Combinatorial Effects of CPP-Modified Antimicrobial Peptides: Synergistic and Additive Interactions Against Pathogenic Bacteria
Abstract
1. Introduction
2. Results
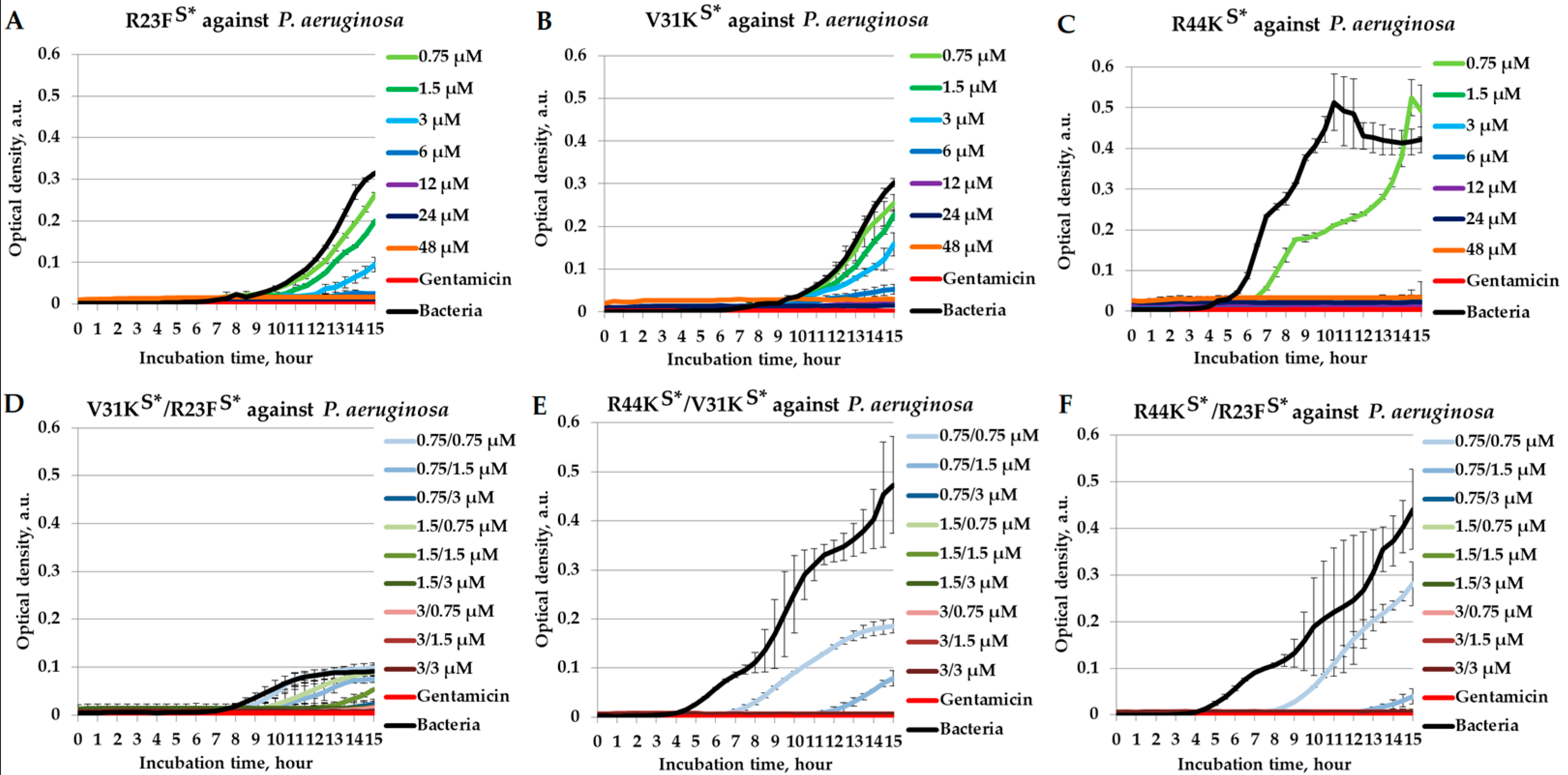
2.1. Combined Antimicrobial Effects of Peptides Against Pseudomonas aeruginosa
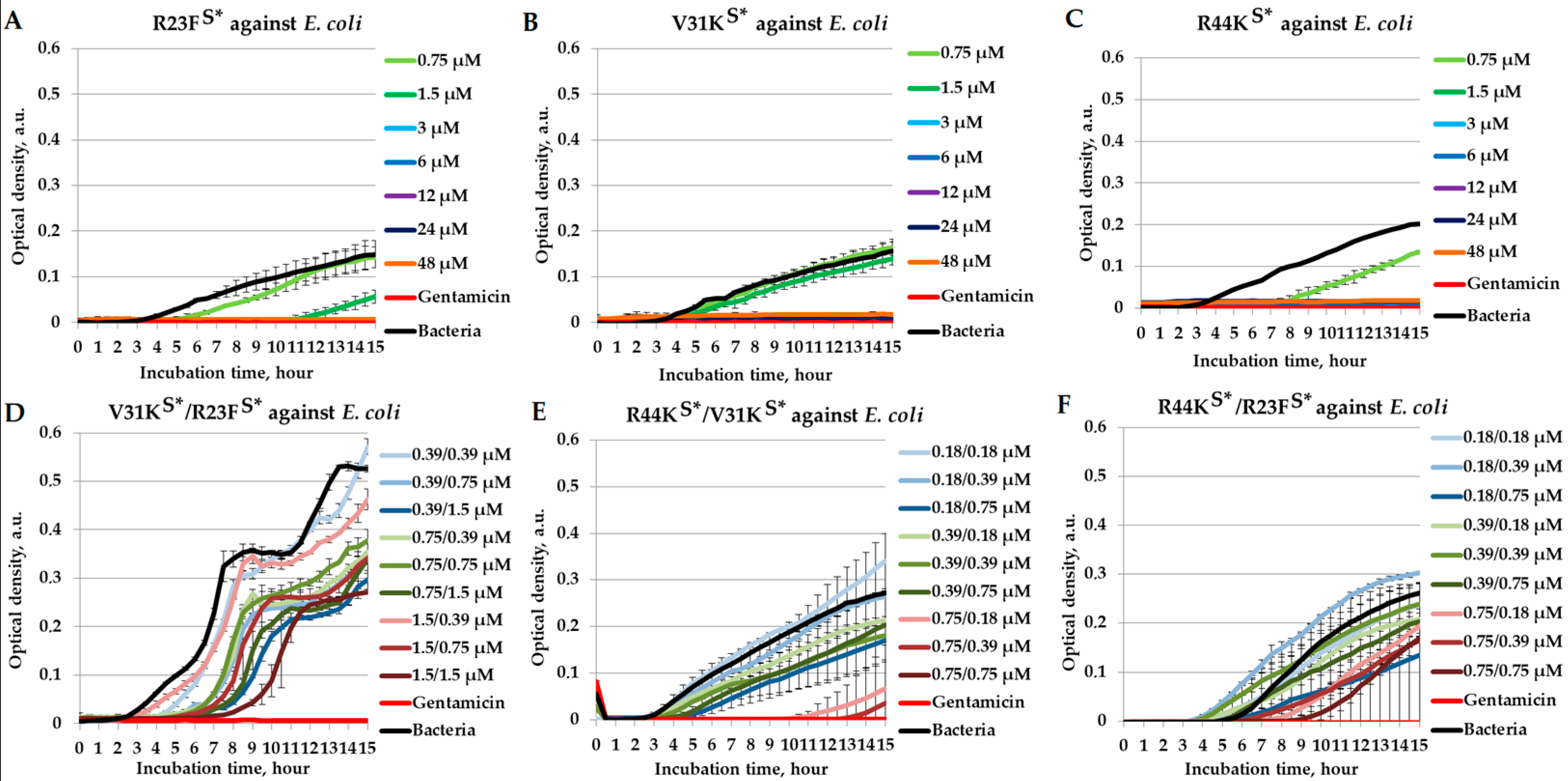
2.2. Combined Antimicrobial Effects of Peptides Against Escherichia coli
2.3. Combined Antimicrobial Effects of Peptides Against Staphylococcus aureus
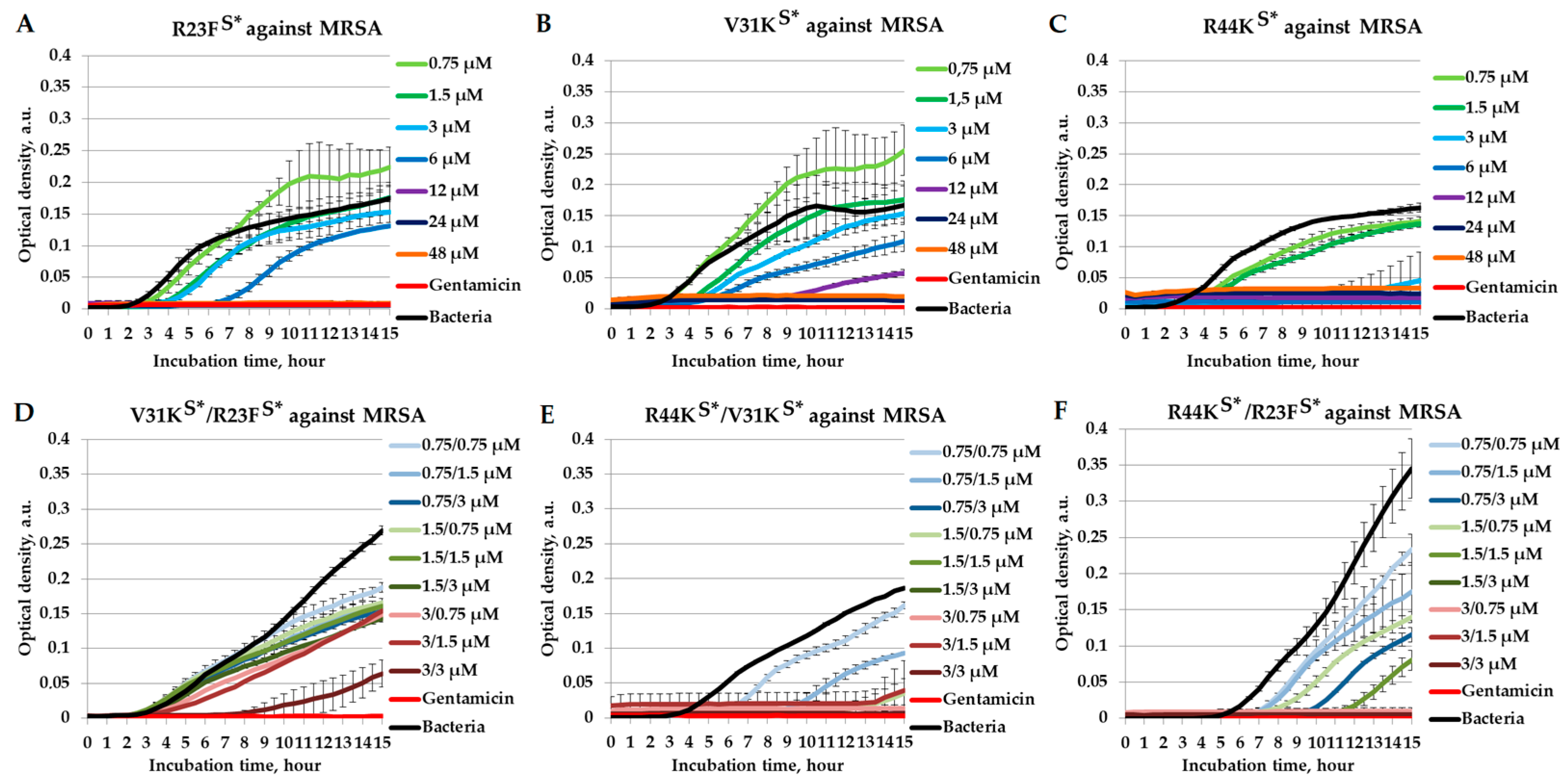
2.4. Combined Antimicrobial Effects of Peptides Against Methicillin-Resistant Staphylococcus aureus
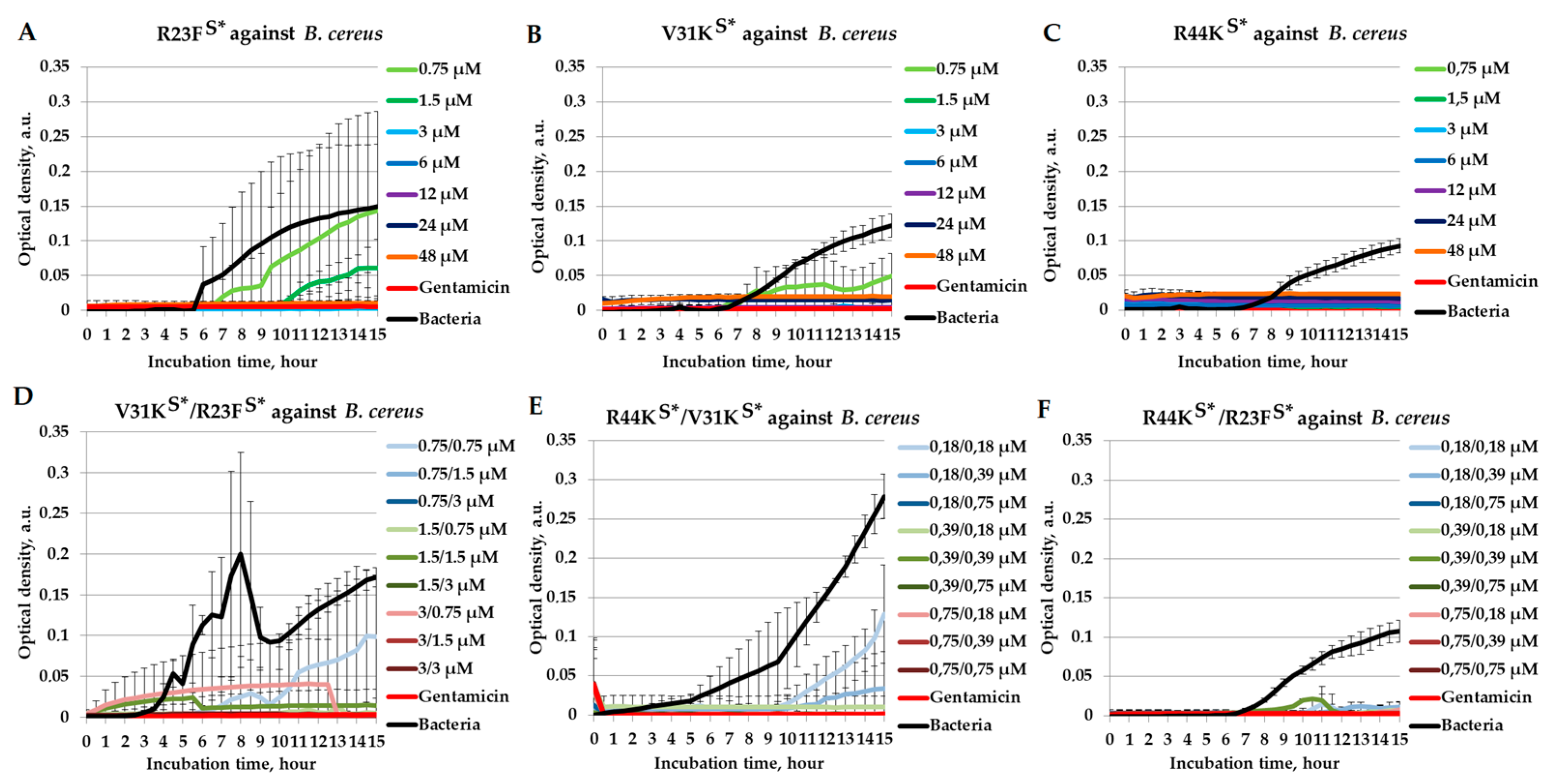
2.5. Combined Antimicrobial Effects of Peptides Against Bacillus cereus
2.6. The Results of the Fractional Inhibitory Concentration Index (FICI) Calculations for Pairwise Peptide Combinations Against E. coli, P. aeruginosa, S. aureus, MRSA, and B. cereus
3. Discussions
4. Materials and Methods
4.1. Synthesis and Purification of Peptides
4.2. Microorganism Strains
4.3. Testing of Synergistic Antimicrobial Activity of Peptides Against E. coli, P. aeruginosa, MRSA, S. aureus, and B. cereus in a Liquid Medium and Calculation Fractional Inhibitory Concentration Indexes (FICIs)
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xuan, J.; Feng, W.; Wang, J.; Wang, R.; Zhang, B.; Bo, L.; Chen, Z.-S.; Yang, H.; Sun, L. Antimicrobial peptides for combating drug-resistant bacterial infections. Drug Resist. Updat. 2023, 68, 100954. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zuo, S.; Wang, B.; Zhang, K.; Wang, Y. Antimicrobial Mechanisms and Clinical Application Prospects of Antimicrobial Peptides. Molecules 2022, 27, 2675. [Google Scholar] [CrossRef]
- Girdhar, M.; Sen, A.; Nigam, A.; Oswalia, J.; Kumar, S.; Gupta, R. Antimicrobial peptide-based strategies to overcome antimicrobial resistance. Arch. Microbiol. 2024, 206, 411. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Wang, D.; He, X.; Cao, M.; Cong, H.; Yu, B. Screening of Short-Chain Peptide RKIIIRW and Its Use as a Hydrogel for Wound Healing and Bacterial Infection Treatment. ACS Appl. Polym. Mater. 2024, 6, 10452–10466. [Google Scholar] [CrossRef]
