Elacridar Inhibits BCRP Protein Activity in 2D and 3D Cell Culture Models of Ovarian Cancer and Re-Sensitizes Cells to Cytotoxic Drugs
Abstract
1. Introduction
2. Results
2.1. The Drug Resistance Characterization of the A2780 Cell Line and TOP-Resistant Cell Lines
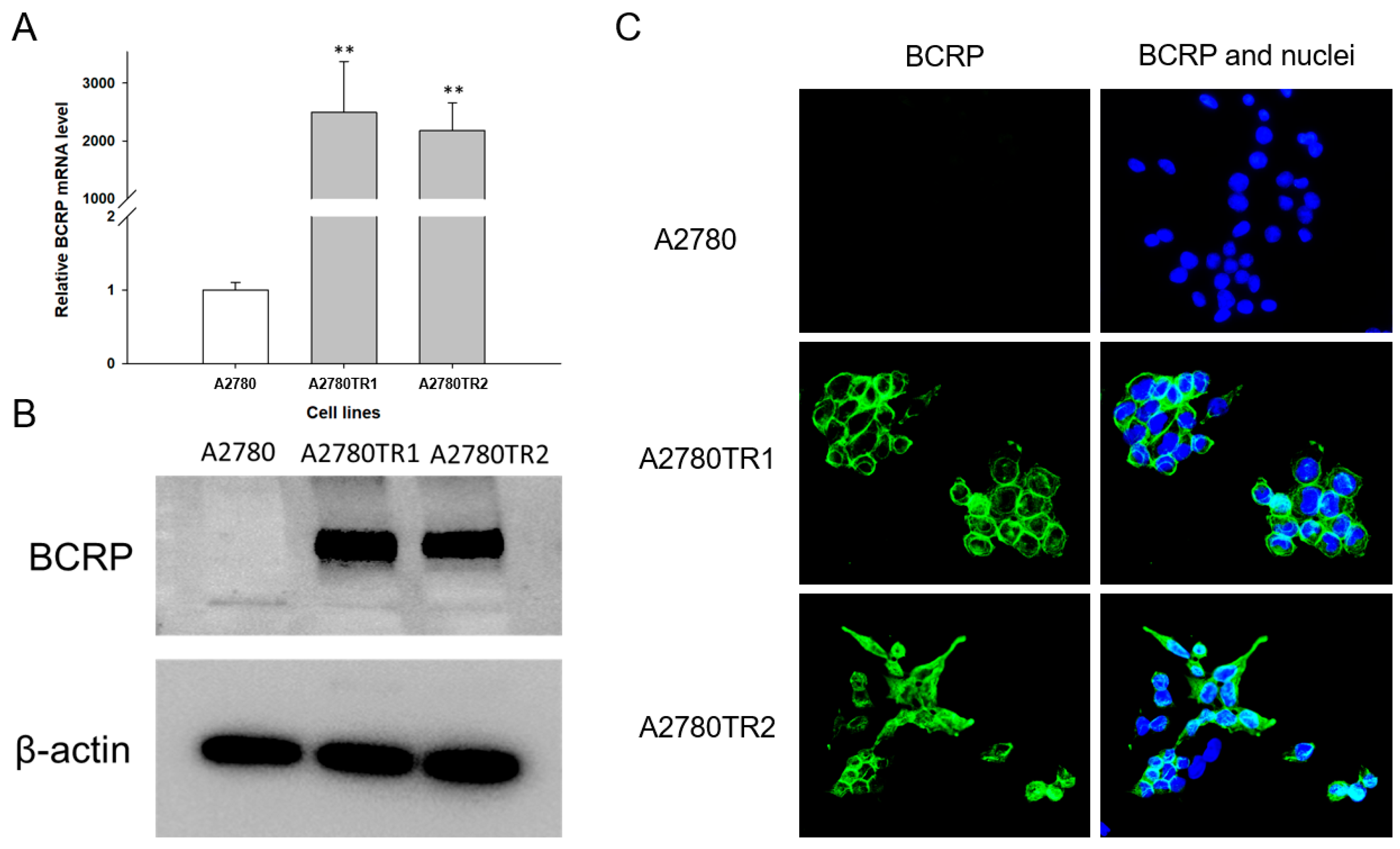
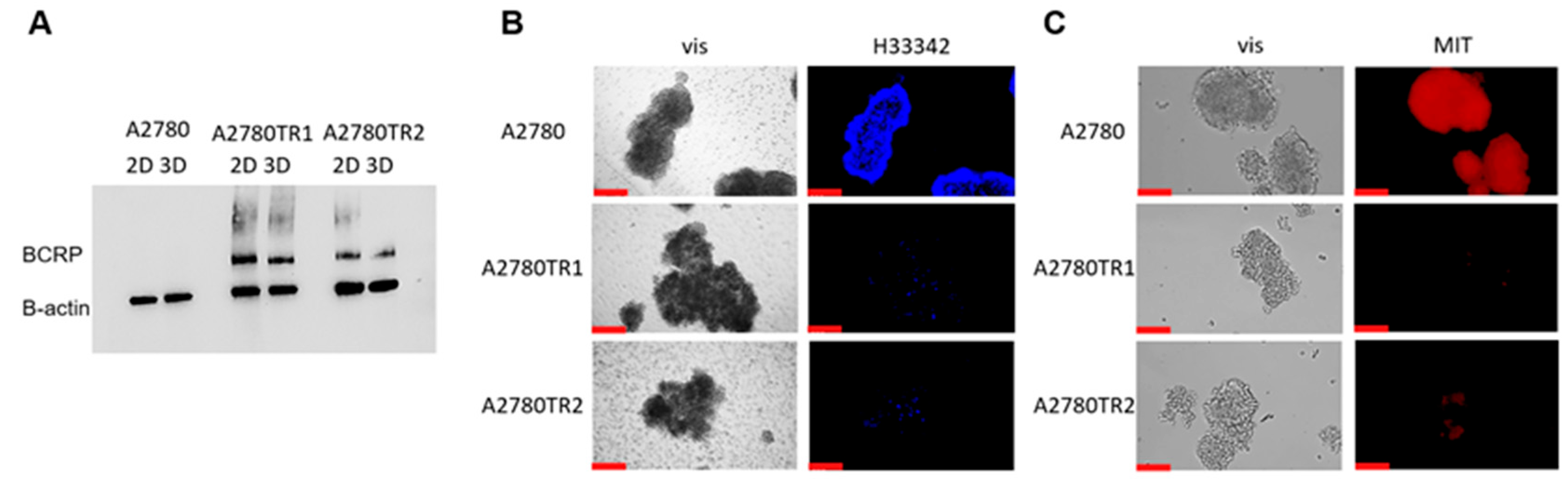
2.2. Characterization of BCRP Protein Expression in A2780 Cell Line and TOP-Resistant Cell Lines
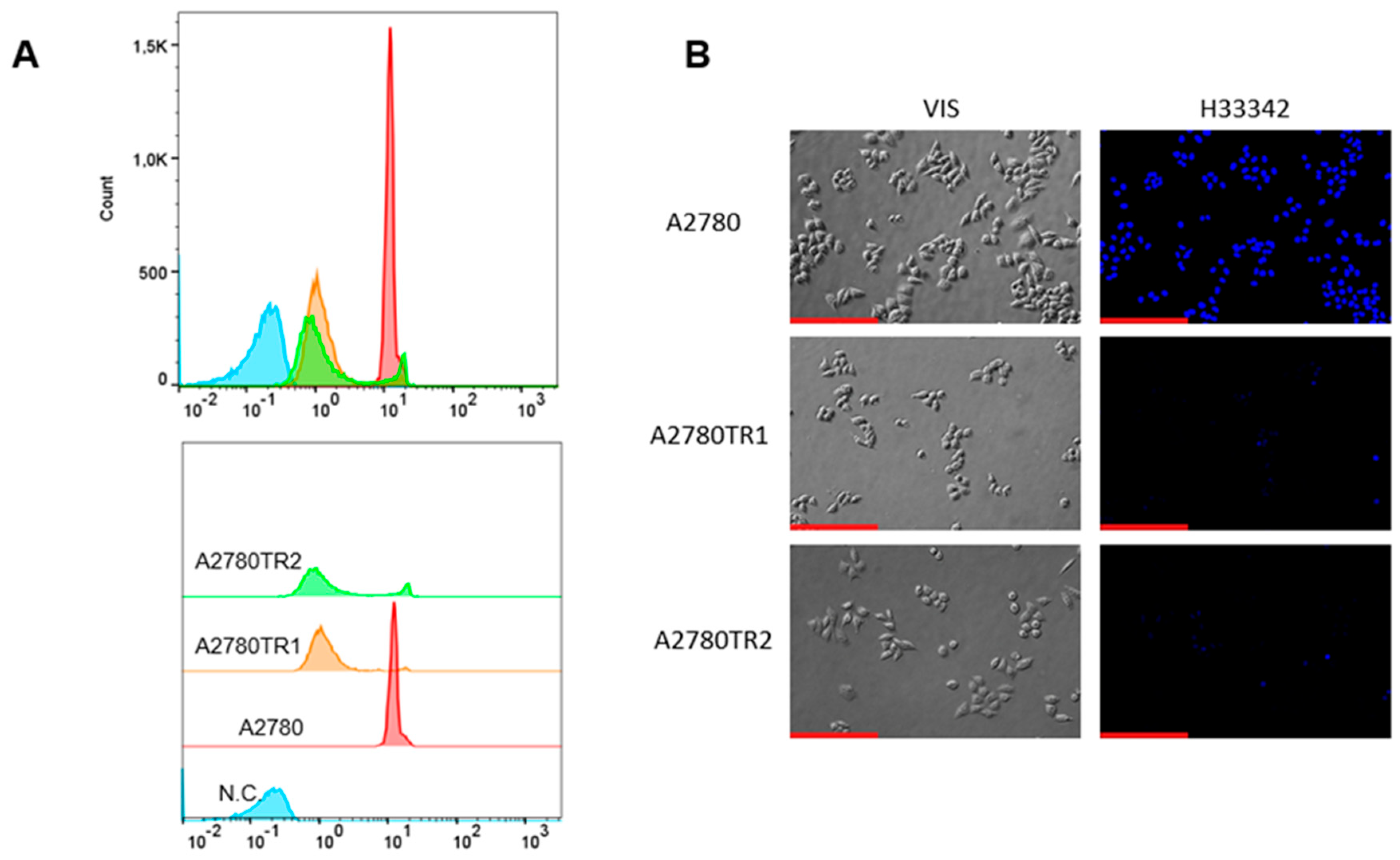
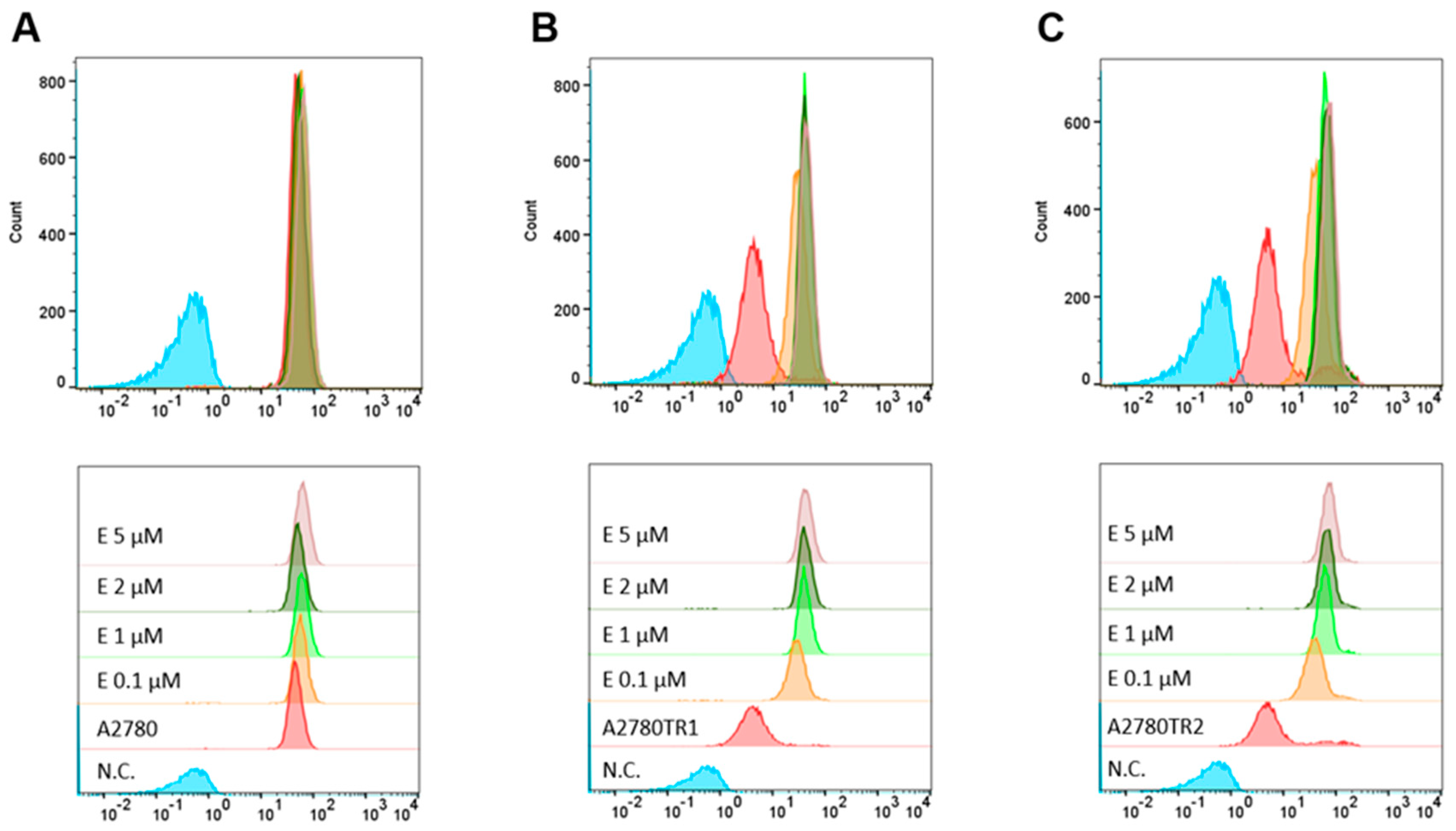
2.3. BCRP Activity Analysis
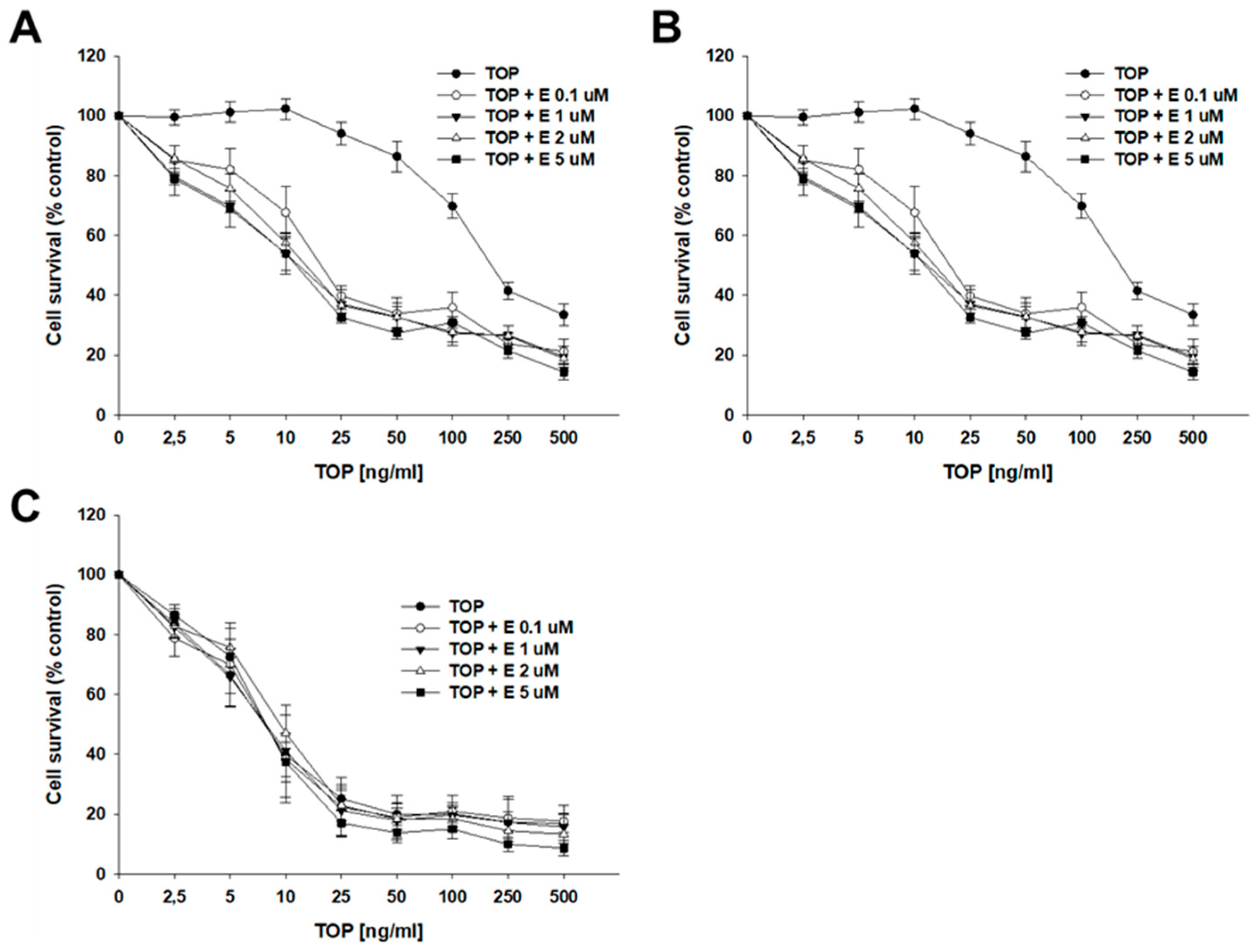
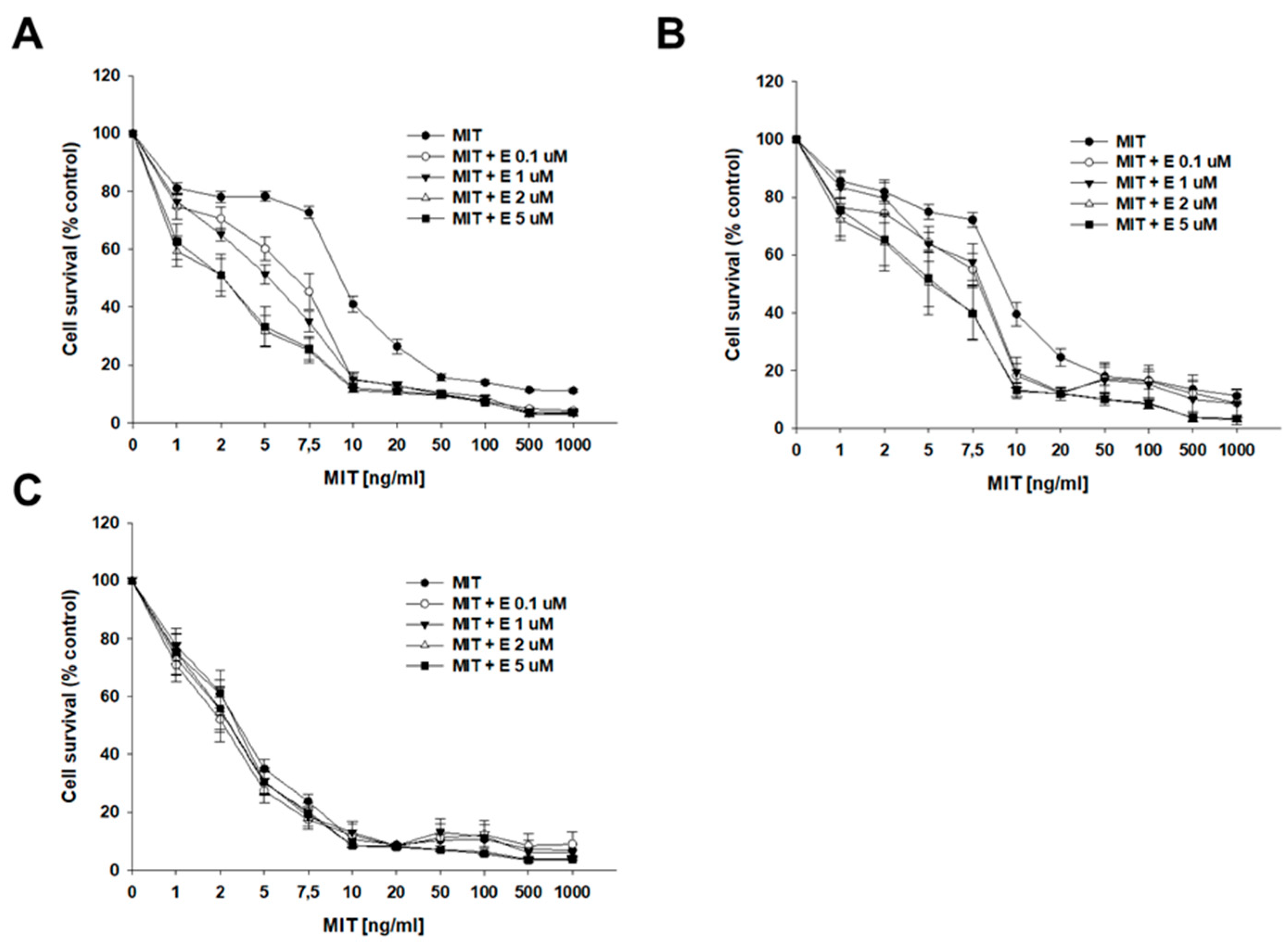
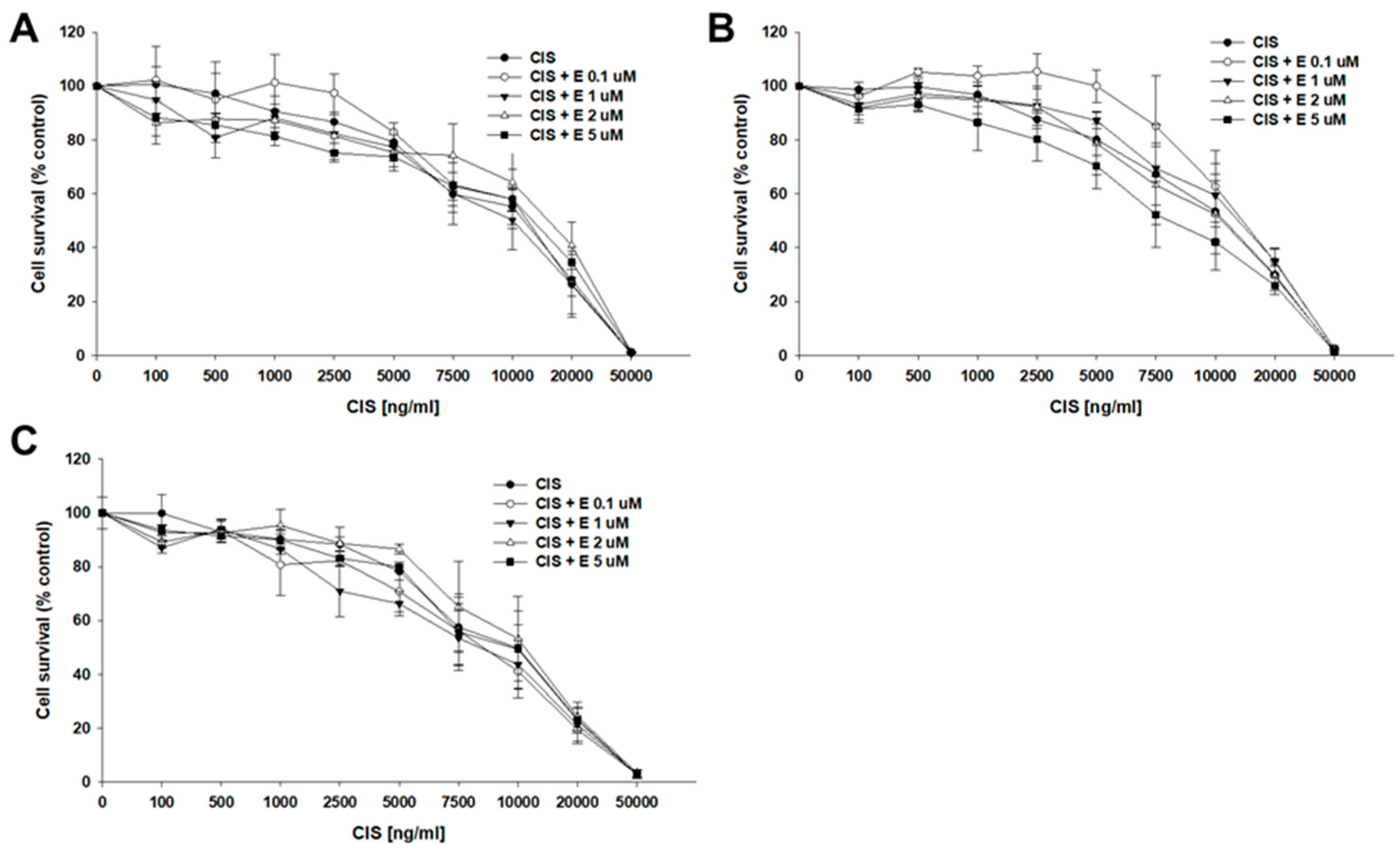
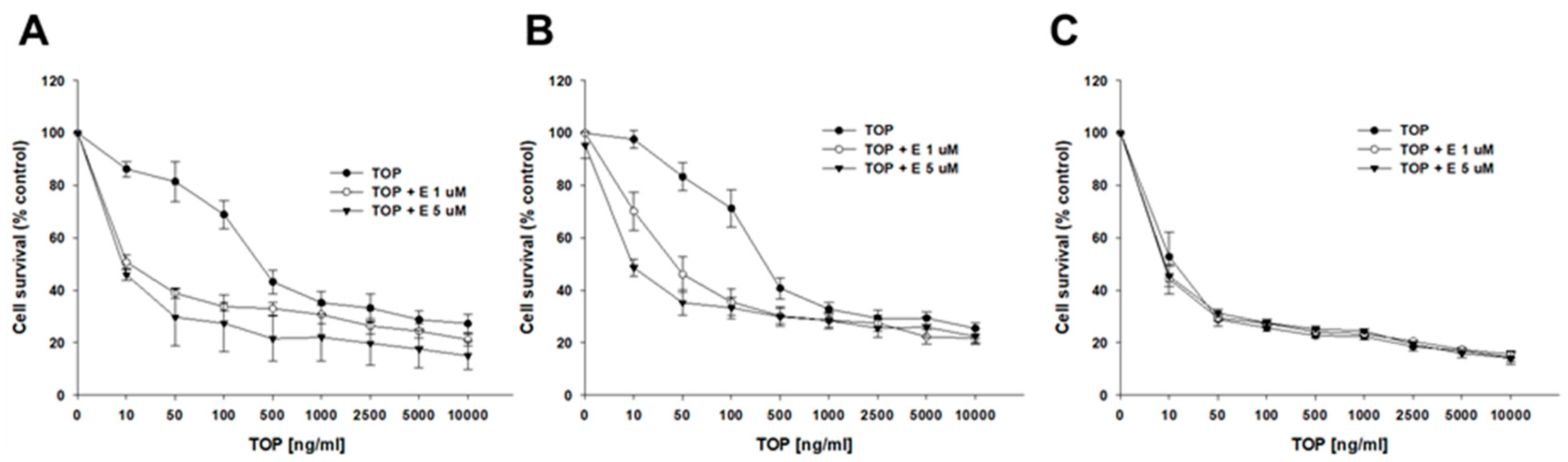
2.4. Analysis of How Elacridar Affects the Resistance to Cytotoxic Drugs
2.5. BCRP Protein Expression After Elacridar Treatment
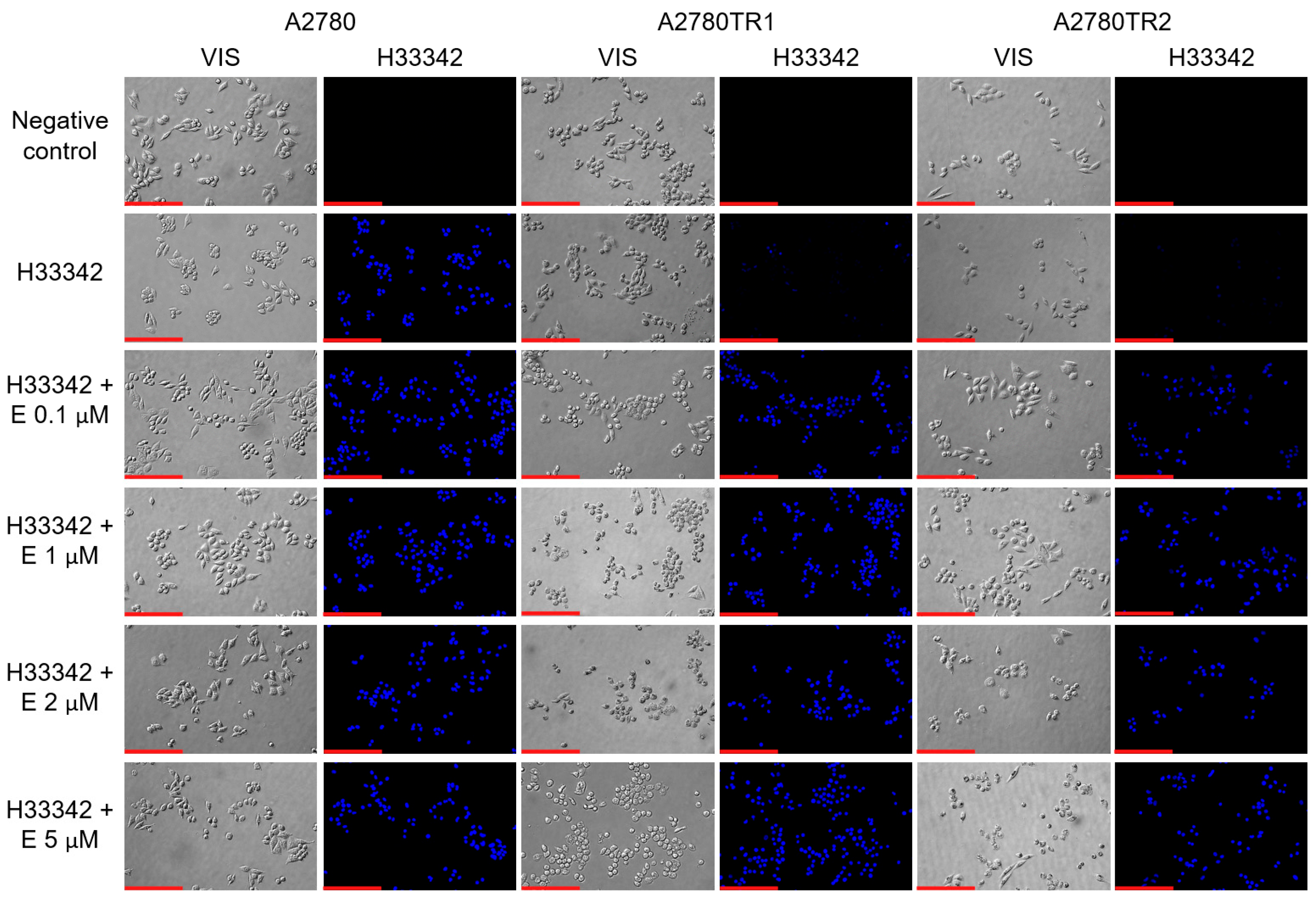
2.6. BCRP Protein Activity After Elacridar Treatment in 2D Culture
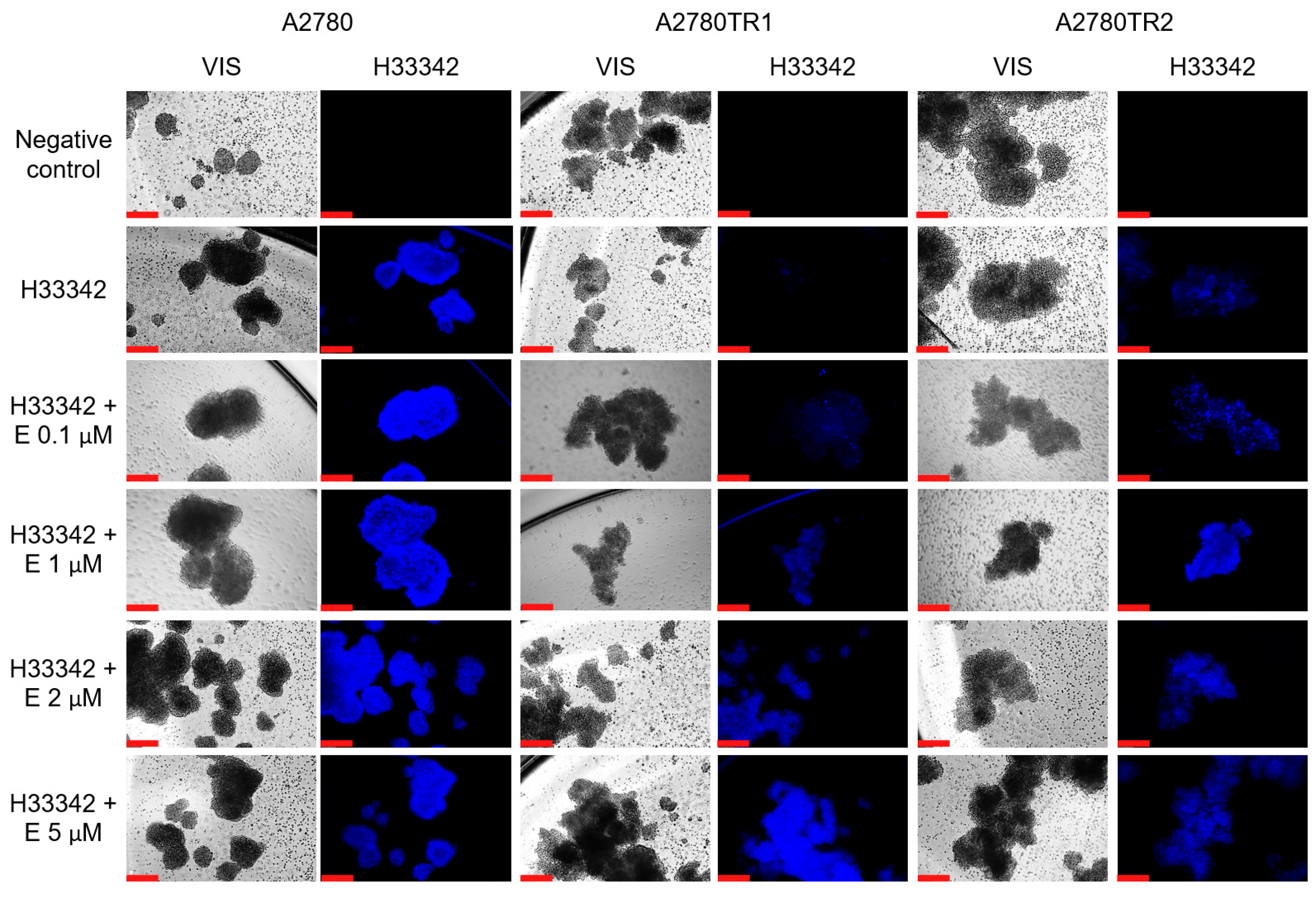
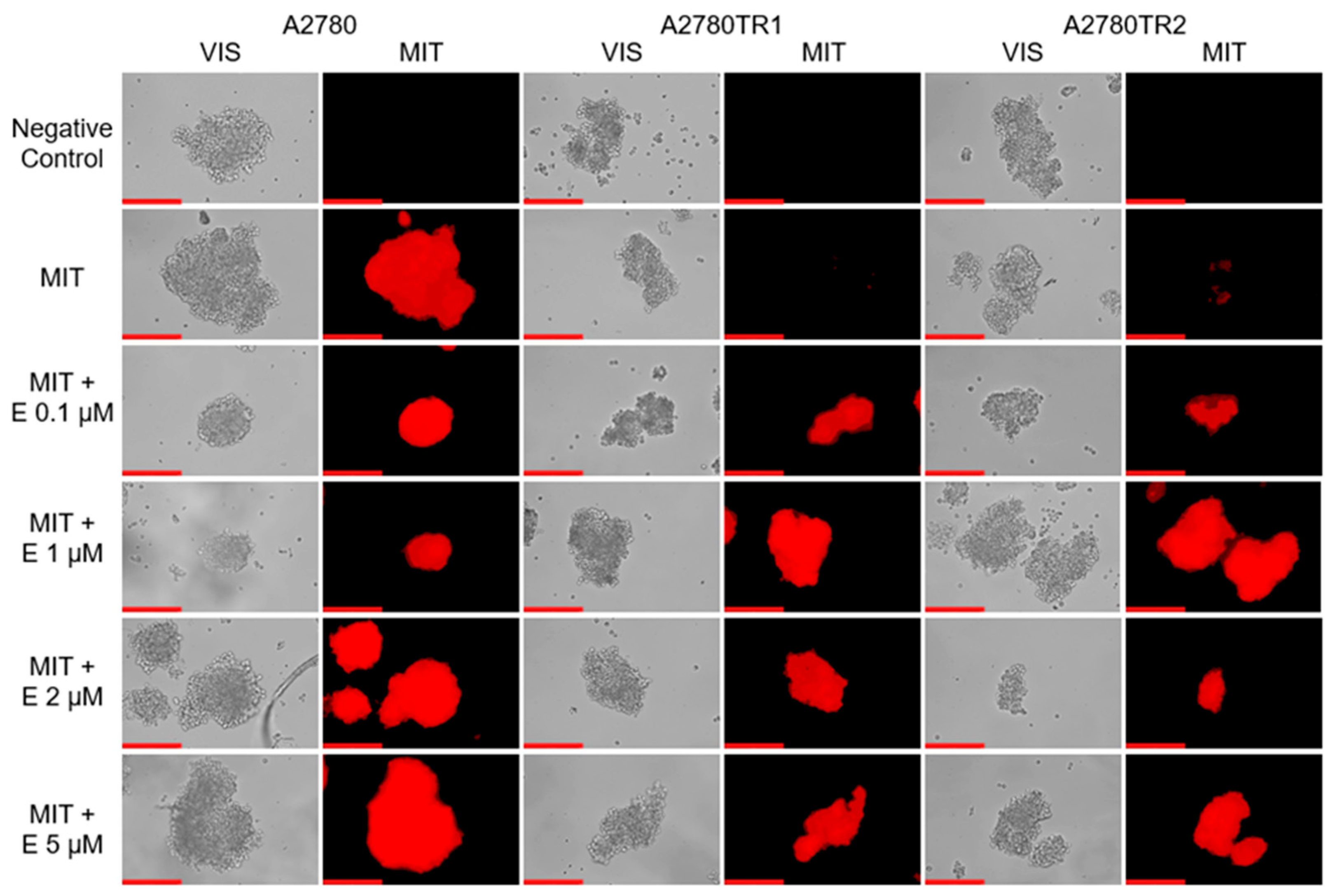
2.7. Analysis of Elacridar’s Effect in a 3D Model
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Cell Lines and Cell Culture
4.3. Isolation of the RNA, cDNA Synthesis, and qPCR
4.4. Treatment of Cells with Elacridar
4.5. Protein Isolation and Western Blot Analysis
4.6. Immunofluorescence
4.7. Live-Cell Immunofluorescence (H33342 Accumulation)
4.8. Flow Cytometry Analysis
4.9. Analysis of MIT and H33342 Accumulation in Spheroids
4.10. 2D MTT Assay
4.11. 3D MTT Assay
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABC | ATP-Binding Cassette |
| ALS | Amyotrophic Lateral Sclerosis |
| AML | Acute Myeloid Leukemia |
| BCRP | Breast Cancer Resistance Protein |
| CIS | Cisplatin |
| CML | Chronic Myeloid Leukemia |
| CNS | Central Nervous System |
| cDNA | Complementary DNA |
| DOX | Doxorubicin |
| DPBS | Dulbecco’s Phosphate-Buffered Saline |
| ECM | Extracellular Matrix |
| EOC | Epithelial Ovarian Cancer |
| FDA | Food and Drug Administration |
| H33342 | Hoechst 33342 |
| HCC | Hepatocellular Carcinoma |
| MIT | Mitoxantrone |
| MDR | Multidrug Resistance |
| MSD | Membrane-Spanning Domain |
| NBD | Nucleotide-Binding Domain |
| P-gp | P-glycoprotein |
| PAC | Paclitaxel |
| PBS | Phosphate-Buffered Saline |
| PhIP | 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine |
| RNA | Ribonucleic Acid |
| RT | Reverse Transcription |
| SCLC | Small Cell Lung Cancer |
| TM | Transmembrane |
| TOP | Topotecan |
References
- Gaona-Luviano, P.; Medina-Gaona, L.A.; Magaña-Pérez, K. Epidemiology of Ovarian Cancer. Chin. Clin. Oncol. 2020, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.R.; Shih, I.-M. Ovarian Cancer. Annu. Rev. Pathol. 2009, 4, 287–313. [Google Scholar] [CrossRef] [PubMed]
- Kossaï, M.; Leary, A.; Scoazec, J.-Y.; Genestie, C. Ovarian Cancer: A Heterogeneous Disease. Pathobiology 2018, 85, 41–49. [Google Scholar] [CrossRef]
- Basta, A.; Bidziński, M.; Bieńkiewicz, A.; Blecharz, P.; Bodnar, L.; Jach, R.; Knapp, P.; Kojs, Z.; Kotarski, J.; Markowska, J.; et al. Recommendations of the Polish Gynecological Oncology Society for the Diagnosis and Treatment of Ovarian Cancer. Curr. Gynecol. Oncol. 2017, 15, 5–23. [Google Scholar] [CrossRef]
- Pignata, S.; Cecere, S.C.; Du Bois, A.; Harter, P.; Heitz, F. Treatment of Recurrent Ovarian Cancer. Ann. Oncol. 2017, 28, viii51–viii56. [Google Scholar] [CrossRef]
