Profiles of Monocyte Subsets and Fibrosis-Related Genes in Patients with Muscular Dystrophy Undergoing Intermittent Prednisone Therapy
Abstract
1. Introduction
2. Results
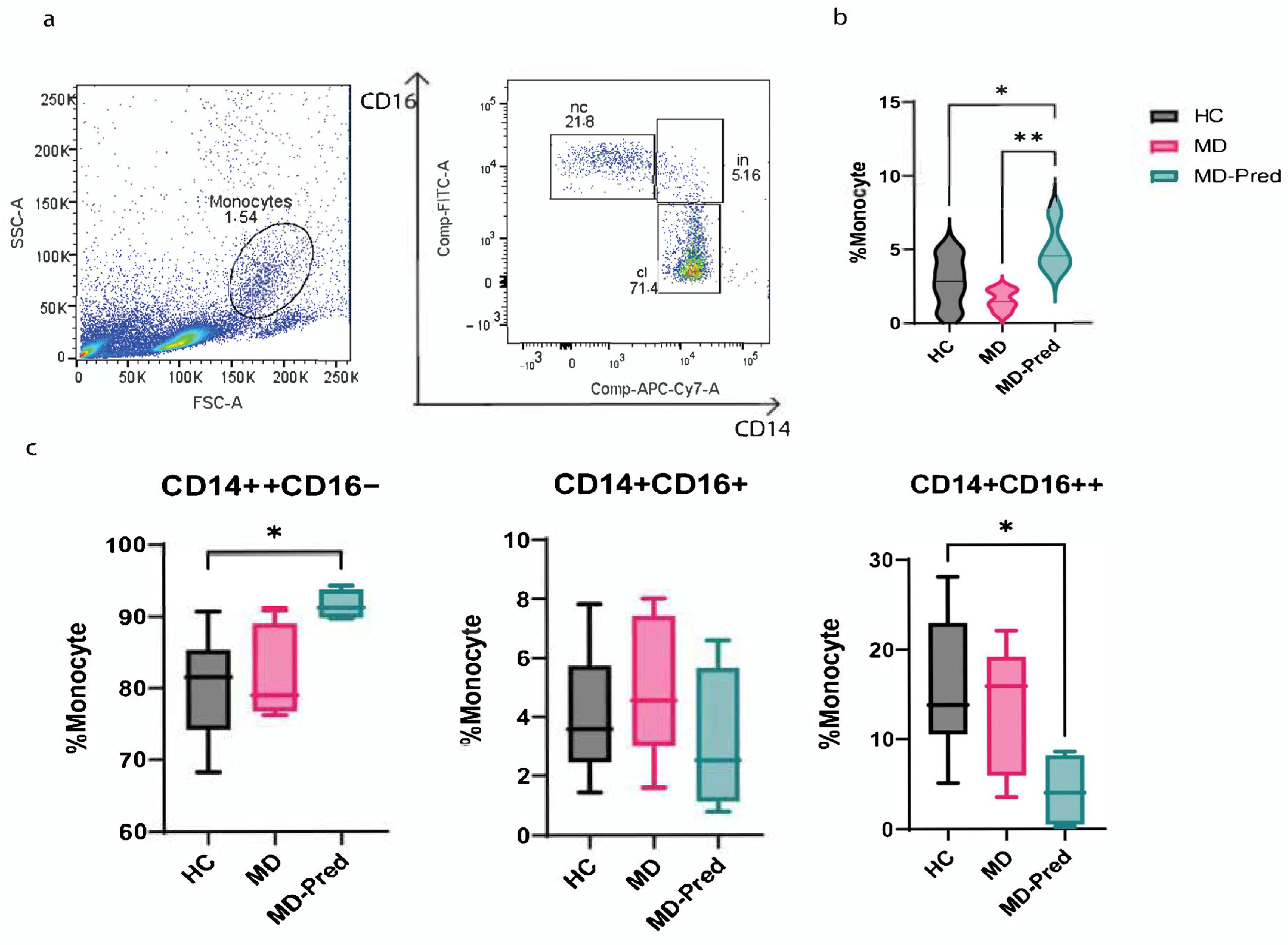
2.1. Intermittent Prednisone Induces an Alterations in Monocyte Subsets in Muscle Dystrophies
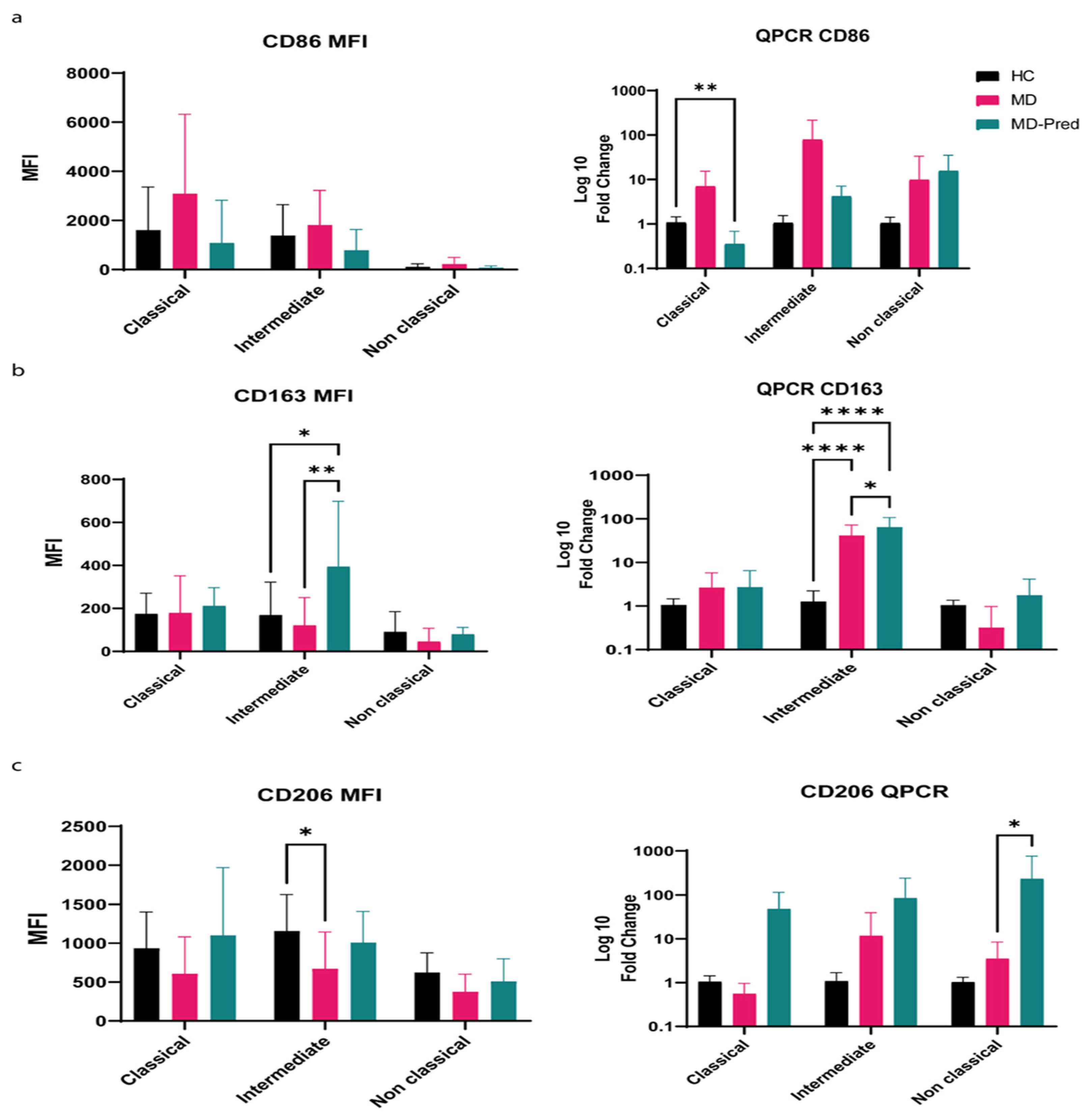
2.2. Expression of Surface Receptors CD86, CD206, and CD163 in Monocyte Subsets Shifts Toward an M2-like Phenotype After Intermittent Prednisone Treatment
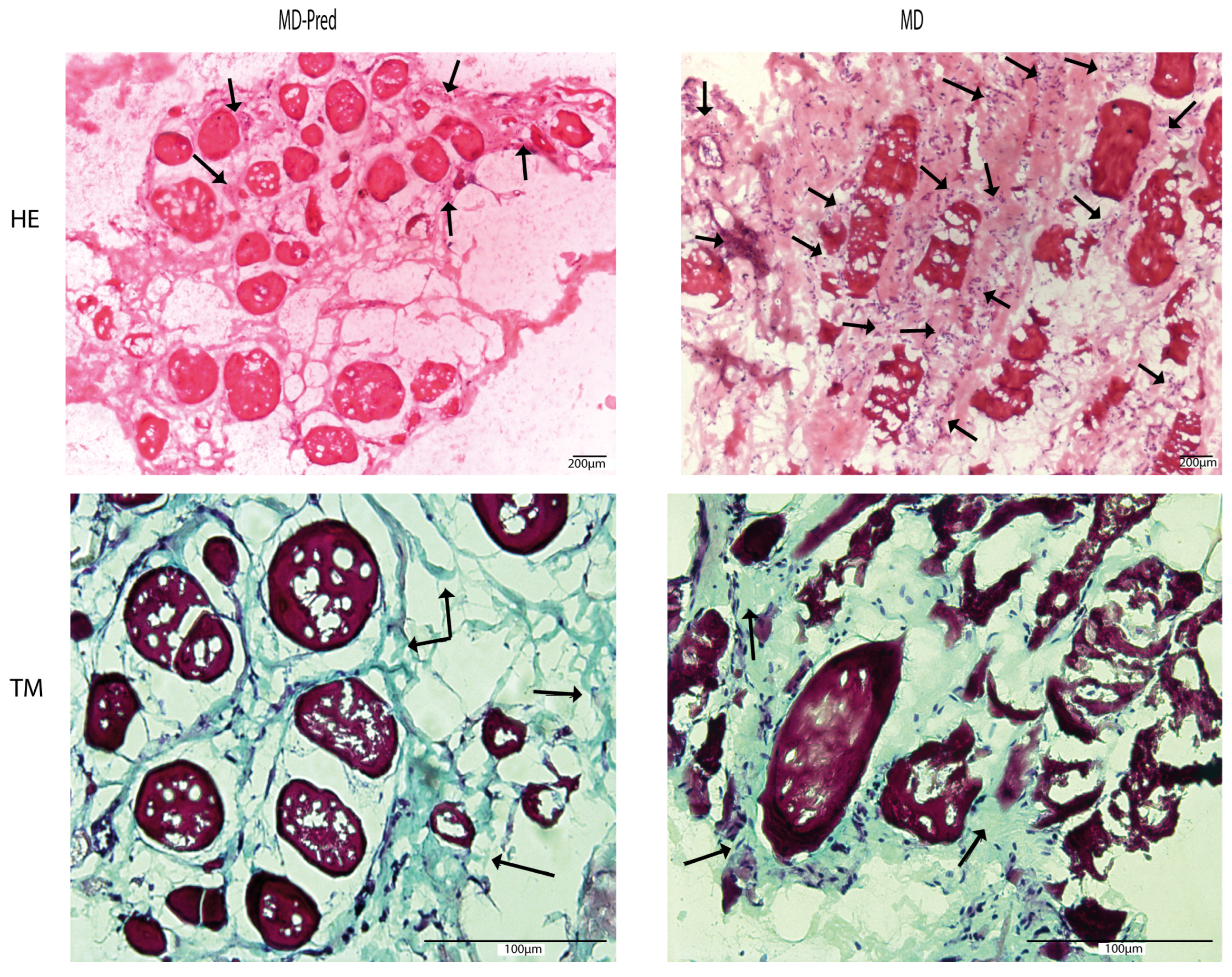
2.3. Assessment of Intermittent Prednisone Treatment Effects on Muscle Biopsies in Patients with Muscular Dystrophy
2.4. Intermittent Prednisone Exposure Induces Dynamic Changes in Gene Expression in Muscle Biopsies
3. Discussion
3.1. Overview of Prednisone Regimens in Muscle Dystrophies
3.2. Intermittent Prednisone Induces Changes in Monocyte Subset Phenotypes
3.3. Intermittent Prednisone Does Not Reduce Fibrosis in MD Muscles in Some Cases
3.4. Prednisone Affects Fibrosis-Related Gene Expression: The Interesting Role of CEBPB
4. Materials and Methods
4.1. Clinical and Genetic Data of Participants
4.2. Preparation of PBMC
4.3. Monocyte Subset Flow Cytometry Analysis
4.4. FACS Sorting of Monocyte
4.5. QPCR Analysis of Sorted Monocytes
4.6. Human Muscle Biopsies
4.7. Hematoxylin and Eosin and Masson’s Trichrome Staining of Muscle Sections
4.8. RNA Extraction from Muscle Biopsies and Related cDNA Synthesis
4.9. Analysis of Fibrosis-Related Genes by RT2 Profiler
4.10. PPI and Public Immunological Gene Expression Data
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DMD | Duchenne muscle dystrophy; |
| MDs | Muscle dystrophies; |
| Pred | Prednisone; |
| GC | Glucocorticoids; |
| LGMD | Limb–girdle muscular dystrophy; |
| MFI | Mean fluorescence intensity. |
References
- Mezzi, N.; Messaoud, O.; Mkaouar, R.; Zitouna, N.; Romdhane, S.; Abdessalem, G.; Charfeddine, C.; Maazoul, F.; Ouerteni, I.; Hamdi, Y. Spectrum of genetic diseases in Tunisia: Current situation and main milestones achieved. Genes 2021, 12, 1820. [Google Scholar] [CrossRef] [PubMed]
- Ben Hamida, M.; Fardeau, M.; Attia, N. Severe childhood muscular dystrophy affecting both sexes and frequent in Tunisia. Muscle Nerve 1983, 6, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Ausems, C.R.M.; van Engelen, B.G.; van Bokhoven, H.; Wansink, D.G. Systemic cell therapy for muscular dystrophies: The ultimate transplantable muscle progenitor cell and current challenges for clinical efficacy. Stem Cell Rev. Rep. 2021, 17, 878–899. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Goemans, N.; Takeda, S.; Mercuri, E.; Aartsma-Rus, A. Duchenne muscular dystrophy. Nat. Rev. Dis. Primers 2021, 7, 13. [Google Scholar] [CrossRef]
- Crisafulli, S.; Sultana, J.; Fontana, A.; Salvo, F.; Messina, S.; Trifirò, G. Global epidemiology of Duchenne muscular dystrophy: An updated systematic review and meta-analysis. Orphanet J. Rare Dis. 2020, 15, 141. [Google Scholar] [CrossRef]
- Kirschner, J.; Lochmüller, H. Sarcoglycanopathies. Handb. Clin. Neurol. 2011, 101, 41–46. [Google Scholar]
- Gibertini, S.; Zanotti, S.; Savadori, P.; Curcio, M.; Saredi, S.; Salerno, F.; Andreetta, F.; Bernasconi, P.; Mantegazza, R.; Mora, M. Fibrosis and inflammation are greater in muscles of beta-sarcoglycan-null mouse than mdx mouse. Cell Tissue Res. 2014, 356, 427–443. [Google Scholar] [CrossRef]
- Hartigan-O’Connor, D.; Kirk, C.J.; Crawford, R.; Mulé, J.J.; Chamberlain, J.S. Immune evasion by muscle-specific gene expression in dystrophic muscle. Mol. Ther. 2001, 4, 525–533. [Google Scholar] [CrossRef]
