The Role of Epithelial-Derived Extracellular Vesicles in Allergic Sensitisation: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Data Extraction and Quality Assessment
3. Results
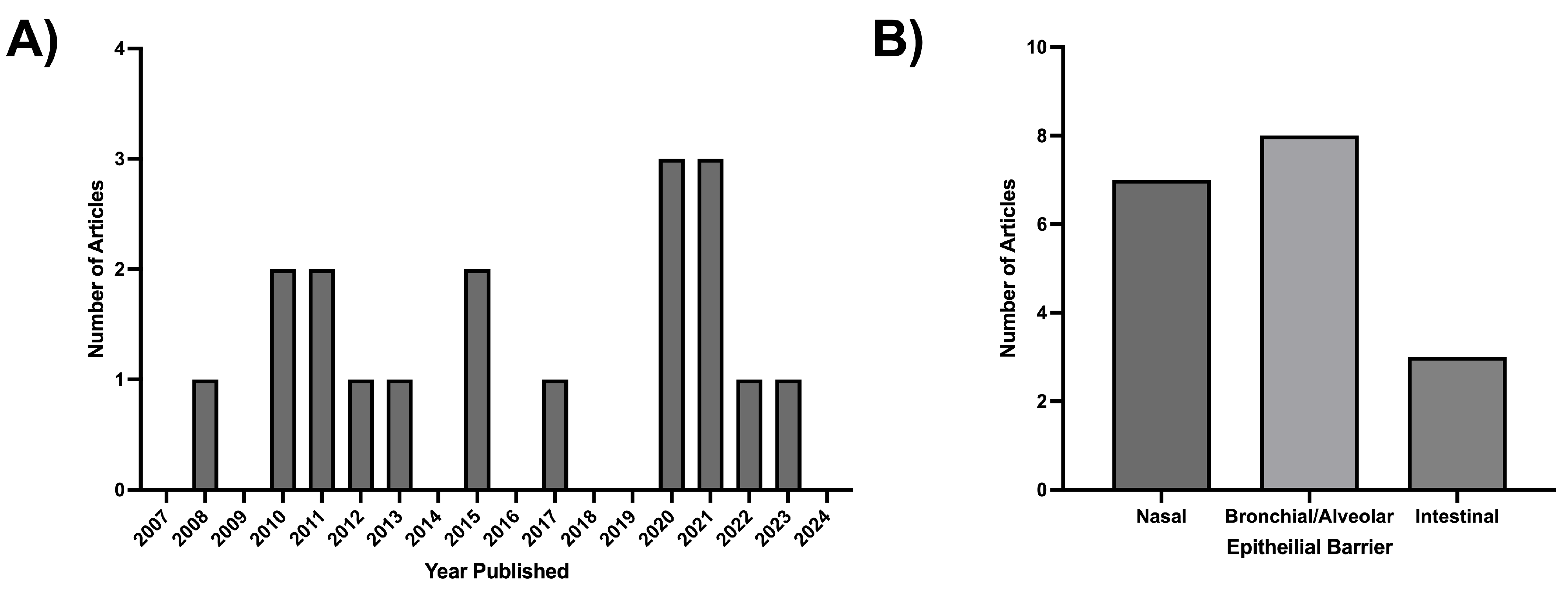
3.1. PRISMA and Publication Selection
3.2. Nasal Epithelial-Derived Extracellular Vesicles (ne-EVs)
3.3. Bronchial/Alveolar Epithelial-Derived Extracellular Vesicles (bae-EVs)
3.4. Intestinal Epithelial-Derived Extracellular Vesicles (ie-EVs)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EV | Extracellular Vesicle |
| DC | Dendritic Cells |
| ne-EVs | Nasal Epithelial-Derived Extracellular Vesicles |
| bae-EVs | Bronchial/Alveolar Epithelial-Derived Extracellular Vesicles |
| ie-EVs | Intestinal Epithelial-Derived Extracellular Vesicles |
| TJ | Tight Junction |
| IL | Interleukin |
| AR | Allergic Rhinitis |
| IgE | Immunoglobulin E |
References
- Pawankar, R. Allergic diseases and asthma: A global public health concern and a call to action. World Allergy Organ. J. 2014, 7, 12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Warren, C.M.; Jiang, J.; Gupta, R.S. Epidemiology and Burden of Food Allergy. Curr. Allergy Asthma Rep. 2020, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, G.V.; Cochrane, S.; Onion, D.; Fairclough, L.C. The Role of Lipids in Allergic Sensitization: A Systematic Review. Front. Mol. Biosci. 2022, 9, 832330. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, G.V.; Cochrane, S.; Onion, D.; Fairclough, L.C. Invariant NKT cells are more abundant in peanut-allergic adults and a subset of CD8+ iNKT cells are depleted after peanut oil exposure. Front. Immunol. 2023, 14, 1293158. [Google Scholar] [CrossRef]
- Tordesillas, L.; Cubells-Baeza, N.; Gómez-Casado, C.; Berin, C.; Esteban, V.; Barcik, W.; O’MAhony, L.; Ramirez, C.; Pacios, L.F.; Garrido-Arandia, M.; et al. Mechanisms underlying induction of allergic sensitization by Pru p 3. Clin. Exp. Allergy 2017, 47, 1398–1408. [Google Scholar] [CrossRef]
- Hils, M.; Wölbing, F.; Hilger, C.; Fischer, J.; Hoffard, N.; Biedermann, T. The History of Carbohydrates in Type I Allergy. Front. Immunol. 2020, 11, 586924. [Google Scholar] [CrossRef]
- Keumatio Doungstop, B.C.; Van Vliet, S.J.; Van Ree, R.; De Jong, E.C.; Van Kooyk, Y. Carbohydrates in allergy: From disease to novel immunotherapies. Trends Immunol. 2021, 42, 635–648. [Google Scholar] [CrossRef]
- Royer, P.-J.; Emara, M.; Yang, C.; Al-Ghouleh, A.; Tighe, P.; Jones, N.; Sewell, H.F.; Shakib, F.; Martinez-Pomares, L.; Ghaemmaghami, A.M. The Mannose Receptor Mediates the Uptake of Diverse Native Allergens by Dendritic Cells and Determines Allergen-Induced T Cell Polarization through Modulation of IDO Activity. J. Immunol. 2010, 185, 1522–1531. [Google Scholar] [CrossRef]
