The Role of MicroRNA in the Pathogenesis of Atopic Dermatitis
Abstract
1. Introduction
2. Materials and Methods
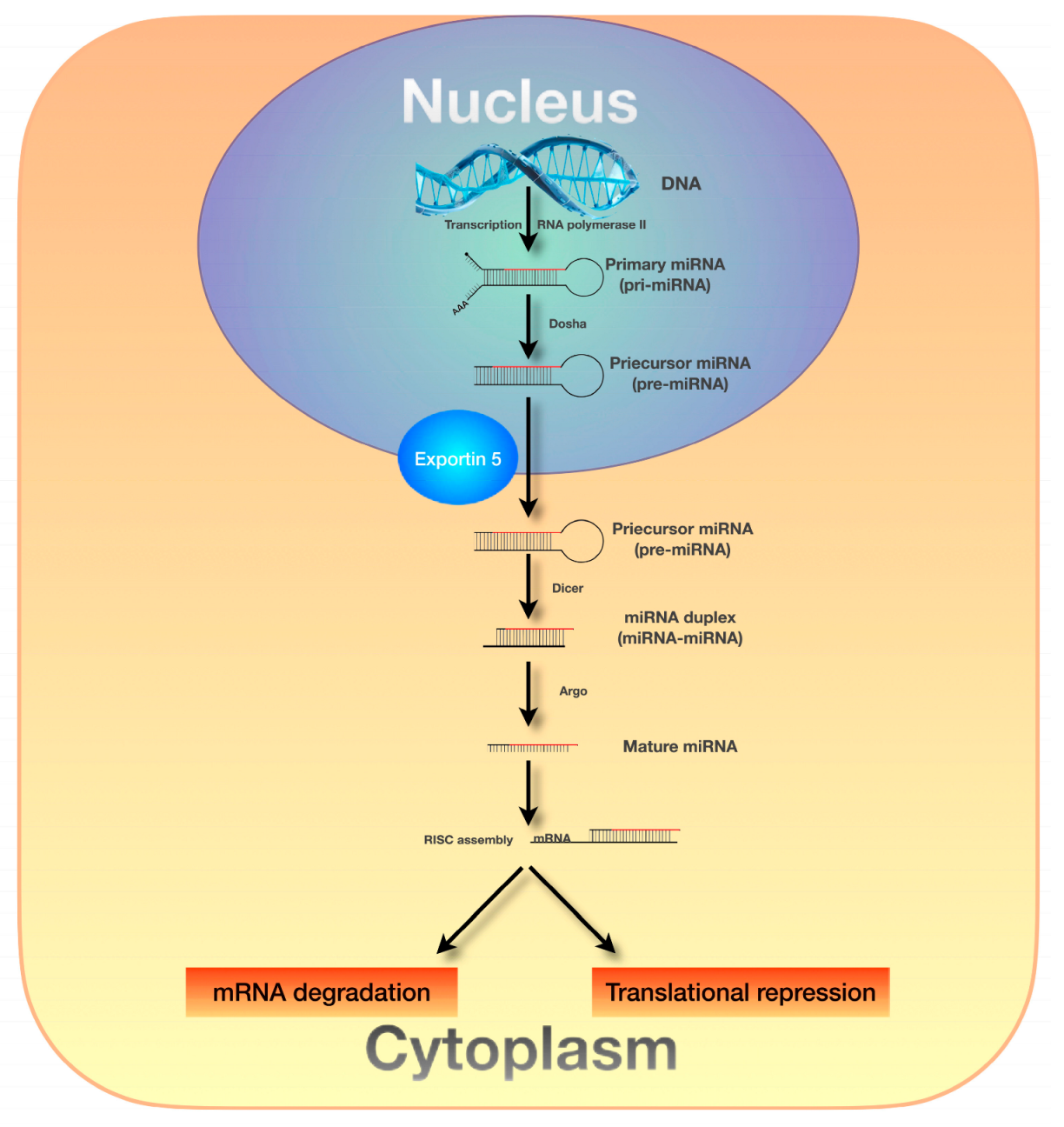
3. MicroRNAs
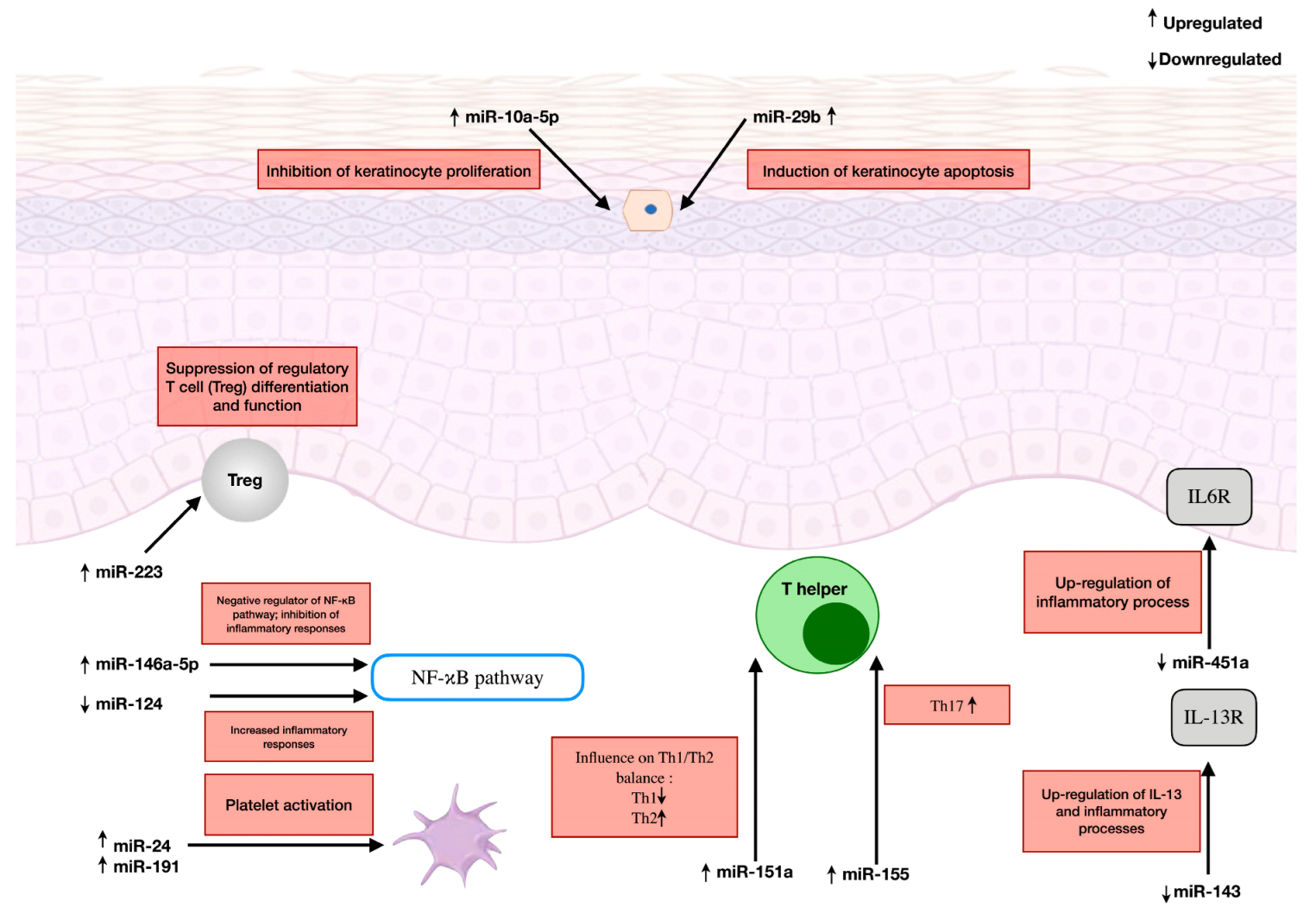
3.1. MicroRNA-223
3.2. MicroRNA-10a-5p
3.3. MicroRNA-29b
3.4. MicroRNA-146a-5p
3.5. MicroRNA-451a
3.6. MicroRNA-124
3.7. MicroRNA-143
3.8. MicroRNA-151a
3.9. MicroRNA-24 and MicroRNA-191
3.10. MicroRNA-155
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Atopic dermatitis |
| miRNA | MicroRNA |
| ILC2 | Type 2 innate lymphoid cells (ILC2) |
| Pri-miRNAs | Primary miRNA transcripts pri-miRNAs |
| Pre-miRNAs | Precursor miRNAs |
| RISC | RNA-induced silencing complex |
| MiR-223 | MicroRNA-223 miR-223 |
| IL-1β | Interleukin 1β |
| IL-4 | Interleukin 4 |
| IL-13 | Interleukin 13 |
| IL-31 | Interleukin 31 |
| IL-5 | Interleukin 5 |
| IL-17 | Interleukin 17 |
| IL-17A | Interleukin 17A |
| IL-8 | Interleukin 8 |
| IL-10 | Interleukin 10 |
| EASI | Eczema Area and Severity Index |
| MiR-10a-5p | MicroRNA-10a-5p |
| HAS3 | Hyaluronic acid synthase 3 |
| MAP3K7 | Protein kinase kinase kinase 7 |
| MiR-29b | MicroRNA-29b |
| IFN-γ | Regulates interferon-γ |
| SCORAD | Scoring Atopic Dermatitis |
| BCL2L2 | BCL2-like 2 |
| MiR-146a-5p | MicroRNA-146a-5p |
| NF-κB | Nuclear factor kappa B |
| IRAK1 | Interleukin-1 receptor-associated kinase 1 |
| TRAF6 | Tumor necrosis factor receptor-associated factor 6 |
| CARD-10 | Caspase recruitment domain-containing protein 10 |
| CCL5 | C-C motif chemokine ligand 5 |
| IL-12p40 | Interleukin 12 subunit p40 |
| MiR-451a | MicroRNA-451a |
| PBMCs | Peripheral blood mononuclear cells |
| PSMB8 | Proteasome subunit beta type 8 |
| IL6R | Interleukin 6 receptor |
| MiR-124 | MicroRNA-124 |
| CCL5 | Chemokine C-C motif ligan |
| CCL8 | Chemokine C-C motif ligand 8 |
| MiR-143 | MicroRNA-143 |
| FLG | Filaggrin |
| LOR | Loricrin |
| IVL, | Involucrin |
| MiR-151a | MicroRNA-151a |
| β2 IL12RB2 | Interleukin-12 receptor |
| MiR-24 | MicroRNA-24 |
| MiR-191 | MicroRNA-191 |
| TARC | Thymus and Activation-Regulated Chemokine |
| PF-4 | Platelet factor 4 and β-TG (beta-thromboglobulin) |
| β-TG | Beta-thromboglobulin |
| MiR-155 | MicroRNA-155 |
| Th17 | Type 17 helper T |
| CTLA-4 | Cytotoxic T-lymphocyte-associated protein 4 |
| GATA3 | GATA binding protein 3 |
References
- Zhou, N.Y.; Nili, A.; Blackwell, C.K.; Ogbuefi, N.; Cummings, P.; Lai, J.; Griffith, J.W.; Paller, A.S.; Wakschlag, L.S.; Fishbein, A.B. Parent report of sleep health and attention regulation in a cross-sectional study of infants and preschool-aged children with atopic dermatitis. Pediatr. Dermatol. 2022, 39, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, S.; Becj, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic Dermatitis. Nat. Rev. Dis. Primers 2018, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.; Kido-Nakahara, M.; Tsuji, G.; Furue, M. Basics and recent advances in the pathophysiology of atopic dermatitis. J. Dermatol. 2021, 48, 130–139. [Google Scholar] [CrossRef]
- Brunner, P.M.; Guttman-Yassky, E.; Leung, D.Y. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J. Allergy Clin. Immunol. 2017, 139, 65–76. [Google Scholar] [CrossRef]
