Abstract
Wild fruits are distributed worldwide, but are consumed mainly in developing countries, where they are an important part of the diet. Still, in many other countries, they are consumed only locally. Blackthorn (Prunus spinosa L.) is an underutilized species rich in fibres and phenolic compounds, making it suitable as a potential functional food for supporting human health. Cold (Cw) and hot (Hw) water-extracted (poly)phenolic polysaccharide–protein complexes, differing in carbohydrate, phenolic and protein contents, were isolated from blackthorn fruits and characterized. The complexes exhibited molecular weights of 235,200 g/mol (Cw) and 218,400 g/mol (Hw), and were rich in pectic polymers containing galacturonic acid, arabinose, galactose and rhamnose, indicating a dominance of homogalacturonan (HG) [→4)-α-D-GalA(1→4)-α-D-GalA(1→]n and a low content of RGI [→2)-α-L-Rha(1→4)-α-D-GalA(1→2)-α-L-Rha(1→]n sequences associated with arabinan or arabinogalactan. Minor content of glucan, probably starch-derived, was also solubilized. Pectic polysaccharides were highly esterified and partly acetylated. Pharmacological testing was performed in male Dunkin–Hartley guinea pigs, a model with human-like airway reflexes. Both complexes affected airway defense mechanisms. Particularly, Hw significantly suppressed citric acid-induced cough, similar to codeine, and reduced bronchoconstriction comparably to salbutamol in a dose-dependent manner. These findings support further exploration of Hw as a natural antitussive and bronchodilatory agent.
1. Introduction
The blackthorn (Prunus spinosa L.) is a spiny deciduous shrub that produces small purple edible plums. This species is mainly found from southern central Europe to southern Scandinavia and east to Asia Minor, growing on forest edges and open woodlands as part of Mediterranean thermophilic plant communities. It is mostly wild-growing or cultivated as an ornamental plant for its fruits and is used to make jams, wine, vinegar and spirits [1]. P. spinosa has several notable medicinal applications due to its rich composition of bioactive compounds found in its flowers, fruits and leaves. The flowers are recommended as a complementary treatment for diseases related to oxidative stress, and they exhibit antioxidant activity against the primary in vivo relevant reactive oxygen species. Their extracts are a rich source of polyphenols, especially flavonoids and phenolic acids [2,3], which are considered bioactive substances with potent anticancer effects [4].
Similarly, their fruits are a rich source of flavonoids, anthocyanins, phenolic acids, glycoconjugates, vitamins, minerals and organic acids, which exhibit significant antioxidant and antibacterial properties [5]. Flavonoids such as catechin, epicatechin and rutin have been reported to have protective effects against diabetes, while other flavonoids, including myricetin, quercetin and kaempferol, exhibit antihypertensive activity [6].
Extracts from leaves have antioxidant properties and show antibacterial effects, especially against Bacillus cereus and Escherichia cloacae [7]. The extracts also show inhibitory activity against enzymes associated with type II diabetes mellitus [8] and antitumor effects on malignant cell lines [9]. The main compounds identified in the P. spinosa leaf and flower extract mixture include chlorogenic acid and ellagic acid, contributing to its antioxidant capacity [7,9].
Further, P. spinosa has been used in traditional medicine for various purposes, including the treatment of gastric ulcers. Ethanol extracts of fruits have shown antioxidant, anti-inflammatory and wound-healing properties in a rat model of indomethacin-induced gastric ulcers [10]. Extracts of flowers are traditionally indicated for the treatment of urinary tract disorders, inflammation and cardiovascular diseases. They have been found to have antioxidant activity, potential anti-inflammatory effects, and cellular safety [11].
Given the existing data in the literature on anti-inflammatory effects associated with various components of P. spinosa fruits, we focused on investigating the influence of phenolic polysaccharide–protein complexes on airway defense reflexes, such as cough and bronchoconstriction. We hypothesized that the fruit extracts, rich in bioactive compounds, would suppress citric acid-induced cough and reduce bronchoconstriction. These reflexes are commonly associated with oxidative stress and inflammation, suggesting a possible interplay with the antioxidant properties of P. spinosa fruit. Guinea pigs were selected for the in vivo experiments due to their well-characterized cough reflex and airway responsiveness, which closely resemble those of humans, making them a relevant model for translational respiratory research.
2. Results and Discussion
2.1. Plant Material and Characterization of (Poly)Phenolic Polysaccharide–Protein Complexes
(Poly)phenolic polysaccharide–protein complexes were isolated from the cell walls of ripe P. spinosa L. fruits by sequential extraction with cold (Cw) and hot (Hw) water in a yield of 1.0 wt% and 3.8 wt%, respectively (Table 1). Both water fractions showed relatively broad molecular weight distribution patterns but differed in the contents of carbohydrates, protein, phenolics and uronic acid. The Hw fraction was richer in proteins and phenolics and had a significantly higher yield. Both blackthorn fractions were rich in uronic acids (~34 wt% (Cw) and 39 wt% (Hw)), while the content of rhamnose residues in fractions was only about 4 wt%. This fact indicates the dominance of pectic polysaccharide–homogalacturonan in these water-extracted fractions. In addition, the second dominant polymers were arabinan or arabinogalactan, which represented 17 wt% of the Cw fraction and about 20 wt% of the Hw fraction. Low levels of glucans, probably derived from starch, were also solubilized in both fractions [5].