- Webb, P.M.; Jordan, S.J. Epidemiology of Epithelial Ovarian Cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Chien, J.; Kuang, R.; Landen, C.; Shridhar, V. Platinum-Sensitive Recurrence in Ovarian Cancer: The Role of Tumor Microenvironment. Front. Oncol. 2013, 3, 251. [Google Scholar] [CrossRef]
- Nitiss, J.L. Targeting DNA Topoisomerase II in Cancer Chemotherapy. Nat. Rev. Cancer 2009, 9, 338–350. [Google Scholar] [CrossRef]
- Almajidi, Y.Q.; Kadhim, M.M.; Alsaikhan, F.; Turki Jalil, A.; Hassan Sayyid, N.; Alexis Ramírez-Coronel, A.; Hassan Jawhar, Z.; Gupta, J.; Nabavi, N.; Yu, W.; et al. Doxorubicin-Loaded Micelles in Tumor Cell-Specific Chemotherapy. Environ. Res. 2023, 227, 115722. [Google Scholar] [CrossRef]
- Pommier, Y.; Leo, E.; Zhang, H.; Marchand, C. DNA Topoisomerases and Their Poisoning by Anticancer and Antibacterial Drugs. Chem. Biol. 2010, 17, 421–433. [Google Scholar] [CrossRef]
- Hartwell, D.; Jones, J.; Loveman, E.; Harris, P.; Clegg, A.; Bird, A. Topotecan for Relapsed Small Cell Lung Cancer: A Systematic Review and Economic Evaluation. Cancer Treat. Rev. 2011, 37, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Abudou, M.; Zhong, D.; Wu, T.; Wu, X. Topotecan for Ovarian Cancer. Cochrane Database Syst. Rev. 2008, 2020. [Google Scholar] [CrossRef]
- Dunton, C. A Review of Topotecan in Combination Chemotherapy for Advanced Cervical Cancer. Ther. Clin. Risk Manag. 2008, 4, 213–218. [Google Scholar] [CrossRef]
- O’Brien, M.; Eckardt, J.; Ramlau, R. Recent Advances with Topotecan in the Treatment of Lung Cancer. Oncologist 2007, 12, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- Berroyer, A.; Kim, N. The Functional Consequences of Eukaryotic Topoisomerase 1 Interaction with G-Quadruplex DNA. Genes 2020, 11, 193. [Google Scholar] [CrossRef]
- Yakkala, P.A.; Penumallu, N.R.; Shafi, S.; Kamal, A. Prospects of Topoisomerase Inhibitors as Promising Anti-Cancer Agents. Pharmaceuticals 2023, 16, 1456. [Google Scholar] [CrossRef]
- Alshammari, M.K.; Alghazwni, M.K.; Alharbi, A.S.; Alqurashi, G.G.; Kamal, M.; Alnufaie, S.R.; Alshammari, S.S.; Alshehri, B.A.; Tayeb, R.H.; Bougeis, R.J.M.; et al. Nanoplatform for the Delivery of Topotecan in the Cancer Milieu: An Appraisal of Its Therapeutic Efficacy. Cancers 2022, 15, 65. [Google Scholar] [CrossRef]
- Rubin, E.H.; Li, T.-K.; Duann, P.; Liu, L.F. Cellular Resistance to Topoisomerase Poisons. In Drug Resistance; Hait, W.N., Ed.; Cancer Treatment and Research; Springer: Boston, MA, USA, 1996; Volume 87, pp. 243–260. ISBN 978-1-4612-8540-3. [Google Scholar]
- Omori, M.; Noro, R.; Seike, M.; Matsuda, K.; Hirao, M.; Fukuizumi, A.; Takano, N.; Miyanaga, A.; Gemma, A. Inhibitors of ABCB1 and ABCG2 Overcame Resistance to Topoisomerase Inhibitors in Small Cell Lung Cancer. Thorac. Cancer 2022, 13, 2142–2151. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.W.; Polgar, O.; Deeken, J.; To, K.W.; Bates, S.E. ABCG2: Determining Its Relevance in Clinical Drug Resistance. Cancer Metastasis Rev. 2007, 26, 39–57. [Google Scholar] [CrossRef]
- Maliepaard, M.; van Gastelen, M.A.; de Jong, L.A.; Pluim, D.; van Waardenburg, R.C.; Ruevekamp-Helmers, M.C.; Floot, B.G.; Schellens, J.H. Overexpression of the BCRP/MXR/ABCP Gene in a Topotecan-Selected Ovarian Tumor Cell Line. Cancer Res. 1999, 59, 4559–4563. [Google Scholar]
