Effects of C-Ring Structural Differences on the Inhibition of Nε-(Carboxyethyl)lysine in the Methylglyoxal-Lysine System by Flavonoids
Abstract
1. Introduction
2. Results and Discussion
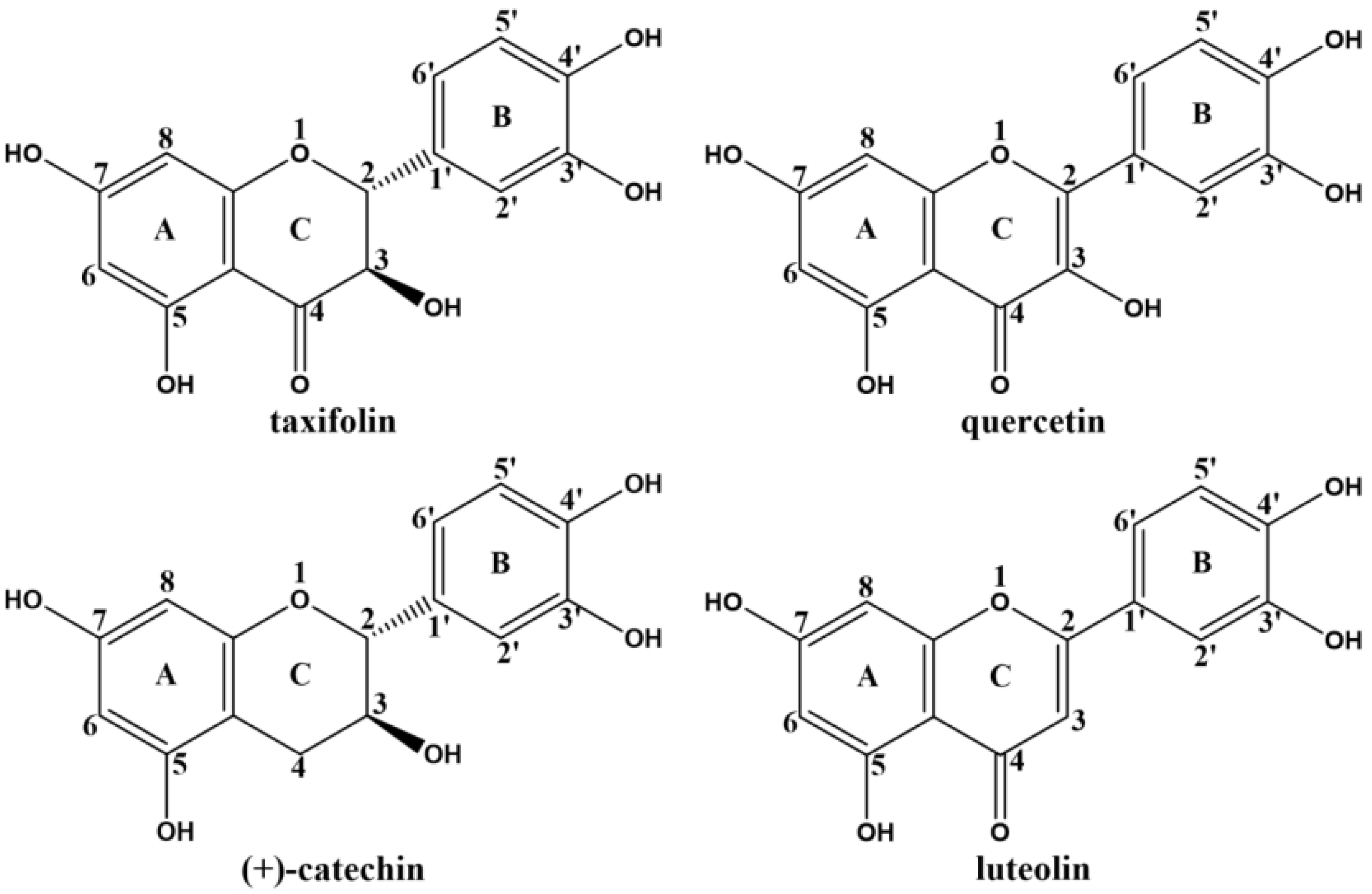
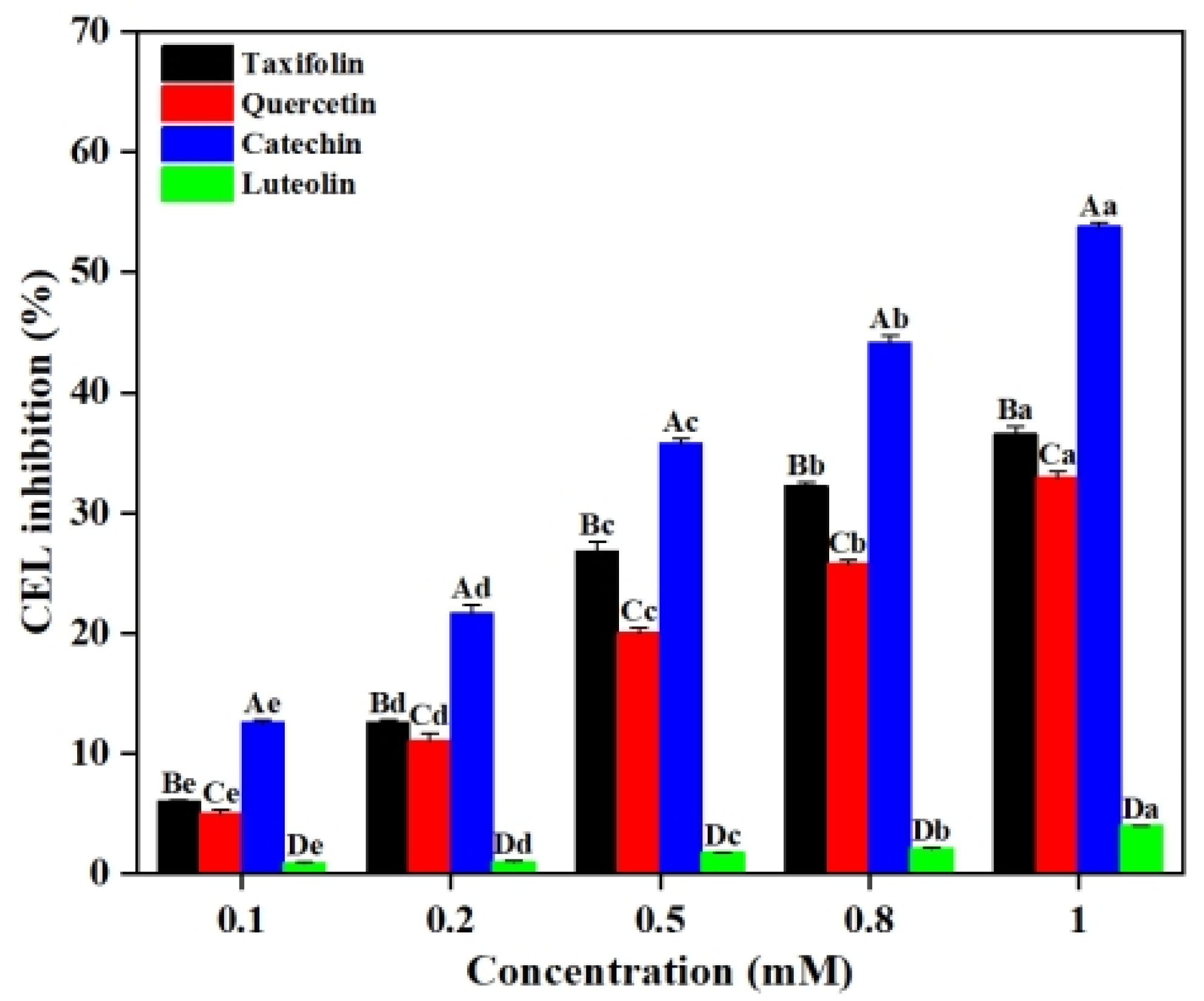
2.1. Effects of Flavonoids on the Formation of CEL
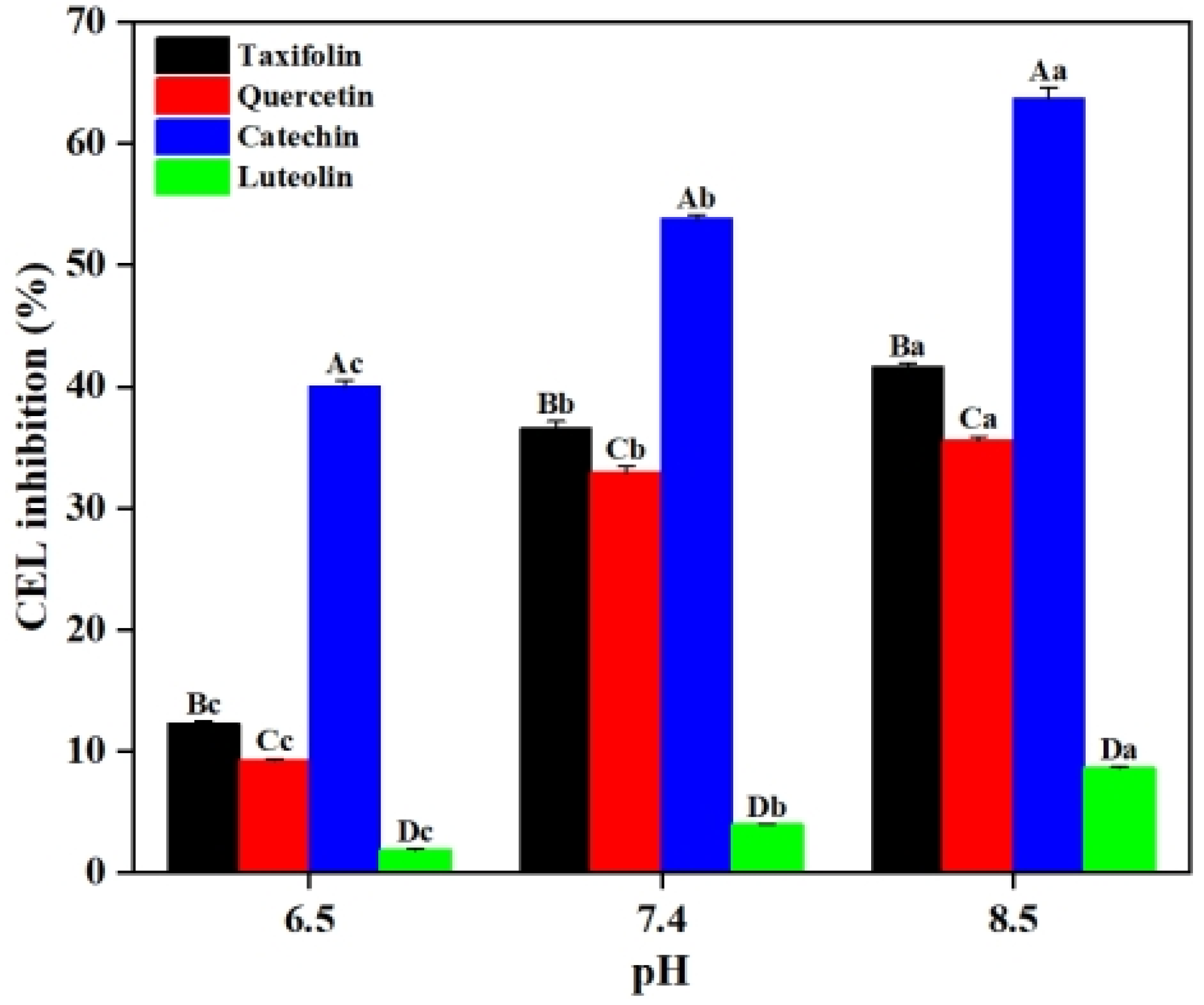
2.2. Effects of pH on CEL Formation
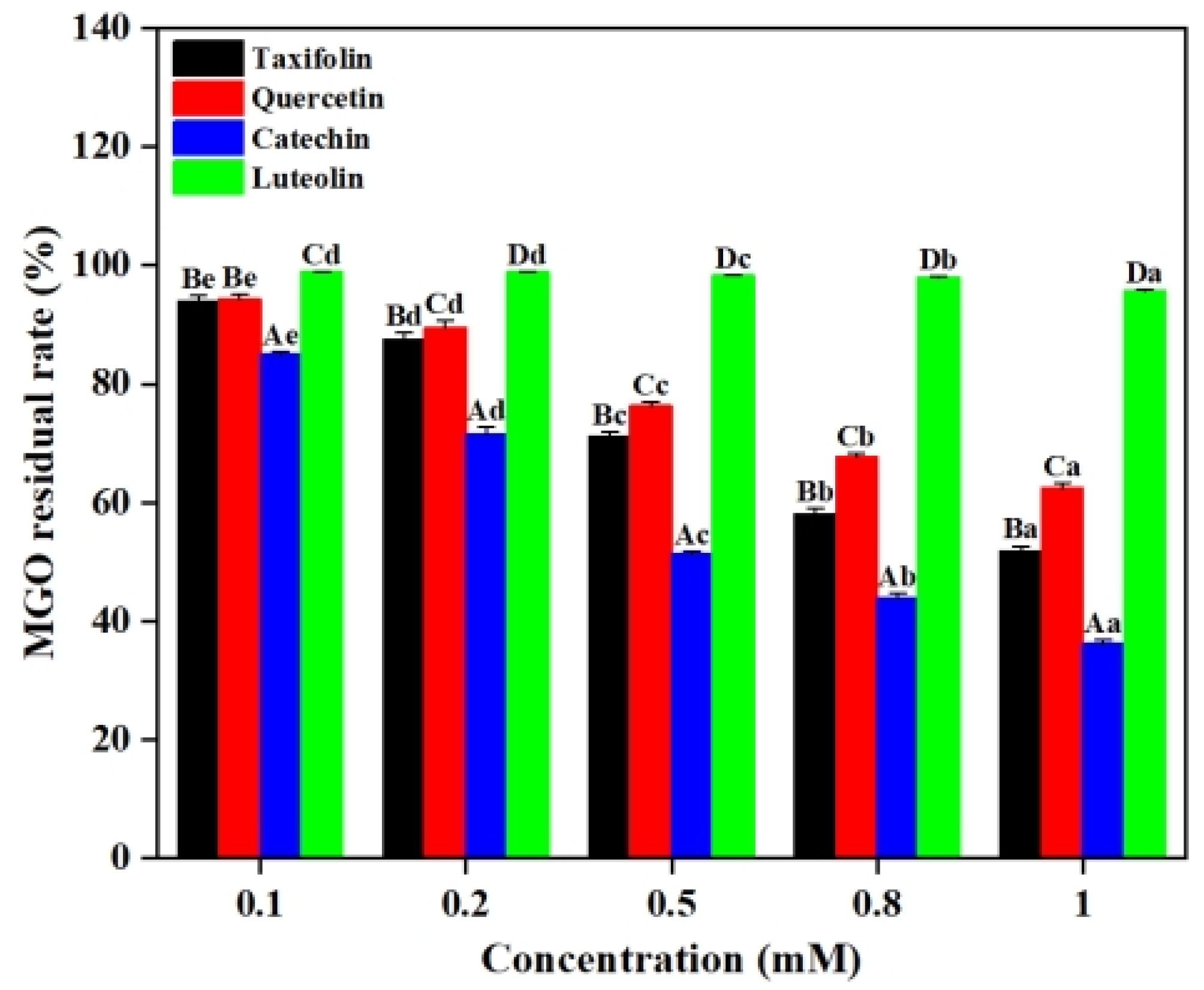
2.3. Effect of Flavonoids on MGO
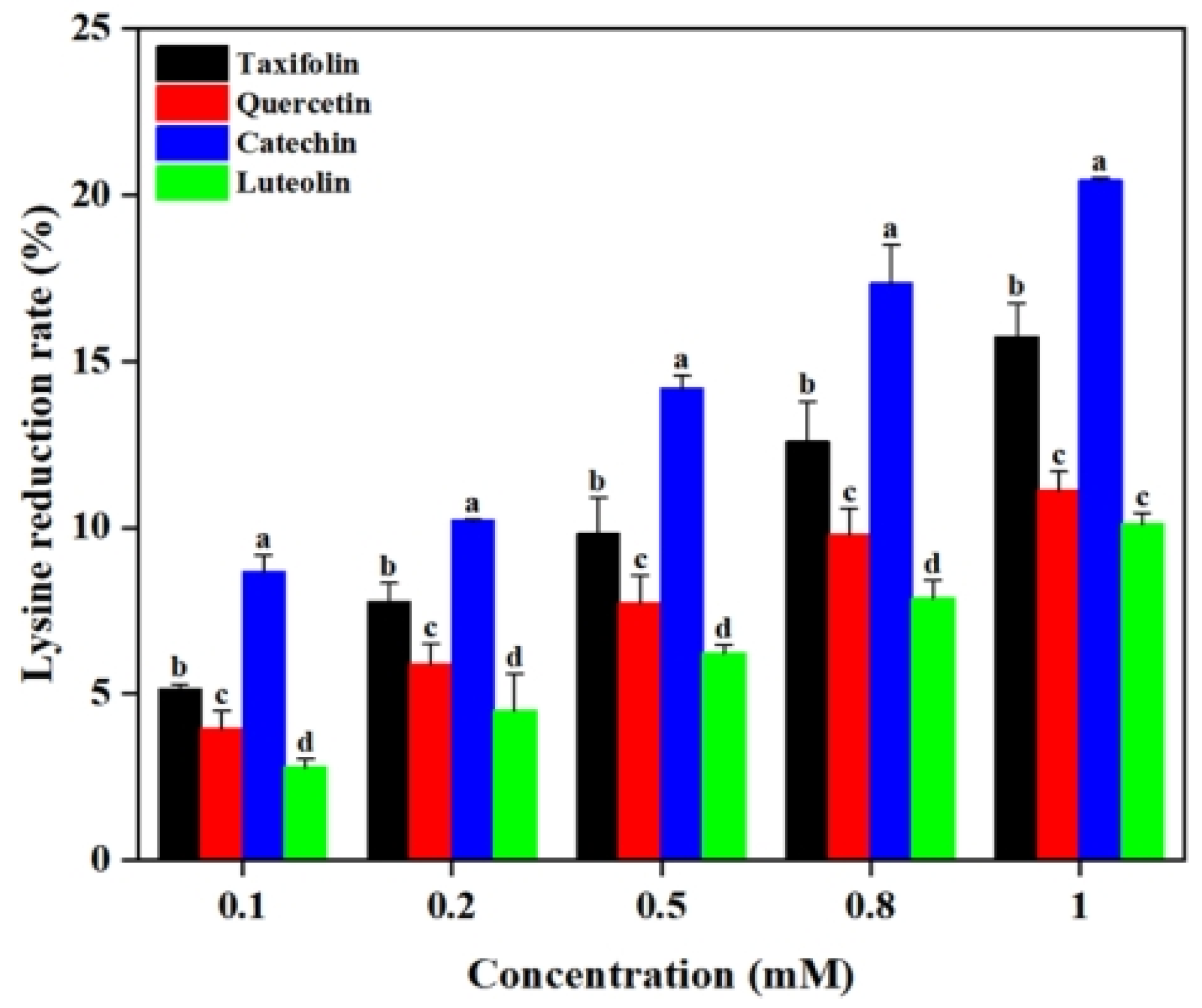
2.4. Effect of Flavonoids on Lysine
2.5. Interactions Between Flavonoids and MGO
2.5.1. Capture Capacity of Flavonoids on MGO
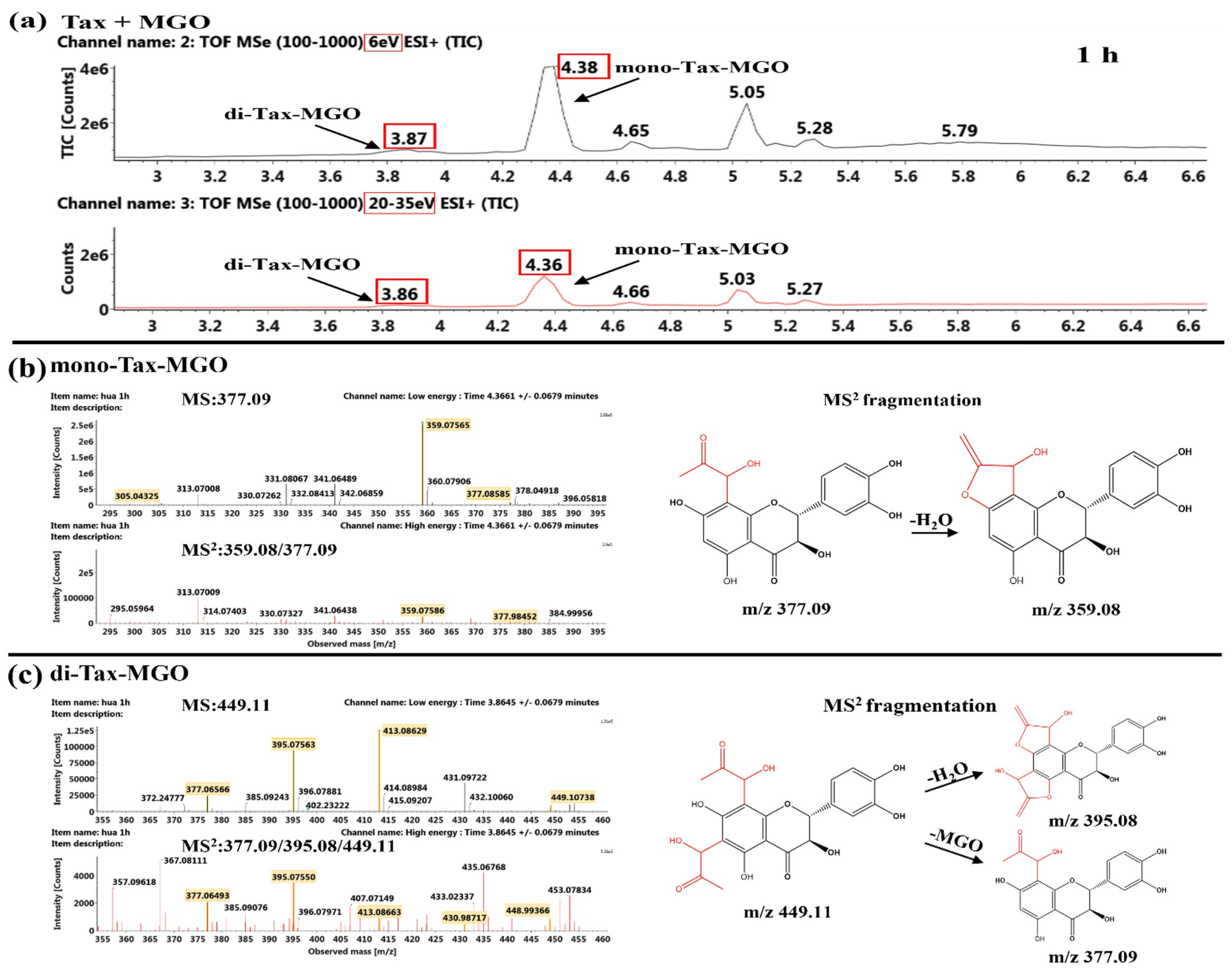
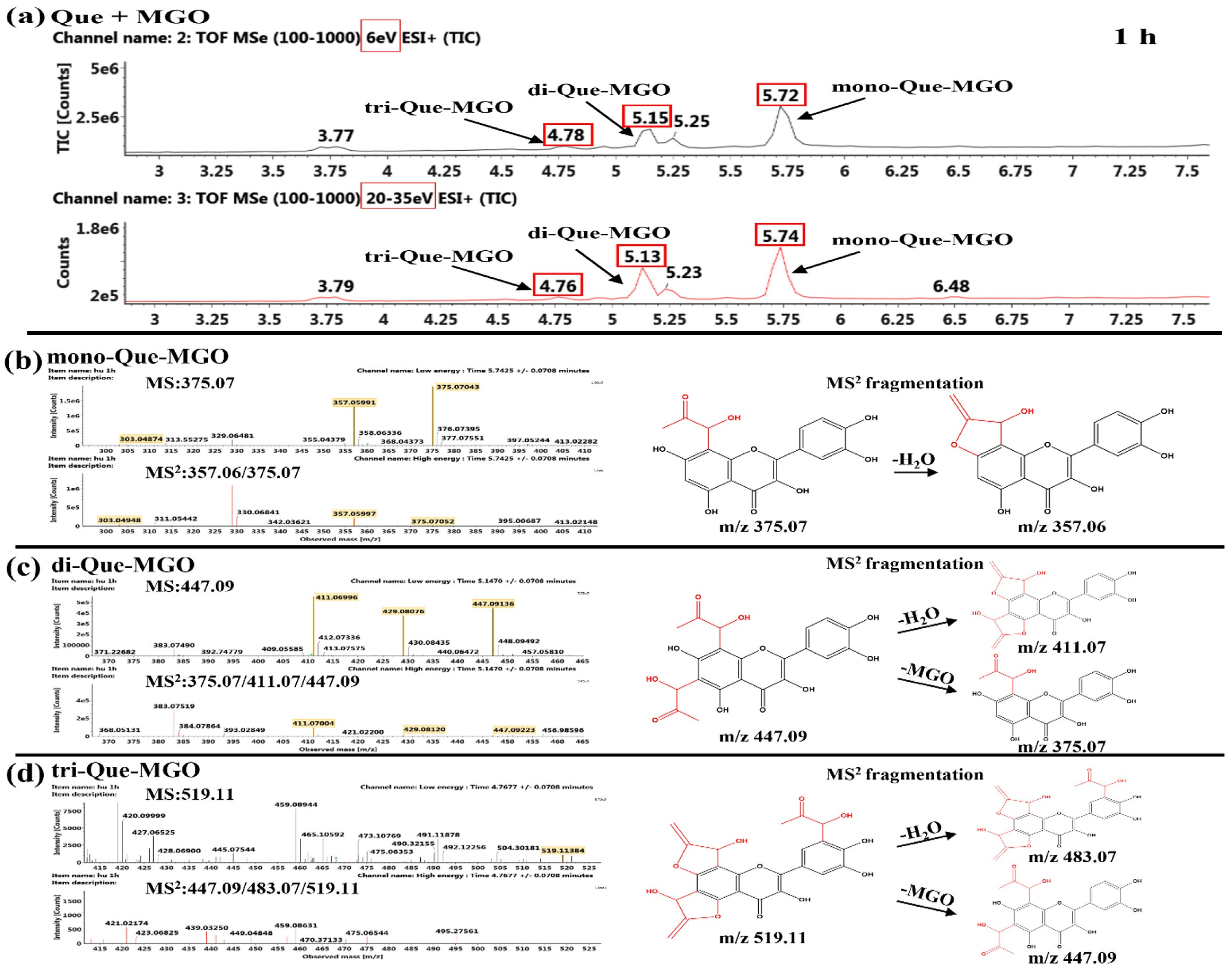
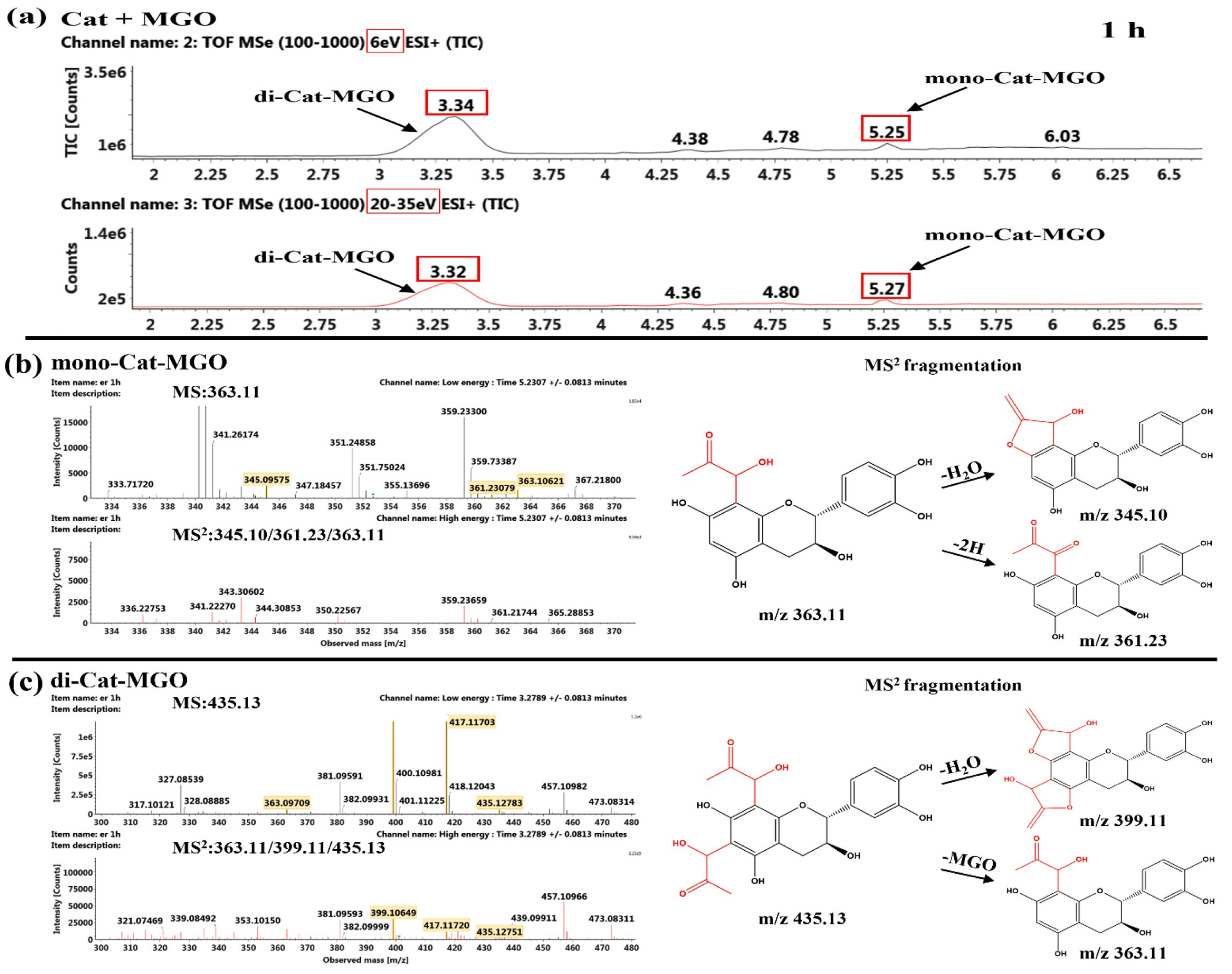
2.5.2. Product Identification of Flavonoids and MGO
Identification of the Reaction Products of Taxifolin and MGO
Identification of the Reaction Products of Quercetin and MGO
Identification of the Reaction Products of Catechin and MGO
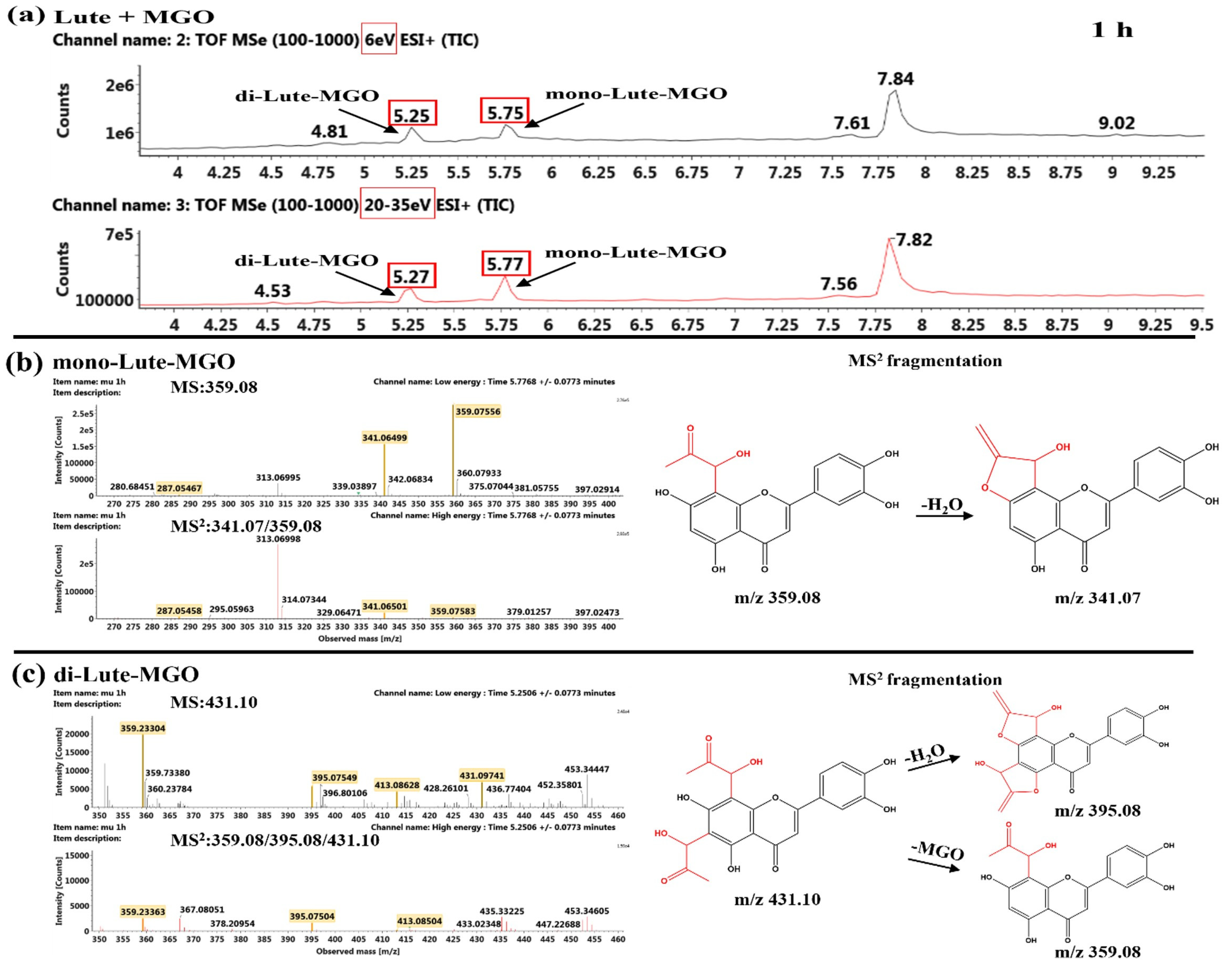
Identification of the Reaction Products of Luteolin and MGO
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Preparation of MGO-Lys-Flavonoid Model
3.3. Determination of CEL
3.4. Effects of pH on the Formation of CEL
3.5. Determination of MGO
3.6. Determination of Lysine
3.7. Determination of MGO Capture Capacity by Flavonoids
3.8. Identification of the Flavonoid-MGO Adducts
3.9. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, S.; Dong, X.; Zhang, Y.; Chen, Y.; Yu, Y.; Huang, M.; Zheng, Y. Formation of advanced glycation end products in raw and subsequently boiled broiler muscle: Biological variation and effects of postmortem ageing and storage. Food Sci. Hum. Wellness 2022, 11, 255–262. [Google Scholar] [CrossRef]