Table 1.
Compositional analyses of cold (Cw) and hot water (Hw) fractions of wild blackthorn fruits.
2.2. NMR of the (Poly)Phenolic Polysaccharide–Protein Complex Hw
The HSQC spectra of both fractions were similar, indicating identical pectin material extracted with cold and hot water. Differences were observed mainly in the yield of the fractions, with the hot water yield being four times higher than that with cold water [5]. The HSQC spectrum (selected regions—Figure 1, and full spectrum—Figure S2 in the Supplementary Data) of the blackthorn hot water extract (Hw) showed characteristic signals of homogalacturonan (HG), rhamnogalacturonan (RG-I), and arabinogalactan (AG), which are characteristic components of pectin [12,13]. In the α-anomeric region, the most intense broad H1/C1 signals at δH1/C1 5.135/102.09, 5.039/102.97, 4.969/102.98 ppm were assigned to 1,4-linked α-D-GalpA residues and its methyl ester present in different environments of galacturonan. The characteristic H5/C5 signal at δH5/C5 5.090/73.38 ppm and the methyl group signal at δH/C 3.819/55.67 ppm confirmed the esterification of α-D-GalpA residues. The other H5/C5 signal at δH5/C5 4.676/74.35 ppm originated from unesterified α-D-GalpA residues. The ratio of esterified to unesterified residues 72:28 indicated a higher degree of esterification. The observed H4/C4 signals at δH4/C4 4.464/81.54, 4.408/81.52 ppm derive from 1,4-linked esterified and unesterified α-D-GalpA, respectively. The remaining skeletal H2/C2 and H3/C3 signals of both esterified and unesterified α-D-GalpA were found at δH2/C2 3.740/70.86, and δH3/C3 3.998/71.0 ppm. In addition, the less intense α-anomeric signal at δH1/C1 5.286/101.20 ppm and skeletal signals at δH2/C2 4.131/79.38 and δH6/C6 1.257/19.48 ppm were attributed to 1,2-linked α-L-Rhap residue, as constituents of rhamnogalacturonan. The presence of acetylated sugar residue is a feature of pectin. In the region of ~ δH 2.227–2.096 ppm, four CH3 signals of the acetyl group were detected, with the dominant one at δH/C 2.096/23.04 ppm, thereby revealing the presence of acetylated α-D-GalpA residue at position O3 [14]. Further, in the α-anomeric region, a group of signals (~ δC1 109–112 ppm), belonging to α-L-Araf residues were observed. We were able to identify 1,5-linked (δH1/C1 5.090/110.40 ppm), 1,3,5-linked (δH1/C1 5.115/110.38 ppm), and four terminally linked (δH1/C1 5.158/109.97, 5.184/109.91, 5.244/109.27, 5.257/112.0 ppm) α-L-Araf residues, suggesting the presence of highly branched arabinan/arabinogalactan, as a side chain of galacturonan. In the skeletal region, characteristic C2-C4 signals of Ara residues in furano-forms were present at ~ δC2–C4 79.5–88 ppm. The 1,5-linkage of α-L-Araf residue was confirmed by its downfield-shifted C5 atom (δC5 69.59 ppm) with respect to the terminally linked Araf residues (δC5 64.05 ppm). Finally, in the β-anomeric region, signals at δH1/C1 4.630/107.14, δH1/C1 4.465/106.18, and δH1/C1 4.512/105.53 ppm were attributed to 1,4-linked and terminally linked β-D-Galp and 1,4-linked β-D-GlcpA residues, respectively, which together with the terminally linked α-L-Rhap residue (δH1/C1 4.753/103.01 ppm) are components of arabinogalactan.
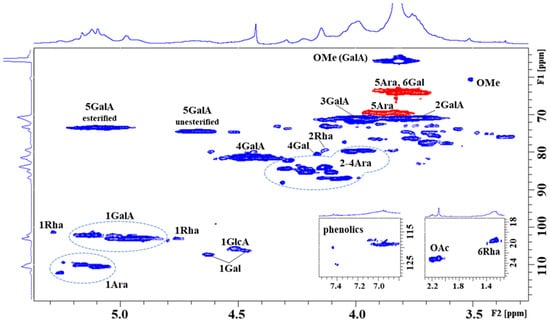
Figure 1.
Selected regions of 1H-13C hetero-correlated HSQC spectra (CH—blue colour; CH2—red colour) of the hot water fraction (Hw) of blackthorn fruits.
