Tear Cytokine Changes up to One Year After Allogeneic Hematopoietic Stem Cell Transplant: Effect of Daily Topical Cyclosporine-A 0.1% Emulsion
Abstract
1. Introduction
2. Methods
2.1. Design
2.2. Study Population
2.3. Eligibility
2.4. Study Intervention
2.5. Assessment of Tolerance/Compliance with Ikervis®
2.6. Study Visits
2.7. Clinical Procedures
2.8. Tear Cytokines
2.9. Statistical Analysis
3. Results
3.1. Clinical Symptom Changes
3.2. Clinical Sign Changes
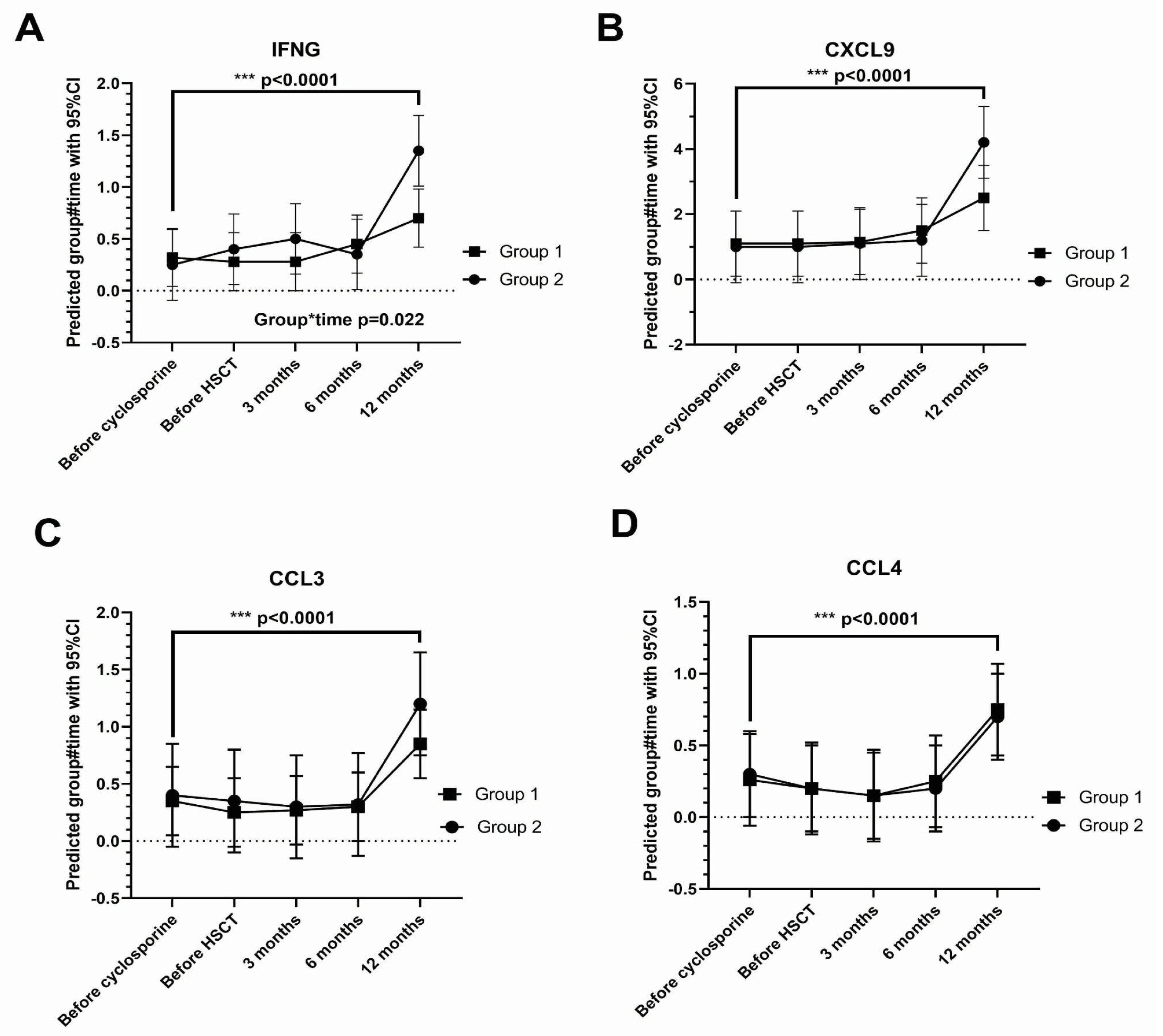
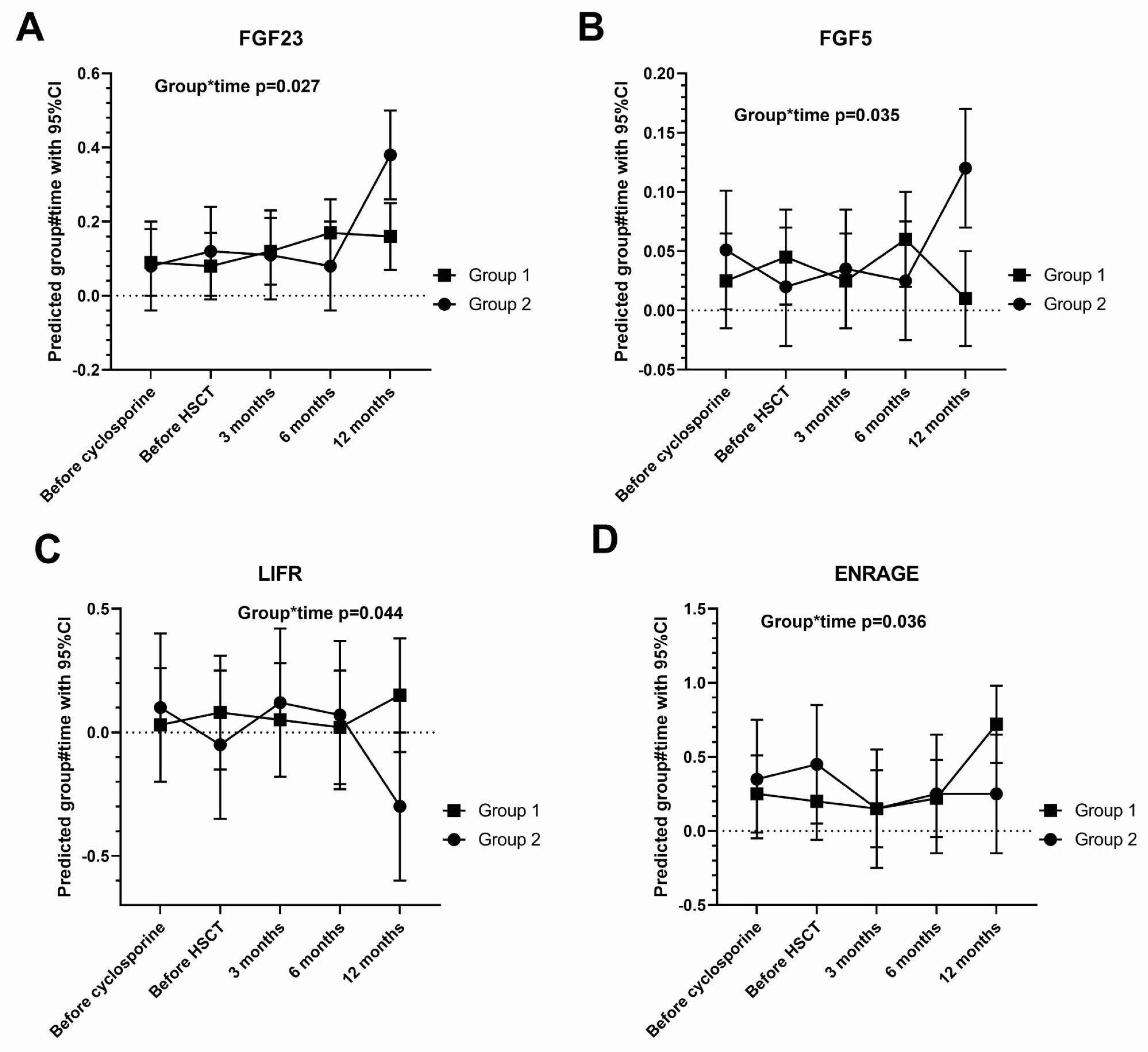
3.3. Tear Cytokine Changes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berchicci, L.; Miserocchi, E.; Iuliano, L.; Bandello, F.; Rabiolo, A.; Marchese, A.; Modorati, G.; Gigliotti, C. Ocular chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation in an Italian referral center. Ocul. Surf. 2018, 16, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Paczesny, S.; Parkman, R.; Cooke, K.R.; Lee, S.; Flowers, M.E.; Griffith, L.M.; Vogelsang, G.; Hakim, F.T.; Russek-Cohen, E.; Hansen, J.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: III. The 2014 Biomarker Working Group Report. Biol. Blood Marrow Transpl. 2015, 21, 780–792. [Google Scholar] [CrossRef] [PubMed]
- Westeneng, A.C.; Verdonck, L.; Hettinga, Y.; Lokhorst, H.; van Dorp, S.; Rothova, A. Ocular graft-versus-host disease after allogeneic stem cell transplantation. Cornea 2010, 29, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Na, K.-S.; Yoo, Y.-S.; Mok, J.W.; Lee, J.W.; Joo, C.-K. Incidence and risk factors for ocular GVHD after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transpl. 2015, 50, 1459–1464. [Google Scholar] [CrossRef]
- Mian, S.I.; De la Parra-Colín, P.; De Melo-Franco, R.; Johnson, C.; Barrientos-Gutierrez, T. Dry Eye Disease Incidence Associated with Chronic Graft-Host Disease: Nonconcurrent Cohort Study, An American Ophthalmological Society Thesis. Trans. Am. Ophthalmol. Soc. 2015, T11, 113. [Google Scholar]
- Ogawa, Y.; Tsubota, K.; Yamada, M.; Wakui, M.; Mashima, Y.; Watanabe, R.; Ono, M.; Oguchi, Y.; Yoshino, M.; Yang, H.-Y. Dry eye after haematopoietic stem cell transplantation. Br. J. Ophthalmol. 1999, 83, 1125–1130. [Google Scholar] [CrossRef]