- Uribarri, J.; Woodruff, S.; Goodman, S.; Cai, W.; Chen, X.; Pyzik, R.; Yong, A.; Striker, G.E.; Vlassara, H. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J. Am. Diet. Assoc. 2010, 110, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Jin, X.; Li, Y.; Yu, Y.; He, L.; Liu, R. Effects of A-type oligomer procyanidins on protein glycation using two glycation models coupled with spectroscopy, chromatography, and molecular docking. Food Res. Int. 2022, 155, 111068. [Google Scholar] [CrossRef]
- Zhou, Y.; Duan, H.; Chen, J.; Ma, S.; Wang, M.; Zhou, X. The mechanism of in vitro non-enzymatic glycosylation inhibition by Tartary buckwheat’s rutin and quercetin. Food Chem. 2023, 406, 134956. [Google Scholar] [CrossRef]
- Chalotra, R.; Gupta, T.; Chib, S.; Amanat, M.; Kumar, P.; Singh, R. Treatment of diabetic complications: Do flavonoids holds the keys? Crit. Rev. Food Sci. Nutr. 2024, 64, 11091–11112. [Google Scholar] [CrossRef] [PubMed]
- Juca, M.M.; Sales Cysne Filho, F.M.; de Almeida, J.C.; Mesquita, D.d.S.; de Moraes Barriga, J.R.; Cilene Ferreira, D.K.; Barbosa, T.M.; Vasconcelos, L.C.; Almeida Moreira Leal, L.K.; Ribeiro Honorio Junior, J.E.; et al. Flavonoids: Biological activities and therapeutic potential. Nat. Prod. Res. 2020, 34, 692–705. [Google Scholar] [CrossRef]
- Rakha, A.; Umar, N.; Rabail, R.; Butt, M.S.; Kieliszek, M.; Hassoun, A.; Aadil, R.M. Anti-inflammatory and anti-allergic potential of dietary flavonoids: A review. Biomed. Pharmacother. 2022, 156, 113945. [Google Scholar] [CrossRef]
