The Interplay Between Exosomes and Gut Microbiota in Neuroinflammation: A New Frontier in Alzheimer’s Disease
Abstract
1. Introduction
2. The Intestinal Microbiota and Its Metabolites in the Pathophysiology of Alzheimer’s Disease
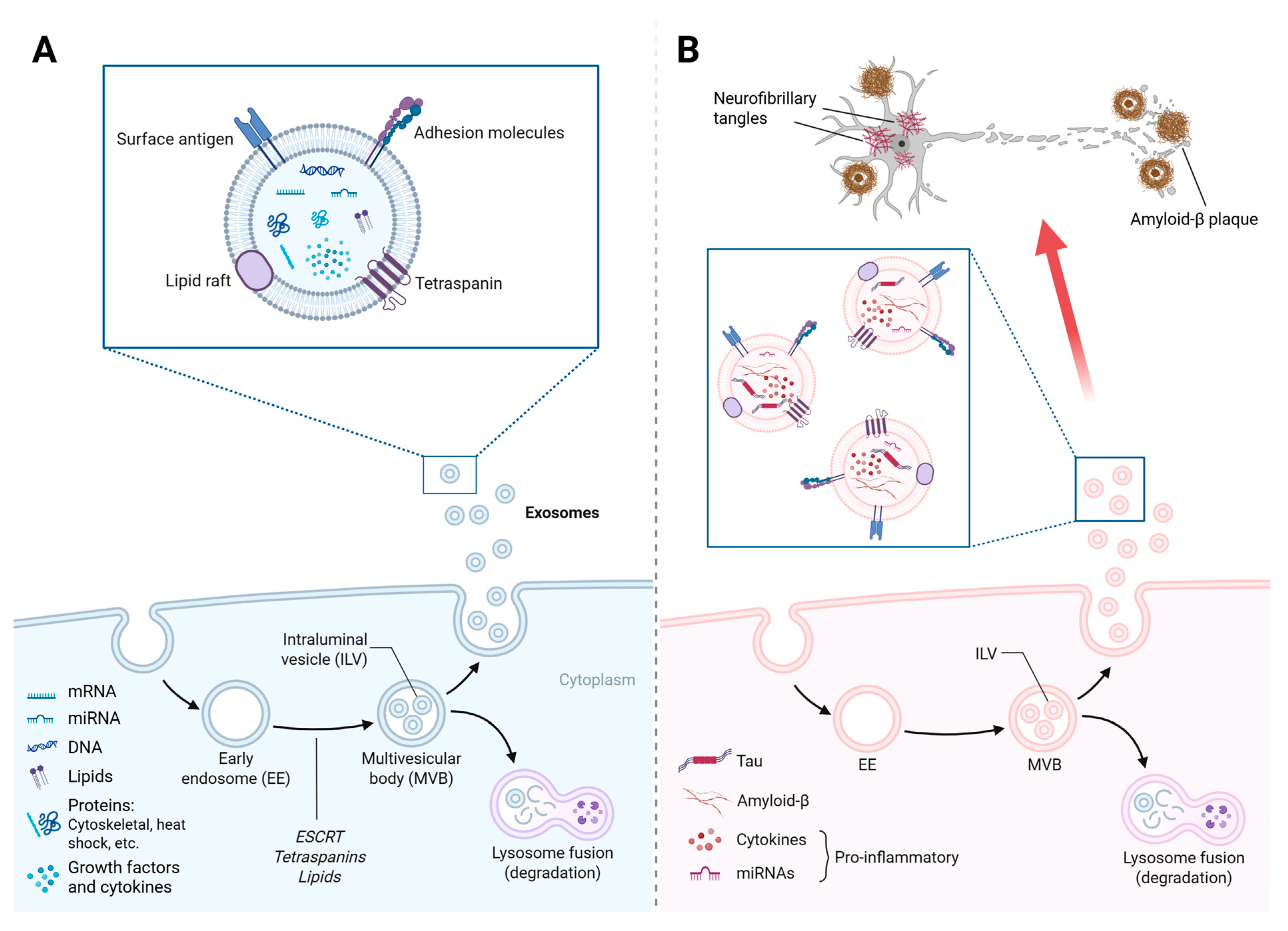
3. Exosomes in the Central Nervous System (CNS) and Their Connection with the Gut Microbiota
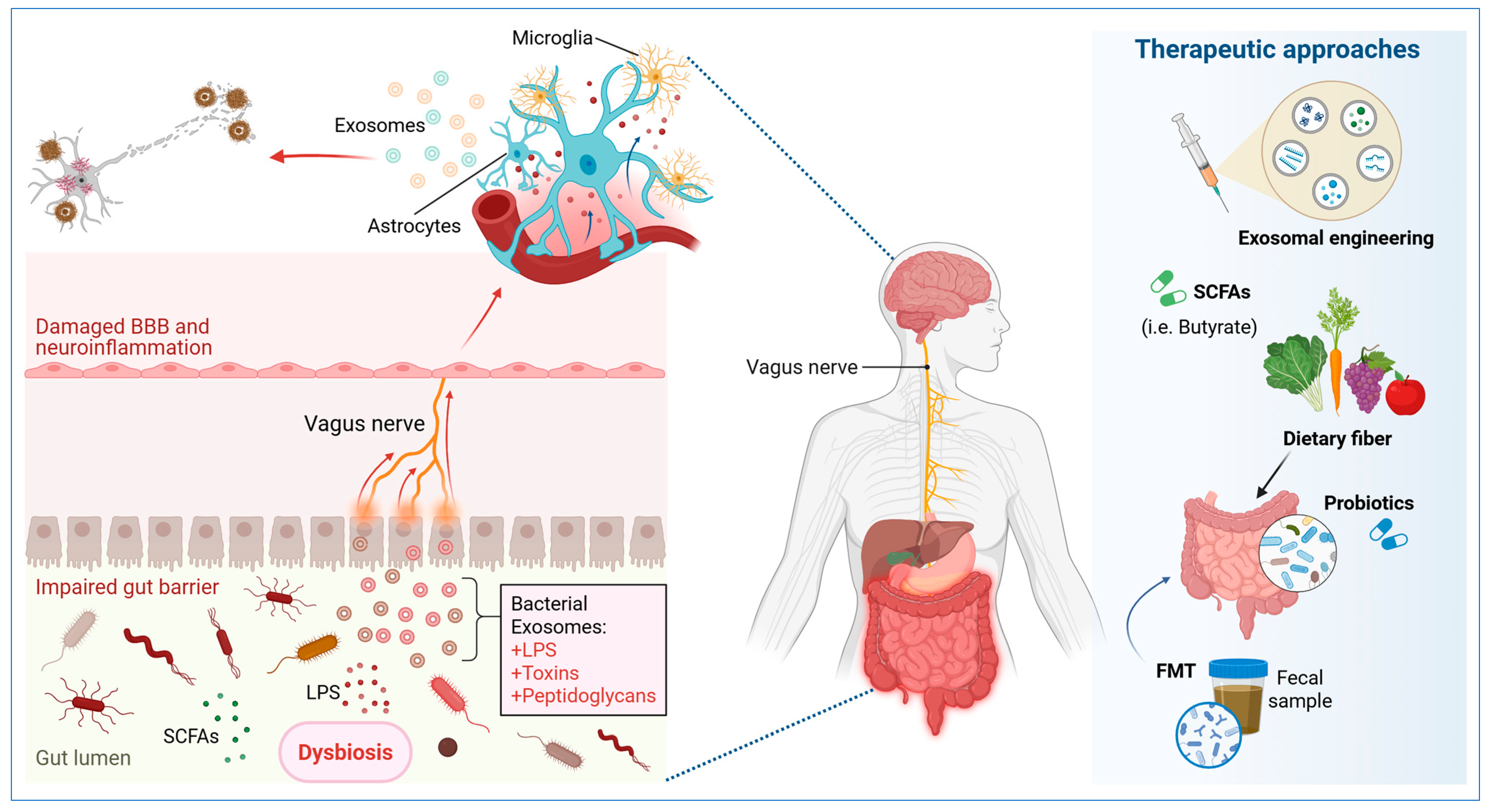
4. Exosome-Mediated Regulation of Neuroinflammation and Gut Dysbiosis in AD
5. Diagnostic and Therapeutic Applications Based on Exosomes and Microbial Metabolites
6. Challenges and Future Perspectives
7. Materials and Methods
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Depp, C.; Sun, T.; Sasmita, A.O.; Spieth, L.; Berghoff, S.A.; Nazarenko, T.; Overhoff, K.; Steixner-Kumar, A.A.; Subramanian, S.; Arinrad, S.; et al. Myelin dysfunction drives amyloid-β deposition in models of Alzheimer’s disease. Nature 2023, 618, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Li, R.; Sterling, K.; Song, W. Amyloid β-based therapy for Alzheimer’s disease: Challenges, successes and future. Signal Transduct. Target. Ther. 2023, 8, 248. [Google Scholar] [PubMed]
- Paroni, G.; Bisceglia, P.; Seripa, D. Understanding the Amyloid Hypothesis in Alzheimer’s Disease. J. Alzheimer’s Dis. 2019, 68, 493–510. [Google Scholar] [CrossRef] [PubMed]
- Marizzoni, M.; Cattaneo, A.; Mirabelli, P.; Festari, C.; Lopizzo, N.; Nicolosi, V.; Mombelli, E.; Mazzelli, M.; Luongo, D.; Naviglio, D.; et al. Short-Chain Fatty Acids and Lipopolysaccharide as Mediators Between Gut Dysbiosis and Amyloid Pathology in Alzheimer’s Disease. J. Alzheimer’s Dis. 2020, 78, 683–697. [Google Scholar] [CrossRef]
- Foster, J.A.; Lyte, M.; Meyer, E.; Cryan, J.F. Gut Microbiota and Brain Function: An Evolving Field in Neuroscience: Table 1. Int. J. Neuropsychopharmacol. 2016, 19, pyv114. [Google Scholar] [CrossRef]
- Alkhalifa, A.E.; Al-Ghraiybah, N.F.; Odum, J.; Shunnarah, J.G.; Austin, N.; Kaddoumi, A. Blood–Brain Barrier Breakdown in Alzheimer’s Disease: Mechanisms and Targeted Strategies. Int. J. Mol. Sci. 2023, 24, 16288. [Google Scholar] [CrossRef]
- Mou, Y.; Du, Y.; Zhou, L.; Yue, J.; Hu, X.; Liu, Y.; Chen, S.; Lin, X.; Zhang, G.; Xiao, H.; et al. Gut Microbiota Interact With the Brain Through Systemic Chronic Inflammation: Implications on Neuroinflammation, Neurodegeneration, and Aging. Front. Immunol. 2022, 13, 796288. [Google Scholar] [CrossRef] [PubMed]
- Raut, S.; Bhalerao, A.; Powers, M.; Gonzalez, M.; Mancuso, S.; Cucullo, L. Hypometabolism, Alzheimer’s Disease, and Possible Therapeutic Targets: An Overview. Cells 2023, 12, 2019. [Google Scholar] [CrossRef]
- De Ture, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef]
- Yin, F. Lipid metabolism and Alzheimer’s disease: Clinical evidence, mechanistic link and therapeutic promise. FEBS J. 2023, 290, 1420–1453. [Google Scholar] [CrossRef]
- Lian, P.; Cai, X.; Wang, C.; Liu, K.; Yang, X.; Wu, Y.; Zhang, Z.; Ma, Z.; Cao, X.; Xu, Y. Identification of metabolism-related subtypes and feature genes in Alzheimer’s disease. J. Transl. Med. 2023, 21, 628. [Google Scholar] [CrossRef] [PubMed]
- Uceda, S.; Echeverry-Alzate, V.; Reiriz-Rojas, M.; Martínez-Miguel, E.; Pérez-Curiel, A.; Gómez-Senent, S.; Beltrán-Velasco, A.I. Gut Microbial Metabolome and Dysbiosis in Neurodegenerative Diseases: Psychobiotics and Fecal Microbiota Transplantation as a Therapeutic Approach—A Comprehensive Narrative Review. Int. J. Mol. Sci. 2023, 24, 13294. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, S.H.; Andrews, A.M.; Paul, D.; Pachter, J.S. Extracellular vesicles: Mediators and biomarkers of pathology along CNS barriers. Fluids Barriers CNS 2018, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Lizarraga-Valderrama, L.R.; Sheridan, G.K. Extracellular vesicles and intercellular communication in the central nervous system. FEBS Lett. 2021, 595, 1391–1410. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Li, L.; Li, M.; Guo, C.; Yao, J.; Mi, S. Exosome and Exosomal MicroRNA: Trafficking, Sorting, and Function. Genom. Proteom. Bioinform. 2015, 13, 17–24. [Google Scholar] [CrossRef]