- Tsakok, T.; Marrs, T.; Mohsin, M.; Baron, S.; du Toit, G.; Till, S.; Flohr, C. Does atopic dermatitis cause food allergy? A systematic review. Pediatr. Allergy Immunol. 2016, 27, 255–277. [Google Scholar] [CrossRef]
- Spergel, J.M. From atopic dermatitis to asthma: The atopic march. Ann. Allergy Asthma Immunol. 2010, 105, 99–106; quiz 107–109, 117. [Google Scholar] [CrossRef]
- Schneider, L.; Tilles, S.; Lio, P.; Boguniewicz, M.; Beck, L.; LeBovidge, J.; Novak, N.; Bernstein, D.; Blessing-Moore, J.; Khan, D.; et al. Atopic dermatitis: A practice parameter update 2012. J. Allergy Clin. Immunol. 2013, 131, 295–299.e1–27. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhuo, F.; Guo, Y.; Wang, S.; Zhang, K.; Li, X.; Dai, W.; Dou, X.; Yu, B. Skin microbiota: Pathogenic roles and implications in atopic dermatitis. Front. Cell. Infec.t Microbiol. 2025, 14, 1518811. [Google Scholar] [CrossRef]
- Czarnowicki, T.; He, H.; Krueger, J.G.; Guttman-Yassky, E. Atopic dermatitis endotypes and implications for targeted therapeutics. J. Allergy Clin. Immunol. 2019, 143, 1–11. [Google Scholar] [CrossRef]
- Thyssen, J.P.; Vestergaard, C.; Barbarot, S.; Deleuran, M.; de Bruin, M.S.; Bieber, T.; Taieb, A.; Seneschal, J.; Cork, M.J.; Paul, C.; et al. European Task Force on Atopic Dermatitis: Position on atopic dermatitis pathophysiology and biomarker use for targeted treatment in adults. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1937–1947. [Google Scholar]
- Kim, B.S. Innate lymphoid cells in the skin. J. Invest. Dermatol. 2015, 135, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Wollenberg, A.; Christen-Zäch, S.; Taieb, A.; Paul, C.; Thyssen, J.; de Bruin-Weller, M.; Vestergaard, C.; Seneschal, J.; Werfel, T.; Cork, M.; et al. ETFAD/EADV Eczema task force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2717–2744. [Google Scholar] [CrossRef]
- Nowicki, R.J. ABC atopowego zapalenia skóry. In AZS w Pytaniach i Odpowiedziach, 2nd ed.; Termedia: Poznań, Poland, 2022; p. 243. [Google Scholar]
- Ramirez-Marin, H.A.; Silverberg, J.I. Differences between pediatric and adult atopic dermatitis. Pediatr. Dermatol. 2022, 39, 345–353. [Google Scholar] [CrossRef]
- Chen, Y.E.; Tsao, H. The skin microbiome: Current perspectives and future challenges. J. Am. Acad. Dermatol. 2013, 69, 143–155. [Google Scholar] [CrossRef]
- Paller, A.S.; Kong, H.H.; Seed, P.; Naik, S.; Scharschmidt, T.C.; Gallo, R.L.; Luger, T.; Irvine, A.D. The microbiome in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019, 143, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Flowers, L.; Grice, E.A. The skin microbiota: Balancing risk and reward. Cell Host Microbe 2020, 28, 190–200. [Google Scholar] [CrossRef]
- Tauber, M.; Balica, S.; Hsu, C.-Y.; Jean-Decoster, C.; Lauze, C.; Redoules, D.; Viodé, C.; Schmitt, A.-M.; Serre, G.; Simon, M.; et al. Staphylococcus aureus density on lesional and nonlesional skin is strongly associated with disease severity in atopic dermatitis. J. Allergy Clin. Immunol. 2016, 137, 1272–1274. [Google Scholar] [CrossRef]
- Slominski, R.M.; Chen, J.Y.; Raman, C.; Slominski, A.T. Photo-neuro-immuno-endocrinology: How the ultraviolet radiation regulates the body, brain, and immune system. Proc. Natl. Acad. Sci. USA 2024, 121, 14. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.M.; Raman, C.; Jetten, A.M.; Slominski, A.T. Neuro-immuno-endocrinology of the skin: How environment regulates body homeostasis. Nat. Rev. Endocrinol. 2025. [Google Scholar] [CrossRef]
