Identification of a Broad Bean Wilt Virus 2 (BBWV2) Isolate (BBWV2-SP) from Spinacia oleracea L.
Abstract
1. Introduction
2. Results
2.1. Viral Disease Phenotype Observation
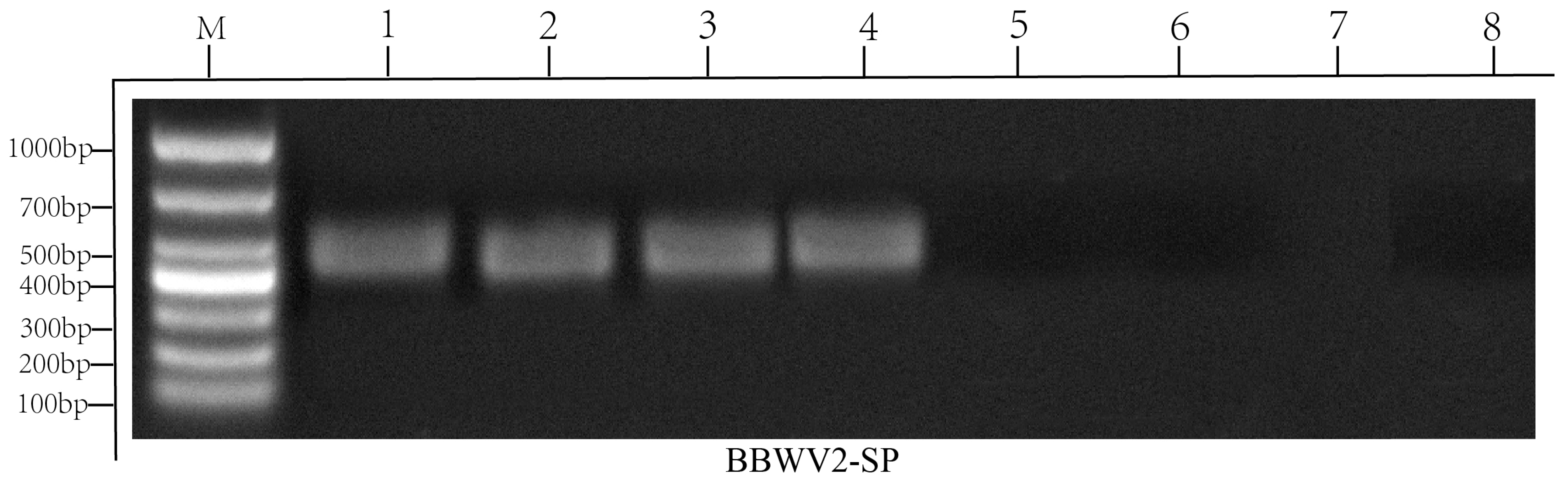
2.2. Virus Detection by RT-PCR
2.3. Identification of Viral Genome-Derived Contigs by Spinach Reads
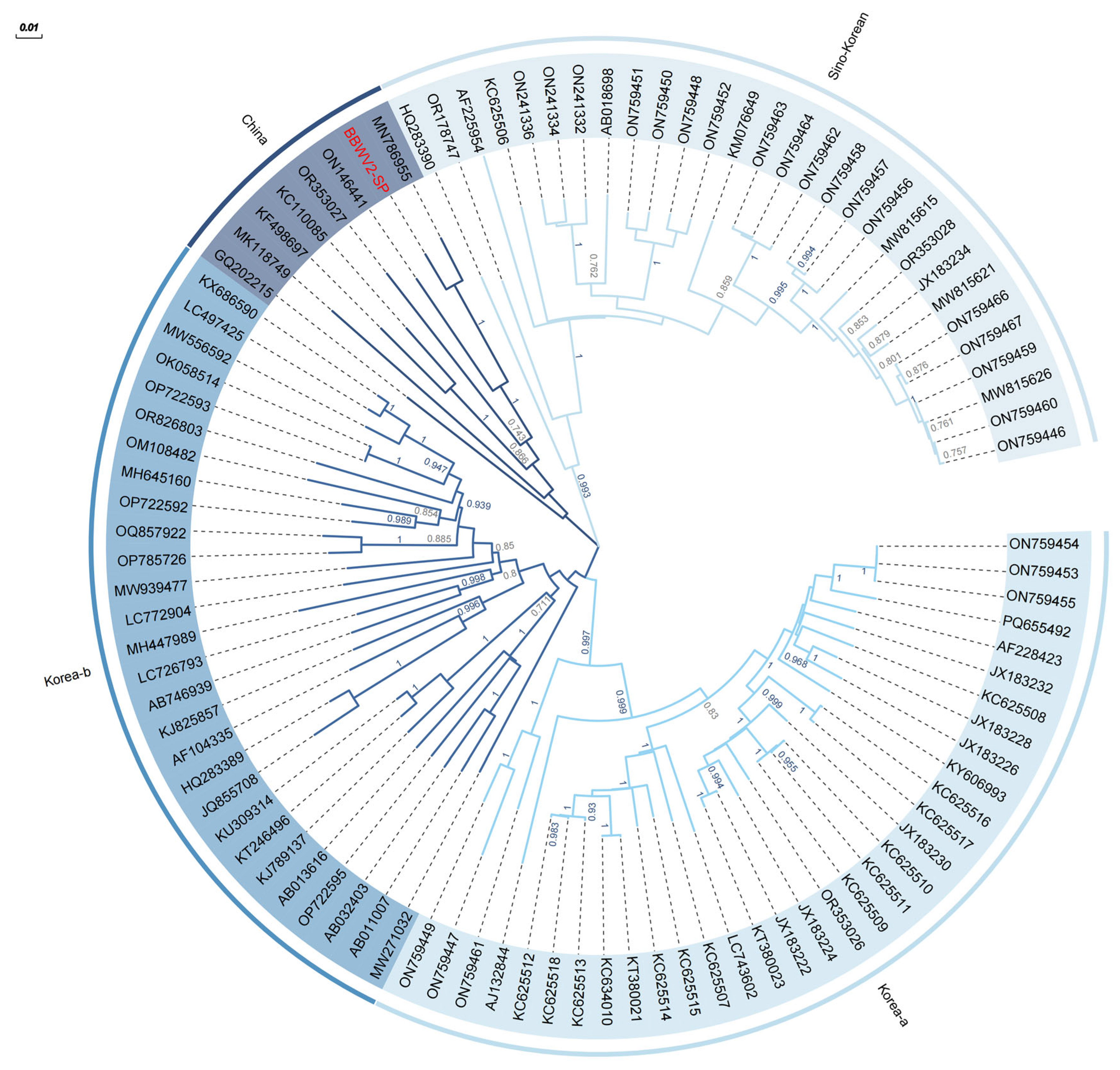
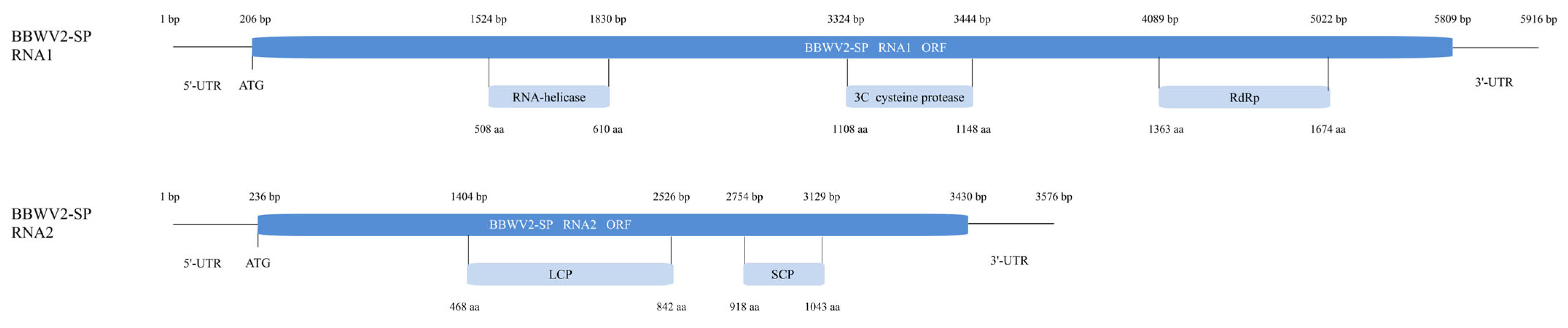
2.4. Evolutionary Characteristics and Genome Structure of BBWV2-SP
2.5. Design and Validation of BBWV2-SP Molecular Primers
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Extraction of Total Nucleic Acids
4.3. Virus Detection by RT-PCR
4.4. Transcriptome Sequencing and Capturing Viral Genomes in Spinach Through RNA Assembly
4.5. Evolutionary Characteristics and Genome Structure of BBWV2-SP
4.6. Design of Molecular Primers Based on the Conserved Sequence of BBWV2 RNA2 Genome
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roberts, J.L.; Moreau, R. Functional properties of spinach (Spinacia oleracea L.) phytochemicals and bioactives. Food Funct. 2016, 7, 3337–3353. [Google Scholar] [CrossRef] [PubMed]
