Abstract
Breast cancer (BC) is the most common cancer in women worldwide. It is one of the main causes of cancer-related mortality. The breast tumor microenvironment (Br-TME) has emerged as an important factor related to BC development and prognosis. Tumor-associated macrophages (TAMs) are the main effector cells in the Br-TME; they play key roles in regulating angiogenesis, immunosuppression, metastasis, and chemoresistance in BC patients. In this review, we introduce the macrophage niche in the Br-TME, particularly emphasizing the origin of TAMs. Next, we summarize the typical pathways and molecular mechanisms of the interactions between TAMs and various other components in the Br-TME. Finally, we provide an overview of drugs that target TAMs and discuss the prevailing technologies for drug delivery in the context of BC treatment. Identification of the dynamic variations in tumor-promoting TAMs will help reveal the key links that drive BC progression. This review provides a theoretical basis for upcoming clinical trials that may substantially benefit patients.
1. Introduction
Breast cancer (BC) is the most commonly diagnosed malignant tumor in women and ranks second among causes of cancer-related death in women [1]. It is currently estimated that 1.6 million BC cases occur worldwide per year, affecting as many as one in eight women in high-income countries [2]. It is expected to account for 4.4 million diagnoses and 685,000 deaths in 2070 [3]. Approximately 18.4% of global BC cases occur in China [4]. Despite the considerable progress achieved with treatment protocols, metastatic breast cancer remains incurable [5].
In clinical practice, BC is recognized as a multifaceted disease [6]. Global gene expression profiling studies have classified BC into four subtypes by hierarchical clustering, namely, the luminal A subtype, luminal B subtype, human epidermal growth factor receptor 2 (HER2)-overexpression subtype, and triple-negative subtype (triple-negative breast cancer, TNBC) [7].
Both the luminal A subtype and luminal B subtype are positive for estrogen receptor (ER) and progesterone receptor (PR) expression. Luminal A tumors are negative for HER2 and have low expression of Ki-67, a nuclear marker of cell proliferation [8]. Luminal B tumors are divided into HER2-negative tumors and HER2-positive tumors with different levels of Ki-67 expression [7]. These two subtypes can be distinguished by the activation of two major biological processes: a proliferation/cell cycle-related pathway and a luminal/hormone-regulated pathway. Prat et al. analyzed the differences in the expression status of a panel of 50 genes between typical luminal A and luminal B subtypes [9]. They reported that the expression of genes involved in cell differentiation (e.g., Krüppel-like factor 4 and jun proto-oncogene) and adhesion (e.g., vinculin and type II collagen) was upregulated in the luminal A subtype, whereas the expression of genes involved in the immune response [e.g., interleukin (IL)-2 receptors and CD86] and the cell cycle (e.g., cyclin B1 and radiation sensitivity 51 recombinase) was upregulated in the luminal B subtype [9]. The hallmark of precancerous lesions and BC is an increased proportion of ER+PR+ cells with increased Ki-67 expression [10]. Patients with high PR expression in luminal A tumors have a better baseline prognosis than those with luminal B tumors [11].
HER2 is encoded by the erb-b2 receptor tyrosine kinase 2 (ERBB2) gene and is a transmembrane glycoprotein epidermal growth factor receptor (EGFR) with tyrosine kinase activity [12]. According to the guidelines of the American Society of Clinical Oncology and the College of American Pathologists in 2018, HER2 positivity is defined by an immunohistochemistry (IHC) score of 3 for HER2 and/or the in situ hybridization-based detection of HER2 amplification [13]. HER2-overexpressing BC is characterized by ER negativity and PR negativity [14]; it has a faster growth rate and a worse prognosis, with a 5-year survival rate of 50–60% [15]. Although HER2-targeting drugs (e.g., trastuzumab, lapatinib, and pertuzumab) can increase the survival rate, HER-2 downregulation and the existence of HER2 receptor variants [e.g., splicing variants of HER2 lacking exon 16 (HER2∆16)] may cause primary or acquired drug resistance [16].
TNBC often occurs in premenopausal women under 40 years of age, who account for approximately 15–20% of all BC patients [17]. TNBC is negative for ER, PR, and HER2 [18]. Compared with that of patients with other BC subtypes, the survival time of TNBC patients is significantly shorter; 40% of patients die within the first 5 years after diagnosis, and the recurrence rate after surgery is as high as 25% [19]. Jiang et al. analyzed clinical, genomic, and transcriptomic data from 465 patients with primary TNBC and proposed a Fudan classification system [20]. With this system, TNBC is classified into four transcriptome-based subtypes: luminal androgen receptor TNBC, immunomodulatory TNBC, basal-like immune-suppressed TNBC, and mesenchymal-like TNBC. Fan et al. conducted a single-center phase 2 study involving 139 untreated women with TNBC and confirmed a significant progression-free survival benefit of treatment based on the Fudan classification [21].
2. Macrophage Niche in the Breast Tumor Microenvironment
BC cells are surrounded by newly formed abnormal blood vessels, stromal cells, fibroblasts, endothelial cells, and innate and adaptive immune cells [22]. BC cells secrete soluble factors (e.g., growth factors and cytokines) to regulate the turnover of normal epithelial cells through interactions with the extracellular matrix (ECM) and cells in the breast tumor microenvironment (Br-TME) [23,24]. The crosstalk between BC cells and the Br-TME leads to a cascade of events that favor BC progression [25].
The Br-TME contains a variety of infiltrating immune cells; macrophages are the primary constituents related to innate immunity in the breast [26]. Higher expression levels of macrophage-related genes are directly correlated with more aggressive BC phenotypes [27]. Colony-stimulating factor 1 (CSF1) is a major growth factor that regulates the survival, differentiation, and proliferation of macrophages [28]. Cassetta et al. performed whole-tumor RNA sequencing for an independent cohort of 47 BC patients [29]. The results indicated that the CSF1high group had a significantly greater macrophage signature score than the CSF1mid group and the CSF1low group did. The sialic acid binding Ig-like lectin 1 (SIGLEC1) gene is one of the most upregulated genes in macrophages of the Br-TME and is correlated with the expression of the pan-macrophage marker CD163 [30]. Cassetta et al. also reported that SIGLEC1+ macrophages were enriched in basal subtypes rather than HER2-overexpressing and luminal subtypes [29]. These macrophages responded to BC by upregulating SIGLEC1 expression and producing tumor necrosis factor-α (TNF-α) to increase SIGLEC1 expression and vice versa. Both a high macrophage signature score and a higher proportion of SIGLEC1+ macrophages were significantly associated with shorter disease-specific survival in BC patients.
Changes in the composition of the ECM are associated with the increased migration of macrophages toward BC cells. In a cohort study of 278 BC patients, the deposition of hyaluronan (HA) was associated with increased macrophage counts and a poor prognosis, regardless of BC subtype [31]. An increase in ECM stiffness was positively correlated with the number of infiltrating macrophages in 20 BC patients [32]. Tenascin-C is an extracellular matrix glycoprotein that is transiently expressed in response to tissue injury to mediate fibrotic processes [33]. Tenascin-C is highly upregulated in primary and metastatic BC, and it is a predictor of metastatic recurrence and short overall survival [34]. Deligne et al. noted that macrophages in the Br-TME were abundant at sites where tenascin-C was overexpressed [35]. After treatment with a combination of an anti-tenascin-C antibody and anti-programmed cell death ligand 1 (PD-L1), macrophages translocated to the lesion edge from the stroma, which suppressed tumor growth and lung metastasis.
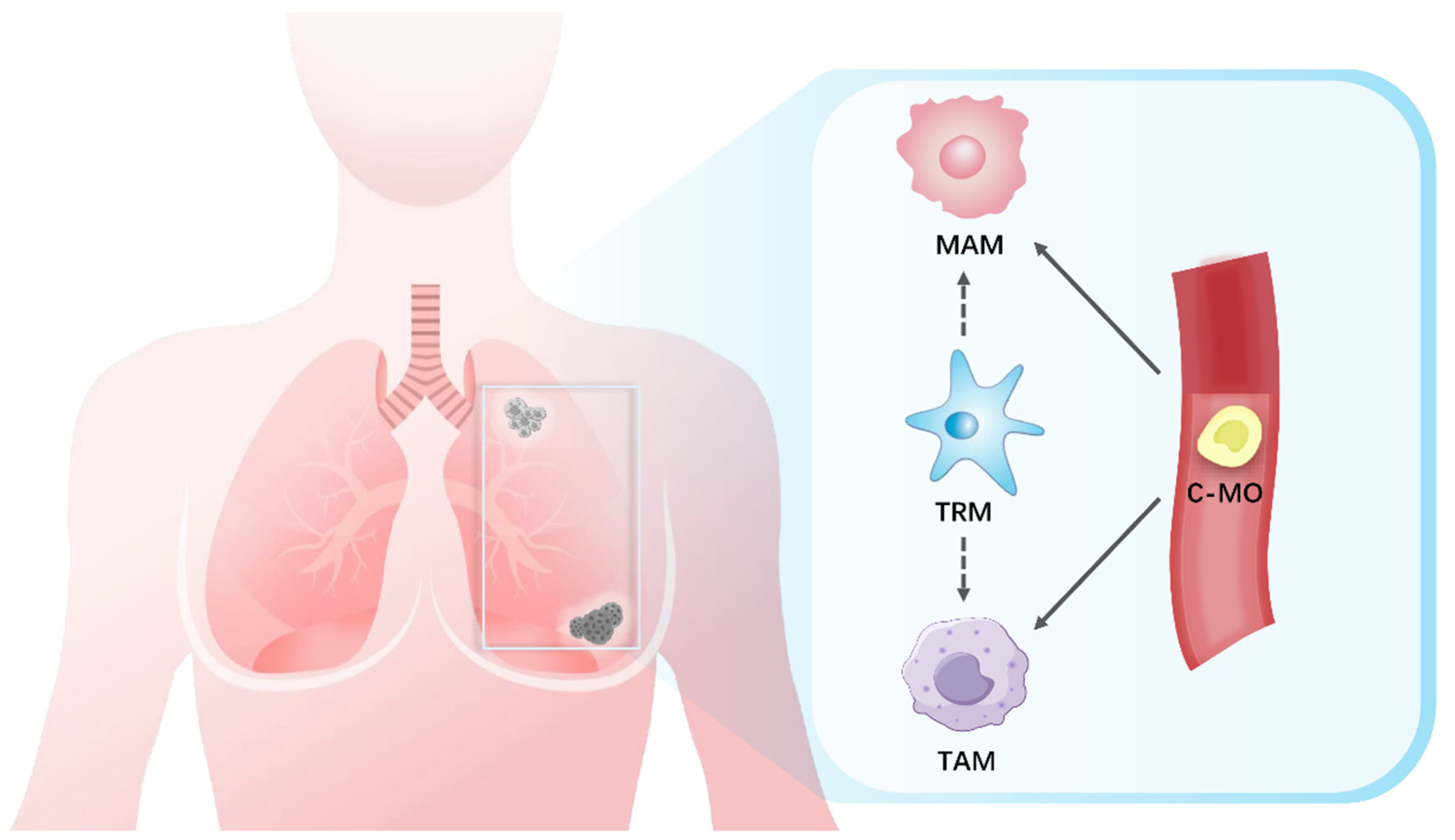
Macrophages constitute an intrinsically heterogeneous population characterized by significant plasticity (Figure 1).

Figure 1.
Macrophages in the Br-TME. The embryonic-derived tissue-resident macrophages (TRMs), the tumor-associated macrophages (TAMs), and the metastasis-associated macrophages (MAMs) are three groups of macrophages that are important components of the Br-TME. TRMs serve as a potential source for both TAMs and MAMs. Circulating classical monocytes (C-MOs) are necessary for TAM accumulation in the Br-TME via tumor cell-secreted factors (e.g., CCL 2, CCL18, CCL20, CSF1, and VEGF). The CCL2/CCR2 signaling axis drives the recruitment of C-MOs to premetastatic niches in distant tumor-challenged lesions and leads to MAM differentiation.
The embryonic-derived tissue-resident macrophages (TRMs or Mϕ) infiltrating tumors are a source of macrophages, and they participate in cancer evolution and metastasis [36]. TRMs originate from the yolk sac during embryonic development and maintain self-sustaining populations to constitute the innate immune system [37,38]. In healthy breast tissues, TRMs are localized within the stroma of adipose tissue adjacent to the ductal epithelium [39]. TRMs participate in organizing the structure of terminal buds and extracellular matrix, and they exert anti-tumor immunity in a CSF1-dependent manner [40]. In fact, the CSF1 response has been found in many BC cases, and TRM reprogramming is associated with higher tumor proliferation rates and tumor grades [41]. Hypoxia and anaerobic glycolysis-induced changes in the hypoxia-inducible factor (HIF)-1α signaling pathway represent another reprogramming mechanism of TRMs that promotes BC [42,43]. Folate receptor beta (FOLR2) is a membrane protein involved in folate metabolism [44]. It has been reported that FOLR2+ TRMs induce naïve CD8+ T cells to transform into granzyme B+TNF-α+interferon-γ (IFN-γ)+ multifunctional effector cells in the Br-TME [45]. The F4/80+FOLR2+ subset is a favorable prognostic factor for hormone receptor-positive BC, and a high density of FOLR2+ TRMs was found to be directly correlated with an increased survival rate in BC patients [46]. However, Linde et al. found that CD206hi intraepithelial TRMs upregulated the wingless-related integration site (Wnt)-1 through chemokine (C-C motif) ligand (CCL) 2 to disrupt E-cadherin junctions [47]. Stromal TRMs infiltrated into the epithelium, triggering early dissemination and subsequent metastasis in HER2+ BC.
In general, chronic inflammation in the TME promotes the polarization of macrophages into tumor-associated macrophages (TAMs) [48]. Clodronate liposomes (CLs) are inducers of macrophage apoptosis [49]. Hirano et al. used a green fluorescent protein-transgenic technology and injected the TNBC cell line E0771 into the fourth mammary gland fat pad (MGFP) of C57BL/6 J mice to assess macrophage distribution and tumor development [50]. In the CL-treated MGFP group, the number of TRMs was significantly reduced compared to the phosphate-buffered saline liposome-treated MGFP group, leading to decreased TAM infiltration, reduced tumor vessel density, and fewer distant metastases. This study suggested that TRMs are a major resource of TAMs and that TRMs contribute to the early development of TNBC. A recent study revealed that TAM heterogeneity in the Br-TME was driven by their territorial localization of pre-tumoral tissues [51]. The stromal/adipose TAMs expressed FOLR2 in both healthy and cancerous tissues, and advanced TAMs in the tumor nest were identified by the expressions of triggering receptors expressed on myeloid cells 2 and secreted phosphoprotein 1. The recruitment of circulating monocytes is also necessary for TAM accumulation in the Br-TME [52]. Peripheral monocytes are recruited to the Br-TME and differentiate into TAMs by factors such as CCL2, CCL18, CCL20, CSF1, and the vascular endothelial growth factor (VEGF), which are derived from both tumor cells and stromal cells [53,54].
The establishment of a premetastatic niche (PMN) represents a pivotal stage in the initiation and advancement of metastasis, which provides a conducive microenvironment for the settlement and colonization of disseminated tumor cells at distant metastatic sites [55]. Both monocyte-derived macrophages and TRMs contribute to metastasis and have been termed metastasis-associated macrophages (MAMs) [56,57]. When compared with TRMs and TAMs, MAMs expressed higher levels of vascular endothelial growth factor receptor 1 (VEGFR1, also known as FLT1) [58]. During angiogenesis, FLT1 binds the VEGF in a non-kinase-dependent manner with a 10-fold higher affinity than VEGFR2 [59]. Qian et al. constructed two independent mouse models of lineage FLT1 ablation and FLT1 kinase domain knockout to demonstrate that MAMs uniquely express FLT1 for BC cell survival after metastatic seeding by stimulating the downstream CSF1 autocrine signaling pathway [60]. In a BC metastasis mouse model, CCL2/C-C motif chemokine receptor (CCR) 2 axis induced the recruitment of circulating classical monocytes (C-MOs) into the PMN. Then, C-MOs preferentially migrated to the tumor-invaded lungs and differentiated into MAMs as early as 18 h [61]. Kitamura et al. used a monocyte tracking technology and revealed that C-MOs (F4/80lowCD11b+Ly6C+) differentiated into a distinct F4/80highCD11bhighLy6Chigh population of myeloid cells (called MAM precursor cells or MAMPCs) after migrating to the lungs with metastatic lesions [56]. MAMPCs and MAMs exhibited analogous mature macrophage markers, including CD14, CD36, CD64, and CD206. When compared to C-MOs, both MAMPCs and MAMs had upregulated expression levels of TAM signature genes. In a BC mouse model of lung metastasis, gene transcriptomic results demonstrated that newly recruited bone marrow-derived monocytes (BMDMs) were converted into precursor CD11bhiLy6Chi cells through the activation of the CCL2 receptor CCR2 [62]. This cell population produced CCL3 to promote the early accumulation of MAMs. The deletion of CCL3 or its receptor CCR1 in BMDMs could reverse this effect.