- Lee, W.; Chaudhary, F.; Agrawal, D.K. Environmental Influences on Atopic Eczema. J. Environ. Sci. Public Health 2024, 8, 101–115. [Google Scholar] [CrossRef]
- Kato, A.; Fukai, K.; Oiso, N.; Hosomi, N.; Murakami, T.; Ishii, M. Association of SPINK5 gene polymorphisms with atopic dermatitis in the Japanese population. Br. J. Dermatol. 2003, 148, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Sandilands, A.; O’Regan, G.M.; Liao, H.; Zhao, Y.; Terron-Kwiatkowski, A.; Watson, R.M.; Cassidy, A.J.; Goudie, D.R.; Smith, F.J.; McLean, W.H.; et al. Prevalent and rare mutations in the gene encoding filaggrin cause ichthyosis vulgaris and predispose individuals to atopic dermatitis. J. Invest. Dermatol. 2006, 126, 1770–1775. [Google Scholar] [CrossRef] [PubMed]
- Dexheimer, P.J.; Cochella, L. MicroRNAs: From mechanism to organism. Front. Cell Dev. Biol 2020, 8, 409. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.X.; Rothenberg, M.E. MicroRNA. J. Allergy Clin. Immunol. 2018, 141, 1202–1207. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Hutvágner, G.; Zamore, P.D. A microRNA in a multiple-turnover RNAi enzyme complex. Science 2002, 297, 2056–2060. [Google Scholar] [CrossRef]
- Perlis, R.H. Translating biomarkers to clinical practice. Mol. Psychiatry 2011, 16, 1076–1087. [Google Scholar] [CrossRef]
- Zen, K.; Zhang, C.Y. Circulating microRNAs: A novel class of biomarkers to diagnose and monitor human cancers. Med. Res. Rev. 2012, 32, 326–348. [Google Scholar] [CrossRef]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; Huang, K.H.; Lee, M.J.; Galas, D.J.; Wang, K. The microRNA spectrum in 12 body fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, S.; Weber, J.; Baxter, D.; Galas, D.J. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res. 2010, 38, 7248–7259. [Google Scholar] [CrossRef]
- Zubakov, D.; Boersma, A.W.; Choi, Y.; van Kuijk, P.F.; Wiemer, E.A.; Kayser, M. MicroRNA markers for forensic body fluid identification obtained from microarray screening and quantitative RT-PCR confirmation. Int. J. Legal. Med. 2010, 124, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Condrat, C.E.; Thompson, D.C.; Barbu, M.G.; Bugnar, O.L.; Boboc, A.; Cretoiu, D.; Suciu, N.; Cretoiu, S.M.; Voinea, S.C. miRNAs as Biomarkers in Disease: Latest Findings Regarding Their Role in Diagnosis and Prognosis. Cells 2020, 9, 276. [Google Scholar] [CrossRef]
- Schneider, M.R. MicroRNAs as novel players in skin development, homeostasis and disease. Br. J. Dermatol. 2012, 166, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Lynam-Lennon, N.; Maher, S.G.; Reynolds, J.V. The roles of microRNA in cancer and apoptosis. Biol. Rev. 2009, 84, 55–71. [Google Scholar] [CrossRef]
- Specjalski, K.; Jassem, E. MicroRNAs: Potential Biomarkers and Targets of Therapy in Allergic Diseases? Arch. Immunol. Ther. Exp 2019, 67, 213–223. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Sen, S. MicroRNA as Biomarkers and Diagnostics. J. Cell. Physiol. 2016, 231, 25–30. [Google Scholar] [CrossRef]