- Li, G.; Deng, H.; Xu, W.; Chen, W.; Lai, Z.; Zhu, Y.; Zhang, L.; Shao, C.; Shan, A. Combating Antibiotic-Resistant Bacterial Infection Using Coassembled Dimeric Antimicrobial Peptide-Based Nanofibers. ACS Nano 2025, 19, 3155–3771. [Google Scholar] [CrossRef]
- Kang, S.-J.; Nam, S.H.; Lee, B.-J. Engineering Approaches for the Development of Antimicrobial Peptide-Based Antibiotics. Antibiotics 2022, 11, 1338. [Google Scholar] [CrossRef] [PubMed]
- Kalmouni, M.; Oh, Y.; Alata, W.; Magzoub, M. Designed Cell-Penetrating Peptide Constructs for Inhibition of Pathogenic Protein Self-Assembly. Pharmaceutics 2024, 16, 1443. [Google Scholar] [CrossRef]
- Huang, X.; Li, G. Antimicrobial Peptides and Cell-Penetrating Peptides: Non-Antibiotic Membrane-Targeting Strategies Against Bacterial Infections. Infect. Drug Resist. 2023, 16, 1203–1219. [Google Scholar] [CrossRef]
- Hu, Y.; Ling, Y.; Qin, Z.; Huang, J.; Jian, L.; Ren, D.F. Isolation, identification, and synergistic mechanism of a novel antimicrobial peptide and phenolic compound from fermented walnut meal and their application in Rosa roxbughii Tratt spoilage fungus. Food Chem. 2024, 433, 137333. [Google Scholar] [CrossRef]
- Mihaylova-Garnizova, R.; Davidova, S.; Hodzhev, Y.; Satchanska, G. Antimicrobial Peptides Derived from Bacteria: Classification, Sources, and Mechanism of Action against Multidrug-Resistant Bacteria. Int. J. Mol. Sci. 2024, 25, 10788. [Google Scholar] [CrossRef]
- Feijoo-Coronel, M.L.; Mendes, B.; Ramírez, D.; Peña-Varas, C.; de los Monteros-Silva, N.Q.E.; Proaño-Bolaños, C.; de Oliveira, L.C.; Lívio, D.F.; da Silva, J.A.; da Silva, J.M.S.F.; et al. Antibacterial and Antiviral Properties of Chenopodin-Derived Synthetic Peptides. Antibiotics 2024, 13, 78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chu, A.; Ouyang, X.; Li, B.; Yang, P.; Ba, Z.; Yang, Y.; Mao, W.; Zhong, C.; Gou, S.; et al. Rationally designed highly amphipathic antimicrobial peptides demonstrating superior bacterial selectivity relative to the corresponding α-helix peptide. Eur. J. Med. Chem. 2025, 286, 117310. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Yang, F.; Wang, C.; Zafir, M.; Gao, Z.; Liu, P.; El-Gohary, F.A.; Zhao, X.; Xue, H. High-level biosynthesis and purification of the antimicrobial peptide Kiadin based on non-chromatographic purification and acid cleavage methods. Biotechnol. Biofuels Bioprod. 