- Januchowski, R.; Sterzyńska, K.; Zaorska, K.; Sosińska, P.; Klejewski, A.; Brązert, M.; Nowicki, M.; Zabel, M. Analysis of MDR Genes Expression and Cross-Resistance in Eight Drug Resistant Ovarian Cancer Cell Lines. J. Ovarian Res. 2016, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Klejewski, A.; Świerczewska, M.; Zaorska, K.; Brązert, M.; Nowicki, M.; Zabel, M.; Januchowski, R. New and Old Genes Associated with Topotecan Resistance Development in Ovarian Cancer Cell Lines. Anticancer Res. 2017, 37, 1625–1636. [Google Scholar] [CrossRef]
- Januchowski, R.; Wojtowicz, K.; Sujka-Kordowska, P.; Andrzejewska, M.; Zabel, M. MDR Gene Expression Analysis of Six Drug-Resistant Ovarian Cancer Cell Lines. BioMed Res. Int. 2013, 2013, 241763. [Google Scholar] [CrossRef] [PubMed]
- Nowacka, M.; Ginter-Matuszewska, B.; Świerczewska, M.; Sterzyńska, K.; Nowicki, M.; Januchowski, R. Effect of ALDH1A1 Gene Knockout on Drug Resistance in Paclitaxel and Topotecan Resistant Human Ovarian Cancer Cell Lines in 2D and 3D Model. Int. J. Mol. Sci. 2022, 23, 3036. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.-H. ABC Transporters as Multidrug Resistance Mechanisms and the Development of Chemosensitizers for Their Reversal. Cancer Cell Int. 2005, 5, 30. [Google Scholar] [CrossRef][Green Version]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug Resistance in Cancer: Role of ATP-Dependent Transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef]
- Thomas, H.; Coley, H.M. Overcoming Multidrug Resistance in Cancer: An Update on the Clinical Strategy of Inhibiting P-Glycoprotein. Cancer Control 2003, 10, 159–165. [Google Scholar] [CrossRef]
- Ni, Z.; Bikadi, Z.; Rosenberg, M.F.; Mao, Q. Structure and Function of the Human Breast Cancer Resistance Protein (BCRP/ABCG2). Curr. Drug Metab. 2010, 11, 603–617. [Google Scholar] [CrossRef]
- Mao, Q.; Unadkat, J.D. Role of the Breast Cancer Resistance Protein (BCRP/ABCG2) in Drug Transport—An Update. AAPS J. 2014, 17, 65–82. [Google Scholar] [CrossRef]
- Lyu, C.; Wang, L.; Stadlbauer, B.; Buchner, A.; Pohla, H. A Pan-Cancer Landscape of ABCG2 across Human Cancers: Friend or Foe? Int. J. Mol. Sci. 2022, 23, 15955. [Google Scholar] [CrossRef]
- Mohrmann, K.; van Eijndhoven, M.A.J.; Schinkel, A.H.; Schellens, J.H.M. Absence of N-Linked Glycosylation Does Not Affect Plasma Membrane Localization of Breast Cancer Resistance Protein (BCRP/ABCG2). Cancer Chemother. Pharmacol. 2005, 56, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Diop, N.K.; Hrycyna, C.A. N-Linked Glycosylation of the Human ABC Transporter ABCG2 on Asparagine 596 Is Not Essential for Expression, Transport Activity, or Trafficking to the Plasma Membrane. Biochemistry 2005, 44, 5420–5429. [Google Scholar] [CrossRef] [PubMed]
- Melis, M. Brightening the Path: Riboflavin Illuminates Breast Cancer Resistance Protein Monitoring. J. Pharmacol. Exp. Ther. 2024, 390, 159–161. [Google Scholar] [CrossRef]
- Zhang, F.; Throm, S.L.; Murley, L.L.; Miller, L.A.; Steven Zatechka, D.; Kiplin Guy, R.; Kennedy, R.; Stewart, C.F. MDM2 Antagonist Nutlin-3a Reverses Mitoxantrone Resistance by Inhibiting Breast Cancer Resistance Protein Mediated Drug Transport. Biochem. Pharmacol. 2011, 82, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Mehendale-Munj, S.; Sawant, S. Breast Cancer Resistance Protein: A Potential Therapeutic Target for Cancer. Curr. Drug Targets 2021, 22, 420–428. [Google Scholar] [CrossRef]
- Ferry, D.R.; Traunecker, H.; Kerr, D.J. Clinical Trials of P-Glycoprotein Reversal in Solid Tumours. Eur. J. Cancer 1996, 32, 1070–1081. [Google Scholar] [CrossRef]
- Karthika, C.; Sureshkumar, R.; Zehravi, M.; Akter, R.; Ali, F.; Ramproshad, S.; Mondal, B.; Tagde, P.; Ahmed, Z.; Khan, F.S.; et al. Multidrug Resistance of Cancer Cells and the Vital Role of P-Glycoprotein. Life 2022, 12, 897. [Google Scholar] [CrossRef]
