Treadmill Exercise-Induced RNA m6A Methylation Modification in the Prevention of High-Fat Diet-Induced MASLD in Mice
Abstract
1. Introduction
2. Results
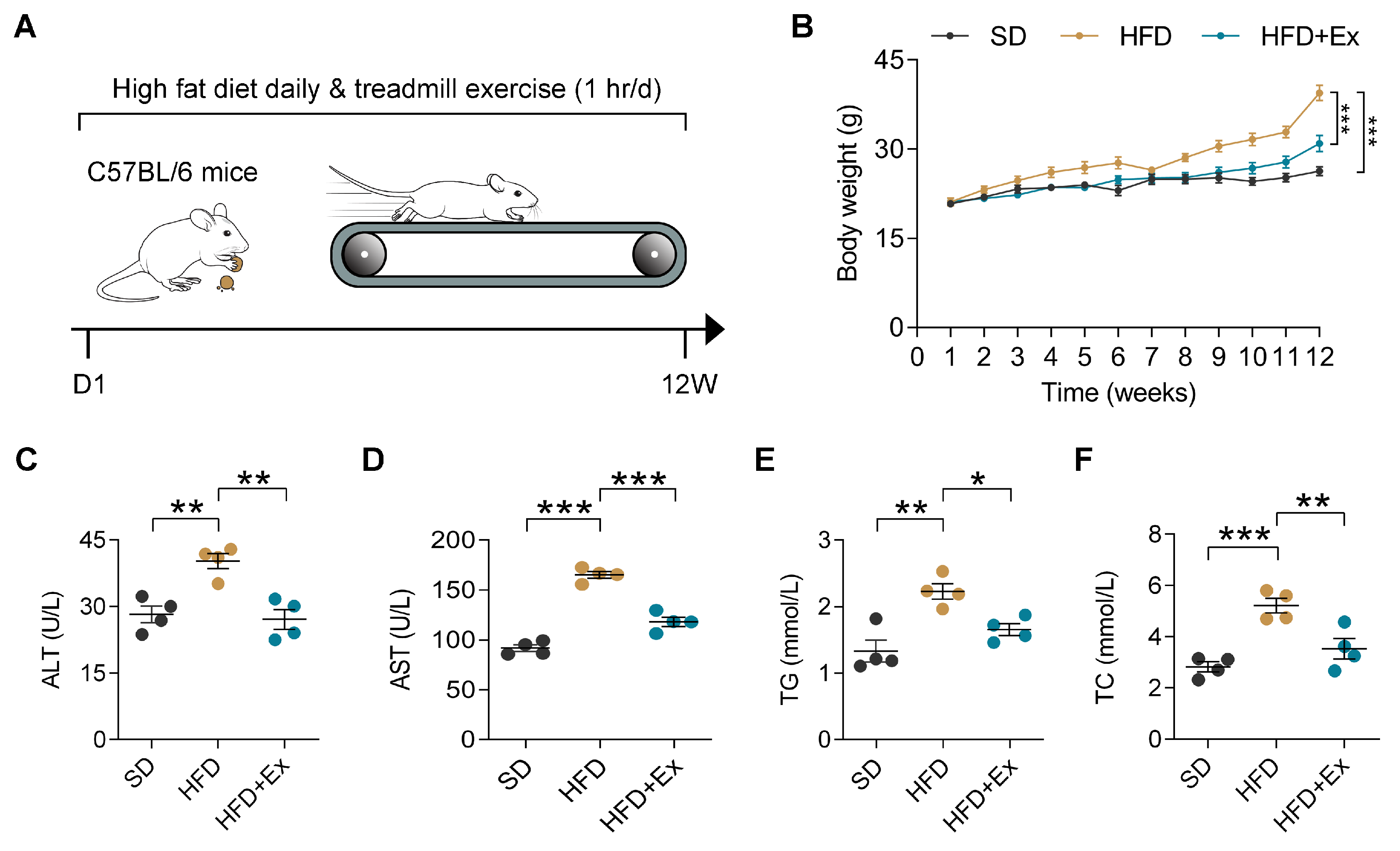
2.1. Treadmill Exercise Mitigates HFD-Induced Weight Gain and Liver Dysfunction in Mice
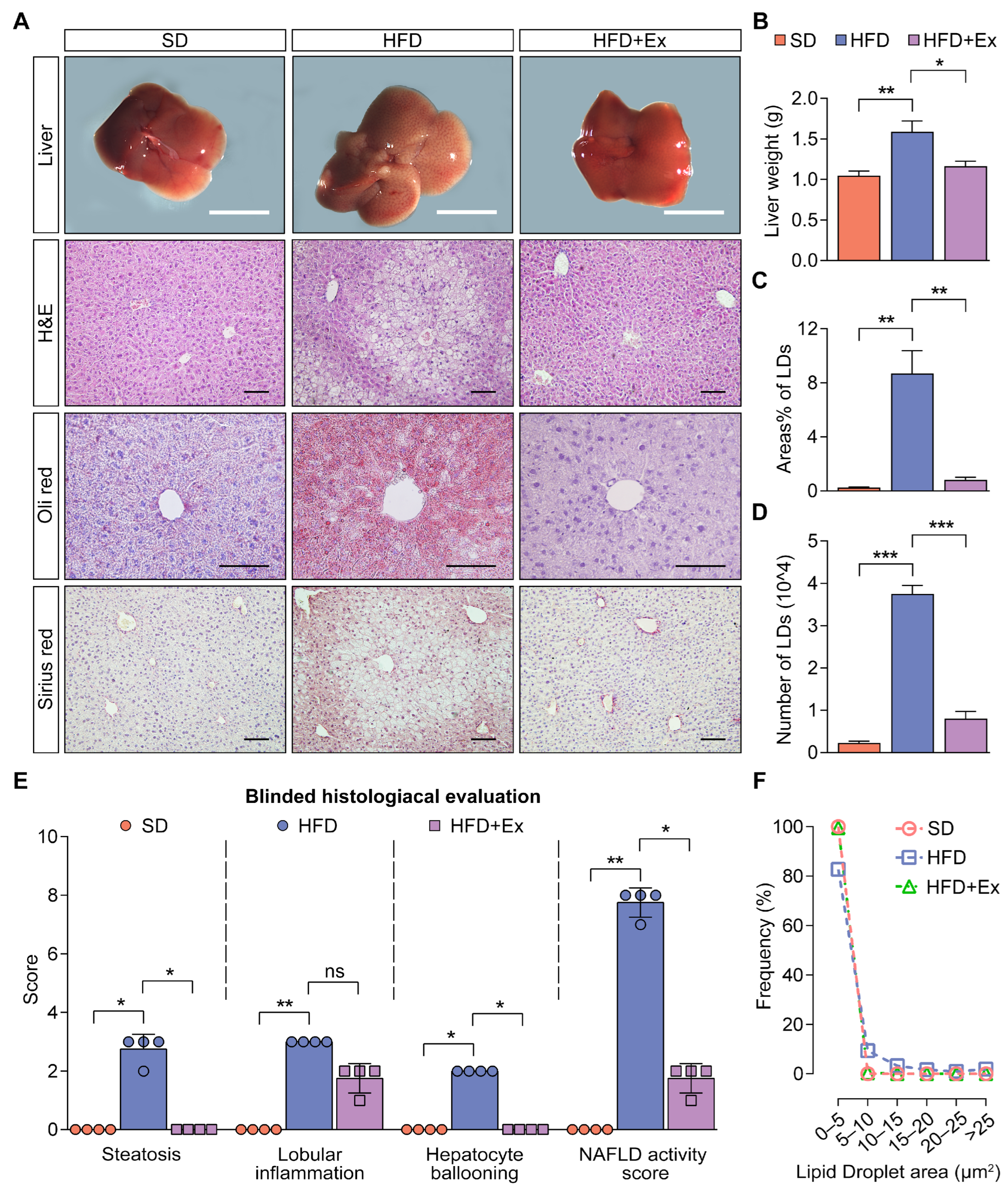
2.2. Treadmill Exercise Suppresses MASLD Development in HFD-Fed Mice
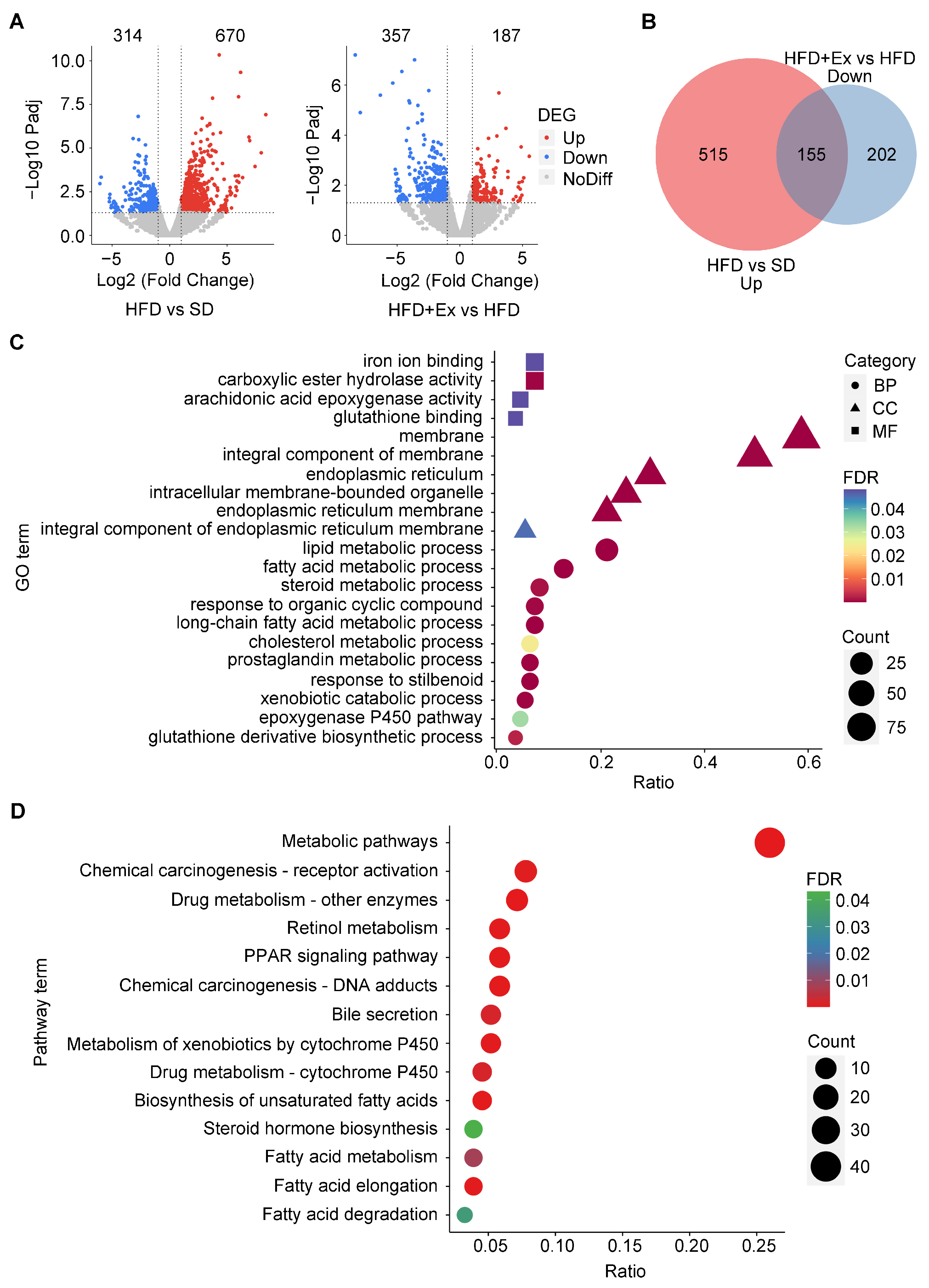
2.3. Treadmill Exercise Modulates Hepatic Gene Expression and Alters Metabolic Pathways in HFD-Induced MASLD
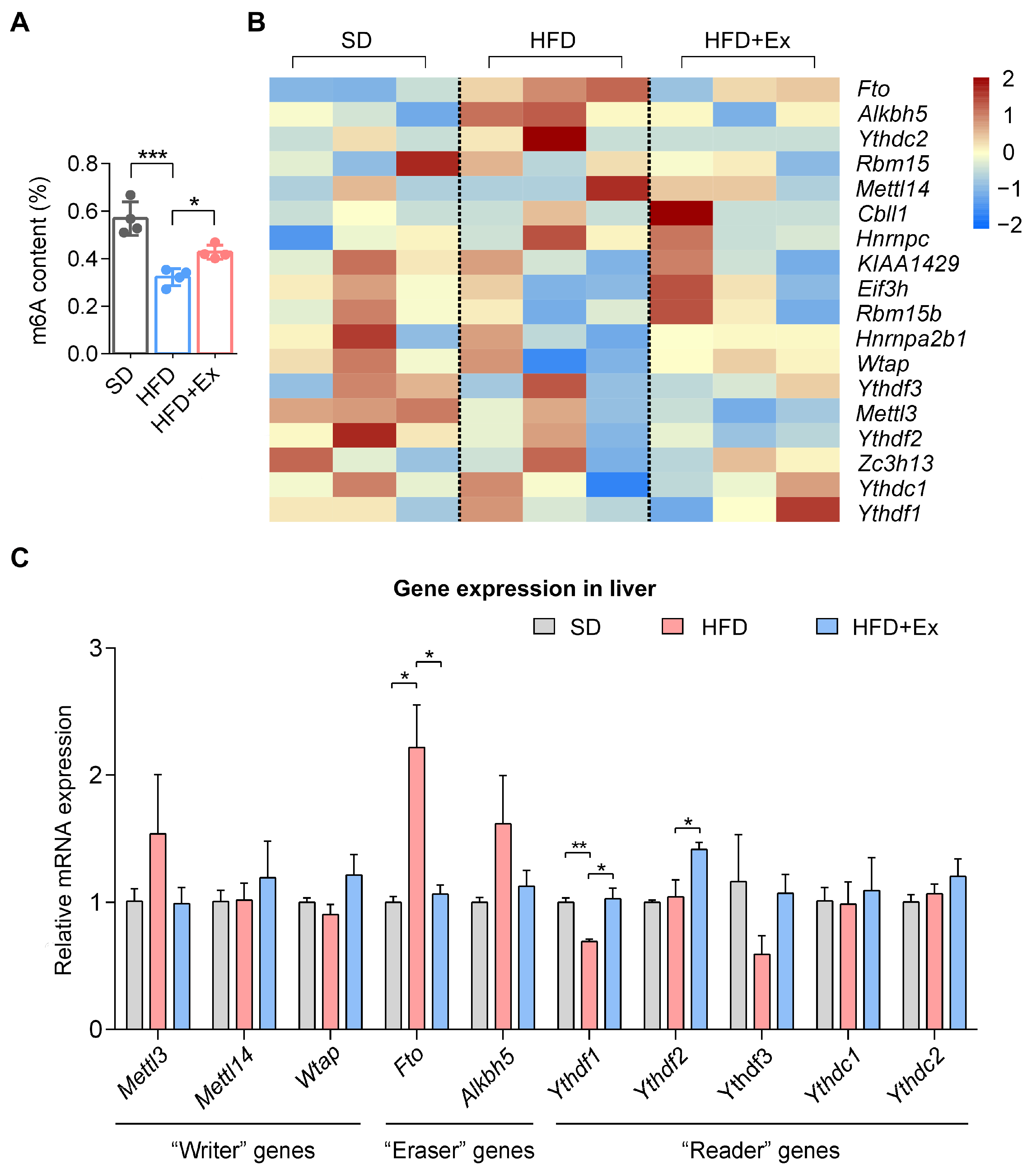
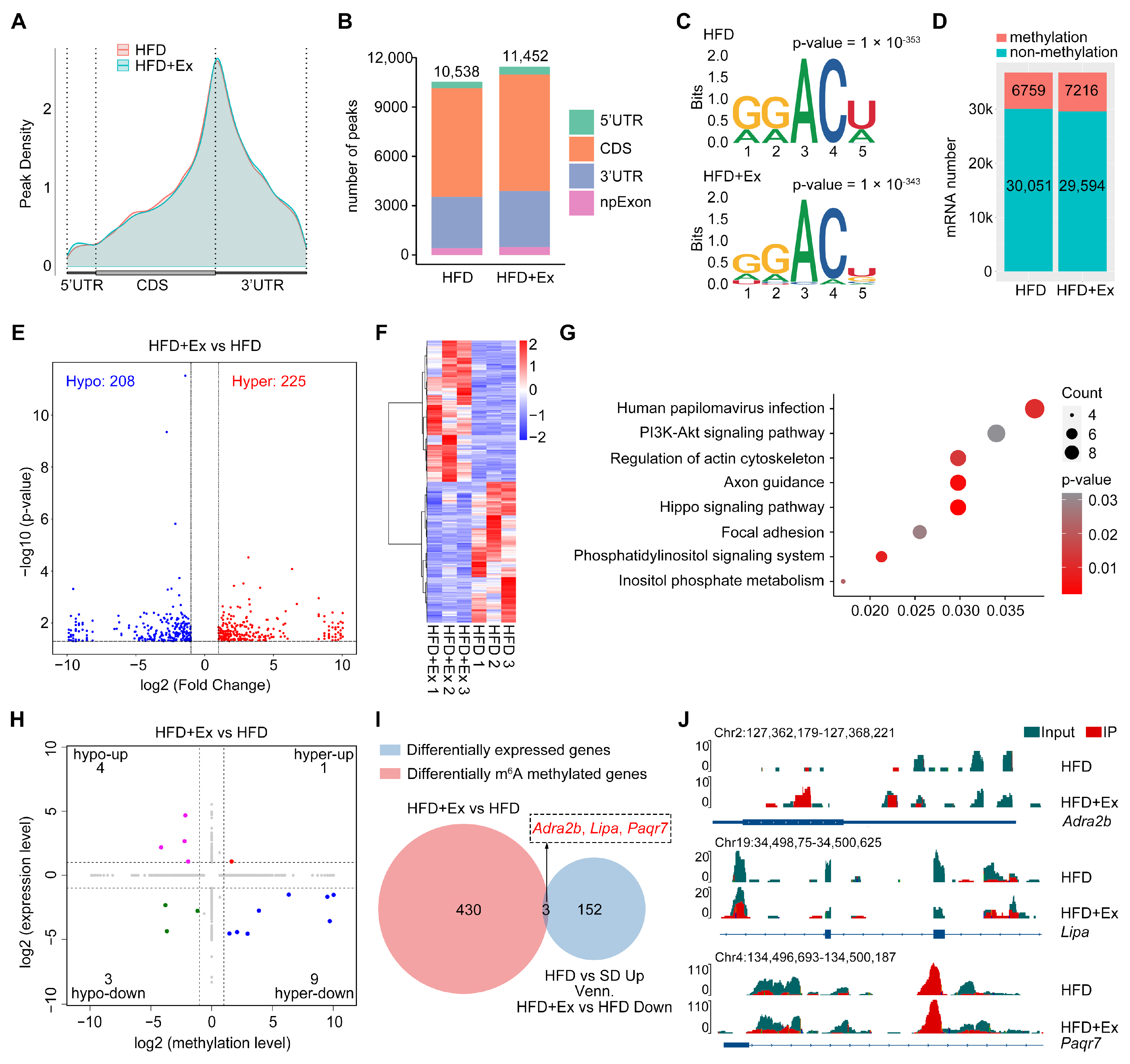
2.4. Treadmill Exercise Modulates Hepatic m6A RNA Methylation in HFD-Induced MASLD
2.5. Treadmill Exercise Modifies the m6A RNA Methylation Landscape to Regulate Liver Function in HFD-Induced MASLD
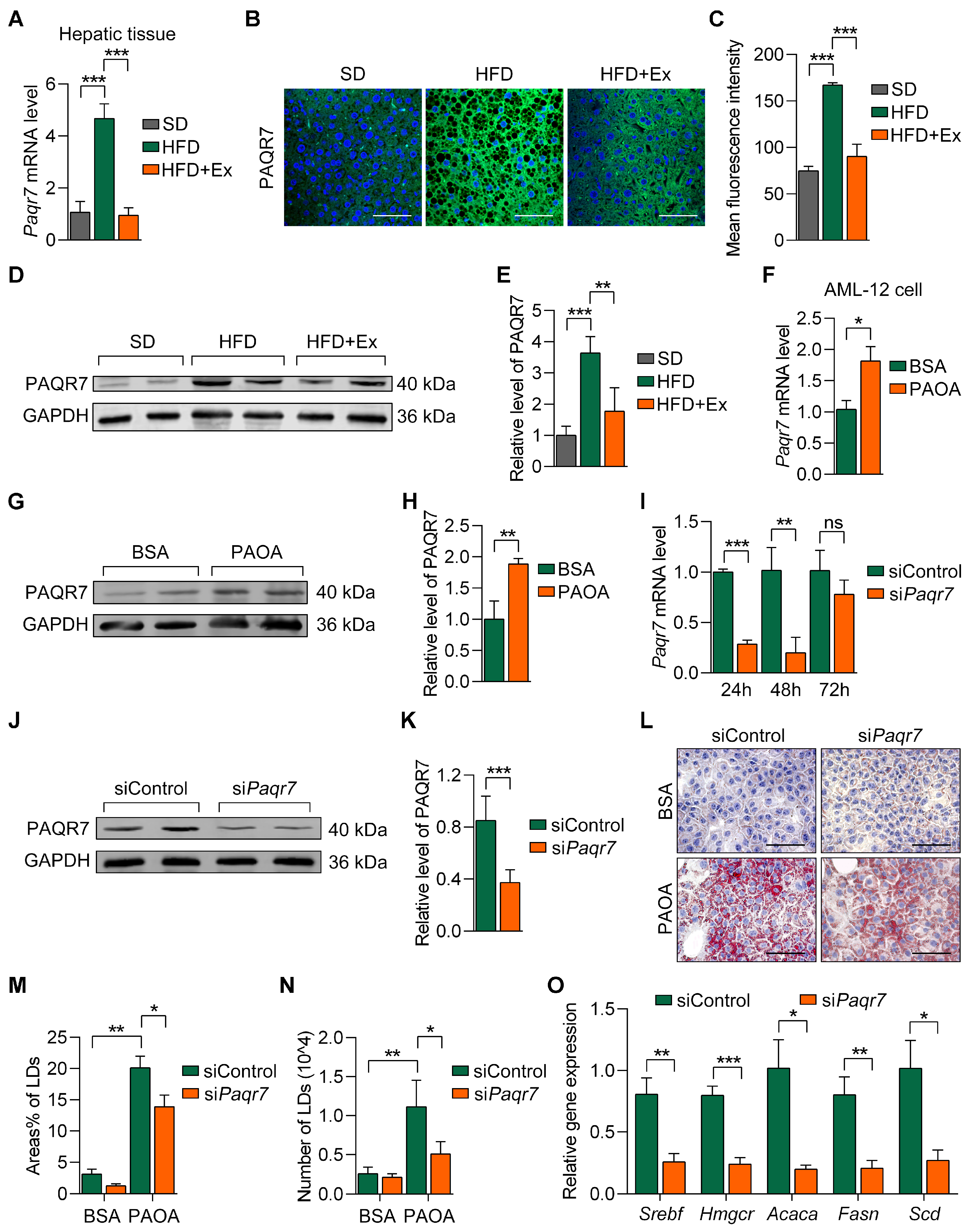
2.6. Treadmill Exercise Modulates PAQR7 Expression and Lipid Metabolism in MASLD
3. Discussion
4. Materials and Methods
4.1. Animals Diet and Exercise Intervention
4.2. Cell Culture and Treatment
4.3. Physiological Measurements
4.4. Histological Analysis
4.5. RNA Sequencing (RNA-Seq) Analysis
4.6. m6A RNA Methylation Quantification
4.7. Reverse Transcription and Quantitative PCR (RT-qPCR)
4.8. Methylated RNA Immunoprecipitation Sequencing (MeRIP-Seq)
4.9. Immunofluorescence
4.10. Western Blot
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| DEG | Differentially expressed gene |
| DMG | Differentially methylated gene |
| HFD | High-fat diet |
