Parvalbumin Neurons in the Basal Forebrain Projecting to the Mammillary Nucleus Ameliorate Age-Related Cognitive Decline
Abstract
1. Introduction
2. Results
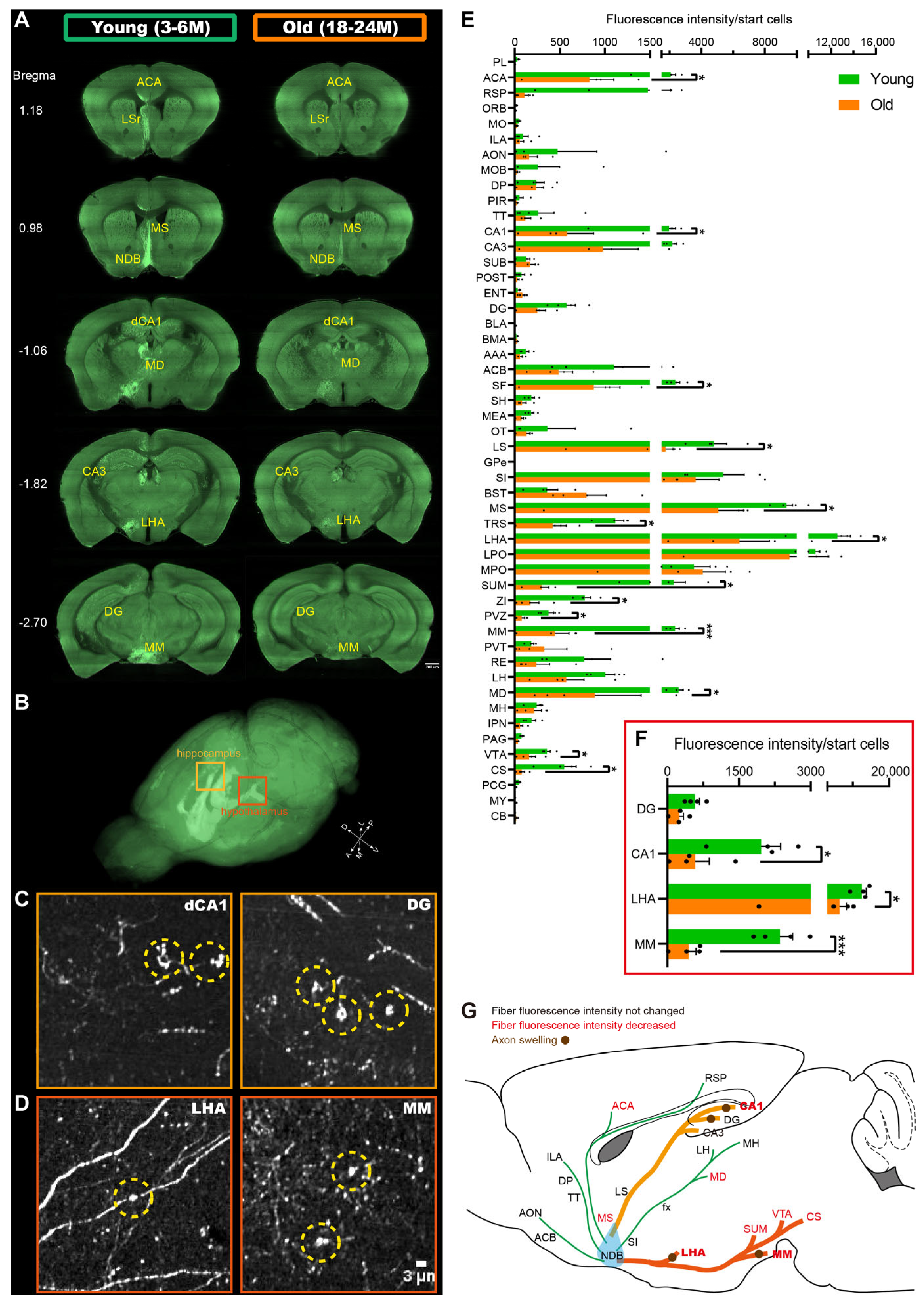
2.1. Vulnerable Projections of BF-PV Neurons in the Aging Mice
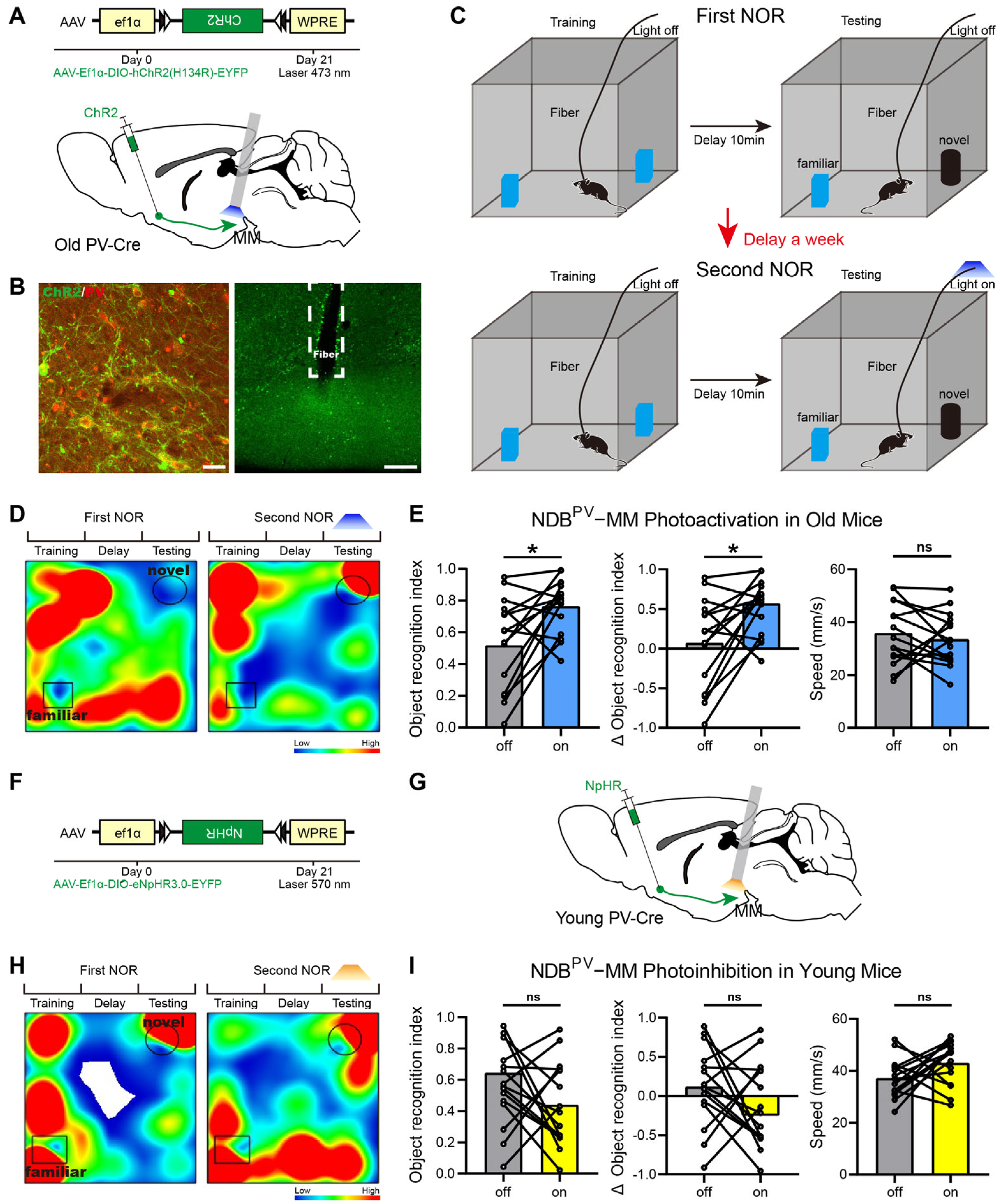
2.2. Roles of BFPV-MM Projections in Cognition
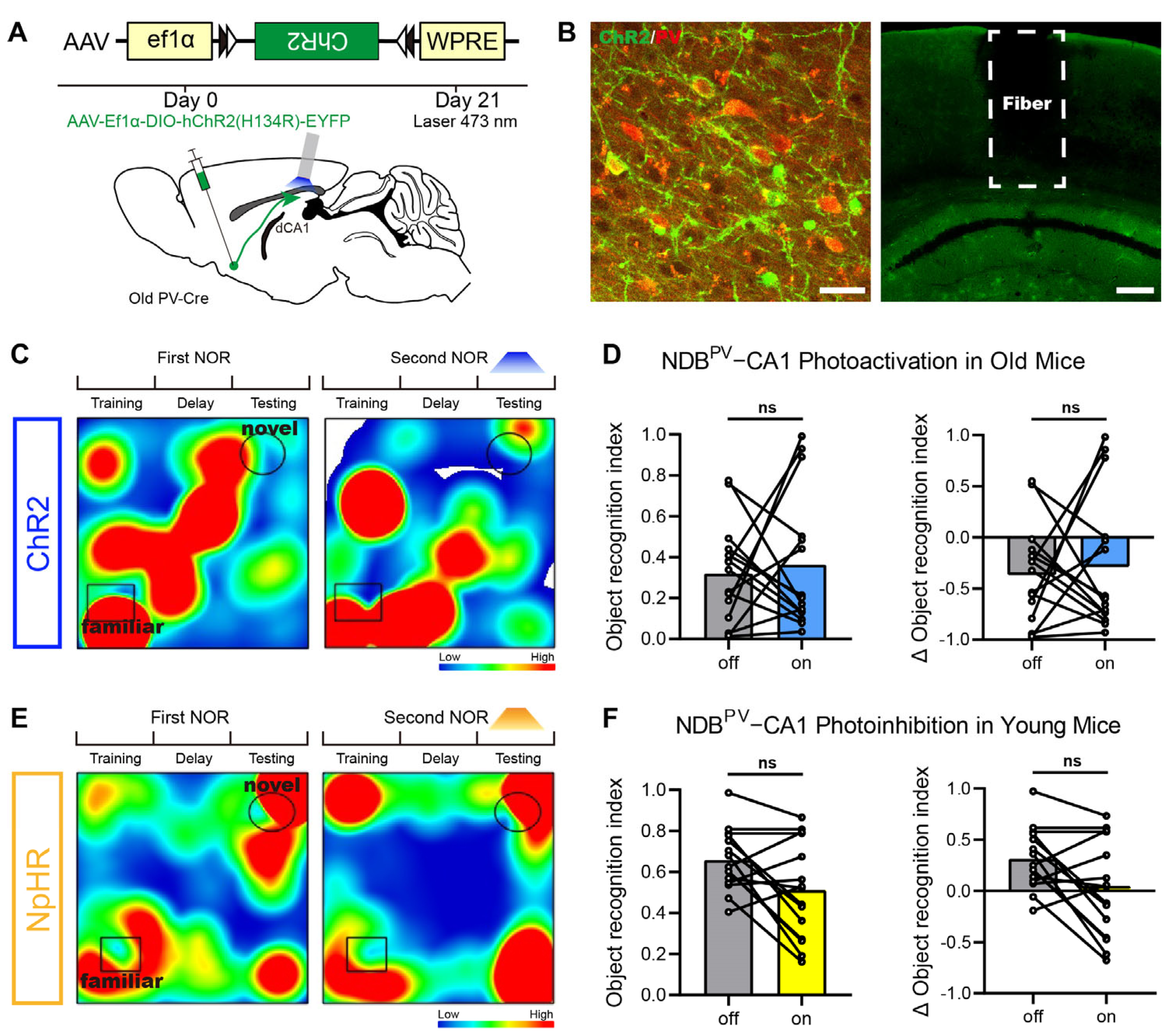
2.3. Roles of BFPV-CA1 Projections in Cognition
2.4. Heterogeneity of MM- and CA1-Projecting PV Neurons
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Viral Vectors and Stereotaxic Injections
4.3. Anterograde Tracing and Array-fMOST Whole-Brain Imaging
4.4. Data Processing and Registration
4.5. Quantification
4.6. Behavioral Assays
4.7. Novel Object Recognition Test
4.8. Optogenetics
4.9. Retrograde Tracing
4.10. Immunohistochemistry
4.11. Sparse Labeling and TDI-fMOST
4.12. Reconstruction and Visualization
4.13. Quantitative Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bañuelos, C.; Kittleson, J.R.; LaNasa, K.H.; Galiano, C.S.; Roth, S.M.; Perez, E.J.; Long, J.M.; Roberts, M.T.; Fong, S.; Rapp, P.R. Cognitive Aging and the Primate Basal Forebrain Revisited: Disproportionate Gabaergic Vulnerability Revealed. J. Neurosci. 2023, 43, 8425–8441. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Thankachan, S.; McCarley, R.W.; Brown, R.E. The Menagerie of the Basal Forebrain: How Many (Neural) Species Are There, What Do They Look Like, How Do They Behave and Who Talks to Whom? Curr. Opin. Neurobiol. 2017, 44, 159–166. [Google Scholar] [CrossRef]
- Tian, J.; Ren, M.; Zhao, P.; Luo, S.; Chen, Y.; Xu, X.; Jiang, T.; Sun, Q.; Li, A.; Gong, H.; et al. Dissection of the Long-Range Projections of Specific Neurons at the Synaptic Level in the Whole Mouse Brain. Proc. Natl. Acad. Sci. USA 2022, 119, e2202536119. [Google Scholar] [CrossRef]