- Herberth, G.; Bauer, M.; Gasch, M.; Hinz, D.; Röder, S.; Olek, S.; Kohajda, T.; Rolle-Kampczyk, U.; von Bergen, M.; Sack, U.; et al. Lifestyle and Environmental Factors and Their Influence on Newborns Allergy Risk study group. Maternal and cord blood miR-223 expression associates with prenatal tobacco smoke exposure and low regulatory T-cell numbers. J. Allergy Clin. Immunol. 2014, 133, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Herberth, G.; Pierzchalski, A.; Feltens, R.; Bauer, M.; Röder, S.; Olek, S.; Hinz, D.; Borte, M.; von Bergen, M.; Lehmann, I.; et al. Prenatal phthalate exposure associates with low regulatory T-cell numbers and atopic dermatitis in early childhood: Results from the LINA mother-child study. J. Allergy Clin. Immunol. 2017, 139, 1376–1379.e8. [Google Scholar] [CrossRef]
- Jia, H.-Z.; Liu, S.-L.; Zou, Y.-F.; Chen, X.-F.; Yu, L.; Wan, J.; Zhang, H.-Y.; Chen, Q.; Xiong, Y.; Yu, B.; et al. MicroRNA-223 is involved in the pathogenesis of atopic dermatitis by affecting histamine-N- methyltransferase. Cell. Mol. Biol. 2018, 64, 103–107. [Google Scholar] [CrossRef]
- Hernández-Rodríguezz, R.; Amezcua-Guerra, L. The potential role of microRNAs as biomarkers in atopic dermatitis: A systematic review. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 11804–11809. [Google Scholar]
- Grafanaki, K.; Antonatos, C.; Maniatis, A.; Petropoulou, A.; Vryzaki, E.; Vasilopoulos, Y.; Georgiou, S.; Gregoriou, S. Intrinsic effects of exposome in atopic dermatitis: Genomics, epigenomics and regulatory layers. J. Clin. Med. 2023, 12, 4000. [Google Scholar] [CrossRef]
- Yasuike, R.; Tamagawa-Mineoka, R.; Nakamura, N.; Masuda, K.; Katoh, N. Plasma miR223 is a possible biomarker for diagnosing patients with severe atopic dermatitis. Allergol. Int. 2021, 70, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Vaher, H.; Runnel, T.; Urgard, E.; Aab, A.; Badosa, G.C.; Maslovskaja, J.; Abram, K.; Raam, L.; Kaldvee, B.; Annilo, T.; et al. miR-10a-5p is increased in atopic dermatitis and has capacity to inhibit keratinocyte proliferation. Allergy 2019, 74, 2146–2156. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, T.; Memezawa, A.I.; Okuyama, R.; Sayo, T.; Sugiyama, Y.; Inoue, S.; Aiba, S. Increased hyaluronan production and decreased E-cadherin expression by cytokine-stimulated keratinocytes lead to spongiosis formation. J. Invest. Dermatol. 2009, 129, 1412–1420. [Google Scholar] [CrossRef]
- Malaisse, J.; Bourguignon, V.; De Vuyst, E.; de Rouvroit, C.L.; Nikkels, A.F.; Flamion, B.; Poumay, Y. Hyaluronan metabolism in human keratinocytes and atopic dermatitis skin is driven by a balance of hyaluronan synthases 1 and 3. J. Invest. Dermatol. 2014, 134, 2174–2182. [Google Scholar] [CrossRef]
- Lee, A.-Y. The role of microRNAs in epidermal barrier. Int. J. Mol. Sci. 2020, 21, 5781. [Google Scholar] [CrossRef]
- Yu, X.; Wang, M.; Li, L.; Zhang, L.; Chan, M.T.V.; Wu, W.K.K. MicroRNAs in atopic dermatitis: A systematic review. J. Cell. Mol. Med. 2020, 24, 5966–5972. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Li, Y.; Wu, J.; Xu, J. IFN-γ-induced microRNA-29b up-regulation contributes to keratinocyte apoptosis in atopic dermatitis through inhibiting Bcl2L2. Int. J. Clin. Exp. Pathol. 2017, 10, 10117–10126. [Google Scholar]
- Rebane, A.; Runnel, T.; Aab, A.; Maslovskaja, J.; Rückert, B.; Zimmermann, M.; Plaas, M.; Kärner, J.; Treis, A.; Pihlap, M.; et al. MicroRNA-146a alleviates chronic skin inflammation in atopic dermatitis through suppression of innate immune responses in keratinocytes. J. Allergy Clin. Immunol. 2014, 134, 836–847.e11. [Google Scholar] [CrossRef]