- Bartosiewicz, D.; Krasowska, A. Inhibitors of ABC Transporters and Biophysical Methods to Study Their Activity. Z. Für Naturforschung C 2009, 64, 454–458. [Google Scholar] [CrossRef]
- Callaghan, R.; Luk, F.; Bebawy, M. Inhibition of the Multidrug Resistance P-Glycoprotein: Time for a Change of Strategy? Drug Metab. Dispos. 2014, 42, 623–631. [Google Scholar] [CrossRef]
- Matsson, P.; Pedersen, J.M.; Norinder, U.; Bergström, C.A.S.; Artursson, P. Identification of Novel Specific and General Inhibitors of the Three Major Human ATP-Binding Cassette Transporters P-Gp, BCRP and MRP2 Among Registered Drugs. Pharm. Res. 2009, 26, 1816–1831. [Google Scholar] [CrossRef]
- Sane, R.; Mittapalli, R.K.; Elmquist, W.F. Development and Evaluation of a Novel Microemulsion Formulation of Elacridar to Improve Its Bioavailability. J. Pharm. Sci. 2013, 102, 1343–1354. [Google Scholar] [CrossRef] [PubMed]
- Hyafil, F.; Vergely, C.; Du Vignaud, P.; Grand-Perret, T. In Vitro and in Vivo Reversal of Multidrug Resistance by GF120918, an Acridonecarboxamide Derivative. Cancer Res. 1993, 53, 4595–4602. [Google Scholar] [PubMed]
- Dumaitre, B.; Dodic, N. Acridine Derivatives. Patent Application WO 92/12132, 23 July 1992. [Google Scholar]
- Dash, R.P.; Jayachandra Babu, R.; Srinivas, N.R. Therapeutic Potential and Utility of Elacridar in comparison to P-Glycoprotein Inhibition: An Insight from the Published In Vitro, Preclinical and Clinical Studies. Eur. J. Drug Metab. Pharmacokinet. 2017, 42, 915–933. [Google Scholar] [CrossRef]
- Schirizzi, A.; Contino, M.; Carrieri, L.; Riganti, C.; De Leonardis, G.; Scavo, M.P.; Perrone, M.G.; Miciaccia, M.; Kopecka, J.; Refolo, M.G.; et al. The Multiple Combination of Paclitaxel, Ramucirumab and Elacridar Reverses the Paclitaxel-Mediated Resistance in Gastric Cancer Cell Lines. Front. Oncol. 2023, 13, 1129832. [Google Scholar] [CrossRef] [PubMed]
- Bar-Zeev, M.; Kelmansky, D.; Assaraf, Y.G.; Livney, Y.D. β-Casein Micelles for Oral Delivery of SN-38 and Elacridar to Overcome BCRP-Mediated Multidrug Resistance in Gastric Cancer. Eur. J. Pharm. Biopharm. 2018, 133, 240–249. [Google Scholar] [CrossRef]
- Alves, R.; Gonçalves, A.C.; Jorge, J.; Almeida, A.M.; Sarmento-Ribeiro, A.B. Combination of Elacridar with Imatinib Modulates Resistance Associated with Drug Efflux Transporters in Chronic Myeloid Leukemia. Biomedicines 2022, 10, 1158. [Google Scholar] [CrossRef]
- Marchetti, S.; Oostendorp, R.L.; Pluim, D.; van Eijndhoven, M.; van Tellingen, O.; Schinkel, A.H.; Versace, R.; Beijnen, J.H.; Mazzanti, R.; Schellens, J.H. In Vitro Transport of Gimatecan (7-t-Butoxyiminomethylcamptothecin) by Breast Cancer Resistance Protein, P-Glycoprotein, and Multidrug Resistance Protein 2. Mol. Cancer Ther. 2007, 6, 3307–3313. [Google Scholar] [CrossRef]
- Karbownik, A.; Sobańska, K.; Płotek, W.; Grabowski, T.; Klupczynska, A.; Plewa, S.; Grześkowiak, E.; Szałek, E. The Influence of the Coadministration of the P-Glycoprotein Modulator Elacridar on the Pharmacokinetics of Lapatinib and Its Distribution in the Brain and Cerebrospinal Fluid. Investig. New Drugs 2020, 38, 574–583. [Google Scholar] [CrossRef]
- Durmus, S.; Sparidans, R.W.; Wagenaar, E.; Beijnen, J.H.; Schinkel, A.H. Oral Availability and Brain Penetration of the B-RAFV600E Inhibitor Vemurafenib Can Be Enhanced by the P-GLYCOprotein (ABCB1) and Breast Cancer Resistance Protein (ABCG2) Inhibitor Elacridar. Mol. Pharm. 2012, 9, 3236–3245. [Google Scholar] [CrossRef]