- Doyle, L.; Wang, M. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Lu, Q.; Kou, D.; Lou, S.; Ashrafizadeh, M.; Aref, A.R.; Canadas, I.; Tian, Y.; Niu, X.; Wang, Y.; Torabian, P.; et al. Nanoparticles in tumor microenvironment remodeling and cancer immunotherapy. J. Hematol. Oncol. 2024, 17, 16. [Google Scholar] [CrossRef]
- Brites, D.; Fernandes, A. Neuroinflammation and Depression: Microglia Activation, Extracellular Microvesicles and microRNA Dysregulation. Front. Cell. Neurosci. 2015, 9, 476. [Google Scholar] [CrossRef]
- Jiang, L.; Dong, H.; Cao, H.; Ji, X.; Luan, S.; Liu, J. Exosomes in Pathogenesis, Diagnosis, and Treatment of Alzheimer’s Disease. Med. Sci. Monitor. 2019, 25, 3329–3335. [Google Scholar] [CrossRef]
- Iranifar, E.; Seresht, B.M.; Momeni, F.; Fadaei, E.; Mehr, M.H.; Ebrahimi, Z.; Rahmati, M.; Kharazinejad, E.; Mirzaei, H. Exosomes and microRNAs: New potential therapeutic candidates in Alzheimer disease therapy. J. Cell. Physiol. 2019, 234, 2296–2305. [Google Scholar] [CrossRef]
- Gonda, A.; Kabagwira, J.; Senthil, G.N.; Wall, N.R. Internalization of Exosomes through Receptor-Mediated Endocytosis. Mol. Cancer Res. 2019, 17, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiao, G.; Ren, S.; Zhang, X.; Li, C.; Wu, W.; Wang, H.; Liu, H.; Zhou, H.; Chen, Y. Exosomes from bone marrow mesenchymal stem cells enhance fracture healing through the promotion of osteogenesis and angiogenesis in a rat model of nonunion. Stem Cell Res. Ther. 2020, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Riazifar, M.; Mohammadi, M.R.; Pone, E.J.; Yeri, A.; Lässer, C.; Segaliny, A.I.; McIntyre, L.L.; Shelke, G.V.; Hutchins, E.; Hamamoto, A.; et al. Stem Cell-Derived Exosomes as Nanotherapeutics for Autoimmune and Neurodegenerative Disorders. ACS Nano 2019, 13, 6670–6688. [Google Scholar] [CrossRef] [PubMed]
- Upadhya, R.; Zingg, W.; Shetty, S.; Shetty, A.K. Astrocyte-derived extracellular vesicles: Neuroreparative properties and role in the pathogenesis of neurodegenerative disorders. J. Control. Release 2020, 323, 225–239. [Google Scholar] [CrossRef]
- Cunha e Rocha, K.; Ying, W.; Olefsky, J.M. Exosome-Mediated Impact on Systemic Metabolism. Annu. Rev. Physiol. 2024, 86, 225–253. [Google Scholar] [CrossRef]
- Liu, W.L.; Lin, H.W.; Lin, M.R.; Yu, Y.; Liu, H.H.; Dai, Y.L.; Chen, L.W.; Jia, W.W.; He, X.J.; Li, X.L.; et al. Emerging blood exosome-based biomarkers for preclinical and clinical Alzheimer’s disease: A meta-analysis and systematic review. Neural Regen. Res. 2022, 17, 2381. [Google Scholar]
- Cai, H.; Pang, Y.; Wang, Q.; Qin, W.; Wei, C.; Li, Y.; Li, T.; Li, F.; Wang, Q.; Li, Y.; et al. Proteomic profiling of circulating plasma exosomes reveals novel biomarkers of Alzheimer’s disease. Alzheimers Res. Ther. 2022, 14, 181. [Google Scholar] [CrossRef]
- Zhao, H.; Zhu, L.; Wang, C.; Yang, Y. Extracellular vesicles-based theranostics for neurodegenerative diseases. WIREs Nanomed. Nanobiotechnol. 2024, 16, e1993. [Google Scholar] [CrossRef]
- Park, C.; Weerakkody, J.S.; Schneider, R.; Miao, S.; Pitt, D. CNS cell-derived exosome signatures as blood-based biomarkers of neurodegenerative diseases. Front Neurosci. 2024, 18, 1426700. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The gut microbiome in neurological disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef]
- Smith, A.R.; Mill, J.; Lunnon, K. The molecular etiology of Alzheimer’s disease. Brain Pathol. 2020, 30, 964–965. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Gao, J.; Zhu, M.; Liu, K.; Zhang, H.L. Gut Microbiota and Dysbiosis in Alzheimer’s Disease: Implications for Pathogenesis and Treatment. Mol. Neurobiol. 2020, 57, 5026–5043. [Google Scholar] [CrossRef]
- Ji, D.; Chen, W.Z.; Zhang, L.; Zhang, Z.H.; Chen, L.J. Gut microbiota, circulating cytokines and dementia: A Mendelian randomization study. J. Neuroinflamm. 2024, 21, 2. [Google Scholar] [CrossRef]
