardien, S.; Hamm, C.; Nef, H.; et al. A mouse model reveals an important role for catecholamine-induced lipotoxicity in the pathogenesis of stress-induced cardiomyopathy. Eur. J. Heart Fail. 2013, 15, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Salerno, N.; Scalise, M.; Marino, F.; Filardo, A.; Chiefalo, A.; Panuccio, G.; Torella, M.; De Angelis, A.; De Rosa, S.; Ellison-Hughes, G.M.; et al. A Mouse Model of Dilated Cardiomyopathy Produced by Isoproterenol Acute Exposure Followed by 5-Fluorouracil Administration. JCDD 2023, 10, 225. [Google Scholar] [CrossRef]
- Wang, W.; Liu, T.; Liu, Y.; Yu, L.; Yan, X.; Weng, W.; Lu, X.; Zhang, C. Astaxanthin attenuates alcoholic cardiomyopathy via inhibition of endoplasmic reticulum stress-mediated cardiac apoptosis. Toxicol. Appl. Pharmacol. 2021, 412, 115378. [Google Scholar] [CrossRef]
- Hughes, W.M.; Rodriguez, W.E.; Rosenberger, D.; Chen, J.; Sen, U.; Tyagi, N.; Moshal, K.S.; Vacek, T.; Kang, Y.J.; Tyagi, S.C. Role of Copper and Homocysteine in Pressure Overload Heart Failure. Cardiovasc. Toxicol. 2008, 8, 137–144. [Google Scholar] [CrossRef]
- Powers, J.D.; Kooiker, K.B.; Mason, A.B.; Teitgen, A.E.; Flint, G.V.; Tardiff, J.C.; Schwartz, S.D.; McCulloch, A.D.; Regnier, M.; Davis, J.; et al. Modulating the tension-time integral of the cardiac twitch prevents dilated cardiomyopathy in murine hearts. JCI Insight 2020, 5, e142446. [Google Scholar] [CrossRef]
- McConnell, B.K.; Singh, S.; Fan, Q.; Hernandez, A.; Portillo, J.P.; Reiser, P.J.; Tikunova, S.B. Knock-in mice harboring a Ca2+ desensitizing mutation in cardiac troponin C develop early onset dilated cardiomyopathy. Front. Physiol. 2015, 6, 242. [Google Scholar] [CrossRef]
- Davis, J.; Davis, L.C.; Correll, R.N.; Makarewich, C.A.; Schwanekamp, J.A.; Moussavi-Harami, F.; Wang, D.; York, A.J.; Wu, H.; Houser, S.R.; et al. A Tension-Based Model Distinguishes Hypertrophic versus Dilated Cardiomyopathy. Cell 2016, 165, 1147–1159. [Google Scholar] [CrossRef]
- Singal, P.K.; Iliskovic, N. Doxorubicin-Induced Cardiomyopathy. N. Engl. J. Med. 1998, 339, 900–905. [Google Scholar] [CrossRef]
- Coppola, C.; Riccio, G.; Barbieri, A.; Monti, M.G.; Piscopo, G.; Rea, D.; Arra, C.; Maurea, C.; De Lorenzo, C.; Maurea, N. Antineoplastic-related cardiotoxicity, morphofunctional aspects in a murine model: Contribution of the new tool 2D-speckle tracking. OTT 2016, 9, 6785–6794. [Google Scholar] [CrossRef]
- Neilan, T.G. Tissue Doppler imaging predicts left ventricular dysfunction and mortality in a murine model of cardiac injury. Eur. Heart J. 2006, 27, 1868–1875. [Google Scholar] [CrossRef] [PubMed]
- Hullin, R.; Métrich, M.; Sarre, A.; Basquin, D.; Maillard, M.; Regamey, J.; Martin, D. Diverging effects of enalapril or eplerenone in primary prevention against doxorubicin-induced cardiotoxicity. Cardiovasc. Res. 2018, 114, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, W.; Hu, T.; Ni, J.; Xu, B.; Huang, W. A Doxorubicin-Induced Murine Model of Dilated Cardiomyopathy In Vivo. JoVE 2020, 159, 61158. [Google Scholar] [CrossRef]
- Tsai, T.H.; Lin, C.J.; Hang, C.L.; Chen, W.Y. Calcitriol Attenuates Doxorubicin-Induced Cardiac Dysfunction and Inhibits Endothelial-to-Mesenchymal Transition in Mice. Cells 2019, 8, 865. [Google Scholar] [CrossRef]
- Seifert, C.F.; Nesser, M.E.; Thompson, D.F. Dexrazoxane in the Prevention of Doxorubicin-Induced Cardiotoxicity. Ann. Pharmacother. 1994, 28, 1063–1072. [Google Scholar] [CrossRef]
- Ghasemi, K.; Vaseghi, G.; Mansourian, M. Pharmacological interventions for preventing anthracycline-induced clinical and subclinical cardiotoxicity: A network meta-analysis of metastatic breast cancer. J. Oncol. Pharm. Pract. 2021, 27, 414–427. [Google Scholar] [CrossRef]
- Aquila, I.; Cianflone, E.; Scalise, M.; Marino, F.; Mancuso, T.; Filardo, A.; Smith, A.J.; Cappetta, D.; De Angelis, A.; Urbanek, K.; et al. c-kit Haploinsufficiency impairs adult cardiac stem cell growth, myogenicity and myocardial regeneration. Cell Death Dis. 2019, 10, 436. [Google Scholar] [CrossRef]
- Ellison, G.M.; Torella, D.; Karakikes, I.; Purushothaman, S.; Curcio, A.; Gasparri, C.; Indolfi, C.; Cable, N.T.; Goldspink, D.F.; Nadal-Ginard, B. Acute β-Adrenergic Overload Produces Myocyte Damage through Calcium Leakage from the Ryanodine Receptor 2 but Spares Cardiac Stem Cells. J. Biol. Chem. 2007, 282, 11397–11409. [Google Scholar] [CrossRef]
