A Randomized Controlled Trial Evaluating the Effects of a Probiotic Containing Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 on Gastrointestinal Symptoms and Metabolomic Profiles in Female Dancers
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics
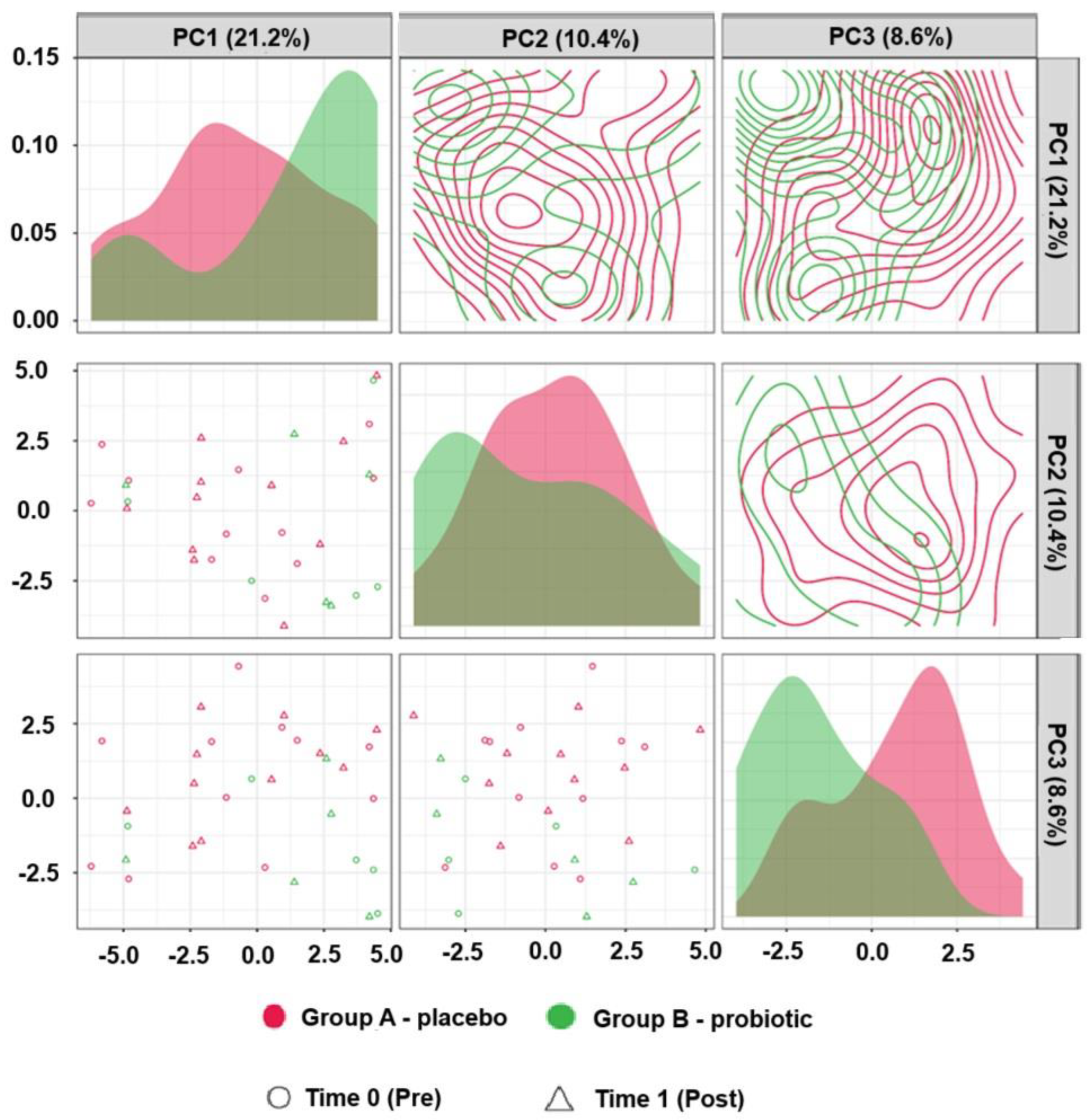
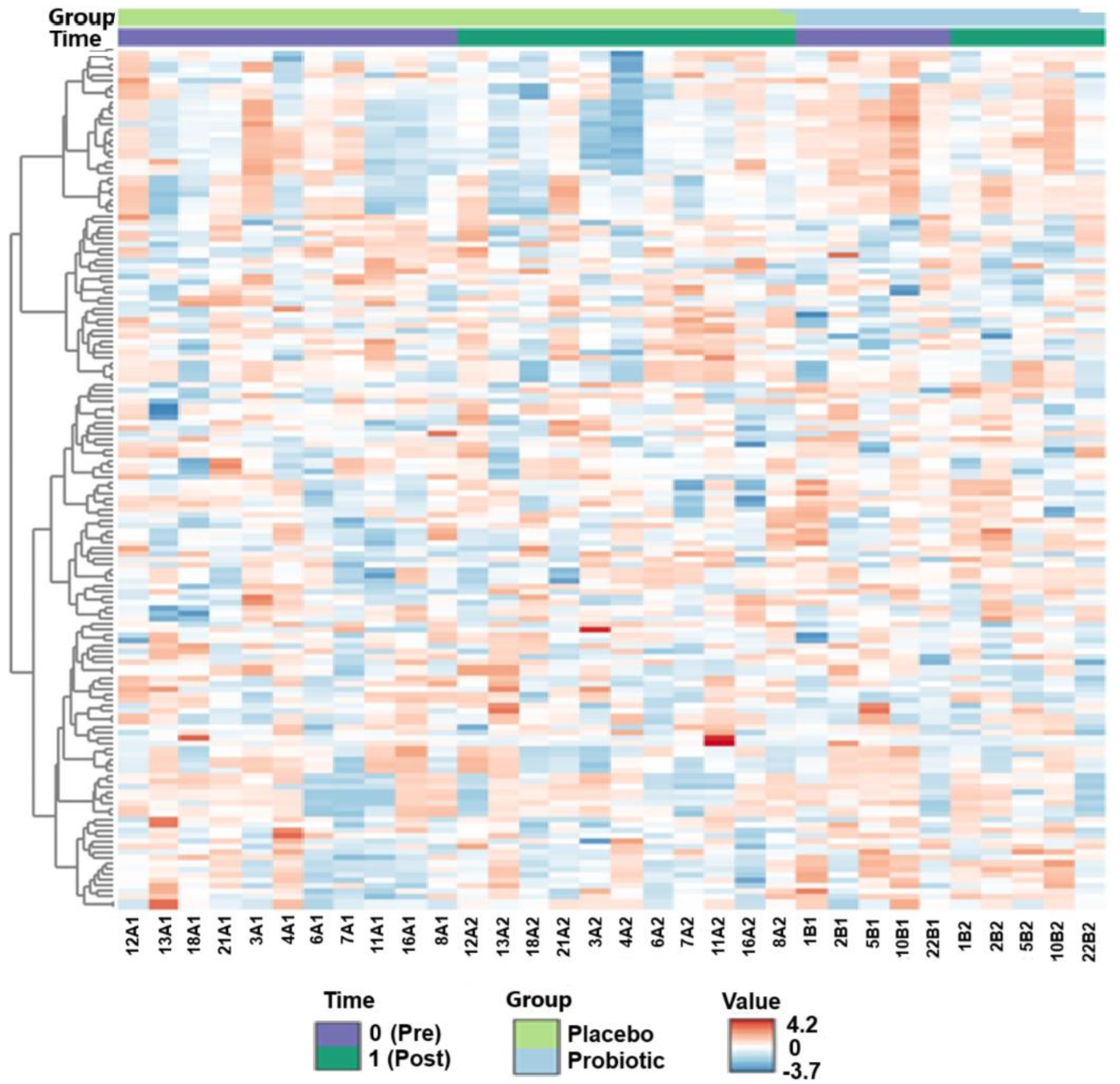
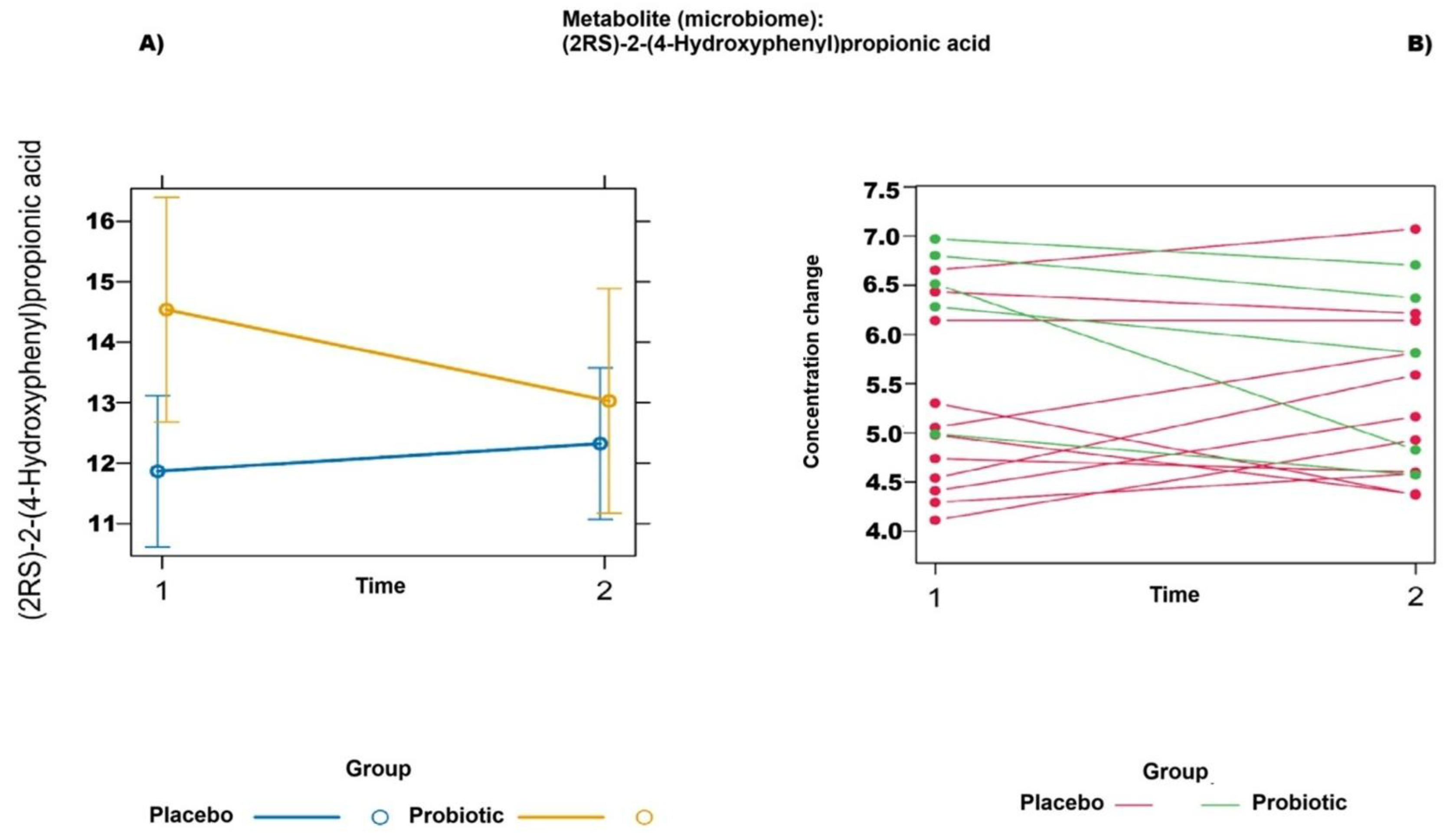
2.2. Untargeted Metabolomics (Liquid Chromatography–Mass Spectrometry)
2.3. Gastrointestinal Complaints (Rome-IV Questionnaire)
3. Discussion
Limitations
4. Materials and Methods
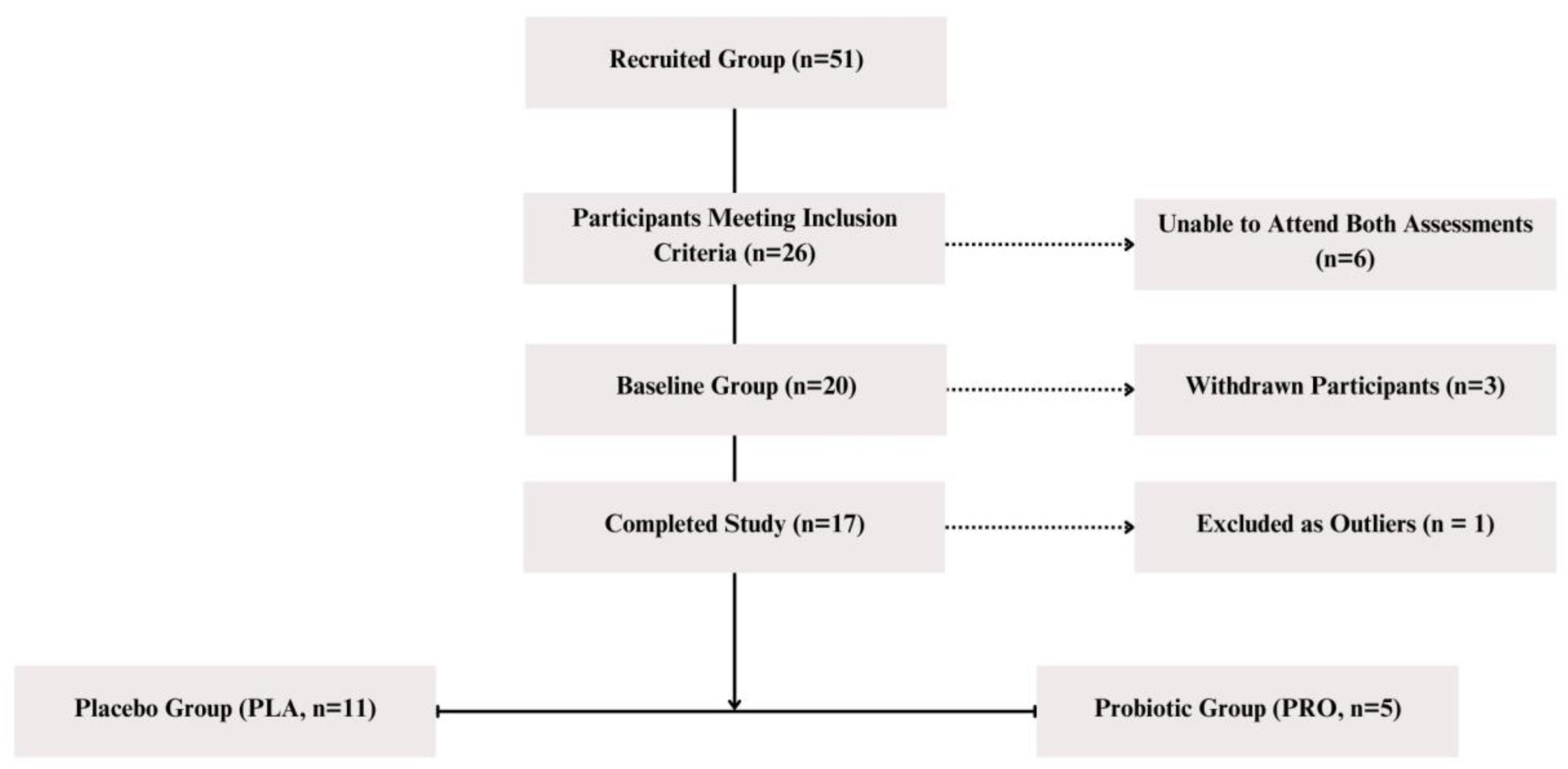
4.1. Study Group
4.2. Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFU | Active fluorescent unit |
| ANOVA | Analysis of variance |
| ASCA | ANOVA–simultaneous component analysis |
| BMI | Body mass index |
| CFU | Colony-forming unit |
| CONSORT | Consolidated Standards of Reporting Trials |
| ETA2 (η2) | Eta-squared (effect size measure) |
| FDR | False discovery rate |
| GABA | Gamma-aminobutyric acid |
| HPLC | High-performance liquid chromatography |
| IBS | Irritable bowel syndrome |
| IQR | Interquartile range (in statistical filtering) |
| MDAT | Mediterranean Diet Assessment Tool |
| MEBA | Multivariate empirical Bayes analysis of variance |
| MS | Mass spectrometry |
| NIST | National Institute of Standards and Technology |
| PCA | Principal component analysis |
| PLA | Placebo group |
| PRO | Probiotic group |
| RCT | Randomized controlled trial |
| Rome IV | Rome IV Diagnostic Criteria |
| SCFA | Short-chain fatty acid |
| SD | Standard deviation |
| SHIME | Simulator of the Human Intestinal Microbial Ecosystem |
| SORT | Strength of Recommendation Taxonomy |
| UHPLC | Ultra-high-performance liquid chromatography |
References
- Koutedakis, Y.; Jamurtas, A. The dancer as a performing athlete: Physiological considerations. Sports Med. 2004, 34, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C. Exercise, infection, and immunity. Int. J. Sports Med. 1994, 15 (Suppl. S3), S131–S141. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.T.; O’Sullivan, O.; Claesson, M.J.; Cotter, P.D. The athlete gut microbiome and its relevance to health and performance: A review. Sports Med. 2022, 52 (Suppl. S1), 119–128. [Google Scholar] [CrossRef]
- Ringham, R.; Klump, K.; Kaye, W.; Stone, D.; Libman, S.; Stowe, S.; Marcus, M. Eating disorder symptomatology among ballet dancers. Int. J. Eat. Disord. 2006, 39, 503–508. [Google Scholar] [CrossRef]
