Pomegranate Extract Modulates Oxidative Stress by Reducing Basal ROS Levels and Protecting White Blood Cells from Induced Oxidative Damage in Aging Mice
Abstract
1. Introduction
2. Results
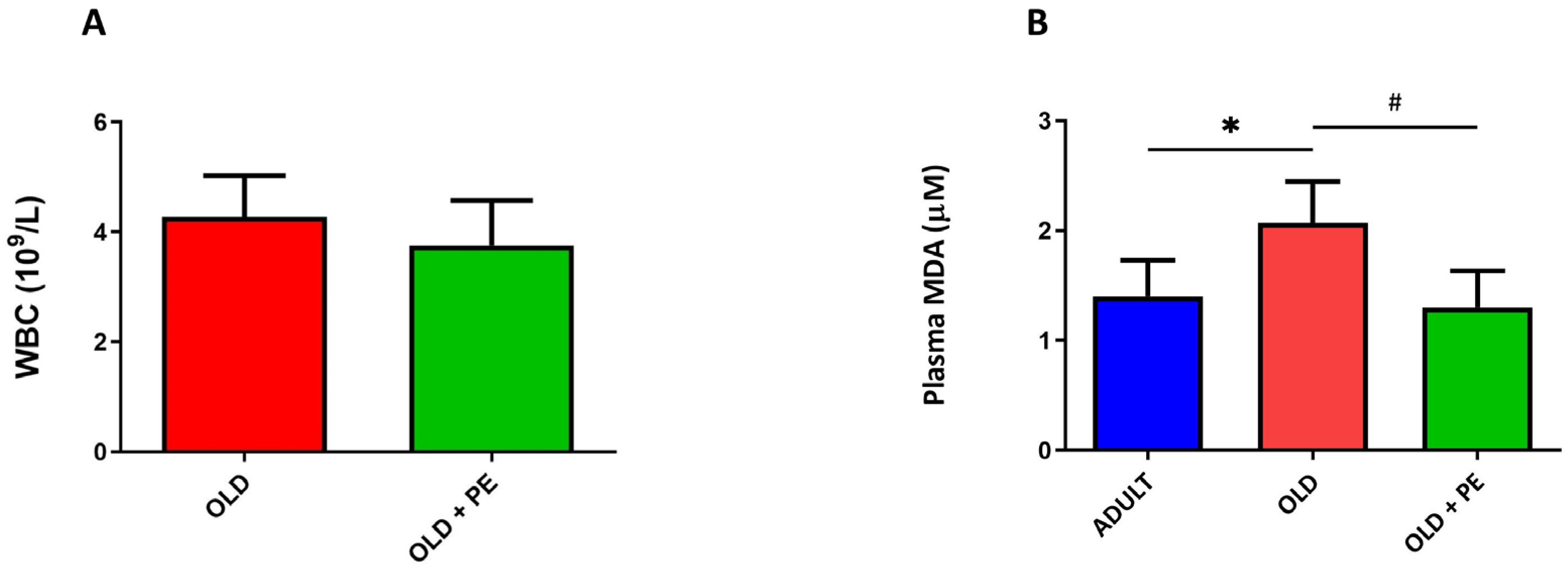
2.1. Effect of Pomegranate Extract on White Blood Cell Count and Lipid Peroxidation in Plasma
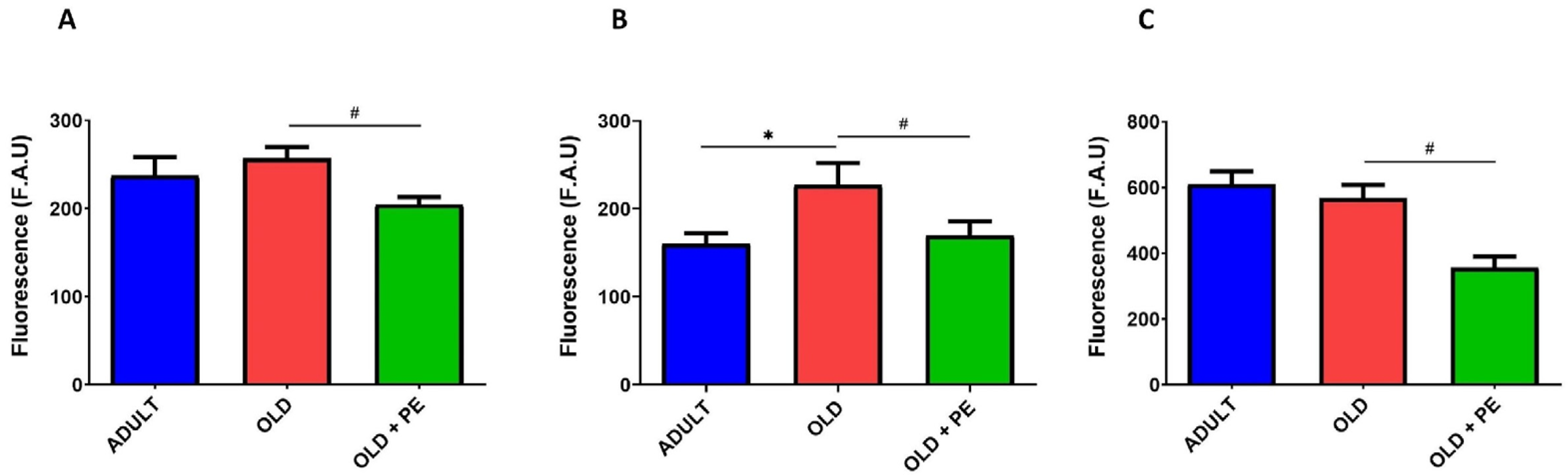
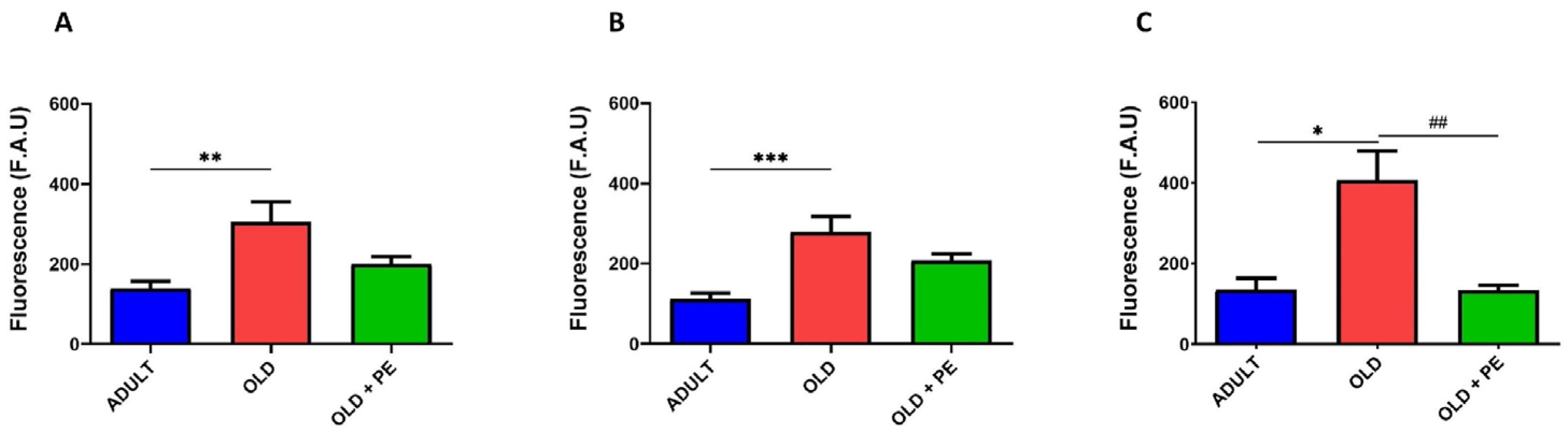
2.2. Study of Intracellular Basal ROS Levels with PE Supplementation
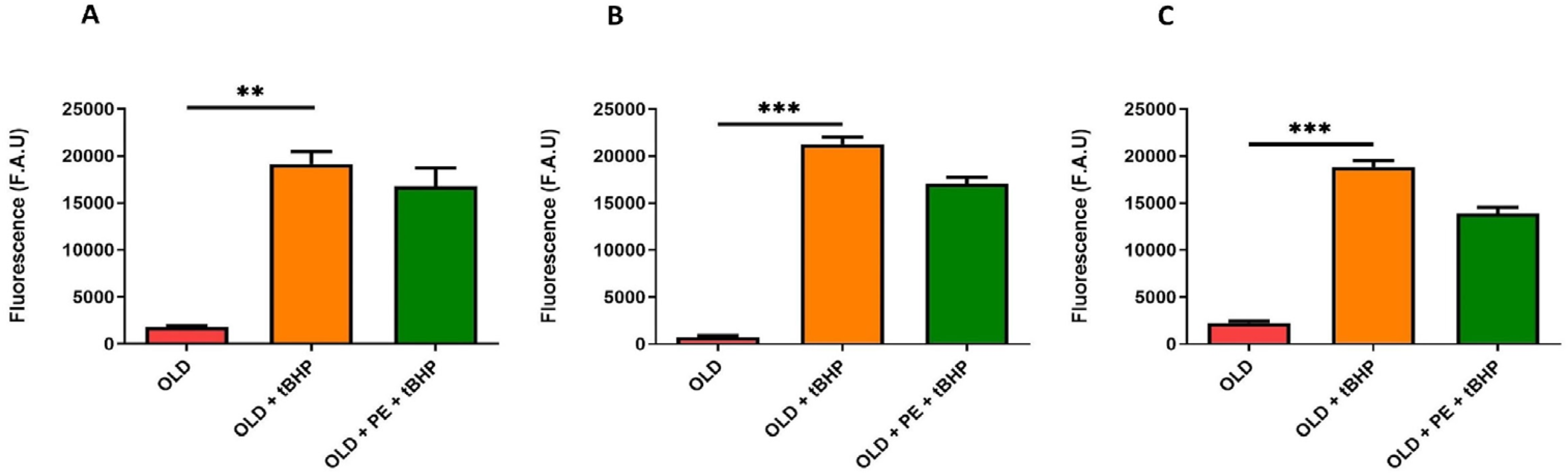
2.3. Study of Intracellular ROS Levels Induced with t-BHP in Aged Mice with PE Supplementation
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Pomegranate Extract Supplementation
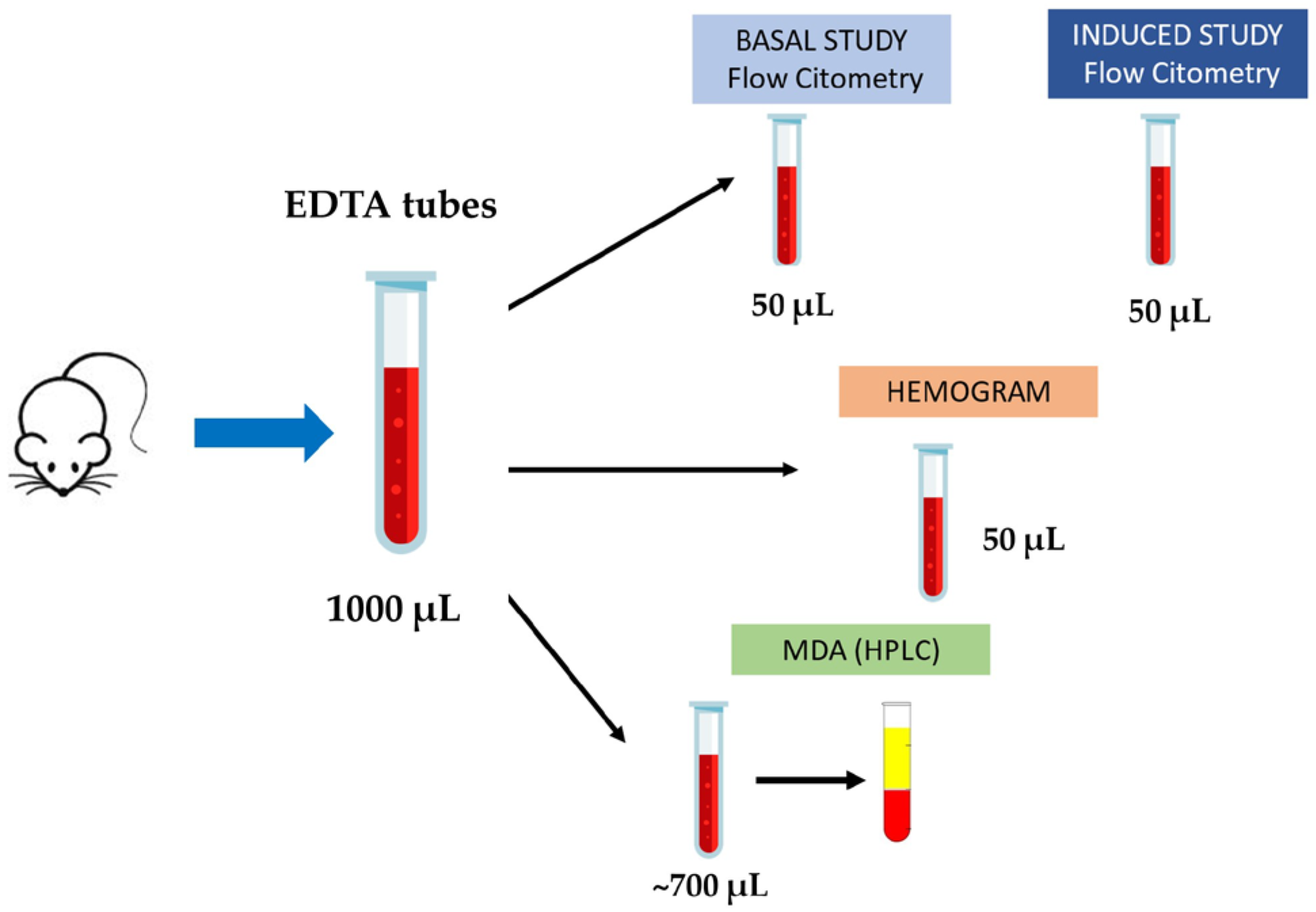
4.3. Blood Collection and Sample Processing
4.4. Hematological Analysis and Lipid Peroxidation Determination
4.5. Flow Cytometry Analysis
- Mitochondria peroxy yellow 1 (MitoPY1) (1 mM, λ excitation = 488 nm, λ emission = 625 nm) to detect mitochondrial hydrogen peroxide [65].
4.6. Statistical Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Prata, C.; Maraldi, T.; Angeloni, C. Strategies to Counteract Oxidative Stress and Inflammation in Chronic-Degenerative Diseases. Int. J. Mol. Sci. 2022, 23, 6439. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Niculescu, A.-G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Viña, J.; Borrás, C.; Miquel, J. Theories of Ageing. IUBMB Life 2007, 59, 249–254. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef]
- Wedel, S.; Martic, I.; Hrapovic, N.; Fabre, S.; Madreiter-Sokolowski, C.T.; Haller, T.; Pierer, G.; Ploner, C.; Jansen-Dürr, P.; Cavinato, M. tBHP Treatment as a Model for Cellular Senescence and Pollution-Induced Skin Aging. Mech. Ageing Dev. 2020, 190, 111318. [Google Scholar] [CrossRef]