- Mendell, J.R.; Moxley, R.; Griggs, R.; Brooke, M.; Fenichel, G.; Miller, J.; King, W.; Signore, L.; Pandya, S.; Florence, J. Randomized, double-blind six-month trial of prednisone in Duchenne’s muscular dystrophy. N. Engl. J. Med. 1989, 320, 1592–1597. [Google Scholar] [CrossRef]
- Fenichel, G.; Florence, J.; Pestronk, A.; Mendell, J.; Moxley, R.T., III; Griggs, R.; Brooke, M.; Miller, J.; Robison, J.; King, W. Long-term benefit from prednisone therapy in Duchenne muscular dystrophy. Neurology 1991, 41, 1874. [Google Scholar] [CrossRef]
- Tidball, J.G.; Dorshkind, K.; Wehling-Henricks, M. Shared signaling systems in myeloid cell-mediated muscle regeneration. Development 2014, 141, 1184–1196. [Google Scholar] [CrossRef] [PubMed]
- Willis, A.B.; Zelikovich, A.S.; Sufit, R.; Ajroud-Driss, S.; Vandenborne, K.; Demonbreun, A.R.; Batra, A.; Walter, G.A.; McNally, E.M. Serum protein and imaging biomarkers after intermittent steroid treatment in muscular dystrophy. Sci. Rep. 2024, 14, 28745. [Google Scholar] [CrossRef] [PubMed]
- Kinali, M.; Mercuri, E.; Main, M.; Muntoni, F.; Dubowitz, V. An effective, low-dosage, intermittent schedule of prednisolone in the long-term treatment of early cases of Duchenne dystrophy. Neuromuscul. Disord. 2002, 12, S169–S174. [Google Scholar] [CrossRef] [PubMed]
- Straub, V.; Bushby, K. Therapeutic possibilities in the autosomal recessive limb-girdle muscular dystrophies. Neurotherapeutics 2008, 5, 619–626. [Google Scholar] [CrossRef]
- Chu, M.L.; Moran, E. The limb–girdle muscular dystrophies: Is treatment on the horizon? Neurotherapeutics 2018, 15, 849–862. [Google Scholar] [CrossRef]
- Sonan-Douayoua, T.; Akani, F.; Assi, B.; Cowppli-Bony, P.; Aka-Diarra, E.; Doumbia-Ouattara, M.; Kouame-Assouan, A.-E.; Félix, B.Y.; Kouassi, E.B. Case report/cas clinique myopathie maghrebine due a une sarcoglycanopathie myopathy from magreb due to a sarcoglycanopathy. Afr. J. Neurol. Sci. 2007, 26, 1. [Google Scholar]
- Hussein, M.R.; Hamed, S.A.; Mostafa, M.G.; Abu-Dief, E.E.; Kamel, N.F.; Kandil, M.R. The effects of glucocorticoid therapy on the inflammatory and dendritic cells in muscular dystrophies. Int. J. Exp. Pathol. 2006, 87, 451–461. [Google Scholar] [CrossRef]
- Belhassen, F.; Gouider-Khouja, N.; Kefi, M.; Amouri, R.; Kobbi, S.; Miladi, N.; Hamida, M.B.; Hentati, F. 179 Results of a trial of prednisone treatment in limb girdle muscular dystrophy 2. Eur. J. Paediatr. Neurol. 1999, 3, A109. [Google Scholar] [CrossRef]
- Guglieri, M.; Bushby, K.; McDermott, M.P.; Hart, K.A.; Tawil, R.; Martens, W.B.; Herr, B.E.; McColl, E.; Speed, C.; Wilkinson, J. Effect of different corticosteroid dosing regimens on clinical outcomes in boys with Duchenne muscular dystrophy: A randomized clinical trial. JAMA 2022, 327, 1456–1468. [Google Scholar] [CrossRef]
- Wintzinger, M.; Panta, M.; Miz, K.; Prabakaran, A.D.; Durumutla, H.B.; Sargent, M.; Peek, C.B.; Bass, J.; Molkentin, J.D.; Quattrocelli, M. Impact of circadian time of dosing on cardiomyocyte-autonomous effects of glucocorticoids. Mol. Metab. 2022, 62, 101528. [Google Scholar] [CrossRef]
- Sali, A.; Guerron, A.D.; Gordish-Dressman, H.; Spurney, C.F.; Iantorno, M.; Hoffman, E.P.; Nagaraju, K. Glucocorticoid-treated mice are an inappropriate positive control for long-term preclinical studies in the mdx mouse. PLoS ONE 2012, 7, e34204. [Google Scholar] [CrossRef] [PubMed]