2.3. The Evaluation of Cw and Hw Impact on the Defence Reflexes of the Airways
2.3.1. The Antitussive Effect of Cw and Hw Complexes
The pharmacodynamic aspect of this study focused on evaluating the effects of P. spinosa-originated cold water-extracted (Cw) and hot water-extracted (Hw) glycoconjugates and codeine on the cough reflex, which was experimentally induced using CA aerosol inhalation. A guinea pig model was employed due to its well-defined cough reflex, which closely resembles that of humans, making it a widely used model in respiratory research [15]. The mechanism behind acid-induced coughing is largely attributed to the activation of vagal afferent C fibers containing tachykinins, which innervate the airways [16].
Cw and Hw were administered orally, Cw at the screening dose of 50 mg/kg and Hw at doses of 50, 75 and 100 mg/kg. Their antitussive potential was compared with codeine, a centrally acting opioid cough suppressant. The findings revealed a significant reduction in the number of cough efforts following both complexes’ administrations, with a more significant impact caused by the Hw (exhibiting a dose-dependent effect). As illustrated in Figure 2, all tested doses of Hw markedly suppressed cough efforts at 60 min (p < 0.05 or p < 0.01, respectively), with this significant effect persisting throughout the experiment (p < 0.01). Similarly, the reference drug codeine effectively reduced cough frequency during the CA aerosol challenge. Notably, as observed in Figure 2, the cough-suppressing properties of Hw were comparable to those of the reference opioid antitussive, codeine.
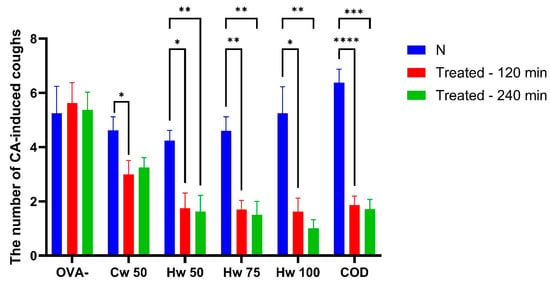
Figure 2.
The effect of orally administered P. spinosa complexes Cw (50 mg/kg bw) and Hw (50, 75 and 100 mg/kg bw), codeine phosphate (10 mg/kg bw, COD), and saline (1 mL/kg bw; OVA-) on the number of citric acid (CA)-induced cough efforts in guinea pigs was evaluated. The baseline measurement (N) was recorded before drug administration. Statistical significance was determined using a one-way ANOVA followed by the Bonferroni post hoc test, with * p < 0.05, ** p < 0.01, *** p < 0.001 and **** p < 0.0001 compared to baseline (N).
2.3.2. The Bronchodilatory Effect of Cw and Hw Complexes
Plethysmographic assessment of specific airway resistance (sRaw) is a well-established and validated technique for evaluating airway obstruction, requiring no active participation from the subject. As a result, it is considered a highly sensitive and appropriate method for fundamental research and clinical monitoring [17]. In this study, sRaw measurements revealed the ability of P. spinosa complexes Cw and Hw to significantly inhibit airway smooth muscle contraction induced by the mediator of allergy—histamine, methacholine—thus mimicking the effect of parasympathetic acetylcholine and CA, which triggered bronchoconstriction through the release of tachykinins (Figure 3, Figure 4 and Figure 5). These mechanisms play a role not only in normal airway smooth muscle responsiveness but also in airway hyperreactivity. For comparative analysis, the commonly used bronchodilator salbutamol, a beta-2 adrenergic receptor agonist, was tested under identical experimental conditions.
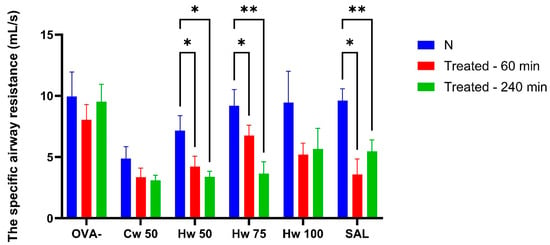
Figure 3.
Alterations in specific airway resistance (sRaw) adjusted for respiratory rate (RR) following AC exposure assessed at baseline and at 60 and 240 min post-administration of a cold water complex (Cw) of P.spinosa at a dose 50 mg/kg (Cw 50) and a hot water complex (Hw) at three different doses (Hw 50, Hw 75, Hw 100), salbutamol (SAL), and saline (OVA-). Statistical analysis was performed using a one-way ANOVA with Bonferroni’s post hoc test, with * p < 0.05 and ** p < 0.01 considered significant compared to baseline (N). The baseline measurement (N) was recorded before drug administration.
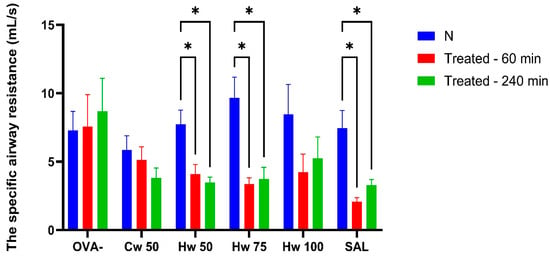
Figure 4.
The effect of Cw and Hw complexes and control drugs on sRaw values induced by histamine. Statistical significance was determined using a one-way ANOVA followed by the Bonferroni post hoc test, with * p < 0.05 compared to baseline (N).