- Xina, W.; Chaohui, W.; Baoming, C.; Shengxiu, L. Nitrate accumulation in petiole and blade of different spinach cultivars and its relation to plant growth. J. Plant Nutr. Fertil. 2005, 11, 675–681. [Google Scholar]
- Prohens-Tomás, J.; Nuez, F. Vegetables I: Asteraceae, Brassicaceae, Chenopodicaceae, and Cucurbitaceae; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007; Volume 1. [Google Scholar]
- Correll, J.C.; Morelock, T.E.; Black, M.C.; Koike, S.T.; Brandenberger, L.P.; Dainello, F.J. Economically important diseases of spinach. Plant Dis. 1994, 78, 653–660. [Google Scholar] [CrossRef]
- Masarapu Hema, M.H.; Pothur Sreenivasulu, P.S.; Kumar, P.L. Cucumber mosaic. In Virus Diseases of Tropical and Subtropical Crops; CABI: Wallingford, UK, 2015; pp. 73–93. [Google Scholar]
- Roossinck, M.J. Cucumber mosaic virus, a model for RNA virus evolution. Mol. Plant Pathol. 2001, 2, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, J. Epidemiology and control of virus diseases of vegetables. Ann. Appl. Biol. 1987, 110, 661–681. [Google Scholar] [CrossRef]
- Provvidenti, R. Turnip mosaic potyvirus. Viruses of Plants; CABI: Wallingford, UK, 1996; pp. 1340–1343. [Google Scholar]
- Nellist, C.F.; Ohshima, K.; Ponz, F.; Walsh, J.A. Turnip mosaic virus, a virus for all seasons. Ann. Appl. Biol. 2022, 180, 312–327. [Google Scholar] [CrossRef]
- Duffus, J.E. Economic significance of Beet western yellows (Radish yellows) on Sugar Beet. Phytopathology 1961, 51, 605–607. [Google Scholar]
- Ohki, S.T.; Yamashita, S.; Arai, K.; Doi, Y.; Yora, K. Beet yellows virus and beet western yellows virus isolated from spinach and fodder beet plants affected by yellowing diseases. Jpn. J. Phytopathol. 1977, 43, 46–54. [Google Scholar] [CrossRef]
- Taylor, R. Broad bean wilt virus. In Descriptions of Plant Viruses; Commonwealth Mycological Institute (CMI): Surrey, UK; Association of Applied Biologists (AAB): Warwick, UK, 1972; No. 81; Available online: https://cir.nii.ac.jp/crid/1572824499190032896 (accessed on 15 February 2025).
