Tetrahydrocurcumin Outperforms Curcumin in Preventing Oxidative Stress-Induced Dysfunction in Tert-Butyl Hydroperoxide-Stimulated Cardiac Fibroblasts
Abstract
1. Introduction
2. Results
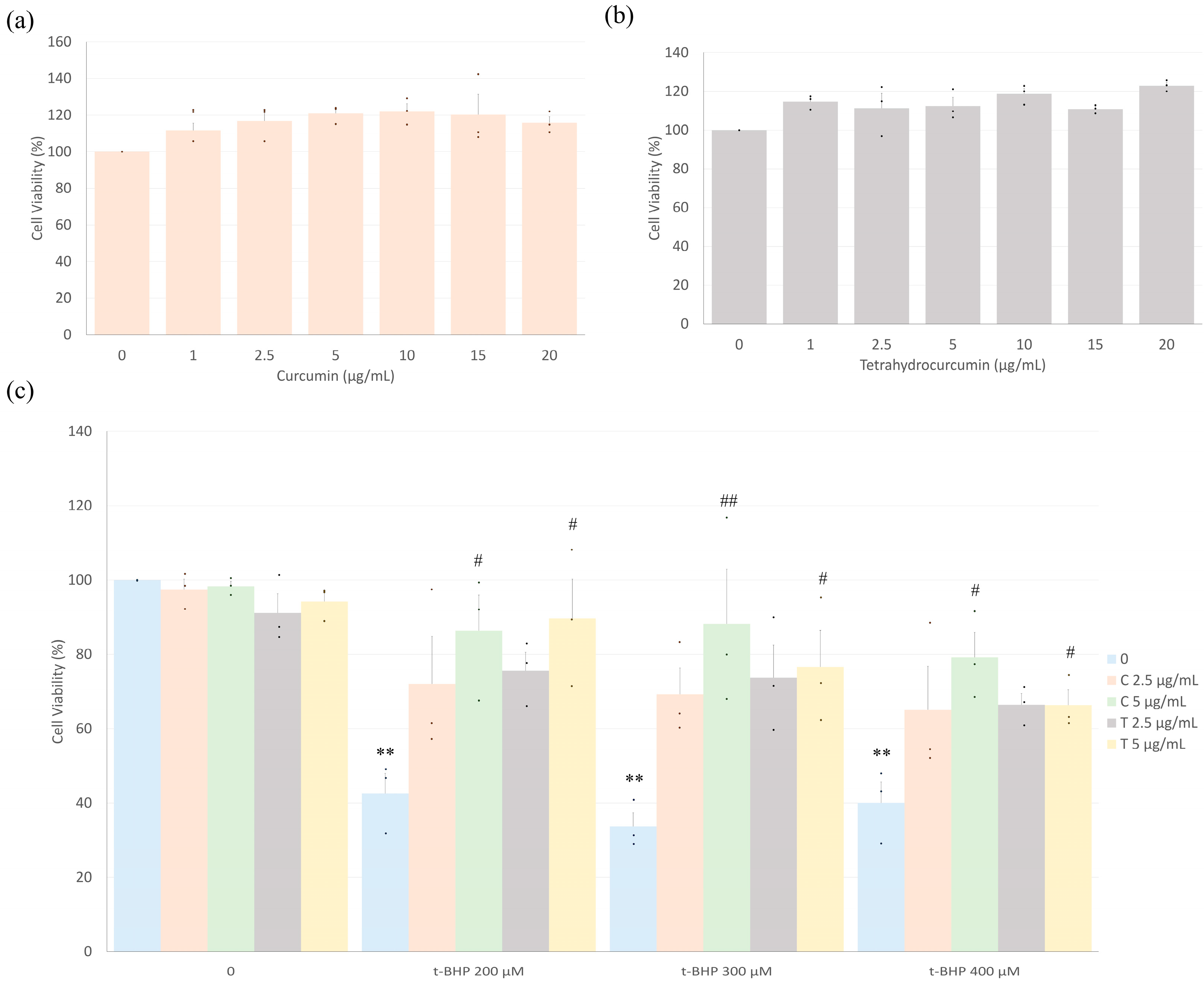
2.1. Curcumin and Tetrahydrocurcumin Prevent t-BHP-Induced Reduction in Cell Viability
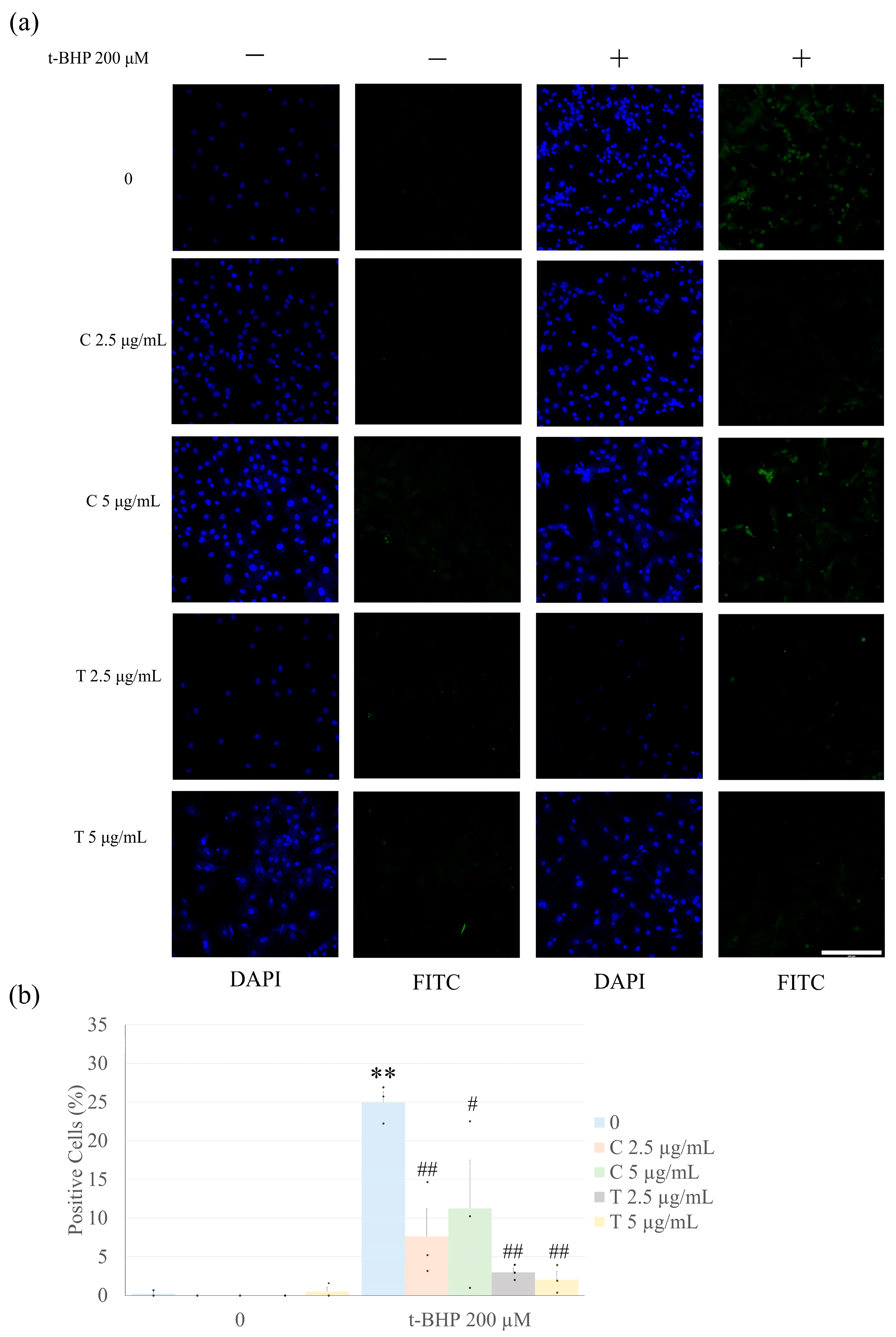
2.2. Pretreatment with Curcumin and Tetrahydrocurcumin Prevents t-BHP-Induced Apoptosis
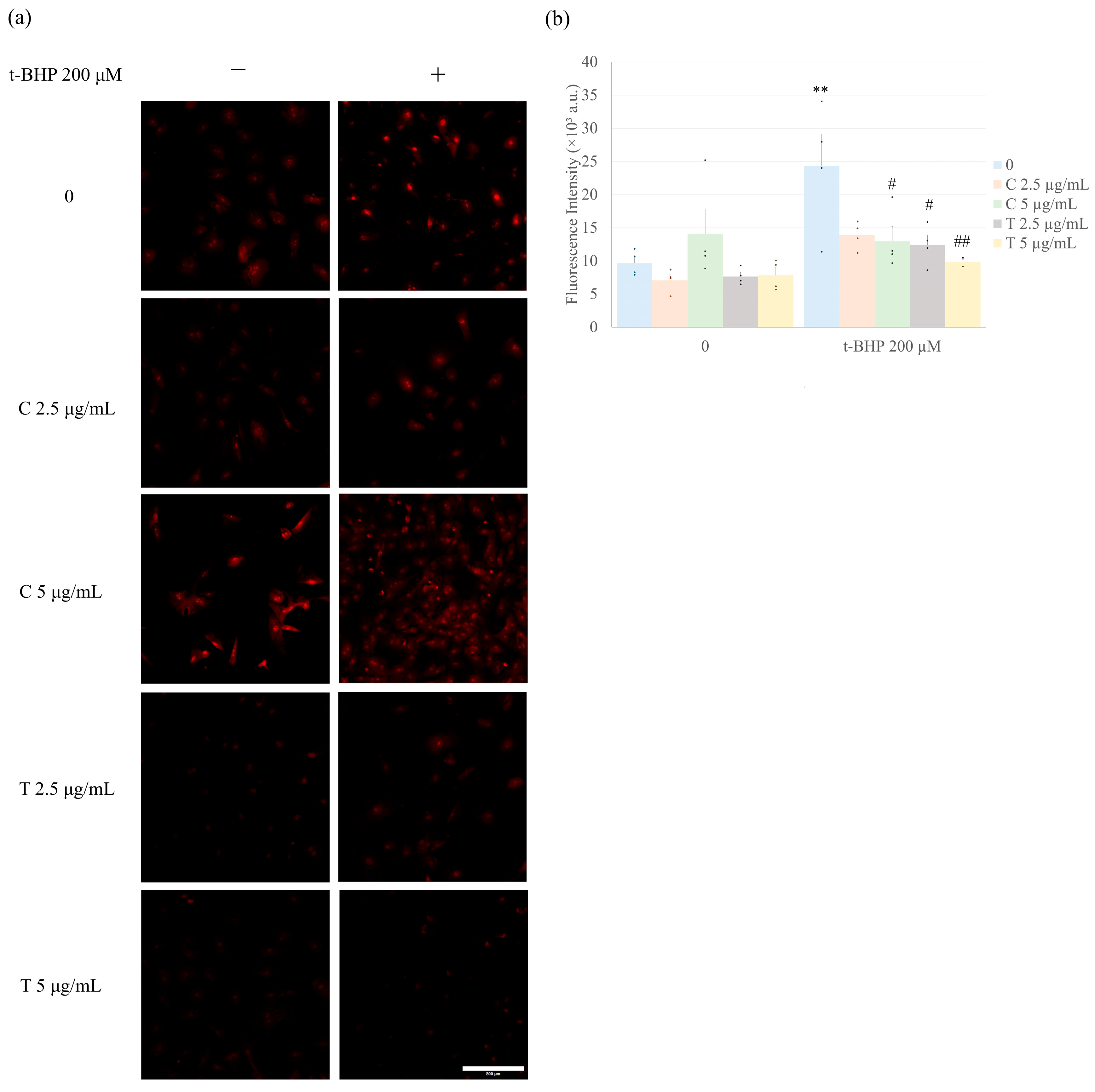
2.3. Curcumin and Tetrahydrocurcumin Pretreatment Prevent Intracellular ROS Production
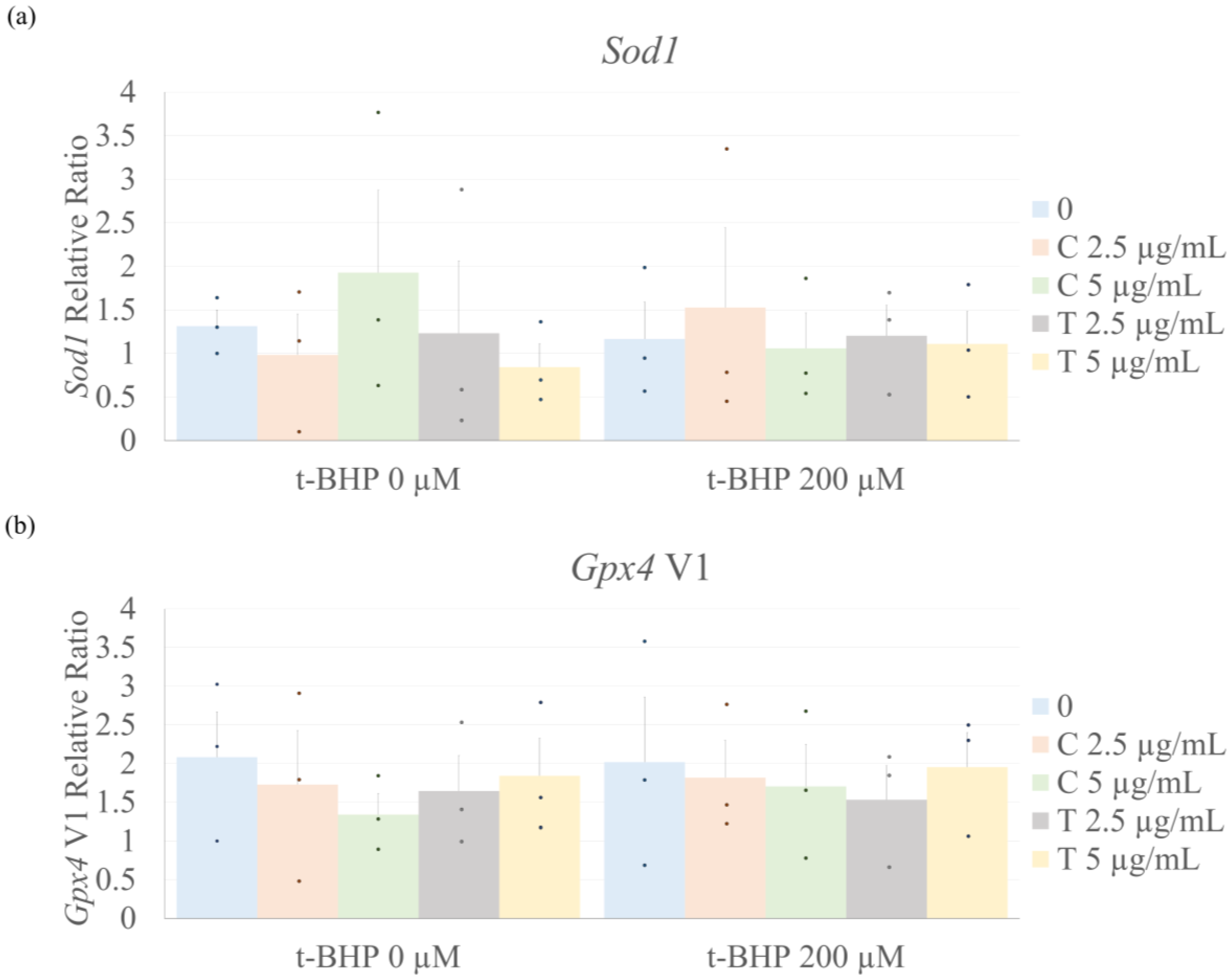
2.4. Antioxidant Enzyme Expression
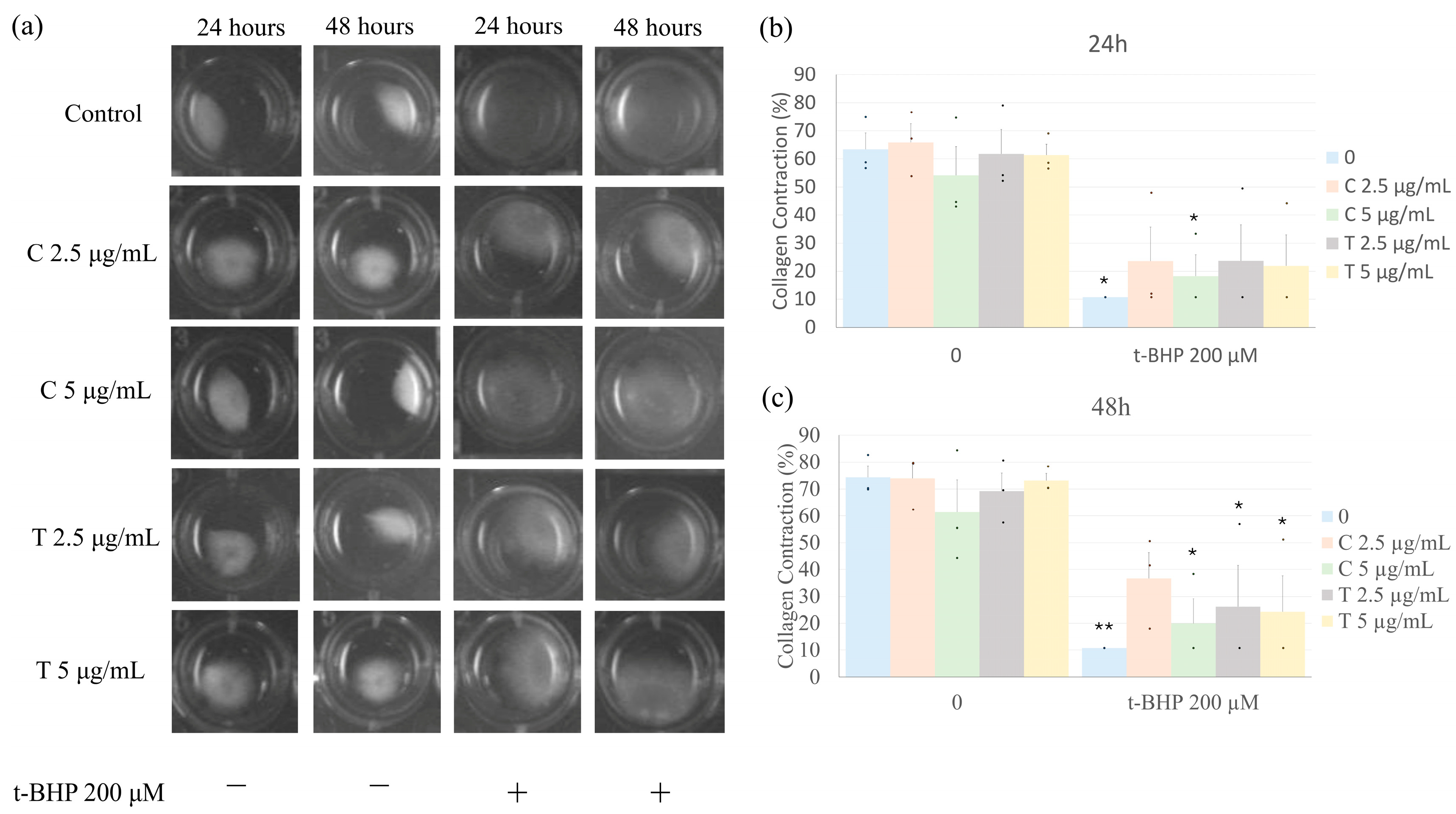
2.5. Effects of Treatment on Collagen Gel Contraction
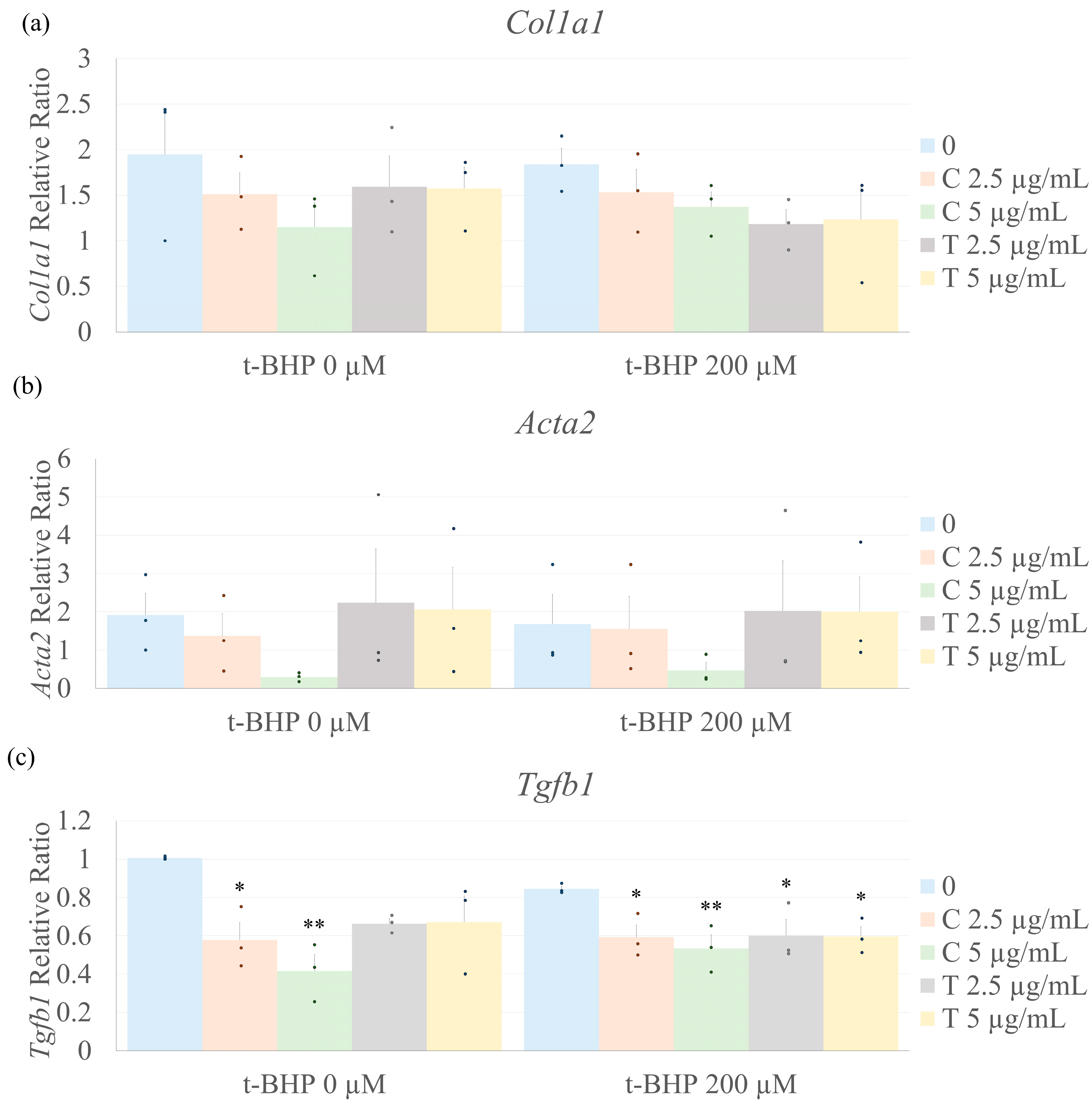
2.6. Biomarkers of Fibrosis
3. Discussion
4. Materials and Methods
4.1. Heart Fibroblasts Extraction and Cell Culture
4.2. Fibroblast Treatment
4.3. Cell Viability Assay
4.4. Oxidative Stress
4.5. Apoptosis
4.6. Collagen Gel Contraction
4.7. Quantitative Reverse Transcriptase-Polymerase Chain Reaction (qRT-PCR)
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| t-BHP | Tert-butyl hydroperoxide |
| ROS | Reactive oxygen species |
| Tgfb1 | Transforming growth factor beta |
| ECM | Extracellular matrix |
| CF | Cardiac fibroblasts |
| DMEM | Dulbecco’s modified Eagle’s medium |
| MTT | Methylthiazol tetrazolium |
| DHE | Dihydroethidium |
| TUNEL | Terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labeling |
| DAPI | 4’,6-diamidino-2-phenylindole |
| qRT-PCR | Quantitative reverse transcriptase-polymerase chain reaction |
| ARBP | Acidic ribosomal binding protein |
| SOD | Superoxide dismutase |
| GPx4 | Glutathione peroxidase 4 |
| α-SMA | α-smooth muscle actin |
| ANOVA | Analysis of variance |
| S.E.M | Standard error of the mean |
References
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Gibbs, B.B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data from the American Heart Association. Circulation 2024, 149, E347–E913. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Cardiac Fibrosis. Cardiovasc. Res. 2021, 117, 1450–1488. [Google Scholar] [CrossRef]