- Xu, M.; Chung, S.; Zhang, S.; Zhong, P.; Ma, C.; Chang, W.C.; Weissbourd, B.; Sakai, N.; Luo, L.; Nishino, S.; et al. Basal Forebrain Circuit for Sleep-Wake Control. Nat. Neurosci. 2015, 18, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Knowland, D.; Lilascharoen, V.; Pacia, C.P.; Shin, S.; Wang, E.H.; Lim, B.K. Distinct Ventral Pallidal Neural Populations Mediate Separate Symptoms of Depression. Cell 2017, 170, 284–297.e18. [Google Scholar] [CrossRef]
- Schiffino, F.L.; McNally, J.M.; Maness, E.B.; McKenna, J.T.; Brown, R.E.; Strecker, R.E. Basal Forebrain Parvalbumin Neurons Modulate Vigilant Attention and Rescue Deficits Produced by Sleep Deprivation. J. Sleep. Res. 2024, 33, e13919. [Google Scholar] [CrossRef]
- Hegedüs, P.; Király, B.; Schlingloff, D.; Lyakhova, V.; Velencei, A.; Szabó, Í.; Mayer, M.I.; Zelenak, Z.; Nyiri, G.; Hangya, B. Parvalbumin-Expressing Basal Forebrain Neurons Mediate Learning from Negative Experience. Nat. Commun. 2024, 15, 4768. [Google Scholar] [CrossRef] [PubMed]
- Bartholome, O.; de la Brassinne Bonardeaux, O.; Neirinckx, V.; Rogister, B. A Composite Sketch of Fast-Spiking Parvalbumin-Positive Neurons. Cereb. Cortex Commun. 2020, 1, tgaa026. [Google Scholar] [CrossRef]
- Hwang, E.; Brown, R.E.; Kocsis, B.; Kim, T.; McKenna, J.T.; McNally, J.M.; Han, H.B.; Choi, J.H. Optogenetic Stimulation of Basal Forebrain Parvalbumin Neurons Modulates the Cortical Topography of Auditory Steady-State Responses. Brain Struct. Funct. 2019, 224, 1505–1518. [Google Scholar] [CrossRef]
- Hwang, E.; Han, H.B.; Kim, J.Y.; Choi, J.H. High-Density Eeg of Auditory Steady-State Responses During Stimulation of Basal Forebrain Parvalbumin Neurons. Sci. Data 2020, 7, 288. [Google Scholar] [CrossRef]