3. TAM Modulation by the Br-TME
3.1. Plasticity of TAMs
Depending on the signals in the microenvironment, TAMs in the Br-TME polarize into two distinct phenotypes: an antitumor subtype (lipopolysaccharide-induced macrophages, M1 TAMs) and a tumor-promoting subtype (IL-4-stimulated macrophages, M2 TAMs) [63]. In the early stages of BC, M1 TAMs activate antitumor immunity and suppress angiogenesis [64]. As tumors progress to advanced stages, the proportion of M2 TAMs increases, and M2 TAMs become the predominant subtype of TAMs in the Br-TME [65]. M2 TAMs are tumor-promoting factors that support BC progression by promoting angiogenesis, immune evasion, tumor stemness, tumor cell invasion, and metastasis [47,66,67].
M1 TAMs are immune effector cells with highly potent proinflammatory effects that release factors including but not limited to IL-6, IL-12, IL-23, and TNF-α to facilitate BC cell elimination [68,69]. Bernsmeier et al. reported that M1 TAMs that accumulated at sites of early oncogenesis produced nitric oxide (NO) and reactive oxygen species (ROS) to limit the proliferation of tumor cells [70]. The novel cysteine histone protease inhibitor GB111-NH2 inhibits BC development and induces the proliferation of M1 TAMs by increasing ROS levels [71]. M1 TAMs also directly engulf BC cells to present tumor antigens to activate cascades of antitumor immunity [72]. However, the prolonged activity of M1 TAMs fosters chronic inflammation and aggravates the genomic instability of malignant cells [73]. In this context, BC cells acquire the ability to induce M1 TAM repolarization toward the M2 phenotype [74,75].
M2 TAMs are rich in arginase-1 (ARG-1), mannose receptor (CD206), and the anti-inflammatory factor IL-10 [76]. M2 TAMs can be further classified into M2a, M2b, M2c, and M2d subsets [77]. The phenotype after polarization depends on interactions between specific ligands and their receptors, highlighting the dynamic status of the Br-TME [78]. The M2a subset (CD86highCD163lowHLA-DRhigh) is induced by the T helper (Th) 2 cytokines IL-4 and IL-13 [79]. Little et al. reported that the concentrations of VEGF and CCL8 in the conditioned medium of M2a macrophages were significantly greater than those in the conditioned medium of M2b or M2c macrophages [80]. The M2b subset (IL-10highIL-12lowCD86+MHCII+) is an intermediate subset between M1 and M2 TAMs [81]. Liu et al. established a BC xenograft model with continuous bevacizumab administration [82]. They revealed that the M2b subset was induced by the cross-communication of Fcγ receptors and Toll-like receptor 4 (TLR4), both of which were involved in acquired resistance to bevacizumab. Glucocorticoids and IL-10 induce the M2c subset (CD86lowCD163highTLR1) [83]. They are closely related to the rapid proliferation and poor differentiation of primary ER− BC [84]. The M2d subset (CD86lowCD163high) is activated by IL-6 and induces angiogenesis and the growth of BC cell clusters [85].
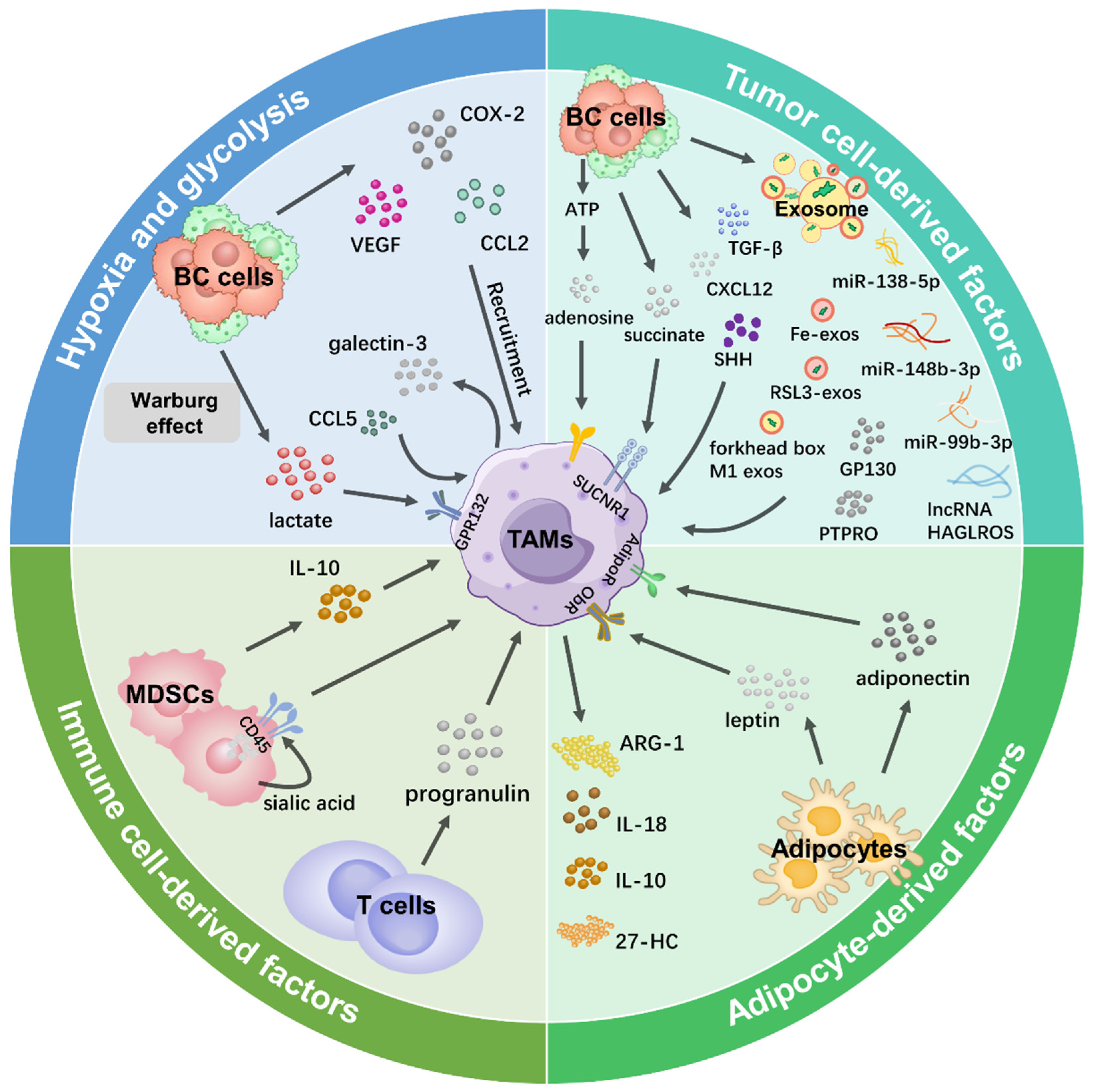
Here, we elucidated the regulatory mechanisms underlying the induction of M1 and M2 TAMs in the Br-TME, with a particular emphasis on M2 TAMs to explore how the regulation of these two macrophage phenotypes occurs in a reciprocal manner (Figure 2).

Figure 2.
TAM modulation by the Br-TME. In four scenarios of the Br-TME, the primary factors that regulate TAMs are shown.
3.2. Hypoxia
Hypoxia is a prominent feature of solid tumors [86]. Long-term hypoxia is a major driving force for cancer progression [87,88]. Hypoxic regions of the Br-TME attract M2 TAMs by releasing the hypoxia-induced chemoattractants vascular endothelial growth factor A (VEGFA), angiopoietin (ANG) 2, CCL18, and prostate cancer-associated transcript 6 (PCAT6). HIF-1 and the nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB) are activated in solid tumors in response to hypoxia to activate the expression of VEGF, COX-2, and monocyte chemoattractant protein 1 (MCP-1, also known as CCL2), which have been shown to recruit macrophages [89,90]. Notably, TAMs are distributed mainly in the hypoxic areas of solid tumors [91,92]. Wang et al. demonstrated that hypoxia induced the synthesis and secretion of galectin-3 from M2 TAMs in an autocrine manner to regulate NF-κB nuclear translocation [93]. By establishing an orthotopic syngeneic mouse model of mammary adenocarcinoma and a metastasis model by tail vein injection, they further confirmed that a galectin-3 inhibitor could suppress BC tumor growth and metastasis in hypoxic areas [94]. The exosomal circular RNA (circ)-0100519 was transcriptionally enhanced by HIF-1α and it was secreted from cancer cells [94]. After engulfed by TAMs, circ-0100519 suppressed nuclear factor-like 2 ubiquitination, leading to M2-TAM polarization.
TAMs synthesize two HIF isotypes in response to hypoxia [95]. HIF-1α is rapidly activated when acute and severe hypoxia (1–2% O2) occurs, and HIF-2α accumulates during prolonged and moderate hypoxia (<5% O2) [96]. HIF-1α and HIF-2α are both upregulated in TAMs within the hypoxic area of BC tumors [97]. Leek et al. reported that TAMs in the Br-TME preferentially migrated to hypoxic regions and strongly expressed HIF-2α, and these phenotypes were associated with high tumor vessel density, high tumor grade, and a poor prognosis in BC patients [98]. HIF-2α expression is an independent prognostic factor for recurrence-free survival and BC-specific survival [99]. Doedens et al. demonstrated in vitro that hypoxia enhanced TAM-mediated T cell suppression by altering HIF-1α expression [100]. Li et al. revealed that the HIF-1α inhibitor Lificiguat inhibited TNBC tumor growth and angiogenesis by transforming M2 TAMs into M1 TAMs in the Br-TME [101].
3.3. Glycolysis
The hypoxia-induced upregulation of glucose transporter-1 promotes glucose uptake for glycolysis to maintain TAM survival [102]. Tumor cells switch to anaerobic glycolysis (the “Warburg effect”) to reduce pyruvate oxidation and increase the excretion of lactate up to 40-fold via monocarboxylate transporter 4 [103]. Lactate and G protein-coupled receptor 132 (GPR132) are key molecules for TAM polarization and BC metastasis [104]. Chen et al. reported that BC cell-derived lactate activated GPR132 in macrophages for M2 phenotype polarization and that the alteration of GPR132 expression could inhibit lung metastasis and prolong the survival of BC patients [104]. Mu et al. reported that lactate stimulated M2 TAMs through activation of the extracellular signal-regulated protein kinase/signal transducer and activator of transcription 3 (STAT3) signaling pathway [105]. Lactate deprivation suppressed M2 phenotype polarization and led to devascularization, highlighting a new strategy for BC treatment. Lin et al. demonstrated that BC cell-derived lactate could also activate the neurogenic locus notch homolog signaling pathway to increase CCL5 secretion from M2 TAMs to form a positive feedback loop of glycolysis between M2 TAMs and BC cells [106]. Zinc finger E-box binding homeobox 1 (Zeb1) is a transcription factor that influences cellular homeostasis [107]. Zeb1 directly upregulates the expression of multiple glycolytic enzymes to promote the “Warburg effect” and BC invasiveness [108]. Jiang et al. reported that the ectopic expression of Zeb1 in BC cells resulted in the secretion of lactate to induce the TAM presentation of CD206, ARG-1, and IL-10 via the protein kinase A/cyclic AMP-responsive element-binding signaling pathway in a conditional knockout mouse model [109]. Niu et al. reported that high expressions of sodium/glucose transporter (SGLT1) in BC cells supported the maintenance of high levels of glycolysis and lactate metabolites, which stimulated the HIF-1α/STAT3 pathway to increase the number of M2 TAMs [110]. M2 TAMs upregulated SGLT1 expression in BC cells via the EGFR/phosphatidylinositol-3-kinase (PI3K)/protein kinase B (AKT) signaling pathway, leading to tamoxifen resistance and accelerating tumor growth both in vitro and in vivo, while the depletion of M2 TAMs induced the opposite effects.
3.4. Other Cell-Derived Factors in the Br-TME
3.4.1. Tumor Cell-Derived Factors
CCL2 is widely expressed in solid tumor cells [111]. CCL2 expression levels are inversely correlated with the expression status of ER and PR [112]. CCL2 release from BC cells mediates the recruitment of peripheral monocytes to the Br-TME, where they differentiate toward the M2 phenotype [61,112]. The enhancer of zeste homolog 2 (EZH2) is a catalytic core subunit of polycomb repressive complex 2, and it interacts with the trimethylation of histone H3 lysine 27 to silence downstream tumor suppressor genes (such as cyclin-dependent kinase inhibitor 1A and E-cadherin) [113]. Archer et al. reported that the EZH2 knockdown in BC cells triggered CCL2 transcription and secretion to promote M2 TAM infiltration into the Br-TME [114]. Sushi domain containing 2 (SUSD2) is a transmembrane protein expressed on BC cells that promotes angiogenesis [115]. Hultgren et al. examined SUSD2 expression and TAM distribution in 175 BC patients by IHC staining and reported that at least 2-fold more M2 TAMs were in the SUSD2high Br-TME [116]. SUSD2-expressing BC cells potentiated angiogenesis indirectly by CCL2 secretion, revealing a positive relationship between SUSD2highCCL2high BC cells and increased proportions of M2 TAMs in BC tissues. Seoane et al. reported that the POU class 1 homeobox 1 transcription factor in BC cells mediated macrophage recruitment and polarization to M2 TAMs by releasing the chemokine (C-X-C motif) ligand (CXCL)12 [117]. Raschioni et al. demonstrated that activation of the C-X-C chemokine receptor 4 (CXCR4)/CXCL12 pathway was associated with the presence of M2 TAMs in primary BC of the luminal B subtype, maintaining the invasiveness of BC cells [118].
Succinate is an intermediate of the tricarboxylic acid cycle within mitochondria whose level is mainly controlled by the tumor suppressor succinate dehydrogenase (SDH) [119]. The gene mutation or activity inhibition of SDH causes the accumulation of succinate in BC cells [120]. Succinate is taken up by the succinate receptor (SUCNR1) on TAMs, and then M2 phenotype polarization is induced via the SUCNR1/PI3K/HIF-1α signaling pathway [121]. BC cells export ATP during hypoxia and chemotherapy [122,123]. Excessive ATP is rapidly degraded to adenosine [124]. Adenosine receptors are expressed on TAMs, and stimulation with adenosine induces M2 TAMs in the Br-TME [125].
Ma et al. demonstrated that BC cells secreted transforming growth factor β (TGF-β) to activate the TGF-β signaling pathway in TAMs, which significantly upregulated the expression of miR-182 in TAMs [126]. MiR-182 directly inhibited TLR4 gene expression in TAMs to inactivate the downstream NF-κB signaling pathway, reducing the release of proinflammatory factors (e.g., TNF-α and IL-12) and promoting the production of the anti-inflammatory factor IL-10.
The hedgehog (Hh) pathway is one of the most active signaling pathways in cancer cells [127]. It is initiated by the binding of one of three sonic hedgehog ligands (SHHs), desert hedgehog, or Indian hedgehog to the patched-1 receptor, followed by smoothened (SMO) release and the transcriptional activation of glioma-associated oncogene homolog 1 (GLI 1) for cell proliferation, differentiation, EMT, and stem cell maintenance [128]. By administering the exogenous Hh/SMO inhibitor vismodegib to female BALB/c mice bearing orthotopic 4T1 tumors, Hanna et al. found that exogenous SHH protein upregulated M2 marker ARG-1 expression and that macrophages were polarized toward the immunosuppressive M2 TAM phenotype [129]. Treatment of TAMs with the small-molecule GLI 1 inhibitor GANT61, which could effectively inhibit the activity of the Hh signaling pathway in BC, restored M1 TAMs, as characterized by the upregulation of inducible nitric oxide synthase (iNOS) and TNF-α expression and the reduction in ARG-1 and CD206 expression, leading to CD8+ T cell trafficking back to the Br-TME where they exerted regulatory effects; these findings provide new ideas and directions for the clinical treatment of BC.
Snail family transcriptional repressor 1 (SNAIL1) is expressed in BC cells as well as in the Br-TME stroma [130]. SNAIL1 expression in primary BC is associated with a higher rate of recurrence, greater tumor aggressiveness, and worse outcomes [131]. Deletion of the SNAIL1 gene significantly inhibits BC metastasis [132]. Brenot et al. established a MMTV-PyMT mouse model in which the SNAIL1 gene was transfected into the TNBC cell line 4T1 [133]. They demonstrated that SNAIL1 inhibited the production of IL-1α, TNF-α, and the granulocyte-macrophage colony-stimulating factor to restrain the proinflammatory responses of TAMs and promote M2 TAM polarization.