- Jonker, J.W.; Smit, J.W.; Brinkhuis, R.F.; Maliepaard, M.; Beijnen, J.H.; Schellens, J.H.; Schinkel, A.H. Role of Breast Cancer Resistance Protein in the Bioavailability and Fetal Penetration of Topotecan. J. Natl. Cancer Inst. 2000, 92, 1651–1656. [Google Scholar] [CrossRef]
- Sun, D.; Liu, J.; Wang, Y.; Dong, J. Co-Administration of MDR1 and BCRP or EGFR/PI3K Inhibitors Overcomes Lenvatinib Resistance in Hepatocellular Carcinoma. Front. Oncol. 2022, 12, 944537. [Google Scholar] [CrossRef] [PubMed]
- Rowbottom, C.; Pietrasiewicz, A.; Tuczewycz, T.; Grater, R.; Qiu, D.; Kapadnis, S.; Trapa, P. Optimization of Dose and Route of Administration of the P-Glycoprotein Inhibitor, Valspodar (PSC-833) and the P-Glycoprotein and Breast Cancer Resistance Protein Dual-Inhibitor, Elacridar (GF120918) as Dual Infusion in Rats. Pharmacol. Res. Perspect. 2021, 9, e00740. [Google Scholar] [CrossRef] [PubMed]
- Jablonski, M.R.; Markandaiah, S.S.; Jacob, D.; Meng, N.J.; Li, K.; Gennaro, V.; Lepore, A.C.; Trotti, D.; Pasinelli, P. Inhibiting Drug Efflux Transporters Improves Efficacy of ALS Therapeutics. Ann. Clin. Transl. Neurol. 2014, 1, 996–1005. [Google Scholar] [CrossRef]
- Kuppens, I.E.L.M.; Witteveen, E.O.; Jewell, R.C.; Radema, S.A.; Paul, E.M.; Mangum, S.G.; Beijnen, J.H.; Voest, E.E.; Schellens, J.H.M. A Phase I, Randomized, Open-Label, Parallel-Cohort, Dose-Finding Study of Elacridar (GF120918) and Oral Topotecan in Cancer Patients. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007, 13, 3276–3285. [Google Scholar] [CrossRef] [PubMed]
- Kruijtzer, C.M.F.; Beijnen, J.H.; Rosing, H.; ten Bokkel Huinink, W.W.; Schot, M.; Jewell, R.C.; Paul, E.M.; Schellens, J.H.M. Increased Oral Bioavailability of Topotecan in Combination with the Breast Cancer Resistance Protein and P-Glycoprotein Inhibitor GF120918. J. Clin. Oncol. 2002, 20, 2943–2950. [Google Scholar] [CrossRef]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D Cell Cultures—A Comparison of Different Types of Cancer Cell Cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef]
- Świerczewska, M.; Sterzyńska, K.; Ruciński, M.; Andrzejewska, M.; Nowicki, M.; Januchowski, R. The Response and Resistance to Drugs in Ovarian Cancer Cell Lines in 2D Monolayers and 3D Spheroids. Biomed. Pharmacother. 2023, 165, 115152. [Google Scholar] [CrossRef]
- Jain, R.K. Determinants of Tumor Blood Flow: A Review. Cancer Res. 1988, 48, 2641–2658. [Google Scholar]
- Chauhan, V.P.; Stylianopoulos, T.; Boucher, Y.; Jain, R.K. Delivery of Molecular and Nanoscale Medicine to Tumors: Transport Barriers and Strategies. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 281–298. [Google Scholar] [CrossRef]
- Nowacka, M.; Sterzynska, K.; Andrzejewska, M.; Nowicki, M.; Januchowski, R. Drug Resistance Evaluation in Novel 3D in Vitro Model. Biomed. Pharmacother. 2021, 138, 111536. [Google Scholar] [CrossRef]
- Januchowski, R.; Zawierucha, P.; Ruciński, M.; Andrzejewska, M.; Wojtowicz, K.; Nowicki, M.; Zabel, M. Drug Transporter Expression Profiling in Chemoresistant Variants of the A2780 Ovarian Cancer Cell Line. Biomed. Pharmacother. 2014, 68, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhou, H.; Tan, L.; Siu, K.T.H.; Guan, X.-Y. Exploring Treatment Options in Cancer: Tumor Treatment Strategies. Signal Transduct. Target. Ther. 2024, 9, 1–44. [Google Scholar] [CrossRef]
