The Lemon Flavonoid Eriomin® Suppresses Pituitary–Adrenal Axis Activity in Aged Rats
Abstract
1. Introduction
2. Results
2.1. Body Mass, Pituitary, and Adrenal Gland Weight
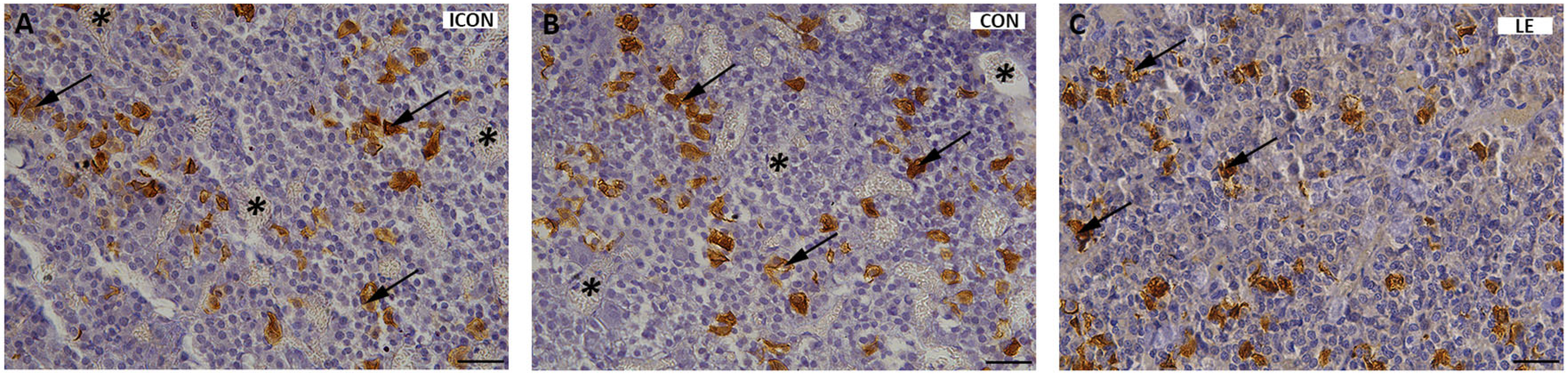
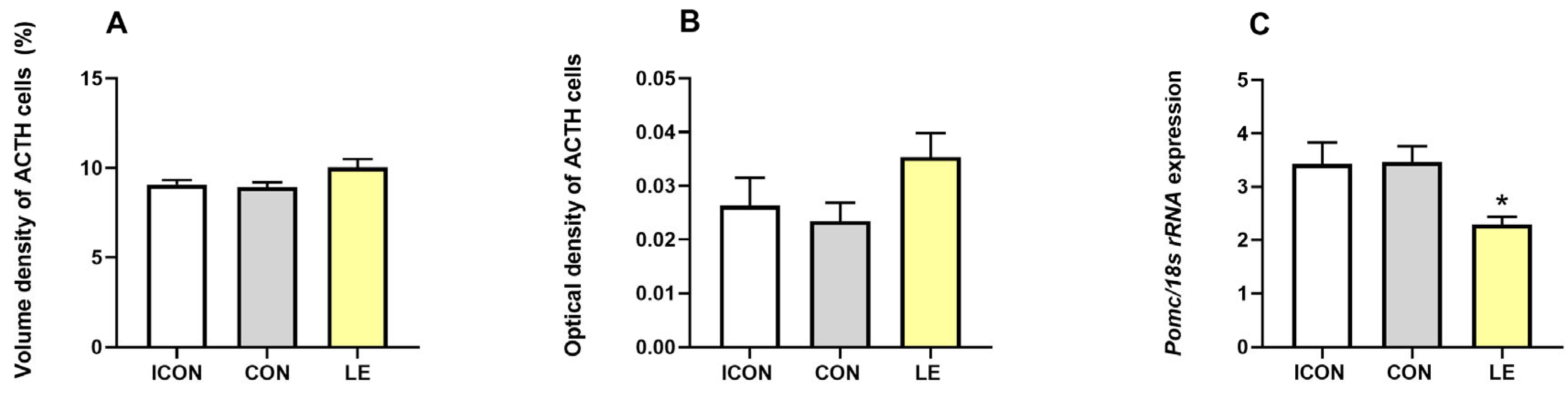
2.2. Histological, Stereological, and Molecular Insights into Pituitary ACTH Cells