- Teo, W.; Newton, M.J.; McGuigan, M.R. Circadian rhythms in exercise performance: Implications for hormonal and muscular adaptation. J. Sports Sci. Med. 2011, 10, 600–606. [Google Scholar]
- Clauss, M.; Gérard, P.; Mosca, A.; Leclerc, M. Interplay between exercise and gut microbiome in the context of human health and performance. Front. Nutr. 2021, 8, 637010. [Google Scholar] [CrossRef]
- Fontana, F.; Longhi, G.; Tarracchini, C.; Mancabelli, L.; Lugli, G.A.; Alessandri, G.; Turroni, F.; Milani, C.; Ventura, M. The human gut microbiome of athletes: Metagenomic and metabolic insights. Microbiome 2023, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef]
- Marttinen, M.; Ala-Jaakkola, R.; Laitila, A.; Lehtinen, M.J. Gut microbiota, probiotics and physical performance in athletes and physically active individuals. Nutrients 2020, 12, 2936. [Google Scholar] [CrossRef]
- Varghese, S.; Rao, S.; Khattak, A.; Zamir, F.; Chaari, A. Physical exercise and the gut microbiome: A bidirectional relationship influencing health and performance. Nutrients 2024, 16, 3663. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Dziewiecka, H.; Buttar, H.S.; Kasperska, A.; Ostapiuk–Karolczuk, J.; Domagalska, M.; Cichoń, J.; Skarpańska-Stejnborn, A. Physical activity induced alterations of gut microbiota in humans: A systematic review. BMC Sports Sci. Med. Rehabil. 2022, 14, 122. [Google Scholar] [CrossRef]
- Clark, A.; Mach, N. Exercise-induced stress behavior, gut-microbiota-brain axis and diet: A systematic review for athletes. J. Int. Soc. Sports Nutr. 2016, 13, 43. [Google Scholar] [CrossRef]
- Hoppe, M.; Önning, G.; Berggren, A.; Hulthén, L. Probiotic strain Lactobacillus plantarum 299v increases iron absorption from an iron-supplemented fruit drink: A double-isotope cross-over single-blind study in women of reproductive age. Br. J. Nutr. 2015, 114, 1195–1202, Erratum in Br. J. Nutr. 2015, 114, 1948. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Metges, C.C. Contribution of microbial amino acids to amino acid homeostasis of the host. J. Nutr. 2000, 130, 1857S–1864S. [Google Scholar] [CrossRef] [PubMed]