- Király, B.; Domonkos, A.; Jelitai, M.; Lopes-Dos-Santos, V.; Martínez-Bellver, S.; Kocsis, B.; Schlingloff, D.; Joshi, A.; Salib, M.; Fiáth, R.; et al. The Medial Septum Controls Hippocampal Supra-Theta Oscillations. Nat. Commun. 2023, 14, 6159. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Thankachan, S.; McKenna, J.T.; McNally, J.M.; Yang, C.; Choi, J.H.; Chen, L.; Kocsis, B.; Deisseroth, K.; Strecker, R.E.; et al. Cortically Projecting Basal Forebrain Parvalbumin Neurons Regulate Cortical Gamma Band Oscillations. Proc. Natl. Acad. Sci. USA 2015, 112, 3535–3540. [Google Scholar] [CrossRef]
- Griffith, W.H.; Dubois, D.W.; Fincher, A.; Peebles, K.A.; Bizon, J.L.; Murchison, D. Characterization of Age-Related Changes in Synaptic Transmission onto F344 Rat Basal Forebrain Cholinergic Neurons Using a Reduced Synaptic Preparation. J. Neurophysiol. 2014, 111, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Takao, K.; Suemitsu, S.; Murakami, S.; Kitamura, N.; Wani, K.; Okamoto, M.; Aoki, S.; Ishihara, T. Age-Dependent and Region-Specific Alteration of Parvalbumin Neurons and Perineuronal Nets in the Mouse Cerebral Cortex. Neurochem. Int. 2018, 112, 59–70. [Google Scholar] [CrossRef]
- Ueno, H.; Fujii, K.; Takao, K.; Suemitsu, S.; Murakami, S.; Kitamura, N.; Wani, K.; Matsumoto, Y.; Okamoto, M.; Ishihara, T. Alteration of Parvalbumin Expression and Perineuronal Nets Formation in the Cerebral Cortex of Aged Mice. Mol. Cell Neurosci. 2019, 95, 31–42. [Google Scholar] [CrossRef]
- Inan, M.; Zhao, M.; Manuszak, M.; Karakaya, C.; Rajadhyaksha, A.M.; Pickel, V.M.; Schwartz, T.H.; Goldstein, P.A.; Manfredi, G. Energy Deficit in Parvalbumin Neurons Leads to Circuit Dysfunction, Impaired Sensory Gating and Social Disability. Neurobiol. Dis. 2016, 93, 35–46. [Google Scholar] [CrossRef]
- Ruden, J.B.; Dugan, L.L.; Konradi, C. Parvalbumin Interneuron Vulnerability and Brain Disorders. Neuropsychopharmacology 2021, 46, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Do, J.P.; Xu, M.; Lee, S.H.; Chang, W.C.; Zhang, S.; Chung, S.; Yung, T.J.; Fan, J.L.; Miyamichi, K.; Luo, L.; et al. Cell Type-Specific Long-Range Connections of Basal Forebrain Circuit. eLife 2016, 5, e13214. [Google Scholar]