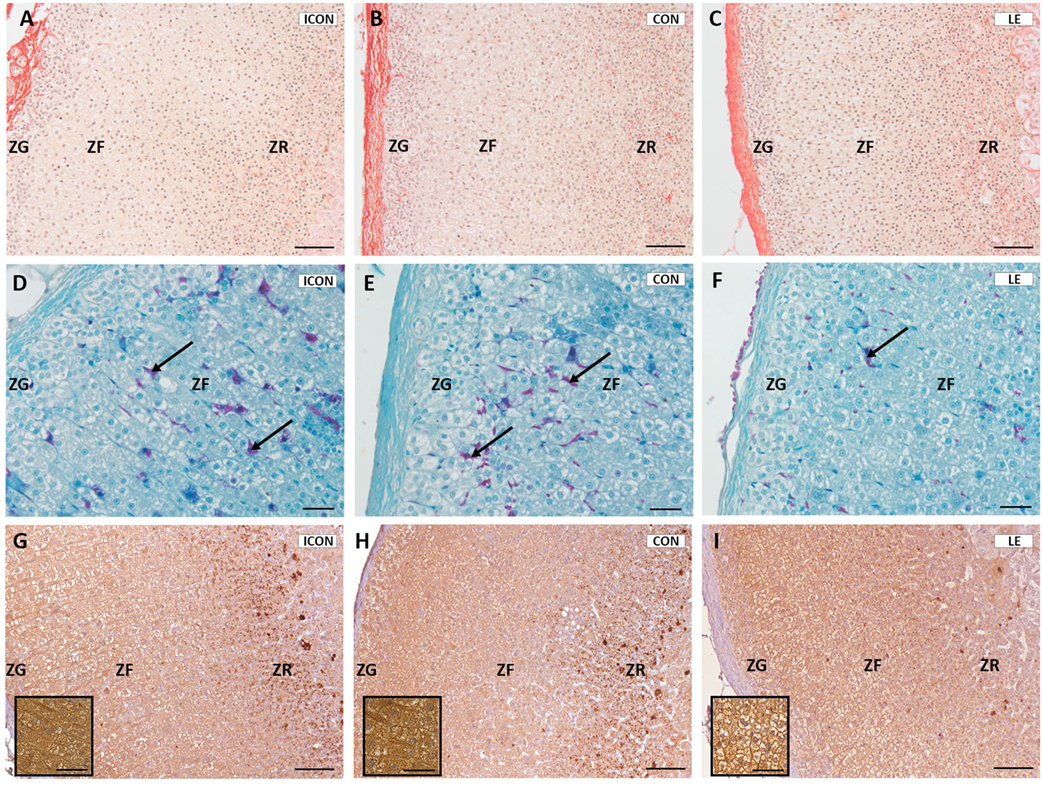
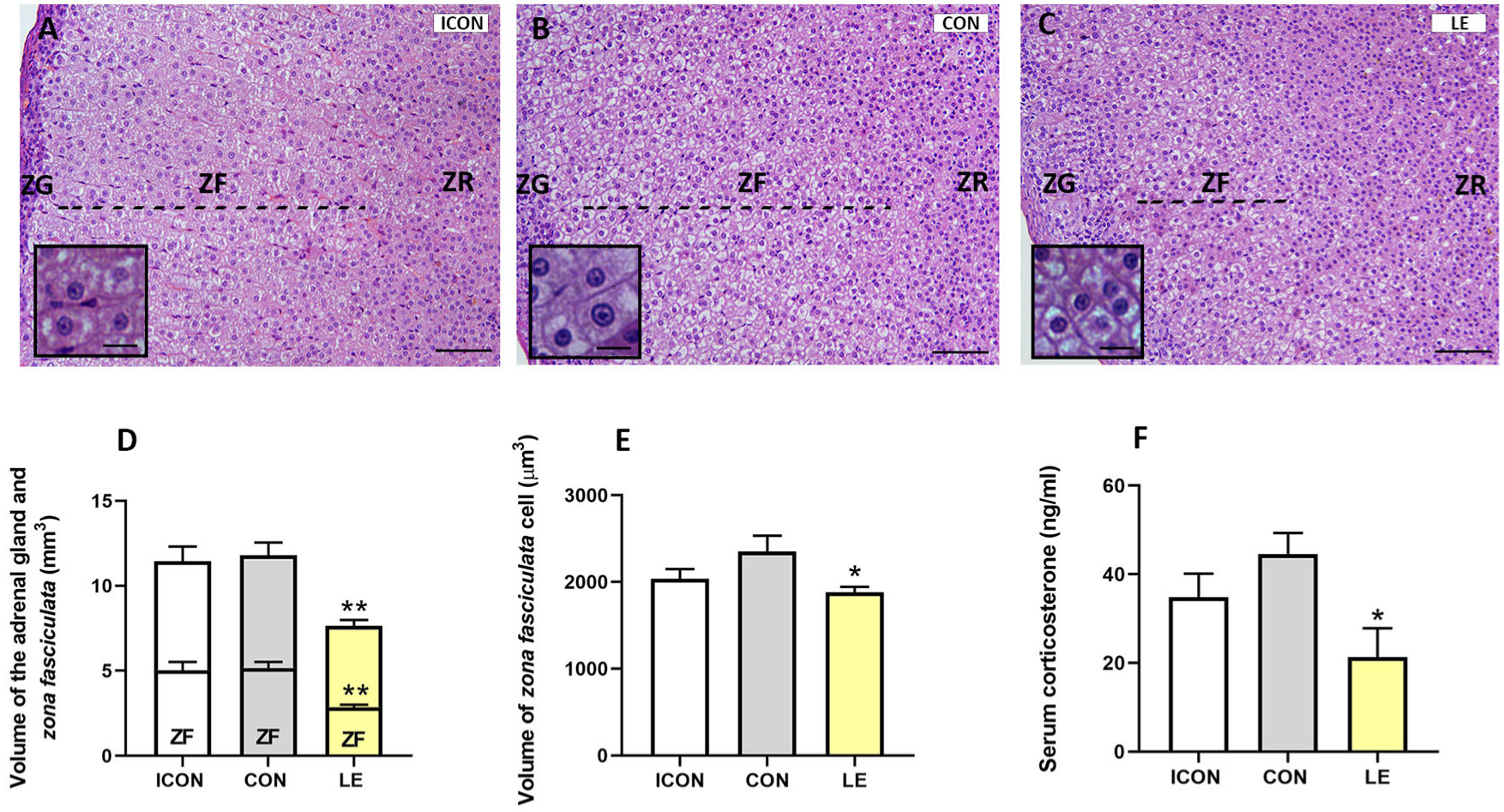
2.3. Histological, Stereological, and Biochemical Insights into the Adrenal Gland
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Sample Collection and Processing
4.3. Histological Staining Procedure
4.4. Immunohistochemical Staining Procedure
4.5. Stereological Analysis
4.6. Quantitative Real-Time PCR Analysis
4.7. Hormonal Analyses
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and Aging-Related Diseases: From Molecular Mechanisms to Interventions and Treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef] [PubMed]
- Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 7 April 2025).