- Straathof, C.S.; Overweg-Plandsoen, W.; van den Burg, G.J.; van der Kooi, A.J.; Verschuuren, J.J.; de Groot, I.J. Prednisone 10 days on/10 days off in patients with Duchenne muscular dystrophy. J. Neurol. 2009, 256, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Ricotti, V.; Ridout, D.A.; Scott, E.; Quinlivan, R.; Robb, S.A.; Manzur, A.Y.; Muntoni, F. Long-term benefits and adverse effects of intermittent versus daily glucocorticoids in boys with Duchenne muscular dystrophy. J. Neurol. Neurosurg. Psychiatry 2013, 84, 698–705. [Google Scholar] [CrossRef]
- Zulfiqar, E.; Hurjkaliani, S.; Umar, S.A.; Shahzad, M.; Ahsan, M.; Tahir, Q.; Nizami, U.; Rizvi, B.; Khan, A.A.; Baloch, A. Comparing intermittent and daily prednisone in duchenne muscular dystrophy: A systematic review and meta-analysis. Ann. Med. Surg. 2025, 87, 1637–1645. [Google Scholar] [CrossRef]
- Wintzinger, M.; Miz, K.; York, A.; Demonbreun, A.R.; Molkentin, J.D.; McNally, E.M.; Quattrocelli, M. Effects of Glucocorticoids in Murine Models of Duchenne and Limb-Girdle Muscular Dystrophy. Methods Mol. Biol. 2023, 2587, 467–478. [Google Scholar] [CrossRef]
- Griggs, R.C.; Herr, B.E.; Reha, A.; Elfring, G.; Atkinson, L.; Cwik, V.; Mccoll, E.; Tawil, R.; Pandya, S.; McDermott, M.P. Corticosteroids in Duchenne muscular dystrophy: Major variations in practice. Muscle Nerve 2013, 48, 27–31. [Google Scholar] [CrossRef]
- Ziemkiewicz, N.; Hilliard, G.; Pullen, N.A.; Garg, K. The Role of Innate and Adaptive Immune Cells in Skeletal Muscle Regeneration. Int. J. Mol. Sci. 2021, 22, 3265. [Google Scholar] [CrossRef]
- Spencer, M.J.; Tidball, J.G. Do immune cells promote the pathology of dystrophin-deficient myopathies? Neuromuscul. Disord. 2001, 11, 556–564. [Google Scholar] [CrossRef]
- Wehling, M.; Spencer, M.J.; Tidball, J.G. A nitric oxide synthase transgene ameliorates muscular dystrophy in mdx mice. J. Cell Biol. 2001, 155, 123. [Google Scholar] [CrossRef]
- Kapellos, T.S.; Bonaguro, L.; Gemünd, I.; Reusch, N.; Saglam, A.; Hinkley, E.R.; Schultze, J.L. Human monocyte subsets and phenotypes in major chronic inflammatory diseases. Front. Immunol. 2019, 10, 2035. [Google Scholar] [CrossRef]
- Ziegler-Heitbrock, L. Blood monocytes and their subsets: Established features and open questions. Front. Immunol. 2015, 6, 423. [Google Scholar] [CrossRef] [PubMed]
- Saclier, M.; Cuvellier, S.; Magnan, M.; Mounier, R.; Chazaud, B. Monocyte/macrophage interactions with myogenic precursor cells during skeletal muscle regeneration. FEBS J. 2013, 280, 4118–4130. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, E.; Zordan, P.; Sciorati, C.; Rovere-Querini, P.; Brunelli, S. Macrophage plasticity in skeletal muscle repair. BioMed Res. Int. 2014, 2014, 560629. [Google Scholar] [CrossRef] [PubMed]
- Chazaud, B.; Brigitte, M.; Yacoub-Youssef, H.; Arnold, L.; Gherardi, R.; Sonnet, C.; Lafuste, P.; Chretien, F. Dual and beneficial roles of macrophages during skeletal muscle regeneration. Exerc. Sport Sci. Rev. 2009, 37, 18–22. [Google Scholar] [CrossRef]
- Rizzo, G.; Di Maggio, R.; Benedetti, A.; Morroni, J.; Bouche, M.; Lozanoska-Ochser, B. Splenic Ly6Chi monocytes are critical players in dystrophic muscle injury and repair. JCI Insight 2020, 5, e130807. [Google Scholar] [CrossRef]
- Lederer, D.J.; Martinez, F.J. Idiopathic pulmonary fibrosis. N. Engl. J. Med. 2018, 378, 1811–1823. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y. Role of matrix metalloproteinases in skeletal muscle: Migration, differentiation, regeneration and fibrosis. Cell Adhes. Migr. 2009, 3, 337–341. [Google Scholar] [CrossRef]
- Babaeijandaghi, F.; Cheng, R.; Kajabadi, N.; Soliman, H.; Chang, C.-K.; Smandych, J.; Tung, L.W.; Long, R.; Ghassemi, A.; Rossi, F.M. Metabolic reprogramming of skeletal muscle by resident macrophages points to CSF1R inhibitors as muscular dystrophy therapeutics. Sci. Transl. Med. 2022, 14, eabg7504. [Google Scholar] [CrossRef]