Semaphorins (SEMAs) are a family of proteins that include the membrane-bound form of SEMA (e.g., SEMA7A, SEMA4C) and the secreted form of SEMA (e.g., SEMA3A, SEMA4D) [134]. Wallerius et al. reported that BC cell-derived SEMA3A worked through its receptor neuropilin 1 to suppress the proliferation of M2 TAMs in the Br-TME and that SEMA3A expression levels were correlated with the expression levels of genes characteristic of M1 TAMs, CD8+ T cells, and natural killer (NK) cells [135]. M2 TAMs are the main source of SEMA4D, which promotes endothelial cell migration, angiogenesis, and tumor growth in BC mice [136]. SEMA4C stimulates the recruitment of M2 TAMs and upregulates the expression of the proangiogenic factors ANG and CSF1 in the Br-TME [137]. Elder et al. demonstrated that SEMA7A overexpression in involutional breast epithelial cells stimulated the expression of myeloid-derived podoplanin (PDPN) on M2 TAMs, leading to the adhesion of PDPN-expressing M2 TAMs to lymphatic endothelial cells, lymphatic remodeling, and BC metastasis [138].
3.4.2. Exosomes
Tumor-derived exosomes are considered important mediators of the crosstalk between BC cells and the Br-TME [139,140]. The role of tumor-derived exosomes in M2 TAM polarization has been emphasized in recent studies [141]. Ham et al. reported the presence of the IL-6 receptor glycoprotein 130 (GP130) in BC cell-derived exosomes [142]. GP130 phosphorylates STAT3 in macrophages, followed by STAT3 translocation to the nucleus to induce the transcription of IL-6, IL-10, CXCR4, and CCL2 to promote BMDM survival and TAM polarization to the M2 subtype. Piao et al. reported that TNBC-derived exosomes promoted the recruitment of CD206+ARG-1+ TAMs to create a favorable niche for lymph node metastasis [143]. Protein tyrosine phosphatase receptor type O (PTPRO) belongs to the receptor protein tyrosine phosphatase family [144]. PTPRO inhibits HER2-positive BC through dephosphorylation, leading to the dual effects of signaling suppression and the endosomal internalization of HER2 [145]. By generating stable PTPRO-overexpressing BC cells, Dong et al. reported that PTPRO in BC-derived exosomes induced M1 TAMs by mediating the dephosphorylation of STAT3 and STAT6 [146].
MiRNAs are a class of regulatory noncoding RNAs in exosomes [147]. MiRNAs regulate macrophage polarization in the Br-TME by regulating signaling pathways and the expression of multiple transcription factors [148]. Hao et al. isolated exosomes from the culture supernatant of BC cells and established an indirect coculture system of BC cells and TAMs and found that miR-148b-3p was overexpressed in BC cell-derived exosomes and induced M2 TAMs via the tuberous sclerosis complex 2/mTORC1 signaling pathway [149]. Xun et al. reported that lysine demethylase 6B (KDM6B) levels in THP-1 cells were significantly reduced after suspension coculture with the BC cell line MDA-MB-231 [150]. BC-derived exosomal miR-138-5p was shown to inhibit KDM6B expression in TAMs by binding to its 3′-untranslated region. The expression levels of the M1-related markers IL-6, TNF-α, and IL-1β were inhibited, and those of the M2-related markers CD163, ARG-1, IL-10, TGF-β, VEGFA, and CD163 were increased; these effects were partially reversed by treatment with a miR-138-5p inhibitor. Mao et al. used miRNA-seq technology to screen the differentially enriched miRNAs in the exosomes of drug-resistant and drug-susceptible BC cell lines [151]. They reported that BC cells and their exosomes contained high levels of miR-99b-3p and that these exosomes were taken up by TAMs in the Br-TME. MiR-99b-3p directly targeted the serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform to induce the M2 polarization of TAMs, promoting the downstream phosphorylation of AKT/mTOR to stimulate the migration and paclitaxel resistance of BC cells. Meng et al. revealed that the lncRNA HAGLROS was highly expressed in the exosomes of BC cells and that this phenotype was related to a poor prognosis [152]. LncRNA HAGLROS, not only acted as competitive endogenous RNA to compete with miR-135b-3p, which increased the expression of its target gene collagen type X alpha 1 chain, but also phosphorylated STAT3 to induce M2 TAM polarization.
Ferroptosis is a novel form of iron-dependent cell death that plays a crucial role in the occurrence and development of BC [153]. Xiong et al. reported that short-term acidosis-induced ferroptosis in BC cells occurred in a zinc finger protein an1 type domain 5 (ZFAND5)/solute carrier family 3 member 2 (SLC3A2)-dependent manner [154]. Acidosis promoted the expression of ZFAND5, which promoted the ubiquitination process of SLC3A2 and inhibited its protein stability, thus decreasing glutathione synthesis in BC cells and inducing changes in mitochondrial morphology, resulting in the ferroptosis of BC cells. The ferroptosis of BC cells induces the increased expression of M1 markers (such as CD86 and iNOS), the secretion of proinflammatory cytokines (such as TNF-α and IL-12), and the decreased expression of M2 markers (such as CD206 and ARG-1) in TAMs, thereby promoting M1-type macrophage polarization and inhibiting tumor growth. Prognostic analysis revealed that high ZFAND5 expression or low SLC3A2 expression was associated with longer overall survival in BC patients. Yi et al. added the ferroptosis inducer erastin to the culture supernatant of the human MDA-MB-231 cell line and the mouse 4T1 cell line to increase Fe2+ levels and ROS production to induce ferroptosis in BC cells [155]. After treatment with erastin, the exosomes were collected and cocultured with macrophage lines (THP-1 cells and RAW 264.7 cells). The results indicated that ferroptosis-induced BC cell-derived exosomes (Fe-exos) significantly reduced the levels of the M2 markers CD206 and ARG-1 and increased the levels of the M1 markers CD68 and iNOS. Wang et al. generated exosomes derived from M1 TAMs and loaded them with a ferroptosis inducer, yielding RSL3-exos [156]. They reported that RSL3-exos spontaneously localized to and accumulated in the Br-TME and significantly promoted M1 TAMs by inhibiting the secretion of IL-10 and TGF-β, blocking the infiltration of regulatory T cells (Tregs), and suppressing fatty acid β-oxidation. Wang et al. reported that TNBC cells transmitted forkhead box M1-containing exosomes into TAMs for the transcriptional activation of indoleamine 2,3-dioxygenase 1, which increased the level of the metabolite quinolinic acid to impair ferroptosis [157].
3.4.3. Adipocyte-Derived Factors
Breast adipose tissue is the main endocrine system of the mammary gland, where a variety of growth factors and enzymes are secreted to communicate with all components of the Br-TME [158]. Breast adipose tissue contains abundant infiltrated macrophages with a gene expression profile indicating inflammation, including CCR5, peroxisome proliferator-activated receptor (PPAR) α, IL-6, and IL-8 [159].
Adipocytes produce adiponectin (APN) in the Br-TME [160,161]. In response to APN, TAMs exhibit an anti-inflammatory M2 phenotype, and the production of proinflammatory cytokines is suppressed by the inhibition of the phosphorylation of inhibitors of NF-κB, c-Jun N-terminal kinase (JNK), p38, and STAT3 [160]. APN interacts with adiponectin receptors (AdipoRs) to promote M2 subtype polarization [162]. Tan et al. compared the signaling pathways stimulated by AdipoR1 and AdipoR2 [163]. AdipoR1 stimulated IL-10 production by activating the adenosine 5′-monophosphate (AMP)-activated protein kinase and p38 signaling pathways, whereas AdipoR2 regulated inflammation by activating COX-2 and PPARγ.
Leptin is a monocyte/macrophage chemoattractant [164]. It promotes tumor-promoting functions through binding with the canonical leptin receptor (ObR) [165]. Leptin exposure during TAM differentiation results in high levels of M2 TAM markers (ARG-1, IL-10, and CXCR4) and low levels of M1 TAM markers (iNOS and IL-12) [166]. Li et al. reported that leptin treatment increased the expression of a variety of cytokines in TAMs to promote the migration and invasion of BC cells; IL-18 regulation by the NF-κB/NF-κB1 signaling pathway was found to be the most significant change induced by this treatment [167].
Sphingomyelin (SM) is an important component of lipid rafts in the plasma membrane [168,169]. Sphingomyelin synthase 2 (SMS2) is a key enzyme that regulates the SM content [170]. Deng et al. reported that abundant SMS2 was associated with high expression levels of CD206 and ARG-1, large tumor sizes, and the development of lung metastasis [171]. SMS2 inhibitors or SMS2 gene knockdown reduced M2 TAM polarization as well as tumor progression in a 4T1-TNBC mouse model.
Lipid accumulation is closely related to the tumor-promoting function of TAMs. Niu et al. demonstrated that caspase-1 induced the cleavage of aspartic acid at position 64 of PPARγ, which was then transported to mitochondria and interacted with medium-chain acyl-CoA dehydrogenase (MCAD) to inhibit fatty acid oxidation, leading to the accumulation of lipid droplets and M2 TAM polarization [172]. Caspase-1 inhibitors disrupt PPARγ/MCAD activity and repolarize TAMs rather than suppressing their activity. 27-Hydroxycholesterol (27-HC) is a metabolite of cholesterol. Shi et al. reported that cytochrome P450 family 27 subfamily A member 1, the synthetase of 27-HC, was more abundant in the THP-1 cell line than in BC cells and promoted the migration of CCR2- and CCR5-expressing monocytes to the Br-TME and their polarization into M2 TAMs [173]. M2 TAMs produced and released 27-HC, which not only promotes the proliferation, migration, and invasion of BC cells but also further promotes monocyte recruitment, ultimately contributing to the development of BC.
3.4.4. TME Infiltration of Immune Cell-Derived Factors
Circulating myeloid-derived suppressor cells (MDSCs) are present in almost all solid tumors and are contributors to immunosuppression [174]. MDSCs are recruited to the Br-TME [175]. MDSCs trigger M2 TAM polarization in the Br-TME by secreting IL-10 [176]. In hypoxic regions, sialic acid from MDSCs induces the defragmentation of CD45 protein dimers to trigger tyrosine phosphatase activity in CD11b+Ly6ChiLy6G− monocytic MDSCs, followed by TAM differentiation through the STAT3 signaling pathway [177].
Progranulin (PGRN) is a growth factor secreted by T lymphocytes, and its overexpression has been identified in a variety of tumors [178]. Fang et al. treated a BC mouse model with PGRN, and the expression levels of the M2 TAM markers ARG-1 and CD206 were increased, with abundant PD-L1 on their surface [179]. This phenomenon might be reversed by treatment with a static STAT3 signaling inhibitor.
4. The Cancer-Promoting Functions of TAMs in the Br-TME
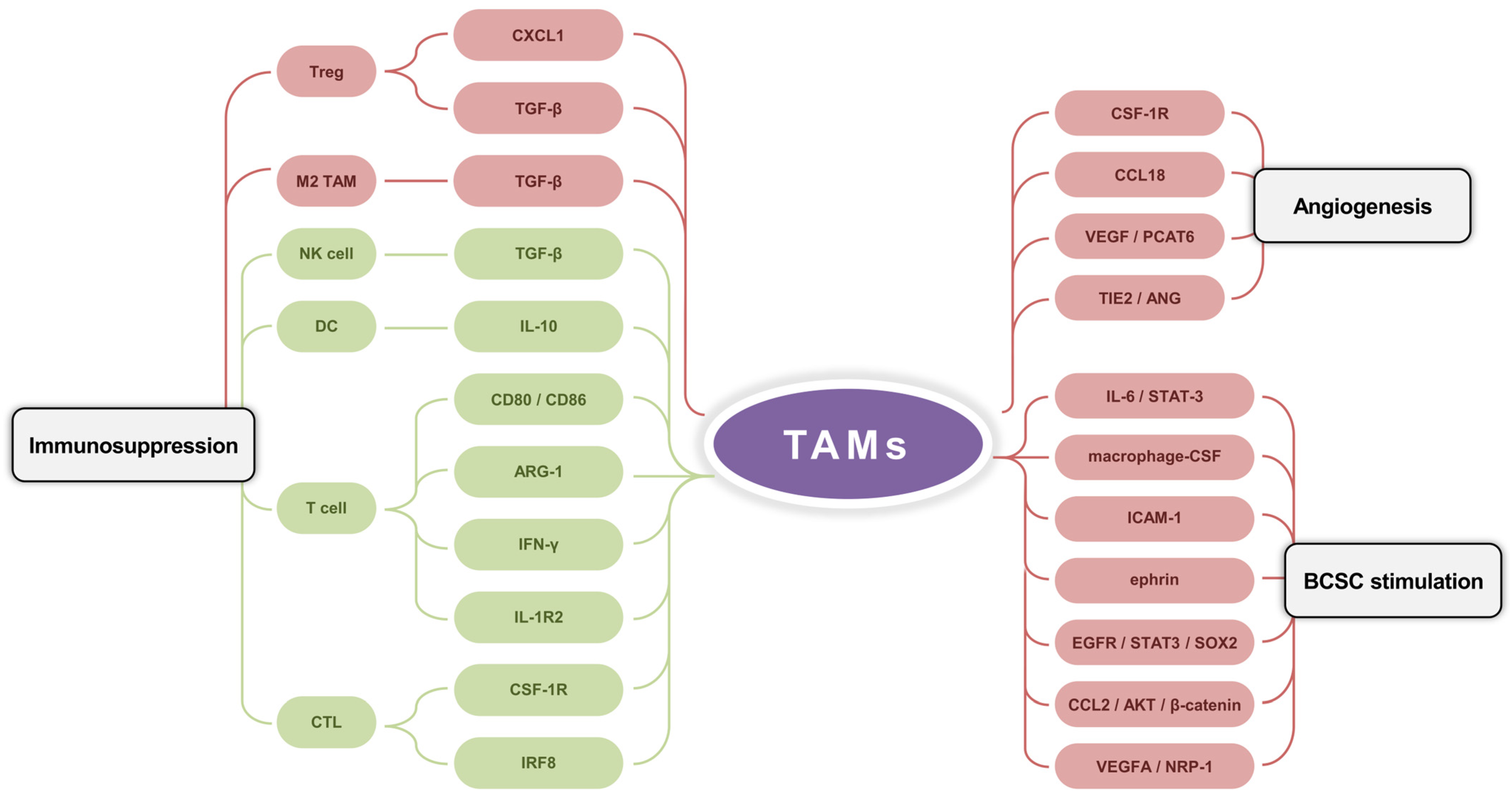
In the Br-TME, TAMs promote tumor progression through the angiogenesis, immunosuppression, and maintenance of BC cell stemness (Figure 3).

Figure 3.
The cancer-promoting functions of TAMs in the Br-TME. TAMs facilitate angiogenesis by multiple factors and pathway cascades (e.g., CSF-1R, CCL18, VEGF/PCAT6, and TIE2/ANG). TAMs stimulate factors (e.g., macrophage-CSF, ICAM-1, and ephrin) and signaling pathways (e.g., IL-6/STAT-3, EGFR/STAT3/SOX2, CCL2/AKT/β-catenin, and VEGFA/NRP-1) to maintain the quantity and functionality of breast cancer stem cells. TAMs regulate the immune microenvironment by enhancing M2 TAM and Treg with TGF-β and CXCL1, and by inhibiting DC, NK cell, T cell, and CTL with TGF-β, IL-10, ARG-1, IFN-γ, IRF8, CD80/CD86, IL-1R2, and CSF-1R.
4.1. Angiogenesis
Angiogenesis is a key limiting step in the processes of tumor growth and metastasis. In the presence of M2 TAMs, angiogenesis is initiated [180]. Lin et al. generated a BC-sensitive transgenic mouse model and found that angiogenic transition and malignant transformation were delayed after knockdown of the CSF1 receptor (CSF-1R) on M2 TAMs [181]. Lin et al. used double IHC staining to assess the expression status of CCL18 in 80 tissue samples from BC patients [182]. The results indicated that the expression level of CCL18 was positively correlated with microvessel density in the Br-TME independent of VEGFR signaling pathway activity. When CCL18 was blocked with a neutralizing antibody, the ability of M2 TAMs to promote migration was suppressed, and angiogenesis was subsequently inhibited.
Dong et al. reported that M2 TAMs secreted the VEGF to stimulate PCAT6 expression and that PCAT6 promoted VEGFR2 expression in TNBC [183]. PCAT6 also participates in the VEGFR/AKT/mTOR signaling pathway to induce angiogenesis in the Br-TME [184]. Tyrosine kinases with immunoglobulin-like and EGF-like domain (TIE) receptors and their ANG ligands have been identified as a second vascular tissue-specific receptor tyrosine kinase system [185,186]. Palma et al. demonstrated that TIE2 was weakly expressed in circulating monocytes but was significantly upregulated during their homing to BC tumors and differentiation into perivascular macrophage subsets [187]. TIE2-expressing macrophages (TEMs) possess characteristics of M2 TAMs and promote angiogenesis and BC development [188]. Mazzieri et al. reported that the binding ability of TEMs to blood vessels was attenuated by the use of ANG blockers in a BC mouse model, followed by the downregulation of TIE2 expression on TEMs and the suppression of blood vessel perfusion in the Br-TME [189].