- Rana, J.N.; Mumtaz, S. Prunin: An emerging anticancer flavonoid. Int. J. Mol. Sci. 2025, 26, 2678. [Google Scholar] [CrossRef]
- Ling, Y.; Zhang, L.; Chen, G.; Euston, S.R.; Peng, B.; Zhang, Z. Effects of C-ring structures on the formations of flavonoid semiquinones and their binding behavior with β-lactoglobulin as revealed by experimental and modeling approaches. Int. J. Biol. Macromol. 2025, 291, 139104. [Google Scholar] [CrossRef]
- Zhou, Q.; Cheng, K.; Xiao, J.; Wang, M. The multifunctional roles of flavonoids against the formation of advanced glycation end products (AGEs) and AGEs-induced harmful effects. Trends Food Sci. Technol. 2020, 103, 333–347. [Google Scholar] [CrossRef]
- Li, L.-F.; Wang, M.-D.; Zhang, C.-Y.; Jin, M.-Y.; Chen, H.-L.; Luo, H.; Hou, T.-Y.; Zhang, Z.-J.; Li, H. Influence of hydroxyl substitution on the inhibition of flavonoids in advanced glycation end-products formation in glucose-lysine-arginine Maillard reaction models. Food Res. Int. 2025, 207, 116068. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Cui, H.; Feng, L.; Yu, J.; Hayat, K.; Jia, C.; Zhang, X.; Ho, C.-T. Effect of the C-ring structure of flavonoids on the yield of adducts formed by the linkage of the active site at the A-ring and amadori rearrangement products during the maillard intermediate preparation. J. Agric. Food Chem. 2022, 70, 3280–3288. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour, A.; Sadeghi, M.; Miroliaei, M. Role of structural peculiarities of flavonoids in suppressing AGEs generated from HSA/Glucose system. Appl. Biochem. Biotechnol. 2024, 196, 6296–6314. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, X. Structures required of polyphenols for inhibiting advanced glycation end products formation. Curr. Drug Metab. 2013, 14, 414–431. [Google Scholar] [CrossRef]