- Villalta, S.A.; Rinaldi, C.; Deng, B.; Liu, G.; Fedor, B.; Tidball, J.G. Interleukin-10 reduces the pathology of mdx muscular dystrophy by deactivating M1 macrophages and modulating macrophage phenotype. Hum. Mol. Genet. 2011, 20, 790–805. [Google Scholar] [CrossRef]
- Kharraz, Y.; Guerra, J.; Mann, C.J.; Serrano, A.L.; Muñoz-Cánoves, P. Macrophage plasticity and the role of inflammation in skeletal muscle repair. Mediat. Inflamm. 2013, 2013, 491497. [Google Scholar] [CrossRef]
- Juban, G.; Saclier, M.; Yacoub-Youssef, H.; Kernou, A.; Arnold, L.; Boisson, C.; Larbi, S.B.; Magnan, M.; Cuvellier, S.; Théret, M. AMPK activation regulates LTBP4-dependent TGF-β1 secretion by pro-inflammatory macrophages and controls fibrosis in Duchenne muscular dystrophy. Cell Rep. 2018, 25, 2163–2176.e2166. [Google Scholar] [CrossRef] [PubMed]
- Parente, L. Deflazacort: Therapeutic index, relative potency and equivalent doses versus other corticosteroids. BMC Pharmacol. Toxicol. 2017, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Quinkler, M.; Oelkers, W.; Diederich, S. Clinical implications of glucocorticoid metabolism by 11beta-hydroxysteroid dehydrogenases in target tissues. Eur. J. Endocrinol. 2001, 144, 87–97. [Google Scholar] [CrossRef] [PubMed]
- DeSilva, S.; Drachman, D.B.; Mellits, D.; Kuncl, R.W. Prednisone treatment in Duchenne muscular dystrophy: Long-term benefit. Arch. Neurol. 1987, 44, 818–822. [Google Scholar] [CrossRef]
- McMillan, H.J. Intermittent glucocorticoid regimes for younger boys with duchenne muscular dystrophy: Balancing efficacy with side effects. Muscle Nerve 2019, 59, 638–639. [Google Scholar] [CrossRef]
- Jobling, A.I.; Augusteyn, R.C. What causes steroid cataracts? A review of steroid-induced posterior subcapsular cataracts. Clin. Exp. Optom. 2002, 85, 61–75. [Google Scholar] [CrossRef]
- Lo Cascio, V.; Kanis, J.; Beneton, M.; Bertoldo, F.; Adami, S.; Poggi, G.; Zanolin, M.E. Acute effects of deflazacort and prednisone on rates of mineralization and bone formation. Calcif. Tissue Int. 1995, 56, 109–112. [Google Scholar] [CrossRef]
- Quattrocelli, M.; Barefield, D.Y.; Warner, J.L.; Vo, A.H.; Hadhazy, M.; Earley, J.U.; Demonbreun, A.R.; McNally, E.M. Intermittent glucocorticoid steroid dosing enhances muscle repair without eliciting muscle atrophy. J. Clin. Investig. 2017, 127, 2418–2432. [Google Scholar] [CrossRef]
- Gordon, S.; Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005, 5, 953–964. [Google Scholar] [CrossRef]
- Liu, P.; Gao, Y.; Luo, P.; Yu, H.; Guo, S.; Liu, F.; Gao, J.; Xu, J.; Wang, S.; Zhang, C. Glucocorticoid-induced expansion of classical monocytes contributes to bone loss. Exp. Mol. Med. 2022, 54, 765–776. [Google Scholar] [CrossRef]
- Barczyk, K.; Ehrchen, J.; Tenbrock, K.; Ahlmann, M.; Kneidl, J.; Viemann, D.; Roth, J. Glucocorticoids promote survival of anti-inflammatory macrophages via stimulation of adenosine receptor A3. Blood J. Am. Soc. Hematol. 2010, 116, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Ehrchen, J.; Steinmüller, L.; Barczyk, K.; Tenbrock, K.; Nacken, W.; Eisenacher, M.; Nordhues, U.; Sorg, C.; Sunderkötter, C.; Roth, J. Glucocorticoids induce differentiation of a specifically activated, anti-inflammatory subtype of human monocytes. Blood 2007, 109, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Gren, S.T.; Rasmussen, T.B.; Janciauskiene, S.; Håkansson, K.; Gerwien, J.G.; Grip, O. A single-cell gene-expression profile reveals inter-cellular heterogeneity within human monocyte subsets. PLoS ONE 2015, 10, e0144351. [Google Scholar] [CrossRef]