4.2. Immunosuppression
M2 TAMs in the Br-TME mainly exhibit immunosuppressive features [190]. The depletion of M2 TAMs using CSF-1R antagonists in resected breast tissue from 179 BC patients not only promoted the functions of cytotoxic T lymphocytes (CTLs) in antitumor immunity but also increased chemosensitivity [191]. The interaction between CD80/CD86 on M2 TAMs and the inhibitory T lymphocyte-associated protein 4 receptor on active T cells leads to cell cycle arrest in T cells [192]. M2 TAMs suppress the cytotoxicity of NK cells by releasing anti-inflammatory cytokines such as TGF-β [193]. Ruffell et al. compared the sorted epithelial cell populations and stromal cell populations in the Br-TME and revealed that M2 TAMs were the primary source of IL-10; these cells were found to inhibit IL-12 production by dendritic cells (DCs) to indirectly block the activation and proliferation of CD8+ CTLs [194]. Li et al. constructed a 4T1-Luc metastasis-tracking model of orthotropic BC and demonstrated that M2 TAM-mediated CXCL1 transcriptionally activates the NF-κB/forkhead box protein P3 signaling pathway to promote CD4+ T cell differentiation into Treg, followed by immune escape and lung metastasis [195]. TGF-β derived from M2 TAMs also facilitates Tregs infiltration into the mouse Br-TME and supports their ability to suppress CTLs [196]. Interferon regulatory factor 8 (IRF8) is a transcription factor required for the ability of M2 TAMs to present antigens to tumor cells. Nixon et al. used murine BC models with the myeloid cell-specific ablation of IRF8 and demonstrated that M2 TAMs caused the depletion of BC cell-reactive CTLs via IRF8 [197]. TAM-specific IRF8 deletion restored the CTL population and inhibited BC tumor growth.
L-arginine is a substrate of iNOS, a molecular marker of M1 TAMs, and it is responsible for the cytotoxic role of macrophages [192]. The increased levels of L-arginine caused the transition from glycolysis to oxidative phosphorylation in activated T cells and promoted the generation of central memory-like T cells with increased survival ability, increasing the antitumor activity of T cells [198]. The expression levels of ARG-1 are relatively high in BC patients [199]. ARG-1 hydrolyzes L-arginine [200]. ARG-1 catalyzes the conversion of L-arginine to urea and L-ornithine in TAMs, which deprives T cells of the raw materials essential for their proliferation [201,202]. L-ornithine promotes cell proliferation and repairs tissue injury through the generation of polyamines and collagen, both of which are highly immunosuppressive in the Br-TME [203].
M2 TAMs regulate the PD-1/PD-L1 axis [204]. M2 TAMs secrete IFN-γ to activate the signaling pathways of Janus kinase/STAT3 and PI3K/AKT to upregulate PD-L1 expression, inducing the apoptosis and immune anergy of tumor-specific T cells [205]. M2 TAMs enhance inhibitory activity through TGF-β-induced M2 TAM polarization and PD-L1 expression [206]. Xia et al. demonstrated that M2 TAMs promoted TNBC tumor growth via IL-1β-activated IL-1 receptor 2 (IL-1R2), whereas the IL-1R2 blockade strongly attenuated macrophage recruitment, M2 TAM polarization, and CD8+ T cell depletion, thereby reducing the tumor burden and prolonging the survival period [207]. Moreover, the use of an anti-IL-1R2 neutralizing antibody further increased the antitumor efficacy of a PD-1 antagonist.
4.3. Stimulation of Breast Cancer Stem Cells
Al-Hajj et al. isolated breast cancer stem cells (BCSCs) from primary or metastatic sites in nine patients and proposed that BCSCs serve as the origin of the tumorigenic population of cancer cells [208]. BCSCs have self-renewal and multidirectional differentiation abilities and proliferation potential. Moreover, BCSCs also possess the general characteristics of cancer cells, such as high tumorigenicity, high metastasis potential, and resistance to chemotherapy [209]. Radharani et al. induced RAW264.7 cell transformation into TAMs via culture with conditioned media from the murine 4T1 cell line; they reported that TAM-conditioned media enriched the BCSC populations and increased the expression levels of the BCSC-specific transcription factors sex determining region Y-box 2 (SOX-2), octamer-binding transcription factor-3, octamer-binding transcription factor-4, and nanog homeobox [210]. They further demonstrated that IL-6 derived from TAMs played a key role in BCSC enrichment through the activation of STAT3. Lu et al. reported that M2 TAMs secreted macrophage-CSF, intercellular cell adhesion molecule-1, and ephrin to increase the survival, renewal, and tumorigenicity of BCSCs [211]. Yang et al. reported that M2 TAMs stimulated the BCSC phenotype and tumorigenesis by activating the EGFR/STAT3/SOX2 signaling pathway [212].
CD44+/CD24− BCSCs are enriched in TNBC and are associated with the invasive behavior of TNBC [213]. β-catenin is a key factor in the Wnt signaling pathway [214]. Silencing β-catenin significantly reduces the proportion of CD44+/CD24− BCSCs and the invasion ability of BC cells, which provides a new strategy for TNBC treatment [215]. Chen et al. demonstrated that M2 TAMs significantly promoted the stemness of the TNBC cell lines BT549 and HCC1937 by activating the CCL2/AKT/β-catenin signaling pathway [216]. Neuropilin-1 (NRP-1) is a transmembrane glycoprotein receptor that is highly expressed in BC and participates in cell proliferation, invasion, migration, and EMT [217,218]. Wang et al. reported that M2 TAMs secreted VEGFA to promote the stemness of TNBC cells and that the VEGFA/NRP-1 axis triggered the downstream Wnt/β-catenin signaling pathway to induce the BCSC phenotype [219].
5. Therapeutic Strategies and Prospects
TAMs are among the most important components of the Br-TME. Targeting TAMs is related to a series of emerging strategies for BC treatment, including limiting monocyte recruitment and TAM reprogramming [220].
Blockade of the CCL2/CCR2 axis markedly reduces the rate at which BC progresses by preventing the accumulation of TAMs in the Br-TME and increasing the antitumor efficacy of surrounding CD8+ T cells [192]. Nevertheless, several clinical trials have shown that CCL2 concentration is only transiently suppressed after the administration of a CCL2 neutralizing antibody (CNTO 888) and that CCL2 levels might exceed pretreatment baseline levels to cause the hypermetastasis of BC cells [221,222]. The blockade of CSF-1R has been shown to decrease M2 TAM infiltration and improve the chemotherapeutic response [191]. Compared with paclitaxel or carboplatin alone, the combination of CSF-1R signaling antagonists with paclitaxel or carboplatin exhibited enhanced antitumor efficacy and suppressed metastasis in preclinical BC models [223]. To date, the inhibition of CSF-1R has yet to translate into objective clinical responses. This is, in part, attributed to the elevated frequency of neutrophils and the depletion of anti-metastatic CD169+ lymph node macrophages [224,225].
Targeting immune inhibitory molecules on TAMs has also been considered as a strategy. RP-182 is a synthetic 10-mer amphipathic analog of host defense peptides that can selectively induce a conformational switch of the mannose receptor CD206 protein on the surface of M2 TAMs for phenotypic reprogramming [226]. CD40 agonists, PI3Kγ inhibitors, CD47 inhibitors, and histone deacetylase inhibitor subgroup II can suppress the growth of primary and metastatic murine BC through the conversion of M2 TAMs to M1 TAMs [203]. Bruton’s tyrosine kinase inhibitors, TLR agonists, STAT3 inhibitors, and inhibitors of interleukin-1 receptor antagonists have also been shown to block BC progression by interfering with signaling pathways related to M2 phenotype polarization [220]. The ROS regulator manganese(III) 5,10,15,20-tetrakis(1-ethyl-2-pyridinio) porphyrin pentachloride inhibits the IL-4-stimulated polarization of M2 TAMs by reducing STAT3 activation [227]. T. mongolicum extract has been used to treat breast inflammation and nodules. It remodels the Br-TME by inhibiting the IL-10/STAT3/PD-L1 immunosuppressive signaling pathway and by promoting the repolarization of M2 TAMs to suppress the proliferation, migration, and invasion of TNBC cells [228]. Poly (ADP–ribose) polymerase (PARP) is involved in the base excision repair process for DNA single-strand breaks. PARP inhibitors (PARPis) induce synthetic lethality in BC patients carrying loss-of-function mutations in breast cancer susceptibility gene 1 (BRCA1) and BRCA2 and are routinely used in the clinical treatment of metastatic BC [229]. However, Litton et al. reported that BC cells with the BRCA1 gene defects strongly promoted M2 TAM infiltration and that the therapeutic response to PARPis was poor [230]. Wang et al. demonstrated that agonists of the small-molecule stimulator of interferon genes effectively reprogramed M2 TAMs into an antitumorigenic state, characterized by the induction of type I IFN responses and the expression of the costimulatory molecule CD86, and that the expression of CD86 could stimulate T cell cross-priming, increasing the sensitivity of BC cells with BRCA1 and BRCA2 mutations to PARPi treatment [231].
In recent years, many drugs have been developed to precisely regulate TAMs. However, the clinical applications remain limited due to the shortcomings of these agents, such as their poor solubility, rapid metabolism, nonselectivity, and off-target effects. It is also a challenge to modulate TAM phenotypes while maintaining their antitumor activities. Nanodrug delivery systems (NDDSs) have emerged as novel technologies for BC treatment. They offer advantages in enhancing the stability of macromolecules and promoting their cellular uptake within the Br-TME [232]. Li et al. devised a reactive oxygen species/glutathione dual-responsive drug conveyance platform (CUR/miR155@DssD-Hb NPs) to codeliver curcumin (CUR) and miR-155 [233]. CUR could promote the transformation of Tregs to type I helper T cells, induce the polarization of M2 TAMs toward M1 TAMs, and prevent the recruitment and aggregation of MDSCs in the Br-TME. The overexpression of miR-155 in M2 TAMs promoted their repolarization. Both in vitro and in vivo results revealed that the platform effectively reduced the proportions of viable BC cells and immunosuppressive cells (including MDSCs, Tregs, M2 TAMs, and exhausted T cells), stimulated DC maturation, and subsequently stimulated CD8+ T cell activation. To improve the chemotherapeutic effect in TNBC, Zhang et al. developed novel PANPs that were shelled with poly(lactic-co-glycolic acid) (PLGA) and loaded with perfluoropentane (PFP) for real-time tracking, paclitaxel, and an anti-miR-221 inhibitor [234]. TAMs were applied to internalize PANPs as RAW-PANPs, and they accumulated around BC cells to deliver PANPs. The results showed that RAW-PANPs inhibited both TNBC cell proliferation in vitro and BC tumor growth and progression in vivo, which had strong potential for treating TNBC patients presenting drug resistance. Clodronate is an important alternative for TAM removal and has been proven to effectively reduce tumor volume and weight in a 4T1 mouse model [235]. CL improves the pharmacokinetics of clodronate. Gu et al. reported that CL could deplete TAMs and significantly inhibit BC recurrence and bone metastasis [236]. Hydrazinocurcumin (HC) is a pyrazole derivative of curcumin that exerts antitumor effects through the repolarization of TAMs in the Br-TME [237]. Kumari et al. synthesized self-assembled amphiphilic PEGylated galactomannan (GM) nanoparticles loaded with HC (PSGM-HCNPs), and the results revealed that PSGM-HCNPs repolarized IL-4-stimulated RAW 264.7 cells by increasing ROS levels, decreasing CD206 and ARG-1 expression, and promoting proinflammatory cytokine secretion, thus reducing the tumor burden and prolonging the survival time of Ehrlich’s ascites carcinoma-bearing mice [238].
The recognition of TAM heterogeneity is becoming increasingly crucial for personalized BC therapy. However, the complexity of the regulatory mechanisms of macrophage polarization is still elusive, and there may be a variety of unknown signaling pathways and regulators; this complexity makes it difficult to identify the core regulatory factors of TAMs and develop new landmark drugs. It is imperative to carry out more comprehensive and in-depth research on the diverse interactions involving TAMs in the Br-TME.