- Thornalley, P.J.; Battah, S.; Ahmed, N.; Karachalias, N.; Agalou, S.; Babaei-Jadidi, R.; Dawnay, A. Quantitative screening of advanced glycation endproducts in cellular and extracellular proteins by tandem mass spectrometry. Biochem. J. 2003, 375, 581–592. [Google Scholar] [CrossRef]
- Sugawa, H.; Ikeda, T.; Tominaga, Y.; Katsuta, N.; Nagai, R. Rapid formation of Nε-(carboxymethyl)lysine (CML) from ribose depends on glyoxal production by oxidation. RSC Chem. Biol. 2024, 5, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, L.; Lund, M.N.; Li, B.; Hu, Y.; Zhang, X. Reduction of Nε-(carboxymethyl) lysine by (−)-epicatechin and (−)-epigallocatechin gallate: The involvement of a possible trapping mechanism by catechin quinones. Food Chem. 2018, 266, 427–434. [Google Scholar] [CrossRef]
- Zhu, H.; Poojary, M.M.; Andersen, M.L.; Lund, M.N. Effect of pH on the reaction between naringenin and methylglyoxal: A kinetic study. Food Chem. 2019, 298, 125086. [Google Scholar] [CrossRef]
- Jia, W.; Guo, A.; Zhang, R.; Shi, L. Mechanism of natural antioxidants regulating advanced glycosylation end products of Maillard reaction. Food Chem. 2023, 404, 134541. [Google Scholar] [CrossRef]
- Ames, J.M. Determination of Nε-(carboxymethyl)lysine in foods and related systems. In Maillard Reaction: Recent Advances in Food and Biomedical Sciences; Schleicher, E., Somoza, V., Shieberle, P., Eds.; New York Academy of Sciences: New York, NY, USA, 2008; Volume 1126, pp. 20–24. [Google Scholar]