- Ye, E.A.; Steinle, J.J. miR-146a attenuates inflammatory pathways mediated by TLR4/NF-κB and TNFα to protect primary human retinal microvascular endothelial cells grown in high glucose. Mediat. Inflamm. 2016, 2016, 3958453. [Google Scholar] [CrossRef]
- Yan, F.; Meng, W.; Ye, S.; Zhang, X.; Mo, X.; Liu, J.; Chen, D.; Lin, Y. MicroRNA-146a as a potential regulator involved in the pathogenesis of atopic dermatitis. Mol. Med. Rep. 2019, 20, 4645–4653. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Huang, Y.; Huang, Y.-Y.; Kuang, Y.-S.; Wei, Y.-J.; Xiang, L.; Zhang, X.-J.; Jia, Z.-C.; Jiang, S.; Li, J.-Y.; et al. MicroRNA-146a promotes IgE class switch in B cells via upregulating 14-3-3σ expression. Mol. Immunol. 2017, 92, 180–189. [Google Scholar] [CrossRef]
- Carreras-Badosa, G.; Runnel, T.; Plaas, M.; Kärner, J.; Rückert, B.; Lättekivi, F.; Kõks, S.; Akdis, C.A.; Kingo, K.; Rebane, A. MicroRNA-146a is linked to the production of IgE in mice but not in atopic dermatitis patients. Allergy 2018, 73, 2400–2403. [Google Scholar] [CrossRef]
- Nousbeck, J.; McAleer, M.A.; Hurault, G.; Kenny, E.; Harte, K.; Kezic, S.; Tanaka, R.J.; Irvine, A.D. MicroRNA analysis of childhood atopic dermatitis reveals a role for miR-451a. Br. J. Dermatol. 2021, 184, 514–523. [Google Scholar] [CrossRef]
- Jeong, D.; Kim, J.; Nam, J.; Sun, H.; Lee, Y.-H.; Lee, T.-J.; Aguiar, R.C.T.; Kim, S.-W. MicroRNA-124 links p53 to the NF-κB pathway in B-cell lymphomas. Leukemia 2015, 29, 1868–1874. [Google Scholar] [CrossRef]
- Yang, C.; Sui, G.; Wang, L.; Chen, Z.; Wang, F. MiR-124 Prevents the Microglial Proinflammatory Response by Inhibiting the Activities of TLR4 and Downstream NLRP3 in Palmitic Acid-Treated BV2 Cells. J. Mol. Neurosci. 2022, 72, 496–506. [Google Scholar] [CrossRef]
- Yang, B.; Ge, Y.; Zhou, Y.; Wang, J.; Xie, X.; Li, S.; Tang, M.; Xu, L.; Tian, J. MiR-124a inhibits the proliferation and inflammation in rheumatoid arthritis fibroblast-like synoviocytes via targeting PIK3/NF-κB pathway. Cell Biochem. Funct. 2019, 37, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zeng, B.; Wang, C.; Wang, H.; Huang, P.; Pan, Y. MicroRNA-124 alleviates chronic skin inflammation in atopic eczema via suppressing innate immune responses in keratinocytes. Cell Immunol. 2017, 319, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Ingram, J.L.; Kraft, M. IL-13 in asthma and allergic disease: Asthma phenotypes and targeted therapies. J. Allergy Clin. Immunol. 2012, 130, 829–842; quiz 843–844. [Google Scholar] [CrossRef]
- Howell, M.D.; Kim, B.E.; Gao, P.; Grant, A.V.; Boguniewicz, M.; Debenedetto, A.; Schneider, L.; Beck, L.A.; Barnes, K.C.; Leung, D.Y. Cytokine modulation of atopic dermatitis filaggrin skin expression. J. Allergy Clin. Immunol. 2007, 120, 150–155. [Google Scholar] [CrossRef]