2025, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Henson, B.A.B.; Li, F.; Álvarez-Huerta, J.A.; Wedamulla, P.G.; Palacios, A.V.; Scott, M.R.M.; Lim, D.T.E.; Scott, W.M.H.; Villanueva, M.T.L.; Ye, E.; et al. Novel active Trp- and Arg-rich antimicrobial peptides with high solubility and low red blood cell toxicity designed using machine learning tools. Int. J. Antimicrob. Agents 2025, 65, 107399. [Google Scholar] [CrossRef]
- Maani, Z.; Rahbarnia, L.; Bahadori, A.; Chollou, K.M.; Farajnia, S. Spotlight on HIV-derived TAT peptide as a molecular shuttle in drug delivery. Drug Discov. Today 2024, 29, 104191. [Google Scholar] [CrossRef]
- Guidotti, G.; Brambilla, L.; Rossi, D. Cell-Penetrating Peptides: From Basic Research to Clinics. Trends Pharmacol. Sci. 2017, 38, 406–424. [Google Scholar] [CrossRef]
- Milletti, F. Cell-penetrating peptides: Classes, origin, and current landscape. Drug Discov. Today 2012, 17, 850–860. [Google Scholar] [CrossRef]
- Derossi, D.; Joliot, A.H.; Chassaing, G.; Prochiantz, A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J. Biol. Chem. 1994, 269, 10444–10450. [Google Scholar] [CrossRef]
- Vivès, E.; Brodin, P.; Lebleu, B. A Truncated HIV-1 Tat Protein Basic Domain Rapidly Translocates through the Plasma Membrane and Accumulates in the Cell Nucleus. J. Biol. Chem. 1997, 272, 16010–16017. [Google Scholar] [CrossRef]
- Zhu, J.; Liang, Z.; Yao, H.; Wu, Z. Identifying Cell-Penetrating Peptides for Effectively Delivering Antimicrobial Molecules into Streptococcus suis. Antibiotics 2024, 13, 725. [Google Scholar] [CrossRef]
- Nooranian, S.; Oskuee, R.K.; Jalili, A. Antimicrobial Peptides, a Pool for Novel Cell Penetrating Peptides Development and Vice Versa. Int. J. Pept. Res. Ther. 2021, 27, 1205–1220. [Google Scholar] [CrossRef]
- Buccini, D.F.; Cardoso, M.H.; Franco, O.L. Antimicrobial Peptides and Cell-Penetrating Peptides for Treating Intracellular Bacterial Infections. Front. Cell. Infect. Microbiol. 2021, 10, 612931. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lim, S.I.; Shin, S.-H.; Lim, Y.; Koh, J.W.; Yang, S. Conjugation of Cell-Penetrating Peptides to Antimicrobial Peptides Enhances Antibacterial Activity. ACS Omega 2019, 4, 15694–15701. [Google Scholar] [CrossRef]
