Metformin Degradation by Advanced Oxidation Processes: Performance, Limitations, and Environmental Concerns
Abstract
1. Introduction
2. Diabetes Mellitus
2.1. Diabetes Mellitus Classification
2.2. Diabetes Mellitus: Cases and Deaths
2.3. Treatment for Diabetes Mellitus
2.4. Occurrence of Metformin in Water Matrices
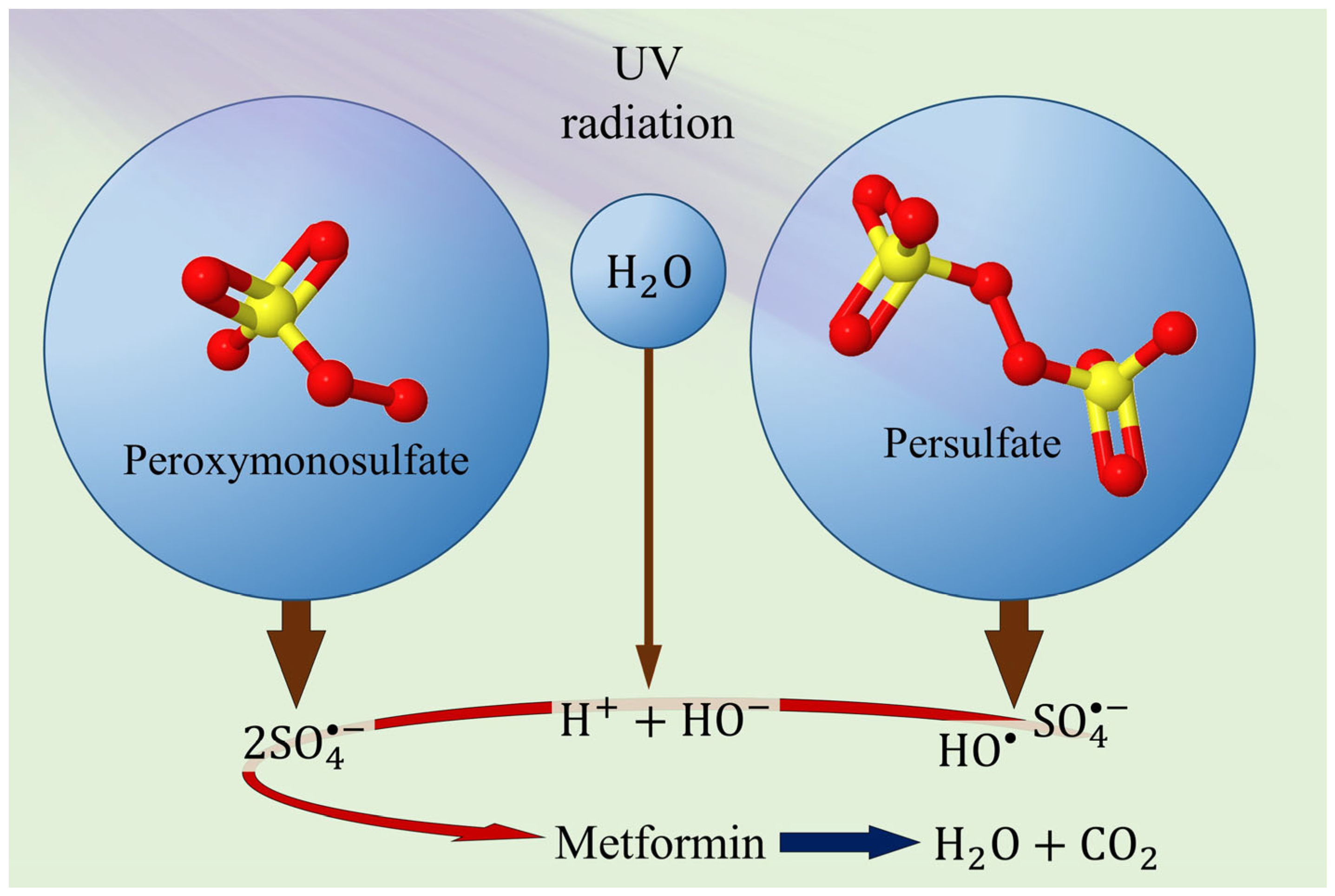
3. Metformin Degradation by Advanced Oxidation Processes
4. Metformin Degradation By-Products
5. Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| •OH | Hydroxyl radicals |
| 2,4-AMT | 2-amino-4 methylamino-1,3,5-triazine |
| 4,2,1-AIMT | 4-amino-2-imino-1-mehtyl-1,2-dihydro-1,3,5-triazine |
| AFR | Africa |
| AOPs | Advanced oxidation processes |
| BTEX | Benzene, toluene, ethylbenzene and xylenes |
| BUF | Buformin |
| CB | Conduction band |
| CNT | Carbon nanotubes |
| diMTF | Dimer of metformin |
| DM | Diabetes mellitus |
| DMA | Dimethylamine |
| DMF | N,N-dimethylformamide |
| DMG | Dimethylguanidine |
| DMU | N,N-dimethylurea |
| DMT1 | Diabetes mellitus type 1 |
| DPP-4 | Dipeptidyl peptidase |
| EBI | Electron beam irradiation |
| ECs | Emerging contaminants |
| EU | European Union |
| EUR | Europe |
| FGZAg | Fe3O4@rGO@ZnO/Ag-NPs |
| GLP-1 | Glucagon-like peptide-1 |
| H2O2 | Hydrogen peroxide |
| IDF | International Diabetes Federation |
| Kow | Octanol–water partition coefficient |
| LDL | Low-density lipoprotein |
| MBG | 1-mehtylbiguanide |
| MENA | Middle East and North Africa |
| MET | Metformin |
| MMA | Monomethylamine |
| MODY | Maturity Onset Diabetes of the Young |
| MTFOOH | Hydroperoxide of metformin |
| NAC | North America and Caribbean |
| NDMA | N-nitrosodimethylamine |
| NPs | Nanoparticles |
| PEDOT | Poly (3,4-ethylenedioxythiophene) |
| PHF | Phenformin |
| PMS | Peroxymonosulfate |
| rGO | Reduced graphene oxide |
| SCA | South and Central America |
| SEA | South-east Asia |
| SGLT2 | Sodium–glucose co-transporter 2 |
| t-BA | Tert-butyl alcohol |
| TiO2 | Titanium dioxide |
| UV | Ultraviolet |
| VB | Valence band |
| VUV | Vacuum ultraviolet |
| WO3 | Tungsten trioxide |
| WP | Western pacific |
| WWTPs | Wastewater treatment plants |
| ZrO2 | Zirconium dioxide |
References
- Bunke, D.; Moritz, S.; Brack, W.; Herráez, D.L.; Posthuma, L.; Nuss, M. Developments in Society and Implications for Emerging Pollutants in the Aquatic Environment. Environ. Sci. Eur. 2019, 31, 32. [Google Scholar] [CrossRef]
- Ahmed, S.; Khan, F.S.A.; Mubarak, N.M.; Khalid, M.; Tan, Y.H.; Mazari, S.A.; Karri, R.R.; Abdullah, E.C. Emerging Pollutants and Their Removal Using Visible-Light Responsive Photocatalysis—A Comprehensive Review. J. Environ. Chem. Eng. 2021, 9, 106643. [Google Scholar] [CrossRef]
- Vasilachi, I.; Asiminicesei, D.; Fertu, D.; Gavrilescu, M. Occurrence and Fate of Emerging Pollutants in Water Environment and Options for Their Removal. Water 2021, 13, 181. [Google Scholar] [CrossRef]
- Dulio, V.; Van Bavel, B.; Brorström-Lundén, E.; Harmsen, J.; Hollender, J.; Schlabach, M.; Slobodnik, J.; Thomas, K.; Koschorreck, J. Emerging Pollutants in the EU: 10 Years of NORMAN in Support of Environmental Policies and Regulations. Environ. Sci. Eur. 2018, 30, 5. [Google Scholar] [CrossRef]
- Tang, Y.; Yin, M.; Yang, W.; Li, H.; Zhong, Y.; Mo, L.; Liang, Y.; Ma, X.; Sun, X. Emerging Pollutants in Water Environment: Occurrence, Monitoring, Fate, and Risk Assessment. Water Environ. Res. 2019, 91, 984–991. [Google Scholar] [CrossRef]
- Arman, N.Z.; Salmiati, S.; Aris, A.; Salim, M.R.; Nazifa, T.H.; Muhamad, M.S.; Marpongahtun, M. A Review on Emerging Pollutants in the Water Environment: Existences, Health Effects and Treatment Processes. Water 2021, 13, 3258. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mentha, S.S.; Misra, Y.; Dwivedi, N. Emerging Pollutants of Severe Environmental Concern in Water and Wastewater: A Comprehensive Review on Current Developments and Future Research. Water-Energy Nexus 2023, 6, 74–95. [Google Scholar] [CrossRef]
- Gondi, R.; Kavitha, S.; Yukesh Kannah, R.; Parthiba Karthikeyan, O.; Kumar, G.; Kumar Tyagi, V.; Rajesh Banu, J. Algal-Based System for Removal of Emerging Pollutants from Wastewater: A Review. Bioresour. Technol. 2022, 344, 126245. [Google Scholar] [CrossRef]
- Peña-Guzmán, C.; Ulloa-Sánchez, S.; Mora, K.; Helena-Bustos, R.; Lopez-Barrera, E.; Alvarez, J.; Rodriguez-Pinzón, M. Emerging Pollutants in the Urban Water Cycle in Latin America: A Review of the Current Literature. J. Environ. Manag. 2019, 237, 408–423. [Google Scholar] [CrossRef]