- Shikari, H.; Antin, J.H.; Dana, R. Ocular graft-versus-host disease: A review. Surv. Ophthalmol. 2013, 58, 233–251. [Google Scholar] [CrossRef]
- Ogawa, Y.; Mori, T.; Yamada, M.; Mashima, Y.; Kuwana, M.; Yamazaki, K.; Oguchi, Y.; Kawakami, Y.; Okamoto, S. Periductal area as the primary site for T-cell activation in lacrimal gland chronic graft-versus-host disease. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1888–1896. [Google Scholar] [CrossRef]
- Townley, J.R.; Dana, R.; Jacobs, D.S. Keratoconjunctivitis sicca manifestations in ocular graft versus host disease: Pathogenesis, presentation, prevention, and treatment. Semin. Ophthalmol. 2011, 26, 251–260. [Google Scholar] [CrossRef]
- Munir, S.Z.; Aylward, J. A Review of Ocular Graft-Versus-Host Disease. Optom. Vis. Sci. 2017, 94, 545–555. [Google Scholar] [CrossRef]
- Tong, L.; Ho, A.; Linn, Y.C.; Hou, A.; Hwang, W.Y.K.; Than, H.; Yeo, S.W.J.; Quek, J.K.S.; Lim, E.W.L.; Lim, F.L.W.I.; et al. Conjunctival T Cell Profile in Allogeneic Hematopoietic Stem Cell Transplant Patients after Instilling Topical Cyclosporine-A 0.1% Cationic Emulsion. Ophthalmol Ther 2023, 12, 1547–1567. [Google Scholar] [CrossRef] [PubMed]
- Lelli, G.J., Jr.; Farjo, A.Q.; Nairus, T.M.; Musch, D.C.; Mian, I.S.; Gupta, A.B. Ophthalmic cyclosporine use in ocular GVHD. Cornea 2006, 25, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Kiang, E.; Tesavibul, N.; Yee, R.; Kellaway, J.; Przepiorka, D. The use of topical cyclosporin A in ocular graft-versus-host-disease. Bone Marrow Transpl. 1998, 22, 147–151. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Y.; Okada, N.; Kawai, M.; Shimazaki, J.; Ogawa, Y.; Uchino, M.; Tatematsu, Y.; Tsubota, K.; Igarashi, A.; Dogru, M.; et al. Ocular surface and tear functions after topical cyclosporine treatment in dry eye patients with chronic graft-versus-host disease. Bone Marrow Transpl. 2008, 41, 293–302. [Google Scholar] [CrossRef]
- Malta, J.B.; Rhoades, W.; Sugar, A.; Shtein, R.M.; Musch, D.C.; Soong, H.K.; Mian, I.S. Treatment of ocular graft-versus-host disease with topical cyclosporine 0.05%. Cornea 2010, 29, 1392–1396. [Google Scholar] [CrossRef]