- Jemimah, S.; Chabib, C.M.M.; Hadjileontiadis, L.; AlShehhi, A. Gut microbiome dysbiosis in Alzheimer’s disease and mild cognitive impairment: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0285346. [Google Scholar] [CrossRef] [PubMed]
- Hickman, S.; Izzy, S.; Sen, P.; Morsett, L.; El Khoury, J. Microglia in neurodegeneration. Nat. Neurosci. 2018, 21, 1359–1369. [Google Scholar] [CrossRef]
- Zakharova, N.V.; Bugrova, A.E.; Indeykina, M.I.; Fedorova, Y.B.; Kolykhalov, I.V.; Gavrilova, S.I.; Nikolaev, E.N.; Kononikhin, A.S. Proteomic Markers and Early Prediction of Alzheimer’s Disease. Biochemistry 2022, 87, 762–776. [Google Scholar] [CrossRef]
- Zhang, T.; Gao, G.; Kwok, L.Y.; Sun, Z. Gut microbiome-targeted therapies for Alzheimer’s disease. Gut Microbes 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Wilton, D.K.; Dissing-Olesen, L.; Stevens, B. Neuron-Glia Signaling in Synapse Elimination. Annu. Rev. Neurosci. 2019, 42, 107–127. [Google Scholar] [CrossRef]
- Jiang, L.; Li, J.; Tang, B.; Guo, J. Associations between gut microbiota and Parkinson disease: A bidirectional Mendelian randomization analysis. Eur. J. Neurol. 2023, 30, 3471–3477. [Google Scholar] [CrossRef]
- Zhong, J.G.; Lan, W.T.; Feng, Y.Q.; Li, Y.H.; Shen, Y.Y.; Gong, J.H.; Zou, Z.; Hou, X. Associations between dysbiosis gut microbiota and changes of neurotransmitters and short-chain fatty acids in valproic acid model rats. Front. Physiol. 2023, 14, 1077821. [Google Scholar] [CrossRef]
- Chen, C.; Liao, J.; Xia, Y.; Liu, X.; Jones, R.; Haran, J.; McCormick, B.; Sampson, T.R.; Alam, A.; Ye, K. Gut microbiota regulate Alzheimer’s disease pathologies and cognitive disorders via PUFA-associated neuroinflammation. Gut 2022, 71, 2233–2252. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.Y.; Lee, H.Y.; Choi, Y.Y.; Mo, S.J.; Jeon, S.; Ha, J.H.; Park, S.D.; Shim, J.J.; Lee, J.; Chung, B.G. Effect of gut microbiota-derived metabolites and extracellular vesicles on neurodegenerative disease in a gut-brain axis chip. Nano Converg. 2024, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Jiang, H.; Liu, T.; Yan, J.; Zhang, F.; Zhang, X.; Zhao, J.; Mu, X.; Jiang, J. Depletion of gut microbiota resistance in 5×FAD mice enhances the therapeutic effect of mesenchymal stem cell-derived exosomes. Biomed. Pharmacother. 2023, 161, 114455. [Google Scholar] [CrossRef]
- Kesika, P.; Suganthy, N.; Sivamaruthi, B.S.; Chaiyasut, C. Role of gut-brain axis, gut microbial composition, and probiotic intervention in Alzheimer’s disease. Life Sci. 2021, 264, 118627. [Google Scholar] [CrossRef]
- Pirolli, N.H.; Bentley, W.E.; Jay, S.M. Bacterial Extracellular Vesicles and the Gut-Microbiota Brain Axis: Emerging Roles in Communication and Potential as Therapeutics. Adv. Biol. 2021, 5, 2000540. [Google Scholar] [CrossRef] [PubMed]
- Haas-Neill, S.; Forsythe, P. A Budding Relationship: Bacterial Extracellular Vesicles in the Microbiota-Gut-Brain Axis. Int. J. Mol. Sci. 2020, 21, 8899. [Google Scholar] [CrossRef]
- Fock, E.; Parnova, R. Mechanisms of Blood–Brain Barrier Protection by Microbiota-Derived Short-Chain Fatty Acids. Cells 2023, 12, 657. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, H.; Zhang, X.; Wang, W.; Chen, Y.; Cai, Z.; Wang, Q.; Wang, J.; Shi, Y. Promotion of astrocyte-neuron glutamate-glutamine shuttle by SCFA contributes to the alleviation of Alzheimer’s disease. Redox Biol. 2023, 62, 102690. [Google Scholar] [CrossRef]
- Dong, Y.; Cui, C. The role of short-chain fatty acids in central nervous system diseases. Mol. Cell. Biochem. 2022, 477, 2595–2607. [Google Scholar] [CrossRef]
- Almeida, C.; Oliveira, R.; Soares, R.; Barata, P. Influence of gut microbiota dysbiosis on brain function: A systematic review. Porto Biomed. J. 2020, 5, 1. [Google Scholar] [CrossRef]