- Pradeau-Phélut, L.; Alvès, S.; Le Tareau, L.; Larralde, C.; Bernard, E.; Schirmer, C.; Lai-Kee-Him, J.; Lepvrier, E.; Bron, P.; Delamarche, C.; et al. Efficient Biochemical Method for Characterizing and Classifying Related Amyloidogenic Peptides. Anal. Chem. 2025, 97, 1078–1086. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Y.; Lin, H.; Chen, D. The effects and mechanisms of novel antibacterial amyloid peptides derived from Streptococcus mutans proteome. Chem. Eng. J. 2024, 497, 154458. [Google Scholar] [CrossRef]
- Kravchenko, S.V.; Domnin, P.A.; Grishin, S.Y.; Vershinin, N.A.; Gurina, E.V.; Zakharova, A.A.; Azev, V.N.; Mustaeva, L.G.; Gorbunova, E.Y.; Kobyakova, M.I.; et al. Enhancing the Antimicrobial Properties of Peptides through Cell-Penetrating Peptide Conjugation: A Comprehensive Assessment. Int. J. Mol. Sci. 2023, 24, 16723. [Google Scholar] [CrossRef]
- Galzitskaya, O.V.; Kurpe, S.R.; Panfilov, A.V.; Glyakina, A.V.; Grishin, S.Y.; Kochetov, A.P.; Deryusheva, E.I.; Machulin, A.V.; Kravchenko, S.V.; Domnin, P.A.; et al. Amyloidogenic Peptides: New Class of Antimicrobial Peptides with the Novel Mechanism of Activity. Int. J. Mol. Sci. 2022, 23, 5463. [Google Scholar] [CrossRef]
- Wani, N.A.; Gazit, E.; Ramamoorthy, A. Interplay between Antimicrobial Peptides and Amyloid Proteins in Host Defense and Disease Modulation. Langmuir 2024, 40, 25355–25366. [Google Scholar] [CrossRef] [PubMed]
- Kravchenko, S.V.; Domnin, P.A.; Grishin, S.Y.; Zakhareva, A.P.; Zakharova, A.A.; Mustaeva, L.G.; Gorbunova, E.Y.; Kobyakova, M.I.; Surin, A.K.; Poshvina, D.V.; et al. Optimizing Antimicrobial Peptide Design: Integration of Cell-Penetrating Peptides, Amyloidogenic Fragments, and Amino Acid Residue Modifications. Int. J. Mol. Sci. 2024, 25, 6030. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Olcay, B.; Ozdemir, G.D.; Ozdemir, M.A.; Ercan, U.K.; Guren, O.; Karaman, O. Prediction of the synergistic effect of antimicrobial peptides and antimicrobial agents via supervised machine learning. BMC Biomed. Eng. 2024, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Fu, L.; Li, S.; Chen, Z.; Ouyang, J.; Shang, X.; Liu, Y.; Gao, L.; Wang, Y. Synergistic collaboration between AMPs and non-direct antimicrobial cationic peptides. Nat. Commun. 2024, 15, 7319. [Google Scholar] [CrossRef]