- Chun, Y.H.; Beak, J.U.; Kim, H.-S.; Na, K.-S. Topical Cyclosporine Pretreatment of Ocular Surface in Allogeneic Hematopoietic Stem Cell Transplant Recipients. J. Ocul. Pharmacol. Ther. 2018, 34, 628–632. [Google Scholar] [CrossRef]
- Cantú-Rodríguez, O.G.; Gómez-Almaguer, D.; Jaime-Pérez, J.C.; González-Llano, O.; Mancías-Guerra, C.; Herrera-Rojas, M.A.; Vázquez-Mellado, A.; León, A.G.-D.; Garza-Acosta, A.C.; González-Cantú, G.A.; et al. Cyclosporine A for the Prevention of Ocular Graft versus Host Disease in Allogeneic Hematopoietic Stem Cell Transplant Recipients Is Safe and Feasible. Acta Haematol. 2020, 143, 425–431. [Google Scholar] [CrossRef]
- Ngo, W.; Blackie, C.; Simpson, T.; Keir, N.; Situ, P.; Korb, D. Psychometric properties and validation of the Standard Patient Evaluation of Eye Dryness questionnaire. Cornea 2013, 32, 1204–1210. [Google Scholar] [CrossRef]
- Fenner, B.J.; Tong, L. Corneal staining characteristics in limited zones compared with whole cornea documentation for the detection of dry eye subtypes. Investig. Ophthalmol. Vis. Sci. 2013, 54, 8013–8019. [Google Scholar] [CrossRef]
- Terry, R.L.; Grant, T.; Holden, B.A.; Cornish, R.; Back, A. CCLRU standards for success of daily and extended wear contact lenses. Optom. Vis. Sci. 1993, 70, 234–243. [Google Scholar] [CrossRef]
- Tong, L.; Teo, C.H.Y.; Lee, R.K.J. Spatial Distribution of Noninvasive Break Up Times and Clinical Relevance in Healthy Participants and Mild Dry Eye. Transl. Vis. Sci. Technol. 2019, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Sun, X.; Qian, T.; Wei, A.; Xu, J.; Cui, X.; Li, Y.; Wang, W.M. Assessment of tear film stability in dry eye with a newly developed keratograph. Cornea 2013, 32, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Sun, X.; Hong, J.; Xu, J.; Cui, X. Assessment of Bulbar Redness with a Newly Developed Keratograph. Optom. Vis. Sci. 2015, 92, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Yeo, S.; Aung, H.T.; Tong, L. Agreement of noninvasive tear break-up time measurement between Tomey RT-7000 Auto Refractor-Keratometer and Oculus Keratograph 5M. Clin. Ophthalmol. 2016, 10, 1785–1790. [Google Scholar]
- Versura, P.; Campos, E.C. TearLab® Osmolarity System for diagnosing dry eye. Expert Rev. Mol. Diagn. 2013, 13, 119–129. [Google Scholar] [CrossRef]
