Immune-Checkpoint Expression in Breast Cancer Patients: Clinicopathological Implications: A Retrospective Case Series Study
Abstract
1. Introduction
2. Results
2.1. Patient and Tumor Characteristics
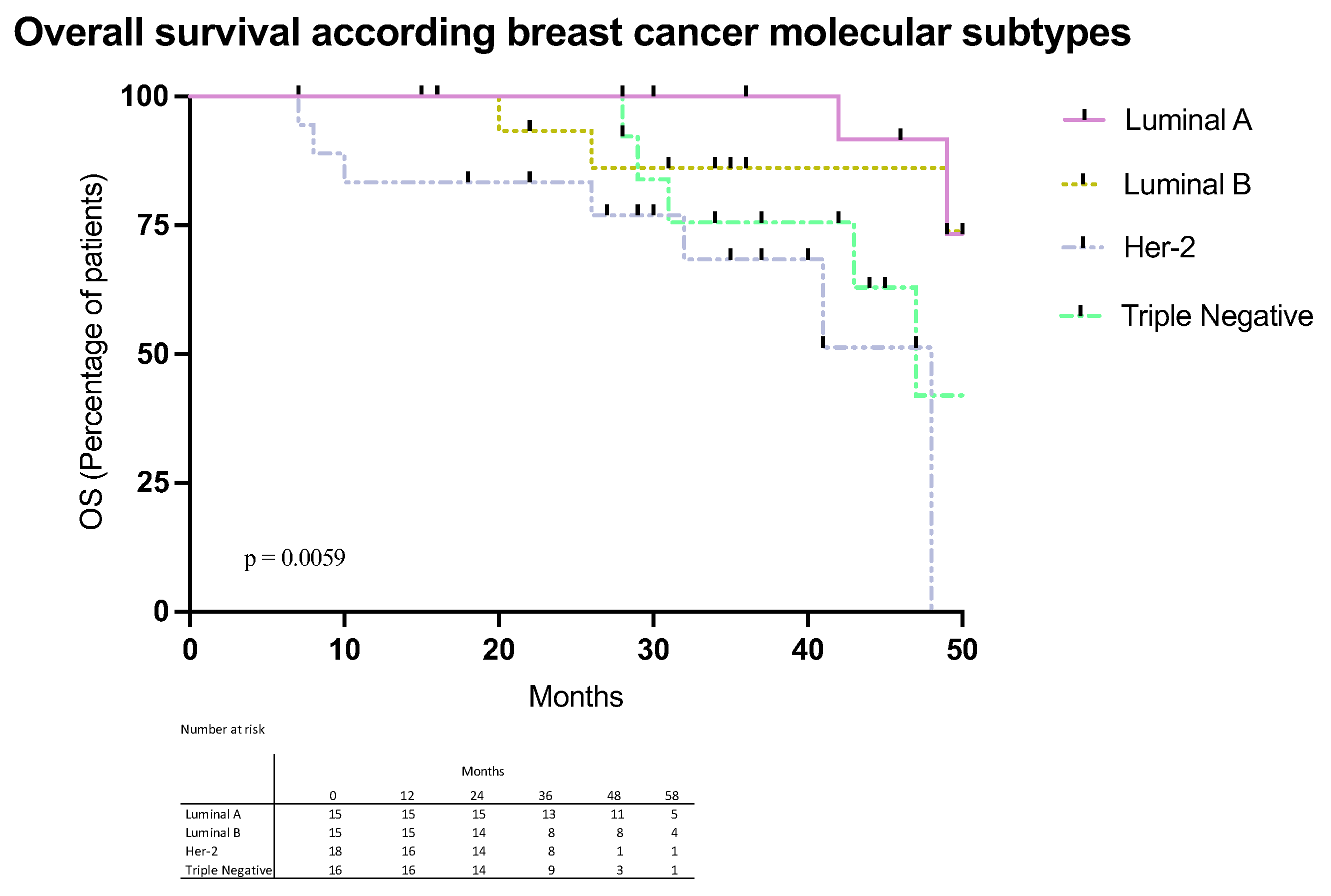
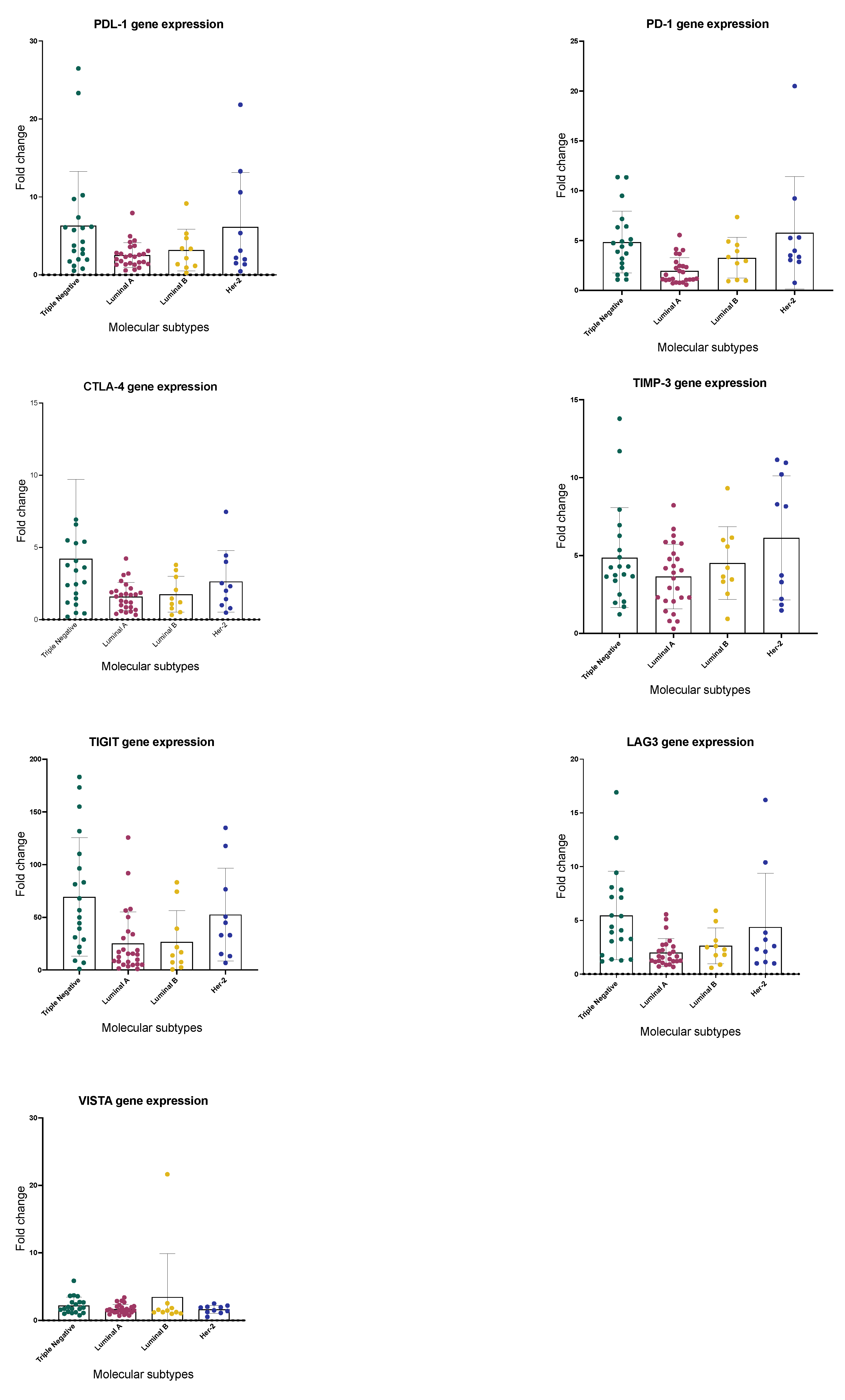
2.2. Immune Checkpoints Gene Expression
2.3. Expression of Immune Checkpoints by Immunohistochemistry
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Biospecimen Collection and Immunohistochemistry Studies
4.3. Gene Expression
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, L.M.; Cortes, S.; Vilensky, M.; Valenzuela, O.; Cortes-Sanabria, L.; de Souza, M.; Barbeito, R.A.; Abdelhay, E.; Artagaveytia, N.; Daneri-Navarro, A. Socioeconomic, Clinical, and Molecular Features of Breast Cancer Influence Overall Survival of Latin American Women. Front. Oncol. 2022, 12, 845527. [Google Scholar] [CrossRef] [PubMed]
- Xiong, N.; Wu, H.; Yu, Z. Advancements and challenges in triple-negative breast cancer: A comprehensive review of therapeutic and diagnostic strategies. Front. Oncol. 2024, 14, 1405491. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Xue, R.; Zhu, Z.; Farrukh, H.; Song, W.; Li, T.; Zheng, L.; Pan, C.X. Increasing cure rates of solid tumors by immune checkpoint inhibitors. Exp. Hematol. Oncol. 2023, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Cortes, J.; Dent, R.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; et al. Event-free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 386, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; He, Y.; Tang, Y.; Dai, W.; Si, Y.; Mao, F.; Xu, J.; Yu, C.; Sun, X. A meta-analysis of application of PD-1/PD-L1 inhibitor-based immunotherapy in unresectable locally advanced triple-negative breast cancer. Immunotherapy 2023, 15, 1073–1088. [Google Scholar] [CrossRef] [PubMed]