- Deblonde, T.; Cossu-Leguille, C.; Hartemann, P. Emerging Pollutants in Wastewater: A Review of the Literature. Int. J. Hyg. Environ. Health 2011, 214, 442–448. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Hameed, B.H. Removal of Emerging Pharmaceutical Contaminants by Adsorption in a Fixed-Bed Column: A Review. Ecotoxicol. Environ. Saf. 2018, 149, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, T.; Bilal, M.; Nabeel, F.; Adeel, M.; Iqbal, H.M.N. Environmentally-Related Contaminants of High Concern: Potential Sources and Analytical Modalities for Detection, Quantification, and Treatment. Environ. Int. 2019, 122, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Kar, P.; Shukla, K.; Jain, P.; Sathiyan, G.; Gupta, R.K. Semiconductor Based Photocatalysts for Detoxification of Emerging Pharmaceutical Pollutants from Aquatic Systems: A Critical Review. Nano Mater. Sci. 2021, 3, 25–46. [Google Scholar] [CrossRef]
- Neha, R.; Adithya, S.; Jayaraman, R.S.; Gopinath, K.P.; M, P.; L, P.; Arun, J. Nano-Adsorbents an Effective Candidate for Removal of Toxic Pharmaceutical Compounds from Aqueous Environment: A Critical Review on Emerging Trends. Chemosphere 2021, 272, 129852. [Google Scholar] [CrossRef]
- Priya, A.K.; Gnanasekaran, L.; Rajendran, S.; Qin, J.; Vasseghian, Y. Occurrences and Removal of Pharmaceutical and Personal Care Products from Aquatic Systems Using Advanced Treatment—A Review. Environ. Res. 2022, 204, 112298. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Jeevanantham, S.; Anubha, M.; Jayashree, S. Degradation of Toxic Agrochemicals and Pharmaceutical Pollutants: Effective and Alternative Approaches toward Photocatalysis. Environ. Pollut. 2022, 298, 118844. [Google Scholar] [CrossRef]
- Quesada, H.B.; Baptista, A.T.A.; Cusioli, L.F.; Seibert, D.; De Oliveira Bezerra, C.; Bergamasco, R. Surface Water Pollution by Pharmaceuticals and an Alternative of Removal by Low-Cost Adsorbents: A Review. Chemosphere 2019, 222, 766–780. [Google Scholar] [CrossRef]
- Eniola, J.O.; Kumar, R.; Barakat, M.A.; Rashid, J. A Review on Conventional and Advanced Hybrid Technologies for Pharmaceutical Wastewater Treatment. J. Clean. Prod. 2022, 356, 131826. [Google Scholar] [CrossRef]
- Khalil, A.M.E.; Memon, F.A.; Tabish, T.A.; Salmon, D.; Zhang, S.; Butler, D. Nanostructured Porous Graphene for Efficient Removal of Emerging Contaminants (Pharmaceuticals) from Water. Chem. Eng. J. 2020, 398, 125440. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Z.; Yao, B.; Zhou, Y. Occurrence, Bioaccumulation, Fate, and Risk Assessment of Emerging Pollutants in Aquatic Environments: A Review. Sci. Total Environ. 2024, 923, 171388. [Google Scholar] [CrossRef]
- Majumdar, A.; Pal, A. Recent Advancements in Visible-Light-Assisted Photocatalytic Removal of Aqueous Pharmaceutical Pollutants. Clean Techn. Env. Policy 2020, 22, 11–42. [Google Scholar] [CrossRef]
- Velempini, T.; Prabakaran, E.; Pillay, K. Recent Developments in the Use of Metal Oxides for Photocatalytic Degradation of Pharmaceutical Pollutants in Water—A Review. Mater. Today Chem. 2021, 19, 100380. [Google Scholar] [CrossRef]
- Issaka, E.; AMU-Darko, J.N.-O.; Yakubu, S.; Fapohunda, F.O.; Ali, N.; Bilal, M. Advanced Catalytic Ozonation for Degradation of Pharmaceutical pollutants―A Review. Chemosphere 2022, 289, 133208. [Google Scholar] [CrossRef] [PubMed]
- Branchet, P.; Arpin-Pont, L.; Piram, A.; Boissery, P.; Wong-Wah-Chung, P.; Doumenq, P. Pharmaceuticals in the Marine Environment: What Are the Present Challenges in Their Monitoring? Sci. Total Environ. 2021, 766, 142644. [Google Scholar] [CrossRef]
- Dias, R.; Sousa, D.; Bernardo, M.; Matos, I.; Fonseca, I.; Vale Cardoso, V.; Neves Carneiro, R.; Silva, S.; Fontes, P.; Daam, M.A.; et al. Study of the Potential of Water Treatment Sludges in the Removal of Emerging Pollutants. Molecules 2021, 26, 1010. [Google Scholar] [CrossRef]
- Rempel, A.; Gutkoski, J.P.; Nazari, M.T.; Biolchi, G.N.; Biduski, B.; Treichel, H.; Colla, L.M. Microalgae Growth with a High Concentration of Emerging Pollutants and Phytotoxicity Evaluation of Cultivation Wastewater. J. Water Process Eng. 2022, 46, 102616. [Google Scholar] [CrossRef]
- World Health Organization. Classification of Diabetes Mellitus; World Health Organization: Geneva, Switzerland, 2019; ISBN 978-92-4-151570-2. [Google Scholar]
- Punthakee, Z.; Goldenberg, R.; Katz, P. Definition, Classification and Diagnosis of Diabetes, Prediabetes and Metabolic Syndrome. Can. J. Diabetes 2018, 42, S10–S15. [Google Scholar] [CrossRef]