- Shing, C.M.; Peake, J.M.; Lim, C.L.; Briskey, D.; Walsh, N.P.; Fortes, M.B.; Ahuja, K.D.; Vitetta, L. Effects of probiotics supplementation on gastrointestinal permeability, inflammation and exercise performance in the heat. Eur. J. Appl. Physiol. 2014, 114, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Gil-Hernández, E.; Ruiz-González, C.; Rodriguez-Arrastia, M.; Ropero-Padilla, C.; Rueda-Ruzafa, L.; Sánchez-Labraca, N.; Roman, P. Effect of gut microbiota modulation on sleep: A systematic review and meta-analysis of clinical trials. Nutr. Rev. 2023, 81, 1556–1570. [Google Scholar] [CrossRef]
- Misera, A.; Liśkiewicz, P.; Łoniewski, I.; Skonieczna-Żydecka, K.; Samochowiec, J. Effect of psychobiotics on psychometric tests and inflammatory markers in major depressive disorder: Meta-analysis of randomized controlled trials with meta-regression. Pharmaceuticals 2021, 14, 952. [Google Scholar] [CrossRef] [PubMed]
- Rode, J.; Carlman, H.M.T.E.; König, J.; Repsilber, D.; Hutchinson, A.N.; Thunberg, P.; Andersson, P.; Persson, J.; Kiselev, A.; Stern, L.L.; et al. Probiotic mixture containing Lactobacillus helveticus, Bifidobacterium longum and Lactiplantibacillus plantarum affects brain responses toward an emotional task in healthy subjects: A randomized clinical trial. Front. Nutr. 2022, 9, 827182. [Google Scholar] [CrossRef]
- Mohr, A.E.; Jäger, R.; Carpenter, K.C.; Kerksick, C.M.; Purpura, M.; Townsend, J.R.; West, N.P.; Black, K.; Gleeson, M.; Pyne, D.B.; et al. The athletic gut microbiota. J. Int. Soc. Sports Nutr. 2020, 17, 24. [Google Scholar] [CrossRef]
- Tang, H.; Huang, W.; Yao, Y.F. The metabolites of lactic acid bacteria: Classification, biosynthesis and modulation of gut microbiota. Microb. Cell. 2023, 10, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Arseneault-Bréard, J.; Rondeau, I.; Gilbert, K.; Girard, S.-A.; Tompkins, T.A.; Godbout, R.; Rousseau, G. Combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 reduces post-myocardial infarction depression symptoms and restores intestinal permeability in a rat model. Br. J. Nutr. 2012, 107, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Partrick, K.A.; Rosenhauer, A.M.; Auger, J.; Arnold, A.R.; Ronczkowski, N.M.; Jackson, L.M.; Lord, M.N.; Abdulla, S.M.; Chassaing, B.; Huhman, K.L. Ingestion of probiotic (Lactobacillus helveticus and Bifidobacterium longum) alters intestinal microbial structure and behavioral expression following social defeat stress. Sci. Rep. 2021, 11, 3763. [Google Scholar] [CrossRef] [PubMed]
- Firtel, M.; Henderson, G.; Sokolov, I. Nanosurgery: Observation of peptidoglycan strands in Lactobacillus helveticus cell walls. Ultramicroscopy 2004, 101, 105–109. [Google Scholar] [CrossRef]