- Singh, K.; Xiao, L.; Remondino, A.; Sawyer, D.B.; Colucci, W.S. Adrenergic regulation of cardiac myocyte apoptosis. J. Cell. Physiol. 2001, 189, 257–265. [Google Scholar] [CrossRef]
- Cheng, M.; Chen, C.; Yu, K.; Lv, X.; Zeng, Q.; Dong, N.; Zhu, F. Ablation of CXCR4 expression in cardiomyocytes exacerbates isoproterenol-induced cell death and heart failure. Int. J. Mol. Med. 2022, 51, 13. [Google Scholar] [CrossRef]
- Rau, C.D.; Wang, J.; Avetisyan, R.; Romay, M.C.; Martin, L.; Ren, S.; Wang, Y.; Lusis, A.J. Mapping Genetic Contributions to Cardiac Pathology Induced by Beta-Adrenergic Stimulation in Mice. Circ. Cardiovasc. Genet. 2015, 8, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Perez-Bonilla, P.; LaViolette, B.; Bhandary, B.; Ullas, S.; Chen, X.; Hirenallur-Shanthappa, D. Isoproterenol induced cardiac hypertrophy: A comparison of three doses and two delivery methods in C57BL/6J mice. PLoS ONE 2024, 19, e0307467. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, D.; Gargoum, R.; Tyagi, N.; Metreveli, N.; Sen, U.; Maldonado, C.; Tyagi, S. Homocysteine enriched diet leads to prolonged QT interval and reduced left ventricular performance in telemetric monitored mice. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 492–498. [Google Scholar] [CrossRef]
- Salerno, N.; Salerno, L.; Marino, F.; Scalise, M.; Chiefalo, A.; Panuccio, G.; De Angelis, A.; Cianflone, E.; Urbanek, K.; Torella, D. Myocardial regeneration protocols towards the routine clinical scenario: An unseemly path from bench to bedside. eClinicalMedicine 2022, 50, 101530. [Google Scholar] [CrossRef]
- Cianflone, E.; Torella, M.; Biamonte, F.; De Angelis, A.; Urbanek, K.; Costanzo, F.S.; Rota, M.; Ellison-Hughes, G.M.; Torella, D. Targeting Cardiac Stem Cell Senescence to Treat Cardiac Aging and Disease. Cells 2020, 9, 1558. [Google Scholar] [CrossRef]
- Kannel, W.B.; Hjortland, M.; Castelli, W.P. Role of diabetes in congestive heart failure: The Framingham study. Am. J. Cardiol. 1974, 34, 29–34. [Google Scholar] [CrossRef]
- Harmancey, R.; Taegtmeyer, H. The complexities of diabetic cardiomyopathy: Lessons from patients and animal models. Curr. Diab Rep. 2008, 8, 243–248. [Google Scholar] [CrossRef]
- Sorrentino, A.; Borghetti, G.; Zhou, Y.; Cannata, A.; Meo, M.; Signore, S.; Anversa, P.; Leri, A.; Goichberg, P.; Qanud, K.; et al. Hyperglycemia induces defective Ca 2+ homeostasis in cardiomyocytes. Am. J. Physiol.-Heart Circ. Physiol. 2017, 312, H150–H161. [Google Scholar] [CrossRef]
- Cersosimo, A.; Salerno, N.; Sabatino, J.; Scatteia, A.; Bisaccia, G.; De Rosa, S.; Dellegrottaglie, S.; Bucciarelli-Ducci, C.; Torella, D.; Leo, I. Underlying mechanisms and cardioprotective effects of SGLT2i and GLP-1Ra: Insights from cardiovascular magnetic resonance. Cardiovasc. Diabetol. 2024, 23, 94. [Google Scholar] [CrossRef]
- Molinaro, C.; Salerno, L.; Marino, F.; Scalise, M.; Salerno, N.; Pagano, L.; De Angelis, A.; Cianflone, E.; Torella, D.; Urbanek, K. Unraveling and Targeting Myocardial Regeneration Deficit in Diabetes. Antioxidants 2022, 11, 208. [Google Scholar] [CrossRef]
- Singh, R.; Gholipourmalekabadi, M.; Shafikhani, S.H. Animal models for type 1 and type 2 diabetes: Advantages and limitations. Front. Endocrinol. 2024, 15, 1359685. [Google Scholar] [CrossRef] [PubMed]
- Hanafusa, T.; Miyagawa, J.-I.; Nakajima, H.; Tomita, K.; Kuwajima, M.; Matsuzawa, Y.; Tarui, S. The NOD mouse. Diabetes Res. Clin. Pract. 1994, 24, S307–S311. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Oudit, G.Y.; Wang, X.; Zhang, L.; Ussher, J.R.; Lopaschuk, G.D.; Kassiri, Z. Type 1 diabetic cardiomyopathy in the Akita (Ins2 WT/C96Y) mouse model is characterized by lipotoxicity and diastolic dysfunction with preserved systolic function. Am. J. Physiol.-Heart Circ. Physiol. 2009, 297, H2096–H2108. [Google Scholar] [CrossRef] [PubMed]
- Marino, F.; Salerno, N.; Scalise, M.; Salerno, L.; Torella, A.; Molinaro, C.; Chiefalo, A.; Filardo, A.; Siracusa, C.; Panuccio, G.; et al. Streptozotocin-Induced Type 1 and 2 Diabetes Mellitus Mouse Models Show Different Functional, Cellular and Molecular Patterns of Diabetic Cardiomyopathy. IJMS 2023, 24, 1132. [Google Scholar] [CrossRef]
- Lenzen, S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 2008, 51, 216–226. [Google Scholar] [CrossRef]