- González, A.; Schelbert, E.B.; Díez, J.; Butler, J. Myocardial Interstitial Fibrosis in Heart Failure: Biological and Translational Perspectives. J. Am. Coll. Cardiol. 2018, 71, 1696–1706. [Google Scholar] [CrossRef] [PubMed]
- Travers, J.G.; Kamal, F.A.; Robbins, J.; Yutzey, K.E.; Blaxall, B.C. Cardiac fibrosis: The fibroblast awakens. Circ. Res. 2016, 118, 1021–1040. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Zhang, Z.; Ling, L.; Liu, X.; Wang, X. Drugs for treating myocardial fibrosis. Front. Pharmacol. 2023, 14, 1221881. [Google Scholar] [CrossRef] [PubMed]
- Webber, M.; Jackson, S.P.; Moon, J.C.; Captur, G. Myocardial Fibrosis in Heart Failure: Anti-Fibrotic Therapies and the Role of Cardiovascular Magnetic Resonance in Drug Trials. Cardiol. Ther. 2020, 9, 363–376. [Google Scholar] [CrossRef]
- Borg, T.K.; Caulfield, J.B. Collagen in the heart. Tex. Rep. Biol. Med. 1979, 39, 321–333. [Google Scholar]
- Kodigepalli, K.M.; Thatcher, K.; West, T.; Howsmon, D.P.; Schoen, F.J.; Sacks, M.S.; Breuer, C.K.; Lincoln, J. Biology and biomechanics of the heart valve extracellular matrix. J. Cardiovasc. Dev. Dis. 2020, 7, 57. [Google Scholar] [CrossRef]
- Varzideh, F.; Kansakar, U.; Donkor, K.; Wilson, S.; Jankauskas, S.S.; Mone, P.; Wang, X.; Lombardi, A.; Santulli, G. Cardiac Remodeling After Myocardial Infarction: Functional Contribution of microRNAs to Inflammation and Fibrosis. Front. Cardiovasc. Med. 2022, 9, 863238. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, J.; Song, J.; Xie, M.; Liu, Y.; Dong, Z.; Liu, X.; Li, X.; Zhang, M.; Chen, Y.; et al. Elabela alleviates ferroptosis, myocardial remodeling, fibrosis and heart dysfunction in hypertensive mice by modulating the IL-6/STAT3/GPX4 signaling. Free Radic. Biol. Med. 2022, 181, 130–142. [Google Scholar] [CrossRef]
- Kanisicak, O.; Khalil, H.; Ivey, M.J.; Karch, J.; Maliken, B.D.; Correll, R.N.; Brody, M.J.; Lin, S.-C.J.; Aronow, B.J.; Tallquist, M.D.; et al. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat. Commun. 2016, 7, 12260. [Google Scholar] [CrossRef]