- Li, M.; Ding, J.; Stanton, C.; Ross, R.P.; Zhao, J.; Yang, B.; Chen, W. Bifidobacterium longum subsp. infantis FJSYZ1M3 ameliorates DSS-induced colitis by maintaining the intestinal barrier, regulating inflammatory cytokines, and modifying gut microbiota. Food Funct. 2023, 14, 354–368. [Google Scholar] [CrossRef]
- Aykut, M.N.; Erdoğan, E.N.; Çelik, M.N.; Gürbüz, M. An updated view of the effect of probiotic supplement on sports performance: A detailed review. Curr. Nutr. Rep. 2024, 13, 251–263. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Oliveira, F.L.; Salgaço, M.K.; de Oliveira, M.T.; Mesa, V.; Sartoratto, A.; Peregrino, A.M.; Ramos, W.S.; Sivieri, K. Exploring the potential of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 as promising psychobiotics using SHIME. Nutrients 2023, 15, 1521. [Google Scholar] [CrossRef]
- Neveu, V.; Pérez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef]
- Hu, S.; Ding, Q.; Zhang, W.; Kang, M.; Ma, J.; Zhao, L. Gut microbial beta-glucuronidase: A vital regulator in female estrogen metabolism. Gut Microbes. 2023, 15, 2236749. [Google Scholar] [CrossRef]
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid metabolism: The interaction of metabolites and gut microbiota. Biosci. Biotechnol. Biochem. 2018, 82, 600–610. [Google Scholar] [CrossRef]
- Hou, S. Genistein: Therapeutic and preventive effects, mechanisms, and clinical application in digestive tract tumor. Evid. Based Complement Altern. Med. 2022, 2022, 5957378. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T.; Yokoyama, S.; Osawa, T. Effect of the genistein metabolite on leptin secretion in murine adipocytes in vitro. Food Chem. 2013, 138, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, P.; Cui, Y.; Hu, X.; Chen, F.; Ma, C. Protective effects of hydroxyphenyl propionic acids on lipid metabolism and gut microbiota in mice fed a high-fat diet. Nutrients 2023, 15, 1043. [Google Scholar] [CrossRef] [PubMed]
- Katanić Stanković, J.S.; Mihailović, N.; Mihailović, V. Genistein: Advances on resources, biosynthesis pathway, bioavailability, bioactivity, and pharmacology. In Handbook of Dietary Flavonoids; Xiao, J., Ed.; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Blaut, M.; Schoefer, L.; Braune, A. Transformation of flavonoids by intestinal microorganisms. Int. J. Vitam Nutr. Res. 2003, 73, 79–87. [Google Scholar] [CrossRef]