- Maraschin, J.D.F. Classification of Diabetes. In Diabetes; Ahmad, S.I., Ed.; Advances in Experimental Medicine and Biology; Springer New York: New York, NY, USA, 2013; Volume 771, pp. 12–19. ISBN 978-1-4614-5440-3. [Google Scholar]
- American Diabetes Association. Classification and Diagnosis of Diabetes. Diabetes Care 2017, 40, S11–S24. [Google Scholar] [CrossRef]
- Antar, S.A.; Ashour, N.A.; Sharaky, M.; Khattab, M.; Ashour, N.A.; Zaid, R.T.; Roh, E.J.; Elkamhawy, A.; Al-Karmalawy, A.A. Diabetes Mellitus: Classification, Mediators, and Complications; A Gate to Identify Potential Targets for the Development of New Effective Treatments. Biomed. Pharmacother. 2023, 168, 115734. [Google Scholar] [CrossRef]
- Baynest, H.W. Classification, Pathophysiology, Diagnosis and Management of Diabetes Mellitus. J. Diabetes Metab. 2015, 6, 1000541. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Magliano, D.; Boyko, E.J. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; ISBN 978-2-930229-98-0. [Google Scholar]
- Da Trindade, M.T.; Kogawa, A.C.; Salgado, H.R.N. Metformin: A Review of Characteristics, Properties, Analytical Methods and Impact in the Green Chemistry. Crit. Rev. Anal. Chem. 2018, 48, 66–72. [Google Scholar] [CrossRef] [PubMed]
- DRUGBANK Phenformin: Uses, Interactions, Mechanism of Action|DrugBank. Available online: https://go.drugbank.com/drugs/DB00914 (accessed on 15 September 2024).
- DRUGBANK Buformin. Available online: https://go.drugbank.com/drugs/DB04830 (accessed on 15 September 2024).
- Metry, M.; Shu, Y.; Abrahamsson, B.; Cristofoletti, R.; Dressman, J.B.; Groot, D.W.; Parr, A.; Langguth, P.; Shah, V.P.; Tajiri, T.; et al. Biowaiver Monographs for Immediate Release Solid Oral Dosage Forms: Metformin Hydrochloride. J. Pharm. Sci. 2021, 110, 1513–1526. [Google Scholar] [CrossRef] [PubMed]
- PubChem Buformin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2468 (accessed on 15 September 2024).
- Yendapally, R.; Sikazwe, D.; Kim, S.S.; Ramsinghani, S.; Fraser-Spears, R.; Witte, A.P.; La-Viola, B. A Review of Phenformin, Metformin, and Imeglimin. Drug. Dev. Res. 2020, 81, 390–401. [Google Scholar] [CrossRef]
- Gallwitz, B. Clinical Use of DPP-4 Inhibitors. Front. Endocrinol. 2019, 10, 389. [Google Scholar] [CrossRef]
- Elizalde-Velázquez, G.A.; Gómez-Oliván, L.M. Occurrence, Toxic Effects and Removal of Metformin in the Aquatic Environments in the World: Recent Trends and Perspectives. Sci. Total Environ. 2020, 702, 134924. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Viollet, B. Metformin: Update on Mechanisms of Action and Repurposing Potential. Nat. Rev. Endocrinol. 2023, 19, 460–476. [Google Scholar] [CrossRef]
- CHEMANALYST Metformin HCL Market Size, Share, Growth & Forecast. Available online: https://www.chemanalyst.com/industry-report/metformin-hcl-market-4156 (accessed on 12 November 2024).
- Ambrosio-Albuquerque, E.P.; Cusioli, L.F.; Bergamasco, R.; Sinópolis Gigliolli, A.A.; Lupepsa, L.; Paupitz, B.R.; Barbieri, P.A.; Borin-Carvalho, L.A.; De Brito Portela-Castro, A.L. Metformin Environmental Exposure: A Systematic Review. Environ. Toxicol. Pharmacol. 2021, 83, 103588. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Sillanpää, M.; Jacob, M.M.; Vo, D.-V.N. Metformin as an Emerging Concern in Wastewater: Occurrence, Analysis and Treatment Methods. Environ. Res. 2022, 213, 113613. [Google Scholar] [CrossRef]
- Briones, R.M.; Sarmah, A.K.; Padhye, L.P. A Global Perspective on the Use, Occurrence, Fate and Effects of Anti-Diabetic Drug Metformin in Natural and Engineered Ecosystems. Environ. Pollut. 2016, 219, 1007–1020. [Google Scholar] [CrossRef]
- European Parliament and Council of the European Union. Directive 2008/105/EC of the European Parliament and of the Council of 16 December 2008 on Environmental Quality Standards in the Field of Water Policy, Amending and Subsequently Repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/491/EEC, 86/280/EEC and Amending Directive 2000/60/EC of the European Parliament and of the Council. Off. J. Eur. Union 2008, 51, 84–97. [Google Scholar]
- European Commission. Commission Implementing Regulation (EU) 2025/439 of 28 February 2025 establishing a watch list of substances for Union-wide monitoring in the field of water policy pursuant to Directive 2008/105/EC of the European Parliament and the Council. Off. J. Eur. Union 2025, 439. Available online: https://eur-lex.europa.eu/eli/dec_impl/2025/439/oj (accessed on 24 May 2025).