- Jakwerth, C.A.; Ordovas-Montanes, J.; Blank, S.; Schmidt-Weber, C.B.; Zissler, U.M. Role of Respiratory Epithelial Cells in Allergic Diseases. Cells 2022, 11, 1387. [Google Scholar] [CrossRef]
- Georas, S.N.; Rezaee, F. Epithelial barrier function: At the front line of asthma immunology and allergic airway inflammation. J. Allergy Clin. Immunol. 2014, 134, 509–520. [Google Scholar] [CrossRef]
- Hellings, P.W.; Steelant, B. Epithelial barriers in allergy and asthma. J. Allergy Clin. Immunol. 2020, 145, 1499–1509. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Winton, H.L.; Soeller, C.; Tovey, E.R.; Gruenert, D.C.; Thompson, P.J.; Stewart, G.A.; Taylor, G.W.; Garrod, D.R.; Cannell, M.B.; et al. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J. Clin. Investig. 1999, 104, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, S.F. Thymic stromal lymphopoietin and allergic disease. J. Allergy Clin. Immunol. 2012, 130, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Grotenboer, N.S.; Ketelaar, M.E.; Koppelman, G.H.; Nawijn, M.C. Decoding asthma: Translating genetic variation in IL33 and IL1RL1 into disease pathophysiology. J. Allergy Clin. Immunol. 2013, 131, 856–865.e9. [Google Scholar] [CrossRef]
- Michaudel, C.; Mackowiak, C.; Maillet, I.; Fauconnier, L.; Akdis, C.A.; Sokolowska, M.; Dreher, A.; Tan, H.-T.T.; Quesniaux, V.F.; Ryffel, B.; et al. Ozone exposure induces respiratory barrier biphasic injury and inflammation controlled by IL-33. J. Allergy Clin. Immunol. 2018, 142, 942–958. [Google Scholar] [CrossRef]
- Sugita, K.; Kabashima, K. Tight junctions in the development of asthma, chronic rhinosinusitis, atopic dermatitis, eosinophilic esophagitis, and inflammatory bowel diseases. J. Leukoc. Biol. 2020, 107, 749–762. [Google Scholar] [CrossRef]
- Akdis, C.A. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat. Rev. Immunol. 2021, 21, 739–751. [Google Scholar] [CrossRef]
- Du Toit, G.; Sampson, H.A.; Plaut, M.; Burks, A.W.; Akdis, C.A.; Lack, G. Food allergy: Update on prevention and tolerance. J. Allergy Clin. Immunol. 2018, 141, 30–40. [Google Scholar] [CrossRef]
- Kulis, M.D.; Smeekens, J.M.; Immormino, R.M.; Moran, T.P. The airway as a route of sensitization to peanut: An update to the dual allergen exposure hypothesis. J. Allergy Clin. Immunol. 2021, 148, 689–693. [Google Scholar] [CrossRef]
- Hovhannisyan, L.; Czechowska, E.; Gutowska-Owsiak, D. The Role of Non-Immune Cell-Derived Extracellular Vesicles in Allergy. Front. Immunol. 2021, 12, 702381. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’DRiscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.; Wang, M. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.M.; Banyard, A.; Smith, C.; Mironov, A.; McCabe, M.G. Large Extracellular Vesicles Can be Characterised by Multiplex Labelling Using Imaging Flow Cytometry. Int. J. Mol. Sci. 2020, 21, 8723. [Google Scholar] [CrossRef] [PubMed]