- Landete, J.M. Flavone, flavanone and flavonol metabolism from soybean and flaxseed extracts by the intestinal microbiota of adults and infants. J. Sci. Food Agric. 2022, 102, 2575–2583. [Google Scholar] [CrossRef]
- Chacar, S.; Tarighi, M.; Fares, N.; Faivre, J.-F.; Louka, N.; Maroun, R.G. Identification of phenolic compounds-rich grape pomace extracts urine metabolites and correlation with gut microbiota modulation. Antioxidants 2018, 7, 75. [Google Scholar] [CrossRef]
- Cao, G.; Zuo, J.; Wu, B.; Wu, Y. Polyphenol supplementation boosts aerobic endurance in athletes: Systematic review. Front. Physiol. 2024, 15, 1369174. [Google Scholar] [CrossRef]
- Ticinesi, A.; Guerra, A.; Nouvenne, A.; Meschi, T.; Maggi, S. Disentangling the complexity of nutrition, frailty and gut microbial pathways during aging: A focus on hippuric Acid. Nutrients 2023, 15, 1138. [Google Scholar] [CrossRef]
- Pallister, T.; Jackson, M.A.; Martin, T.C.; Zierer, J.; Jennings, A.; Mohney, R.P.; MacGregor, A.; Steves, C.J.; Cassidy, A.; Spector, T.D.; et al. Hippurate as a metabolomic marker of gut microbiome diversity: Modulation by diet and relationship to metabolic syndrome. Sci. Rep. 2017, 7, 13670. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, M. Absorption and metabolism of flavonoids in the caco-2 cell culture model and a perused rat intestinal model. Drug Metab. Dispos. 2002, 30, 370–377. [Google Scholar] [CrossRef]
- Ahmadi, S.; Wang, S.; Nagpal, R.; Wang, B.; Jain, S.; Razazan, A.; Mishra, S.P.; Zhu, X.; Wang, Z.; Kavanagh, K.; et al. A human-origin probiotic cocktail ameliorates aging-related leaky gut and inflammation via modulating the microbiota/taurine/tight junction axis. JCI Insight. 2020, 5, e132055. [Google Scholar] [CrossRef] [PubMed]
- Sivamaruthi, B.S.; Fern, L.A.; Rashidah Pg Hj Ismail, D.S.N.; Chaiyasut, C. The influence of probiotics on bile acids in diseases and aging. Biomed. Pharmacother. 2020, 128, 110310. [Google Scholar] [CrossRef]
- Yang, M.; Gu, Y.; Li, L.; Liu, T.; Song, X.; Sun, Y.; Cao, X.; Wang, B.; Jiang, K.; Cao, H. Bile acid–gut microbiota axis in inflammatory bowel disease: From bench to dedside. Nutrients 2021, 13, 3143. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Filho, J.G.; Carvalho, A.S.E.S.; Alves, J.D.S.; Egea, M.B. Next-generation probiotics as a therapeutic strategy for the treatment of phenylketonuria: A review. Nutr. Rev. 2022, 80, 2100–2112. [Google Scholar] [CrossRef]