- Thankachan, S.; Katsuki, F.; McKenna, J.T.; Yang, C.; Shukla, C.; Deisseroth, K.; Uygun, D.S.; Strecker, R.E.; Brown, R.E.; McNally, J.M.; et al. Thalamic Reticular Nucleus Parvalbumin Neurons Regulate Sleep Spindles and Electrophysiological Aspects of Schizophrenia in Mice. Sci. Rep. 2019, 9, 3607. [Google Scholar] [CrossRef]
- Etter, G.; van der Veldt, S.; Manseau, F.; Zarrinkoub, I.; Trillaud-Doppia, E.; Williams, S. Optogenetic Gamma Stimulation Rescues Memory Impairments in an Alzheimer’s Disease Mouse Model. Nat. Commun. 2019, 10, 5322. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, J.; Li, A.; Yao, M.; Liu, G.; Chen, S.; Luo, Y.; Wang, Z.; Gong, H.; Li, X.; et al. Acetylcholine Deficiency Disrupts Extratelencephalic Projection Neurons in the Prefrontal Cortex in a Mouse Model of Alzheimer’s Disease. Nat. Commun. 2022, 13, 998. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Wang, D.; Yang, Q.; Yan, T.; Wang, Z.; Yan, Y.; Zhao, J.; Xie, Z.; Liu, Y.; Ke, Z.; et al. The Lateralization of Left Hippocampal Ca3 During the Retrieval of Spatial Working Memory. Nat. Commun. 2020, 11, 2901. [Google Scholar] [CrossRef]
- Balak, N.; Balkuv, E.; Karadag, A.; Basaran, R.; Biceroglu, H.; Erkan, B.; Tanriover, N. Mammillothalamic and Mammillotegmental Tracts as New Targets for Dementia and Epilepsy Treatment. World Neurosurg. 2018, 110, 133–144. [Google Scholar] [CrossRef]
- Vann, S.D.; Aggleton, J.P. The Mammillary Bodies: Two Memory Systems in One? Nat. Rev. Neurosci. 2004, 5, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Dillingham, C.M.; Milczarek, M.M.; Perry, J.C.; Vann, S.D. Time to Put the Mammillothalamic Pathway into Context. Neurosci. Biobehav. Rev. 2021, 121, 60–74. [Google Scholar] [CrossRef]