Author Contributions
L.M. and Y.J. contributed equally and share first authorship to this work. Conceptualization, J.Z. and J.X.; writing—original draft preparation, L.M.; writing—review and editing, Y.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Nos. 81501817 and 82202610) and the Jiangsu Province Capability Improvement Project through Science, Technology and Education (No: ZDXK202239).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Grabinski, V.F.; Brawley, O.W. Disparities in Breast Cancer. Obstet. Gynecol. Clin. N. Am. 2022, 49, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Britt, K.L.; Cuzick, J.; Phillips, K.A. Key steps for effective breast cancer prevention. Nat. Rev. Cancer 2020, 20, 417–436. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Lei, S.; Zheng, R.; Zhang, S.; Wang, S.; Chen, R.; Sun, K.; Zeng, H.; Zhou, J.; Wei, W. Global patterns of breast cancer incidence and mortality: A population-based cancer registry data analysis from 2000 to 2020. Cancer Commun. 2021, 41, 1183–1194. [Google Scholar] [CrossRef]
- Liu, Y.-M.; Ge, J.-Y.; Chen, Y.-F.; Liu, T.; Chen, L.; Liu, C.-C.; Ma, D.; Chen, Y.-Y.; Cai, Y.-W.; Xu, Y.-Y. Combined Single-Cell and Spatial Transcriptomics Reveal the Metabolic Evolvement of Breast Cancer during Early Dissemination. Adv. Sci. 2023, 10, e2205395. [Google Scholar] [CrossRef]
- Neagu, A.N.; Whitham, D.; Buonanno, E.; Jenkins, A.; Alexa-Stratulat, T.; Tamba, B.I.; Darie, C.C. Proteomics and its applications in breast cancer. Am. J. Cancer Res. 2021, 11, 4006–4049. [Google Scholar]
- Tsang, J.Y.S.; Tse, G.M. Molecular Classification of Breast Cancer. Adv. Anat. Pathol. 2020, 27, 27–35. [Google Scholar] [CrossRef]
- De Azambuja, E.; Cardoso, F.; de Castro, G., Jr.; Colozza, M.; Mano, M.S.; Durbecq, V.; Sotiriou, C.; Larsimont, D.; Piccart-Gebhart, M.J.; Paesmans, M. Ki-67 as prognostic marker in early breast cancer: A meta-analysis of published studies involving 12,155 patients. Br. J. Cancer 2007, 96, 1504–1513. [Google Scholar] [CrossRef]
- Prat, A.; Cheang, M.C.; Martín, M.; Parker, J.S.; Carrasco, E.; Caballero, R.; Tyldesley, S.; Gelmon, K.; Bernard, P.S.; Nielsen, T.O.; et al. Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal A breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 203–209. [Google Scholar] [CrossRef]
- Khan, S.A.; Rogers, M.A.; Obando, J.A.; Tamsen, A. Estrogen receptor expression of benign breast epithelium and its association with breast cancer. Cancer Res. 1994, 54, 993–997. [Google Scholar]
- Creighton, C.J.; Kent Osborne, C.; van de Vijver, M.J.; Foekens, J.A.; Klijn, J.G.; Horlings, H.M.; Nuyten, D.; Wang, Y.; Zhang, Y.; Chamness, G.C.; et al. Molecular profiles of progesterone receptor loss in human breast tumors. Breast Cancer Res. Treat. 2009, 114, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Corti, C.; Giugliano, F.; Nicolò, E.; Ascione, L.; Curigliano, G. Antibody-Drug Conjugates for the Treatment of Breast Cancer. Cancers 2021, 13, 2898. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef] [PubMed]
- Gennari, A.; André, F.; Barrios, C.H.; Cortés, J.; de Azambuja, E.; DeMichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.A.; et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef]
- Dwivedi, S.; Purohit, P.; Misra, R.; Lingeswaran, M.; Vishnoi, J.R.; Pareek, P.; Misra, S.; Sharma, P. Single Cell Omics of Breast Cancer: An Update on Characterization and Diagnosis. Indian J. Clin. Biochem. 2019, 34, 3–18. [Google Scholar] [CrossRef]
- Vernieri, C.; Milano, M.; Brambilla, M.; Mennitto, A.; Maggi, C.; Cona, M.S.; Prisciandaro, M.; Fabbroni, C.; Celio, L.; Mariani, G.; et al. Resistance mechanisms to anti-HER2 therapies in HER2-positive breast cancer: Current knowledge, new research directions and therapeutic perspectives. Crit. Rev. Oncol./Hematol. 2019, 139, 53–66. [Google Scholar] [CrossRef]
- Bou Zerdan, M.; Ghorayeb, T.; Saliba, F.; Allam, S.; Bou Zerdan, M.; Yaghi, M.; Bilani, N.; Jaafar, R.; Nahleh, Z. Triple Negative Breast Cancer: Updates on Classification and Treatment in 2021. Cancers 2022, 14, 1253. [Google Scholar] [CrossRef]
- Vagia, E.; Mahalingam, D.; Cristofanilli, M. The Landscape of Targeted Therapies in TNBC. Cancers 2020, 12, 916. [Google Scholar] [CrossRef]
- Pusztai, L.; Denkert, C.; O’Shaughnessy, J.; Cortes, J.; Dent, R.; McArthur, H.; Kümmel, S.; Bergh, J.; Park, Y.H.; Hui, R.; et al. Event-free survival by residual cancer burden with pembrolizumab in early-stage TNBC: Exploratory analysis from KEYNOTE-522. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2024, 35, 429–436. [Google Scholar] [CrossRef]
- Jiang, Y.-Z.; Ma, D.; Suo, C.; Shi, J.; Xue, M.; Hu, X.; Xiao, Y.; Yu, K.-D.; Liu, Y.-R.; Yu, Y.; et al. Genomic and Transcriptomic Landscape of Triple-Negative Breast Cancers: Subtypes and Treatment Strategies. Cancer Cell 2019, 35, 428–440.e425. [Google Scholar] [CrossRef]
- Fan, L.; Wang, Z.-H.; Ma, L.-X.; Wu, S.-Y.; Wu, J.; Yu, K.-D.; Sui, X.-Y.; Xu, Y.; Liu, X.-Y.; Chen, L.; et al. Optimising first-line subtyping-based therapy in triple-negative breast cancer (FUTURE-SUPER): A multi-cohort, randomised, phase 2 trial. Lancet Oncol. 2024, 25, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Mehraj, U.; Dar, A.H.; Wani, N.A.; Mir, M.A. Tumor microenvironment promotes breast cancer chemoresistance. Cancer Chemother. Pharmacol. 2021, 87, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Bahcecioglu, G.; Basara, G.; Ellis, B.W.; Ren, X.; Zorlutuna, P. Breast cancer models: Engineering the tumor microenvironment. Acta Biomater. 2020, 106, 1–21. [Google Scholar] [CrossRef]
- Soysal, S.D.; Tzankov, A.; Muenst, S.E. Role of the Tumor Microenvironment in Breast Cancer. Pathobiology 2015, 82, 142–152. [Google Scholar] [CrossRef]
- Junttila, M.R.; de Sauvage, F.J. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 2013, 501, 346–354. [Google Scholar] [CrossRef]
- Deepak, K.G.K.; Vempati, R.; Nagaraju, G.P.; Dasari, V.R.; Nagini, S.; Rao, D.N.; Malla, R.R. Tumor microenvironment: Challenges and opportunities in targeting metastasis of triple negative breast cancer. Pharmacol. Res. 2020, 153, 104683. [Google Scholar] [CrossRef]
- Mukhtar, R.A.; Nseyo, O.; Campbell, M.J.; Esserman, L.J. Tumor-associated macrophages in breast cancer as potential biomarkers for new treatments and diagnostics. Expert Rev. Mol. Diagn. 2011, 11, 91–100. [Google Scholar] [CrossRef]
- Beck, A.H.; Espinosa, I.; Edris, B.; Li, R.; Montgomery, K.; Zhu, S.; Varma, S.; Marinelli, R.J.; van de Rijn, M.; West, R.B. The macrophage colony-stimulating factor 1 response signature in breast carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 778–787. [Google Scholar] [CrossRef]
- Cassetta, L.; Fragkogianni, S.; Sims, A.H.; Swierczak, A.; Forrester, L.M.; Zhang, H.; Soong, D.Y.H.; Cotechini, T.; Anur, P.; Lin, E.Y.; et al. Human Tumor-Associated Macrophage and Monocyte Transcriptional Landscapes Reveal Cancer-Specific Reprogramming, Biomarkers, and Therapeutic Targets. Cancer Cell 2019, 35, 588–602.e510. [Google Scholar] [CrossRef]
- Finak, G.; Bertos, N.; Pepin, F.; Sadekova, S.; Souleimanova, M.; Zhao, H.; Chen, H.; Omeroglu, G.; Meterissian, S.; Omeroglu, A.; et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat. Med. 2008, 14, 518–527. [Google Scholar] [CrossRef]
- Tiainen, S.; Tumelius, R.; Rilla, K.; Hämäläinen, K.; Tammi, M.; Tammi, R.; Kosma, V.-M.; Oikari, S.; Auvinen, P. High numbers of macrophages, especially M2-like (CD163-positive), correlate with hyaluronan accumulation and poor outcome in breast cancer. Histopathology 2015, 66, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Acerbi, I.; Cassereau, L.; Dean, I.; Shi, Q.; Au, A.; Park, C.; Chen, Y.Y.; Liphardt, J.; Hwang, E.S.; Weaver, V.M. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr. Biol. Quant. Biosci. Nano Macro 2015, 7, 1120–1134. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, G.; Liu, H. Tenascin-C: A Key Regulator in Angiogenesis during Wound Healing. Biomolecules 2022, 12, 1689. [Google Scholar] [CrossRef] [PubMed]
- Hongu, T.; Pein, M.; Insua-Rodríguez, J.; Gutjahr, E.; Mattavelli, G.; Meier, J.; Decker, K.; Descot, A.; Bozza, M.; Harbottle, R.; et al. Perivascular tenascin C triggers sequential activation of macrophages and endothelial cells to generate a pro-metastatic vascular niche in the lungs. Nat. Cancer 2022, 3, 486–504. [Google Scholar] [CrossRef]
- Deligne, C.; Murdamoothoo, D.; Gammage, A.N.; Gschwandtner, M.; Erne, W.; Loustau, T.; Marzeda, A.M.; Carapito, R.; Paul, N.; Velazquez-Quesada, I.; et al. Matrix-Targeting Immunotherapy Controls Tumor Growth and Spread by Switching Macrophage Phenotype. Cancer Immunol. Res. 2020, 8, 368–382. [Google Scholar] [CrossRef]
- Davies, L.C.; Taylor, P.R. Tissue-resident macrophages: Then and now. Immunology 2015, 144, 541–548. [Google Scholar] [CrossRef]
- Dawson, C.A.; Pal, B.; Vaillant, F.; Gandolfo, L.C.; Liu, Z.; Bleriot, C.; Ginhoux, F.; Smyth, G.K.; Lindeman, G.J.; Mueller, S.N.; et al. Tissue-resident ductal macrophages survey the mammary epithelium and facilitate tissue remodelling. Nat. Cell Biol. 2020, 22, 546–558. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Biswas, M. Understanding tissue-resident macrophages unlocks the potential for novel combinatorial strategies in breast cancer. Front. Immunol. 2024, 15, 1375528. [Google Scholar] [CrossRef]
- Ingman, W.V.; Wyckoff, J.; Gouon-Evans, V.; Condeelis, J.; Pollard, J.W. Macrophages promote collagen fibrillogenesis around terminal end buds of the developing mammary gland. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2006, 235, 3222–3229. [Google Scholar] [CrossRef]
- Sullivan, A.R.; Pixley, F.J. CSF-1R signaling in health and disease: A focus on the mammary gland. J. Mammary Gland Biol. Neoplasia 2014, 19, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Johnson, K.C.C.; Gatti-Mays, M.E.; Li, Z. Emerging strategies in targeting tumor-resident myeloid cells for cancer immunotherapy. J. Hematol. Oncol. 2022, 15, 118. [Google Scholar] [CrossRef] [PubMed]
- Güç, E.; Pollard, J.W. Redefining macrophage and neutrophil biology in the metastatic cascade. Immunity 2021, 54, 885–902. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. FOLR2+ Macrophages Are Associated with T-cell Infiltration and Improved Prognosis. Cancer Discov. 2022, 12, 1407. [Google Scholar] [CrossRef]
- Nalio Ramos, R.; Missolo-Koussou, Y.; Gerber-Ferder, Y.; Bromley, C.P.; Bugatti, M.; Núñez, N.G.; Tosello Boari, J.; Richer, W.; Menger, L.; Denizeau, J.; et al. Tissue-resident FOLR2(+) macrophages associate with CD8(+) T cell infiltration in human breast cancer. Cell 2022, 185, 1189–1207.e1125. [Google Scholar] [CrossRef]
- Corgnac, S.; Boutet, M.; Kfoury, M.; Naltet, C.; Mami-Chouaib, F. The Emerging Role of CD8(+) Tissue Resident Memory T (T(RM)) Cells in Antitumor Immunity: A Unique Functional Contribution of the CD103 Integrin. Front. Immunol. 2018, 9, 1904. [Google Scholar] [CrossRef]
- Linde, N.; Casanova-Acebes, M.; Sosa, M.S.; Mortha, A.; Rahman, A.; Farias, E.; Harper, K.; Tardio, E.; Torres, I.R.; Jones, J.; et al. Macrophages orchestrate breast cancer early dissemination and metastasis. Nat. Commun. 2018, 9, 21. [Google Scholar] [CrossRef]
- Ginhoux, F.; Jung, S. Monocytes and macrophages: Developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 2014, 14, 392–404. [Google Scholar] [CrossRef]
- Van Rooijen, N.; Sanders, A.; van den Berg, T.K. Apoptosis of macrophages induced by liposome-mediated intracellular delivery of clodronate and propamidine. J. Immunol. Methods 1996, 193, 93–99. [Google Scholar] [CrossRef]
- Hirano, R.; Okamoto, K.; Shinke, M.; Sato, M.; Watanabe, S.; Watanabe, H.; Kondoh, G.; Kadonosono, T.; Kizaka-Kondoh, S. Tissue-resident macrophages are major tumor-associated macrophage resources, contributing to early TNBC development, recurrence, and metastases. Commun. Biol. 2023, 6, 144. [Google Scholar] [CrossRef]
- Laviron, M.; Petit, M.; Weber-Delacroix, E.; Combes, A.J.; Arkal, A.R.; Barthélémy, S.; Courau, T.; Hume, D.A.; Combadière, C.; Krummel, M.F.; et al. Tumor-associated macrophage heterogeneity is driven by tissue territories in breast cancer. Cell Rep. 2022, 39, 110865. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef] [PubMed]
- Panni, R.Z.; Linehan, D.C.; DeNardo, D.G. Targeting tumor-infiltrating macrophages to combat cancer. Immunotherapy 2013, 5, 1075–1087. [Google Scholar] [CrossRef]
- Mondini, M.; Loyher, P.L.; Hamon, P.; Gerbé de Thoré, M.; Laviron, M.; Berthelot, K.; Clémenson, C.; Salomon, B.L.; Combadière, C.; Deutsch, E.; et al. CCR2-Dependent Recruitment of Tregs and Monocytes Following Radiotherapy Is Associated with TNFα-Mediated Resistance. Cancer Immunol. Res. 2019, 7, 376–387. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Lin, L.; Du, L.; Ding, Y.; Zheng, F.; Xie, H.; Wang, Y.; Hu, M.; Liu, B.; et al. Macrophages promote pre-metastatic niche formation of breast cancer through aryl hydrocarbon receptor activity. Signal Transduct. Target. Ther. 2024, 9, 352. [Google Scholar] [CrossRef]
- Kitamura, T.; Doughty-Shenton, D.; Cassetta, L.; Fragkogianni, S.; Brownlie, D.; Kato, Y.; Carragher, N.; Pollard, J.W. Monocytes Differentiate to Immune Suppressive Precursors of Metastasis-Associated Macrophages in Mouse Models of Metastatic Breast Cancer. Front. Immunol. 2017, 8, 2004. [Google Scholar] [CrossRef]
- Cotechini, T.; Atallah, A.; Grossman, A. Tissue-Resident and Recruited Macrophages in Primary Tumor and Metastatic Microenvironments: Potential Targets in Cancer Therapy. Cells 2021, 10, 960. [Google Scholar] [CrossRef]
- Qian, B.; Deng, Y.; Im, J.H.; Muschel, R.J.; Zou, Y.; Li, J.; Lang, R.A.; Pollard, J.W. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS ONE 2009, 4, e6562. [Google Scholar] [CrossRef]
- Lee, C.; Kim, M.J.; Kumar, A.; Lee, H.W.; Yang, Y.; Kim, Y. Vascular endothelial growth factor signaling in health and disease: From molecular mechanisms to therapeutic perspectives. Signal Transduct. Target. Ther. 2025, 10, 170. [Google Scholar] [CrossRef]
- Qian, B.Z.; Zhang, H.; Li, J.; He, T.; Yeo, E.J.; Soong, D.Y.; Carragher, N.O.; Munro, A.; Chang, A.; Bresnick, A.R.; et al. FLT1 signaling in metastasis-associated macrophages activates an inflammatory signature that promotes breast cancer metastasis. J. Exp. Med. 2015, 212, 1433–1448. [Google Scholar] [CrossRef]