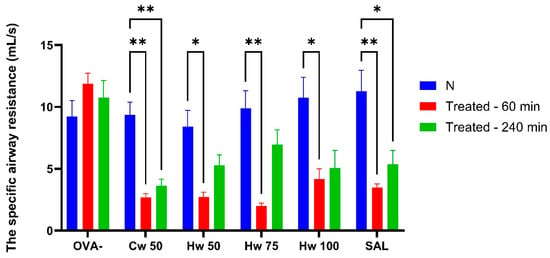
Figure 5.
The effect of glycoconjugates and control drugs on sRaw values induced by methacholine. Statistical significance was determined using a one-way ANOVA followed by the Bonferroni post hoc test, with * p < 0.05 and ** p < 0.01 compared to baseline (N).
Figure 3 illustrates that Hw significantly and dose-dependently lowered sRaw values that had been elevated due to short-term airway irritation caused by CA, and this effect was similar to that of the standard bronchodilator, salbutamol. The role of acidic mediators in the development of both acute and chronic airway inflammation is generally accepted [18], and reducing their impact on airway responsiveness is beneficial in conditions such as asthma and other allergic diseases. These results pointed to the potential benefit of the P. spinosa Hw complex.
The different effects of Cw and Hw on airway smooth muscle reactivity elicited by histamine are demonstrated in Figure 4. Cw showed no significant impact on histamine-induced bronchoconstriction, suggesting a lack of interaction with histaminergic pathways. In contrast, Hw exerted a pronounced bronchodilatory effect at doses of 50 mg/kg and 75 mg/kg body weight, with efficacy comparable to the control drug salbutamol. Since Hw is structurally distinct from salbutamol, it likely acts through mechanisms independent of beta-2 receptor activation, potentially offering therapeutic value in patients with allergic asthma refractory to beta-2 agonists as an adjunctive treatment, especially for those seeking natural therapeutic alternatives.
Methacholine mimics the effect of the natural parasympathetic mediator acetylcholine. However, methacholine is preferred over acetylcholine in airway research because it is more stable, selectively stimulates M3 muscarinic receptors, and produces a reproducible bronchoconstrictive response suitable for diagnostic and experimental use [19]. Unlike bronchoconstriction elicited by CA or histamine, the Cw complex demonstrated a significant inhibitory effect on methacholine-induced airway reactivity that lasted 240 min after its peroral administration. Additionally, the observation that the Hw significantly reduces methacholine-induced airway hyperresponsiveness (Figure 5) at all doses tested, achieving a level of bronchoprotection comparable to that of salbutamol, confirms a clinically relevant bronchodilatory effect of P. spinosa complexes and supports their potential therapeutic application in obstructive airway diseases [20].
Despite these promising findings, further studies are needed to evaluate the efficacy of the Hw complex in models of allergen-induced airway inflammation. Such investigations will be necessary to determine therapeutic value under conditions mimicking chronic respiratory diseases.
3. Materials and Methods
3.1. Chemicals
The citric acid (CA) p.a., histamine, methacholine, salbutamol, and codeine phosphate were obtained from Sigma-Aldrich Chemicals (Lambda Life, Bratislava, Slovakia) and before the experimental procedures were dissolved in 0.9% saline according to the manufacturer’s instructions.
3.2. Plant Material and Isolation of (Poly)Phenolic Polysaccharide–Protein Complexes
Ripe dark blue fruits of Prunus spinosa L. were harvested in the Kysuce region of Slovakia (Svrčinovec-Bahno district) in November 2015. Botanical identification of blackthorn (Prunus spinosa L.) was performed by Dr. Pavol Mereďa from Institute of Botany, Center for Plant Biology and Biodiversity, Slovak Academy of Sciences, Bratislava, Slovakia. Freshly harvested fruits of Prunus spinosa L. (~12.5 L) were pitted, blended, and juiced (3.6 kg). The fruit residues were further boiled in 96% ethanol (3 L, 30 min), washed thoroughly with ethanol, and after mixing with distilled water, lyophilized to obtain cell wall material (510 g) for next extraction steps.
The cell wall material (500 g) was stirred three times in distilled water (3 L) containing 0.02% sodium azide at room temperature for 20 h. The extracts were filtered, gradually concentrated, combined, and precipitated into 96% ethanol (1:5 v/v) containing 1% acetic acid. They were left overnight in a cold room, and precipitates were isolated by centrifugation, washed with ethanol, suspended in distilled water, dialyzed, and freeze-dried to obtain the cold water fraction designated Cw.
The residues after cold water extraction were further extracted three times in hot distilled water (70 °C, 3 L) for 1 h. The extracts were filtered, gradually concentrated, combined, and precipitated in 96% ethanol (1:5 v/v) containing 1% acetic acid. They were left overnight in a cold room, and precipitates were separated by centrifugation, washed with ethanol, suspended in distilled water, dialyzed, and lyophilized to obtain the hot water fraction designated Hw [5].