- Ravassa, S.; López, B.; Treibel, T.A.; San José, G.; Losada-Fuentenebro, B.; Tapia, L.; Bayés-Genís, A.; Díez, J.; González, A. Cardiac Fibrosis in heart failure: Focus on non-invasive diagnosis and emerging therapeutic strategies. Mol. Asp. Med. 2023, 93, 101194. [Google Scholar] [CrossRef]
- Yang, E.Y.; Ghosn, M.G.; Khan, M.A.; Gramze, N.L.; Brunner, G.; Nabi, F.; Nambi, V.; Nagueh, S.F.; Nguyen, D.T.; Graviss, E.A.; et al. Myocardial Extracellular Volume Fraction Adds Prognostic Information beyond Myocardial Replacement Fibrosis. Circ. Cardiovasc. Imaging 2019, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, B.; Li, L.; Zhou, X.; Li, Q. Effects of Resveratrol on Pulmonary Fibrosis via TGF-β/Smad/ERK Signaling Pathway. Am. J. Chin. Med. 2023, 51, 651–676. [Google Scholar] [CrossRef]
- Wang, C.-J.; Wang, J.-M.; Lin, W.-L.; Chu, C.-Y.; Chou, F.-P.; Tseng, T.-H. Research Section Protective Eect of Hibiscus Anthocyanins Against tert-butyl Hydroperoxide-induced Hepatic Toxicity in Rats. Food Chem. Toxicol. 2000, 38, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Alía, M.; Ramos, S.; Mateos, R.; Bravo, L.; Goya, L. Response of the antioxidant defense system to tert-butyl hydroperoxide and hydrogen peroxide in a human hepatoma cell line (HepG2). J. Biochem. Mol. Toxicol. 2005, 19, 119–128. [Google Scholar] [CrossRef]
- Ebadollahi, S.; Zabihi, E.; Golpour, M.; Aghajanpour-Mir, M. The effect of arbutin on the expression of tumor suppressor P53, BAX/BCL-2 ratio and oxidative stress induced by tert-butyl hydroperoxide in fibroblast and LNcap cell lines. Cell J. 2021, 22, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Caligo, M.A.; Bonatti, F.; Guidugli, L.; Aretini, P.; Galli, A. A yeast recombination assay to characterize human BRCA1 missense variants of unknown pathological significance. Hum. Mutat. 2009, 30, 123–133. [Google Scholar] [CrossRef]
- Dashtaki, A.; Mahjoub, S.; Zabihi, E.; Pourbagher, R. The Effects of Pre-Treatment and Post-Treatment of Thymol against tert-Butyl Hydroperoxide (t-BHP) Cytotoxicity in MCF-7 Cell Line and Fibroblast Derived Foreskin. Rep. Biochem. Mol. Biol. 2020, 9, 338. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Report on Traditional and Complementary Medicine 2019. 2019. Available online: https://www.who.int/publications/i/item/978924151536 (accessed on 25 February 2025).