- Sheta, M.; Taha, E.A.; Lu, Y.; Eguchi, T. Extracellular Vesicles: New Classification and Tumor Immunosuppression. Biology 2023, 12, 110. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e18. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Mohammadipoor, A.; Hershfield, M.R.; Linsenbardt, H.R.; Smith, J.; Mack, J.; Natesan, S.; Averitt, D.L.; Stark, T.R.; Sosanya, N.M. Biological function of Extracellular Vesicles (EVs): A review of the field. Mol. Biol. Rep. 2023, 50, 8639–8651. [Google Scholar] [CrossRef]
- El Andaloussi, S.; Mäger, I.; Breakefield, X.O.; Wood, M.J.A. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013, 12, 347–357. [Google Scholar] [CrossRef]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef]
- Gatti, S.; Bruno, S.; Deregibus, M.C.; Sordi, A.; Cantaluppi, V.; Tetta, C.; Camussi, G. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol. Dial. Transplant. 2011, 26, 1474–1483. [Google Scholar] [CrossRef]
- Del Conde, I.; Shrimpton, C.N.; Thiagarajan, P.; LóPez, J.A. Tissue-factor–bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood 2005, 106, 1604–1611. [Google Scholar] [CrossRef] [PubMed]
- Sangaphunchai, P.; Todd, I.; Fairclough, L.C. Extracellular vesicles and asthma: A review of the literature. Clin. Exp. Allergy 2020, 50, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Harvey-Seutcheu, C.; Hopkins, G.; Fairclough, L.C. The Role of Extracellular Vesicles in Atopic Dermatitis. Int. J. Mol. Sci. 2024, 25, 3255. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fang, S.-B.; Zhou, Z.-R.; Peng, Y.-Q.; Liu, X.-Q.; He, B.-X.; Chen, D.-H.; Chen, D.; Fu, Q.-L. Plasma EVs Display Antigen-Presenting Characteristics in Patients With Allergic Rhinitis and Promote Differentiation of Th2 Cells. Front. Immunol. 2021, 12, 710372. [Google Scholar] [CrossRef]
- Qiu, S.; Du, Y.; Duan, X.; Geng, X.; Xie, J.; Gao, H.; Yang, P.-C. Cytotoxic T lymphocytes mediate chronic inflammation of the nasal mucosa of patients with atypical allergic rhinitis. N. Am. J. Med. Sci. 2011, 3, 378–383. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qiu, S.Q.; Duan, X.B.; Geng, X.R.; Xie, J.X.; Gao, H. Antigen-specific activities of CD8+T cells in the nasal mucosa of patients with nasal allergy. Asian Pac. J. Allergy Immunol. 2012, 30, 107–113. [Google Scholar]
- Luo, X.; Han, M.; Liu, J.; Wang, Y.; Luo, X.; Zheng, J.; Wang, S.; Liu, Z.; Liu, D.; Yang, P.-C.; et al. Epithelial cell-derived micro RNA-146a generates interleukin-10-producing monocytes to inhibit nasal allergy. Sci. Rep. 2015, 5, 10. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, X.; Wang, Y.; Zhao, Y. Altered microRNA Expression Profiles of Extracellular Vesicles in Nasal Mucus From Patients With Allergic Rhinitis. Allergy Asthma Immunol. Res. 2015, 7, 449–457. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, X.; Wang, X.; Wang, Y.; Zhao, Y. Exosomal long non-coding RNA GAS5 suppresses Th1 differentiation and promotes Th2 differentiation via downregulating EZH2 and T-bet in allergic rhinitis. Mol. Immunol. 2020, 118, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Cai, W.; Wu, Q.; Chen, D.; Wang, P.; Xu, Z. Exosomal lncRNA Nuclear Paraspeckle Assembly Transcript 1 (NEAT1)contributes to the progression of allergic rhinitis via modulating microRNA-511/Nuclear Receptor Subfamily 4 Group A Member 2 (NR4A2) axis. Bioengineered 2021, 12, 8067–8079. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, H.; Wang, Y.M.; Han, X.L. ESP-B4 promotes nasal epithelial cell-derived extracellular vesicles containing miR-146a-5p to modulate Smad3/GATA-3 thus relieving allergic rhinitis ESP-B4/miR-146a-5p in AR. Phytomedicine 2023, 108, 13. [Google Scholar] [CrossRef] [PubMed]
- Prado, N.; Marazuela, E.G.; Segura, E.; FernánDez-García, H.; Villalba, M.; ThérY, C.; RodrígUez, R.; Batanero, E. Exosomes from bronchoalveolar fluid of tolerized mice prevent allergic reaction. J. Immunol. 2008, 181, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Prado, N.; Cañamero, M.; Villalba, M.; Rodríguez, R.; Batanero, E. Bystander suppression to unrelated allergen sensitization through intranasal administration of tolerogenic exosomes in mouse. Mol. Immunol. 2010, 47, 2148–2151. [Google Scholar] [CrossRef] [PubMed]
- Shin, T.; Kim, J.H.; Jeon, S.G.; Zhu, Z.; Gho, Y.S.; Kim, Y. Extracellular vesicles are key intercellular mediators in the development of immune dysfunction to allergens in the airways. Allergy 2010, 65, 1256–1265. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kulshreshtha, A.; Ahmad, T.; Agrawal, A.; Ghosh, B. Proinflammatory role of epithelial cell-derived exosomes in allergic airway inflammation. J. Allergy Clin. Immunol. 2013, 131, 1194–1203.e14. [Google Scholar] [CrossRef] [PubMed]
- Gon, Y.; Maruoka, S.; Inoue, T.; Kuroda, K.; Yamagishi, K.; Kozu, Y.; Shikano, S.; Soda, K.; Lötvall, J.; Hashimoto, S. Selective release of miRNAs via extracellular vesicles is associated with house-dust mite allergen-induced airway inflammation. Clin. Exp. Allergy 2017, 47, 1586–1598. [Google Scholar] [CrossRef]
- Bartel, S.; La Grutta, S.; Cilluffo, G.; Perconti, G.; Bongiovanni, A.; Giallongo, A.; Behrends, J.; Kruppa, J.; Hermann, S.; Chiang, D.; et al. Human airway epithelial extracellular vesicle miRNA signature is altered upon asthma development. Allergy 2020, 75, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhou, Y.; Di, C.; Zhao, C.; Chen, J.; Su, W.; Wu, Q.; Wu, M.; Su, X.; Xia, Z. Increased airway epithelial cell-derived exosomes activate macrophage-mediated allergic inflammation via CD100 shedding. J. Cell. Mol. Med. 2021, 25, 8850–8862. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yu, Q.; Tang, W.; Wu, Y.; Lv, J.; Sun, L.; Shi, G.; Wu, M.; Qu, J.; Di, C.; et al. Epithelial exosomal contactin-1 promotes monocyte-derived dendritic cell-dominant T-cell responses in asthma. J. Allergy Clin. Immunol. 2021, 148, 1545–1558. [Google Scholar] [CrossRef]