- Lemp, M.A.; Foulks, G.N.; Geffen, D.; Sullivan, B.D.; Pepose, J.S.; Tauber, J.; del Castillo, J.M.B.; Baudouin, C. Tear osmolarity in the diagnosis and management of dry eye disease. Am. J. Ophthalmol. 2011, 151, 792–798.e1. [Google Scholar] [CrossRef]
- Ambaw, Y.A.; Torta, F.; Ji, S.; Chao, C.; Wenk, M.R.; Raida, M. Tear eicosanoids in healthy people and ocular surface disease. Sci. Rep. 2018, 8, 11296. [Google Scholar] [CrossRef]
- Wik, L.; Pettersson, E.; Grundberg, I.; Assarsson, E.; Lundberg, M.; Nordberg, N.; Henriksson, S.; Broberg, J.; Björkesten, J.; Westerberg, C. Proximity Extension Assay in Combination with Next-Generation Sequencing for High-throughput Proteome-wide Analysis. Mol. Cell. Proteomics 2021, 20, 100168. [Google Scholar] [CrossRef]
- Eckstein, L.A.; Van Quill, K.R.; Bui, S.K.; Uusitalo, M.S.; O’Brien, J.M. Cyclosporin a inhibits calcineurin/nuclear factor of activated T-cells signaling and induces apoptosis in retinoblastoma cells. Investig. Ophthalmol. Vis. Sci. 2005, 46, 782–790. [Google Scholar] [CrossRef]
- Paavonen, T.; Häyry, P. Effect of cyclosporin A on T-dependent and T-independent immunoglobulin synthesis in vitro. Nature 1980, 287, 542–544. [Google Scholar] [CrossRef]
- Williams, G.P.; Oswal, K.S.; Tomlins, P.J.; Curnow, S.J.; Rauz, S.; Barry, R.J.; Denniston, A.K.O. The dominant human conjunctival epithelial CD8αβ+ T cell population is maintained with age but the number of CD4+ T cells increases. Age 2012, 34, 1517–1528. [Google Scholar] [CrossRef] [PubMed]
- Knop, N.; Knop, E. Conjunctiva-associated lymphoid tissue in the human eye. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1270–1279. [Google Scholar]
- Bose, T.; Lee, R.; Hou, A.; Tong, L.; Chandy, K.G. Tissue resident memory T cells in the human conjunctiva and immune signatures in human dry eye disease. Sci. Rep. 2017, 7, 45312. [Google Scholar] [CrossRef] [PubMed]
- Arnous, R.; Cunningham, A.L.; Sandgren, K.; Carnt, N.; Arshad, S.; White, A. Tissue resident memory T cells inhabit the deep human conjunctiva. Sci. Rep. 2022, 12, 6077. [Google Scholar] [CrossRef]
- Hingorani, M.; Metz, D.; Lightman, S.L. Characterisation of the normal conjunctival leukocyte population. Exp. Eye Res. 1997, 64, 905–912. [Google Scholar] [CrossRef]