| KEGG | Kyoto encyclopedia of genes and genomes |
| m6A | N6-methyladenine |
| MASLD | Metabolic-associated steatotic liver disease |
| NASH | Non-alcoholic steatohepatitis |
| NCC | Hepatocellular carcinoma |
| PAOA | Palmitic acid and oleic acid |
| SD | Standard diet |
| TC | Total cholesterol |
| TG | Triglycerides |
References
- Powell, E.E.; Wong, V.W.; Rinella, M. Non-Alcoholic Fatty Liver Disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Vazquez-Montesino, L.M.; Li, A.A.; Cholankeril, G.; Ahmed, A. Inadequate Physical Activity and Sedentary Behavior Are Independent Predictors of Nonalcoholic Fatty Liver Disease. Hepatology 2020, 72, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Cotter, T.G.; Rinella, M. Nonalcoholic Fatty Liver Disease 2020: The State of the Disease. Gastroenterology 2020, 158, 1851–1864. [Google Scholar] [CrossRef]
- Rinella, M.E. Nonalcoholic Fatty Liver Disease: A Systematic Review. JAMA 2015, 313, 2263–2273. [Google Scholar] [CrossRef]
- Romero-Gómez, M.; Zelber-Sagi, S.; Trenell, M. Treatment of NAFLD with Diet, Physical Activity and Exercise. J. Hepatol. 2017, 67, 829–846. [Google Scholar] [CrossRef]
- Hejazi, K.; Hackett, D. Effect of Exercise on Liver Function and Insulin Resistance Markers in Patients with Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2023, 12, 3011. [Google Scholar] [CrossRef]
- Nam, H.; Yoo, J.J.; Cho, Y.; Kang, S.H.; Ahn, S.B.; Lee, H.W.; Jun, D.W.; Song, D.S.; Choi, M. Effect of Exercise-Based Interventions in Nonalcoholic Fatty Liver Disease: A Systematic Review with Meta-Analysis. Dig. Liver Dis. 2023, 55, 1178–1186. [Google Scholar] [CrossRef]