- Żakowski, W.; Braszka, Ł.; Zawistowski, P.; Orzeł-Gryglewska, J.; Jurkowlaniec, E. Inactivation of the Medial Mammillary Nucleus Attenuates Theta Rhythm Activity in the Hippocampus in Urethane-Anesthetized Rats. Neurosci. Lett. 2017, 645, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Dillingham, C.M.; Frizzati, A.; Nelson, A.J.; Vann, S.D. How Do Mammillary Body Inputs Contribute to Anterior Thalamic Function? Neurosci. Biobehav. Rev. 2015, 54, 108–119. [Google Scholar] [CrossRef]
- Vann, S.D. Dismantling the Papez Circuit for Memory in Rats. Elife 2013, 2, e00736. [Google Scholar] [CrossRef]
- Vann, S.D.; Erichsen, J.T.; O’Mara, S.M.; Aggleton, J.P. Selective Disconnection of the Hippocampal Formation Projections to the Mammillary Bodies Produces Only Mild Deficits on Spatial Memory Tasks: Implications for Fornix Function. Hippocampus 2011, 21, 945–957. [Google Scholar] [CrossRef]
- Vann, S.D.; Nelson, A.J. The Mammillary Bodies and Memory: More Than a Hippocampal Relay. Prog. Brain Res. 2015, 219, 163–185. [Google Scholar]
- Chen, S.; Liu, G.; Li, A.; Liu, Z.; Long, B.; Yang, X.; Gong, H.; Li, X. Three-Dimensional Mapping in Multi-Samples with Large-Scale Imaging and Multiplexed Post Staining. Commun. Biol. 2023, 6, 148. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Xu, D.; Yuan, J.; Li, X.; Guo, C.; Peng, J.; Li, Y.; Schwarz, L.A.; Li, A.; Hu, B.; et al. High-Throughput Dual-Colour Precision Imaging for Brain-Wide Connectome with Cytoarchitectonic Landmarks at the Cellular Level. Nat. Commun. 2016, 7, 12142. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Tan, C.; Feng, Z.; Chen, S.; Zhang, Z.; Li, W.; Guan, Y.; Gong, H.; Luo, Q.; Li, A. A Robust Image Registration Interface for Large Volume Brain Atlas. Sci. Rep. 2020, 10, 2139. [Google Scholar] [CrossRef]