- Jiao, Y.; Quan, W.; He, Z.; Gao, D.; Qin, F.; Zeng, M.; Chen, J. Effects of catechins on Nε-(carboxymethyl)lysine and Nε-(Carboxyethyl)lysine formation in green tea and model systems. J. Agric. Food Chem. 2019, 67, 1254–1260. [Google Scholar] [CrossRef]
- Gruber, P.; Hofmann, T. Chemoselective synthesis of peptides containing major advanced glycation end-products of lysine and arginine. J. Pept. Res. 2005, 66, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, L.; Xie, J.; Shen, M. Formation mechanism of AGEs in Maillard reaction model systems containing ascorbic acid. Food Chem. 2022, 378, 132108. [Google Scholar] [CrossRef]
- Zhao, L.; Zhu, X.; Yu, Y.; He, L.; Li, Y.; Zhang, L.; Liu, R. Comprehensive analysis of the anti-glycation effect of peanut skin extract. Food Chem. 2021, 362, 130169. [Google Scholar] [CrossRef] [PubMed]
- Cömert, E.D.; Gökmen, V. Kinetic evaluation of the reaction between methylglyoxal and certain scavenging compounds and determination of their in vitro dicarbonyl scavenging activity. Food Res. Int. 2019, 121, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.-Y.; Hsiao, W.-T.; Chen, X.-Y. Efficiency of trapping methylglyoxal by phenols and phenolic acids. J. Food Sci. 2011, 76, H90–H96. [Google Scholar] [CrossRef]
- Peng, X.; Ma, J.; Chen, F.; Wang, M. Naturally occurring inhibitors against the formation of advanced glycation end-products. Food Funct. 2011, 2, 289–301. [Google Scholar] [CrossRef]