- Qian, B.Z.; Li, J.; Zhang, H.; Kitamura, T.; Zhang, J.; Campion, L.R.; Kaiser, E.A.; Snyder, L.A.; Pollard, J.W. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 2011, 475, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, T.; Qian, B.Z.; Soong, D.; Cassetta, L.; Noy, R.; Sugano, G.; Kato, Y.; Li, J.; Pollard, J.W. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J. Exp. Med. 2015, 212, 1043–1059. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Dev, K.; Agarwal, B.; Das, P.; Syed, M.A. Macrophages: Their role, activation and polarization in pulmonary diseases. Immunobiology 2018, 223, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Fan, Z.; Liu, P.; Chen, L.; Guan, Z.; Liu, Y.; Luo, Y. Anemoside A3 activates TLR4-dependent M1-phenotype macrophage polarization to represses breast tumor growth and angiogenesis. Toxicol. Appl. Pharmacol. 2021, 432, 115755. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.; Li, Z.; Zhu, B. Metabolic regulatory crosstalk between tumor microenvironment and tumor-associated macrophages. Theranostics 2021, 11, 1016–1030. [Google Scholar] [CrossRef]
- Yu, T.; Di, G. Role of tumor microenvironment in triple-negative breast cancer and its prognostic significance. Chin. J. Cancer Res. = Chung-Kuo Yen Cheng Yen Chiu 2017, 29, 237–252. [Google Scholar] [CrossRef]
- Salmaninejad, A.; Valilou, S.F.; Soltani, A.; Ahmadi, S.; Abarghan, Y.J.; Rosengren, R.J.; Sahebkar, A. Tumor-associated macrophages: Role in cancer development and therapeutic implications. Cell. Oncol. 2019, 42, 591–608. [Google Scholar] [CrossRef]
- Malekghasemi, S.; Majidi, J.; Baghbanzadeh, A.; Abdolalizadeh, J.; Baradaran, B.; Aghebati-Maleki, L. Tumor-Associated Macrophages: Protumoral Macrophages in Inflammatory Tumor Microenvironment. Adv. Pharm. Bull. 2020, 10, 556–565. [Google Scholar] [CrossRef]
- Rhee, I. Diverse macrophages polarization in tumor microenvironment. Arch. Pharmacal Res. 2016, 39, 1588–1596. [Google Scholar] [CrossRef]
- Bernsmeier, C.; van der Merwe, S.; Périanin, A. Innate immune cells in cirrhosis. J. Hepatol. 2020, 73, 186–201. [Google Scholar] [CrossRef]
- Salpeter, S.J.; Pozniak, Y.; Merquiol, E.; Ben-Nun, Y.; Geiger, T.; Blum, G. A novel cysteine cathepsin inhibitor yields macrophage cell death and mammary tumor regression. Oncogene 2015, 34, 6066–6078. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P. The interaction of anticancer therapies with tumor-associated macrophages. J. Exp. Med. 2015, 212, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Canli, O.; Nicolas, A.M.; Gupta, J.; Finkelmeier, F.; Goncharova, O.; Pesic, M.; Neumann, T.; Horst, D.; Löwer, M.; Sahin, U.; et al. Myeloid Cell-Derived Reactive Oxygen Species Induce Epithelial Mutagenesis. Cancer Cell 2017, 32, 869–883.e865. [Google Scholar] [CrossRef] [PubMed]
- Henze, A.T.; Mazzone, M. The impact of hypoxia on tumor-associated macrophages. J. Clin. Investig. 2016, 126, 3672–3679. [Google Scholar] [CrossRef]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Obeid, E.; Nanda, R.; Fu, Y.X.; Olopade, O.I. The role of tumor-associated macrophages in breast cancer progression. Int. J. Oncol. 2013, 43, 5–12. [Google Scholar] [CrossRef]
- Ambarus, C.A.; Krausz, S.; van Eijk, M.; Hamann, J.; Radstake, T.R.; Reedquist, K.A.; Tak, P.P.; Baeten, D.L.P. Systematic validation of specific phenotypic markers for in vitro polarized human macrophages. J. Immunol. Methods. 2012, 375, 196–206. [Google Scholar] [CrossRef]
- Little, A.C.; Pathanjeli, P.; Wu, Z.; Bao, L.; Goo, L.E.; Yates, J.A.; Oliver, C.R.; Soellner, M.B.; Merajver, S.D. IL-4/IL-13 Stimulated Macrophages Enhance Breast Cancer Invasion Via Rho-GTPase Regulation of Synergistic VEGF/CCL-18 Signaling. Front. Oncol. 2019, 9, 456. [Google Scholar] [CrossRef]
- Wang, L.X.; Zhang, S.X.; Wu, H.J.; Rong, X.L.; Guo, J. M2b macrophage polarization and its roles in diseases. J. Leukoc. Biol. 2019, 106, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ji, X.; Kang, N.; Zhou, J.; Liang, X.; Li, J.; Han, T.; Zhao, C.; Yang, T. Tumor necrosis factor α inhibition overcomes immunosuppressive M2b macrophage-induced bevacizumab resistance in triple-negative breast cancer. Cell Death Dis. 2020, 11, 993. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef]
- Sousa, S.; Brion, R.; Lintunen, M.; Kronqvist, P.; Sandholm, J.; Mönkkönen, J.; Kellokumpu-Lehtinen, P.L.; Lauttia, S.; Tynninen, O.; Joensuu, H.; et al. Human breast cancer cells educate macrophages toward the M2 activation status. Breast Cancer Res. 2015, 17, 101. [Google Scholar] [CrossRef]
- Cao, W.; Peters, J.H.; Nieman, D.; Sharma, M.; Watson, T.; Yu, J. Macrophage subtype predicts lymph node metastasis in oesophageal adenocarcinoma and promotes cancer cell invasion in vitro. Br. J. Cancer 2015, 113, 738–746. [Google Scholar] [CrossRef]
- Rickard, A.G.; Palmer, G.M.; Dewhirst, M.W. Clinical and Pre-clinical Methods for Quantifying Tumor Hypoxia. Adv. Exp. Med. Biol. 2019, 1136, 19–41. [Google Scholar] [CrossRef]
- Jing, X.; Yang, F.; Shao, C.; Wei, K.; Xie, M.; Shen, H.; Shu, Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer 2019, 18, 157. [Google Scholar] [CrossRef]
- Schito, L.; Semenza, G.L. Hypoxia-Inducible Factors: Master Regulators of Cancer Progression. Trends Cancer 2016, 2, 758–770. [Google Scholar] [CrossRef]
- Campillo, N.; Falcones, B.; Otero, J.; Colina, R.; Gozal, D.; Navajas, D.; Farré, R.; Almendros, I. Differential Oxygenation in Tumor Microenvironment Modulates Macrophage and Cancer Cell Crosstalk: Novel Experimental Setting and Proof of Concept. Front. Oncol. 2019, 9, 43. [Google Scholar] [CrossRef]
- Li, N.; Li, Y.; Li, Z.; Huang, C.; Yang, Y.; Lang, M.; Cao, J.; Jiang, W.; Xu, Y.; Dong, J.; et al. Hypoxia Inducible Factor 1 (HIF-1) Recruits Macrophage to Activate Pancreatic Stellate Cells in Pancreatic Ductal Adenocarcinoma. Int. J. Mol. Sci. 2016, 17, 799. [Google Scholar] [CrossRef]
- Yang, M.; McKay, D.; Pollard, J.W.; Lewis, C.E. Diverse Functions of Macrophages in Different Tumor Microenvironments. Cancer Res. 2018, 78, 5492–5503. [Google Scholar] [CrossRef] [PubMed]
- DeNardo, D.G.; Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 2019, 19, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y.S.; Yu, L.G.; Zhang, X.K.; Zhao, L.; Gong, F.L.; Yang, X.X.; Guo, X.L. Galectin-3 expression and secretion by tumor-associated macrophages in hypoxia promotes breast cancer progression. Biochem. Pharmacol. 2020, 178, 114113. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, M.; Zhang, X.; Ji, J.; Zhang, H.; Shen, L.; Zhu, Y.; Liu, X. Exosomal circ-0100519 promotes breast cancer progression via inducing M2 macrophage polarisation by USP7/NRF2 axis. Clin. Transl. Med. 2024, 14, e1763. [Google Scholar] [CrossRef]
- Imtiyaz, H.Z.; Williams, E.P.; Hickey, M.M.; Patel, S.A.; Durham, A.C.; Yuan, L.J.; Hammond, R.; Gimotty, P.A.; Keith, B.; Simon, M.C. Hypoxia-inducible factor 2alpha regulates macrophage function in mouse models of acute and tumor inflammation. J. Clin. Investig. 2010, 120, 2699–2714. [Google Scholar] [CrossRef]
- Cowman, S.J.; Koh, M.Y. Revisiting the HIF switch in the tumor and its immune microenvironment. Trends Cancer 2022, 8, 28–42. [Google Scholar] [CrossRef]
- Lewis, C.; Murdoch, C. Macrophage responses to hypoxia: Implications for tumor progression and anti-cancer therapies. Am. J. Pathol. 2005, 167, 627–635. [Google Scholar] [CrossRef]
- Leek, R.D.; Talks, K.L.; Pezzella, F.; Turley, H.; Campo, L.; Brown, N.S.; Bicknell, R.; Taylor, M.; Gatter, K.C.; Harris, A.L. Relation of hypoxia-inducible factor-2 alpha (HIF-2 alpha) expression in tumor-infiltrative macrophages to tumor angiogenesis and the oxidative thymidine phosphorylase pathway in Human breast cancer. Cancer Res. 2002, 62, 1326–1329. [Google Scholar]
- Helczynska, K.; Larsson, A.M.; Holmquist Mengelbier, L.; Bridges, E.; Fredlund, E.; Borgquist, S.; Landberg, G.; Påhlman, S.; Jirström, K. Hypoxia-inducible factor-2alpha correlates to distant recurrence and poor outcome in invasive breast cancer. Cancer Res. 2008, 68, 9212–9220. [Google Scholar] [CrossRef]
- Doedens, A.L.; Stockmann, C.; Rubinstein, M.P.; Liao, D.; Zhang, N.; DeNardo, D.G.; Coussens, L.M.; Karin, M.; Goldrath, A.W.; Johnson, R.S. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res. 2010, 70, 7465–7475. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, M.Z.; Zhang, S.J.; Sun, X.; Zhou, C.; Li, J.; Liu, J.; Feng, J.; Lu, S.Y.; Liu, P.J.; et al. HIF-1α inhibitor YC-1 suppresses triple-negative breast cancer growth and angiogenesis by targeting PlGF/VEGFR1-induced macrophage polarization. Biomed. Pharmacother. 2023, 161, 114423. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, S.M.; Zhang, F.; Ding, C.; Montoya-Durango, D.E.; Hu, X.; Yang, C.; Wang, Z.; Yuan, F.; Fox, M.; Zhang, H.G.; et al. Tumor-derived exosomes drive immunosuppressive macrophages in a pre-metastatic niche through glycolytic dominant metabolic reprogramming. Cell Metab. 2021, 33, 2040–2058.e2010. [Google Scholar] [CrossRef]
- Vaitheesvaran, B.; Xu, J.; Yee, J.; Lu, Q.-Y.; Go, V.L.; Xiao, G.G.; Lee, W.N. The Warburg effect: A balance of flux analysis. Metabolomics Off. J. Metabolomic Soc. 2015, 11, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zuo, H.; Xiong, H.; Kolar, M.J.; Chu, Q.; Saghatelian, A.; Siegwart, D.J.; Wan, Y. Gpr132 sensing of lactate mediates tumor-macrophage interplay to promote breast cancer metastasis. Proc. Natl. Acad. Sci. USA 2017, 114, 580–585. [Google Scholar] [CrossRef]
- Mu, X.; Shi, W.; Xu, Y.; Xu, C.; Zhao, T.; Geng, B.; Yang, J.; Pan, J.; Hu, S.; Zhang, C.; et al. Tumor-derived lactate induces M2 macrophage polarization via the activation of the ERK/STAT3 signaling pathway in breast cancer. Cell Cycle 2018, 17, 428–438. [Google Scholar] [CrossRef]
- Lin, S.; Sun, L.; Lyu, X.; Ai, X.; Du, D.; Su, N.; Li, H.; Zhang, L.; Yu, J.; Yuan, S. Lactate-activated macrophages induced aerobic glycolysis and epithelial-mesenchymal transition in breast cancer by regulation of CCL5-CCR5 axis: A positive metabolic feedback loop. Oncotarget 2017, 8, 110426–110443. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, L.; Zhang, Y.; Chen, K. Micro ribonucleic acid-448 regulates zinc finger e-box binding homeobox 1 to inhibit the growth of breast cancer cells and increase their sensitivity to chemotherapy. Clinics 2022, 77, 100089. [Google Scholar] [CrossRef]
- Jiang, H.; Zhou, C.; Zhang, Z.; Wang, Q.; Wei, H.; Shi, W.; Li, J.; Wang, Z.; Ou, Y.; Wang, W.; et al. Jagged1-Notch1-deployed tumor perivascular niche promotes breast cancer stem cell phenotype through Zeb1. Nat. Commun. 2020, 11, 5129. [Google Scholar] [CrossRef]
- Jiang, H.; Wei, H.; Wang, H.; Wang, Z.; Li, J.; Ou, Y.; Xiao, X.; Wang, W.; Chang, A.; Sun, W.; et al. Zeb1-induced metabolic reprogramming of glycolysis is essential for macrophage polarization in breast cancer. Cell Death Dis. 2022, 13, 206. [Google Scholar] [CrossRef]
- Niu, X.; Ma, J.; Li, J.; Gu, Y.; Yin, L.; Wang, Y.; Zhou, X.; Wang, J.; Ji, H.; Zhang, Q. Sodium/glucose cotransporter 1-dependent metabolic alterations induce tamoxifen resistance in breast cancer by promoting macrophage M2 polarization. Cell Death Dis. 2021, 12, 509. [Google Scholar] [CrossRef]
- Pollard, J.W. Tumour-educated macrophages promote tumour progression and metastasis. Nat. Rev. Cancer 2004, 4, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhuang, Z.G.; Xu, S.F.; He, Q.; Shao, Y.G.; Ji, M.; Yang, L.; Bao, W. Expression of CCL2 is significantly different in five breast cancer genotypes and predicts patient outcome. Int. J. Clin. Exp. Med. 2015, 8, 15684–15691. [Google Scholar] [PubMed]
- Duan, R.; Du, W.; Guo, W. EZH2: A novel target for cancer treatment. J. Hematol. Oncol. 2020, 13, 104. [Google Scholar] [CrossRef] [PubMed]
- Archer, M.; Bernhardt, S.M.; Hodson, L.J.; Woolford, L.; Van der Hoek, M.; Dasari, P.; Evdokiou, A.; Ingman, W.V. CCL2-Mediated Stromal Interactions Drive Macrophage Polarization to Increase Breast Tumorigenesis. Int. J. Mol. Sci. 2023, 24, 7385. [Google Scholar] [CrossRef]
- Watson, A.P.; Evans, R.L.; Egland, K.A. Multiple functions of sushi domain containing 2 (SUSD2) in breast tumorigenesis. Mol. Cancer Res. MCR 2013, 11, 74–85. [Google Scholar] [CrossRef]
- Hultgren, E.M.; Patrick, M.E.; Evans, R.L.; Stoos, C.T.; Egland, K.A. SUSD2 promotes tumor-associated macrophage recruitment by increasing levels of MCP-1 in breast cancer. PLoS ONE 2017, 12, e0177089. [Google Scholar] [CrossRef]
- Seoane, S.; Martinez-Ordoñez, A.; Eiro, N.; Cabezas-Sainz, P.; Garcia-Caballero, L.; Gonzalez, L.O.; Macia, M.; Sanchez, L.; Vizoso, F.; Perez-Fernandez, R. POU1F1 transcription factor promotes breast cancer metastasis via recruitment and polarization of macrophages. J. Pathol. 2019, 249, 381–394. [Google Scholar] [CrossRef]
- Raschioni, C.; Bottai, G.; Sagona, A.; Errico, V.; Testori, A.; Gatzemeier, W.; Corsi, F.; Tinterri, C.; Roncalli, M.; Santarpia, L.; et al. CXCR4/CXCL12 Signaling and Protumor Macrophages in Primary Tumors and Sentinel Lymph Nodes Are Involved in Luminal B Breast Cancer Progression. Dis. Markers 2018, 2018, 5018671. [Google Scholar] [CrossRef]
- Mills, E.; O’Neill, L.A. Succinate: A metabolic signal in inflammation. Trends Cell Biol. 2014, 24, 313–320. [Google Scholar] [CrossRef]
- Jiménez-Morales, S.; Pérez-Amado, C.J.; Langley, E.; Hidalgo-Miranda, A. Overview of mitochondrial germline variants and mutations in human disease: Focus on breast cancer (Review). Int. J. Oncol. 2018, 53, 923–936. [Google Scholar] [CrossRef]
- Selak, M.A.; Armour, S.M.; MacKenzie, E.D.; Boulahbel, H.; Watson, D.G.; Mansfield, K.D.; Pan, Y.; Simon, M.C.; Thompson, C.B.; Gottlieb, E. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell 2005, 7, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Di Virgilio, F.; Sarti, A.C.; Falzoni, S.; De Marchi, E.; Adinolfi, E. Extracellular ATP and P2 purinergic signalling in the tumour microenvironment. Nat. Rev. Cancer 2018, 18, 601–618. [Google Scholar] [CrossRef] [PubMed]
- Locher, K.P. Mechanistic diversity in ATP-binding cassette (ABC) transporters. Nat. Struct. Mol. Biol. 2016, 23, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Mittal, D.; Stagg, J.; Smyth, M.J. Targeting cancer-derived adenosine: New therapeutic approaches. Cancer Discov. 2014, 4, 879–888. [Google Scholar] [CrossRef]
- Vijayan, D.; Young, A.; Teng, M.W.L.; Smyth, M.J. Targeting immunosuppressive adenosine in cancer. Nat. Rev. Cancer 2017, 17, 709–724. [Google Scholar] [CrossRef]