- Stine, J.G.; DiJoseph, K.; Pattison, Z.; Harrington, A.; Chinchilli, V.M.; Schmitz, K.H.; Loomba, R. Exercise Training Is Associated With Treatment Response in Liver Fat Content by Magnetic Resonance Imaging Independent of Clinically Significant Body Weight Loss in Patients with Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2023, 118, 1204–1213. [Google Scholar] [CrossRef] [PubMed]
- McGee, S.L.; Hargreaves, M. Epigenetics and Exercise. Trends Endocrinol. Metab. 2019, 30, 636–645. [Google Scholar] [CrossRef]
- Dupont, C.; Armant, D.R.; Brenner, C.A. Epigenetics: Definition, Mechanisms and Clinical Perspective. Semin. Reprod. Med. 2009, 27, 351–357. [Google Scholar] [CrossRef]
- Zaccara, S.; Ries, R.J.; Jaffrey, S.R. Reading, Writing and Erasing mRNA Methylation. Nat. Rev. Mol. Cell Biol. 2023, 24, 770. [Google Scholar] [CrossRef]
- Roundtree, I.A.; Evans, M.E.; Pan, T.; He, C. Dynamic RNA Modifications in Gene Expression Regulation. Cell 2017, 169, 1187–1200. [Google Scholar] [CrossRef]
- Qin, Y.; Li, B.; Arumugam, S.; Lu, Q.; Mankash, S.M.; Li, J.; Sun, B.; Li, J.; Flavell, R.A.; Li, H.B.; et al. m6A mRNA Methylation-Directed Myeloid Cell Activation Controls Progression of Nafld and Obesity. Cell Rep. 2021, 37, 109968. [Google Scholar] [CrossRef]