- European Commission. Directorate-General for Environment Communication from the Commission to the European Parliament, the Council and the European Economic and Social Committee: European Union Strategic Approach to Pharmaceuticals in the Environment. Off. J. Eur. Union 2019, 128. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52019DC0128 (accessed on 26 May 2025).
- European Commission. Directorate-General for Health and Food Safety Communication from the Commission to the Council and the European Parliament: A European One Health Action Plan against Antimicrobial Resistance (AMR). Off. J. Eur. Union 2017, 339. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52017DC0339 (accessed on 26 May 2025).
- Lertxundi, U.; Domingo-Echaburu, S.; Barros, S.; Santos, M.M.; Neuparth, T.; Quintana, J.B.; Rodil, R.; Montes, R.; Orive, G. Is the Environmental Risk of Metformin Underestimated? Environ. Sci. Technol. 2023, 57, 8463–8466. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Y.; Dionysiou, D.D. Advanced Oxidation Processes for Removal of Emerging Contaminants in Water. Water 2023, 15, 398. [Google Scholar] [CrossRef]
- Chinnaiyan, P.; Thampi, S.G.; Bharath Krishnaa, A.; Sasi, M.; Tejashwi, L. Optimisation of System Parameters for the Removal of Metformin in a Photocatalytic Reactor Employing TiO2. IOP Conf. Ser. Mater. Sci. Eng. 2019, 561, 12087. [Google Scholar] [CrossRef]
- Carbuloni, C.F.; Savoia, J.E.; Santos, J.S.P.; Pereira, C.A.A.; Marques, R.G.; Ribeiro, V.A.S.; Ferrari, A.M. Degradation of Metformin in Water by TiO2–ZrO2 Photocatalysis. J. Environ. Manag. 2020, 262, 110347. [Google Scholar] [CrossRef]
- Chinnaiyan, P.; Thampi, S.G.; Kumar, M.; Balachandran, M. Photocatalytic Degradation of Metformin and Amoxicillin in Synthetic Hospital Wastewater: Effect of Classical Parameters. Int. J. Environ. Sci. Technol. 2019, 16, 5463–5474. [Google Scholar] [CrossRef]
- Nezar, S.; Laoufi, N.A. Electron Acceptors Effect on Photocatalytic Degradation of Metformin under Sunlight Irradiation. Sol. Energy 2018, 164, 267–275. [Google Scholar] [CrossRef]
- Quintão, F.J.O.; Freitas, J.R.L.; De Fátima Machado, C.; Aquino, S.F.; De Queiroz Silva, S.; De Cássia Franco Afonso, R.J. Characterization of Metformin By-products under Photolysis, Photocatalysis, Ozonation and Chlorination by High-performance Liquid Chromatography Coupled to High-resolution Mass Spectrometry. Rapid Comm Mass Spectrom. 2016, 30, 2360–2368. [Google Scholar] [CrossRef]
- Tominaga, F.K.; De Jesus, J.M.S.; Klanovicz, N.; Redígolo, M.M.; Silva, T.T.; Lebre, D.T.; Teixeira, A.C.S.C.; Leo, P.; Borrely, S.I. Ecotoxicological Assessment of Metformin as an Antidiabetic Water Residue Treated by Electron Beam Accelerator Irradiation. Discov. Water 2024, 4, 5. [Google Scholar] [CrossRef]
- Pandey, P.; Mohanan, S.; Surenjan, A. Photocatalytic Degradation of Metformin on a Rectangular Baffled Reactor: CFD Modeling and Validation Investigation. Chem. Eng. Process.-Process Intensif. 2024, 202, 109833. [Google Scholar] [CrossRef]
- Parra-Marfil, A.; López-Ramón, M.V.; Aguilar-Aguilar, A.; García-Silva, I.A.; Rosales-Mendoza, S.; Romero-Cano, L.A.; Bailón-García, E.; Ocampo-Pérez, R. An Efficient Removal Approach for Degradation of Metformin from Aqueous Solutions with Sulfate Radicals. Environ. Res. 2023, 217, 114852. [Google Scholar] [CrossRef] [PubMed]
- Nematolahi, S.Z.; Dehghani, M.; Yousefinejad, S.; Hashemi, H.; Golaki, M.; Mohammadpour, A.; Abdollahi, S.H. Removal of Metformin from Aqueous Solution Using Fe3+ Doped TiO2 Nanoparticles under UV Irradiation. Desalination Water Treat. 2021, 236, 182–189. [Google Scholar] [CrossRef]
- Karimian, S.; Moussavi, G.; Fanaei, F.; Mohammadi, S.; Shekoohiyan, S.; Giannakis, S. Shedding Light on the Catalytic Synergies between Fe (II) and PMS in Vacuum UV (VUV/Fe/PMS) Photoreactors for Accelerated Elimination of Pharmaceuticals: The Case of Metformin. Chem. Eng. J. 2020, 400, 125896. [Google Scholar] [CrossRef]
- Fujinami, S.C. Metformin Degradation with Combined Ozone/UV/H2O2 Process; Nanyang Technological University: Singapore, 2020. [Google Scholar]
- Minh, T.D.; Ncibi, M.C.; Srivastava, V.; Thangaraj, S.K.; Jänis, J.; Sillanpää, M. Gingerbread Ingredient-Derived Carbons-Assembled CNT Foam for the Efficient Peroxymonosulfate-Mediated Degradation of Emerging Pharmaceutical Contaminants. Appl. Catal. B Environ. 2019, 244, 367–384. [Google Scholar] [CrossRef]
- Cheshme Khavar, A.H.; Moussavi, G.; Mahjoub, A.; Yaghmaeian, K.; Srivastava, V.; Sillanpää, M.; Satari, M. Novel Magnetic Fe3 O4 @rGO@ZnO Onion-like Microspheres Decorated with Ag Nanoparticles for the Efficient Photocatalytic Oxidation of Metformin: Toxicity Evaluation and Insights into the Mechanisms. Catal. Sci. Technol. 2019, 9, 5819–5837. [Google Scholar] [CrossRef]