3.3. General Methods
The contents of carbohydrates, proteins, uronic acids and phenolic substances were determined in the fractions colorimetrically using phenol–sulfuric acid [21], Bradford reagent [22], m-hydroxydiphenyl reagent [23], and Folin–Ciocalteu assays [24].
The quantitative neutral sugar determinations of fractions Cw and Hw were carried out by gas chromatography after their hydrolyses with 2M TFA for 1 h at 120 °C in the form of their alditol acetates [25]. Samples were analysed on a TRACE Ultra Gas Chromatograph (Thermo Scientific, Waltham, MA, USA) equipped with a TG-SQC (Thermo Scientific, Waltham, MA, USA) capillary column (30 m × 0.25 mm × 0.2 µm) at a temperature program of 80 °C (4 min)—(8 °C/min)—160 °C (4 min)—(4 °C/min)—250 °C (20 min), and the flow rate of helium was 0.4 mL/min. The gas chromatograph was coupled with an ITQ 900 mass spectrometer (Thermo Scientific, Waltham, MA, USA) with EI ionization under standard 70 eV electron energy, an emission current of 25 μA, and an ion source temperature of 200 °C. Alditol acetates of monosaccharides Rha, Fuc, Rib, Ara, Xyl, Man, Gal, and Glc were used as standards.
Molecular weight distribution patterns of samples were obtained by SEC-HPLC using an Agilent Technologies 1260 Infinity (Agilent Technologies Inc., Santa Clara, CA, USA), RI and DAD detectors; a tandem of columns HEMA-BIO 1000 and HEMA-BIO 300 (8 mm × 250 mm); a mobile phase of 0.1M NaNO3 with a flow rate of 0.4 mL/min; and an injection volume of 25 μL). A set of dextran standards (Mw 5220; 25,500; 72,700; 158,100; 344,800 and 759,400 g/mol, American Polymer Standards Corporation (APSC, Mentor, OH, USA) were used for columns’ calibration. The average molecular weight (Mw) was calculated using Agilent Cirrus GPC software (version 1.2) (Agilent Technologies, Inc., Santa Clara, CA, USA).
NMR spectra of fractions were measured in D2O, at 60 °C on a Bruker AVANCE III HD 400 MHz spectrometer equipped with a broad-band BB-(H-F)-D-05-Z liquid N2 Prodigy probe (Bruker BioSpin, Germany). For 1H and 13C NMR spectra, chemical shifts were referenced to TSP-d4 (δ 0/0 ppm). For the assignment of signals, advanced techniques of 1D and 2D homo- and hetero-correlated spectroscopy from the Bruker pulse sequence library were applied.
3.4. Experimental Animals
This study utilized male Dunkin–Hartley guinea pigs (n = 56) with a body weight ranging from 250 to 300 g. The animals were sourced from a certified breeding facility, Velaz Ltd., in Prague, Czech Republic (CZ 21760118). They were housed in an accredited animal facility under controlled conditions (air-conditioned rooms maintained at 23 ± 2 °C, humidity levels between 50 and 60%, a 12 h light/dark cycle, and continuous access to water and standard laboratory chow), in compliance with Directive 2010/63/EU on the protection of animals used for scientific purposes [26]. Prior to the experiment, the guinea pigs underwent an acclimatization period to minimize stress responses. Animals were kept in groups and provided with paper bedding and shelters as environmental enrichment to support natural behaviour and reduce stress.
Specific inclusion criteria included good general health status and a clearly elicitable cough reflex prior to the experiment. Animals showing signs of ongoing illness or lacking a reliable cough reflex were excluded. These criteria were established a priori. The animals were randomly assigned to three control groups (two positive and one negative) and four experimental groups, with each group consisting of eight animals. The treatment groups were as follows.
- Negative control group OVA-, which were administered saline orally at a dose of 1 mL/kg body weight (bw).
- Positive control group COD, which received codeine phosphate orally at a dose of 10 mg/kg bw.
- Positive control group SAL, which were treated with inhaled salbutamol (5 min treatment with 4 µM solution).
- Experimental group Cw 50, which were administered Cw glycoconjugate at a dose of 50 mg/kg bw.
- Experimental group Hw 50, which were treated with Hw sample at a dose of 50 mg/kg bw.
- Experimental group Hw 75, which received Hw glycoconjugate at a dose of 75 mg/kg bw.
- Experimental group Hw 100, which were treated with Hw at a dose of 100 mg/kg bw.
All experimental procedures were approved by the local Ethics Committee (IRB00005636, Decision No. EK 40/2018) and conducted in compliance with Directive 2010/63/EU of the European Parliament and the Council and Slovak regulations on the ethical use of animals in research [26].
3.5. The Methodology for Evaluating the Effects of Cw and Hw on Airway Defence Reflexes
3.5.1. Assessment of Antitussive Effect
The methodology used to evaluate the cough reflex, experimentally induced by a chemical tussigen (0.3 M citric acid), has been extensively described in previous studies [27,28]. This method has also been adapted to assess the antitussive effects of plant-derived substances [29,30].