- Gan, X.; Dai, Z.; Ge, C.; Yin, H.; Wang, Y.; Tan, J.; Sun, S.; Zhou, W.; Yuan, S.; Yang, F. Fto Promotes Liver Inflammation by Suppressing m6A mRNA Methylation of Il-17RA. Front. Oncol. 2022, 12, 989353. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Sun, C.; Yan, Y.; Niu, Z.; Li, Y.; Xu, X.; Zhang, J.; Wu, Y.; Li, Y.; Wang, L.; et al. Aberrant Elevation of Fto Levels Promotes Liver Steatosis by Decreasing the m6A Methylation and Increasing the Stability of SREBF1 and ChREBP mRNAs. J. Mol. Cell. Biol. 2023, 14, mjac061. [Google Scholar] [CrossRef] [PubMed]
- Danaher, J.; Stathis, C.G.; Wilson, R.A.; Moreno-Asso, A.; Wellard, R.M.; Cooke, M.B. High Intensity Exercise Downregulates FTO mRNA Expression During the Early Stages of Recovery in Young Males and Females. Nutr. Metab. 2020, 17, 68. [Google Scholar] [CrossRef]
- Liu, S.; Cai, T.; Fang, C.; Lin, S.; Yang, W.; Wei, Y.; Zhou, F.; Liu, L.; Luo, Y.; Guo, Z.; et al. Long-Term Exercise Training Down-Regulates m6A RNA Demethylase FTO Expression in the Hippocampus and Hypothalamus: An Effective Intervention for Epigenetic Modification. BMC Neurosci. 2022, 23, 54. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, J.; Yu, P.; Feng, J.; Xu, G.E.; Zhao, X.; Wang, T.; Lehmann, H.I.; Li, G.; Sluijter, J.P.G.; et al. METTL14 Is Required for Exercise-Induced Cardiac Hypertrophy and Protects Against Myocardial Ischemia-Reperfusion Injury. Nat. Commun. 2022, 13, 6762. [Google Scholar] [CrossRef]
- Xu, Z.; Qin, Y.; Lv, B.; Tian, Z.; Zhang, B. Effects of Moderate-Intensity Continuous Training and High-Intensity Interval Training on Testicular Oxidative Stress, Apoptosis and m6A Methylation in Obese Male Mice. Antioxidants 2022, 11, 1874. [Google Scholar] [CrossRef]