- Van Den Beld, A.W.; Kaufman, J.-M.; Zillikens, M.C.; Lamberts, S.W.J.; Egan, J.M.; Van Der Lely, A.J. The Physiology of Endocrine Systems with Ageing. Lancet Diabetes Endocrinol. 2018, 6, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Stamou, M.I.; Colling, C.; Dichtel, L.E. Adrenal Aging and Its Effects on the Stress Response and Immunosenescence. Maturitas 2023, 168, 13–19. [Google Scholar] [CrossRef]
- Gaffey, A.E.; Bergeman, C.S.; Clark, L.A.; Wirth, M.M. Aging and the HPA Axis: Stress and Resilience in Older Adults. Neurosci. Biobehav. Rev. 2016, 68, 928–945. [Google Scholar] [CrossRef]
- Goncharova, N.; Bowden, D.; Johnson, E. Editorial: The HPA Axis and Aging: Individual Features, Age-Related Pathology. Front. Endocrinol. 2023, 14, 1222033. [Google Scholar] [CrossRef]
- Kobayashi, N.; Machida, T.; Takahashi, T.; Takatsu, H.; Shinkai, T.; Abe, K.; Urano, S. Elevation by Oxidative Stress and Aging of Hypothalamic-Pituitary-Adrenal Activity in Rats and Its Prevention by Vitamin E. J. Clin. Biochem. Nutr. 2009, 45, 207–213. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Juszczyk, G.; Mikulska, J.; Kasperek, K.; Pietrzak, D.; Mrozek, W.; Herbet, M. Chronic Stress and Oxidative Stress as Common Factors of the Pathogenesis of Depression and Alzheimer’s Disease: The Role of Antioxidants in Prevention and Treatment. Antioxidants 2021, 10, 1439. [Google Scholar] [CrossRef]
- Signorello, M.G.; Ravera, S.; Leoncini, G. Oxidative Stress Induced by Cortisol in Human Platelets. Int. J. Mol. Sci. 2024, 25, 3776. [Google Scholar] [CrossRef]
- Godonu, S.S.; Francis-Lyons, N. Role Of Cortisol in The Synthesis of Glutamate During Oxidative Stress. Int. J. Sci. Res. Technol. 2025, 2, 100–104. [Google Scholar] [CrossRef]
- Ribeiro, C.B.; Ramos, F.M.; Manthey, J.A.; Cesar, T.B. Effectiveness of Eriomin® in Managing Hyperglycemia and Reversal of Prediabetes Condition: A Double-blind, Randomized, Controlled Study. Phytother. Res. 2019, 33, 1921–1933. [Google Scholar] [CrossRef]
- Mohammadi, N.; Dos Santos Lima, A.; Azevedo, L.; Granato, D. Bridging the Gap in Antioxidant Activity of Flavonoids: Correlating the Oxidation of Human Plasma with Chemical and Cellular Assays. Curr. Res. Food Sci. 2024, 8, 100714. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Liu, W.; Bashir, M.; Nisar, M.F.; Wan, C. (Craig) Eriocitrin: A Review of Pharmacological Effects. Biomed. Pharmacother. 2022, 154, 113563. [Google Scholar] [CrossRef]
- Ji, M.; Deng, Z.; Rong, X.; Li, R.; You, Z.; Guo, X.; Cai, C.; Zhao, Y.; Gao, P.; Cao, G.; et al. Naringenin Prevents Oxidative Stress and Inflammation in LPS-Induced Liver Injury through the Regulation of LncRNA-mRNA in Male Mice. Molecules 2022, 28, 198. [Google Scholar] [CrossRef]
- Madureira, M.B.; Concato, V.M.; Cruz, E.M.S.; Bitencourt De Morais, J.M.; Inoue, F.S.R.; Concimo Santos, N.; Gonçalves, M.D.; Cremer De Souza, M.; Basso Scandolara, T.; Fontana Mezoni, M.; et al. Naringenin and Hesperidin as Promising Alternatives for Prevention and Co-Adjuvant Therapy for Breast Cancer. Antioxidants 2023, 12, 586. [Google Scholar] [CrossRef]