- Xu, Z.; Feng, Z.; Zhao, M.; Sun, Q.; Deng, L.; Jia, X.; Jiang, T.; Luo, P.; Chen, W.; Tudi, A.; et al. Whole-Brain Connectivity Atlas of Glutamatergic and Gabaergic Neurons in the Mouse Dorsal and Median Raphe Nuclei. eLife 2021, 10, e65502. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, Z.; Li, A.; Gong, H.; Long, B.; Li, X. High-Throughput Strategy for Profiling Sequential Section with Multiplex Staining of Mouse Brain. Front. Neuroanat. 2021, 15, 771229. [Google Scholar] [CrossRef]
- Quan, T.; Zhou, H.; Li, J.; Li, S.; Li, A.; Li, Y.; Lv, X.; Luo, Q.; Gong, H.; Zeng, S. Neurogps-Tree: Automatic Reconstruction of Large-Scale Neuronal Populations with Dense Neurites. Nat. Methods 2016, 13, 51–54. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, T.; Li, Q.; Liu, B.; Chen, J.; Li, A.; Jiang, T.; Gong, H.; Li, X. Parvalbumin Neurons in the Basal Forebrain Projecting to the Mammillary Nucleus Ameliorate Age-Related Cognitive Decline. Int. J. Mol. Sci. 2025, 26, 5934. https://doi.org/10.3390/ijms26135934
Sun T, Li Q, Liu B, Chen J, Li A, Jiang T, Gong H, Li X. Parvalbumin Neurons in the Basal Forebrain Projecting to the Mammillary Nucleus Ameliorate Age-Related Cognitive Decline. International Journal of Molecular Sciences. 2025; 26(13):5934. https://doi.org/10.3390/ijms26135934
Chicago/Turabian StyleSun, Tingting, Qianqian Li, Bimin Liu, Jiale Chen, Anan Li, Tao Jiang, Hui Gong, and Xiangning Li. 2025. "Parvalbumin Neurons in the Basal Forebrain Projecting to the Mammillary Nucleus Ameliorate Age-Related Cognitive Decline" International Journal of Molecular Sciences 26, no. 13: 5934. https://doi.org/10.3390/ijms26135934
APA StyleSun, T., Li, Q., Liu, B., Chen, J., Li, A., Jiang, T., Gong, H., & Li, X. (2025). Parvalbumin Neurons in the Basal Forebrain Projecting to the Mammillary Nucleus Ameliorate Age-Related Cognitive Decline. International Journal of Molecular Sciences, 26(13), 5934. https://doi.org/10.3390/ijms26135934