- Mhlongo, J.T.; Waddad, A.Y.; Albericio, F.; de la Torre, B.G. Antimicrobial Peptide Synergies for Fighting Infectious Diseases. Adv. Sci. 2023, 10, 2300472. [Google Scholar] [CrossRef] [PubMed]
- Lennard, P.R.; Hiemstra, P.S.; Nibbering, P.H. Complementary Activities of Host Defence Peptides and Antibiotics in Combating Antimicrobial Resistant Bacteria. Antibiotics 2023, 12, 1518. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Jo, Y.; Shin, S. Understanding Antimicrobial Peptide Synergy: Differential Binding Interactions and Their Impact on Membrane Integrity. J. Phys. Chem. B 2024, 128, 9756–9771. [Google Scholar] [CrossRef]
- Jahangiri, A.; Neshani, A.; Mirhosseini, S.A.; Ghazvini, K.; Zare, H.; Sedighian, H. Synergistic effect of two antimicrobial peptides, Nisin and P10 with conventional antibiotics against extensively drug-resistant Acinetobacter baumannii and colistin-resistant Pseudomonas aeruginosa isolates. Microb. Pathog. 2021, 150, 104700. [Google Scholar] [CrossRef]
- Salama, A.H. Combined action of two synthetic ultrashort antimicrobial peptides exhibiting synergistic effects against clinically significant resistant bacteria. Vet. World 2024, 17, 2725–2730. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef]
- Hadjicharalambous, A.; Bournakas, N.; Newman, H.; Skynner, M.J.; Beswick, P. Antimicrobial and Cell-Penetrating Peptides: Understanding Penetration for the Design of Novel Conjugate Antibiotics. Antibiotics 2022, 11, 1636. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Yan, Z.-B.; Meng, Y.-M.; Hong, X.-Y.; Shao, G.; Ma, J.-J.; Cheng, X.-R.; Liu, J.; Kang, J.; Fu, C.-Y. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef]
- Kurpe, S.R.; Grishin, S.Y.; Surin, A.K.; Panfilov, A.V.; Slizen, M.V.; Chowdhury, S.D.; Galzitskaya, O.V. Antimicrobial and Amyloidogenic Activity of Peptides. Can Antimicrobial Peptides Be Used against SARS-CoV-2? Int. J. Mol. Sci. 2020, 21, 9552. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Q.; Ren, K.; Xu, T.; Zhang, Z.; Xu, M.; Rao, Z.; Zhang, X. A Review of Antimicrobial Peptides: Structure, Mechanism of Action, and Molecular Optimization Strategies. Fermentation 2024, 10, 540. [Google Scholar] [CrossRef]