- Pilard, C.; Ancion, M.; Delvenne, P.; Jerusalem, G.; Hubert, P.; Herfs, M. Cancer immunotherapy: It’s time to better predict patients’ response. Br. J. Cancer 2021, 125, 927–938. [Google Scholar] [CrossRef]
- Llera, A.S.; Abdelhay, E.S.F.W.; Artagaveytia, N.; Daneri-Navarro, A.; Müller, B.; Velazquez, C.; Alcoba, E.B.; Alonso, I.; da Quinta, D.B.A.; Binato, R. The Transcriptomic Portrait of Locally Advanced Breast Cancer and Its Prognostic Value in a Multi-Country Cohort of Latin American Patients. Front. Oncol. 2022, 12, 835626. [Google Scholar] [CrossRef] [PubMed]
- Swoboda, A.; Nanda, R. Immune Checkpoint Blockade for Breast Cancer. Cancer Treat. Res. 2018, 173, 155–165. [Google Scholar] [CrossRef]
- Lan, H.R.; Chen, M.; Yao, S.Y.; Chen, J.X.; Jin, K.T. Novel immunotherapies for breast cancer: Focus on 2023 findings. Int. Immunopharmacol. 2024, 128, 111549. [Google Scholar] [CrossRef] [PubMed]
- Emens, L.A.; Loi, S. Immunotherapy Approaches for Breast Cancer Patients in 2023. Cold Spring Harb. Perspect. Med. 2023, 13, a041332. [Google Scholar] [CrossRef] [PubMed]
- Mollavelioglu, B.; Cetin Aktas, E.; Cabioglu, N.; Abbasov, A.; Onder, S.; Emiroglu, S.; Tukenmez, M.; Muslumanoglu, M.; Igci, A.; Deniz, G.; et al. High co-expression of immune checkpoint receptors PD-1, CTLA-4, LAG-3, TIM-3, and TIGIT on tumor-infiltrating lymphocytes in early-stage breast cancer. World J. Surg. Oncol. 2022, 20, 349. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.Y.; Wu, H.W.; Lu, J.L.; Zhang, Y.H.; Luo, Y.F.; Xu, Q.Q.; Shen, S.J.; Liang, Z.Y. PD1 protein expression in tumor infiltrated lymphocytes rather than PDL1 in tumor cells predicts survival in triple-negative breast cancer. Cancer Biol. Ther. 2018, 19, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Baessler, A.; Vignali, D.A.A. T Cell Exhaustion. Annu. Rev. Immunol. 