- Zhang, H.; Zhai, Z.; Wang, Y.; Zhang, J.; Wu, H.; Wang, Y.; Li, C.; Li, D.; Lu, L.; Wang, X.; et al. Resveratrol Ameliorates Ionizing Irradiation-Induced Long-Term Hematopoietic Stem Cell Injury in Mice. Free Radic. Biol. Med. 2013, 54, 40–50. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Jiang, K.; Han, W.; Zhang, J.; Xie, L.; Liu, Y.; Xiao, J.; Wang, X. Mangiferin Prevents TBHP-Induced Apoptosis and ECM Degradation in Mouse Osteoarthritic Chondrocytes via Restoring Autophagy and Ameliorates Murine Osteoarthritis. Oxid. Med. Cell. Longev. 2019, 2019, 8783197. [Google Scholar] [CrossRef]
- Nielsen, F.; Mikkelsen, B.B.; Nielsen, J.B.; Andersen, H.R.; Grandjean, P. Plasma Malondialdehyde as Biomarker for Oxidative Stress: Reference Interval and Effects of Life-Style Factors. Clin. Chem. 1997, 43, 1209–1214. [Google Scholar] [CrossRef] [PubMed]
- Viña, J.; Borras, C.; Gomez-Cabrera, M.C. A Free Radical Theory of Frailty. Free Radic. Biol. Med. 2018, 124, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Dan Dunn, J.; Alvarez, L.A.; Zhang, X.; Soldati, T. Reactive Oxygen Species and Mitochondria: A Nexus of Cellular Homeostasis. Redox Biol. 2015, 6, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Harrington, J.S.; Ryter, S.W.; Plataki, M.; Price, D.R.; Choi, A.M.K. Mitochondria in Health, Disease, and Aging. Physiol. Rev. 2023, 103, 2349–2422. [Google Scholar] [CrossRef]
- Guo, Y.; Guan, T.; Shafiq, K.; Yu, Q.; Jiao, X.; Na, D.; Li, M.; Zhang, G.; Kong, J. Mitochondrial Dysfunction in Aging. Ageing Res. Rev. 2023, 88, 101955. [Google Scholar] [CrossRef]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, Oxidants, and Aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef]