- Chaabene, R.; Khannous, L.; Samet, Y. Electrochemical Degradation of Aqueous Metformin at Boron-Doped Diamond Electrode: Kinetic Study and Phytotoxicity Tests. Int. J. Environ. Sci. Technol. 2023, 20, 5169–5182. [Google Scholar] [CrossRef]
- Kumar, R.; Akbarinejad, A.; Jasemizad, T.; Fucina, R.; Travas-Sejdic, J.; Padhye, L.P. The Removal of Metformin and Other Selected PPCPs from Water by Poly (3,4-Ethylenedioxythiophene) Photocatalyst. Sci. Total Environ. 2021, 751, 142302. [Google Scholar] [CrossRef]
- Gu, Y.; Liu, X.; Han, Q.; Wang, F. Insights into the Efficient Degradation of Metformin—An Emerging Antidiabetic Medicine by UV/Sulfite Process. E3S Web Conf. 2024, 536, 3012. [Google Scholar] [CrossRef]
- Iqbal, T.; Munir, R.M.; Younas, A.; Afsheen, S.; Mansha, M.S.; Ahsan, S.; AlObaid, A.A.; Al-Zaqri, N. Facile Green Synthesis of La-Doped WO3 Nanomaterials for Their Photocatalytic Activity against Hazardous Pharmaceutical Compounds: Experimental and COMSOL Simulation. J. Inorg. Organomet. Polym. 2024, 34, 3300–3313. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El-Nemr, M.A.; Elkatory, M.R.; Ragab, S.; Niculescu, V.-C.; El Nemr, A. Principles of Photocatalysts and Their Different Applications: A Review. Top. Curr. Chem. 2023, 381, 31. [Google Scholar] [CrossRef]
- Silerio-Vázquez, F.D.J.; González-Burciaga, L.A.; Antileo, C.; Núñez-Núñez, C.M.; Proal-Nájera, J.B. Photocatalytic Degradation of Antibiotics in Water via TiO2-x: Research Needs for Technological Advancements. J. Hazard. Mater. Adv. 2024, 16, 100506. [Google Scholar] [CrossRef]
- Beil, S.B.; Bonnet, S.; Casadevall, C.; Detz, R.J.; Eisenreich, F.; Glover, S.D.; Kerzig, C.; Næsborg, L.; Pullen, S.; Storch, G.; et al. Challenges and Future Perspectives in Photocatalysis: Conclusions from an Interdisciplinary Workshop. JACS Au 2024, 4, 2746–2766. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Tan, X.; Liu, Z.; Hu, B.; Wang, X. Recent Progress on Metal-Enhanced Photocatalysis: A Review on the Mechanism. Research 2021, 2021, 9794329. [Google Scholar] [CrossRef] [PubMed]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why Is Anatase a Better Photocatalyst than Rutile?—Model Studies on Epitaxial TiO2 Films. Sci. Rep. 2014, 4, 4043. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New Understanding of the Difference of Photocatalytic Activity among Anatase, Rutile and Brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef]
- Habibi, M.H.; Vosooghian, H. Photocatalytic Degradation of Some Organic Sulfides as Environmental Pollutants Using Titanium Dioxide Suspension. J. Photochem. Photobiol. A Chem. 2005, 174, 45–52. [Google Scholar] [CrossRef]
- Tabatabai-Yazdi, F.; Ebrahimian Pirbazari, A.; Esmaeili Khalilsaraei, F.; Asasian Kolur, N.; Gilani, N. Photocatalytic Treatment of Tetracycline Antibiotic Wastewater by Silver/TiO2 Nanosheets/Reduced Graphene Oxide and Artificial Neural Network Modeling. Water Environ. Res. 2020, 92, 662–676. [Google Scholar] [CrossRef]
- Naeem, H.; Tofil, H.M.; Soliman, M.; Hai, A.; Zaidi, S.H.H.; Kizilbash, N.; Alruwaili, D.; Ajmal, M.; Siddiq, M. Reduced Graphene Oxide-Zinc Sulfide Nanocomposite Decorated with Silver Nanoparticles for Wastewater Treatment by Adsorption, Photocatalysis and Antimicrobial Action. Molecules 2023, 28, 926. [Google Scholar] [CrossRef]
- Hemmati, S.; Heravi, M.M.; Karmakar, B.; Veisi, H. Green Fabrication of Reduced Graphene Oxide Decorated with Ag Nanoparticles (rGO/Ag NPs) Nanocomposite: A Reusable Catalyst for the Degradation of Environmental Pollutants in Aqueous Medium. J. Mol. Liq. 2020, 319, 114302. [Google Scholar] [CrossRef]
- Gaggero, E.; Calza, P.; Cerrato, E.; Paganini, M.C. Cerium-, Europium- and Erbium-Modified ZnO and ZrO2 for Photocatalytic Water Treatment Applications: A Review. Catalysts 2021, 11, 1520. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; El-Ghanam, A.M.; Saad, S.R.; Mohamed, R.H.A. Promoted Removal of Metformin Hydrochloride Anti-Diabetic Drug from Water by Fabricated and Modified Nanobiochar from Artichoke Leaves. Sustain. Chem. Pharm. 2020, 18, 100336. [Google Scholar] [CrossRef]
- Vieira, Y.; Ribeiro, T.H.; Leichtweis, J.; Dotto, G.L.; Foletto, E.L.; Georgin, J.; Franco, D.S.P.; Lima, E.C. A Critical Review of the Current Environmental Risks Posed by the Antidiabetic Metformin and the Status, Advances, and Trends in Adsorption Technologies for Its Remediation. J. Water Process Eng. 2023, 54, 103943. [Google Scholar] [CrossRef]
- Heidari, Z.; Alizadeh, R.; Ebadi, A.; Oturan, N.; Oturan, M.A. Efficient Photocatalytic Degradation of Furosemide by a Novel Sonoprecipited ZnO over Ion Exchanged Clinoptilolite Nanorods. Sep. Purif. Technol. 2020, 242, 116800. [Google Scholar] [CrossRef]
- Suwannaruang, T.; Kidkhunthod, P.; Chanlek, N.; Soontaranon, S.; Wantala, K. High Anatase Purity of Nitrogen-Doped TiO2 Nanorice Particles for the Photocatalytic Treatment Activity of Pharmaceutical Wastewater. Appl. Surf. Sci. 2019, 478, 1–14. [Google Scholar] [CrossRef]