- Surwit, R.S.; Kuhn, C.M.; Cochrane, C.; McCubbin, J.A.; Feinglos, M.N. Diet-Induced Type II Diabetes in C57BL/6J Mice. Diabetes 1988, 37, 1163–1167. [Google Scholar] [CrossRef]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef]
- Chen, H.; Charlat, O.; Tartaglia, L.A.; Woolf, E.A.; Weng, X.; Ellis, S.J.; Lakey, N.D.; Culpepper, J.; More, K.J.; Breitbart, R.E.; et al. Evidence That the Diabetes Gene Encodes the Leptin Receptor: Identification of a Mutation in the Leptin Receptor Gene in db/db Mice. Cell 1996, 84, 491–495. [Google Scholar] [CrossRef]
- Kikutani, H.; Makino, S. The Murine Autoimmune Diabetes Model: NOD and Related Strains. In Advances in Immunology; Elsevier: Amsterdam, The Netherlands, 1992; Volume 51, pp. 285–322. [Google Scholar] [CrossRef]
- Chen, D.; Thayer, T.C.; Wen, L.; Wong, F.S. Mouse Models of Autoimmune Diabetes: The Nonobese Diabetic (NOD) Mouse. In Animal Models of Diabetes; King, A.J.F., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2020; Volume 2128, pp. 87–92. [Google Scholar] [CrossRef]
- Mathews, C.E.; Langley, S.H.; Leiter, E.H. New mouse model to study islet transplantation in insulin-dependent diabetes mellitus. Transplantation 2002, 73, 1333–1336. [Google Scholar] [CrossRef]
- Al-Awar, A.; Kupai, K.; Veszelka, M.; Szűcs, G.; Attieh, Z.; Murlasits, Z.; Török, S.; Pósa, A.; Varga, C. Experimental Diabetes Mellitus in Different Animal Models. J. Diabetes Res. 2016, 2016, 9051426. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, C.; Zhang, X.; Li, J.; Li, J.; Zheng, L.; Huang, K. Apelin alleviates diabetes-associated endoplasmic reticulum stress in the pancreas of Akita mice. Peptides 2011, 32, 1634–1639. [Google Scholar] [CrossRef] [PubMed]
- Nerup, J.; Mandrap-Poulsen, T.; Helqvist, S.; Andersen, H.U.; Pociot, F.; Reimers, J.I.; Cuartero, B.G.; Karlsen, A.E.; Bjerre, U.; Lorenzen, T. On the pathogenesis of IDDM. Diabetologia 1994, 37, S82–S89. [Google Scholar] [CrossRef] [PubMed]
- Sandler, S.; Swenne, I. Streptozotocin, but not alloxan, induces DNA repair synthesis in mouse pancreatic islets in vitro. Diabetologia 1983, 25, 444–447. [Google Scholar] [CrossRef]
- Marino, F.; Scalise, M.; Salerno, N.; Salerno, L.; Molinaro, C.; Cappetta, D.; Torella, M.; Greco, M.; Foti, D.; Sasso, F.C.; et al. Diabetes-Induced Cellular Senescence and Senescence-Associated Secretory Phenotype Impair Cardiac Regeneration and Function Independently of Age. Diabetes 2022, 71, 1081–1098. [Google Scholar] [CrossRef]
- Cohen, C.D.; De Blasio, M.J.; Lee, M.K.S.; Farrugia, G.E.; Prakoso, D.; Krstevski, C.; Deo, M.; Donner, D.G.; Kiriazis, H.; Flynn, M.C.; et al. Diastolic dysfunction in a pre-clinical model of diabetes is associated with changes in the cardiac non-myocyte cellular composition. Cardiovasc. Diabetol. 2021, 20, 116. [Google Scholar] [CrossRef]
- Chandramouli, C.; Reichelt, M.E.; Curl, C.L.; Varma, U.; Bienvenu, L.A.; Koutsifeli, P.; Raaijmakers, A.J.A.; De Blasio, M.J.; Qin, C.X.; Jenkins, A.J.; et al. Diastolic dysfunction is more apparent in STZ-induced diabetic female mice, despite less pronounced hyperglycemia. Sci. Rep. 2018, 8, 2346. [Google Scholar] [CrossRef]
- Donahue, R.P.; Dorn, J.M.; Stranges, S.; Swanson, M.; Hovey, K.; Trevisan, M. Impaired fasting glucose and recurrent cardiovascular disease among survivors of a first acute myocardial infarction: Evidence of a sex difference? The Western New York experience. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 504–511. [Google Scholar] [CrossRef]
- Heydemann, A. An Overview of Murine High Fat Diet as a Model for Type 2 Diabetes Mellitus. J. Diabetes Res. 2016, 2016, 2902351. [Google Scholar] [CrossRef]
- Hutchinson, K.R.; Lord, C.K.; West, T.A.; Stewart, J.A. Cardiac Fibroblast-Dependent Extracellular Matrix Accumulation Is Associated with Diastolic Stiffness in Type 2 Diabetes. PLoS ONE 2013, 8, e72080. [Google Scholar] [CrossRef]
- Van Den Bergh, A.; Flameng, W.; Herijgers, P. Type II diabetic mice exhibit contractile dysfunction but maintain cardiac output by favourable loading conditions. Eur. J. Heart Fail. 2006, 8, 777–783. [Google Scholar] [CrossRef]