- Zhu, H.; Poojary, M.M.; Andersen, M.L.; Lund, M.N. The effect of molecular structure of polyphenols on the kinetics of the trapping reactions with methylglyoxal. Food Chem. 2020, 319, 126500. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhan, L.; Wen, Q.; Feng, Y.; Luo, Y.; Tan, T. Trapping methylglyoxal by taxifolin and its metabolites in mice. J. Agric. Food Chem. 2022, 70, 5026–5038. [Google Scholar] [CrossRef]
- Liu, G.; Xia, Q.; Lu, Y.; Zheng, T.; Sang, S.; Lv, L. Influence of quercetin and its methylglyoxal adducts on the formation of α-dicarbonyl compounds in a lysine/glucose model system. J. Agric. Food Chem. 2017, 65, 2233–2239. [Google Scholar] [CrossRef]
- Zhu, Q.; Zheng, Z.; Cheng, K.; Wu, J.; Zhang, S.; Tang, Y.; Sze, K.-H.; Chen, J.; Chen, F.; Wang, M. Natural polyphenols as direct trapping agents of lipid peroxidation-derived acrolein and 4-hydroxy-trans-2-nonenal. Chem. Res. Toxicol. 2009, 22, 1721–1727. [Google Scholar] [CrossRef]
- Meade, S.J.; Miller, A.G.; Gerrard, J.A. The role of dicarbonyl compounds in non-enzymatic crosslinking: A structure-activity study. Bioorg. Med. Chem. 2003, 11, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Shao, X.; Bai, N.; Lo, C.-Y.; Yang, C.S.; Ho, C.-T. Tea polyphenol (−)-epigallocatechin-3-gallate: A new trapping agent of reactive dicarbonyl species. Chem. Res. Toxicol. 2007, 20, 1862–1870. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Yang, X.; Zhu, L.; Wu, G.; Qi, X.; Zhang, H. Trapping of reactive carbonyl species by fiber-bound polyphenols from whole grains under simulated physiological conditions. Food Res. Int. 2022, 156, 111142. [Google Scholar] [CrossRef] [PubMed]
- Lund, M.N. Reactions of plant polyphenols in foods: Impact of molecular structure. Trends Food Sci. Technol. 2021, 112, 241–251. [Google Scholar] [CrossRef]