- Tan, S.; Yu, C.Y.; Sim, Z.W.; Low, Z.S.; Lee, B.; See, F.; Min, N.; Gautam, A.; Chu, J.J.H.; Ng, K.W.; et al. Pomegranate Activates TFEB to Promote Autophagy-Lysosomal Fitness and Mitophagy. Sci. Rep. 2019, 9, 727. [Google Scholar] [CrossRef]
- Deepika; Maurya, P.K. Ellagic Acid: Insight into Its Protective Effects in Age-Associated Disorders. 3 Biotech 2022, 12, 340. [Google Scholar] [CrossRef]
- Stockton, A.; Farhat, G.; McDougall, G.J.; Al-Dujaili, E.A.S. Effect of Pomegranate Extract on Blood Pressure and Anthropometry in Adults: A Double-Blind Placebo-Controlled Randomised Clinical Trial. J. Nutr. Sci. 2017, 6, e39. [Google Scholar] [CrossRef]
- Dormal, V.; Pachikian, B.; Debock, E.; Buchet, M.; Copine, S.; Deldicque, L. Evaluation of a Dietary Supplementation Combining Protein and a Pomegranate Extract in Older People: A Safety Study. Nutrients 2022, 14, 5182. [Google Scholar] [CrossRef]
- Attia, H.; ElBanna, S.A.; Khattab, R.A.; Farag, M.A.; Yassin, A.S.; Aziz, R.K. Integrating Microbiome Analysis, Metabolomics, Bioinformatics, and Histopathology to Elucidate the Protective Effects of Pomegranate Juice against Benzo-Alpha-Pyrene-Induced Colon Pathologies. Int. J. Mol. Sci. 2023, 24, 10691. [Google Scholar] [CrossRef] [PubMed]
- Sommer, F.; Anderson, J.M.; Bharti, R.; Raes, J.; Rosenstiel, P. The Resilience of the Intestinal Microbiota Influences Health and Disease. Nat. Rev. Microbiol. 2017, 15, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Faye, C.; Mcgowan, J.C.; Denny, C.A.; David, D.J. Neurobiological Mechanisms of Stress Resilience and Implications for the Aged Population. Curr. Neuropharmacol. 2018, 16, 234–270. [Google Scholar] [CrossRef] [PubMed]
- Firth, J.; Gangwisch, J.E.; Borisini, A.; Wootton, R.E.; Mayer, E.A. Food and Mood: How Do Diet and Nutrition Affect Mental Wellbeing? BMJ 2020, 369, m2382. [Google Scholar] [CrossRef]