- Geldasa, F.T.; Kebede, M.A.; Shura, M.W.; Hone, F.G. Experimental and Computational Study of Metal Oxide Nanoparticles for the Photocatalytic Degradation of Organic Pollutants: A Review. RSC Adv. 2023, 13, 18404–18442. [Google Scholar] [CrossRef]
- Khaki, M.R.D.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Application of Doped Photocatalysts for Organic Pollutant Degradation—A Review. J. Environ. Manag. 2017, 198, 78–94. [Google Scholar] [CrossRef]
- Mehtab, A.; Ahmed, J.; Alshehri, S.M.; Mao, Y.; Ahmad, T. Rare Earth Doped Metal Oxide Nanoparticles for Photocatalysis: A Perspective. Nanotechnology 2022, 33, 142001. [Google Scholar] [CrossRef]
- Ahmad, I. Comparative Study of Metal (Al, Mg, Ni, Cu and Ag) Doped ZnO/g-C3N4 Composites: Efficient Photocatalysts for the Degradation of Organic Pollutants. Sep. Purif. Technol. 2020, 251, 117372. [Google Scholar] [CrossRef]
- Katančić, Z.; Chen, W.-T.; Waterhouse, G.I.N.; Kušić, H.; Lončarić Božić, A.; Hrnjak-Murgić, Z.; Travas-Sejdic, J. Solar-Active Photocatalysts Based on TiO2 and Conductive Polymer PEDOT for the Removal of Bisphenol A. J. Photochem. Photobiol. A Chem. 2020, 396, 112546. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B.; Wang, Y.; Liu, J.; Sillanpää, M. Synergistic Degradation of Organic Pollutants by Poly (3,4-Ethylenedioxythiophene) Based Photo-Electrocatalysis. J. Water Process Eng. 2022, 45, 102494. [Google Scholar] [CrossRef]
- Li, K.; Zhang, S.; Li, Y.; Fan, J.; Lv, K. MXenes as Noble-Metal-Alternative Co-Catalysts in Photocatalysis. Chin. J. Catal. 2021, 42, 3–14. [Google Scholar] [CrossRef]
- Koe, W.S.; Lee, J.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C. An Overview of Photocatalytic Degradation: Photocatalysts, Mechanisms, and Development of Photocatalytic Membrane. Environ. Sci. Pollut. Res. 2020, 27, 2522–2565. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Zhao, H.; Pan, F.; Feng, X.; Jung, B.; Abdel-Wahab, A.; Batchelor, B.; Li, Y. Visible-Light-Driven Photocatalytic Degradation of Organic Water Pollutants Promoted by Sulfite Addition. Environ. Sci. Technol. 2017, 51, 13372–13379. [Google Scholar] [CrossRef]
- Li, K.; Gong, K.; Liu, J.; Yang, Y.; Nabi, I.; Bacha, A.-U.-R.; Cheng, H.; Han, J.; Zhang, L. New Insights into the Role of Sulfite in BiOX Photocatalytic Pollutants Elimination: In-Operando Generation of Plasmonic Bi Metal and Oxygen Vacancies. J. Hazard. Mater. 2021, 418, 126207. [Google Scholar] [CrossRef]
- Wei, Y.; Zou, Q.; Ye, P.; Wang, M.; Li, X.; Xu, A. Photocatalytic Degradation of Organic Pollutants in Wastewater with G-C3N4/Sulfite System under Visible Light Irradiation. Chemosphere 2018, 208, 358–365. [Google Scholar] [CrossRef]
- Zhang, B.-T.; Zhang, Y.; Teng, Y.; Fan, M. Sulfate Radical and Its Application in Decontamination Technologies. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1756–1800. [Google Scholar] [CrossRef]
- Xiao, R.; Luo, Z.; Wei, Z.; Luo, S.; Spinney, R.; Yang, W.; Dionysiou, D.D. Activation of Peroxymonosulfate/Persulfate by Nanomaterials for Sulfate Radical-Based Advanced Oxidation Technologies. Curr. Opin. Chem. Eng. 2018, 19, 51–58. [Google Scholar] [CrossRef]
- Milh, H.; Yu, X.; Cabooter, D.; Dewil, R. Degradation of Ciprofloxacin Using UV-Based Advanced Removal Processes: Comparison of Persulfate-Based Advanced Oxidation and Sulfite-Based Advanced Reduction Processes. Sci. Total Environ. 2021, 764, 144510. [Google Scholar] [CrossRef]
- Wu, S.; Shen, L.; Lin, Y.; Yin, K.; Yang, C. Sulfite-Based Advanced Oxidation and Reduction Processes for Water Treatment. Chem. Eng. J. 2021, 414, 128872. [Google Scholar] [CrossRef]
- Urán-Duque, L.; Saldarriaga-Molina, J.C.; Rubio-Clemente, A. Advanced Oxidation Processes Based on Sulfate Radicals for Wastewater Treatment: Research Trends. Water 2021, 13, 2445. [Google Scholar] [CrossRef]
- Duan, X.; Yang, S.; Wacławek, S.; Fang, G.; Xiao, R.; Dionysiou, D.D. Limitations and Prospects of Sulfate-Radical Based Advanced Oxidation Processes. J. Environ. Chem. Eng. 2020, 8, 103849. [Google Scholar] [CrossRef]
- Kumari, M.; Pulimi, M. Sulfate Radical-Based Degradation of Organic Pollutants: A Review on Application of Metal-Organic Frameworks as Catalysts. ACS Omega 2023, 8, 34262–34280. [Google Scholar] [CrossRef] [PubMed]
- Boczkaj, G.; Fernandes, A. Wastewater Treatment by Means of Advanced Oxidation Processes at Basic pH Conditions: A Review. Chem. Eng. J. 2017, 320, 608–633. [Google Scholar] [CrossRef]
- Ahmed, H.R. Heterogeneous Catalysts in Advanced Oxidation Processes: A Comprehensive Review on Antibiotic Removal from Wastewater. Sep. Purif. Technol. 2025, 374, 133670. [Google Scholar] [CrossRef]