- Yan, L.; Wei, J.A.; Yang, F.; Wang, M.; Wang, S.; Cheng, T.; Liu, X.; Jia, Y.; So, K.F.; Zhang, L. Physical Exercise Prevented Stress-Induced Anxiety via Improving Brain RNA Methylation. Adv. Sci. 2022, 9, e2105731. [Google Scholar] [CrossRef]
- Keating, S.E.; Hackett, D.A.; George, J.; Johnson, N.A. Exercise and Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. J. Hepatol. 2012, 57, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Sung, K.C.; Ryu, S.; Lee, J.Y.; Kim, J.Y.; Wild, S.H.; Byrne, C.D. Effect of Exercise on the Development of New Fatty Liver and the Resolution of Existing Fatty Liver. J. Hepatol. 2016, 65, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Peng, Y.; Zhang, L.; Ba, Y.; Jin, G.; Liu, G. Effect of Different Exercise Modalities on Nonalcoholic Fatty Liver Disease: A Systematic Review and Network Meta-Analysis. Sci. Rep. 2024, 14, 6212. [Google Scholar] [CrossRef] [PubMed]
- Kazeminasab, F.; Baharlooie, M.; Rezazadeh, H.; Soltani, N.; Rosenkranz, S.K. The Effects of Aerobic Exercise on Liver Function, Insulin Resistance, and Lipid Profiles in Prediabetic and Type 2 Diabetic Mice. Physiol. Behav. 2023, 271, 114340. [Google Scholar] [CrossRef]
- Farzanegi, P.; Dana, A.; Ebrahimpoor, Z.; Asadi, M.; Azarbayjani, M.A. Mechanisms of Beneficial Effects of Exercise Training on Non-Alcoholic Fatty Liver Disease (NAFLD): Roles of Oxidative Stress and Inflammation. Eur. J. Sport Sci. 2019, 19, 994–1003. [Google Scholar] [CrossRef]