2024, 42, 179–206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, J.; Gu, T.; Meng, X.; Cai, X.; Zhang, J.; Chen, Y.; Zhang, D.; Wu, Y. Enhanced antitumor efficacy of bispecific antibody blocking PD-L1 and LAG-3 with doxorubicin: Mechanism and safety evaluation. Breast Cancer Res. Treat. 2025, 211, 637–648. [Google Scholar] [CrossRef] [PubMed]

| Parameter | |||||
|---|---|---|---|---|---|
| H(+)Her2(−) | H(+)Her2(+) | H(−)Her2(+) | Triple-Negative | Analysis p | |
| Age | NS | ||||
| Mean (SD) | 57.65 ± 12.42 | 56.44 ± 23.42 | 55.35 ± 12.76 | 54.00 ± 14.60 | |
| Range | 33−89 | 35−86 | 33−93 | 28−85 | |
| Menopause N (%) | NS | ||||
| Pre-menopause | 37 (25) | 4(22.22) | 0 (27.02) | 15 (34.09) | |
| Post-Menopause | 111 (75) | 14(77.78) | 27 (77.98) | 29 (65.91) | |
| Clinical stage N (%) | NS | ||||
| I | 26 (19.12) | 4 (25.00) | 13 (9.09) | 5 (12.50) | |
| II | 67 (49.27) | 10 (62.50) | 12 (36.36) | 19 (47.50) | |
| III | 37 (27.20) | 1 (12.25) | 13 (39.39) | 15 (37.50) | |
| IV | 6 (4.41) | 1 (12.25) | 5 (15.15) | 1 (2.50) | |
| Grade | 0.0001 | ||||
| Low | 30 (25.42) | 3 (20.00) | 2 (6.06) | 2 (6.45) | |
| Intermediate | 72 (61.01) | 8 (53.33) | 15 (45.45) | 5 (16.13) | |
| High | 16 (13.56) | 4 (26.67) | 16 (48.49) | 24 (77.42) | |
| Ki67 | 0.0001 | ||||
| <10% | 62 (51.24) | 1(5,55) | 6 (20.00) | 8 (21.05) | |
| 10–19% | 20 (16.53) | 1 (5.55) | 6 (20.00) | 7 (18.42) | |
| ≥20% | 39 (32.23) | 16 (88.00) | 18 (60.00) | 23 (60.53) |
| Immune Checkpoint (% Tumors > 2-Fold Change) | Low | Grade Intermediate | High |
|---|---|---|---|
| PD-L1 | 57.1 | 45.5 | 87.5 |
| PD-1 | 57.4 | 51.5 | 80.0 |
| CTLA-4 | 42.9 | 24.2 | 68.0 |
| LAG-3 | 57.1 | 45.5 | 79.2 |
| TIM-3 | 76.9 | 76.5 | 95.8 |
| TIGIT | 92.3 | 90.9 | 96.0 |