- Chakraborty, S.; Karmenyan, A.; Tsai, J.W.; Chiou, A. Inhibitory effects of curcumin and cyclocurcumin in 1-methyl-4-phenylpyridinium (MPP+) induced neurotoxicity in differentiated PC12 cells. Sci. Rep. 2017, 7, 16977. [Google Scholar] [CrossRef]
- Yu, Y.; Sun, J.; Wang, R.; Liu, J.; Wang, P.; Wang, C. Curcumin Management of Myocardial Fibrosis and its Mechanisms of Action: A Review. Am. J. Chin. Med. 2019, 47, 1675–1710. [Google Scholar] [CrossRef] [PubMed]
- Sadoughi, F.; Hallajzadeh, J.; Mirsafaei, L.; Asemi, Z.; Zahedi, M.; Mansournia, M.A.; Yousefi, B. Cardiac fibrosis and curcumin: A novel perspective on this natural medicine. Mol. Biol. Rep. 2021, 48, 7597–7608. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhai, M.; Jiang, L.; Song, F.; Zhang, B.; Li, J.; Li, H.; Li, B.; Xia, L.; Xu, L.; et al. Tetrahydrocurcumin ameliorates diabetic cardiomyopathy by attenuating high glucose-induced oxidative stress and fibrosis via activating the SIRT1 pathway. Oxidative Med. Cell. Longev. 2019, 2019, 6746907. [Google Scholar] [CrossRef]
- Lau, W.L.; Khazaeli, M.; Savoj, J.; Manekia, K.; Bangash, M.; Thakurta, R.G.; Dang, A.; Vaziri, N.D.; Singh, B. Dietary tetrahydrocurcumin reduces renal fibrosis and cardiac hypertrophy in 5/6 nephrectomized rats. Pharmacol. Res. Perspect. 2018, 6, e00385. [Google Scholar] [CrossRef]
- Shen, C.Y.; Jiang, J.G.; Yang, L.; Wang, D.W.; Zhu, W. Anti-ageing active ingredients from herbs and nutraceuticals used in traditional Chinese medicine: Pharmacological mechanisms and implications for drug discovery. Br. J. Pharmacol. 2017, 174, 1395–1425. [Google Scholar] [CrossRef]
- Osawa, T.; Kato, Y. Protective role of antioxidative food factors in oxidative stress caused by hyperglycemia. Ann. N. Y. Acad. Sci. 2005, 1043, 440–451. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Deb, L.; Prasad, S. Curcumin differs from tetrahydrocurcumin for molecular targets, signaling pathways and cellular responses. Molecules 2015, 20, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.C.; Tsai, M.L.; Lai, C.S.; Wang, Y.J.; Ho, C.T.; Pan, M.H. Chemopreventative effects of tetrahydrocurcumin on human diseases. Food Funct. 2014, 5, 12–17. [Google Scholar] [CrossRef]