- Di Costanzo, A.; Indolfi, C.; Franzone, A.; Esposito, G.; Spaccarotella, C.A.M. Lp(a) in the Pathogenesis of Aortic Stenosis and Approach to Therapy with Antisense Oligonucleotides or Short Interfering RNA. IJMS 2023, 24, 14939. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, L.; Weisheit, C.K.; Gestrich, C.; Peukert, K.; Duerr, G.D.; Ayub, M.A.; Erdfelder, F.; Stöckigt, F. A Closed-chest Model to Induce Transverse Aortic Constriction in Mice. JoVE 2018, 134, 57397. [Google Scholar] [CrossRef]
- Fard, A.; Wang, C.Y.; Takuma, S.; Skopicki, H.A.; Pinsky, D.J.; Di Tullio, M.R.; Homma, S. Noninvasive Assessment and Necropsy Validation of Changes in Left Ventricular Mass in Ascending Aortic Banded Mice. J. Am. Soc. Echocardiogr. 2000, 13, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Richards, D.A.; Aronovitz, M.J.; Calamaras, T.D.; Tam, K.; Martin, G.L.; Liu, P.; Bowditch, H.K.; Zhang, P.; Huggins, G.S.; Blanton, R.M. Distinct Phenotypes Induced by Three Degrees of Transverse Aortic Constriction in Mice. Sci. Rep. 2019, 9, 5844. [Google Scholar] [CrossRef]
- Rockman, H.A.; Ross, R.S.; Harris, A.N.; Knowlton, K.U.; Steinhelper, M.E.; Field, L.J.; Ross, J.; Chien, K.R. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc. Natl. Acad. Sci. USA 1991, 88, 8277–8281, Erratum in Proc. Natl. Acad. Sci. USA 1991, 88, 9907. [Google Scholar] [CrossRef]
- Angela, C.D.; van Oort, R.J.; Wehrens, X.H. Transverse Aortic Constriction in Mice. JoVE 2010, 38, 1729. [Google Scholar] [CrossRef]
- Mohammed, S.F.; Storlie, J.R.; Oehler, E.A.; Bowen, L.A.; Korinek, J.; Lam, C.S.; Simari, R.D.; Burnett, J.C.; Redfield, M.M. Variable phenotype in murine transverse aortic constriction. Cardiovasc. Pathol. 2012, 21, 188–198. [Google Scholar] [CrossRef]
- Bosch, L.; de Haan, J.J.; Bastemeijer, M.; van der Burg, J.; van der Worp, E.; Wesseling, M.; Viola, M.; Odille, C.; el Azzouzi, H.; Pasterkamp, G.; et al. The transverse aortic constriction heart failure animal model: A systematic review and meta-analysis. Heart Fail. Rev. 2021, 26, 1515–1524. [Google Scholar] [CrossRef]
- Huang, J.; Wu, J.; Wang, S.; You, J.; Ye, Y.; Ding, Z.; Yang, F.; Wang, X.; Guo, J.; Ma, L.; et al. Ultrasound biomicroscopy validation of a murine model of cardiac hypertrophic preconditioning: Comparison with a hemodynamic assessment. Am. J. Physiol.-Heart Circ. Physiol. 2017, 313, H138–H148. [Google Scholar] [CrossRef]
- Zhang, L.; Jaswal, J.S.; Ussher, J.R.; Sankaralingam, S.; Wagg, C.; Zaugg, M.; Lopaschuk, G.D. Cardiac Insulin-Resistance and Decreased Mitochondrial Energy Production Precede the Development of Systolic Heart Failure After Pressure-Overload Hypertrophy. Circ: Heart Fail. 2013, 6, 1039–1048. [Google Scholar] [CrossRef]
- Tarnavski, O.; McMullen, J.R.; Schinke, M.; Nie, Q.; Kong, S.; Izumo, S. Mouse cardiac surgery: Comprehensive techniques for the generation of mouse models of human diseases and their application for genomic studies. Physiol. Genom. 2004, 16, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Thastrup, O.L.E.; Cullen, P.J.; Drøbak, B.K.; Hanley, M.R.; Dawson, A.P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc. Natl. Acad. Sci. USA 1990, 87, 2466–2470. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Cheng, S.; Unno, K.; Lin, F.-C.; Liao, R. Regional Cardiac Dysfunction and Dyssynchrony in a Murine Model of Afterload Stress. PLoS ONE 2013, 8, e59915. [Google Scholar] [CrossRef]
- Furihata, T.; Kinugawa, S.; Fukushima, A.; Homma, T.; Masaki, Y.; Takada, S.; Kadoguchi, T.; Matsushima, S.; Yokota, T.; Tsutsui, H. The Transition from Compensated Cardiac Hypertrophy to Failure Created by Transverse Aortic Constriction in Mice. J. Card. Fail. 2014, 20, S204. [Google Scholar] [CrossRef]
- Furihata, T.; Kinugawa, S.; Takada, S.; Fukushima, A.; Takahashi, M.; Homma, T.; Masaki, Y.; Tsuda, M.; Matsumoto, J.; Mizushima, W.; et al. The experimental model of transition from compensated cardiac hypertrophy to failure created by transverse aortic constriction in mice. IJC Heart Vasc. 2016, 11, 24–28. [Google Scholar] [CrossRef]
- Platt, M.J.; Huber, J.S.; Romanova, N.; Brunt, K.R.; Simpson, J.A. Pathophysiological Mapping of Experimental Heart Failure: Left and Right Ventricular Remodeling in Transverse Aortic Constriction Is Temporally, Kinetically and Structurally Distinct. Front. Physiol. 2018, 9, 472. [Google Scholar] [CrossRef]
- Lee, S.-R.; Thorn, S.; Guerrera, N.; Gonzalez, L.; Taniguchi, R.; Langford, J.; Sinusas, A.J.; Dardik, A. Arteriovenous fistula-induced cardiac remodeling shows cardioprotective features in mice. JVS-Vasc. Sci. 2021, 2, 110–128. [Google Scholar] [CrossRef]