- Power, W.J.; Mullaney, P.; Farrell, M.; Collum, L.M. Effect of topical cyclosporin A on conjunctival T cells in patients with secondary Sjögren’s syndrome. Cornea 1993, 12, 507–511. [Google Scholar] [CrossRef]
- Kunert, K.S.; Tisdale, A.S.; Stern, M.E.; Smith, J.A.; Gipson, I.K. Analysis of topical cyclosporine treatment of patients with dry eye syndrome: Effect on conjunctival lymphocytes. Arch. Ophthalmol. 2000, 118, 1489–1496. [Google Scholar] [CrossRef]
- Hingorani, M.; Calder, V.L.; Buckley, R.J.; Lightman, S. The immunomodulatory effect of topical cyclosporin A in atopic keratoconjunctivitis. Investig. Ophthalmol. Vis. Sci. 1999, 40, 392–399. [Google Scholar]
- Zhang, X.; De Paiva, C.S.; Su, Z.; Volpe, E.A.; Li, D.-Q.; Pflugfelder, S.C. Topical interferon-gamma neutralization prevents conjunctival goblet cell loss in experimental murine dry eye. Exp. Eye Res. 2014, 118, 117–124. [Google Scholar] [CrossRef]
- Zhang, X.; Li, D.-Q.; Farley, W.J.; Corrales, R.M.; McClellan, A.J.; Pflugfelder, S.C.; Volpe, E.A.; Chen, W.; De Paiva, C.S. Interferon-γ exacerbates dry eye-induced apoptosis in conjunctiva through dual apoptotic pathways. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6279–6285. [Google Scholar] [CrossRef]
- Ko, J.H.; Kim, S.; Ryu, J.S.; Song, H.J.; Oh, J.Y. Interferon-γ elicits the ocular surface pathology mimicking dry eye through direct modulation of resident corneal cells. Cell Death Discov. 2023, 9, 209. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.C.; Wong, C.Y.; Zeng, W.; Ang, C.-S.; Downie, L.E.; Mifsud, E.J.; Vingrys, A.J. Tear Interferon-Gamma as a Biomarker for Evaporative Dry Eye Disease. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4824–4830. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Shimizu, E.; Tsubota, K. Interferons and Dry Eye in Sjögren’s Syndrome. Int. J. Mol. Sci. 2018, 19, 3548. [Google Scholar] [CrossRef] [PubMed]
- VanDerMeid, K.R.; Su, S.P.; Ward, K.W.; Zhang, J.-Z. Correlation of tear inflammatory cytokines and matrix metalloproteinases with four dry eye diagnostic tests. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1512–1518. [Google Scholar] [CrossRef]