- Claridge, B.; Drack, A.; Pinto, A.R.; Greening, D.W. Defining cardiac fibrosis complexity and regulation towards therapeutic development. Clin. Transl. Discov. 2023, 3, e163. [Google Scholar] [CrossRef]
- Azeredo, P.d.S.; Fan, D.; Murphy, E.A.; Carver, W.E. Potential of Plant-Derived Compounds in Preventing and Reversing Organ Fibrosis and the Underlying Mechanisms. Cells 2024, 13, 421. [Google Scholar] [CrossRef]
- Xie, Q.F.; Cheng, J.J.; Chen, J.F.; Feng, Y.C.; Lin, G.S.; Xu, Y. Comparison of Anti-Inflammatory and Antioxidantactivities of Curcumin, Tetrahydrocurcuminand Octahydrocurcuminin LPS-Stimulated RAW264.7 Macrophages. Evid.-Based Complement. Altern. Med. 2020, 2020, 8856135. [Google Scholar] [CrossRef]
- Ramos-Tovar, E.; Muriel, P. Molecular mechanisms that link oxidative stress, inflammation, and fibrosis in the liver. Antioxidants 2020, 9, 1279. [Google Scholar] [CrossRef]
- Bae, S.J.; Lee, J.S.; Kim, J.M.; Lee, E.K.; Han, Y.K.; Kim, H.J.; Choi, J.; Ha, Y.M.; No, J.-K.; Kim, Y.H.; et al. 5-hydroxytrytophan inhibits tert-butylhydroperoxide (t -BHP)-induced oxidative damage via the suppression of reactive species (RS) and nuclear factor-κB (NF-κB) activation on human fibroblast. J. Agric. Food Chem. 2010, 58, 6387–6394. [Google Scholar] [CrossRef] [PubMed]
- Wedel, S.; Martic, I.; Hrapovic, N.; Fabre, S.; Madreiter-Sokolowski, C.T.; Haller, T.; Pierer, G.; Ploner, C.; Jansen-Dürr, P.; Cavinato, M. tBHP treatment as a model for cellular senescence and pollution-induced skin aging. Mech. Ageing Dev. 2020, 190, 111318. [Google Scholar] [CrossRef] [PubMed]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, pyroptosis and apoptosis: An intricate game of cell death. Cell. Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef]