- Anderson, P.; Bishop, S.; Digerness, S. Caronary vascular function and morphology in hydralazine treated DOCA salt rats. J. Mol. Cell. Cardiol. 1988, 20, 955–967. [Google Scholar] [CrossRef]
- Silberman, G.A.; Fan, T.-H.M.; Liu, H.; Jiao, Z.; Xiao, H.D.; Lovelock, J.D.; Boulden, B.M.; Widder, J.; Fredd, S.; Bernstein, K.E.; et al. Uncoupled Cardiac Nitric Oxide Synthase Mediates Diastolic Dysfunction. Circulation 2010, 121, 519–528. [Google Scholar] [CrossRef]
- Haudek, S.B.; Cheng, J.; Du, J.; Wang, Y.; Hermosillo-Rodriguez, J.; Trial, J.; Taffet, G.E.; Entman, M.L. Monocytic fibroblast precursors mediate fibrosis in angiotensin-II-induced cardiac hypertrophy. J. Mol. Cell. Cardiol. 2010, 49, 499–507. [Google Scholar] [CrossRef]
- Takayanagi, T.; Kawai, T.; Forrester, S.J.; Obama, T.; Tsuji, T.; Fukuda, Y.; Elliott, K.J.; Tilley, D.G.; Davisson, R.L.; Park, J.-Y.; et al. Role of Epidermal Growth Factor Receptor and Endoplasmic Reticulum Stress in Vascular Remodeling Induced by Angiotensin II. Hypertension 2015, 65, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Okamoto, H.; Akino, M.; Onozuka, H.; Matsui, Y.; Tsutsui, H. Pravastatin Attenuates Left Ventricular Remodeling and Diastolic Dysfunction in Angiotensin II-Induced Hypertensive Mice. J. Cardiovasc. Pharmacol. 2008, 51, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, Y.; Mano, T.; Sakata, Y.; Ohtani, T.; Takeda, Y.; Tamaki, S.; Omori, Y.; Ikeya, Y.; Saito, Y.; Ishii, R.; et al. A novel heart failure mice model of hypertensive heart disease by angiotensin II infusion, nephrectomy, and salt loading. Am. J. Physiol.-Heart Circ. Physiol. 2013, 305, H1658–H1667. [Google Scholar] [CrossRef] [PubMed]
- Maass, A.H.; Ikeda, K.; Oberdorf-Maass, S.; Maier, S.K.; Leinwand, L.A. Hypertrophy, Fibrosis, and Sudden Cardiac Death in Response to Pathological Stimuli in Mice With Mutations in Cardiac Troponin T. Circulation 2004, 110, 2102–2109. [Google Scholar] [CrossRef]
- Ren, S.; Chang, S.; Tran, A.; Mandelli, A.; Wang, Y.; Wang, J.J. Implantation of an Isoproterenol Mini-Pump to Induce Heart Failure in Mice. J. Vis. Exp. 2019, 152, 59646. [Google Scholar] [CrossRef]
- Ma, S.; Ma, J.; Tu, Q.; Zheng, C.; Chen, Q.; Lv, W. Isoproterenol Increases Left Atrial Fibrosis and Susceptibility to Atrial Fibrillation by Inducing Atrial Ischemic Infarction in Rats. Front. Pharmacol. 2020, 11, 493. [Google Scholar] [CrossRef]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef]
- Jelinek, M.; Wallach, C.; Ehmke, H.; Schwoerer, A.P. Genetic background dominates the susceptibility to ventricular arrhythmias in a murine model of β-adrenergic stimulation. Sci. Rep. 2018, 8, 2312. [Google Scholar] [CrossRef]
- Harris, S.P.; Bartley, C.R.; Hacker, T.A.; McDonald, K.S.; Douglas, P.S.; Greaser, M.L.; Powers, P.A.; Moss, R.L. Hypertrophic Cardiomyopathy in Cardiac Myosin Binding Protein-C Knockout Mice. Circ. Res. 2002, 90, 594–601. [Google Scholar] [CrossRef]
- Heusch, G.; Gersh, B.J. The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: A continual challenge. Eur. Heart J. 2016, 38, 774–784. [Google Scholar] [CrossRef]
- Salerno, N.; Marino, F.; Scalise, M.; Salerno, L.; Molinaro, C.; Filardo, A.; Chiefalo, A.; Panuccio, G.; De Angelis, A.; Urbanek, K.; et al. Pharmacological clearance of senescent cells improves cardiac remodeling and function after myocardial infarction in female aged mice. Mech. Ageing Dev. 2022, 208, 111740. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, M.L.; Bolli, R.; Canty, J.M., Jr.; Du, X.-J.; Frangogiannis, N.G.; Frantz, S.; Gourdie, R.G.; Holmes, J.W.; Jones, S.P.; Kloner, R.A.; et al. Guidelines for experimental models of myocardial ischemia and infarction. Am. J. Physiol.-Heart Circ. Physiol. 2018, 314, H812–H838. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, C.; Cater, G.; McMahon, B.; Guo, L.; Nouraie, S.M.; Wu, Y.; Villanueva, F.; Kaufman, B.A. Commercial 4-dimensional echocardiography for murine heart volumetric evaluation after myocardial infarction. Cardiovasc. Ultrasound 2020, 18, 9. [Google Scholar] [CrossRef] [PubMed]
- Haubner, B.J.; Adamowicz-Brice, M.; Khadayate, S.; Tiefenthaler, V.; Metzler, B.; Aitman, T.; Penninger, J.M. Complete cardiac regeneration in a mouse model of myocardial infarction. Aging 2012, 4, 966–977. [Google Scholar] [CrossRef]