- Strasser, B.; Geiger, D.; Schauer, M.; Gostner, J.M.; Gatterer, H.; Burtscher, M.; Fuchs, D. Probiotic supplements beneficially affect tryptophan-kynurenine metabolism and reduce the incidence of upper respiratory tract infections in trained athletes: A randomized, double-blinded, placebo-controlled trial. Nutrients 2016, 8, 752. [Google Scholar] [CrossRef] [PubMed]
- Lindheimer, J.B.; Beedie, C.; Raglin, J.S. Placebo effects in sports performance and exercise outcomes. In Placebo Effects Through the Lens of Translational Research; Colloca, L., Noel, J., Franklin, P.D., Seneviratne, C., Eds.; Oxford Academic: Oxford, UK, 2023. [Google Scholar] [CrossRef]
- Konstantis, G.; Efstathiou, S.; Pourzitaki, C.; Kitsikidou, E.; Germanidis, G.; Chourdakis, M. Efficacy and safety of probiotics in the treatment of irritable bowel syndrome: A systematic review and meta-analysis of randomised clinical trials using ROME IV criteria. Clin. Nutr. 2023, 42, 800–809. [Google Scholar] [CrossRef]
- Li, B.; Liang, L.; Deng, H.; Guo, J.; Shu, H.; Zhang, L. Efficacy and safety of probiotics in irritable bowel syndrome: A systematic review and meta-analysis. Front. Pharmacol. 2020, 11, 332. [Google Scholar] [CrossRef]
- Ait-Belgnaoui, A.; Payard, I.; Rolland, C.; Harkat, C.; Braniste, V.; Théodorou, V.; Tompkins, T.A. Bifidobacterium longum and Lactobacillus helveticus synergistically suppress stress-related visceral hypersensitivity through hypothalamic-pituitary-adrenal axis modulation. J. Neurogastroenterol. Motil. 2018, 24, 138–146. [Google Scholar] [CrossRef]
| Placebo (n = 11) Mean ± SD | Probiotic (n = 5) Mean ± SD | p-Value * | |
|---|---|---|---|
| Anthropometric characteristics | |||
| Age [years] | 20.55 ± 1.04 | 20.00 ± 1.30 | 0.55 b |
| Body mass [kg] | 58.07 ± 6.95 | 60.10 ± 7.31 | 0.99 a |
| BMI (body mass index) [kg/m2] | 21.05 ± 2.18 | 20.80 ± 2.29 | 0.93 a |
| Fat [% body mass] | 27 ± 4 | 27 ± 3 | 0.84 a |
| Physical activity level [hours per week] | 17.11 ± 6.98 | 16.00 ± 9.77 | 0.69 b |
| Diet composition | |||
| Energy [kcal] | 1999.23 ± 279.81 | 2325.54 ± 425.00 | 0.26 a |
| Proteins [g] | 85.29 ± 30.13 | 100.47 ± 21.09 | 0.51 a |
| Fats [g] | 74.56 ± 13.93 | 90.47 ± 18.72 | 0.41 a |
| Total cholesterol [mg] | 216.73 ± 104.36 | 330.21 ± 140.77 | 0.40 a |
| Carbohydrates [g] | 271.12 ± 53.14 | 298.79 ± 57.98 | 0.75 a |