- Crouchet, E.; Dachraoui, M.; Jühling, F.; Roehlen, N.; Oudot, M.A.; Durand, S.C.; Ponsolles, C.; Gadenne, C.; Meiss-Heydmann, L.; Moehlin, J.; et al. Targeting the liver clock improves fibrosis by restoring TGF-β signaling. J. Hepatol. 2024, 82, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Saadat, S.; Noureddini, M.; Mahjoubin-Tehran, M.; Nazemi, S.; Shojaie, L.; Aschner, M.; Maleki, B.; Abbasi-Kolli, M.; Moghadam, H.R.; Alani, B.; et al. Pivotal Role of TGF-β/Smad Signaling in Cardiac Fibrosis: Non-coding RNAs as Effectual Players. Front. Cardiovasc. Med. 2021, 7, 588347. [Google Scholar] [CrossRef]
- Peng, D.; Fu, M.; Wang, M.; Wei, Y.; Wei, X. Targeting TGF-β signal transduction for fibrosis and cancer therapy. Mol. Cancer 2022, 21, 104. [Google Scholar] [CrossRef]
- Monika, P.; Chandraprabha, M.N.; Murthy, K.N.C. Catechin, epicatechin, curcumin, garlic, pomegranate peel and neem extracts of Indian origin showed enhanced anti-inflammatory potential in human primary acute and chronic wound derived fibroblasts by decreasing TGF-β and TNF-α expression. BMC Complement. Med. Ther. 2023, 23, 181. [Google Scholar] [CrossRef]
- Li, N.; Liu, T.-H.; Yu, J.-Z.; Li, C.-X.; Liu, Y.; Wu, Y.-Y.; Yang, Z.-S.; Yuan, J.-L. Curcumin and Curcumol Inhibit NF- B and TGF- β 1/Smads Signaling Pathways in CSE-Treated RAW246.7 Cells. Evid.-Based Complement. Altern. Med. 2019, 2019, 3035125. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, J.; Ma, T.; Yang, X.; Yuan, Y.; Guo, Y. The role and mechanism of TGF-β1 in the antidepressant-like effects of tetrahydrocurcumin. Eur. J. Pharmacol. 2023, 959, 176075. [Google Scholar] [CrossRef]
- Xu, L.-H.; Tan, R.-Z.; Lin, J.-Y.; Li, T.; Jia, J.; Wu, L.-H.; Wang, R.; He, Y.-H.; Su, H.-W.; Li, P.; et al. Chaihuang Yishen Granule ameliorates mitochondrial homeostasis by upregulating PRDX5/TFAM axis to inhibit renal fibrosis in CKD. Phytomedicine 2025, 139, 156426. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.Y.; Hsieh, P.L.; Chao, S.C.; Fang, C.Y.; Ohiro, Y.; Liao, Y.W.; Yu, C.C.; Chang, M.T. Targeting MetaLnc9/miR-143/FSCN1 axis inhibits oxidative stress and myofibroblast transdifferentiation in oral submucous fibrosis. J. Dent. Sci. 2024, 19, 1416–1425. [Google Scholar] [CrossRef]