- Reichert, K.; Colantuono, B.; McCormack, I.; Rodrigues, F.; Pavlov, V.; Abid, M.R. Murine Left Anterior Descending (LAD) Coronary Artery Ligation: An Improved and Simplified Model for Myocardial Infarction. JoVE 2017, 122, 55353. [Google Scholar] [CrossRef]
- Brooks, W.W.; Garibaldi, B.A.; Conrad, C.H. Myocardial injury in the mouse induced by transthoracic cauterization. Lab. Anim. Sci. 1998, 48, 374–378. [Google Scholar]
- Van Amerongen, M.J.; Harmsen, M.C.; Petersen, A.H.; Popa, E.R.; Van Luyn, M.J.A. Cryoinjury: A model of myocardial regeneration. Cardiovasc. Pathol. 2008, 17, 23–31. [Google Scholar] [CrossRef]
- Lugrin, J.; Parapanov, R.; Krueger, T.; Liaudet, L. Murine Myocardial Infarction Model using Permanent Ligation of Left Anterior Descending Coronary Artery. JoVE 2019, 150, 59591. [Google Scholar] [CrossRef]
- Chen, J.; Ceholski, D.K.; Liang, L.; Fish, K.; Hajjar, R.J. Variability in coronary artery anatomy affects consistency of cardiac damage after myocardial infarction in mice. Am. J. Physiol.-Heart Circ. Physiol. 2017, 313, H275–H282. [Google Scholar] [CrossRef]
- Benavides-Vallve, C.; Corbacho, D.; Iglesias-Garcia, O.; Pelacho, B.; Albiasu, E.; Castaño, S.; Muñoz-Barrutia, A.; Prosper, F.; Ortiz-De-Solorzano, C.; Katare, R.G. New Strategies for Echocardiographic Evaluation of Left Ventricular Function in a Mouse Model of Long-Term Myocardial Infarction. PLoS ONE 2012, 7, e41691. [Google Scholar] [CrossRef]
- Johny, E.; Dutta, P. Left Coronary Artery Ligation: A Surgical Murine Model of Myocardial Infarction. JoVE 2022, 186, 64387. [Google Scholar] [CrossRef]
- Bauer, M.; Cheng, S.; Jain, M.; Ngoy, S.; Theodoropoulos, C.; Trujillo, A.; Lin, F.-C.; Liao, R. Echocardiographic Speckle-Tracking Based Strain Imaging for Rapid Cardiovascular Phenotyping in Mice. Circ. Res. 2011, 108, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Bhan, A.; Sirker, A.; Zhang, J.; Protti, A.; Catibog, N.; Driver, W.; Botnar, R.; Monaghan, M.J.; Shah, A.M. High-frequency speckle tracking echocardiography in the assessment of left ventricular function and remodeling after murine myocardial infarction. Am. J. Physiol.-Heart Circ. Physiol. 2014, 306, H1371–H1383. [Google Scholar] [CrossRef]
- De Villiers, C.; Riley, P.R. Mouse models of myocardial infarction: Comparing permanent ligation and ischae-mia-reperfusion. Dis. Models Mech. 2020, 13, dmm046565. [Google Scholar] [CrossRef]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Cell Biology of Ischemia/Reperfusion Injury. In International Review of Cell and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2012; Volume 298, pp. 229–317. [Google Scholar] [CrossRef]
- Nossuli, T.O.; Frangogiannis, N.G.; Knuefermann, P.; Lakshminarayanan, V.; Dewald, O.; Evans, A.J.; Peschon, J.; Mann, D.L.; Michael, L.H.; Entman, M.L. Brief murine myocardial I/R induces chemokines in a TNF-α-independent manner: Role of oxygen radicals. Am. J. Physiol.-Heart Circ. Physiol. 2001, 281, H2549–H2558. [Google Scholar] [CrossRef]
- Nossuli, T.O.; Lakshminarayanan, V.; Baumgarten, G.; Taffet, G.E.; Ballantyne, C.M.; Michael, L.H.; Entman, M.L. A chronic mouse model of myocardial ischemia-reperfusion: Essential in cytokine studies. Am. J. Physiol.-Heart Circ. Physiol. 2000, 278, H1049–H1055. [Google Scholar] [CrossRef]
- Dewald, O.; Zymek, P.; Winkelmann, K.; Koerting, A.; Ren, G.; Abou-Khamis, T.; Michael, L.H.; Rollins, B.J.; Entman, M.L.; Frangogiannis, N.G. CCL2/Monocyte Chemoattractant Protein-1 Regulates Inflammatory Responses Critical to Healing Myocardial Infarcts. Circ. Res. 2005, 96, 881–889. [Google Scholar] [CrossRef]
- Christia, P.; Bujak, M.; Gonzalez-Quesada, C.; Chen, W.; Dobaczewski, M.; Reddy, A.; Frangogiannis, N.G. Systematic Characterization of Myocardial Inflammation, Repair, and Remodeling in a Mouse Model of Reperfused Myocardial Infarction. J. Histochem. Cytochem. 2013, 61, 555–570. [Google Scholar] [CrossRef]
- Longacre, L.S.; Kloner, R.A.; Arai, A.E.; Baines, C.P.; Bolli, R.; Braunwald, E.; Downey, J.; Gibbons, R.J.; Gottlieb, R.A.; Heusch, G.; et al. New Horizons in Cardioprotection: Recommendations From the 2010 National Heart, Lung, and Blood Institute Workshop. Circulation 2011, 124, 1172–1179. [Google Scholar] [CrossRef]