In brief, awake guinea pigs were placed in a double-chamber body plethysmograph designed for small laboratory animals (HSE type 855, Hugo Sachs Elektronik, March, Germany). The plethysmograph consists of separate nasal and thoracic chambers, divided by an elastic ring positioned around the animal’s neck. Changes in pressure recorded within each chamber, corresponding to respiratory and cough-related movements, were displayed as a flow curve.
A citric acid (CA) aerosol was generated using a PARI jet nebulizer (Paul Ritzau, Pari-Werk GmbH, Starnberg, Germany; output 5 L/s, mass median particle diameter 1.2 μm) and applied to the nasal chamber for three minutes. CA induces coughing by stimulating rapidly adapting receptors (RARs), TRPV-1, acid-sensing ion channels, and/or extrapulmonary cough receptors [31]. The number of coughs was recorded during inhalation.
Coughing events were identified based on characteristic changes in the flow curve, visible coughing movements, and the corresponding sound. Two independent trained observers monitored and confirmed each cough event. The number of coughs was assessed before administration of the tested substances and then re-evaluated at 120 and 240 minutes post-administration. The antitussive effects of Cw and Hw were compared to the standard antitussive drug, codeine.
3.5.2. Assessment of the Bronchodilatory Effect
The airway reactivity and bronchodilatory effects of the tested substances were evaluated by measuring changes in specific airway resistance (sRaw). These measurements were recorded before and after administration of the test substances at the same time intervals as the cough assessments.
The sRaw values were calculated using the PULMODYN Data Acquisition Software for Respiratory Studies version 2.0 (Harvard Apparatus, Holliston, MA, USA), based on the method described by Pennock et al. [32]. The calculation was derived from recorded airflow changes in both chambers of the body plethysmograph and the time delay between them.
During the experiment, conscious guinea pigs were exposed to aerosolized contractile mediators—histamine or methacholine (both at a concentration of 1 μM/L)—for 30 s. Following this exposure, sRaw values were recorded for one minute. A three-minute interval was maintained between the administration of the broncho-contracting agent and the sRaw measurement, during which fresh air was insufflated into the nasal chamber.
Baseline sRaw values were recorded before the application of any agent. Subsequent measurements, taken at 60 and 240 min post-administration, were used to assess the effects of Cw, Hw, and salbutamol on airway reactivity.
3.6. Statistical Analysis
All data are presented as mean ± standard deviation (SD). Statistical comparisons between experimental groups were performed using a one-way analysis of variance (ANOVA) followed by the Bonferroni’s post hoc test for multiple comparisons. A significance level of p < 0.05 was considered statistically significant. Normality of data distribution was assessed by descriptive statistics and graphical methods. As the data met the assumptions for parametric testing, no data transformations were required. Statistical analysis was conducted using GraphPad Prism version 8.0 (GraphPad Software, San Diego, CA, USA).
4. Conclusions
Underutilized wild blackthorn (Prunus spinose L.) berries, which are a significant source of fibers and phenolic compounds, could represent an alternative source of functional foods with a beneficial effect on human health. (Poly)phenolic polysaccharide–protein complexes were isolated from blackthorn berries by cold and hot water extraction. The complexes differed in their carbohydrate, phenolic, protein and uronic acid contents, while their molecular weights were similar. Both complexes were rich in acidic and highly esterified homogalacturonan, which is usually associated with arabinan or arabinogalactan, components of pectin material. Some galacturonic acids were partly acetylated at the O3 position. Studies of P. spinosa (poly)phenolic polysaccharide–protein complexes have provided insights into their composition and have also revealed previously unknown pharmacodynamic properties. One of the key findings of this research is the confirmation of previously undocumented pharmacodynamic effects of the hot water complex (Hw), particularly its ability to suppress cough (similar to the conventional centrally acting antitussive codeine) and to induce bronchodilation (similar to salbutamol). The observed reduction in cough and bronchoconstriction in an experimental guinea pig model suggests that this P. spinosa complex could be used as an adjunct in the symptomatic treatment of obstructive airway diseases, e.g., bronchial asthma or COPD. Despite the promising findings, further studies are needed to evaluate the efficacy of Hw complexes in models of allergen-induced airway inflammation. Such investigations will be necessary to determine their therapeutic value under conditions mimicking chronic respiratory diseases.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms26135993/s1.
Author Contributions
Conceptualization, Š.M., P.C. and I.U.; methodology, Š.M., P.C. and I.U.; formal analysis, M.M., J.M., P.C. and I.U.; investigation, Š.M., P.C. and I.U.; writing—original draft preparation, Š.M., P.C. and I.U.; writing—review and editing, M.M., J.M., P.C. and I.U.; supervision, Š.M. and P.C.; project administration, Š.M. and P.C.; funding acquisition, Š.M. and P.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Scientific Grant Agency (VEGA) of the Ministry of Education, Slovak Republic (Grant No. 2/0054/22), and the Slovak Research and Development Agency APVV (grant no. APVV-23-0261).