- Alghamdi, S.A.; Alissa, M.; Alsuwat, M.A. Dermal derived matrix hydrogel loaded with curcumin improved wound healing in a diabetic rat model. Tissue Cell 2024, 90, 102495. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, J.; Jiang, W.; Zhao, Y.; Yang, C.; Wu, Y.; Li, Q.; Zhu, C. Multiplexing Nanodrug Ameliorates Liver Fibrosis via ROS Elimination and Inflammation Suppression. Small 2022, 18, 2102848. [Google Scholar] [CrossRef]
- Ye, S.; Li, S.; Ma, Y.; Hu, D.; Xiao, F. Curcumin hinders PBDE-47-induced neutrophil extracellular traps release via Nrf2-associated ROS inhibition. Ecotoxicol. Environ. Saf. 2021, 225, 112779. [Google Scholar] [CrossRef]
- Rodrigues, H.C.N.; Martins, T.F.P.; Santana, N.C.F.e.S.; Braga, C.C.; Silva, M.A.C.; da Cunha, L.C.; Sugizaki, C.S.d.A.; Freitas, A.T.V.d.S.; Costa, N.A.; Peixoto, M.D.R.G. Antioxidant and anti-inflammatory response to curcumin supplementation in hemodialysis patients: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. ESPEN 2021, 44, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Batty, M.; Bennett, M.R.; Yu, E. The Role of Oxidative Stress in Atherosclerosis. Cells 2022, 11, 3843. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Matsuda, T.; Sugiyama, K.-I.; Izawa, S.; Kimura, A. Genetic Analysis of Glutathione Peroxidase in Oxidative Stress Response of Saccharomyces cerevisiae. J. Biol. Chem. 1999, 274, 27002–27009. [Google Scholar] [CrossRef]
- Stewart, J.A.; Massey, E.P.; Fix, C.; Zhu, J.; Goldsmith, E.C.; Carver, W. Temporal alte rations in cardiac fibroblast function following induction of pressure overload. Cell Tissue Res. 2010, 340, 117–126. [Google Scholar] [CrossRef]
- Bell, E.; Ivarsson, B.; Merrill, C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro (tissue structure/hydrated collagen lattice/fibroblast function/cell aging/contractility). Proc. Natl. Acad. Sci. USA 1979, 76, 1274–1278. [Google Scholar] [CrossRef] [PubMed]
- Carver, W.; Fix, E.; Fix, C.; Fan, D.; Chakrabarti, M.; Azhar, M. Effects of emodin, a plant-derived anthraquinone, on TGF-β1-induced cardiac fibroblast activation and function. J. Cell. Physiol. 2021, 236, 7440–7449. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
| Primer | Sequence |
|---|---|
| Collagen type I alpha 1 chain (Col1a1) | Forward: 5’-GCGAAGGCAACAGTCGATTC-3’ |