- Yona, S.; Kim, K.W.; Wolf, Y.; Mildner, A.; Varol, D.; Breker, M.; Strauss-Ayali, D.; Viukov, S.; Guilliams, M.; Misharin, A.; et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 2013, 38, 79–91. [Google Scholar] [CrossRef]
- Gómez-Olarte, S.; Bolaños, N.I.; Echeverry, M.; Rodríguez, A.N.; Cuéllar, A.; Puerta, C.J.; Mariño, A.; González, J.M. Intermediate Monocytes and Cytokine Production Associated With Severe Forms of Chagas Disease. Front. Immunol. 2019, 10, 1671. [Google Scholar] [CrossRef]
- Narasimhan, P.B.; Marcovecchio, P.; Hamers, A.A.J.; Hedrick, C.C. Nonclassical Monocytes in Health and Disease. Annu. Rev. Immunol. 2019, 37, 439–456. [Google Scholar] [CrossRef]
- Moratal, C.; Raffort, J.; Arrighi, N.; Rekima, S.; Schaub, S.; Dechesne, C.A.; Chinetti, G.; Dani, C. IL-1β- and IL-4-polarized macrophages have opposite effects on adipogenesis of intramuscular fibro-adipogenic progenitors in humans. Sci. Rep. 2018, 8, 17005. [Google Scholar] [CrossRef]
- Coulis, G.; Jaime, D.; Guerrero-Juarez, C.; Kastenschmidt, J.M.; Farahat, P.K.; Nguyen, Q.; Pervolarakis, N.; McLinden, K.; Thurlow, L.; Movahedi, S.; et al. Single-cell and spatial transcriptomics identify a macrophage population associated with skeletal muscle fibrosis. Sci. Adv. 2023, 9, eadd9984. [Google Scholar] [CrossRef]
- Ward, L.M.; Weber, D.R. Growth, pubertal development, and skeletal health in boys with Duchenne muscular dystrophy. Curr. Opin. Endocrinol. Diabetes Obes. 2019, 26, 39–48. [Google Scholar] [CrossRef]
- Rogacev, K.S.; Zawada, A.M.; Emrich, I.; Seiler, S.; Böhm, M.; Fliser, D.; Woollard, K.J.; Heine, G.H. Lower Apo AI and lower HDL-C levels are associated with higher intermediate CD14++ CD16+ monocyte counts that predict cardiovascular events in chronic kidney disease. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2120–2127. [Google Scholar] [CrossRef]
- Torres-Ruiz, J.; Carrillo-Vazquez, D.A.; Padilla-Ortiz, D.M.; Vazquez-Rodriguez, R.; Nuñez-Alvarez, C.; Juarez-Vega, G.; Gomez-Martin, D. TLR expression in peripheral monocyte subsets of patients with idiopathic inflammatory myopathies: Association with clinical and immunological features. J. Transl. Med. 2020, 18, 125. [Google Scholar] [CrossRef] [PubMed]
- Linsley, P.S.; Greene, J.L.; Brady, W.; Bajorath, J.; Ledbetter, J.A.; Peach, R. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity 1994, 1, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.L.; Stimpson, M.L.; Lait, P.J.P.; Schewitz-Bowers, L.P.; Jones, L.V.; Dhanda, A.D.; Lee, R.W.J.; Bradbury, C.A. Glucocorticoid treatment in patients with newly diagnosed immune thrombocytopenia switches CD14++ CD16+ intermediate monocytes from a pro-inflammatory to an anti-inflammatory phenotype. Br. J. Haematol. 2021, 192, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.Y.; Son, Y.; Kim, M.S.; Kim, K. Prednisolone suppresses the immunostimulatory effects of 27-hydroxycholesterol. Exp. Ther. Med. 2020, 19, 2335–2342. [Google Scholar] [CrossRef]
- Lit, L.; Sharp, F.R.; Apperson, M.; Liu, D.Z.; Walker, W.L.; Liao, I.; Xu, H.; Ander, B.P.; Wong, B. Corticosteroid effects on blood gene expression in Duchenne muscular dystrophy. Pharmacogenomics J. 2009, 9, 411–418. [Google Scholar] [CrossRef]
- Liu, B.; Dhanda, A.; Hirani, S.; Williams, E.L.; Sen, H.; Martinez Estrada, F.; Ling, D.; Thompson, I.; Casady, M.; Li, Z. CD14++ CD16+ monocytes are enriched by glucocorticoid treatment and are functionally attenuated in driving effector T cell responses. J. Immunol. 2015, 194, 5150–5160. [Google Scholar] [CrossRef]