- Iyyappan, J.; Gaddala, B.; Gnanasekaran, R.; Gopinath, M.; Yuvaraj, D.; Kumar, V. Critical Review on Wastewater Treatment Using Photo Catalytic Advanced Oxidation Process: Role of Photocatalytic Materials, Reactor Design and Kinetics. Case Stud. Chem. Environ. Eng. 2024, 9, 100599. [Google Scholar] [CrossRef]
- Iqbal, J.; Shah, N.S.; Ali Khan, J.; Naushad, M.; Boczkaj, G.; Jamil, F.; Khan, S.; Li, L.; Murtaza, B.; Han, C. Pharmaceuticals Wastewater Treatment via Different Advanced Oxidation Processes: Reaction Mechanism, Operational Factors, Toxicities, and Cost Evaluation—A Review. Sep. Purif. Technol. 2024, 347, 127458. [Google Scholar] [CrossRef]
- Zawadzki, P. Visible Light–Driven Advanced Oxidation Processes to Remove Emerging Contaminants from Water and Wastewater: A Review. Water Air Soil Pollut. 2022, 233, 374. [Google Scholar] [CrossRef]
- Straub, J.O.; Caldwell, D.J.; Davidson, T.; D’Aco, V.; Kappler, K.; Robinson, P.F.; Simon-Hettich, B.; Tell, J. Environmental Risk Assessment of Metformin and Its Transformation Product Guanylurea. I. Environmental Fate. Chemosphere 2019, 216, 844–854. [Google Scholar] [CrossRef]
- Estrada-Arriaga, E.B.; García-Sánchez, L.; Falcón-Rojas, A. Experimental Design Approach for the Removal of the Antidiabetic Drug, Metformin at a High Concentration by Photocatalytic Processes. Water Environ. J. 2021, 35, 759–771. [Google Scholar] [CrossRef]
- Liao, X.; Shen, L.; Jiang, Z.; Gao, M.; Qiu, Y.; Qi, H.; Chen, C. NDMA Formation during Ozonation of Metformin: Roles of Ozone and Hydroxyl Radicals. Sci. Total Environ. 2021, 796, 149010. [Google Scholar] [CrossRef] [PubMed]
- Trouillas, P.; Marchetti, C.; Bonnefont-Rousselot, D.; Lazzaroni, R.; Jore, D.; Gardès-Albert, M.; Collin, F. Mechanism of One-Electron Oxidation of Metformin in Aqueous Solution. Phys. Chem. Chem. Phys. 2013, 15, 9871. [Google Scholar] [CrossRef] [PubMed]
- Badran, I.; Manasrah, A.D.; Nassar, N.N. A Combined Experimental and Density Functional Theory Study of Metformin Oxy-Cracking for Pharmaceutical Wastewater Treatment. RSC Adv. 2019, 9, 13403–13413. [Google Scholar] [CrossRef] [PubMed]
- Egerić, M.; Matović, L.; Savić, M.; Stanković, S.; Wu, Y.; Li, F.; Vujasin, R. Gamma Irradiation Induced Degradation of Organic Pollutants: Recent Advances and Future Perspective. Chemosphere 2024, 352, 141437. [Google Scholar] [CrossRef]
- Thakur, S.; Ojha, A.; Kansal, S.K.; Gupta, N.K.; Swart, H.C.; Cho, J.; Kuznetsov, A.; Sun, S.; Prakash, J. Advances in Powder Nano-Photocatalysts as Pollutant Removal and as Emerging Contaminants in Water: Analysis of Pros and Cons on Health and Environment. Adv. Powder Mater. 2024, 3, 100233. [Google Scholar] [CrossRef]
- Adeyemi, J.O.; Ajiboye, T.; Onwudiwe, D.C. Mineralization of Antibiotics in Wastewater Via Photocatalysis. Water Air Soil Pollut. 2021, 232, 219. [Google Scholar] [CrossRef]
- Pandis, P.K.; Kalogirou, C.; Kanellou, E.; Vaitsis, C.; Savvidou, M.G.; Sourkouni, G.; Zorpas, A.A.; Argirusis, C. Key Points of Advanced Oxidation Processes (AOPs) for Wastewater, Organic Pollutants and Pharmaceutical Waste Treatment: A Mini Review. ChemEngineering 2022, 6, 8. [Google Scholar] [CrossRef]




| Rank | IDF 1 Region | 2021 | 2045 | ||||
|---|---|---|---|---|---|---|---|
| People with DM 2 (Millions) | DM Prevalence | Comparative DM Prevalence | People with DM (Millions) | DM Prevalence | Comparative DM Prevalence | ||
| World | 536.6 | 10.5 | 9.8 | 783.2 | 12.2 | 11.2 | |
| 1 | MENA 3 | 72.7 | 16.2 | 18.1 | 135.7 | 19.3 | 20.4 |
| 2 | NAC 4 | 50.5 | 14.0 | 11.9 | 62.8 | 15.2 | 14.2 |
| 3 | SEA 5 | 90.2 | 8.7 | 10.0 | 151.5 | 11.3 | 11.3 |
| 4 | WP 6 | 205.6 | 11.9 | 9.9 | 260.2 | 14.4 | 11.5 |
| 5 | SCA 7 | 32.5 | 9.5 | 8.2 | 48.9 | 11.9 | 9.8 |
| 6 | EUR 8 | 61.4 | 9.2 | 7.0 | 69.2 | 10.4 | 8.7 |
| 7 | AFR 9 | 23.6 | 4.5 | 5.3 | 54.9 | 5.2 | 5.6 |
| Region | DMT1 1 Children and Adolescents 0–19 Years (2021) | Diabetes-Related Deaths in Adults 20–79 Years (2011) | Diabetes-Related Deaths in Adults 20–79 Years (2021) |
|---|---|---|---|
| WP 2 | 2839 | 1,708,300 | 2,281,732 |
| EUR 3 | 4977 | 600,000 | 1,111,201 |
| NAC 4 | 8371 | 280,800 | 930,692 |
| MENA 5 | 9166 | 276,400 | 796,362 |
| SEA 6 | 34,930 | 1,156,000 | 747,367 |
| AFR 7 | 1240 | 344,500 | 416,163 |
| SCA 8 | 6385 | 227,200 | 410,206 |
| Compound | Molecular Weight | Chemical Formula | Kow 1 | pKa 2 | Solubility in Water | Reference |
|---|---|---|---|---|---|---|
| Metformin | 129.16 | C4H11N5 | −1.43 | 2.8 11.5 | 300 mg/mL | [35,38] |
| Buformin | 157.22 | C6H15N5 | −1.2 | 11.52 18.1 | 1.41 mg/mL | [37,39] |
| Phenformin | 205.27 | C10H15N5 | −1.02 | 11.97 | 210 mg/mL | [36,40] |
| Advanced Oxidation Processes | Conditions and Parameters | Removal Rate | Reference |
|---|---|---|---|
| Heterogeneous photocatalysis-UV/titanium dioxide (TiO2) | Reaction Time = 30 min [MET]0 = 100 mg/L [TiO2] = 1000 mg/L | 81% | [54] |