- Qin, C.; Li, Y.; Zhang, Y.; Liu, L.; Wu, Z.; Weng, P. Insights into oat polyphenols constituent against advanced glycation end products mechanism by spectroscopy and molecular interaction. Food Biosci. 2021, 43, 101313. [Google Scholar] [CrossRef]
- Khalifa, I.; Du, X.; Dutta, K.; Peng, J.; Jia, Y.; Li, C. Mulberry anthocyanins exert anti-AGEs effects by selectively trapping glyoxal and structural-dependently blocking the lysyl residues of β-lactoglobulins. Bioorg. Chem. 2020, 96, 103615. [Google Scholar] [CrossRef]
- Peng, X.; Ma, J.; Cheng, K.-W.; Jiang, Y.; Chen, F.; Wang, M. The effects of grape seed extract fortification on the antioxidant activity and quality attributes of bread. Food Chem. 2010, 119, 49–53. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Lin, Y.-Y.; Pan, M.-H.; Ho, C.-T.; Hung, W.-L. Inhibitory effects of rooibos (Aspalathus linearis) against reactive carbonyl species and advanced glycation end product formation in a glucose-bovine serum albumin model and cookies. Food Chem. X 2022, 16, 100515. [Google Scholar] [CrossRef]
- Khalifa, I.; Peng, J.; Jia, Y.; Li, J.; Zhu, W.; Xu, Y.; Li, C. Anti-glycation and anti-hardening effects of microencapsulated mulberry polyphenols in high-protein-sugar ball models through binding with some glycation sites of whey proteins. Int. J. Biol. Macromol. 2019, 123, 10–19. [Google Scholar] [CrossRef]