- Burgdorf, S.; Lukacs-Kornek, V.; Kurts, C. The mannose receptor mediates uptake of soluble but not of cell-associated antigen for cross-presentation. J. Immunol. 2006, 176, 6770–6776. [Google Scholar] [CrossRef]
- Weber, D.R.; Hadjiyannakis, S.; McMillan, H.J.; Noritz, G.; Ward, L.M. Obesity and endocrine management of the patient with Duchenne muscular dystrophy. Pediatrics 2018, 142 (Suppl. 2), S43–S52. [Google Scholar] [CrossRef]
- Embgenbroich, M.; van der Zande, H.J.P.; Hussaarts, L.; Schulte-Schrepping, J.; Pelgrom, L.R.; García-Tardón, N.; Schlautmann, L.; Stoetzel, I.; Händler, K.; Lambooij, J.M.; et al. Soluble mannose receptor induces proinflammatory macrophage activation and metaflammation. Proc. Natl. Acad. Sci. USA 2021, 118, e2103304118. [Google Scholar] [CrossRef]
- San Emeterio, C.L.; Olingy, C.E.; Chu, Y.; Botchwey, E.A. Selective recruitment of non-classical monocytes promotes skeletal muscle repair. Biomaterials 2017, 117, 32–43. [Google Scholar] [CrossRef]
- Maniecki, M.B.; Etzerodt, A.; Moestrup, S.K.; Møller, H.J.; Graversen, J.H. Comparative assessment of the recognition of domain-specific CD163 monoclonal antibodies in human monocytes explains wide discrepancy in reported levels of cellular surface CD163 expression. Immunobiology 2011, 216, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Stahl, P.; Schlesinger, P.H.; Sigardson, E.; Rodman, J.S.; Lee, Y. Receptor-mediated pinocytosis of mannose glycoconjugates by macrophages: Characterization and evidence for receptor recycling. Cell 1980, 19, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.C.; Andersen, M.N.; Rittig, N.; Rødgaard-Hansen, S.; Grønbaek, H.; Moestrup, S.K.; Møller, H.J.; Etzerodt, A. The macrophage-related biomarkers sCD163 and sCD206 are released by different shedding mechanisms. J. Leukoc. Biol. 2019, 106, 1129–1138. [Google Scholar] [CrossRef]
- Plevriti, A.; Lamprou, M.; Mourkogianni, E.; Skoulas, N.; Giannakopoulou, M.; Sajib, M.S.; Wang, Z.; Mattheolabakis, G.; Chatzigeorgiou, A.; Marazioti, A.; et al. The Role of Soluble CD163 (sCD163) in Human Physiology and Pathophysiology. Cells 2024, 13, 1679. [Google Scholar] [CrossRef]
- Järvinen, T.A.; Järvinen, T.L.; Kääriäinen, M.; Kalimo, H.; Järvinen, M. Muscle injuries: Biology and treatment. Am. J. Sports Med. 2005, 33, 745–764. [Google Scholar] [CrossRef]
- Peverelli, L.; Testolin, S.; Villa, L.; D’Amico, A.; Petrini, S.; Favero, C.; Magri, F.; Morandi, L.; Mora, M.; Mongini, T.; et al. Histologic muscular history in steroid-treated and untreated patients with Duchenne dystrophy. Neurology 2015, 85, 1886–1893. [Google Scholar] [CrossRef]
- Fisher, I.; Abraham, D.; Bouri, K.; Hoffman, E.P.; Muntoni, F.; Morgan, J. Prednisolone-induced changes in dystrophic skeletal muscle. FASEB J. 2005, 19, 834–836. [Google Scholar] [CrossRef]
- Narasimhulu, C.A.; Singla, D.K. BMP-7 Attenuates Sarcopenia and Adverse Muscle Remodeling in Diabetic Mice via Alleviation of Lipids, Inflammation, HMGB1, and Pyroptosis. Antioxidants 2023, 12, 331. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Homma, S.T.; Wang, Y.; Smith, G.R.; Ruf-Zamojski, F.; Sealfon, S.C.; Zhou, L. Diverse effector and regulatory functions of fibro/adipogenic progenitors during skeletal muscle fibrosis in muscular dystrophy. iScience 2023, 26, 105775. [Google Scholar] [CrossRef]
- Villalta, S.A.; Nguyen, H.X.; Deng, B.; Gotoh, T.; Tidball, J.G. Shifts in macrophage phenotypes and macrophage competition for arginine metabolism affect the severity of muscle pathology in muscular dystrophy. Hum. Mol. Genet. 2009, 18, 482–496. [Google Scholar] [CrossRef]