- Chen, X.; Song, C.-H.; Feng, B.-S.; Li, T.-L.; Li, P.; Zheng, P.-Y.; Chen, X.-M.; Xing, Z.; Yang, P.-C. Intestinal epithelial cell-derived integrin αβ6 plays an important role in the induction of regulatory T cells and inhibits an antigen-specific Th2 response. J. Leukoc. Biol. 2011, 90, 751–759. [Google Scholar] [CrossRef]
- Zeng, H.-T.; Liu, J.-Q.; Zhao, M.; Yu, D.; Yang, G.; Mo, L.-H.; Liu, Z.-Q.; Wang, S.; Yang, P.-C. Exosomes carry IL-10 and antigen/MHC II complexes to induce antigen-specific oral tolerance. Cytokine 2020, 133, 9. [Google Scholar] [CrossRef] [PubMed]
- Shin, T.S.; Park, J.Y.; Kim, Y.K.; Kim, J.G. Extracellular vesicles derived from small intestinal lamina propria reduce antigen-specific immune response. Korean J. Intern. Med. 2022, 37, 85–95. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gutowska-Ślesik, J.; Samoliński, B.; Krzych-Fałta, E. The increase in allergic conditions based on a review of literature. Adv. Dermatol. Allergol. 2023, 40, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Löhning, M.; Gao, Z.; Assenmacher, M.; Ranganath, S.; Radbruch, A.; Murphy, K.M. Stat6-Independent GATA-3 Autoactivation Directs IL-4-Independent Th2 Development and Commitment. Immunity 2000, 12, 27–37. [Google Scholar] [CrossRef]
- Mowen, K.A.; Glimcher, L.H. Signaling pathways in Th2 development. Immunol. Rev. 2004, 202, 203–222. [Google Scholar] [CrossRef]
- Beavitt, S.-J.E.; Harder, K.W.; Kemp, J.M.; Jones, J.; Quilici, C.; Casagranda, F.; Lam, E.; Turner, D.; Brennan, S.; Sly, P.D.; et al. Lyn-Deficient Mice Develop Severe, Persistent Asthma: Lyn Is a Critical Negative Regulator of Th2 Immunity. J. Immunol. 2005, 175, 1867–1875. [Google Scholar] [CrossRef]
- Lamiable, O.; Mayer, J.U.; Munoz-Erazo, L.; Ronchese, F. Dendritic cells in Th2 immune responses and allergic sensitization. Immunol. Cell Biol. 2020, 98, 807–818. [Google Scholar] [CrossRef]
- Pothoven, K.L.; Schleimer, R.P. The barrier hypothesis and Oncostatin M: Restoration of epithelial barrier function as a novel therapeutic strategy for the treatment of type 2 inflammatory disease. Tissue Barriers 2017, 5, e1341367. [Google Scholar] [CrossRef]
- Krohn, I.K.; Seys, S.F.; Lund, G.; Jonckheere, A.; de Casterlé, I.D.; Ceuppens, J.L.; Steelant, B.; Hellings, P.W. Nasal epithelial barrier dysfunction increases sensitization and mast cell degranulation in the absence of allergic inflammation. Allergy 2020, 75, 1155–1164. [Google Scholar] [CrossRef]