- Andersen, J.V.; Schousboe, A.; Verkhratsky, A. Astrocyte energy and neurotransmitter metabolism in Alzheimer’s disease: Integration of the glutamate/GABA-glutamine cycle. Prog. Neurobiol. 2022, 217, 102331. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, L.Y.; Xu, Y.; Wang, W.Z.; Qiu, N.Z.; Zhang, F.F.; Zhang, F.; Wang, X.D.; Chen, W.; Xu, X.; et al. Imbalance of multiple neurotransmitter pathways leading to depression-like behavior and cognitive dysfunction in the triple transgenic mouse model of Alzheimer disease. Metab. Brain Dis. 2023, 38, 2465–2476. [Google Scholar] [CrossRef] [PubMed]
- Mondal, J.; Pillarisetti, S.; Junnuthula, V.; Saha, M.; Hwang, S.R.; Park, I.K.; Lee, Y.K. Hybrid exosomes, exosome-like nanovesicles and engineered exosomes for therapeutic applications. J. Control. Release 2023, 353, 1127–1149. [Google Scholar] [CrossRef]
- Song, Y.; Li, Z.; He, T.; Qu, M.; Jiang, L.; Li, W.; Shi, X.; Pan, J.; Zhang, L.; Wang, Y.; et al. M2 microglia-derived exosomes protect the mouse brain from ischemia-reperfusion injury via exosomal miR-124. Theranostics 2019, 9, 2910–2923. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Hu, S.; Wu, Z.; He, M. Therapeutic effects of engineered exosome-based miR-25 and miR-181a treatment in spinocerebellar ataxia type 3 mice by silencing ATXN3. Mol. Med. 2023, 29, 96. [Google Scholar] [CrossRef]
- Winston, C.N.; Aulston, B.; Rockenstein, E.M.; Adame, A.; Prikhodko, O.; Dave, K.N.; Mishra, P.; Rissman, R.A.; Yuan, S.H. Neuronal Exosome-Derived Human Tau is Toxic to Recipient Mouse Neurons in vivo. J. Alzheimer’s Dis. 2019, 67, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhashi, S.; Feldbrügge, L.; Csizmadia, E.; Mitsuhashi, M.; Robson, S.C.; Moss, A.C. Luminal Extracellular Vesicles (EVs) in Inflammatory Bowel Disease (IBD) Exhibit Proinflammatory Effects on Epithelial Cells and Macrophages. Inflamm. Bowel Dis. 2016, 22, 1587–1595. [Google Scholar] [CrossRef]
- Shen, Q.; Huang, Z.; Yao, J.; Jin, Y. Extracellular vesicles-mediated interaction within intestinal microenvironment in inflammatory bowel disease. J. Adv. Res. 2022, 37, 221–233. [Google Scholar] [CrossRef]
- Garcia-Martin, R.; Brandao, B.B.; Thomou, T.; Altindis, E.; Kahn, C.R. Tissue differences in the exosomal/small extracellular vesicle proteome and their potential as indicators of altered tissue metabolism. Cell Rep. 2022, 38, 110277. [Google Scholar] [CrossRef]
- Su, L.; Li, R.; Zhang, Z.; Liu, J.; Du, J.; Wei, H. Identification of altered exosomal microRNAs and mRNAs in Alzheimer’s disease. Ageing Res. Rev. 2022, 73, 101497. [Google Scholar] [CrossRef]
- Cheng, Z.; Yang, L.; Chu, H. The role of gut microbiota, exosomes, and their interaction in the pathogenesis of ALD. J. Adv. Res. 2024, 31, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Luh, F.; Ho, Y.S.; Yen, Y. Exosomes: A review of biologic function, diagnostic and targeted therapy applications, and clinical trials. J. Biomed. Sci. 2024, 31, 67. [Google Scholar] [CrossRef]
- Wang, J.; Barr, M.M.; Wehman, A.M. Extracellular vesicles. Genetics 2024, 227, iyae088. [Google Scholar] [CrossRef] [PubMed]
- Si, Q.; Wu, L.; Pang, D.; Jiang, P. Exosomes in brain diseases: Pathogenesis and therapeutic targets. MedComm 2023, 4, e287. [Google Scholar] [CrossRef]
- Shah, P.; Cho, S.K.; Thulstrup, P.W.; Bjerrum, M.J.; Lee, P.H.; Kang, J.H.; Bhang, Y.J.; Yang, S.W. MicroRNA Biomarkers in Neurodegenerative Diseases and Emerging Nano-Sensors Technology. J. Mov. Disord. 2017, 10, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Gómez, J.A.; Kavanagh, E.; Engskog-Vlachos, P.; Engskog, M.K.R.; Herrera, A.J.; Espinosa-Oliva, A.M.; Joseph, B.; Hajji, N.; Venero, J.L.; Burguillos, M.A. Microglia: Agents of the CNS Pro-Inflammatory Response. Cells 2020, 9, 1717. [Google Scholar] [CrossRef]
- Wang, C.; Zong, S.; Cui, X.; Wang, X.; Wu, S.; Wang, L.; Liu, Y.; Lu, Z. The effects of microglia-associated neuroinflammation on Alzheimer’s disease. Front. Immunol. 2023, 14, 1117172. [Google Scholar] [CrossRef]
- Gayen, M.; Bhomia, M.; Balakathiresan, N.; Knollmann-Ritschel, B. Exosomal MicroRNAs Released by Activated Astrocytes as Potential Neuroinflammatory Biomarkers. Int. J. Mol. Sci. 2020, 21, 2312. [Google Scholar] [CrossRef]