- Benner, C.; Kuhn, C.; Demski, J.; Dobson, J.; Colditz, P.; Nutter, F., Jr. Identification and incidence of pepper viruses in northeastern Georgia. CABI Databases 1985, 69, 999–1001. [Google Scholar]
- Brunt, A.A.; Crabtree, K.; Dallwitz, M.; Gibbs, A.; Watson, L. Viruses of plants. In Descriptions and Lists from the VIDE Database; CABI: Wallingford, UK, 1996. [Google Scholar]
- Kobayashi, Y.O.; Kobayashi, A.; Nakano, M.; Hagiwara, K.; Honda, Y.; Omura, T. Analysis of genetic relations between Broad bean wilt virus 1 and Broad bean wilt virus 2. J. Gen. Plant Pathol. 2003, 69, 320–326. [Google Scholar] [CrossRef]
- Uyemoto, J.; Provvidenti, R. Isolation and identification of two serotypes of broad bean wilt virus. Phytopathology 1974, 64, 1547–1548. [Google Scholar] [CrossRef]
- Lisa, V. Fabaviruses: Broad bean wilt and allied viruses. Plant Viruses 1996, 5, 229–250. [Google Scholar]
- Dong, Z.; Abuduwaili, A.; Wang, X.; Yin, H.; Lu, S.; Xia, F.; Bi, Y.; Li, Y. First Report of Broad Bean Wilt Virus 2 in Marigold (Tagetes erecta) in China. Plant Dis. 2021, 105, 3767. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, H.; Wang, K.; Wang, H.; Wang, D.; Xia, Y.; Wen, C.; Xu, X. First Report of Broad Bean Wilt Virus 2 Infecting Balsam (Impatiens balsamina) in China. Plant Dis. 2024, 108, 232. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, Y.; Gu, T.; Guo, X.; Zhuang, X.; Zhang, K. First Report of Broad Bean Wilt Virus 2 on Mirabilis jalapa in China. Plant Dis. 2023, 107, 1957. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, M.; Li, Y.; Yan, Z.; Tang, W.; Gong, H.; Li, X. First report of Broad bean wilt virus 2 infecting sesame in China. Plant Dis. 2017, 101, 1556. [Google Scholar] [CrossRef]
- Li, Z.; Qin, J.; Zhu, Y.; Zhou, M.; Zhao, N.; Zhou, E.; Wang, X.; Chen, X.; Cui, X. Occurrence, distribution, and genetic diversity of faba bean viruses in China. Front. Microbiol. 2024, 15, 1424699. [Google Scholar] [CrossRef]
- Kalimuthu, K.; Arivalagan, J.; Mohan, M.; Christyraj, J.R.S.S.; Arockiaraj, J.; Muthusamy, R.; Ju, H.-J. Point of care diagnosis of plant virus: Current trends and prospects. Mol. Cell. Probes 2022, 61, 101779. [Google Scholar] [CrossRef]
- Yoshida, N.; Tamada, T. Host range and molecular analysis of Beet leaf yellowing virus, Beet western yellows virus-JP and Brassica yellows virus in Japan. Plant Pathol. 2019, 68, 1045–1058. [Google Scholar] [CrossRef]
- Xu, H.; D’Aubin, J.; Nie, J. Genomic variability in Potato virus M and the development of RT-PCR and RFLP procedures for the detection of this virus in seed potatoes. Virol. J. 2010, 7, 25. [Google Scholar] [CrossRef]
- Chalam, V.; Arif, M.; Caasi, D.; Fletcher, J.; Ochoa-Corona, F. Discrimination among CLRV, GFLV and ToRSV using multiplex RT-PCR. Phytopathology 2012, 102, 21. [Google Scholar]
- Chalam, V.; Khetarpal, R. A critical appraisal of challenges in exclusion of plant viruses during transboundary movement of seeds. CABI Databases 2008, 19, 139–149. [Google Scholar]
- Bashir, N.; Sanger, M.; Järlfors, U.; Ghabrial, S. Expression of the Peanut stunt virus coat protein gene is essential and sufficient for production of host-dependent ribbon-like inclusions in infected plants. Phytopathology 2004, 94, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Elbeaino, T.; Marais, A.; Faure, C.; Trioano, E.; Candresse, T.; Parrella, G. High-throughput sequencing reveals Cyclamen persicum mill. As a natural host for fig mosaic virus. Viruses 2018, 10, 684. [Google Scholar] [CrossRef]