- Jyväkorpi, S.K.; Urtamo, A.; Pitkälä, K.H.; Strandberg, T.E. Nutrition, Daily Walking and Resilience Are Associated with Physical Function in the Oldest Old Men. J. Nutr. Health Aging 2018, 22, 1176–1182. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, Oxidative Stress and the Biology of Ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Sohal, R.S.; Orr, W.C. The Redox Stress Hypothesis of Aging. Free Radic. Biol. Med. 2012, 52, 539–555. [Google Scholar] [CrossRef]
- De la Fuente, M. Effects of Antioxidants on Immune System Ageing. Eur. J. Clin. Nutr. 2002, 56 (Suppl. S3), S5–S8. [Google Scholar] [CrossRef]
- Labunskyy, V.M.; Gladyshev, V.N. Role of Reactive Oxygen Species-Mediated Signaling in Aging. Antioxid. Redox Signal. 2013, 19, 1362–1372. [Google Scholar] [CrossRef]
- Harman, D. Aging: Overview. Ann. N. Y. Acad. Sci. 2001, 928, 1–21. [Google Scholar] [CrossRef]
- Kučera, O.; Endlicher, R.; Roušar, T.; Lotková, H.; Garnol, T.; Drahota, Z.; Cervinková, Z. The Effect of Tert-Butyl Hydroperoxide-Induced Oxidative Stress on Lean and Steatotic Rat Hepatocytes In Vitro. Oxid. Med. Cell. Longev. 2014, 2014, 752506. [Google Scholar] [CrossRef]
- Sapey, E.; Greenwood, H.; Walton, G.; Mann, E.; Love, A.; Aaronson, N.; Insall, R.H.; Stockley, R.A.; Lord, J.M. Phosphoinositide 3-Kinase Inhibition Restores Neutrophil Accuracy in the Elderly: Toward Targeted Treatments for Immunosenescence. Blood 2014, 123, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Sapey, E.; Patel, J.M.; Greenwood, H.L.; Walton, G.M.; Hazeldine, J.; Sadhra, C.; Parekh, D.; Dancer, R.C.A.; Nightingale, P.; Lord, J.M.; et al. Pulmonary Infections in the Elderly Lead to Impaired Neutrophil Targeting, Which Is Improved by Simvastatin. Am. J. Respir. Crit. Care Med. 2017, 196, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Wenisch, C.; Patruta, S.; Daxböck, F.; Krause, R.; Hörl, W. Effect of Age on Human Neutrophil Function. J. Leukoc. Biol. 2000, 67, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.C.; Goldstein, D.R.; Montgomery, R.R. Age-Dependent Dysregulation of Innate Immunity. Nat. Rev. Immunol. 