- Hernandez, P.; Rackles, E.; Alboniga, O.E.; Martínez-Lage, P.; Camacho, E.N.; Onaindia, A.; Fernandez, M.; Talamillo, A.; Falcon-Perez, J.M. Metabolic Profiling of Brain Tissue and Brain-Derived Extracellular Vesicles in Alzheimer’s Disease. J. Extracell. Vesicles 2025, 14, e70043. [Google Scholar] [CrossRef]
- Nakashima, M.; Suga, N.; Fukumoto, A.; Yoshikawa, S.; Matsuda, S. Exosomes, Endosomes, and Caveolae as Encouraging Targets with Favorable Gut Microbiota for the Innovative Treatment of Alzheimer’s Diseases. Discov. Med. 2024, 36, 2132. [Google Scholar] [CrossRef]
- Liu, S.; Gao, J.; Liu, K.; Zhang, H.L. Microbiota-gut-brain axis and Alzheimer’s disease: Implications of the blood-brain barrier as an intervention target. Mech. Ageing Dev. 2021, 199, 111560. [Google Scholar] [CrossRef] [PubMed]
- Nila, I.S.; Sumsuzzman DMd Khan, Z.A.; Jung, J.H.; Kazema, A.S.; Kim, S.J.; Hong, Y. Identification of exosomal biomarkers and its optimal isolation and detection method for the diagnosis of Parkinson’s disease: A systematic review and meta-analysis. Ageing Res. Rev. 2022, 82, 101764. [Google Scholar] [CrossRef] [PubMed]
- Bhusal, A.; Afridi, R.; Lee, W.H.; Suk, K. Bidirectional Communication Between Microglia and Astrocytes in Neuroinflammation. Curr. Neuropharmacol. 2023, 21, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- Brites, D.; Vaz, A.R. Microglia centered pathogenesis in ALS: Insights in cell interconnectivity. Front. Cell. Neurosci. 2014, 8, 117. [Google Scholar] [CrossRef]
- Kaur, D.; Sharma, V.; Deshmukh, R. Activation of microglia and astrocytes: A roadway to neuroinflammation and Alzheimer’s disease. Inflammopharmacology 2019, 27, 663–677. [Google Scholar] [CrossRef]
- Hassanpour, P.; Sadeghsoltani, F.; Safari, M.; Haiaty, S.; Rahbarghazi, R.; Mota, A.; Rahmati, M. Role of Toll-like receptors in exosome biogenesis and angiogenesis capacity. BioImpacts 2024, 15, 30333. [Google Scholar] [CrossRef]
- Pascual, M.; Calvo-Rodriguez, M.; Núñez, L.; Villalobos, C.; Ureña, J.; Guerri, C. Toll-like receptors in neuroinflammation, neurodegeneration, and alcohol-induced brain damage. IUBMB Life 2021, 73, 900–915. [Google Scholar] [CrossRef]
- Paschon, V.; Takada, S.H.; Ikebara, J.M.; Sousa, E.; Raeisossadati, R.; Ulrich, H.; Kihara, A.H. Interplay Between Exosomes, microRNAs and Toll-Like Receptors in Brain Disorders. Mol. Neurobiol. 2016, 53, 2016–2028. [Google Scholar] [CrossRef]
- Chen, J.; Chen, J.; Cheng, Y.; Fu, Y.; Zhao, H.; Tang, M.; Zhao, H.; Lin, N.; Shi, X.; Lei, Y.; et al. Mesenchymal stem cell-derived exosomes protect beta cells against hypoxia-induced apoptosis via miR-21 by alleviating ER stress and inhibiting p38 MAPK phosphorylation. Stem Cell Res. Ther. 2020, 11, 97. [Google Scholar] [CrossRef]
- Li, J.; Huang, Y.; Sun, H.; Yang, L. Mechanism of mesenchymal stem cells and exosomes in the treatment of age-related diseases. Front Immunol. 2023, 14, 1181308. [Google Scholar] [CrossRef]
- Bischoff, J.P.; Schulz, A.; Morrison, H. The role of exosomes in intercellular and inter-organ communication of the peripheral nervous system. FEBS Lett. 2022, 596, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Noonin, C.; Thongboonkerd, V. Exosome-inflammasome crosstalk and their roles in inflammatory responses. Theranostics 2021, 11, 4436–4451. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kang, M.H.; Kim, J.H. A Comprehensive Review on Factors Influences Biogenesis, Functions, Therapeutic and Clinical Implications of Exosomes. Int. J. Nanomed. 2021, 16, 1281–1312. [Google Scholar] [CrossRef] [PubMed]
- Inotsuka, R.; Udono, M.; Yamatsu, A.; Kim, M.; Katakura, Y. Exosome-Mediated Activation of Neuronal Cells Triggered by γ-Aminobutyric Acid (GABA). Nutrients 2021, 13, 2544. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Li, C.; Szalad, A.; Wang, L.; Pan, W.; Zhang, R.; Chopp, M.; Zhang, Z.G.; Liu, X.S. Mesenchymal stromal cell-derived exosomes ameliorate peripheral neuropathy in a mouse model of diabetes. Diabetologia 2020, 63, 431–443. [Google Scholar] [CrossRef]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in neurodegenerative diseases: Mechanism and potential therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 359. [Google Scholar] [CrossRef]