- Miler, M.; Živanović, J.; Ajdžanović, V.; Oreščanin-Dušić, Z.; Milenković, D.; Konić-Ristić, A.; Blagojević, D.; Milošević, V.; Šošić-Jurjević, B. Citrus Flavanones Naringenin and Hesperetin Improve Antioxidant Status and Membrane Lipid Compositions in the Liver of Old-Aged Wistar Rats. Exp. Gerontol. 2016, 84, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Miler, M.; Živanović, J.; Kovačević, S.; Vidović, N.; Djordjevic, A.; Filipović, B.; Ajdžanović, V. Citrus Flavanone Effects on the Nrf2-Keap1/GSK3/NF-κB/NLRP3 Regulation and Corticotroph-Stress Hormone Loop in the Old Pituitary. Int. J. Mol. Sci. 2024, 25, 8918. [Google Scholar] [CrossRef]
- Miler, M.; Živanović, J.; Ajdžanović, V.; Milenkovic, D.; Jarić, I.; Šošić-Jurjević, B.; Milošević, V. Citrus Flavanones Upregulate Thyrotroph Sirt1 and Differently Affect Thyroid Nrf2 Expressions in Old-Aged Wistar Rats. J. Agric. Food Chem. 2020, 68, 8242–8254. [Google Scholar] [CrossRef] [PubMed]
- Šošić-Jurjević, B.; Borković-Mitić, S.; Pavlović, S.; Vlahović, D.; Miler, M.; Cesar, T.; Ajdžanović, V.; Milenkovic, D.; Stellaard, F.; Trifunović, S.; et al. Lemon Flavonoid Extract Eriomin Improves Pro/Antioxidant Status and Interferes with Cholesterol Metabolism without Affecting Serum Cholesterol Levels in Aged Rats. Int. J. Mol. Sci. 2024, 25, 5221. [Google Scholar] [CrossRef]
- Miler, M.; Živanović, J.; Ajdžanović, V.; Milenković, D.; Cesar, T.; Filipović, M.R.; Milošević, V. Lemon Extract Reduces the Hepatic Oxidative Stress and Persulfidation Levels by Upregulating the Nrf2 and Trx1 Expression in Old Rats. BioFactors 2024, 50, 756–771. [Google Scholar] [CrossRef]
- Garrido, P.; De Blas, M.; Del Arco, A.; Segovia, G.; Mora, F. Aging Increases Basal but Not Stress-Induced Levels of Corticosterone in the Brain of the Awake Rat. Neurobiol. Aging 2012, 33, 375–382. [Google Scholar] [CrossRef]
- Tesic, V.; Ciric, J.; Jovanovic Macura, I.; Zogovic, N.; Milanovic, D.; Kanazir, S.; Perovic, M. Corticosterone and Glucocorticoid Receptor in the Cortex of Rats during Aging—The Effects of Long-Term Food Restriction. Nutrients 2021, 13, 4526. [Google Scholar] [CrossRef] [PubMed]
- Trifunović, S.; Manojlović-Stojanoski, M.; Ajdžanović, V.; Nestorović, N.; Ristić, N.; Medigović, I.; Milošević, V. Genistein Stimulates the Hypothalamo-Pituitary-Adrenal Axis in Adult Rats: Morphological and Hormonal Study. Histol. Histopathol. 2012, 27, 627–640. [Google Scholar] [CrossRef] [PubMed]
- Gamo, K.; Shiraki, T.; Matsuura, N.; Miyachi, H. Mechanism of Peroxisome Proliferator-Activated Receptor Gamma (PPARγ) Transactivation by Hesperetin Glucuronides Is Distinct from That by a Thiazolidine-2,4-Dione Agent. Chem. Pharm. Bull. 2014, 62, 491–493. [Google Scholar] [CrossRef][Green Version]