- Suzuki, M.; Ye, F.; Godbold, J.; Vallabhajosyula, M.; Elfassy, T.; Massingale, M.L.; Asbell, P.A. Tear osmolarity as a biomarker for dry eye disease severity. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4557–4561. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, H. Upregulation of the IL-33/ST2 pathway in dry eye. Mol. Vis. 2019, 25, 583–592. [Google Scholar]
- Roda, M.; Reggiani, M.L.B.; Corazza, I.; Pellegrini, M.; Giannaccare, G.; Versura, P.; Taroni, L. Dry eye disease and tear cytokine levels-A meta-analysis. Int. J. Mol. Sci. 2020, 21, 3111. [Google Scholar] [CrossRef]
- Wu, J.; Li, G.-J.; Niu, J.; Wen, F.; Han, L. Analyze interleukin-1β, interleukin-6, and tumor necrosis factor-α levels in dry eye and the therapeutic effect of cyclosporine A. World J. Clin. Cases 2024, 12, 5665–5672. [Google Scholar] [CrossRef]
- Yu, H.; Zeng, W.; Zhao, G.; Hong, J.; Feng, Y. Response of tear cytokines following intense pulsed light combined with meibomian gland expression for treating meibomian gland dysfunction-related dry eye. Front. Endocrinol. 2022, 13, 973962. [Google Scholar] [CrossRef]
- Joossen, C.; Baán, A.; Moreno-Cinos, C.; Joossens, J.; Cools, N.; Lanckacker, E.; Moons, L.; Lemmens, K.; Lambeir, A.M.; Fransen, E.; et al. A novel serine protease inhibitor as potential treatment for dry eye syndrome and ocular inflammation. Sci. Rep. 2020, 10, 17268. [Google Scholar] [CrossRef]
- Schultz, K.R.; Miklos., D.B.; Fowler, D.; Cooke, K.; Shizuru, J.; Zorn, E.; Holler, E.; Ferrara, J.; Shulman, H.; Lee, S.J.; et al. Toward biomarkers for chronic graft-versus-host disease: National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: III. Biomarker Working Group Report. Biol. Blood Marrow Transpl. 2006, 12, 126–137. [Google Scholar] [CrossRef]


| Interleukin | Beta (Coefficient) | p-Value | 95% Confidence Interval (CI) | |
|---|---|---|---|---|
| Lower End | Upper End | |||
| BETANGF | 281.7 | 0.04 | 19.71 | 543.69 |
| IL12B | 83.95 | 0.01 | 24.33 | 143.57 |
| TRANCE | 64.58 | 0.04 | 4.07 | 125.09 |
| TNFSF14 | 32.96 | 0 | 11.32 | 54.6 |
| CCL3 | 24.51 | 0.01 | 7.26 | 41.77 |
| CCL4 | 23.07 | 0.01 | 5.45 | 40.68 |
| IL1α | 20.24 | 0.01 | 5.95 | 34.53 |
| TGFα | 13.62 | 0.03 | 1.17 | 26.07 |
| MMP1 | 5.83 | 0.01 | 1.33 | 10.33 |