- Al Rwahnih, M.; Rowhani, A.; Westrick, N.; Stevens, K.; Diaz-Lara, A.; Trouillas, F.P.; Preece, J.; Kallsen, C.; Farrar, K.; Golino, D. Discovery of viruses and virus-like pathogens in pistachio using high-throughput sequencing. Plant Dis. 2018, 102, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, H.; Huo, Z.; Pang, J.; Zhang, G. Detection of cucumber mosaic virus by RT-PCR. J. Chang. Veg. 2004, 11, 40–41. [Google Scholar]
- Zhao, L.; Feng, C.; Hao, X.; Wang, R.; Hu, L.; Wang, Q.; Wu, Y. Detection and Molecular Variability of T urnip mosaic virus (T UMV) in Shanxi, China. J. Phytopathol. 2014, 162, 519–522. [Google Scholar] [CrossRef]
- Kwak, H.-R.; Byun, H.-S.; Kim, H.-S.; Lee, B.-C.; Choi, H.-S. First Report of Beet Western Yellows Virus in Radish in Korea. Plant Dis. 2023, 107, 3324. [Google Scholar] [CrossRef]
- Du, G.; Wang, X.; Zhou, G. Digoxigeni-labelled cDNA probes for the detection of Tobacco mosaic virus, Cucumber mosaic virus and Potato virus Y. ACTA Phytopathol. Sinca 2004, 34, 75–79. [Google Scholar]
- Jiang, Y. The Molecular Detection and The Complete Genomic Sequencing of Tomato mosaic virus of Processing Tomato in Xinjiang. Master’s Theses, Shihezi University, Shihezi, China, 2008. [Google Scholar]
- Zheng, X.; Cheng, J.; Zhao, Z.; Li, Z.; Wang, W. Simultaneous detection of five viruses (TMV, CMV, TEV, PVY and TVBMV) infecting tobacco by multiplex RT-PCR. ACTA Phytopathol. Sinca 2011, 41, 146–153. [Google Scholar]
- Zhang, L.; Yang, C.; Yu, C.; Yuan, W.; Ma, Q. Study on multiplex RT-PCR method to synchronouslydetect 3kinds of viruses damaging Gerbera j amesonii Bolus. Acta Agric. Shanghai 2009, 25, 20–24. [Google Scholar]
- Kong, B.; Cai, H.; Chen, H.; Mu, Y.; Tang, H.; Liu, J. The Study on Detection Tomato Ring spot Virus (TomRSV) by RT-PCR. J. Yunnan Agric. Univ. 2001, 16, 96–98. [Google Scholar]
- Yang, C.; Yu, C.; Song, S.; Tao, T. The research for the RT-PCR and IC-RT-PCR detection method of Tomato black ring virus (TBRV). Plant Quarantne 2006, 20, 275–278. [Google Scholar]
- Yao, Y.; Chen, G.; Feng, L.; Xie, B. Molecular Detection of Pepper Viruses in Southern Vegetable ProductionBases. China Veg. 2013, 10, 84–89. [Google Scholar]
- Tian, Y.; Yu, L.; Yi, J.; Yang, C.; Wu, J.; Zhou, X.; Yu, C. Detection and identification of Tomato brown rugose fruit virus from imported tomato seeds. CABI Databases 2021, 35, 35–38. [Google Scholar]
- Wang, Q.; Xu, C.; Zheng, Y.; Cai, X.; Ge, C.; Wang, Q.; Wang, X.; Collins, K.; Fei, Z.; Dai, S.; et al. SpinachBase: A central portal for spinach genomics. Database 2019, 2019, baz072. [Google Scholar]
- Kurt, Ş.; Uysal, A.; Akgül, D.S. First Report of Anthracnose Caused by Colletotrichum spinaciae on Spinach in the Mediterranean Region of Turkey. Plant Dis. 2016, 100, 219. [Google Scholar] [CrossRef]
- Liu, B.; Stein, L.; Cochran, K.; du Toit, L.J.; Feng, C.; Dhillon, B.; Correll, J.C. Characterization of Leaf Spot Pathogens from Several Spinach Production Areas in the United States. Plant Dis. 2020, 104, 1994–2004. [Google Scholar] [CrossRef]