- Duong, L.; Gross, S.P.; Siryaporn, A. Developing Antimicrobial Synergy With AMPs. Front. Med. Technol. 2021, 3, 640981. [Google Scholar] [CrossRef] [PubMed]
- Acar, J.F. Antibiotic synergy and antagonism. Med. Clin. North Am. 2000, 84, 1391–1406. [Google Scholar] [CrossRef] [PubMed]
- Marquette, A.; Bechinger, B. Biophysical Investigations Elucidating the Mechanisms of Action of Antimicrobial Peptides and Their Synergism. Biomolecules 2018, 8, 18. [Google Scholar] [CrossRef]
- Gopikrishnan, M.; Haryini, S.; Doss, C.P.G. Emerging strategies and therapeutic innovations for combating drug resistance in Staphylococcus aureus strains: A comprehensive review. J. Basic Microbiol. 2024, 64, 2300579. [Google Scholar] [CrossRef]
- Kravchenko, S.V.; Domnin, P.A.; Grishin, S.Y.; Panfilov, A.V.; Azev, V.N.; Mustaeva, L.G.; Gorbunova, E.Y.; Kobyakova, M.I.; Surin, A.K.; Glyakina, A.V.; et al. Multiple Antimicrobial Effects of Hybrid Peptides Synthesized Based on the Sequence of Ribosomal S1 Protein from Staphylococcus aureus. Int. J. Mol. Sci. 2022, 23, 524. [Google Scholar] [CrossRef]
- Odunitan, T.T.; Oyaronbi, A.O.; Adebayo, F.A.; Adekoyeni, P.A.; Apanisile, B.T.; Oladunni, T.D.; Saibu, O.A. Antimicrobial peptides: A novel and promising arsenal against methicillin-resistant Staphylococcus aureus (MRSA) infections. Pharm. Sci. Adv. 2024, 2, 100034. [Google Scholar] [CrossRef]
- Andrä, J.; Goldmann, T.; Ernst, C.M.; Peschel, A.; Gutsmann, T. Multiple Peptide Resistance Factor (MprF)-mediated Resistance of Staphylococcus aureus against Antimicrobial Peptides Coincides with a Modulated Peptide Interaction with Artificial Membranes Comprising Lysyl-Phosphatidylglycerol. J. Biol. Chem. 2011, 286, 18692–18700. [Google Scholar] [CrossRef]
- Weidenmaier, C.; Peschel, A.; Kempf, V.A.J.; Lucindo, N.; Yeaman, M.R.; Bayer, A.S. DltABCD- and MprF-mediated cell envelope modifications of Staphylococcus aureus confer resistance to platelet microbicidal proteins and contribute to virulence in a rabbit endocarditis model. Infect. Immun. 2005, 73, 8033–8038. [Google Scholar] [CrossRef]
- Joo, H.-S.; Otto, M. Mechanisms of resistance to antimicrobial peptides in staphylococci. Biochim. Biophys. Acta Biomembr. 2015, 1848, 3055–3061. [Google Scholar] [CrossRef]