| CCL11 | −77.52 | 0.02 | −140.51 | −14.53 |
| SLAMF1 | −71.25 | 0.02 | −132.85 | −9.65 |
| IL7 | −62.41 | 0.02 | −112.88 | −11.93 |
| IL10RA | −46.35 | 0.03 | −87.58 | −5.12 |
| Cytokine | p-Value (from Linear Mixed Model Regression) |
|---|---|
| CXCL9 | <0.0001 |
| CCL4 | <0.0001 |
| CCL3 | <0.0001 |
| IFN-gamma | <0.0001 |
| CXCL11 | 0.0004 |
| CXCL10 | 0.0005 |
| TRANCE | 0.0014 |
| ADA | 0.0018 |
| IL12B | 0.0022 |
| FGF23 | 0.0024 |
| CD5 | 0.0024 |
| IL2 | 0.0037 |
| IL2RB | 0.0042 |
| Trail | 0.0068 |
| NRTN | 0.0069 |
| TNFSF9 | 0.0072 |
| UPA | 0.0083 |
| TGFAlpha | 0.0086 |
| TNFSF14 | 0.0106 |
| IL18 | 0.0115 |
| IL18R1 | 0.0122 |
| IL22RA1 | 0.0133 |
| IL8 | 0.0176 |
| CDCP1 | 0.0182 |
| CD40 | 0.0183 |
| IL20 | 0.0219 |
| SIRT2 | 0.0252 |
| HGF | 0.0261 |
| CASP8 | 0.0275 |
| CSF1 | 0.0285 |
| IL6 | 0.0304 |
| STAMBP | 0.0308 |
| CCL20 | 0.0312 |
| EBP1 | 0.032 |
| CXC11 | 0.0336 |
| FLT31 | 0.038 |
| FGF19 | 0.0455 |
| IL1A | 0.0493 |
|
Participant Number | Systemic Drug | Dosage | ||
|---|---|---|---|---|
| After HSCT | 3 Months Post-HSCT | 6 Months Post-HSCT | ||
| 1 | Predisolone | - | - | 5 mg (EOD, 2×/wk) |
| Mycophenolic acid EC (Myfortic) | 720 mg, 360 mg | 180 mg | - | |
| Tacrolimus | nil | - | - | |
| Ciclosporin (NEORAL) | 150 mg, 125 mg, 175 mg, 125 mg, 75 mg, 10 mg, 3 mg | 50 mg, 75 mg, 50 mg | 40 mg, 25 mg | |
| 2 | Tacrolimus | 1 mg | - | - |
| 3 | Ciclosporin (NEORAL) | 125 mg | 75 mg, 50 mg | - |
| 4 | Mycophenolic acid EC (Myfortic) | 720 mg, 540 mg, 360 mg | - | - |
| Ciclosporin (NEORAL) | 200 mg, 225 mg, 125 mg | 50 mg, 25 mg | - | |
| 5 | Predisolone | 50 mg, 40 mg | 10 mg, 25 mg | - |
| Mycophenolic acid EC (Myfortic) | 720 mg | - | - | |
| Tacrolimus | 1 mg | 0.5 mg (4×/wk), 0.2 mg (2×/wk) | - | |
| 6 | Dexamethasone | 5 mg | 4 mg | - |
| Mycophenolic acid EC (Myfortic) | 720 mg | - | - | |
| Ciclosporin (NEORAL) | 150 mg, 50 mg, 100 mg | 75 mg | - | |
| 7 | Mycophenolic acid EC (Myfortic) | 360 mg | 180 mg, 360 mg | - |
| Ciclosporin (NEORAL) | 250 mg, 100 mg, 25 mg | - | - | |
| 8 | Mycophenolic acid EC (Myfortic) | 720 mg | - | - |
| Ciclosporin (NEORAL) | 175 mg, 50 mg | 50 mg, 25 mg, 25 mg (3×/wk) | - | |
| Participant Number | Systemic Drug | Dosage | ||