2013, 13, 875–887. [Google Scholar] [CrossRef]
- Van Avondt, K.; Strecker, J.-K.; Tulotta, C.; Minnerup, J.; Schulz, C.; Soehnlein, O. Neutrophils in Aging and Aging-Related Pathologies. Immunol. Rev. 2023, 314, 357–375. [Google Scholar] [CrossRef]
- Kiani-Esfahani, A.; Tavalaee, M.; Deemeh, M.R.; Hamiditabar, M.; Nasr-Esfahani, M.H. DHR123: An Alternative Probe for Assessment of ROS in Human Spermatozoa. Syst. Biol. Reprod. Med. 2012, 58, 168–174. [Google Scholar] [CrossRef]
- Zarfeshany, A.; Asgary, S.; Javanmard, S.H. Potent Health Effects of Pomegranate. Adv. Biomed. Res. 2014, 3, 100. [Google Scholar] [CrossRef]
- Park, S.; Seok, J.K.; Kwak, J.Y.; Suh, H.-J.; Kim, Y.M.; Boo, Y.C. Anti-Inflammatory Effects of Pomegranate Peel Extract in THP-1 Cells Exposed to Particulate Matter PM10. Evid.-Based Complement. Altern. Med. 2016, 2016, 6836080. [Google Scholar] [CrossRef]
- Haseeb, A.; Khan, N.M.; Ashruf, O.S.; Haqqi, T.M. A Polyphenol-Rich Pomegranate Fruit Extract Suppresses NF-κB and IL-6 Expression by Blocking the Activation of IKKβ and NIK in Primary Human Chondrocytes. Phytother. Res. 2017, 31, 778–782. [Google Scholar] [CrossRef]
- Arranz, L.; Fernández, C.; Rodríguez, A.; Ribera, J.M.; De la Fuente, M. The Glutathione Precursor N-Acetylcysteine Improves Immune Function in Postmenopausal Women. Free Radic. Biol. Med. 2008, 45, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- Møller, P.; Viscovich, M.; Lykkesfeldt, J.; Loft, S.; Jensen, A.; Poulsen, H.E. Vitamin C Supplementation Decreases Oxidative DNA Damage in Mononuclear Blood Cells