- Kim, B.E.; Leung, D.Y.; Boguniewicz, M.; Howell, M.D. Lori- crin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin. Immunol. 2008, 126, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Pellerin, L.; Henry, J.; Hsu, C.Y.; Balica, S.; Jean-Decoster, C.; Mechin, M.C.; Hansmann, B.; Rodriguez, E.; Weindinger, S.; Schmitt, A.M.; et al. Defects of filaggrin-like pro- teins in both lesional and nonlesional atopic skin. J. Allergy Clin. Immunol. 2013, 131, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.P.; Nguyen, G.H.; Jin, H.Z. MicroRNA-143 inhibits IL-13- induced dysregulation of the epidermal barrier-related proteins in skin keratinocytes via targeting to IL-13Rα1. Mol. Cell. Biochem. 2016, 416, 63–70. [Google Scholar] [CrossRef]
- Chen, X.F.; Zhang, L.J.; Zhang, J.; Dou, X.; Shao, Y.; Jia, X.J.; Zhang, W.; Yu, B. MiR-151a is involved in the pathogenesis of atopic dermatitis by regulating interleukin-12 receptor β2. Exp. Dermatol. 2018, 27, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Maeno, M.; Tamagawa-Mineoka, R.; Arakawa, Y.; Nishigaki, H.; Yasuike, R.; Masuda, K.; Katoh, N. Increased plasma miR-24 and miR-191 levels in patients with severe atopic dermatitis: Possible involvement of platelet activation. Clin. Immunol. 2022, 237, 108983. [Google Scholar] [CrossRef]
- Kakinuma, T.; Nakamura, K.; Wakugawa, M.; Mitsui, H.; Tada, Y.; Saeki, H.; Torii, H.; Asahina, A.; Onai, N.; Matsushima, K.; et al. Thymus and activation-regulated chemokine in atopic dermatitis: Serum thymus and activation-regulated chemokine level is closely related with disease activity. J. Allergy Clin. Immunol. 2001, 107, 535–541. [Google Scholar] [CrossRef]
- Slungaard, A. Platelet factor 4: A chemokine enigma. Int. J. Biochem. Cell Biol. 2005, 37, 1162–1167. [Google Scholar] [CrossRef]
- Wachowicz, B.; Morel, A.; Miller, E.; Saluk, J. The physiology of blood platelets and changes of their biological activities in multiple sclerosis. Acta Neurobiol. Exp. 2016, 76, 269–281. [Google Scholar] [CrossRef]
- Scheuerer, B.; Ernst, M.; DürRbaum-Landmann, I.; Fleischer, J.; Grage-Griebenow, E.; Brandt, E.; Flad, H.-D.; Petersen, F. The CXC-chemokine platelet factor 4 promotes monocyte survival and induces monocyte differentiation into macrophages. Blood 2000, 95, 1158–1166. [Google Scholar] [CrossRef]
- Schiemann, F.; Grimm, T.A.; Hoch, J.; Gross, R.; Lindner, B.; Petersen, F.; Bulfone-Paus, S.; Brandt, E. Mast cells and neutrophils proteolytically activate chemokine precursor CTAP-III and are subject to counterregulation by PF-4 through inhibition of chymase and cathepsin G. Blood 2006, 107, 2234–2242. [Google Scholar] [CrossRef]
- Kasperska-Zajac, A.; Nowakowski, M.; Rogala, B. Enhanced platelet activation in patients with atopic eczema/dermatitis syndrome. Inflammation 2004, 28, 299–302. [Google Scholar] [CrossRef]
- Tamagawa-Mineoka, R.; Katoh, N.; Ueda, E.; Masuda, K.; Kishimoto, S. Platelet-derived microparticles and soluble P-selectin as platelet activation markers in patients with atopic dermatitis. Clin. Immunol. 2009, 131, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Sonkoly, E.; Janson, P.; Majuri, M.-L.; Savinko, T.; Fyhrquist, N.; Eidsmo, L.; Xu, N.; Meisgen, F.; Wei, T.; Bradley, M.; et al. MiR-155 is overexpressed in patients with atopic dermatitis and modulates T-cell proliferative responses by targeting cytotoxic T lymphocyte–associated antigen 4. J. Allergy Clin. Immunol. 2010, 126, 581–589.e20. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Xue, H.; Wang, F.; Shu, C.; Zhang, J. MicroRNA-155 may be involved in the pathogenesis of atopic dermatitis by modulating the differentiation and function of T helper type 17 (Th17) cells. Clin. Exp. Immunol. 2015, 181, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Na, S.Y.; Park, M.J.; Park, S.; Lee, E.S. MicroRNA-155 regulates the Th17 immune response by targeting Ets-1 in Behçet’s disease. Clin. Exp. Rheumatol. 2016, 34 (Suppl. 102), 56–63. [Google Scholar]