| Photocatalysis-TiO2/zirconium dioxide (ZrO2) | Reaction Time = 30 min [MET]0 = 10 mg/L [TiO2] = 1000 mg/L pH = 8 | 55% | [55] |
| Photocatalysis | Reaction Time = 150 min [MET]0 = 10 mg/L [TiO2] = 563 mg/L pH = 7.6 | 95.2% | [56] |
| Photocatalysis-TiO2/Na2S2O8 electron acceptors | Reaction Time = 180 min [MET]0 = 10 mg/L [TiO2] = 1000 mg/L Catalyst = Degussa P25 | 99% | [57] |
| Ozonation | Reaction Time = 30 min [MET]0 = 10 mg/L pH = 9 | 60% | [58] |
| Photolysis-UV/C | Reaction Time = 30 min [MET]0 = 10 mg/L | 9.20% | |
| Photocatalysis-TiO2/UV-C | Reaction Time = 30 min [MET]0 = 10 mg/L [TiO2] = 120 mg/L pH = 6 | 31% | |
| EBI 1 and persulfate addition | [MET]0 = 8.2 mg/L Irradiation = 5.0 kGy | 99.41% | [59] |
| Solar-irradiated photocatalysis | Reaction Time = 90 min [MET]0 = 10 mg/L [TiO2] = 750 mg/L pH = 8 | 78.33% | [60] |
| Sulfate radicals (UV/S2O82− system) | Reaction Time= 60 min [MET]0 = 5 mg/L [K2S2O8] = 2750 µM pH = 6.5 | 87.3% | [61] |
| Photocatalysis with Fe3+ doped TiO2 nanoparticles under UV irradiation | Reaction Time = 150 min [MET]0 = 35 mg/L [Fe3+/TiO2] = 75 mg/L pH = 11 | 93.8% | [62] |
| VUV 2/Fe/PMS 3 process | Reaction Time = 60 min [MET]0 = 50 mg/L [Fe] = 0.05 mg/L [PMS] = 20 mg/L pH = 6.3 | 99% | [63] |
| Ozonation/UV/H2O2 | Reaction Time = 10 min [MET]0 = 4 mg/L UV Intensity = 7 W [H2O2] = 250 mg/L pH = 7 O3 Rate = 100 mg/h | 100% | [64] |
| PMS–gingerbread ingredient-derived carbon-assembled CNT 4 foam as the catalyst | Reaction Time = 120 min [MET]0 = 7.8 mg/L Catalyst = Gingerbread ingredient-derived carbon-assembled CNT foam Oxidant = PMS | 68% | [65] |
| Heterogeneous photocatalytic oxidation—Fe3O4@rGO@ZnO/Ag-NPs catalyst | Reaction Time = 60 min [MET]0 = 20 mg/L [FGZ10Ag8] = 1 g/L pH = 5.4 | 100% | [66] |
| Galvanostatic electrolysis | Reaction Time = 60 min [Na2SO4] = 2000 mg/L [NaCl] = 800 mg/L pH = 2 T = 343.15 K | 99.9% | [67] |
| Photocatalysis/PEDOT 5 | Reaction Time = 60 min [MET]0 = 1 mg/L [PEDOT] = 0.5 g/L pH = 5.6 UV-B region | >99% | [68] |
| UV/sulfite process | Reaction Time = 30 min [MET]0 = 15 mg/L [Sulfite reagent] = 10 mM pH = 6 | 97% | [69] |
| Photocatalysis with La-doped tungsten trioxide (WO3) NPs 6 | Reaction Time = 150 min [MET]0 = 200 mg/L [La-doped WO3] = 150 mg/L | 93% | [70] |
| Advanced Oxidation Processes | Identified By-Products | Reference |
|---|---|---|
| TiO2-based photocatalysis | Ammonium (NH4+-N), nitrite (NO2−-N), nitrate (NO3−-N) | [110] |
| UV/sulfite | 4,2,1-AIMT 1, methylbiguanide, metformin hydroperoxide, 2,4-AMT 2 | [69] |
| Ozonation | NDMA 3, cyanamide | [111] |
| Advanced oxidation using sulfate radicals | N-cyanoguanidine, N,N-dimethyl-urea, N,N-dimethyl-cyanamide, N,N-dimethyl-formamide, glycolonitrile, guanidine | [61] |
| Photocatalysis with PEDOT | methylbiguanide, 4,2,1-AIMT | [68] |
| Photocatalysis and ozonation | Guanidine, aminoguanidine, biguanidine | [58] |
| Gamma radiolysis | Metformin hydroperoxide, methylbiguanide, 2,4-AMT, 4,2,1-AIMT | [112] |
| Heterogeneous photocatalytic oxidation—Fe3O4@rGO@ZnO/Ag-NPs catalyst | Guanidine, N,N-dimethylformimidamide, N-carbamimidoylformimidamide, 1,1-dimethylguanidine, N-(N,N-dimethylcarbamimidoyl)formimidamide | [66] |
| Photocatalysis—TiO2/ZrO2 | Guanylurea, MBG 4, diMTF 5, MTFOOH 6, 4,2,1-AIMT | [55] |
| Oxy-cracking | NH3, monomethylamine (MMA) and dimethylamine (DMA), NDMA, DMF 7, DMU 8, DMG 9, hydroxyacetonitrile, guanylurea | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castañeda-Sánchez, J.M.; Silerio-Vázquez, F.d.J.; Villanueva-Fierro, I.; García-Prieto, J.C.; González-Burciaga, L.A.; Proal-Nájera, J.B. Metformin Degradation by Advanced Oxidation Processes: Performance, Limitations, and Environmental Concerns. Int. J. Mol. Sci. 2025, 26, 5925. https://doi.org/10.3390/ijms26135925
Castañeda-Sánchez JM, Silerio-Vázquez FdJ, Villanueva-Fierro I, García-Prieto JC, González-Burciaga LA, Proal-Nájera JB. Metformin Degradation by Advanced Oxidation Processes: Performance, Limitations, and Environmental Concerns. International Journal of Molecular Sciences. 2025; 26(13):5925. https://doi.org/10.3390/ijms26135925
Chicago/Turabian StyleCastañeda-Sánchez, Jaime M., Felipe de J. Silerio-Vázquez, Ignacio Villanueva-Fierro, Juan Carlos García-Prieto, Luis A. González-Burciaga, and José B. Proal-Nájera. 2025. "Metformin Degradation by Advanced Oxidation Processes: Performance, Limitations, and Environmental Concerns" International Journal of Molecular Sciences 26, no. 13: 5925. https://doi.org/10.3390/ijms26135925
APA StyleCastañeda-Sánchez, J. M., Silerio-Vázquez, F. d. J., Villanueva-Fierro, I., García-Prieto, J. C., González-Burciaga, L. A., & Proal-Nájera, J. B. (2025). Metformin Degradation by Advanced Oxidation Processes: Performance, Limitations, and Environmental Concerns. International Journal of Molecular Sciences, 26(13), 5925. https://doi.org/10.3390/ijms26135925