- Buratta, S.; Tancini, B.; Sagini, K.; Delo, F.; Chiaradia, E.; Urbanelli, L.; Emiliani, C. Lysosomal Exocytosis, Exosome Release and Secretory Autophagy: The Autophagic- and Endo-Lysosomal Systems Go Extracellular. Int. J. Mol. Sci. 2020, 21, 2576. [Google Scholar] [CrossRef]
- Soares Martins, T.; Trindade, D.; Vaz, M.; Campelo, I.; Almeida, M.; Trigo, G.; da Cruz E Silva, O.A.B.; Henriques, A.G. Diagnostic and therapeutic potential of exosomes in Alzheimer’s disease. J. Neurochem. 2021, 156, 162–181. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, B.; Wang, J.; Xu, L.; Yu, S.; Fu, J.; Yan, X.; Su, J. Aβ and Tau Regulate Microglia Metabolism via Exosomes in Alzheimer’s Disease. Biomedicines 2022, 10, 1800. [Google Scholar] [CrossRef]
- He, A.; Wang, M.; Li, X.; Chen, H.; Lim, K.; Lu, L.; Zhang, C. Role of Exosomes in the Pathogenesis and Theranostic of Alzheimer’s Disease and Parkinson’s Disease. Int. J. Mol. Sci. 2023, 24, 11054. [Google Scholar] [CrossRef]
- Sharma, A. Mitochondrial cargo export in exosomes: Possible pathways and implication in disease biology. J. Cell. Physiol. 2023, 238, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Satarker, S.; Bojja, S.L.; Gurram, P.C.; Mudgal, J.; Arora, D.; Nampoothiri, M. Astrocytic Glutamatergic Transmission and Its Implications in Neurodegenerative Disorders. Cells 2022, 11, 1139. [Google Scholar] [CrossRef] [PubMed]
- Arnold, F.J.; Putka, A.F.; Raychaudhuri, U.; Hsu, S.; Bedlack, R.S.; Bennett, C.L.; La Spada, A.R. Revisiting Glutamate Excitotoxicity in Amyotrophic Lateral Sclerosis and Age-Related Neurodegeneration. Int. J. Mol. Sci. 2024, 25, 5587. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Garrido, N.; Cordero, C.; Olivo-Martinez, Y.; Badia, J.; Baldomà, L. Cell-to-Cell Communication by Host-Released Extracellular Vesicles in the Gut: Implications in Health and Disease. Int. J. Mol. Sci. 2021, 22, 2213. [Google Scholar] [CrossRef]
- Lin, L.; Tang, R.; Liu, Y.; Li, Z.; Li, H.; Yang, H. The brain-protective mechanism of fecal microbiota transplantation from young donor mice in the natural aging process via exosome, gut microbiota, and metabolomics analyses. Pharmacol. Res. 2024, 207, 107323. [Google Scholar] [CrossRef]
- Li, Q.; Xiao, N.; Zhang, H.; Liang, G.; Lin, Y.; Qian, Z.; Yang, X.; Yang, J.; Fu, Y.; Zhang, C.; et al. Systemic aging and aging-related diseases. FASEB J. 2025, 39, e70430. [Google Scholar] [CrossRef]
- Sun, K.; Zheng, X.; Jin, H.; Yu, F.; Zhao, W. Exosomes as CNS Drug Delivery Tools and Their Applications. Pharmaceutics 2022, 14, 2252. [Google Scholar] [CrossRef]
- Mukerjee, N.; Bhattacharya, A.; Maitra, S.; Kaur, M.; Ganesan, S.; Mishra, S.; Ashraf, A.; Rizwan, M.; Kesari, K.K.; Tabish, T.A.; et al. Exosome isolation and characterization for advanced diagnostic and therapeutic applications. Mater. Today Bio. 2025, 31, 101613. [Google Scholar] [CrossRef]
- Hornung, S.; Dutta, S.; Bitan, G. CNS-Derived Blood Exosomes as a Promising Source of Biomarkers: Opportunities and Challenges. Front. Mol. Neurosci. 2020, 13, 38. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Z.; Li, N.; Tian, C.; Yang, H.; Huo, Y.; Li, Y.; Zhang, J.; Yu, Z. Parkinson’s Disease Derived Exosomes Aggravate Neuropathology in SNCA*A53T Mice. Ann. Neurol. 2022, 92, 230–245. [Google Scholar] [CrossRef]
- Xylaki, M.; Chopra, A.; Weber, S.; Bartl, M.; Outeiro, T.F.; Mollenhauer, B. Extracellular Vesicles for the Diagnosis of Parkinson’s Disease: Systematic Review and Meta-Analysis. Mov. Disord. 2023, 38, 1585–1597. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Y.; Zheng, J.C. Extracellular vesicles, from the pathogenesis to the therapy of neurodegenerative diseases. Transl. Neurodegener. 2022, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Lopes, D.; Lopes, J.; Pereira-Silva, M.; Peixoto, D.; Rabiee, N.; Veiga, F.; Moradi, O.; Guo, Z.H.; Wang, X.D.; Conde, J.; et al. Bioengineered exosomal-membrane-camouflaged abiotic nanocarriers: Neurodegenerative diseases, tissue engineering and regenerative medicine. Mil. Med. Res. 2023, 10, 19. [Google Scholar] [CrossRef]