- Ma, C.; He, D.; Tian, P.; Wang, Y.; He, Y.; Wu, Q.; Jia, Z.; Zhang, X.; Zhang, P.; Ying, H.; et al. miR-182 targeting reprograms tumor-associated macrophages and limits breast cancer progression. Proc. Natl. Acad. Sci. USA 2022, 119, e2114006119. [Google Scholar] [CrossRef]
- Jiang, J. Hedgehog signaling mechanism and role in cancer. Semin. Cancer Biol. 2022, 85, 107–122. [Google Scholar] [CrossRef]
- Hanna, A.; Shevde, L.A. Hedgehog signaling: Modulation of cancer properies and tumor mircroenvironment. Mol. Cancer 2016, 15, 24. [Google Scholar] [CrossRef]
- Hanna, A.; Metge, B.J.; Bailey, S.K.; Chen, D.; Chandrashekar, D.S.; Varambally, S.; Samant, R.S.; Shevde, L.A. Inhibition of Hedgehog signaling reprograms the dysfunctional immune microenvironment in breast cancer. Oncoimmunology 2019, 8, 1548241. [Google Scholar] [CrossRef]
- Francí, C.; Takkunen, M.; Dave, N.; Alameda, F.; Gómez, S.; Rodríguez, R.; Escrivà, M.; Montserrat-Sentís, B.; Baró, T.; Garrido, M.; et al. Expression of Snail protein in tumor-stroma interface. Oncogene 2006, 25, 5134–5144. [Google Scholar] [CrossRef]
- Guan, T.; Yang, X.; Liang, H.; Chen, J.; Chen, Y.; Zhu, Y.; Liu, T. Deubiquitinating enzyme USP9X regulates metastasis and chemoresistance in triple-negative breast cancer by stabilizing Snail1. J. Cell Physiol. 2022, 237, 2992–3000. [Google Scholar] [CrossRef] [PubMed]
- Ni, T.; Li, X.Y.; Lu, N.; An, T.; Liu, Z.P.; Fu, R.; Lv, W.C.; Zhang, Y.W.; Xu, X.J.; Grant Rowe, R.; et al. Snail1-dependent p53 repression regulates expansion and activity of tumour-initiating cells in breast cancer. Nat. Cell Biol. 2016, 18, 1221–1232. [Google Scholar] [CrossRef] [PubMed]
- Brenot, A.; Knolhoff, B.L.; DeNardo, D.G.; Longmore, G.D. SNAIL1 action in tumor cells influences macrophage polarization and metastasis in breast cancer through altered GM-CSF secretion. Oncogenesis 2018, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Bica, C.; Tirpe, A.; Nutu, A.; Ciocan, C.; Chira, S.; Gurzau, E.S.; Braicu, C.; Berindan-Neagoe, I. Emerging roles and mechanisms of semaphorins activity in cancer. Life Sci. 2023, 318, 121499. [Google Scholar] [CrossRef]
- Wallerius, M.; Wallmann, T.; Bartish, M.; Östling, J.; Mezheyeuski, A.; Tobin, N.P.; Nygren, E.; Pangigadde, P.; Pellegrini, P.; Squadrito, M.L.; et al. Guidance Molecule SEMA3A Restricts Tumor Growth by Differentially Regulating the Proliferation of Tumor-Associated Macrophages. Cancer Res. 2016, 76, 3166–3178. [Google Scholar] [CrossRef]
- Thielman, N.R.J.; Funes, V.; Davuluri, S.; Ibanez, H.E.; Sun, W.C.; Fu, J.; Li, K.; Muth, S.; Pan, X.; Fujiwara, K.; et al. Semaphorin 3D promotes pancreatic ductal adenocarcinoma progression and metastasis through macrophage reprogramming. Sci. Adv. 2024, 10, eadp0684. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, Z.; Qiao, L.; Jiang, X.; Ma, J.; Wang, J.; Ye, S.; Ma, Q.; Wei, J.; Wu, M.; et al. Semaphorin 4C Promotes Macrophage Recruitment and Angiogenesis in Breast Cancer. Mol. Cancer Res. MCR 2019, 17, 2015–2028. [Google Scholar] [CrossRef]
- Elder, A.M.; Tamburini, B.A.J.; Crump, L.S.; Black, S.A.; Wessells, V.M.; Schedin, P.J.; Borges, V.F.; Lyons, T.R. Semaphorin 7A Promotes Macrophage-Mediated Lymphatic Remodeling during Postpartum Mammary Gland Involution and in Breast Cancer. Cancer Res. 2018, 78, 6473–6485. [Google Scholar] [CrossRef]
- Jabbari, N.; Akbariazar, E.; Feqhhi, M.; Rahbarghazi, R.; Rezaie, J. Breast cancer-derived exosomes: Tumor progression and therapeutic agents. J. Cell Physiol. 2020, 235, 6345–6356. [Google Scholar] [CrossRef]
- Huang, S.; Dong, M.; Chen, Q. Tumor-Derived Exosomes and Their Role in Breast Cancer Metastasis. Int. J. Mol. Sci. 2022, 23, 13993. [Google Scholar] [CrossRef]
- Baig, M.S.; Roy, A.; Rajpoot, S.; Liu, D.; Savai, R.; Banerjee, S.; Kawada, M.; Faisal, S.M.; Saluja, R.; Saqib, U.; et al. Tumor-derived exosomes in the regulation of macrophage polarization. Inflamm. Res. 2020, 69, 435–451. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.; Lima, L.G.; Chai, E.P.Z.; Muller, A.; Lobb, R.J.; Krumeich, S.; Wen, S.W.; Wiegmans, A.P.; Möller, A. Breast Cancer-Derived Exosomes Alter Macrophage Polarization via gp130/STAT3 Signaling. Front. Immunol. 2018, 9, 871. [Google Scholar] [CrossRef] [PubMed]
- Piao, Y.J.; Kim, H.S.; Hwang, E.H.; Woo, J.; Zhang, M.; Moon, W.K. Breast cancer cell-derived exosomes and macrophage polarization are associated with lymph node metastasis. Oncotarget 2018, 9, 7398–7410. [Google Scholar] [CrossRef]
- Xie, F.; Dong, H.; Zhang, H. Regulatory Functions of Protein Tyrosine Phosphatase Receptor Type O in Immune Cells. Front. Immunol. 2021, 12, 783370. [Google Scholar] [CrossRef]
- Dong, H.; Ma, L.; Gan, J.; Lin, W.; Chen, C.; Yao, Z.; Du, L.; Zheng, L.; Ke, C.; Huang, X.; et al. PTPRO represses ERBB2-driven breast oncogenesis by dephosphorylation and endosomal internalization of ERBB2. Oncogene 2017, 36, 410–422. [Google Scholar] [CrossRef]
- Dong, H.; Xie, C.; Jiang, Y.; Li, K.; Lin, Y.; Pang, X.; Xiong, X.; Zheng, J.; Ke, X.; Chen, Y.; et al. Tumor-Derived Exosomal Protein Tyrosine Phosphatase Receptor Type O Polarizes Macrophage to Suppress Breast Tumor Cell Invasion and Migration. Front. Cell Dev. Biol. 2021, 9, 703537. [Google Scholar] [CrossRef]
- Wilson, R.C.; Doudna, J.A. Molecular mechanisms of RNA interference. Annu. Rev. Biophys. 2013, 42, 217–239. [Google Scholar] [CrossRef]
- Androulidaki, A.; Iliopoulos, D.; Arranz, A.; Doxaki, C.; Schworer, S.; Zacharioudaki, V.; Margioris, A.N.; Tsichlis, P.N.; Tsatsanis, C. The kinase Akt1 controls macrophage response to lipopolysaccharide by regulating microRNAs. Immunity 2009, 31, 220–231. [Google Scholar] [CrossRef]
- Hao, C.; Sheng, Z.; Wang, W.; Feng, R.; Zheng, Y.; Xiao, Q.; Zhang, B. Tumor-derived exosomal miR-148b-3p mediates M2 macrophage polarization via TSC2/mTORC1 to promote breast cancer migration and invasion. Thorac. Cancer 2023, 14, 1477–1491. [Google Scholar] [CrossRef]
- Xun, J.; Du, L.; Gao, R.; Shen, L.; Wang, D.; Kang, L.; Chen, C.; Zhang, Z.; Zhang, Y.; Yue, S.; et al. Cancer-derived exosomal miR-138-5p modulates polarization of tumor-associated macrophages through inhibition of KDM6B. Theranostics 2021, 11, 6847–6859. [Google Scholar] [CrossRef]
- Mao, H.; Ye, R.; Tang, G.; Tao, S.; Wei, K.; Wu, Y.; Pang, S.; Wang, J.; Shi, J.; Ji, Y.; et al. Drug-resistant exosome miR-99b-3p induces macrophage polarization and confers chemoresistance on sensitive cells by targeting PPP2CA. Int. Immunopharmacol. 2024, 142, 113168. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Zhang, R.; Wu, X.; Piao, Z.; Zhang, M.; Jin, T. LncRNA HAGLROS promotes breast cancer evolution through miR-135b-3p/COL10A1 axis and exosome-mediated macrophage M2 polarization. Cell Death Dis. 2024, 15, 633. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Chen, X.; Liu, C.; Ge, W.; Wang, Q.; Hao, X.; Wang, M.; Chen, Y.; Zhang, Q. Identification of a small molecule as inducer of ferroptosis and apoptosis through ubiquitination of GPX4 in triple negative breast cancer cells. J. Hematol. Oncol. 2021, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Zhai, Y.; Meng, Y.; Wu, Z.; Qiu, A.; Cai, Y.; Wang, G.; Yang, L. Acidosis activates breast cancer ferroptosis through ZFAND5/SLC3A2 signaling axis and elicits M1 macrophage polarization. Cancer Lett. 2024, 587, 216732. [Google Scholar] [CrossRef]
- Yi, C.; Wu, S.; Duan, Q.; Liu, L.; Li, L.; Luo, Y.; Wang, A. Ferroptosis-dependent breast cancer cell-derived exosomes inhibit migration and invasion of breast cancer cells by suppressing M2 macrophage polarization. PeerJ 2023, 11, e15060. [Google Scholar] [CrossRef]
- Wang, D.; Qiu, G.; Zhu, X.; Wang, Q.; Zhu, C.; Fang, C.; Liu, J.; Zhang, K.; Liu, Y. Macrophage-inherited exosome excise tumor immunosuppression to expedite immune-activated ferroptosis. J. Immunother. Cancer 2023, 11, e006516. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Y.; Liu, H.; Wu, J. FOXM1 derived from Triple negative breast cancer exosomes promotes cancer progression by activating IDO1 transcription in macrophages to suppress ferroptosis and induce M2 polarization of Tumor-associated macrophages. Genes Genet. Syst. 2024, 100, 24-00079. [Google Scholar] [CrossRef]
- Kothari, C.; Diorio, C.; Durocher, F. The Importance of Breast Adipose Tissue in Breast Cancer. Int. J. Mol. Sci. 2020, 21, 5760. [Google Scholar] [CrossRef]
- Sun, X.; Casbas-Hernandez, P.; Bigelow, C.; Makowski, L.; Joseph Jerry, D.; Smith Schneider, S.; Troester, M.A. Normal breast tissue of obese women is enriched for macrophage markers and macrophage-associated gene expression. Breast Cancer Res. Treat. 2012, 131, 1003–1012. [Google Scholar] [CrossRef]
- Folco, E.J.; Rocha, V.Z.; López-Ilasaca, M.; Libby, P. Adiponectin inhibits pro-inflammatory signaling in human macrophages independent of interleukin-10. J. Biol. Chem. 2009, 284, 25569–25575. [Google Scholar] [CrossRef]
- Nakajima, T.E.; Yamada, Y.; Hamano, T.; Furuta, K.; Matsuda, T.; Fujita, S.; Kato, K.; Hamaguchi, T.; Shimada, Y. Adipocytokines as new promising markers of colorectal tumors: Adiponectin for colorectal adenoma, and resistin and visfatin for colorectal cancer. Cancer Sci. 2010, 101, 1286–1291. [Google Scholar] [CrossRef] [PubMed]
- Parker-Duffen, J.L.; Nakamura, K.; Silver, M.; Zuriaga, M.A.; MacLauchlan, S.; Aprahamian, T.R.; Walsh, K. Divergent roles for adiponectin receptor 1 (AdipoR1) and AdipoR2 in mediating revascularization and metabolic dysfunction in vivo. J. Biol. Chem. 2014, 289, 16200–16213. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.H.; Tyrrell, H.E.; Gao, L.; Xu, D.; Quan, J.; Gill, D.; Rai, L.; Ding, Y.; Plant, G.; Chen, Y.; et al. Adiponectin receptor signaling on dendritic cells blunts antitumor immunity. Cancer Res. 2014, 74, 5711–5722. [Google Scholar] [CrossRef] [PubMed]
- Gruen, M.L.; Hao, M.; Piston, D.W.; Hasty, A.H. Leptin requires canonical migratory signaling pathways for induction of monocyte and macrophage chemotaxis. Am. J. Physiol.-Cell Physiol. 2007, 293, C1481–C1488. [Google Scholar] [CrossRef]
- Barone, I.; Giordano, C.; Bonofiglio, D.; Andò, S.; Catalano, S. The weight of obesity in breast cancer progression and metastasis: Clinical and molecular perspectives. Semin. Cancer Biol. 2020, 60, 274–284. [Google Scholar] [CrossRef]
- Acedo, S.C.; Gambero, S.; Cunha, F.G.; Lorand-Metze, I.; Gambero, A. Participation of leptin in the determination of the macrophage phenotype: An additional role in adipocyte and macrophage crosstalk. Vitr. Cell. Dev. Biol.-Anim. 2013, 49, 473–478. [Google Scholar] [CrossRef]
- Li, K.; Wei, L.; Huang, Y.; Wu, Y.; Su, M.; Pang, X.; Wang, N.; Ji, F.; Zhong, C.; Chen, T. Leptin promotes breast cancer cell migration and invasion via IL-18 expression and secretion. Int. J. Oncol. 2016, 48, 2479–2487. [Google Scholar] [CrossRef]
- Kolesnick, R.N. Sphingomyelin and derivatives as cellular signals. Prog. Lipid Res. 1991, 30, 1–38. [Google Scholar] [CrossRef]
- Zama, K.; Mitsutake, S.; Okazaki, T.; Igarashi, Y. Sphingomyelin in microdomains of the plasma membrane regulates amino acid-stimulated mTOR signal activation. Cell Biol. Int. 2018, 42, 823–831. [Google Scholar] [CrossRef]
- Breslow, D.K.; Weissman, J.S. Membranes in balance: Mechanisms of sphingolipid homeostasis. Mol. Cell 2010, 40, 267–279. [Google Scholar] [CrossRef]
- Deng, Y.; Hu, J.C.; He, S.H.; Lou, B.; Ding, T.B.; Yang, J.T.; Mo, M.G.; Ye, D.Y.; Zhou, L.; Jiang, X.C.; et al. Sphingomyelin synthase 2 facilitates M2-like macrophage polarization and tumor progression in a mouse model of triple-negative breast cancer. Acta Pharmacol. Sin. 2021, 42, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Shi, Q.; Zhang, W.; Shu, Y.; Yang, N.; Chen, B.; Wang, Q.; Zhao, X.; Chen, J.; Cheng, N.; et al. Caspase-1 cleaves PPARγ for potentiating the pro-tumor action of TAMs. Nat. Commun. 2017, 8, 766. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.Z.; Lee, E.J.; Lin, Y.J.; Chen, L.; Zheng, H.Y.; He, X.Q.; Peng, J.Y.; Noonepalle, S.K.; Shull, A.Y.; Pei, F.C.; et al. Recruitment of monocytes and epigenetic silencing of intratumoral CYP7B1 primarily contribute to the accumulation of 27-hydroxycholesterol in breast cancer. Am. J. Cancer Res. 2019, 9, 2194–2208. [Google Scholar]
- Gabrilovich, D.I.; Bronte, V.; Chen, S.H.; Colombo, M.P.; Ochoa, A.; Ostrand-Rosenberg, S.; Schreiber, H. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007, 67, 425. [Google Scholar] [CrossRef]
- Christofides, A.; Strauss, L.; Yeo, A.; Cao, C.; Charest, A.; Boussiotis, V.A. The complex role of tumor-infiltrating macrophages. Nat. Immunol. 2022, 23, 1148–1156. [Google Scholar] [CrossRef]
- Sinha, P.; Clements, V.K.; Bunt, S.K.; Albelda, S.M.; Ostrand-Rosenberg, S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J. Immunol. 2007, 179, 977–983. [Google Scholar] [CrossRef]
- Kumar, V.; Cheng, P.; Condamine, T.; Mony, S.; Languino, L.R.; McCaffrey, J.C.; Hockstein, N.; Guarino, M.; Masters, G.; Penman, E.; et al. CD45 Phosphatase Inhibits STAT3 Transcription Factor Activity in Myeloid Cells and Promotes Tumor-Associated Macrophage Differentiation. Immunity 2016, 44, 303–315. [Google Scholar] [CrossRef]
- Purrahman, D.; Mahmoudian-Sani, M.R.; Saki, N.; Wojdasiewicz, P.; Kurkowska-Jastrzębska, I.; Poniatowski, Ł.A. Involvement of progranulin (PGRN) in the pathogenesis and prognosis of breast cancer. Cytokine 2022, 151, 155803. [Google Scholar] [CrossRef]
- Fang, W.; Zhou, T.; Shi, H.; Yao, M.; Zhang, D.; Qian, H.; Zeng, Q.; Wang, Y.; Jin, F.; Chai, C.; et al. Progranulin induces immune escape in breast cancer via up-regulating PD-L1 expression on tumor-associated macrophages (TAMs) and promoting CD8(+) T cell exclusion. J. Exp. Clin. Cancer Res. CR 2021, 40, 4. [Google Scholar] [CrossRef]
- Murdoch, C.; Muthana, M.; Coffelt, S.B.; Lewis, C.E. The role of myeloid cells in the promotion of tumour angiogenesis. Nat. Rev. Cancer 2008, 8, 618–631. [Google Scholar] [CrossRef]
- Lin, E.Y.; Li, J.F.; Gnatovskiy, L.; Deng, Y.; Zhu, L.; Grzesik, D.A.; Qian, H.; Xue, X.N.; Pollard, J.W. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006, 66, 11238–11246. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Chen, Y.S.; Yao, Y.D.; Chen, J.Q.; Chen, J.N.; Huang, S.Y.; Zeng, Y.J.; Yao, H.R.; Zeng, S.H.; Fu, Y.S.; et al. CCL18 from tumor-associated macrophages promotes angiogenesis in breast cancer. Oncotarget 2015, 6, 34758–34773. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Ruan, S.; Wang, J.; Xia, Y.; Le, K.; Xiao, X.; Hu, T.; Wang, Q. M2 macrophage-induced lncRNA PCAT6 facilitates tumorigenesis and angiogenesis of triple-negative breast cancer through modulation of VEGFR2. Cell Death Dis. 2020, 11, 728. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.J.; Xu, Z.; Wang, Z.; Zhang, H.; Zhuang, Z.W.; Simons, M.; Min, W. Author Correction: SUMOylation of VEGFR2 regulates its intracellular trafficking and pathological angiogenesis. Nat. Commun. 2019, 10, 3679. [Google Scholar] [CrossRef]