| Sucrose [% of carbohydrates] | 10.27 ± 3.89 | 6.75 ± 2.81 | 0.09 a |
| Fiber [g] | 28.96 ± 15.13 | 21.36 ± 12.67 | 0.91 b |
| Meditarrean diet adherence [0–14] | 5.46 ± 1.86 | 6.20 ± 2.49 | 0.42 a |
| Fruits [150–200 g portions per day] | 1.64 ± 0.67 | 1.60 ± 0.55 | 1.00 b |
| Vegetables [200 g portions per day] | 2.36 ± 0.92 | 3.20 ± 0.84 | 0.10 b |
| Legumes [150 g portions per day] | 1.55 ± 1.51 | 2.40 ± 2.51 | 0.60 b |
| Nuts [30 g portions per day] | 2.46 ± 1.44 | 3.00 ± 1.23 | 0.56 b |
| Hematologic markers | |||
| WBC (white blood cells) [×109/L] | 5.54 ± 0.84 | 5.94 ± 1.17 | 0.50 a |
| Lymphocytes [×109/L] | 2.34 ± 0.79 | 2.60 ± 0.46 | 0.41 a |
| Metabolite | Fold Change | log2(FC) |
|---|---|---|
| Hippuric acid [M-H]− | 8938.800000 | 13.1260 |
| Curcumin (NIST EL) [M+H]+ | 354.050000 | 8.4678 |
| Hyocholic acid (microbiome) [M-H]− | 0.036575 | −4.7730 |
| Quinic acid [M+AcO-H]− | 23.103000 | 4.5300 |
| Genistein [M-H]− | 21.530000 | 4.4283 |
| Estriol [M-H]− | 18.119000 | 4.1795 |
| Naringenin (microbiome) [M-H]− | 12.869000 | 3.6858 |
| Taurine [M-H]− | 0.119930 | −3.0597 |
| Alpha-linolenic acid [M-H]− | 6.776500 | 2.7605 |
| Alpha-linolenic acid [M+FA-H]− | 6.574100 | 2.7168 |
| 3a,12a-dihydroxy-7-oxo-5b-cholan-24-oic acid (microbiome) [M-H]− | 0.172040 | −2.5392 |
| Peimine [M+H]+ | 5.497000 | 2.4586 |
| Kaurenoic acid [M-H]− | 0.202690 | −2.3027 |
| 4-Methyl-5-thiazoleethanol (microbiome) [M+H]+ | 4.839600 | 2.2749 |
| Cholic acid [M-H]− | 0.222740 | −2.1666 |
| Valproic acid [M-H]− | 4.270200 | 2.0943 |
| Metabolite | t-Test | p-Value | −log10 (p) | FDR |
|---|---|---|---|---|
| Hyocholic acid (microbiome) [M-H]− | −2.8552 | 0.012719 | 1.8955 | 0.45892 |
| L-phenylalanine [M-H]− | −2.7714 | 0.015002 | 1.8239 | 0.45892 |
| L-tryptophan [M-H]− | −2.6018 | 0.020902 | 1.6798 | 0.45892 |
| 1-Aminocyclohexanecarboxylic acid (NIST EL) [M+H]+ | 2.5913 | 0.021336 | 1.6709 | 0.45892 |
| L-phenylalanine [M+H]+ | −2.4408 | 0.028545 | 1.5445 | 0.45892 |
| 3a,12a-dihydroxy-7-oxo-5b-cholan-24-oic acid (microbiome) [M-H]− | −2.4157 | 0.029951 | 1.5236 | 0.45892 |
| Kaurenoic acid [M-H]− | −2.3055 | 0.036961 | 1.4323 | 0.45892 |
| D-citramalic acid lithium salt (microbiome) [M-H]− | 2.2485 | 0.041167 | 1.3855 | 0.45892 |
| 5-Aminopentanoic acid [M-H]− | −2.1452 | 0.049965 | 1.3013 | 0.45892 |
| PLA (n = 11) Mean ± SD | PRO (n = 5) Mean ± SD | 2-Way ANOVA p-Value (η2) | |||||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Group | Ttime | Group × Time | |