- Liu, R.; Wang, J.; Chen, Y.; Collier, J.M.; Capuk, O.; Jin, S.; Sun, M.; Mondal, S.K.; Whiteside, T.L.; Stolz, D.B.; et al. NOX activation in reactive astrocytes regulates astrocytic LCN2 expression and neurodegeneration. Cell Death Dis. 2022, 13, 371. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Fan, M.; Xu, J.X.; Yang, L.J.; Qi, C.C.; Xia, Q.R.; Ge, J.F. Exosomes derived from bone-marrow mesenchymal stem cells alleviate cognitive decline in AD-like mice by improving BDNF-related neuropathology. J. Neuroinflamm. 2022, 19, 35. [Google Scholar] [CrossRef] [PubMed]
- Hua, T.; Yang, M.; Song, H.; Kong, E.; Deng, M.; Li, Y.; Li, J.; Liu, Z.; Fu, H.; Wang, Y.; et al. Huc-MSCs-derived exosomes attenuate inflammatory pain by regulating microglia pyroptosis and autophagy via the miR-146a-5p/TRAF6 axis. J. Nanobiotechnol. 2022, 20, 324. [Google Scholar] [CrossRef]
- Ocansey, D.K.W.; Zhang, L.; Wang, Y.; Yan, Y.; Qian, H.; Zhang, X.; Xu, W.; Mao, F. Exosome-mediated effects and applications in inflammatory bowel disease. Biol. Rev. 2020, 95, 1287–1307. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Czapski, G.A.; Strosznajder, J.B. Glutamate and GABA in Microglia-Neuron Cross-Talk in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 11677. [Google Scholar] [CrossRef]
- Conn, K.A.; Borsom, E.M.; Cope, E.K. Implications of microbe-derived ɣ-aminobutyric acid (GABA) in gut and brain barrier integrity and GABAergic signaling in Alzheimer’s disease. Gut Microbes 2024, 16, 2371950. [Google Scholar] [CrossRef]
- O’Riordan, K.J.; Collins, M.K.; Moloney, G.M.; Knox, E.G.; Aburto, M.R.; Fülling, C.; Morley, S.J.; Clarke, G.; Schellekens, H.; Cryan, J.F. Short chain fatty acids: Microbial metabolites for gut-brain axis signalling. Mol. Cell. Endocrinol. 2022, 546, 111572. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Dallas, S.L. Exosomes and Extracellular RNA in Muscle and Bone Aging and Crosstalk. Curr. Osteoporos. Rep. 2019, 17, 548–559. [Google Scholar] [CrossRef]
- Zhu, Z.; Huang, J.; Li, X.; Xing, J.; Chen, Q.; Liu, R.; Hua, F.; Qiu, Z.; Song, Y.; Bai, C.; et al. Gut microbiota regulate tumor metastasis via circRNA/miRNA networks. Gut Microbes. 2020, 12, 1788891. [Google Scholar] [CrossRef]
- Yu, L.; Zheng, J.; Li, J.; Wang, Y.; Lu, X.; Fan, X. Integrating serum exosomal microRNA and liver microRNA profiles disclose the function role of autophagy and mechanisms of Fructus Meliae Toosendan-induced hepatotoxicity in mice. Biomed. Pharmacother. 2020, 123, 109709. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.J.; Chau, Z.L.; Chen, S.; Hill, J.J.; Korpany, K.V.; Liang, N.; Lin, L.H.; Lin, Y.H.; Liu, J.K.; Liu, Y.C.; et al. Exosome Processing and Characterization Approaches for Research and Technology Development. Adv. Sci. 2022, 9, e2103222. [Google Scholar] [CrossRef]
- Sadeghi, S.; Tehrani, F.R.; Tahmasebi, S.; Shafiee, A.; Hashemi, S.M. Exosome engineering in cell therapy and drug delivery. Inflammopharmacology 2023, 31, 145–169. [Google Scholar] [CrossRef] [PubMed]
- Yi, Q.; Xu, Z.; Thakur, A.; Zhang, K.; Liang, Q.; Liu, Y.; Yan, Y. Current understanding of plant-derived exosome-like nanoparticles in regulating the inflammatory response and immune system microenvironment. Pharmacol. Res. 2023, 190, 106733. [Google Scholar] [CrossRef]
- Iyaswamy, A.; Thakur, A.; Guan, X.J.; Krishnamoorthi, S.; Fung, T.Y.; Lu, K.; Gaurav, I.; Yang, Z.; Su, C.F.; Lau, K.F.; et al. Fe65-engineered neuronal exosomes encapsulating corynoxine-B ameliorate cognition and pathology of Alzheimer’s disease. Signal Transduct. Target Ther. 2023, 8, 404. [Google Scholar] [CrossRef]
- Ozansoy, M.; Mikati, H.; Velioglu, H.A.; Yulug, B. Exosomes: A missing link between chronic systemic inflammation and Alzheimer’s disease? Biomed. Pharmacother. 2023, 159, 114161. [Google Scholar] [CrossRef]