- Tamaki, A.; Ierano, C.; Szakacs, G.; Robey, R.W.; Bates, S.E. The Controversial Role of ABC Transporters in Clinical Oncology. Essays Biochem. 2011, 50, 209–232. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Schuetz, J.D.; Bunting, K.D.; Colapietro, A.-M.; Sampath, J.; Morris, J.J.; Lagutina, I.; Grosveld, G.C.; Osawa, M.; Nakauchi, H.; et al. The ABC Transporter Bcrp1/ABCG2 Is Expressed in a Wide Variety of Stem Cells and Is a Molecular Determinant of the Side-Population Phenotype. Nat. Med. 2001, 7, 1028–1034. [Google Scholar] [CrossRef]
- Diestra, J.E.; Scheffer, G.L.; Català, I.; Maliepaard, M.; Schellens, J.H.M.; Scheper, R.J.; Germà-Lluch, J.R.; Izquierdo, M.A. Frequent Expression of the Multi-Drug Resistance-Associated Protein BCRP/MXR/ABCP/ABCG2 in Human Tumours Detected by the BXP-21 Monoclonal Antibody in Paraffin-Embedded Material. J. Pathol. 2002, 198, 213–219. [Google Scholar] [CrossRef]
- Zattoni, I.F.; Delabio, L.C.; Dutra, J.d.P.; Kita, D.H.; Scheiffer, G.; Hembecker, M.; Pereira, G.d.S.; Moure, V.R.; Valdameri, G. Targeting Breast Cancer Resistance Protein (BCRP/ABCG2): Functional Inhibitors and Expression Modulators. Eur. J. Med. Chem. 2022, 237, 114346. [Google Scholar] [CrossRef]
- Vethanayagam, R.R.; Wang, H.; Gupta, A.; Zhang, Y.; Lewis, F.; Unadkat, J.D.; Mao, Q. Functional Analysis of the Human Variants of Breast Cancer Resistance Protein: I206L, N590Y, and D620N. Drug Metab. Dispos. 2005, 33, 697–705. [Google Scholar] [CrossRef]
- Mogi, M.; Yang, J.; Lambert, J.-F.; Colvin, G.A.; Shiojima, I.; Skurk, C.; Summer, R.; Fine, A.; Quesenberry, P.J.; Walsh, K. Akt Signaling Regulates Side Population Cell Phenotype via Bcrp1 Translocation. J. Biol. Chem. 2003, 278, 39068–39075. [Google Scholar] [CrossRef]
- van der Kolk, D.M.; Vellenga, E.; Scheffer, G.L.; Müller, M.; Bates, S.E.; Scheper, R.J.; de Vries, E.G.E. Expression and Activity of Breast Cancer Resistance Protein (BCRP) in de Novo and Relapsed Acute Myeloid Leukemia. Blood 2002, 99, 3763–3770. [Google Scholar] [CrossRef]
- Ifergan, I.; Shafran, A.; Jansen, G.; Hooijberg, J.H.; Scheffer, G.L.; Assaraf, Y.G. Folate Deprivation Results in the Loss of Breast Cancer Resistance Protein (BCRP/ABCG2) Expression: A Role for BCRP in Cellular Folate Homeostasis. J. Biol. Chem. 2004, 279, 25527–25534. [Google Scholar] [CrossRef]
- Juvale, K.; Stefan, K.; Wiese, M. Synthesis and Biological Evaluation of Flavones and Benzoflavones as Inhibitors of BCRP/ABCG2. Eur. J. Med. Chem. 2013, 67, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Bharthuar, A.; Saif Ur Rehman, S.; Black, J.D.; Levea, C.; Malhotra, U.; Mashtare, T.L.; Iyer, R. Breast Cancer Resistance Protein (BCRP) and Excision Repair Cross Complement-1 (ERCC1) Expression in Esophageal Cancers and Response to Cisplatin and Irinotecan Based Chemotherapy. J. Gastrointest. Oncol. 2014, 5, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Burger, H.; van Tol, H.; Boersma, A.W.M.; Brok, M.; Wiemer, E.A.C.; Stoter, G.; Nooter, K. Imatinib Mesylate (STI571) Is a Substrate for the Breast Cancer Resistance Protein (BCRP)/ABCG2 Drug Pump. Blood 2004, 104, 2940–2942. [Google Scholar] [CrossRef] [PubMed]
- Maliepaard, M.; van Gastelen, M.A.; Tohgo, A.; Hausheer, F.H.; van Waardenburg, R.C.A.M.; de Jong, L.A.; Pluim, D.; Beijnen, J.H.; Schellens, J.H.M. Circumvention of Breast Cancer Resistance Protein (BCRP)-Mediated Resistance to Camptothecins in Vitro Using Non-Substrate Drugs or the BCRP Inhibitor GF1209181. Clin. Cancer Res. 2001, 7, 935–941. [Google Scholar]