- Brouwers, B.; Hesselink, M.K.; Schrauwen, P.; Schrauwen-Hinderling, V.B. Effects of Exercise Training on Intrahepatic Lipid Content in Humans. Diabetologia 2016, 59, 2068–2079. [Google Scholar] [CrossRef]
- Piguet, A.C.; Guarino, M.; Potaczek, D.P.; Garn, H.; Dufour, J.F. Hepatic Gene Expression in Mouse Models of Non-Alcoholic Fatty Liver Disease After Acute Exercise. Hepatol. Res. 2019, 49, 637–652. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Cui, G.; Zhao, F.; Tian, X.; Sun, B.F.; Yang, Y.; Li, W. m6A Regulates Liver Metabolic Disorders and Hepatogenous Diabetes. Genom. Proteom. Bioinform. 2020, 18, 371–383. [Google Scholar] [CrossRef]
- Wang, S.; Gao, S.; Ye, W.; Li, Y.; Luan, J.; Lv, X. The Emerging Importance Role of M6a Modification in Liver Disease. Biomed. Pharmacother. 2023, 162, 114669. [Google Scholar] [CrossRef]
- Ma, W.; Wu, T. RNA M6a Modification in Liver Biology and Its Implication in Hepatic Diseases and Carcinogenesis. Am. J. Physiol. Cell Physiol. 2022, 323, C1190–C1205. [Google Scholar] [CrossRef]
- Madsen, O.; Willemsen, D.; Ursing, B.M.; Arnason, U.; de Jong, W.W. Molecular Evolution of the Mammalian Alpha 2b Adrenergic Receptor. Mol. Biol. Evol. 2002, 19, 2150–2160. [Google Scholar] [CrossRef] [PubMed]
- Leopold, C.; Duta-Mare, M.; Sachdev, V.; Goeritzer, M.; Maresch, L.K.; Kolb, D.; Reicher, H.; Wagner, B.; Stojakovic, T.; Ruelicke, T.; et al. Hepatocyte-Specific Lysosomal Acid Lipase Deficiency Protects Mice from Diet-Induced Obesity but Promotes Hepatic Inflammation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 500–511. [Google Scholar] [CrossRef]