Institutional Review Board Statement
All experimental procedures were approved by the local Ethics Committee (IRB00005636, Decision No. EK 40/2018) and conducted in compliance with Directive 2010/63/EU of the European Parliament and the Council and Slovak regulations on the ethical use of animals in research.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ANOVA | One-way analysis of variance |
| Ara | Arabinose |
| AG | Arabinogalactan |
| ASM | Airway smooth muscle |
| BC | Butamirate citrate |
| CA | Citric acid |
| COD | Codeine |
| COPD | Chronic obstructive pulmonary disease |
| Cw | Cold water extract |
| D2O | Deuterium oxide |
| EI | Electron ionization |
| ERS | European Respiratory Society |
| Gal | Galactose |
| GC-MS | Gas chromatography–mass spectrometry |
| Glc | Glucose |
| He | Helium |
| HPLC | High-performance liquid chromatography |
| HG | Homogalacturonan |
| Hw | Hot water extract |
| HSQC | Heteronuclear single quantum coherence spectroscopy |
| i.p. | Intraperitoneally |
| Man | Mannose |
| Mw | Molecular weight |
| NaBH4 | Sodium borohydride |
| NaNO3 | Sodium nitrate |
| NMR | Nuclear magnetic resonance |
| p.o. | Perorally |
| Rha | Rhamnose |
| RG-I | Rhamnogalacturonan |
| RR | Respiratory rate |
| SAL | Salbutamol |
| SEC-HPLC | Size exclusion–high-performance liquid chromatography |
| sRaw | Specific airway resistance |
References
- Popescu, I.; Caudullo, G. Prunus spinosa in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publication Office of the European Union: Luxembourg, 2016; p. 145. [Google Scholar]
- Marchelak, A.; Owczarek, A.; Matczak, M.; Pawlak, A.; Kolodziejczyk-Czepas, J.; Nowak, P.; Olszewska, M.A. Bioactivity potential of Prunus spinosa L. flower extracts: Phytochemical profiling, cellular safety, pro-inflammatory enzymes inhibition and protective effects against oxidative stress in vitro. Front. Pharmacol. 2017, 8, 680. [Google Scholar] [CrossRef] [PubMed]
- Marchelak, A.; Kolodziejczyk-Czepas, J.; Wasielewska, P.; Nowak, P.; Olszewska, M.A. The effects of Prunus spinosa L. flower extracts, model polyphenols and phenolic metabolites on oxidative/nitrative modifications of human plasma components with particular emphasis on fibrinogen in vitro. Antioxidants 2021, 10, 581. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Kumar, A.; Kumar Singh, A.; Singh, H.; Thareja, S.; Kumar, P. A comprehensive review on pharmacognosy, phytochemistry and pharmacological activities of 8 potent species of southeast Asia. J. Tradit. Chin. Med. 2024, 44, 620–628. [Google Scholar] [CrossRef]
- Capek, P.; Košťálová, Z. Isolation, chemical characterization and antioxidant activity of Prunus spinosa L. fruit phenolic polysaccharide-proteins. Carbohydr. Res. 2022, 515, 108547. [Google Scholar] [CrossRef]
- Negrean, O.R.; Farcas, A.C.; Pop, O.L.; Socaci, S.A. Blackthorn-A valuable source of phenolic antioxidants with potential health benefits. Molecules 2023, 28, 3456. [Google Scholar] [CrossRef]
- Veličković, I.; Žižak, Ž.; Rajčević, N.; Ivanov, M.; Soković, M.; Marin, P.D.; Grujić, S. Prunus spinosa L. leaf extracts: Polyphenol profile and bioactivities. Not. Bot. Horti Agrobot. 2021, 49, 12137. [Google Scholar] [CrossRef]
- Temiz, M.A.; Okumus, E.; Yaman, T.; Keles, O.F. Mixture of leaf and flower extract of Prunus spinosa L. alleviates hyperglycemia and oxidative stress in streptozotocin-induced diabetic rats. S. Afr. J. Bot. 2021, 141, 145–151. [Google Scholar] [CrossRef]
- Murati, T.; Miletić, M.; Štefanko, A.; Landeka Jurčević, I.; Elez Garofulić, I.; Dragović-Uzelac, V.; Kmetič, I. Comparative assessment of Prunus spinosa L. flower extract in non-neoplastic hepatocytes and hepatoblastoma cells. S. Afr. J. Bot. 2019, 123, 36–42. [Google Scholar] [CrossRef]
- Cetin, N.; Menevse, E.; Ceylan, C.; Celik, Z.E.; Akdam, N.; Rama, S.T.; Buyukyildirim, T.; Pasayeva, L.; Tugay, O.; Gumus, M. Histopathological and biochemical evaluation of the protective efficacy of Prunus spinosa L. extract in a rat model of indomethacin-induced gastric ulcer. Iran. J. Basic Med. Sci. 2024, 27, 1464–1474. [Google Scholar] [CrossRef]