- Takahata, T.; Ookawa, K.; Suto, K.; Tanaka, M.; Yano, H.; Nakashima, O.; Kojiro, M.; Tamura, Y.; Tateishi, T.; Sakata, Y.; et al. Chemosensitivity Determinants of Irinotecan Hydrochloride in Hepatocellular Carcinoma Cell Lines. Basic Clin. Pharmacol. Toxicol. 2008, 102, 399–407. [Google Scholar] [CrossRef]
- de Bruin, M.; Miyake, K.; Litman, T.; Robey, R.; Bates, S.E. Reversal of Resistance by GF120918 in Cell Lines Expressing the ABC Half-Transporter, MXR. Cancer Lett. 1999, 146, 117–126. [Google Scholar] [CrossRef]
- Curran, S.; Achilli, T.-M.; Leary, E.; Wilks, B.T.; Vantangoli, M.M.; Boekelheide, K.; Morgan, J.R. A 3D Spheroid System to Evaluate Inhibitors of the ABCG2 Transporter in Drug Uptake and Penetration. Technology 2015, 3, 54–63. [Google Scholar] [CrossRef]
- Kühnle, M.; Egger, M.; Müller, C.; Mahringer, A.; Bernhardt, G.; Fricker, G.; König, B.; Buschauer, A. Potent and Selective Inhibitors of Breast Cancer Resistance Protein (ABCG2) Derived from the p-Glycoprotein (ABCB1) Modulator Tariquidar. J. Med. Chem. 2009, 52, 1190–1197. [Google Scholar] [CrossRef]
- Weiswald, L.-B.; Bellet, D.; Dangles-Marie, V. Spherical Cancer Models in Tumor Biology. Neoplasia 2015, 17, 1–15. [Google Scholar] [CrossRef]
- Świerczewska, M.; Nowacka, M.; Stasiak, P.; Iżycki, D.; Sterzyńska, K.; Płóciennik, A.; Nowicki, M.; Januchowski, R. Doxorubicin and Topotecan Resistance in Ovarian Cancer: Gene Expression and Microenvironment Analysis in 2D and 3D Models. Biomed. Pharmacother. 2025, 183, 117804. [Google Scholar] [CrossRef]
- Stasiak, P.; Sopel, J.; Lipowicz, J.M.; Rawłuszko-Wieczorek, A.A.; Korbecki, J.; Januchowski, R. The Role of Elacridar, a P-Gp Inhibitor, in the Re-Sensitization of PAC-Resistant Ovarian Cancer Cell Lines to Cytotoxic Drugs in 2D and 3D Cell Culture Models. Int. J. Mol. Sci. 2025, 26, 1124. [Google Scholar] [CrossRef] [PubMed]
- Lagas, J.S.; van Waterschoot, R.A.B.; van Tilburg, V.A.C.J.; Hillebrand, M.J.; Lankheet, N.; Rosing, H.; Beijnen, J.H.; Schinkel, A.H. Brain Accumulation of Dasatinib Is Restricted by P-Glycoprotein (ABCB1) and Breast Cancer Resistance Protein (ABCG2) and Can Be Enhanced by Elacridar Treatment. Clin. Cancer Res. 2009, 15, 2344–2351. [Google Scholar] [CrossRef] [PubMed]




| Cell Line | TOP IC50 (ng/mL) | MIT IC50 (ng/mL) | CIS IC50 (ng/mL) |
|---|---|---|---|
| A2780 | 9.79 (3.93–17.4) 1.00 | 2.98 (1.61–4.99) 1.00 | 11,259 (5719–17,384) 1.00 |
| A2780TR1 | 200.68 (140.79–241.43) 20.50 ↑** | 9.60 (8.52–11.8) 3.22 ↑*** | 12,213 (7330–17,583) 1.08 ↑ |
| A2780TR2 | 132.00 (48.27–229.44) 13.48 ↑** | 9.65 (8.10–13.7) 3.24 ↑*** | 12,625 (5528–22,025) 1.12 ↑ |
| Cell Line | Control TOP IC50 (ng/mL) | Elacridar 0.1 μM TOP IC50 (ng/mL) | Elacridar 1 μM TOP IC50 (ng/mL) | Elacridar 2 μM TOP IC50 (ng/mL) | Elacridar 5 μM TOP IC50 (ng/mL) |
|---|---|---|---|---|---|
| A2780 | 9.79 (3.93–17.43) 1.00 | 6.69 (4.53–18.49) 1.46 ↓ | 10.69 (4.85–17.28) 1.09 ↑ | 11.18 (5.21–15.3) 1.14 ↑ | 8.32 (5.19–9.77) 1.17 ↓ |
| A2780TR1 | 204.68 (140.79–241.43) 1.00 | 18.81 (13.64–22.04) 10.88 ↓*** | 15.29 (8.27–21.4) 13.38 ↓*** | 14.99 (8.34–18.4) 13.65 ↓*** | 12.05 (4.86–18.85) 16.98 ↓*** |
| A2780TR2 | 132.00 (48.27–229.44) 1.00 | 19.11 (9.29–35.64) 6.91 ↓** | 11.11 (6.78–15.54) 11.88 ↓*** | 10.02 (6.03–15.05) 13.17 ↓*** | 7.61 (6.23–9.52) 17.59 ↓*** |
| Cell Line | Control MIT IC50 (ng/mL) | Elacridar 0.1 μM MIT IC50 (ng/mL) | Elacridar 1 μM MIT IC50 (ng/mL) | Elacridar 2 μM MIT IC50 (ng/mL) | Elacridar 5 μM MIT IC50 (ng/mL) |