- Gangadhariah, M.; Pardhi, T.; Ravilla, J.; Chandra, S.; Singh, S.A. Citrus Nutraceutical Eriocitrin and Its Metabolites Are Partial Agonists of Peroxisome Proliferator-Activated Receptor Gamma (PPARγ): A Molecular Docking and Molecular Dynamics Study. J. Biomol. Struct. Dyn. 2023, 45, 11373–11393. [Google Scholar] [CrossRef]
- Qi, W.; Zhong, L.; Li, X.; Li, G.; Liu, Z.; Hu, J.; Chen, N. Hyperglycemia Induces the Variations of 11 β -Hydroxysteroid Dehydrogenase Type 1 and Peroxisome Proliferator-Activated Receptor- γ Expression in Hippocampus and Hypothalamus of Diabetic Rats. J. Diabetes Res. 2012, 2012, 107130. [Google Scholar] [CrossRef]
- Wiesner, G.; Morash, B.; Ur, E.; Wilkinson, M. Food Restriction Regulates Adipose-Specific Cytokines in Pituitary Gland but Not in Hypothalamus. J. Endocrinol. 2004, 180, R1–R6. [Google Scholar] [CrossRef][Green Version]
- Zaripheh, S.; Nara, T.Y.; Nakamura, M.T.; Erdman, J.W. Dietary Lycopene Downregulates Carotenoid 15,15′-Monooxygenase and PPAR-γ in Selected Rat Tissues. J. Nutr. 2006, 136, 932–938. [Google Scholar] [CrossRef]
- Mannelli, M.; Cantini, G.; Poli, G.; Mangoni, M.; Nesi, G.; Canu, L.; Rapizzi, E.; Borgogni, E.; Ercolino, T.; Piccini, V.; et al. Role of the PPAR-γ System in Normal and Tumoral Pituitary Corticotropic Cells and Adrenal Cells. Neuroendocrinology 2010, 92, 23–27. [Google Scholar] [CrossRef]
- Escribano, L.; Simón, A.-M.; Pérez-Mediavilla, A.; Salazar-Colocho, P.; Río, J.D.; Frechilla, D. Rosiglitazone Reverses Memory Decline and Hippocampal Glucocorticoid Receptor Down-Regulation in an Alzheimer’s Disease Mouse Model. Biochem. Biophys. Res. Commun. 2009, 379, 406–410. [Google Scholar] [CrossRef]
- Torres, R.C.; Magalhaes, N.S.; Silva, P.; Martins, M.A.; Carvalho, V.F. Activation of PPAR-γ Reduces HPA Axis Activity in Diabetic Rats by up-Regulating PI3K Expression. Exp. Mol. Pathol. 2016, 101, 290–301. [Google Scholar] [CrossRef]
- Harno, E.; Gali Ramamoorthy, T.; Coll, A.P.; White, A. POMC: The Physiological Power of Hormone Processing. Physiol. Rev. 2018, 98, 2381–2430. [Google Scholar] [CrossRef] [PubMed]
- Karpac, J.; Czyzewska, K.; Kern, A.; Brush, R.S.; Anderson, R.E.; Hochgeschwender, U. Failure of Adrenal Corticosterone Production in Pomc-Deficient Mice Results from Lack of Integrated Effects of Pomc Peptides on Multiple Factors. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E446–E455. [Google Scholar] [CrossRef][Green Version]
- Conran, R.M.; Nickerson, P.A. Atrophy of the zona fasciculata in the adrenal cortex of thyroparathyroidectomized rats: A quantitative study. Am. J. Anat. 1982, 164, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Hinson, J.P.; Vinson, G.P.; Kapas, S.; Teja, R. The Relationship Between Adrenal Vascular Events and Steroid Secretion: The Role of Mast Cells and Endothelin. J. Steroid Biochem. Mol. Biol. 1991, 40, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.-Y.; Kwak, J.-E.; Ahn, M.-R. Eriocitrin Inhibits Angiogenesis by Targeting VEGFR2-Mediated PI3K/AKT/mTOR Signaling Pathways. Nutrients 2024, 16, 1091. [Google Scholar] [CrossRef]