| VISTA | 23.1 | 21.2 | 32.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quiroz-Bolaños, A.; Quintero-Ramos, A.; Godínez-Rubí, J.M.; Franco-Topete, R.; González, P.G.; Gutiérrez-Zepeda, B.M.; Becerra-Loaiza, D.S.; Topete, A.; de Loera-Rodriguez, C.; Toro-Arreola, A.D.; et al. Immune-Checkpoint Expression in Breast Cancer Patients: Clinicopathological Implications: A Retrospective Case Series Study. Int. J. Mol. Sci. 2025, 26, 5851. https://doi.org/10.3390/ijms26125851
Quiroz-Bolaños A, Quintero-Ramos A, Godínez-Rubí JM, Franco-Topete R, González PG, Gutiérrez-Zepeda BM, Becerra-Loaiza DS, Topete A, de Loera-Rodriguez C, Toro-Arreola AD, et al. Immune-Checkpoint Expression in Breast Cancer Patients: Clinicopathological Implications: A Retrospective Case Series Study. International Journal of Molecular Sciences. 2025; 26(12):5851. https://doi.org/10.3390/ijms26125851
Chicago/Turabian StyleQuiroz-Bolaños, Angel, Antonio Quintero-Ramos, Juliana Marisol Godínez-Rubí, Ramon Franco-Topete, Porfirio Gutiérrez González, Bricia M. Gutiérrez-Zepeda, Denisse S. Becerra-Loaiza, Antonio Topete, Cesar de Loera-Rodriguez, Alicia Del Toro-Arreola, and et al. 2025. "Immune-Checkpoint Expression in Breast Cancer Patients: Clinicopathological Implications: A Retrospective Case Series Study" International Journal of Molecular Sciences 26, no. 12: 5851. https://doi.org/10.3390/ijms26125851
APA StyleQuiroz-Bolaños, A., Quintero-Ramos, A., Godínez-Rubí, J. M., Franco-Topete, R., González, P. G., Gutiérrez-Zepeda, B. M., Becerra-Loaiza, D. S., Topete, A., de Loera-Rodriguez, C., Toro-Arreola, A. D., & Daneri-Navarro, A. (2025). Immune-Checkpoint Expression in Breast Cancer Patients: Clinicopathological Implications: A Retrospective Case Series Study. International Journal of Molecular Sciences, 26(12), 5851. https://doi.org/10.3390/ijms26125851