- Tanaka, N.; Dalton, N.; Mao, L.; Rockman, H.A.; Peterson, K.L.; Gottshall, K.R.; Hunter, J.J.; Chien, K.R.; Ross, J. Transthoracic Echocardiography in Models of Cardiac Disease in the Mouse. Circulation 1996, 94, 1109–1117. [Google Scholar] [CrossRef]
- Lindsey, B.; Rojas, J.; Martin, K.; Shelton, S.; Dayton, P. Acoustic characterization of contrast-to-tissue ratio and axial resolution for dual-frequency contrast-specific acoustic angiography imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2014, 61, 1668–1687. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-P.; Liu, Y.-H.; Rhaleb, N.-E.; Kurihara, N.; Kim, H.E.; Carretero, O.A. Echocardiographic assessment of cardiac function in conscious and anesthetized mice. Am. J. Physiol.-Heart Circ. Physiol. 1999, 277, H1967–H1974. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; Samtani, R.; Dhanantwari, P.; Lee, E.; Yamada, S.; Shiota, K.; Donofrio, M.T.; Leatherbury, L.; Lo, C.W. A detailed comparison of mouse and human cardiac development. Pediatr. Res. 2014, 76, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Johri, A.M.; Picard, M.H.; Newell, J.; Marshall, J.E.; King, M.E.E.; Hung, J. Can a Teaching Intervention Reduce Interobserver Variability in LVEF Assessment. JACC: Cardiovasc. Imaging 2011, 4, 821–829. [Google Scholar] [CrossRef]
- Crowley, A.L.; Yow, E.; Barnhart, H.X.; Daubert, M.A.; Bigelow, R.; Sullivan, D.C.; Pencina, M.; Douglas, P.S. Critical Review of Current Approaches for Echocardiographic Reproducibility and Reliability Assessment in Clinical Research. J. Am. Soc. Echocardiogr. 2016, 29, 1144–1154.e7. [Google Scholar] [CrossRef]
- Henderson, V.C.; Kimmelman, J.; Fergusson, D.; Grimshaw, J.M.; Hackam, D.G. Threats to Validity in the Design and Conduct of Preclinical Efficacy Studies: A Systematic Review of Guidelines for In Vivo Animal Experiments. PLoS Med. 2013, 10, e1001489. [Google Scholar] [CrossRef]
- Lara-Pezzi, E.; Menasché, P.; Trouvin, J.-H.; Badimón, L.; Ioannidis, J.P.A.; Wu, J.C.; Hill, J.A.; Koch, W.J.; De Felice, A.F.; de Waele, P.; et al. Guidelines for Translational Research in Heart Failure. J. Cardiovasc. Trans. Res. 2015, 8, 3–22. [Google Scholar] [CrossRef]
- Zhang, B.; Jin, H.; Duan, X. Physical education movement and comprehensive health quality intervention under the background of artificial intelligence. Front. Public Health 2022, 10, 947731. [Google Scholar] [CrossRef]
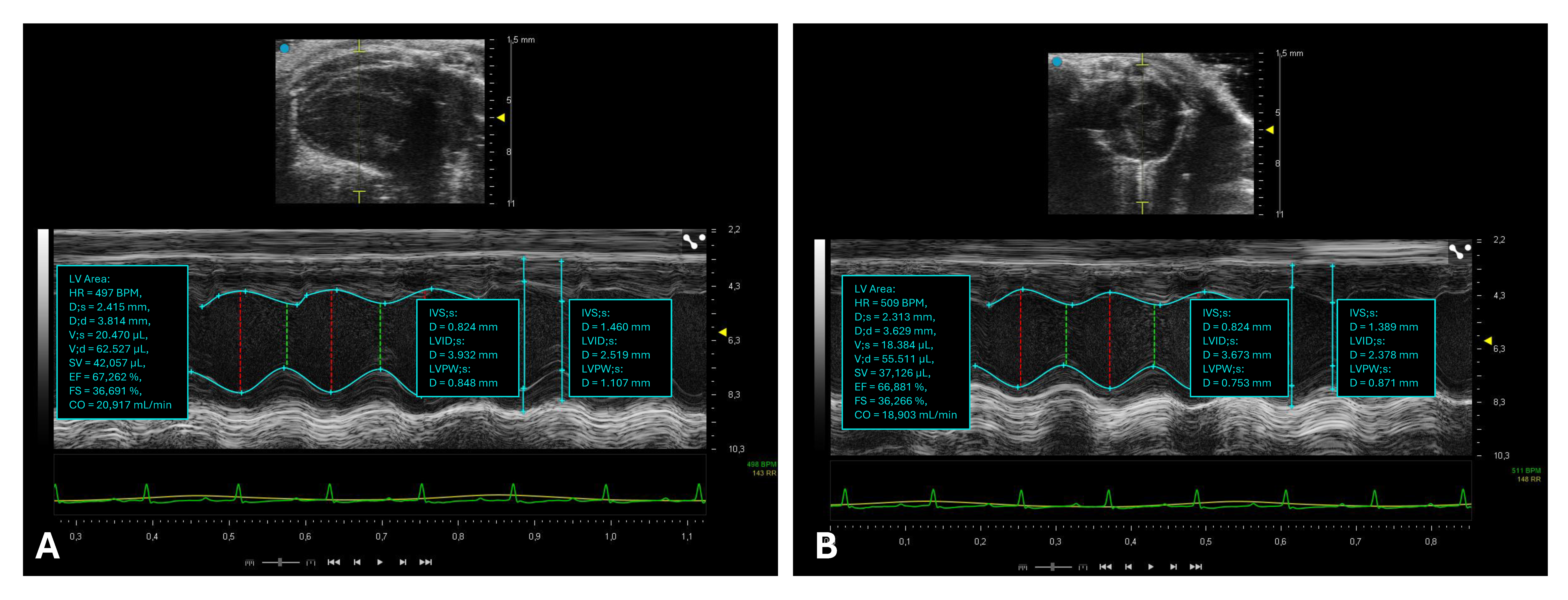



| Echocardiographic Parameters | Adult C57BL/6 | |
|---|---|---|
| Morphology [6] | IVSd (mm) | 0.71 ± 0.15 |
| LVIDd (mm) | 3.69 ± 0.41 | |
| LVPWd (mm) | 0.79 ± 0.22 | |
| IVSs (mm) | 0.97 ± 0.19 | |
| LVIDs (mm) | 2.20 ± 0.50 | |
| LVPWs (mm) | 1.12 ± 0.33 | |
| Systolic function [6] | EF (%) | 71 ± 11 |
| FS (%) | 43 ± 9 | |
| Diastolic function [6] | E (mm/s) | 718 ± 109 |
| A (mm/s) | 455 ± 105 | |
| E slope (mm/s) | 19.87 ± 1.67 | |
| E/A | 1.52 ± 0.40 | |
| e′ (mm/s) | 43.2 ± 10.9 | |
| E/e′ | 15.2 ± 6.7 | |
| Strain [7] | GLS (%) | −22 |
| GRS (%) | 35 | |
| GCS (%) | −30 | |
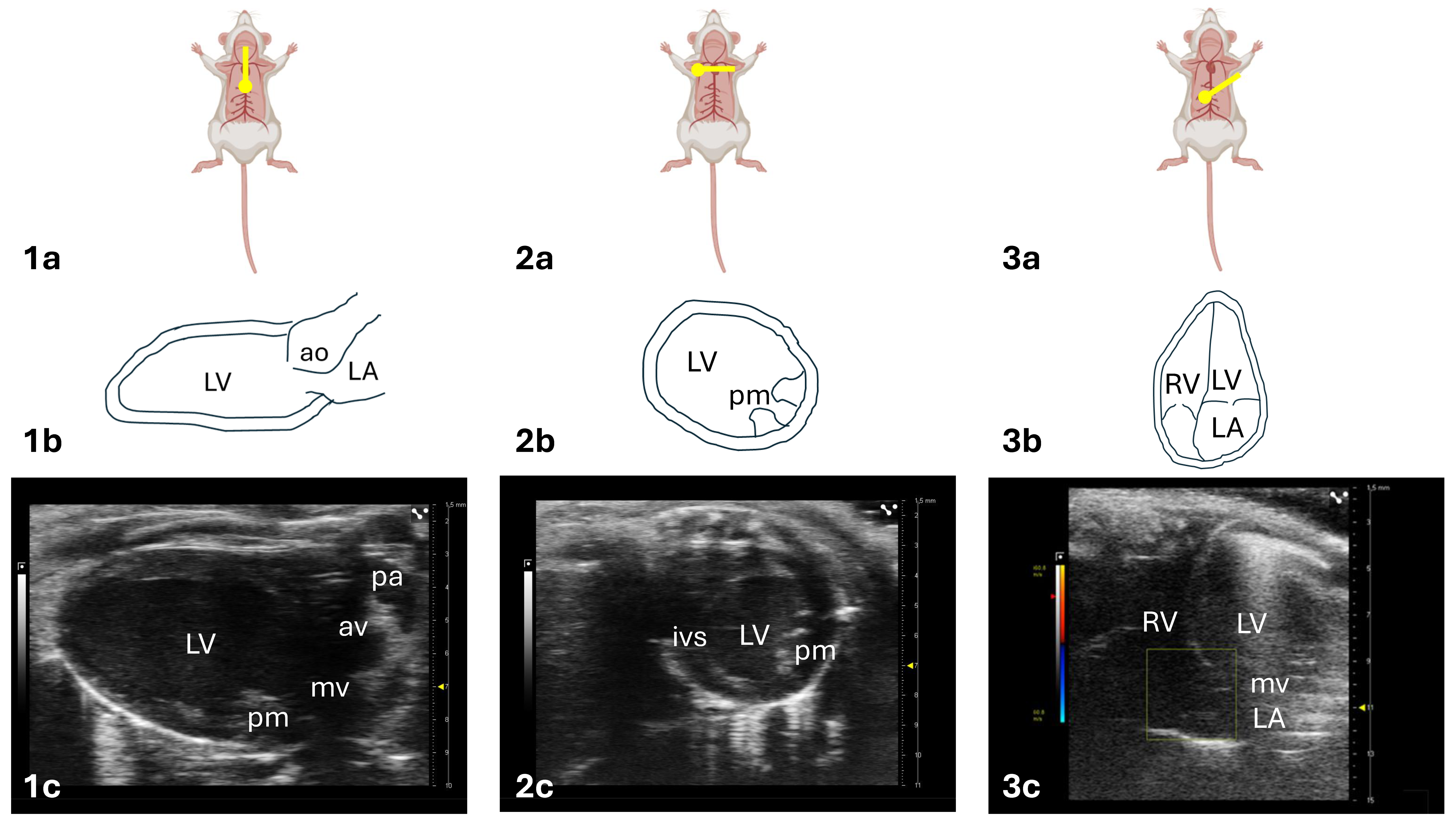
| View | Modality | Anatomy | Measurement ± Explanayory Note |
|---|---|---|---|
PLAX [16]
| 2D [2] | LV outflow tract, MV Ao, LV, and LA | LV assessment:
|
| MM [13] | LV | Morphology:
| |
| STE [17] | LV | GLS:
| |
PSAX [16]
| 2D [2] | LV and RV | LV assessment:
|
| MM [13] | LV | Morphology:
| |
| STE [17] | LV | GRS:
| |
| STE [17] | LV | GCS:
| |
A4C [16]
| 2D [2] CFM [18] | Full heart sweep (LA, LV, MV, RA, RV, TV) | Full heart visualization:
|
| PWD [19] | MV | Diastolic function:
| |
| TDI [20] | MV | Diastolic function:
|
| Mouse Model | Mechanism | Model | Main Features | Echocardiographic Assessment |
|---|---|---|---|---|
| Non-ischemic dilated cardiomyopathy | Drug-induced toxic cardiomyopathy | Single high-dose doxorubicin (15–20 mg/kg) [50] | Acute cardiotoxic action (5 days) through the formation of reactive oxygen species and mitochondrial damage by DNA breakage due to the doxorubicin–topoisomerase 2β complex:
| Acute dilated cardiomyopathy + reduced sistolic function + diastolic dysfunction:
|
| Multiple low-dose doxorubicin (4–5 mg/kg for 5 weeks) [51] | Chronic cardiotoxic action (4–8 weeks) through the formation of reactive oxygen species and mitochondrial damage by DNA breakage due to the doxorubicin–topoisomerase 2β complex:
| Chronic dilated cardiomyopathy + reduced sistolic function + dyastolic disfunction:
| ||
| Isoproterenol(400 mg/kg) [52] | Stress-induced cardiomyopathy due to excessive adrenergic stimulation:
| Acute and reversible dilated cardiomyopathy + reduced sistolic function + dyastolic disfunction:
| ||
| Isoproterenol(200 mg/kg) + 5-fluorouracile (15 mg/kg/day) [53] | Dilated cardiomyopathy resulting from acute Isoproterenol exposure followed by 5-fluorouracil administration (anti-mitotic agent 5-F):
| Chronic dilated cardiomyopathy + reduced sistolic function:
| ||
| Ethanol [54] | Direct cardiotoxicity of ethanol and its metabolites, oxidative stress, and accumulation of fatty acid ethyl esters:
| Chronic dilated cardiomyopathy + reduced sistolic function:
| ||
| Homocistein [55] | Chelates copper and impairs copper-dependent enzymes:
| Chronic dilated cardiomyopathy + left ventricular hypertrophy + reduced sistolic function:
| ||
| Genetically induced | D230NcTm [56] | Reduced Ca2+ sensitivity of troponin C in the sarcomere, resulting in reduced cardiac contractility | Dilated cardiomyopathy + reduced sistolic function + dyastolic disfunction:
| |
| D73NcTnC [57] | Reduced Ca2+ sensitivity of troponin C in the sarcomere, resulting in reduced cardiac contractility | |||
| I61QcTnC [58] | Reduced Ca2+ sensitivity of troponin C in the sarcomere, resulting in reduced cardiac contractility |
| Mouse Model | Mechanism | Model | Main Features | Echocardiographic Assessment |
|---|---|---|---|---|
| Diabetic Cardiomyopathy (T1D) | Spontaneous autoimmune | NOD mice [82] | Infiltration and destruction of β cells by T cells (CD4 and CD8), NK cells, and B cells (insulitis):
| Preserved sistolic function + early dyastolic disfunction:
|
| Genetically induced | AKITA mice [83] | Mutation in insulin 2 gene (Ins2/Cys96Tyr) and overload of misfolded insulin (ER stess):
| ||
| Chemical induction | High-dose streptozocin (100–200 mg/kg) [84] | Rapid ablation of β cells (DNA damage):
| Ventricular dilation ± dyastolic disfunction ± sistolic disfunction:
| |
| Alloxan (50–200 mg/kg) [85] | Infiltration of β cells and formation of free radicals (redox cicle):
| |||
| Diabetic Cardiomyopathy (T2D) | Chemical induction | Multiple low-dose streptozocin (20–40 mg/kg for 5 days) [84] | Infiltration of β cells by macrophages and T and B cells and reduction in islet numbers:
| Preserved sistolic function + early dyastolic disfunction:
|
| Induced obesity | High-fat diet [86] | Normal chow exchanged with a high-fat diet (58% fat):
| Left ventricular hypertrophy + preserved sistolic function + early dyastolic disfunction:
| |
| Obese models | Lepob/ob mice [87] | Deficient in leptin:
| Left ventricular hypertrophy + preserved sistolic function + early dyastolic disfunction:
| |
| Leprdb/db mice [88] | Autosomal recessive mutation in the leptin receptor:
|
| Technique | Advantages | Disadvantages |
|---|---|---|
| Echocardiography [155,157,158] |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salerno, N.; Di Costanzo, A.; Marino, F.; Scalise, M.; Leo, I.; Sabatino, J.; Canino, G.; Leccia, A.; De Angelis, A.; Urbanek, K.; et al. Echocardiographic Assessment of Cardiac Function in Mouse Models of Heart Disease. Int. J. Mol. Sci. 2025, 26, 5995. https://doi.org/10.3390/ijms26135995
Salerno N, Di Costanzo A, Marino F, Scalise M, Leo I, Sabatino J, Canino G, Leccia A, De Angelis A, Urbanek K, et al. Echocardiographic Assessment of Cardiac Function in Mouse Models of Heart Disease. International Journal of Molecular Sciences. 2025; 26(13):5995. https://doi.org/10.3390/ijms26135995
Chicago/Turabian StyleSalerno, Nadia, Assunta Di Costanzo, Fabiola Marino, Mariangela Scalise, Isabella Leo, Jolanda Sabatino, Giovanni Canino, Antonio Leccia, Antonella De Angelis, Konrad Urbanek, and et al. 2025. "Echocardiographic Assessment of Cardiac Function in Mouse Models of Heart Disease" International Journal of Molecular Sciences 26, no. 13: 5995. https://doi.org/10.3390/ijms26135995
APA StyleSalerno, N., Di Costanzo, A., Marino, F., Scalise, M., Leo, I., Sabatino, J., Canino, G., Leccia, A., De Angelis, A., Urbanek, K., Torella, D., & Cianflone, E. (2025). Echocardiographic Assessment of Cardiac Function in Mouse Models of Heart Disease. International Journal of Molecular Sciences, 26(13), 5995. https://doi.org/10.3390/ijms26135995