- Bilimoria, J.; Singh, H. The Angiopoietin ligands and Tie receptors: Potential diagnostic biomarkers of vascular disease. J. Recept. Signal Transduct. Res. 2019, 39, 187–193. [Google Scholar] [CrossRef]
- Augustin, H.G.; Koh, G.Y.; Thurston, G.; Alitalo, K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat. Rev. Mol. Cell Biol. 2009, 10, 165–177. [Google Scholar] [CrossRef]
- De Palma, M.; Venneri, M.A.; Roca, C.; Naldini, L. Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cells. Nat. Med. 2003, 9, 789–795. [Google Scholar] [CrossRef]
- De Palma, M.; Venneri, M.A.; Galli, R.; Sergi Sergi, L.; Politi, L.S.; Sampaolesi, M.; Naldini, L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 2005, 8, 211–226. [Google Scholar] [CrossRef]
- Mazzieri, R.; Pucci, F.; Moi, D.; Zonari, E.; Ranghetti, A.; Berti, A.; Politi, L.S.; Gentner, B.; Brown, J.L.; Naldini, L.; et al. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell 2011, 19, 512–526. [Google Scholar] [CrossRef]
- Huang, X.; Cao, J.; Zu, X. Tumor-associated macrophages: An important player in breast cancer progression. Thorac. Cancer 2022, 13, 269–276. [Google Scholar] [CrossRef]
- DeNardo, D.G.; Brennan, D.J.; Rexhepaj, E.; Ruffell, B.; Shiao, S.L.; Madden, S.F.; Gallagher, W.M.; Wadhwani, N.; Keil, S.D.; Junaid, S.A.; et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011, 1, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.B.; Yeh, E.S.; Soloff, A.C. Tumor-associated macrophages: Unwitting accomplices in breast cancer malignancy. NPJ Breast Cancer 2016, 2, 15025. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.A.; Li, M.O. TGF-β: Guardian of T cell function. J. Immunol. 2013, 191, 3973–3979. [Google Scholar] [CrossRef] [PubMed]
- Ruffell, B.; Chang-Strachan, D.; Chan, V.; Rosenbusch, A.; Ho, C.M.; Pryer, N.; Daniel, D.; Hwang, E.S.; Rugo, H.S.; Coussens, L.M. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell 2014, 26, 623–637. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Wang, N.; Zheng, Y.; Yang, B.; Wang, X.; Zhang, J.; Pan, B.; Wang, Z. Aiduqing formula inhibits breast cancer metastasis by suppressing TAM/CXCL1-induced Treg differentiation and infiltration. Cell Commun. Signal. CCS 2021, 19, 89. [Google Scholar] [CrossRef]
- Sawa-Wejksza, K.; Kandefer-Szerszeń, M. Tumor-Associated Macrophages as Target for Antitumor Therapy. Arch. Immunol. Ther. Exp. 2018, 66, 97–111. [Google Scholar] [CrossRef]
- Nixon, B.G.; Kuo, F.; Ji, L.; Liu, M.; Capistrano, K.; Do, M.; Franklin, R.A.; Wu, X.; Kansler, E.R.; Srivastava, R.M.; et al. Tumor-associated macrophages expressing the transcription factor IRF8 promote T cell exhaustion in cancer. Immunity 2022, 55, 2044–2058.e2045. [Google Scholar] [CrossRef]
- Geiger, R.; Rieckmann, J.C.; Wolf, T.; Basso, C.; Feng, Y.; Fuhrer, T.; Kogadeeva, M.; Picotti, P.; Meissner, F.; Mann, M.; et al. L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-tumor Activity. Cell 2016, 167, 829–842.e813. [Google Scholar] [CrossRef]
- Dieci, M.V.; Radosevic-Robin, N.; Fineberg, S.; van den Eynden, G.; Ternes, N.; Penault-Llorca, F.; Pruneri, G.; D’Alfonso, T.M.; Demaria, S.; Castaneda, C.; et al. Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: A report of the International Immuno-Oncology Biomarker Working Group on Breast Cancer. Semin. Cancer Biol. 2018, 52, 16–25. [Google Scholar] [CrossRef]
- De Boniface, J.; Mao, Y.; Schmidt-Mende, J.; Kiessling, R.; Poschke, I. Expression patterns of the immunomodulatory enzyme arginase 1 in blood, lymph nodes and tumor tissue of early-stage breast cancer patients. Oncoimmunology 2012, 1, 1305–1312. [Google Scholar] [CrossRef]
- Geeraerts, X.; Bolli, E.; Fendt, S.M.; Van Ginderachter, J.A. Macrophage Metabolism As Therapeutic Target for Cancer, Atherosclerosis, and Obesity. Front. Immunol. 2017, 8, 289. [Google Scholar] [CrossRef] [PubMed]
- O’Carrigan, B.; Wong, M.H.; Willson, M.L.; Stockler, M.R.; Pavlakis, N.; Goodwin, A. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst. Rev. 2017, 10, Cd003474. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.K.; Kadel, S.; Townsend, M.G.; Oliwa, M.; Guerriero, J.L. Macrophage Biology and Mechanisms of Immune Suppression in Breast Cancer. Front. Immunol. 2021, 12, 643771. [Google Scholar] [CrossRef]
- Noman, M.Z.; Desantis, G.; Janji, B.; Hasmim, M.; Karray, S.; Dessen, P.; Bronte, V.; Chouaib, S. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J. Exp. Med. 2014, 211, 781–790. [Google Scholar] [CrossRef]
- Parveen, S.; Siddharth, S.; Cheung, L.S.; Kumar, A.; Shen, J.; Murphy, J.R.; Sharma, D.; Bishai, W.R. Therapeutic targeting with DABIL-4 depletes myeloid suppressor cells in 4T1 triple-negative breast cancer model. Mol. Oncol. 2021, 15, 1330–1344. [Google Scholar] [CrossRef]
- Lucas, C.L.; Workman, C.J.; Beyaz, S.; LoCascio, S.; Zhao, G.; Vignali, D.A.; Sykes, M. LAG-3, TGF-β, and cell-intrinsic PD-1 inhibitory pathways contribute to CD8 but not CD4 T-cell tolerance induced by allogeneic BMT with anti-CD40L. Blood 2011, 117, 5532–5540. [Google Scholar] [CrossRef]
- Xia, J.; Zhang, L.; Peng, X.; Tu, J.; Li, S.; He, X.; Li, F.; Qiang, J.; Dong, H.; Deng, Q.; et al. IL1R2 Blockade Alleviates Immunosuppression and Potentiates Anti-PD-1 Efficacy in Triple-Negative Breast Cancer. Cancer Res. 2024, 84, 2282–2296. [Google Scholar] [CrossRef]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef]
- Butti, R.; Gunasekaran, V.P.; Kumar, T.V.S.; Banerjee, P.; Kundu, G.C. Breast cancer stem cells: Biology and therapeutic implications. Int. J. Biochem. Cell Biol. 2019, 107, 38–52. [Google Scholar] [CrossRef]
- Radharani, N.N.V.; Yadav, A.S.; Nimma, R.; Kumar, T.V.S.; Bulbule, A.; Chanukuppa, V.; Kumar, D.; Patnaik, S.; Rapole, S.; Kundu, G.C. Tumor-associated macrophage derived IL-6 enriches cancer stem cell population and promotes breast tumor progression via Stat-3 pathway. Cancer Cell Int. 2022, 22, 122. [Google Scholar] [CrossRef]
- Lu, H.; Clauser, K.R.; Tam, W.L.; Fröse, J.; Ye, X.; Eaton, E.N.; Reinhardt, F.; Donnenberg, V.S.; Bhargava, R.; Carr, S.A.; et al. Addendum: A breast cancer stem cell niche supported by juxtacrine signalling from monocytes and macrophages. Nat. Cell Biol. 2015, 17, 1607. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liao, D.; Chen, C.; Liu, Y.; Chuang, T.H.; Xiang, R.; Markowitz, D.; Reisfeld, R.A.; Luo, Y. Tumor-associated macrophages regulate murine breast cancer stem cells through a novel paracrine EGFR/Stat3/Sox-2 signaling pathway. Stem Cells 2013, 31, 248–258. [Google Scholar] [CrossRef] [PubMed]
- O’Conor, C.J.; Chen, T.; González, I.; Cao, D.; Peng, Y. Cancer stem cells in triple-negative breast cancer: A potential target and prognostic marker. Biomark. Med. 2018, 12, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Klarmann, G.J.; Decker, A.; Farrar, W.L. Epigenetic gene silencing in the Wnt pathway in breast cancer. Epigenetics 2008, 3, 59–63. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, Q.; Lei, K.; Zhu, Q.; Zeng, D.; Liu, Y.; Lu, Y.; Kang, T.; Tang, N.; Huang, L.; et al. Targeting MEX3A attenuates metastasis of breast cancer via β-catenin signaling pathway inhibition. Cancer Lett. 2021, 521, 50–63. [Google Scholar] [CrossRef]
- Chen, X.; Yang, M.; Yin, J.; Li, P.; Zeng, S.; Zheng, G.; He, Z.; Liu, H.; Wang, Q.; Zhang, F.; et al. Tumor-associated macrophages promote epithelial-mesenchymal transition and the cancer stem cell properties in triple-negative breast cancer through CCL2/AKT/β-catenin signaling. Cell Commun. Signal. CCS 2022, 20, 92. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Sun, X.L.; Lu, Y.C.; Chen, S.; Pei, D.S.; Zhang, L.S. NRP1 contributes to stemness and potentiates radioresistance via WTAP-mediated m6A methylation of Bcl-2 mRNA in breast cancer. Apoptosis Int. J. Program. Cell Death 2023, 28, 233–246. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Li, Z.; Wang, Q.; Shi, Y.; Jiang, X.; Sun, X. The miR-124-3p/Neuropilin-1 Axis Contributes to the Proliferation and Metastasis of Triple-Negative Breast Cancer Cells and Co-Activates the TGF-β Pathway. Front. Oncol. 2021, 11, 654672. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Zhao, L.; Shao, S.; Ning, Q.; Jing, X.; Zhang, Y.; Zhao, F.; Liu, X.; Gu, S.; et al. VEGFA/NRP-1/GAPVD1 axis promotes progression and cancer stemness of triple-negative breast cancer by enhancing tumor cell-macrophage crosstalk. Int. J. Biol. Sci. 2024, 20, 446–463. [Google Scholar] [CrossRef]
- Tariq, M.; Zhang, J.; Liang, G.; Ding, L.; He, Q.; Yang, B. Macrophage Polarization: Anti-Cancer Strategies to Target Tumor-Associated Macrophage in Breast Cancer. J. Cell. Biochem. 2017, 118, 2484–2501. [Google Scholar] [CrossRef]
- Yao, M.; Smart, C.; Hu, Q.; Cheng, N. Continuous Delivery of Neutralizing Antibodies Elevate CCL2 Levels in Mice Bearing MCF10CA1d Breast Tumor Xenografts. Transl. Oncol. 2017, 10, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Kan, E.L.; Humayun, M.; Gurvich, N.; Offeddu, G.S.; Wan, Z.; Coughlin, M.F.; Renteria, D.C.; Loew, A.; Wilson, S.; et al. Patient-specific vascularized tumor model: Blocking monocyte recruitment with multispecific antibodies targeting CCR2 and CSF-1R. Biomaterials 2025, 312, 122731. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.Y.; Choi, J.; Choi, K.; Park, C.; Kim, Y.H.; Suh, K.H.; Ham, Y.J.; Jang, S.Y.; Lee, K.H.; Hwang, K.W. Synthesis and evaluation of thieno [3,2-d]pyrimidine derivatives as novel FMS inhibitors. Bioorganic Med. Chem. Lett. 2019, 29, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Tacconi, C.; Commerford, C.D.; Dieterich, L.C.; Schwager, S.; He, Y.; Ikenberg, K.; Friebel, E.; Becher, B.; Tugues, S.; Detmar, M. CD169(+) lymph node macrophages have protective functions in mouse breast cancer metastasis. Cell Rep. 2021, 35, 108993. [Google Scholar] [CrossRef]
- Salvagno, C.; Ciampricotti, M.; Tuit, S.; Hau, C.S.; van Weverwijk, A.; Coffelt, S.B.; Kersten, K.; Vrijland, K.; Kos, K.; Ulas, T.; et al. Therapeutic targeting of macrophages enhances chemotherapy efficacy by unleashing type I interferon response. Nat. Cell Biol. 2019, 21, 511–521. [Google Scholar] [CrossRef]
- Jaynes, J.M.; Sable, R.; Ronzetti, M.; Bautista, W.; Knotts, Z.; Abisoye-Ogunniyan, A.; Li, D.; Calvo, R.; Dashnyam, M.; Singh, A.; et al. Mannose receptor (CD206) activation in tumor-associated macrophages enhances adaptive and innate antitumor immune responses. Sci. Transl. Med. 2020, 12, eaax6337. [Google Scholar] [CrossRef]
- Griess, B.; Mir, S.; Datta, K.; Teoh-Fitzgerald, M. Scavenging reactive oxygen species selectively inhibits M2 macrophage polarization and their pro-tumorigenic function in part, via Stat3 suppression. Free Radic. Biol. Med. 2020, 147, 48–60. [Google Scholar] [CrossRef]
- Deng, X.X.; Jiao, Y.N.; Hao, H.F.; Xue, D.; Bai, C.C.; Han, S.Y. Taraxacum mongolicum extract inhibited malignant phenotype of triple-negative breast cancer cells in tumor-associated macrophages microenvironment through suppressing IL-10/STAT3/PD-L1 signaling pathways. J. Ethnopharmacol. 2021, 274, 113978. [Google Scholar] [CrossRef]
- Layman, R.M.; Arun, B. PARP Inhibitors in Triple-Negative Breast Cancer Including Those With BRCA Mutations. Cancer J. 2021, 27, 67–75. [Google Scholar] [CrossRef]
- Litton, J.K.; Hurvitz, S.A.; Mina, L.A.; Rugo, H.S.; Lee, K.H.; Gonçalves, A.; Diab, S.; Woodward, N.; Goodwin, A.; Yerushalmi, R.; et al. Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: Final overall survival results from the EMBRACA trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 1526–1535. [Google Scholar] [CrossRef]
- Wang, Q.; Bergholz, J.S.; Ding, L.; Lin, Z.; Kabraji, S.K.; Hughes, M.E.; He, X.; Xie, S.; Jiang, T.; Wang, W.; et al. STING agonism reprograms tumor-associated macrophages and overcomes resistance to PARP inhibition in BRCA1-deficient models of breast cancer. Nat. Commun. 2022, 13, 3022. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Chen, X.; Bi, Q.; Xiang, Y.; Jin, R.; Ai, H.; Nie, Y. A parallel and cascade control system: Magnetofection of miR125b for synergistic tumor-association macrophage polarization regulation and tumor cell suppression in breast cancer treatment. Nanoscale 2020, 12, 22615–22627. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, J.; Xie, Y.; Lu, Z.; Sun, W.; Wang, K.; Liang, J.; Chen, X. Reactive oxygen species/glutathione dual sensitive nanoparticles with encapsulation of miR155 and curcumin for synergized cancer immunotherapy. J. Nanobiotechnol. 2024, 22, 400. [Google Scholar] [CrossRef]
- Zhang, L.; Ren, Z.; Lü, J.; Mo, X.; Lin, J.; Li, Y.; Ma, W.; Liu, P.; Shen, Y.; Zhao, Q.; et al. Nanoparticles carrying paclitaxel and anti-miR-221 for breast cancer therapy triggered by ultrasound. Cell Death Discov. 2023, 9, 298. [Google Scholar] [CrossRef]
- Zhang, Q.; Le, K.; Xu, M.; Zhou, J.; Xiao, Y.; Yang, W.; Jiang, Y.; Xi, Z.; Huang, T. Combined MEK inhibition and tumor-associated macrophages depletion suppresses tumor growth in a triple-negative breast cancer mouse model. Int. Immunopharmacol. 2019, 76, 105864. [Google Scholar] [CrossRef]
- Gu, Y.; Taifour, T.; Bui, T.; Zuo, D.; Pacis, A.; Poirier, A.; Attalla, S.; Fortier, A.M.; Sanguin-Gendreau, V.; Pan, T.C.; et al. Osteopontin is a therapeutic target that drives breast cancer recurrence. Nat. Commun. 2024, 15, 9174. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, W.; Cai, X.; Wang, X.; Dang, W.; Tang, H.; Cao, H.; Wang, L.; Chen, T. Hydrazinocurcumin Encapsuled nanoparticles “re-educate” tumor-associated macrophages and exhibit anti-tumor effects on breast cancer following STAT3 suppression. PLoS ONE 2013, 8, e65896. [Google Scholar] [CrossRef]
- Kumari, M.; Purohit, M.P.; Pahuja, R.; Patnaik, S.; Shukla, Y.; Kumar, P.; Gupta, K.C. Pro-inflammatory macrophage polarization enhances the anti-cancer efficacy of self-assembled galactomannan nanoparticles entrapped with hydrazinocurcumin. Drug Deliv. Transl. Res. 2019, 9, 1159–1188. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).