|---|---|---|---|---|
| After HSCT | 3 Months Post-HSCT | 6 Months Post-HSCT | ||
| 9 | Mycophenolic acid EC (Myfortic) | - | 720 mg, 540 mg | 360 mg |
| Tacrolimus | 1 mg | - | - | |
| 10 | Predisolone | 60 mg, 20 mg, 15 mg, 10 mg | 10 mg, 5 mg, 5 mg (EOD) | 10 mg, 5 mg |
| Mycophenolic acid EC (Myfortic) | 360 mg, 180 mg, 180 mg EOD, 180 mg (2·/week) | - | - | |
| Tacrolimus | 2.5 mg, 3 mg, 2 mg, 0.5 mg, 1.5 mg | 1 mg, 0.5 mg | - | |
| 11 | Predisolone | 30 mg, 20 mg, 10 mg | - | 10 mg, 5 mg |
| Tacrolimus | 2 mg, 1.5 mg | 1 mg, 0.5 mg (3×/wk), 0.5 mg (2×/wk) | - | |
| 12 | Tacrolimus | 1.5 mg | - | - |
| 13 | Tacrolimus | 1.5 mg, 1 mg | 0.5 mg | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, L.; Liu, Y.-C.; Yeo, S.W.J.; Liu, C.; Lee, I.X.Y.; Linn, Y.C.; Ho, A.; Than, H.; Quek, J.K.S.; Hwang, W.Y.K.; et al. Tear Cytokine Changes up to One Year After Allogeneic Hematopoietic Stem Cell Transplant: Effect of Daily Topical Cyclosporine-A 0.1% Emulsion. Int. J. Mol. Sci. 2025, 26, 5915. https://doi.org/10.3390/ijms26125915
Tong L, Liu Y-C, Yeo SWJ, Liu C, Lee IXY, Linn YC, Ho A, Than H, Quek JKS, Hwang WYK, et al. Tear Cytokine Changes up to One Year After Allogeneic Hematopoietic Stem Cell Transplant: Effect of Daily Topical Cyclosporine-A 0.1% Emulsion. International Journal of Molecular Sciences. 2025; 26(12):5915. https://doi.org/10.3390/ijms26125915
Chicago/Turabian StyleTong, Louis, Yu-Chi Liu, Sharon Wan Jie Yeo, Chang Liu, Isabelle Xin Yu Lee, Yeh Ching Linn, Aloysius Ho, Hein Than, Jeffrey Kim Siang Quek, William Ying Khee Hwang, and et al. 2025. "Tear Cytokine Changes up to One Year After Allogeneic Hematopoietic Stem Cell Transplant: Effect of Daily Topical Cyclosporine-A 0.1% Emulsion" International Journal of Molecular Sciences 26, no. 12: 5915. https://doi.org/10.3390/ijms26125915
APA StyleTong, L., Liu, Y.-C., Yeo, S. W. J., Liu, C., Lee, I. X. Y., Linn, Y. C., Ho, A., Than, H., Quek, J. K. S., Hwang, W. Y. K., Lim, F. L. W. I., & Lim, L. (2025). Tear Cytokine Changes up to One Year After Allogeneic Hematopoietic Stem Cell Transplant: Effect of Daily Topical Cyclosporine-A 0.1% Emulsion. International Journal of Molecular Sciences, 26(12), 5915. https://doi.org/10.3390/ijms26125915