- Wu, Y.; Hirakawa, H.; Masuta, C.; Onodera, Y. Characterization of genetic resistance to cucumber mosaic virus (CMV) in spinach (Spinacia oleracea L.). BMC Res. Notes 2024, 17, 335. [Google Scholar] [CrossRef]
- Bailiss, K.; Okonkwo, V. Virus infection of spinach (Spinacia oleracea L.) in Britain. J. Hortic. Sci. 1979, 54, 289–297. [Google Scholar] [CrossRef]
- He, Z.; Dong, Z.; Qin, L.; Gan, H. Phylodynamics and codon usage pattern analysis of broad bean wilt virus 2. Viruses 2021, 13, 198. [Google Scholar] [CrossRef] [PubMed]
- Yong, L.; Fan, L.; Yueyue, L.; Songbai, Z. Identification, Distribution and Occurrence of Viruses in the Main Vegetables of China. Sci. Agric. Sin. 2019, 52, 239–261. [Google Scholar]
- Murphy, F.A.; Fauquet, C.M.; Bishop, D.H.; Ghabrial, S.A.; Jarvis, A.W.; Martelli, G.P.; Mayo, M.A.; Summers, M.D. Virus Taxonomy: Classification and Nomenclature of Viruses; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 10. [Google Scholar]
- Li, L.; Yang, C.; Hou, Q.; Wang, J.; Yu, M. Identification of broad bean wilt virus 2 on Chenopodium album L. in Liaoning. CABI Databases 2020, 51, 446–453. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]



| Virus | Primer | Primer Sequence (5′ to 3′) | Tm/°C |
|---|---|---|---|
| CMV | CMVF | TAATTACAGGCCCTTACCCGC | 58 |
| CMVR | TGAGTGGGCAGAGTCGAGTC | ||
| TuMV | TuMVF | CAAGCAATCTTTGAGGATTATG | 54 |
| TuMVR | TATTTCCCATAAGCGAGAATA | ||
| BWYV | BWYVF | CGAATCTTGAACACAGCAGAG | 55 |
| BWYVR | TGTGGG ATCTTGAAGGATAGG | ||
| TMV | TMVF | TTCTTGTCATCAGCGTGGGCCGA | 55 |
| TMVR | AAGTTGCAGGACCAGAGGTCCA | ||
| ToMV | ToMVF | CCGGATCCATGTCTTACTCAATCAC | 52 |
| ToMVR | GTAAGCTTGTTAACTGGGCCCCAACCGGGGGT | ||
| TEV | TEVF | TGATGGATGGTGAGGAG | 47 |
| TEVR | GTGCCGTTCAGTGTCTT | ||
| TRV | TRVF | ATGGGTGACATGTACGATG | 54 |
| TRVR | GAGGCGTCATCGAATTTGT | ||
| TSWV | TSWVF | ATCGGATCCATGTCTAAGGTTAAGCTCAC | 55 |
| TSWVR | ATCCTCGAGTTAAGCAAGTTCTGTGAGTTTTGC | ||
| ToRSV | ToRSVF | GATGTCCTCCATTTGTTTCGCC | 60 |
| ToRSVR | GGAATGTGTCTCCGTCGTTAA | ||
| TBRV | TBRVF | GCAACTAGTGCGAGTGGTAG | 58 |
| TBRVR | CATAAAATTGGAAGCCATCATG | ||
| TYLCV | TYLCVF | AGTCTGAGGCTGTAATGTCGTCC | 62 |
| TYLCVR | CTGTTCGCAAGTATCAATCAAGGT | ||
| ToBRFV | ToBRFV591R | GACAGGTGAATGGAATTTGCCAGATAATTG | 55 |
| ToBRFV591F | AGACATATTTAATACGAATCTGAATCGGCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Liu, Z.; She, H.; Xu, Z.; Zhang, H.; Gao, W.; Qian, W. Identification of a Broad Bean Wilt Virus 2 (BBWV2) Isolate (BBWV2-SP) from Spinacia oleracea L. Int. J. Mol. Sci. 2025, 26, 5946. https://doi.org/10.3390/ijms26135946
Zhao X, Liu Z, She H, Xu Z, Zhang H, Gao W, Qian W. Identification of a Broad Bean Wilt Virus 2 (BBWV2) Isolate (BBWV2-SP) from Spinacia oleracea L. International Journal of Molecular Sciences. 2025; 26(13):5946. https://doi.org/10.3390/ijms26135946
Chicago/Turabian StyleZhao, Xu, Zhiyuan Liu, Hongbing She, Zhaosheng Xu, Helong Zhang, Wujun Gao, and Wei Qian. 2025. "Identification of a Broad Bean Wilt Virus 2 (BBWV2) Isolate (BBWV2-SP) from Spinacia oleracea L." International Journal of Molecular Sciences 26, no. 13: 5946. https://doi.org/10.3390/ijms26135946
APA StyleZhao, X., Liu, Z., She, H., Xu, Z., Zhang, H., Gao, W., & Qian, W. (2025). Identification of a Broad Bean Wilt Virus 2 (BBWV2) Isolate (BBWV2-SP) from Spinacia oleracea L. International Journal of Molecular Sciences, 26(13), 5946. https://doi.org/10.3390/ijms26135946