- Lind, E.F.; Ohashi, P.S. Mir-155, a central modulator of T-cell responses. Eur. J. Immunol. 2014, 44, 11–15. [Google Scholar] [CrossRef]
- Okoye, I.S.; Czieso, S.; Ktistaki, E.; Roderick, K.; Coomes, S.M.; Pelly, V.S.; Kannan, Y.; Perez-Lloret, J.; Zhao, J.L.; Baltimore, D.; et al. Transcriptomics identified a critical role for Th2 cell-intrinsic miR-155 in mediating allergy and antihelminth immunity. Proc. Natl. Acad. Sci. USA 2014, 111, 30813090. [Google Scholar] [CrossRef]
- Xu, W.D.; Feng, S.Y.; Huang, A.F. Role of miR-155 in inflammatory autoimmune diseases: A comprehensive review. Inflamm. Res. 2022, 71, 1501–1517. [Google Scholar] [CrossRef]
- Zitzer, N.C.; Snyder, K.; Meng, X.; Taylor, P.A.; Efebera, Y.A.; Devine, S.M.; Blazar, B.R.; Garzon, R.; Ranganathan, P. MicroRNA-155 Modulates Acute Graft-versus-Host Disease by Impacting T Cell Expansion, Migration, and Effector Function. J. Immunol. 2018, 200, 4170–4179. [Google Scholar] [CrossRef]
- Liang, Z.; Tang, F. The potency of lncRNA MALAT1/miR-155/CTLA4 axis in altering Th1/Th2 balance of asthma. Biosci. Rep. 2020, 40, BSR20190397. [Google Scholar] [CrossRef]
- Rodriguez, A.; Vigorito, E.; Clare, S.; Warren, M.V.; Couttet, P.; Soond, D.R.; van Dongen, S.; Grocock, R.J.; Das, P.P.; Miska, E.A.; et al. Requirement of tabela bic/microRNA-155 for normal immune function. Science 2007, 316, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Daniel, E.; Roff, A.; Hsu, M.H.; Panganiban, R.; Lambert, K.; Ishmael, F. Effects of allergic stimulation and glucocorticoids on miR-155 in CD4+T-cells. Am. J. Clin. Exp. Immunol. 2018, 7, 57–66. [Google Scholar] [PubMed]
- Zhang, Y.; Sun, E.; Li, X.; Zhang, M.; Tang, Z.; He, L.; Lv, K. MiR-155 contributes to Df1-induced asthma by increasing the prolifera- tive response of Th cells via CTLA-4 downregulation. Cell. Immunol. 2017, 314, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Malmhäll, C.; Alawieh, S.; Lu, Y.; Sjöstrand, M.; Bossios, A.; Eldh, M.; Rådinger, M. MicroRNA-155 is essential for T(H)2-mediated allergen-induced eosinophilic inflammation in the lung. J. Allergy Clin. Immunol. 2014, 133, 1429–1438. [Google Scholar] [CrossRef]
- Valdman-Grinshpoun, Y.; Ben-Amitai, D.; Zvulunov, A. Barrier- restoring therapies in atopic dermatitis: Current approaches and future perspectives. Dermatol. Res. Pract. 2012, 2012, 923134. [Google Scholar] [CrossRef]
- Tanaka, A.; Muto, S.; Jung, K.; Itai, A.; Matsuda, H. Topical application with a new NF-kappaB inhibitor improves atopic dermatitis in NC/ NgaTnd mice. J. Invest. Dermatol. 2007, 127, 855–863. [Google Scholar] [CrossRef]
- Renert-Yuval, Y.; Guttman-Yassky, E. New treatments for atopic der- matitis targeting beyond IL-4/IL-13 cytokines. Ann. Allergy Asthma Immunol. 2020, 124, 28–35. [Google Scholar] [CrossRef]
- Agrawal, R.; Wisniewski, J.A.; Woodfolk, J.A. The role of regulatory T cells in atopic dermatitis. Curr. Probl. Dermatol. 2011, 41, 112–124. [Google Scholar]