of Smokers. Eur. J. Nutr. 2004, 43, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, C.; Alvarez, P.; Puerto, M.; Gausserès, N.; Jiménez, L.; De la Fuente, M. Dietary Supplementation with Antioxidants Improves Functions and Decreases Oxidative Stress of Leukocytes from Prematurely Aging Mice. Nutrition 2006, 22, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Pearson, K.J.; Baur, J.A.; Lewis, K.N.; Peshkin, L.; Price, N.L.; Labinskyy, N.; Swindell, W.R.; Kamara, D.; Minor, R.K.; Perez, E.; et al. Resveratrol Delays Age-Related Deterioration and Mimics Transcriptional Aspects of Dietary Restriction without Extending Life Span. Cell Metab. 2008, 8, 157–168. [Google Scholar] [CrossRef]
- Giorgi, C.; Marchi, S.; Simoes, I.C.M.; Ren, Z.; Morciano, G.; Perrone, M.; Patalas-Krawczyk, P.; Borchard, S.; Jędrak, P.; Pierzynowska, K.; et al. Mitochondria and Reactive Oxygen Species in Aging and Age-Related Diseases. Int. Rev. Cell Mol. Biol. 2018, 340, 209–344. [Google Scholar] [CrossRef]
- Cui, H.; Kong, Y.; Zhang, H. Oxidative Stress, Mitochondrial Dysfunction, and Aging. J. Signal Transduct. 2012, 2012, 646354. [Google Scholar] [CrossRef]
- Patergnani, S.; Bouhamida, E.; Leo, S.; Pinton, P.; Rimessi, A. Mitochondrial Oxidative Stress and “Mito-Inflammation”: Actors in the Diseases. Biomedicines 2021, 9, 216. [Google Scholar] [CrossRef]
- Campisi, J.; d’Adda di Fagagna, F. Cellular Senescence: When Bad Things Happen to Good Cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef]
- Childs, B.G.; Durik, M.; Baker, D.J.; van Deursen, J.M. Cellular Senescence in Aging and Age-Related Disease: From Mechanisms to Therapy. Nat. Med. 2015, 21, 1424–1435. [Google Scholar] [CrossRef]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant Activity of Pomegranate Juice and Its Relationship with Phenolic Composition and Processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef]