- Li, X.; Zheng, T.; Sang, S.; Lv, L. Quercetin inhibits advanced glycation end product formation by trapping methylglyoxal and glyoxal. J. Agric. Food Chem. 2014, 62, 12152–12158. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, S.; Chen, Y.; Zhang, R.; Zhou, M.; Wang, C.; Feng, N.; Wu, Q. Structure-activity relationship of procyanidins on advanced glycation end products formation and corresponding mechanisms. Food Chem. 2019, 272, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Khalifa, I.; Du, X.; Li, K.; Xu, Y.; Li, C. Effects of anthocyanins on β-lactoglobulin glycoxidation: A study of mechanisms and structure-activity relationship. Food Funct. 2021, 12, 10550–10562. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ling, Y.; Zhang, L.; Peng, B.; Zhang, Z. Effects of C-Ring Structural Differences on the Inhibition of Nε-(Carboxyethyl)lysine in the Methylglyoxal-Lysine System by Flavonoids. Int. J. Mol. Sci. 2025, 26, 5914. https://doi.org/10.3390/ijms26125914
Ling Y, Zhang L, Peng B, Zhang Z. Effects of C-Ring Structural Differences on the Inhibition of Nε-(Carboxyethyl)lysine in the Methylglyoxal-Lysine System by Flavonoids. International Journal of Molecular Sciences. 2025; 26(12):5914. https://doi.org/10.3390/ijms26125914
Chicago/Turabian StyleLing, Yating, Linlin Zhang, Bangzhu Peng, and Zhuo Zhang. 2025. "Effects of C-Ring Structural Differences on the Inhibition of Nε-(Carboxyethyl)lysine in the Methylglyoxal-Lysine System by Flavonoids" International Journal of Molecular Sciences 26, no. 12: 5914. https://doi.org/10.3390/ijms26125914
APA StyleLing, Y., Zhang, L., Peng, B., & Zhang, Z. (2025). Effects of C-Ring Structural Differences on the Inhibition of Nε-(Carboxyethyl)lysine in the Methylglyoxal-Lysine System by Flavonoids. International Journal of Molecular Sciences, 26(12), 5914. https://doi.org/10.3390/ijms26125914