|---|---|---|---|---|---|
| A2780 | 2.98 (1.61–4.99) 1.00 | 2.56 (1.80–3.57) 1.16 ↓ | 3.08 (2.07–4.22) 1.03 ↑ | 2.54 (0.91–4.20) 1.17 ↑ | 2.55 (0.95–4.32) 1.17 ↑ |
| A2780TR1 | 9.60 (8.52–11.8) 1.00 | 6.73 (5.20–8.12) 1.43 ↓* | 5.25 (4.09–6.29) 1.85 ↓*** | 2.44 (0.90–4.48) 3.93 ↓*** | 2.71 (0.93–4.81) 3.55 ↓*** |
| A2780TR2 | 9.65 (8.10–13.7) 1.00 | 7.32 (6.48–8.19) 1.32 ↓** | 7.40 (6.08–8.39) 1.30 ↓* | 5.10 (0.98–8.19) 1.90 ↓* | 5.00 (1.37–8.32) 1.93 ↓* |
| Cell Line | Control CIS IC50 (ng/mL) | Elacridar 0.1 μM CIS IC50 (ng/mL) | Elacridar 1 μM CIS IC50 (ng/mL) | Elacridar 2 μM CIS IC50 (ng/mL) | Elacridar 5 μM CIS IC50 (ng/mL) |
|---|---|---|---|---|---|
| A2780 | 11,259 (5719–17,384) 1.00 | 9228 (5174–16,051) 1.22 ↓ | 9470 (6001–14,572) 1.19 ↓ | 11,494 (6232–16,100) 1.02 ↑ | 11,089 (6489–14,751) 1.01 ↓ |
| A2780TR1 | 12,213 (7330–17,583) 1.00 | 12,599 (8247–19,394) 1.03 ↑ | 11,430 (6418–19,332) 1.06 ↓ | 16,123 (8281–21,191) 1.32 ↑ | 13,686 (10,139–16,732) 1.12 ↑ |
| A2780TR2 | 12,625 (5528–22,025) 1.00 | 13,744 (7392–18,169) 1.08 ↑ | 13,443 (6763–18,048) 1.08 ↑ | 11,971 (5840–15,251) 1.06 ↓ | 9045 (5325–11,274) 1.39 ↓ |
| Cell Line | Control TOP IC50 (ng/mL) | Elacridar 1 μM TOP IC50 (ng/mL) | Elacridar 5 μM TOP IC50 (ng/mL) |
|---|---|---|---|
| A2780 | 17.61 (7.46–32.48) 1.00 | 11.71 (7.92–18.93) 1.49 ↓ | 10.19 (8.00–12.22) 1.73 ↓ |
| A2780TR1 | 447.80 (159.01–774.23) 1.00 | 15.10 (9.26–20.36) 29.66 ↓** | 9.27 (8.70–9.97) 48.31 ↓** |
| A2780TR2 | 404.38 (237.89–601.68) 1.00 | 62.63 (22.30–155.11) 6.46 ↓** | 16.02 (8.46–29.91) 25.24 ↓** |
| Transcript | Sequence (5′–3′ Direction) Forward | Sequence (5′–3′ Direction) Reverse | ENST Number http://www.ensembl.org | Product Size (bp) |
|---|---|---|---|---|
| BCRP | TTCGGCTTGCAACAACTATG | TCCAGACACACCACGGATAA | 00000237612 | 128 |
| GAPDH | GAAGGTGAAGGTCGGAGTCA | GACAAGCTTCCCGTTCTCAG | 00000229239 | 199 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stasiak, P.; Sopel, J.; Płóciennik, A.; Musielak, O.; Lipowicz, J.M.; Rawłuszko-Wieczorek, A.A.; Sterzyńska, K.; Korbecki, J.; Januchowski, R. Elacridar Inhibits BCRP Protein Activity in 2D and 3D Cell Culture Models of Ovarian Cancer and Re-Sensitizes Cells to Cytotoxic Drugs. Int. J. Mol. Sci. 2025, 26, 5800. https://doi.org/10.3390/ijms26125800
Stasiak P, Sopel J, Płóciennik A, Musielak O, Lipowicz JM, Rawłuszko-Wieczorek AA, Sterzyńska K, Korbecki J, Januchowski R. Elacridar Inhibits BCRP Protein Activity in 2D and 3D Cell Culture Models of Ovarian Cancer and Re-Sensitizes Cells to Cytotoxic Drugs. International Journal of Molecular Sciences. 2025; 26(12):5800. https://doi.org/10.3390/ijms26125800
Chicago/Turabian StyleStasiak, Piotr, Justyna Sopel, Artur Płóciennik, Oliwia Musielak, Julia Maria Lipowicz, Agnieszka Anna Rawłuszko-Wieczorek, Karolina Sterzyńska, Jan Korbecki, and Radosław Januchowski. 2025. "Elacridar Inhibits BCRP Protein Activity in 2D and 3D Cell Culture Models of Ovarian Cancer and Re-Sensitizes Cells to Cytotoxic Drugs" International Journal of Molecular Sciences 26, no. 12: 5800. https://doi.org/10.3390/ijms26125800
APA StyleStasiak, P., Sopel, J., Płóciennik, A., Musielak, O., Lipowicz, J. M., Rawłuszko-Wieczorek, A. A., Sterzyńska, K., Korbecki, J., & Januchowski, R. (2025). Elacridar Inhibits BCRP Protein Activity in 2D and 3D Cell Culture Models of Ovarian Cancer and Re-Sensitizes Cells to Cytotoxic Drugs. International Journal of Molecular Sciences, 26(12), 5800. https://doi.org/10.3390/ijms26125800