- Salimi, A.; Roudkenar, M.H.; Sadeghi, L.; Mohseni, A.; Seydi, E.; Pirahmadi, N.; Pourahmad, J. Ellagic Acid, a Polyphenolic Compound, Selectively Induces ROS-Mediated Apoptosis in Cancerous B-Lymphocytes of CLL Patients by Directly Targeting Mitochondria. Redox Biol. 2015, 6, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, X.; Zeng, X.; Bao, H.; Liu, X. The Punicalagin Compound Mitigates Bronchial Epithelial Cell Senescence Induced by Cigarette Smoke Extract through the PAR2/mTOR Pathway. Curr. Med. Chem. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Molani-Gol, R.; Foroumandi, E.; Alizadeh, M.; Kheirouri, S. Pomegranate and Cognitive Performance: A Systematic Review. Curr. Pharm. Des. 2023, 29, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Siddarth, P.; Li, Z.; Miller, K.J.; Ercoli, L.M.; Merril, D.A.; Henning, S.M.; Heber, D.; Small, G.W. Randomized Placebo-Controlled Study of the Memory Effects of Pomegranate Juice in Middle-Aged and Older Adults. Am. J. Clin. Nutr. 2020, 111, 170–177. [Google Scholar] [CrossRef]
- Ahles, S.; Cuijpers, I.; Hartgens, F.; Troost, F.J. The Effect of a Citrus and Pomegranate Complex on Physical Fitness and Mental Well-Being in Healthy Elderly: A Randomized Placebo-Controlled Trial. J. Nutr. Health Aging 2022, 26, 839–846. [Google Scholar] [CrossRef]
- Verdú, D.; Valls, A.; Díaz, A.; Carretero, A.; Dromant, M.; Kuligowski, J.; Serna, E.; Viña, J. Pomegranate Extract Administration Reverses Loss of Motor Coordination and Prevents Oxidative Stress in Cerebellum of Aging Mice. Antioxidants 2023, 12, 1991. [Google Scholar] [CrossRef]
- European Patent EP1967079. Available online: https://patents.google.com/patent/EP1967079B1/en (accessed on 19 June 2025).