- Messing, M.; Theret, M.; Hughes, M.R.; Wu, J.; Syed, O.H.; Li, F.F.; Li, Y.; Rossi, F.M.; McNagny, K.M. Type-2 innate signals are dispensable for skeletal muscle regeneration and pathology linked to Duchenne muscular dystrophy. EMBO Rep. 2025, 26, 1406–1421. [Google Scholar] [CrossRef] [PubMed]
- Howell, I.; Yang, F.; Brown, V.; Cane, J.; Marchi, E.; Azim, A.; Busby, J.; McDowell, P.; Diver, S.; Borg, C. Anti-inflammatory effects of oral prednisolone at stable state in people treated with mepolizumab: A proteomic and bulk transcriptomics analysis. medRxiv 2024. [Google Scholar] [CrossRef]
- Raghavan, K.; Dedeepiya, V.D.; Srinivasan, S.; Pushkala, S.; Bharatidasan, S.S.; Ikewaki, N.; Iwasaki, M.; Senthilkumar, R.; Preethy, S.; Abraham, S.J.K. Beneficial immune-modulatory effects of the N-163 strain of Aureobasidium pullulans-produced 1,3-1,6 Beta glucans in Duchenne muscular dystrophy: Results of an open-label, prospective, exploratory case-control clinical study. IBRO Neurosci. Rep. 2023, 15, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, K.C.; Alspach, E.; Pazolli, E.; Parajuli, S.; Ren, Q.; Arthur, L.L.; Tapia, R.; Stewart, S.A. c-Myb and C/EBPβ regulate OPN and other senescence-associated secretory phenotype factors. Oncotarget 2018, 9, 21–36. [Google Scholar] [CrossRef]
- Salotti, J.; Johnson, P.F. Regulation of senescence and the SASP by the transcription factor C/EBPβ. Exp. Gerontol. 2019, 128, 110752. [Google Scholar] [CrossRef]
- Huber, R.; Pietsch, D.; Panterodt, T.; Brand, K. Regulation of C/EBPβ and resulting functions in cells of the monocytic lineage. Cell Signal. 2012, 24, 1287–1296. [Google Scholar] [CrossRef]
- Yi, Z.; Geng, S.; Li, L. Comparative analyses of monocyte memory dynamics from mice to humans. Inflamm. Res. 2023, 72, 1539–1549. [Google Scholar] [CrossRef]
- Ruffell, D.; Mourkioti, F.; Gambardella, A.; Kirstetter, P.; Lopez, R.G.; Rosenthal, N.; Nerlov, C. A CREB-C/EBPβ cascade induces M2 macrophage-specific gene expression and promotes muscle injury repair. Proc. Natl. Acad. Sci. USA 2009, 106, 17475–17480. [Google Scholar] [CrossRef]
- Fu, D.; Lala-Tabbert, N.; Lee, H.; Wiper-Bergeron, N. Mdm2 promotes myogenesis through the ubiquitination and degradation of CCAAT/enhancer-binding protein β. J. Biol. Chem. 2015, 290, 10200–10207. [Google Scholar] [CrossRef]
- Gonnella, P.; Alamdari, N.; Tizio, S.; Aversa, Z.; Petkova, V.; Hasselgren, P.O. C/EBPβ regulates dexamethasone-induced muscle cell atrophy and expression of atrogin-1 and MuRF1. J. Cell Biochem. 2011, 112, 1737–1748. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, L.; Liu, J. Bioinformatics and machine learning approaches to explore key biomarkers in muscle aging linked to adipogenesis. BMC Musculoskelet. Disord. 2025, 26, 285. [Google Scholar] [CrossRef] [PubMed]
- Norwood, F.L.; Harling, C.; Chinnery, P.F.; Eagle, M.; Bushby, K.; Straub, V. Prevalence of genetic muscle disease in Northern England: In-depth analysis of a muscle clinic population. Brain 2009, 132, 3175–3186. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, E.P. Causes of clinical variability in Duchenne and Becker muscular dystrophies and implications for exon skipping therapies. Acta Myol. 2020, 39, 179. [Google Scholar]
- Salamone, I.M.; Quattrocelli, M.; Barefield, D.Y.; Page, P.G.; Tahtah, I.; Hadhazy, M.; Tomar, G.; McNally, E.M. Intermittent glucocorticoid treatment enhances skeletal muscle performance through sexually dimorphic mechanisms. J. Clin. Investig. 2022, 132, e149828. [Google Scholar] [CrossRef]
- Nandeesh, B.N.; Narayanappa, G.; Yasha, T.C. Basic requirements to establish a neuromuscular laboratory. Indian J. Pathol. Microbiol. 2022, 65 (Suppl. 1), 233–240. [Google Scholar]