- Pajed, L.; Wagner, C.; Taschler, U.; Schreiber, R.; Kolleritsch, S.; Fawzy, N.; Pototschnig, I.; Schoiswohl, G.; Pusch, L.M.; Wieser, B.I.; et al. Hepatocyte-Specific Deletion of Lysosomal Acid Lipase Leads to Cholesteryl Ester but Not Triglyceride or Retinyl Ester Accumulation. J. Biol. Chem. 2019, 294, 9118–9133. [Google Scholar] [CrossRef]
- Sueldo, C.; Liu, X.; Peluso, J.J. Progestin and adipoQ Receptor 7, Progesterone Membrane Receptor Component 1 (Pgrmc1), and Pgrmc2 and Their Role in Regulating Progesterone’s Ability to Suppress Human Granulosa/Luteal Cells from Entering into the Cell Cycle. Biol. Reprod. 2015, 93, 63. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, T.; Zheng, L.; Huang, J.; Zheng, Y.; Li, J. PAQR7: An Intermediary Mediating Nongenomic Progesterone Action in Female Reproductive Tissue. Reprod. Biol. 2021, 21, 100529. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; He, J.; Wu, Z.; Wang, F.; Huang, J.; Zheng, L.; Luo, T. Progestin and adipoQ Receptor 7 (PAQR7) Mediate the Anti-Apoptotic Effect of P4 on Human Granulosa Cells and Its Deficiency Reduces Ovarian Function in Female Mice. J. Ovarian Res. 2024, 17, 35. [Google Scholar] [CrossRef] [PubMed]
- Jaenisch, R.; Bird, A. Epigenetic Regulation of Gene Expression: How the Genome Integrates Intrinsic and Environmental Signals. Nat. Genet. 2003, 33 (Suppl. S3), 245–254. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, Z.; Qin, C.; Momeni, M.R. The Effects of Exercise on Epigenetic Modifications: Focus on DNA Methylation, Histone Modifications and Non-Coding RNAs. Hum. Cell 2024, 37, 887–903. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and Validation of a Histological Scoring System for Nonalcoholic Fatty Liver Disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zhong, Y.; Guo, Y.; Xu, J.; Wang, S.; Liu, Y.; Lv, Y.; Zheng, X. Treadmill Exercise-Induced RNA m6A Methylation Modification in the Prevention of High-Fat Diet-Induced MASLD in Mice. Int. J. Mol. Sci. 2025, 26, 5810. https://doi.org/10.3390/ijms26125810
Liu X, Zhong Y, Guo Y, Xu J, Wang S, Liu Y, Lv Y, Zheng X. Treadmill Exercise-Induced RNA m6A Methylation Modification in the Prevention of High-Fat Diet-Induced MASLD in Mice. International Journal of Molecular Sciences. 2025; 26(12):5810. https://doi.org/10.3390/ijms26125810
Chicago/Turabian StyleLiu, Xueli, Yuanming Zhong, Yuqian Guo, Jianhua Xu, Shaobing Wang, Yiping Liu, Yi Lv, and Xi Zheng. 2025. "Treadmill Exercise-Induced RNA m6A Methylation Modification in the Prevention of High-Fat Diet-Induced MASLD in Mice" International Journal of Molecular Sciences 26, no. 12: 5810. https://doi.org/10.3390/ijms26125810
APA StyleLiu, X., Zhong, Y., Guo, Y., Xu, J., Wang, S., Liu, Y., Lv, Y., & Zheng, X. (2025). Treadmill Exercise-Induced RNA m6A Methylation Modification in the Prevention of High-Fat Diet-Induced MASLD in Mice. International Journal of Molecular Sciences, 26(12), 5810. https://doi.org/10.3390/ijms26125810