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose Translation from Animal to Human Studies Revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
- Torregrosa-García, A.; Ávila-Gandía, V.; Luque-Rubia, A.J.; Abellán-Ruiz, M.S.; Querol-Calderón, M.; López-Román, F.J. Pomegranate Extract Improves Maximal Performance of Trained Cyclists after an Exhausting Endurance Trial: A Randomised Controlled Trial. Nutrients 2019, 11, 721. [Google Scholar] [CrossRef]
- Wong, S.H.; Knight, J.A.; Hopfer, S.M.; Zaharia, O.; Leach, C.N.; Sunderman, F.W. Lipoperoxides in Plasma as Measured by Liquid-Chromatographic Separation of Malondialdehyde-Thiobarbituric Acid Adduct. Clin. Chem. 1987, 33, 214–220. [Google Scholar] [CrossRef]
- Kalyanaraman, B.; Darley-Usmar, V.; Davies, K.J.A.; Dennery, P.A.; Forman, H.J.; Grisham, M.B.; Mann, G.E.; Moore, K.; Roberts, L.J.; Ischiropoulos, H. Measuring Reactive Oxygen and Nitrogen Species with Fluorescent Probes: Challenges and Limitations. Free Radic. Biol. Med. 2012, 52, 1–6. [Google Scholar] [CrossRef]
- Gomes, A.; Fernandes, E.; Lima, J.L.F.C. Fluorescence Probes Used for Detection of Reactive Oxygen Species. J. Biochem. Biophys. Methods 2005, 65, 45–80. [Google Scholar] [CrossRef] [PubMed]
- Wardman, P. Fluorescent and Luminescent Probes for Measurement of Oxidative and Nitrosative Species in Cells and Tissues: Progress, Pitfalls, and Prospects. Free Radic. Biol. Med. 2007, 43, 995–1022. [Google Scholar] [CrossRef] [PubMed]
- Rothe, G.; Oser, A.; Valet, G. Dihydrorhodamine 123: A New Flow Cytometric Indicator for Respiratory Burst Activity in Neutrophil Granulocytes. Naturwissenschaften 1988, 75, 354–355. [Google Scholar] [CrossRef] [PubMed]
- Jávega, B.; Herrera, G.; O’Connor, J.-E. Flow Cytometric Analysis of Oxidative Stress in Escherichia Coli B Strains Deficient in Genes of the Antioxidant Defence. Int. J. Mol. Sci. 2022, 23, 6537. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verdú, D.; Valls, A.; Serna-García, M.; Herrera, G.; Ezzeddin-Ayoub, M.; Mauricio, M.D.; Viña, J.; Serna, E. Pomegranate Extract Modulates Oxidative Stress by Reducing Basal ROS Levels and Protecting White Blood Cells from Induced Oxidative Damage in Aging Mice. Int. J. Mol. Sci. 2025, 26, 5957. https://doi.org/10.3390/ijms26135957
Verdú D, Valls A, Serna-García M, Herrera G, Ezzeddin-Ayoub M, Mauricio MD, Viña J, Serna E. Pomegranate Extract Modulates Oxidative Stress by Reducing Basal ROS Levels and Protecting White Blood Cells from Induced Oxidative Damage in Aging Mice. International Journal of Molecular Sciences. 2025; 26(13):5957. https://doi.org/10.3390/ijms26135957
Chicago/Turabian StyleVerdú, David, Alicia Valls, Marta Serna-García, Guadalupe Herrera, Mustafa Ezzeddin-Ayoub, Maria D. Mauricio, José Viña, and Eva Serna. 2025. "Pomegranate Extract Modulates Oxidative Stress by Reducing Basal ROS Levels and Protecting White Blood Cells from Induced Oxidative Damage in Aging Mice" International Journal of Molecular Sciences 26, no. 13: 5957. https://doi.org/10.3390/ijms26135957
APA StyleVerdú, D., Valls, A., Serna-García, M., Herrera, G., Ezzeddin-Ayoub, M., Mauricio, M. D., Viña, J., & Serna, E. (2025). Pomegranate Extract Modulates Oxidative Stress by Reducing Basal ROS Levels and Protecting White Blood Cells from Induced Oxidative Damage in Aging Mice. International Journal of Molecular Sciences, 26(13), 5957. https://doi.org/10.3390/ijms26135957