| Postprandial fullness [0–10] | 2.91 ± 1.38 | 2.32 ± 1.31 | 3.00 ± 1.54 | 3.00 ± 1.83 | 0.508 (0.032) | 0.592 (0.021) | 0.592 (0.021) |
| Abdominal pain [0–10] | 5.00 ± 1.55 | 4.45 ± 1.37 | 4.00 ± 1.58 | 3.80 ± 1.79 | 0.308 (0.074) | 0.182 (0.124) | 0.525 (0.294) |
| Menstrual pain [0–10] | 7.27 ± 4.05 | 5.55 ± 3.21 | 6.80 ± 4.21 | 5.60 ± 4.28 | 0.905 (0.001) | 0.224 (0.104) | 0.822 (0.004) |
| Constipation [0–10] | 4.16 ± 1.39 | 3.36 ± 1.67 | 3.92 ± 0.95 | 2.84 ± 1.85 | 0.603 (0.020) | 0.030 (0.295) | 0.724 (0.009) |
| Diarrhea [0–10] | 3.64 ± 2.01 | 2.73 ± 1.42 | 2.40 ± 1.67 | 2.20 ± 0.84 | 0.262 (0.089) | 0.242 (0.096) | 0.448 (0.042) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiącek, J.; Skonieczna-Żydecka, K.; Łoniewski, I.; Styburski, D.; Kaczmarczyk, M.; Karolkiewicz, J. A Randomized Controlled Trial Evaluating the Effects of a Probiotic Containing Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 on Gastrointestinal Symptoms and Metabolomic Profiles in Female Dancers. Int. J. Mol. Sci. 2025, 26, 5823. https://doi.org/10.3390/ijms26125823
Wiącek J, Skonieczna-Żydecka K, Łoniewski I, Styburski D, Kaczmarczyk M, Karolkiewicz J. A Randomized Controlled Trial Evaluating the Effects of a Probiotic Containing Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 on Gastrointestinal Symptoms and Metabolomic Profiles in Female Dancers. International Journal of Molecular Sciences. 2025; 26(12):5823. https://doi.org/10.3390/ijms26125823
Chicago/Turabian StyleWiącek, Jakub, Karolina Skonieczna-Żydecka, Igor Łoniewski, Daniel Styburski, Mariusz Kaczmarczyk, and Joanna Karolkiewicz. 2025. "A Randomized Controlled Trial Evaluating the Effects of a Probiotic Containing Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 on Gastrointestinal Symptoms and Metabolomic Profiles in Female Dancers" International Journal of Molecular Sciences 26, no. 12: 5823. https://doi.org/10.3390/ijms26125823
APA StyleWiącek, J., Skonieczna-Żydecka, K., Łoniewski, I., Styburski, D., Kaczmarczyk, M., & Karolkiewicz, J. (2025). A Randomized Controlled Trial Evaluating the Effects of a Probiotic Containing Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 on Gastrointestinal Symptoms and Metabolomic Profiles in Female Dancers. International Journal of Molecular Sciences, 26(12), 5823. https://doi.org/10.3390/ijms26125823