- Nair, A.; Jacob, S. A Simple Practice Guide for Dose Conversion between Animals and Human. J. Basic Clin. Pharm. 2016, 7, 27. [Google Scholar] [CrossRef]
- Šošić-Jurjević, B.; Filipović, B.; Renko, K.; Miler, M.; Trifunović, S.; Ajdžanovič, V.; Kӧhrle, J.; Milošević, V. Testosterone and Estradiol Treatments Differently Affect Pituitary-Thyroid Axis and Liver Deiodinase 1 Activity in Orchidectomized Middle-Aged Rats. Exp. Gerontol. 2015, 72, 85–98. [Google Scholar] [CrossRef]
- Zivkovic, A.; Trifunovic, S.; Savic, D.; Milosevic, K.; Lavrnja, I. Experimental Autoimmune Encephalomyelitis Influences GH-Axis in Female Rats. Int. J. Mol. Sci. 2024, 25, 5837. [Google Scholar] [CrossRef]
- Šošić-Jurjević, B.; Ajdžanović, V.; Filipović, B.; Trifunović, S.; Jarić, I.; Ristić, N.; Milošević, V. Functional Morphology of Pituitary -Thyroid and -Adrenocortical Axes in Middle-Aged Male Rats Treated with Vitex Agnus Castus Essential Oil. Acta Histochem. 2016, 118, 736–745. [Google Scholar] [CrossRef]
- Jensen, E.B.V.; Gundersen, H.J.G. The Rotator. J. Microsc. 1993, 170, 35–44. [Google Scholar] [CrossRef]
| ICON | CON | LE | |
|---|---|---|---|
| Body mass (g) | 438.5 ± 47.3 | 432.8 ± 21.3 | 440.5 ± 26.1 |
| Absolute pituitary weight (mg) | 11.0 ± 1.3 | 12.9 ± 1.1 | 12.8 ± 1.5 |
| Relative pituitary weight (%) | 2.5 ± 0.3 | 2.9 ± 0.2 | 3.0 ± 0.4 |
| Absolute adrenal weight (mg) | 26.4 ± 1.4 | 27.2 ± 1.4 | 22.2 ± 0.8 *** |
| Relative adrenal weight (%) | 5.5 ± 0.5 | 6.4 ± 0.2 | 5.1 ± 0.2 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trifunović, S.; Gizdović, I.; Ristić, N.; Filipović, B.; Ajdžanović, V.; Miler, M.; Cesar, T.; Šošić-Jurjević, B. The Lemon Flavonoid Eriomin® Suppresses Pituitary–Adrenal Axis Activity in Aged Rats. Int. J. Mol. Sci. 2025, 26, 5818. https://doi.org/10.3390/ijms26125818
Trifunović S, Gizdović I, Ristić N, Filipović B, Ajdžanović V, Miler M, Cesar T, Šošić-Jurjević B. The Lemon Flavonoid Eriomin® Suppresses Pituitary–Adrenal Axis Activity in Aged Rats. International Journal of Molecular Sciences. 2025; 26(12):5818. https://doi.org/10.3390/ijms26125818
Chicago/Turabian StyleTrifunović, Svetlana, Ivona Gizdović, Nataša Ristić, Branko Filipović, Vladimir Ajdžanović, Marko Miler, Thais Cesar, and Branka Šošić-Jurjević. 2025. "The Lemon Flavonoid Eriomin® Suppresses Pituitary–Adrenal Axis Activity in Aged Rats" International Journal of Molecular Sciences 26, no. 12: 5818. https://doi.org/10.3390/ijms26125818
APA StyleTrifunović, S., Gizdović, I., Ristić, N., Filipović, B., Ajdžanović, V., Miler, M., Cesar, T., & Šošić-Jurjević, B. (2025). The Lemon Flavonoid Eriomin® Suppresses Pituitary–Adrenal Axis Activity in Aged Rats. International Journal of Molecular Sciences, 26(12), 5818. https://doi.org/10.3390/ijms26125818