| Reverse: 5’CCCAAGTTCCGGTGTGACTC-3’ | |
| Alpha-smooth muscle actin (Acta2) | Forward: 5’-GGAGTGATGGTTGGAATGG-3’ |
| Reverse: 5’-ATGATGCCGTGTTCTATCG-3’ | |
| Transforming growth factor beta 1 (Tgfb1) | Forward: 5’TGCCCTCTACAACCAACACA-3’ |
| Reverse: 5’GTTGGACAACTGCTCCACCT-3’ | |
| Superoxide dismutase 1 (Sod1) | Forward: 5’-GGTGTGGCCAATGTGTCCATTGAA-3’ |
| Reverse: 5’-CGGCTTCCAGCATTTCCAGTCTTT-3’ | |
| Glutathione peroxidase 4 (Gpx4) | Forward: 5’-AGGCAGGAGCCAGGAAGTAATCAA-3’ |
| Reverse: 5’-CCTTGGGCTGGACTTTCATCCATT-3’ | |
| Acidic ribosomal phosphoprotein P0 (Arbp) | Forward: 5’-TAGAGGGTGTCCGCAATG-3’ |
| Reverse: 5’-GAAGGTGTAGTCAGTCTCC-3’ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azeredo, P.d.S.; Fix, C.; Pernomian, L.; Wenceslau, C.F.; Piroli, G.G.; Vicente, C.P.; Carver, W.E. Tetrahydrocurcumin Outperforms Curcumin in Preventing Oxidative Stress-Induced Dysfunction in Tert-Butyl Hydroperoxide-Stimulated Cardiac Fibroblasts. Int. J. Mol. Sci. 2025, 26, 5964. https://doi.org/10.3390/ijms26135964
Azeredo PdS, Fix C, Pernomian L, Wenceslau CF, Piroli GG, Vicente CP, Carver WE. Tetrahydrocurcumin Outperforms Curcumin in Preventing Oxidative Stress-Induced Dysfunction in Tert-Butyl Hydroperoxide-Stimulated Cardiac Fibroblasts. International Journal of Molecular Sciences. 2025; 26(13):5964. https://doi.org/10.3390/ijms26135964
Chicago/Turabian StyleAzeredo, Patrícia dos Santos, Charity Fix, Laena Pernomian, Camilla F. Wenceslau, Gerardo G. Piroli, Cristina Pontes Vicente, and Wayne E. Carver. 2025. "Tetrahydrocurcumin Outperforms Curcumin in Preventing Oxidative Stress-Induced Dysfunction in Tert-Butyl Hydroperoxide-Stimulated Cardiac Fibroblasts" International Journal of Molecular Sciences 26, no. 13: 5964. https://doi.org/10.3390/ijms26135964
APA StyleAzeredo, P. d. S., Fix, C., Pernomian, L., Wenceslau, C. F., Piroli, G. G., Vicente, C. P., & Carver, W. E. (2025). Tetrahydrocurcumin Outperforms Curcumin in Preventing Oxidative Stress-Induced Dysfunction in Tert-Butyl Hydroperoxide-Stimulated Cardiac Fibroblasts. International Journal of Molecular Sciences, 26(13), 5964. https://doi.org/10.3390/ijms26135964