- Magiera, A.; Czerwińska, M.E.; Owczarek, A.; Marchelak, A.; Granica, S.; Olszewska, M.A. Polyphenol-enriched extracts of Prunus spinosa fruits: Anti-Inflammatory and antioxidant effects in human immune cells ex vivo in relation to phytochemical profile. Molecules 2022, 27, 1691. [Google Scholar] [CrossRef]
- Capek, P.; Uhliariková, I. Antioxidant active polysaccharides extracted with oxalate from wild blackthorn fruits (Prunus spinosa L.). Int. J. Mol. Sci. 2024, 25, 4519. [Google Scholar] [CrossRef] [PubMed]
- Golovchenko, V.V.; Khlopin, V.A.; Patova, O.A.; Vityazev, F.V.; Dmitrenok, A.S.; Shashkov, A.S. Structural characterization of arabinogalactan-II and pectin from Urtica cannabina. Carbohydr. Polym. 2025, 348, 122868. [Google Scholar] [CrossRef]
- Patova, O.A.; Smirnov, V.V.; Golovchenko, V.V.; Vityazev, F.V.; Shashkov, A.S.; Popov, S.V. Structural, rheological and antioxidant properties of pectins from Equisetum arvense L. and Equisetum sylvaticum L. Carbohydr. Polym. 2019, 209, 239–249. [Google Scholar] [CrossRef]
- Sterusky, M.; Plevkova, J.; Grendar, M.; Buday, T. Female guinea pig model for cough studies and its response to most common tussive substances. Physiol. Res. 2020, 69 (Suppl. 1), S171–S179. [Google Scholar] [CrossRef] [PubMed]
- Canning, B.J.; Chang, A.B.; Bolser, D.C.; Smith, J.A.; Mazzone, S.B.; McGarvey, L. CHEST Expert Cough Panel. Anatomy and neurophysiology of cough: CHEST Guideline and Expert Panel report. Chest 2014, 146, 1633–1648. [Google Scholar] [CrossRef]
- Adner, M.; Canning, B.J.; Meurs, H.; Ford, W.; Ramos Ramírez, P.; van den Berg, M.P.M.; Birrell, M.A.; Stoffels, E.; Lundblad, L.K.A.; Nilsson, G.P.; et al. Back to the future: Re-establishing guinea pig in vivo asthma models. Clin. Sci. 2020, 134, 1219–1242. [Google Scholar] [CrossRef] [PubMed]
- Monga, N.; Sethi, G.S.; Kondepudi, K.K.; Naura, A.S. Lipid mediators and asthma: Scope of therapeutics. Biochem. Pharmacol. 2020, 179, 113925. [Google Scholar] [CrossRef] [PubMed]
- Cockcroft, D.W.; Davis, B.E. Mechanisms of airway hyperresponsiveness. J. Allergy Clin. Immunol. 2006, 118, 551–559. [Google Scholar] [CrossRef]
- Marques, L.; Vale, N. Salbutamol in the management of asthma: A review. Int. J. Mol. Sci. 2022, 23, 14207. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Sedmak, J.J.; Grossberg, S.E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal. Biochem. 1977, 79, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Englyst, H.N.; Cummings, J.H. Simplified method for the measurement of total non-starch polysaccharides by gas-liquid chromatography of constituent sugars as alditol acetates. Analyst 1984, 109, 937–942. [Google Scholar] [CrossRef]
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Off. J. Eur. Union 2010, L276, 33–79. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32010L0063 (accessed on 21 June 2025).
- Sutovska, M.; Nosalova, G.; Franova, S. The role of potassium ion channels in cough and other reflexes of the airways. J. Physiol. Pharmacol. 2007, 58 Pt 2 (Suppl. 5), 673–683. [Google Scholar]
- Kocmalova, M.; Oravec, M.; Adamkov, M.; Sadlonova, V.; Kazimierova, I.; Medvedova, I.; Joskova, M.; Franova, S.; Sutovska, M. Potassium ion channels and allergic asthma. Adv. Exp. Med. Biol. 2015, 838, 35–45. [Google Scholar] [CrossRef]
- Šutovská, M.; Capek, P.; Kazimierová, I.; Pappová, L.; Jošková, M.; Matulová, M.; Fraňová, S.; Pawlaczyk, I.; Gancarz, R. Echinacea complex-chemical view and anti-asthmatic profile. J. Ethnopharmacol. 2015, 175, 163–171. [Google Scholar] [CrossRef]
- Barboríková, J.; Šutovská, M.; Kazimierová, I.; Jošková, M.; Fraňová, S.; Kopecký, J.; Capek, P. Extracellular polysaccharide produced by Chlorella vulgaris—Chemical characterization and anti-asthmatic profile. Int. J. Biol. Macromol. 2019, 135, 1–11. [Google Scholar] [CrossRef]
- Turcotte, S.E.; Lougheed, M.D. Cough in asthma. Curr. Opin. Pharmacol. 2011, 11, 231–237. [Google Scholar] [CrossRef]
- Pennock, B.E.; Cox, C.P.; Rogers, R.M.; Cain, W.A.; Wells, J.H. A noninvasive technique for measurement of changes in specific airway resistance. J. Appl. Physiol. 1979, 46, 399–406. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).