- Maron, B.; Rolff, J.; Friedman, J.; Hayouka, Z. Antimicrobial Peptide Combination Can Hinder Resistance Evolution. Microbiol. Spectr. 2022, 10, e00973-22. [Google Scholar] [CrossRef] [PubMed]
- Sadasivam, D.; Nambiar, P.; Dutta, A.; Mitra, D. Rational design of antimicrobial peptides: An optimization approach. Mol. Syst. Des. Eng. 2024, 9, 311–322. [Google Scholar] [CrossRef]
- Verma, D.P.; Tripathi, A.K.; Thakur, A.K. Innovative Strategies and Methodologies in Antimicrobial Peptide Design. J. Funct. Biomater. 2024, 15, 320. [Google Scholar] [CrossRef] [PubMed]
- Mercer, D.K.; Torres, M.D.T.; Duay, S.S.; Lovie, E.; Simpson, L.; von Köckritz-Blickwede, M.; de la Fuente-Nunez, C.; O’Neil, D.A.; Angeles-Boza, A.M. Antimicrobial Susceptibility Testing of Antimicrobial Peptides to Better Predict Efficacy. Front. Cell. Infect. Microbiol. 2020, 10, 326. [Google Scholar] [CrossRef]
- Gurtovenko, A.A.; Anwar, J. Modulating the Structure and Properties of Cell Membranes: The Molecular Mechanism of Action of Dimethyl Sulfoxide. J. Phys. Chem. B 2007, 111, 10453–10460. [Google Scholar] [CrossRef]
- Gironi, B.; Kahveci, Z.; McGill, B.; Lechner, B.-D.; Pagliara, S.; Metz, J.; Morresi, A.; Palombo, F.; Sassi, P.; Petrov, P.G. Effect of DMSO on the Mechanical and Structural Properties of Model and Biological Membranes. Biophys. J. 2020, 119, 274–286. [Google Scholar] [CrossRef]
- Grishin, S.Y.; Domnin, P.A.; Kravchenko, S.V.; Azev, V.N.; Mustaeva, L.G.; Gorbunova, E.Y.; Kobyakova, M.I.; Surin, A.K.; Makarova, M.A.; Kurpe, S.R.; et al. Is It Possible to Create Antimicrobial Peptides Based on the Amyloidogenic Sequence of Ribosomal S1 Protein of P. aeruginosa? Int. J. Mol. Sci. 2021, 22, 9776. [Google Scholar] [CrossRef]
- Recktenwald, M.; Kaur, M.; Benmassaoud, M.M.; Copling, A.; Khanna, T.; Curry, M.; Cortes, D.; Fleischer, G.; Carabetta, V.J.; Vega, S.L. Antimicrobial Peptide Screening for Designing Custom Bactericidal Hydrogels. Pharmaceutics 2024, 16, 860. [Google Scholar] [CrossRef]
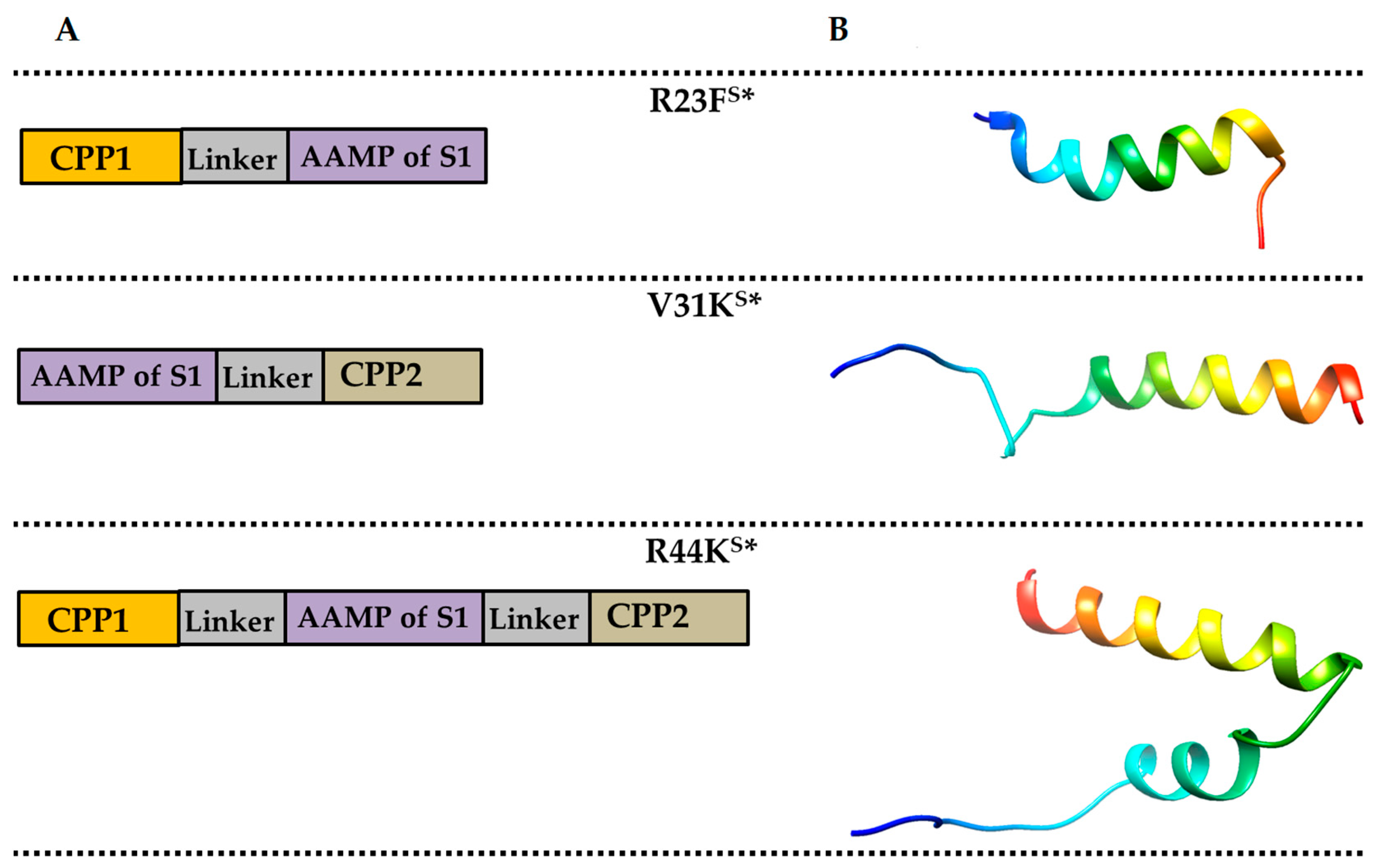

| Bacteria | Peptide 1 | Peptide 2 | Peptide 1 MIC in Combination | Peptide 1 MIC | Peptide 2 MIC in Combination | Peptide 2 MIC | FICI # |
|---|---|---|---|---|---|---|---|
| P. aeruginosa | V31KS* | R23FS* | 1.5 | 12 | 3 | 6 | 0.63 |
| E. coli | V31KS* | R23FS* | No data | 3 | No data | 3 | No data |