| MicroRNA | Expression in AD | Mechanism of Action | Molecular Targets | Potential Clinical Significance | Experimental Model of Molecular Targets |
|---|---|---|---|---|---|
| miR-223 | Upregulated | Suppression of regulatory T cell (Treg) differentiation and function | Not precisely determined | Biomarker of AD severity; associated with prenatal tobacco smoke exposure | Human |
| miR-10a-5p | Upregulated | Inhibition of keratinocyte proliferation; modulation of cytokine expression (IL-1β, IL-4, IL-8, IL-17A, CCL5) | Hyaluronic acid synthase 3 (HAS3); MAP3K7 kinase | Regulator of skin barrier integrity | In vitro |
| miR-29b | Upregulated | Regulation of IFN-γ-induced keratinocyte apoptosis | BCL2L2 (BCL2-like 2) | Biomarker correlating with AD severity (SCORAD) | In vitro |
| miR-146a-5p | Upregulated | Negative regulator of NF-κB pathway; inhibition of inflammatory responses | IRAK1, TRAF6, CARD-10, CCL5 | Potential regulator inhibiting type 2 immune responses | Human |
| miR-151a | Upregulated | Influence on Th1/Th2 balance | Interleukin-12 receptor β2 (IL12RB2) | Potential AD biomarker | In vitro |
| miR-24 | Upregulated | Association with platelet activation | Likely associated with TARC (CCL17) | Biomarker of AD severity; correlation with platelet activation | Human |
| miR-191 | Upregulated | Association with platelet activation | Likely associated with TARC (CCL17) | Biomarker of AD severity; correlation with platelet activation | Human |
| miR-155 | Upregulated | Regulation of immune response; influence on Th17cell differentiation | CTLA-4; transcription factor c-Maf; transcription factor PU.1 | Potential diagnostic marker; complex effect on Th2 cells | Animal (mice) |
| miR-451a | Downregulated | Regulation of inflammatory processes | Interleukin 6 receptor (IL6R); proteasome subunit beta type 8 (PSMB8) | Potential diagnostic marker of AD in infants | Human |
| miR-124 | Downregulated | Control of NF-κB-dependent inflammatory responses | p65 subunit (NF-κB) | Potential therapeutic target | In vitro |
| miR-143 | Downregulated | Inhibition of IL-13 activity and inflammatory processes | Interleukin-13 receptor alpha 1 (IL-13Rα1) | Regulator of inflammatory cascade in AD | In vitro |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gołuchowska, N.; Ząber, A.; Będzichowska, A.; Tomaszewska, A.; Rustecka, A.; Kalicki, B. The Role of MicroRNA in the Pathogenesis of Atopic Dermatitis. Int. J. Mol. Sci. 2025, 26, 5846. https://doi.org/10.3390/ijms26125846
Gołuchowska N, Ząber A, Będzichowska A, Tomaszewska A, Rustecka A, Kalicki B. The Role of MicroRNA in the Pathogenesis of Atopic Dermatitis. International Journal of Molecular Sciences. 2025; 26(12):5846. https://doi.org/10.3390/ijms26125846
Chicago/Turabian StyleGołuchowska, Natalia, Aldona Ząber, Agata Będzichowska, Agata Tomaszewska, Agnieszka Rustecka, and Bolesław Kalicki. 2025. "The Role of MicroRNA in the Pathogenesis of Atopic Dermatitis" International Journal of Molecular Sciences 26, no. 12: 5846. https://doi.org/10.3390/ijms26125846
APA StyleGołuchowska, N., Ząber, A., Będzichowska, A., Tomaszewska, A., Rustecka, A., & Kalicki, B. (2025). The Role of MicroRNA in the Pathogenesis of Atopic Dermatitis. International Journal of Molecular Sciences, 26(12), 5846. https://doi.org/10.3390/ijms26125846