- D’Acunzo, P.; Kim, Y.; Ungania, J.M.; Pérez-González, R.; Goulbourne, C.N.; Levy, E. Isolation of mitochondria-derived mitovesicles and subpopulations of microvesicles and exosomes from brain tissues. Nat. Protoc. 2022, 17, 2517–2549. [Google Scholar] [CrossRef]
- Melnik, M.; Miyoshi, E.; Ma, R.; Corrada, M.; Kawas, C.; Bohannan, R.; Caraway, C.; Miller, C.A.; Hinman, J.D.; John, V.; et al. Simultaneous isolation of intact brain cells and cell-specific extracellular vesicles from cryopreserved Alzheimer’s disease cortex. J. Neurosci. Methods 2024, 406, 110137. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.J.; Tafti, H.A.; Amanzadeh, A.; Rabbani, S.; Shokrgozar, M.A.; Heidari, R.; Behroozi, J.; Eyni, H.; Uversky, V.N.; Ghanbari, H. Comparison of the efficiency of ultrafiltration, precipitation, and ultracentrifugation methods for exosome isolation. Biochem. Biophys. Rep. 2024, 38, 101668. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Li, H.; Xu, G.; Hu, Y.; Zhang, W.; Tian, K. Current Knowledge and Future Perspectives of Exosomes as Nanocarriers in Diagnosis and Treatment of Diseases. Int. J. Nanomed. 2023, 18, 4751–4778. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liang, A.; Huang, J. Exendin-4-enriched exosomes from hUCMSCs alleviate diabetic nephropathy via gut microbiota and immune modulation. Front. Microbiol. 2024, 15, 1399632. [Google Scholar] [CrossRef]
- Ji, X.Y.; Guo, Y.X.; Wang, L.B.; Wu, W.C.; Wang, J.Q.; He, J.; Gao, R.; Rasouli, J.; Gao, M.Y.; Wang, Z.H.; et al. Microglia-derived exosomes modulate myelin regeneration via miR-615-5p/MYRF axis. J. Neuroinflam. 2024, 21, 29. [Google Scholar] [CrossRef]
- Hung, C.C.; Chang, C.C.; Huang, C.W.; Nouchi, R.; Cheng, C.H. Gut microbiota in patients with Alzheimer’s disease spectrum: A systematic review and meta-analysis. Aging 2022, 14, 477–496. [Google Scholar] [CrossRef]
- Soares Martins, T.; Catita, J.; Martins Rosa, I.A.B.; da Cruz e Silva, O.; Henriques, A.G. Exosome isolation from distinct biofluids using precipitation and column-based approaches. PLoS ONE 2018, 13, e0198820. [Google Scholar] [CrossRef]
- Hasan, I.; Guo, B.; Zhang, J.; Chang, C. Advances in Antioxidant Nanomedicines for Imaging and Therapy of Alzheimer’s Disease. Antioxid. Redox Signal. 2024, 40, 863–888. [Google Scholar] [CrossRef]
- Ribeiro, J.; Lopes, I.; Gomes, A.C. A New Perspective for the Treatment of Alzheimer’s Disease: Exosome-like Liposomes to Deliver Natural Compounds and RNA Therapies. Molecules 2023, 28, 6015. [Google Scholar] [CrossRef]
- Cano, A.; Ettcheto, M.; Bernuz, M.; Puerta, R.; de Antonio, E.; Sánchez-López, E.; Souto, E.B.; Camins, A.; Martí, M.; Pividori, M.I.; et al. Extracellular vesicles, the emerging mirrors of brain physiopathology. Int. J. Biol. Sci. 2023, 19, 721–743. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uceda, S.; Reiriz, M.; Echeverry-Alzate, V.; Beltrán-Velasco, A.I. The Interplay Between Exosomes and Gut Microbiota in Neuroinflammation: A New Frontier in Alzheimer’s Disease. Int. J. Mol. Sci. 2025, 26, 5828. https://doi.org/10.3390/ijms26125828
Uceda S, Reiriz M, Echeverry-Alzate V, Beltrán-Velasco AI. The Interplay Between Exosomes and Gut Microbiota in Neuroinflammation: A New Frontier in Alzheimer’s Disease. International Journal of Molecular Sciences. 2025; 26(12):5828. https://doi.org/10.3390/ijms26125828
Chicago/Turabian StyleUceda, Sara, Manuel Reiriz, Víctor Echeverry-Alzate, and Ana Isabel Beltrán-Velasco. 2025. "The Interplay Between Exosomes and Gut Microbiota in Neuroinflammation: A New Frontier in Alzheimer’s Disease" International Journal of Molecular Sciences 26, no. 12: 5828. https://doi.org/10.3390/ijms26125828
APA StyleUceda, S., Reiriz, M., Echeverry-Alzate, V., & Beltrán-Velasco, A. I. (2025). The Interplay Between Exosomes and Gut Microbiota in Neuroinflammation: A New Frontier in Alzheimer’s Disease. International Journal of Molecular Sciences, 26(12), 5828. https://doi.org/10.3390/ijms26125828