- Trabelsi, N.; Othman, H.; Bedhioufi, H.; Chouk, H.; El Mabrouk, H.; Mahdouani, M.; Gribaa, M.; Saad, A.; H’Mida, D. Is Tunisia ready for precision medicine? Challenges of medical genomics within a LMIC healthcare system. J. Community Genet. 2024, 15, 339–350. [Google Scholar] [CrossRef]
- Taneva, A.; Gresham, D.; Guergueltcheva, V.; Chamova, T.; Bojinova, V.; Gospodinova, M.; Katzarova, M.; Petkov, R.; Voit, T.; Aneva, L. Phenotypic Variability of LGMD 2C/R5 in a Genetically Homogenous Group of Bulgarian Muslim Roma. Genes 2024, 15, 1144. [Google Scholar] [CrossRef]
- Abida, Y.; Younes, T.B.; Miladi, Z.; Hedia, K.; Ichraf, K.; Benrhouma, H.; Youssef-Turki, I.B. Facteurs prédictifs du déclin moteur dans la dystrophie musculaire de Duchenne chez les patients traités par corticoïdes. Rev. Neurol. 2022, 178, S78. [Google Scholar] [CrossRef]
- Matthews, E.; Brassington, R.; Kuntzer, T.; Jichi, F.; Manzur, A.Y. Corticosteroids for the treatment of Duchenne muscular dystrophy. Cochrane Database Syst. Rev. 2016, 2016, Cd003725. [Google Scholar] [CrossRef]

| Groupe | ID | Age (y) | Age at Diagnosis (y) | Loss of Ambulation (Yes/No) | Genetic Anomalies |
|---|---|---|---|---|---|
| MD | DMD1 | 6 | 3 | No | DMD deletion exon 45–50 |
| DMD6 | 10 | 6 | No | DMD deletion exon 44 | |
| DMD9 | 9 | 8 | Yes | DMD deletion exon 8–48 | |
| DMD11 | 7 | 6 | No | DMD deletion exon 48–50 | |
| DMD5 | 9 | 8 | Yes | DMD deletion exon 3–11 | |
| LGMD5 | 10 | 6 | No | LGMD2C/R5 patient SGCG c.525delTC | |
| LGMD3EA | 14 | 7 | No | LGMD2C/R5 patient | |
| LGMD3EA2 | 14 | 7 | No | LGMD2C/R5 patient | |
| LGMD7EA | 12 | 10 | Yes | LGMD2C/R5 patient SGCG c.525delTC | |
| LGMD7EA | 8 | 6 | No | LGMD2C/R5 patient SGCG c.525delTC | |
| MD-Pred | DMD7 | 8 | 5 | No | DMD deletion exon 45–54 |
| DMD10 | 11 | 9 | Yes | DMD deletion exon 45–55 | |
| DMD3 | 14 | 8 | No | DMD deletion exon 45–47 | |
| DMD8 | 9 | 4 | No | DMD deletion exon 45 | |
| LGMD2 | 11 | 2 | Yes | LGMD2C/R5 patient SGCG c.525delTC | |
| LGMD6 | 11 | 9 | Yes | LGMD2C/R5 patient SGCG c.525delTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chikhaoui, A.; Najjar, D.; Bouchoucha, S.; Boussetta, R.; Achour, N.B.; Tizaoui, K.; Kraoua, I.; Turki, I.; Yacoub-Youssef, H. Profiles of Monocyte Subsets and Fibrosis-Related Genes in Patients with Muscular Dystrophy Undergoing Intermittent Prednisone Therapy. Int. J. Mol. Sci. 2025, 26, 5992. https://doi.org/10.3390/ijms26135992
Chikhaoui A, Najjar D, Bouchoucha S, Boussetta R, Achour NB, Tizaoui K, Kraoua I, Turki I, Yacoub-Youssef H. Profiles of Monocyte Subsets and Fibrosis-Related Genes in Patients with Muscular Dystrophy Undergoing Intermittent Prednisone Therapy. International Journal of Molecular Sciences. 2025; 26(13):5992. https://doi.org/10.3390/ijms26135992
Chicago/Turabian StyleChikhaoui, Asma, Dorra Najjar, Sami Bouchoucha, Rim Boussetta, Nadia Ben Achour, Kalthoum Tizaoui, Ichraf Kraoua, Ilhem Turki, and Houda Yacoub-Youssef. 2025. "Profiles of Monocyte Subsets and Fibrosis-Related Genes in Patients with Muscular Dystrophy Undergoing Intermittent Prednisone Therapy" International Journal of Molecular Sciences 26, no. 13: 5992. https://doi.org/10.3390/ijms26135992
APA StyleChikhaoui, A., Najjar, D., Bouchoucha, S., Boussetta, R., Achour, N. B., Tizaoui, K., Kraoua, I., Turki, I., & Yacoub-Youssef, H. (2025). Profiles of Monocyte Subsets and Fibrosis-Related Genes in Patients with Muscular Dystrophy Undergoing Intermittent Prednisone Therapy. International Journal of Molecular Sciences, 26(13), 5992. https://doi.org/10.3390/ijms26135992