| S. aureus | V31KS* | R23FS* | 1.5 | 12 | 3 | 6 | 0.63 |
| MRSA | V31KS* | R23FS* | No data | 24 | No data | 12 | No data |
| B. cereus | V31KS* | R23FS* | 1.5 | 1.5 | 3 | 3 | 2 |
| P. aeruginosa | R44KS* | V31KS* | 0.75 | 1.5 | 3 | 12 | 0.75 |
| E. coli | R44KS* | V31KS* | 0.75 | 1.5 | 0.75 | 3 | 0.75 |
| S. aureus | R44KS* | V31KS* | 0.75 | 3 | 0.75 | 12 | 0.31 |
| MRSA | R44KS* | V31KS* | 3 | 6 | 0.75 | 24 | 0.53 |
| B. cereus | R44KS* | V31KS* | 0.18 | 0.75 | 0.75 | 1.5 | 0.74 |
| P. aeruginosa | R44KS* | R23FS* | 0.75 | 1.5 | 3 | 6 | 1 |
| E. coli | R44KS* | R23FS* | No data | 1.5 | No data | 3 | No data |
| S. aureus | R44KS* | R23FS* | 0.75 | 3 | 3 | 6 | 0.75 |
| MRSA | R44KS* | R23FS* | 1.5 | 6 | 3 | 12 | 0.5 |
| B. cereus | R44KS* | R23FS* | 0.18 | 0.75 | 0.75 | 3 | 0.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galzitskaya, O.V.; Kravchenko, S.V.; Grishin, S.Y.; Zakhareva, A.P.; Mustaeva, L.G.; Gorbunova, E.Y.; Surin, A.K.; Azev, V.N. Combinatorial Effects of CPP-Modified Antimicrobial Peptides: Synergistic and Additive Interactions Against Pathogenic Bacteria. Int. J. Mol. Sci. 2025, 26, 5968. https://doi.org/10.3390/ijms26135968
Galzitskaya OV, Kravchenko SV, Grishin SY, Zakhareva AP, Mustaeva LG, Gorbunova EY, Surin AK, Azev VN. Combinatorial Effects of CPP-Modified Antimicrobial Peptides: Synergistic and Additive Interactions Against Pathogenic Bacteria. International Journal of Molecular Sciences. 2025; 26(13):5968. https://doi.org/10.3390/ijms26135968
Chicago/Turabian StyleGalzitskaya, Oxana V., Sergey V. Kravchenko, Sergei Y. Grishin, Alena P. Zakhareva, Leila G. Mustaeva, Elena Y. Gorbunova, Alexey K. Surin, and Viacheslav N. Azev. 2025. "Combinatorial Effects of CPP-Modified Antimicrobial Peptides: Synergistic and Additive Interactions Against Pathogenic Bacteria" International Journal of Molecular Sciences 26, no. 13: 5968. https://doi.org/10.3390/ijms26135968
APA StyleGalzitskaya, O. V., Kravchenko, S. V., Grishin, S. Y., Zakhareva, A. P., Mustaeva, L. G., Gorbunova, E. Y., Surin, A. K., & Azev, V. N. (2025). Combinatorial Effects of CPP-Modified Antimicrobial Peptides: Synergistic and Additive Interactions Against Pathogenic Bacteria. International Journal of Molecular Sciences, 26(13), 5968. https://doi.org/10.3390/ijms26135968