- Miranda-Waldetario, M.C.G.; Maria, A. Oral tolerance to dietary antigens and Foxp3+ regulatory T cells. Immunol. Rev. 2024, 326, 8–16. [Google Scholar] [CrossRef]
- Battaglia, M.; Gianfrani, C.; Gregori, S.; Roncaroloa, M.G. IL-10-Producing T Regulatory Type 1 Cells and Oral Tolerance. Ann. N. Y. Acad. Sci. 2004, 1029, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Hadis, U.; Wahl, B.; Schulz, O.; Hardtke-Wolenski, M.; Schippers, A.; Wagner, N.; Müller, W.; Sparwasser, T.; Förster, R.; Pabst, O. Intestinal Tolerance Requires Gut Homing and Expansion of FoxP3+ Regulatory T Cells in the Lamina Propria. Immunity 2011, 34, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Mucida, D. Oral tolerance in the absence of naturally occurring Tregs. J. Clin. Investig. 2005, 115, 1923–1933. [Google Scholar] [CrossRef] [PubMed]
- Mestas, J.; Hughes, C.C.W. Of Mice and Not Men: Differences between Mouse and Human Immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar] [CrossRef]
- Aun, M.; Bonamichi-Santos, R.; Arantes-Costa, F.M.; Kalil, J.; Giavina-Bianchi, P. Animal models of asthma: Utility and limitations. J. Asthma Allergy 2017, 10, 293–301. [Google Scholar] [CrossRef]
- Krieger, S.M.; Boverhof, D.R.; Woolhiser, M.R.; Hotchkiss, J.A. Assessment of the respiratory sensitization potential of proteins using an enhanced mouse intranasal test (MINT). Food Chem. Toxicol. 2013, 59, 165–176. [Google Scholar] [CrossRef]
- Liu, A.H. Endotoxin exposure in allergy and asthma: Reconciling a paradox. J. Allergy Clin. Immunol. 2002, 109, 379–392. [Google Scholar] [CrossRef]
- Trivedi, B.; Valerio, C.; Slater, J.E. Endotoxin content of standardized allergen vaccines. J. Allergy Clin. Immunol. 2003, 111, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Janssen, R.; de Kleer, J.W.; Heming, B.; Bastiaan-Net, S.; Garssen, J.; Willemsen, L.E.; Masereeuw, R. Food allergen sensitization on a chip: The gut–immune–skin axis. Trends Biotechnol. 2024, 42, 119–134. [Google Scholar] [CrossRef]
- He, R.; Oyoshi, M.K.; Garibyan, L.; Kumar, L.; Ziegler, S.F.; Geha, R.S. TSLP acts on infiltrating effector T cells to drive allergic skin inflammation. Proc. Natl. Acad. Sci. USA 2008, 105, 11875–11880. [Google Scholar] [CrossRef]
- Simmons, J.; Gallo, R.L. The Central Roles of Keratinocytes in Coordinating Skin Immunity. J. Investig. Dermatol. 2024, 144, 2377–2398. [Google Scholar] [CrossRef] [PubMed]
- Tucis, D.; Hopkins, G.; Browne, W.; James, V.; Onion, D.; Fairclough, L.C. The Role of Extracellular Vesicles in Allergic Sensitization: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 4492. [Google Scholar] [CrossRef] [PubMed]
- Lambring, C.B.; Siraj, S.; Patel, K.; Sankpal, U.T.; Mathew, S.; Basha, R. Impact of the Microbiome on the Immune System. Crit. Rev. Immunol. 2019, 39, 313–328. [Google Scholar] [CrossRef] [PubMed]
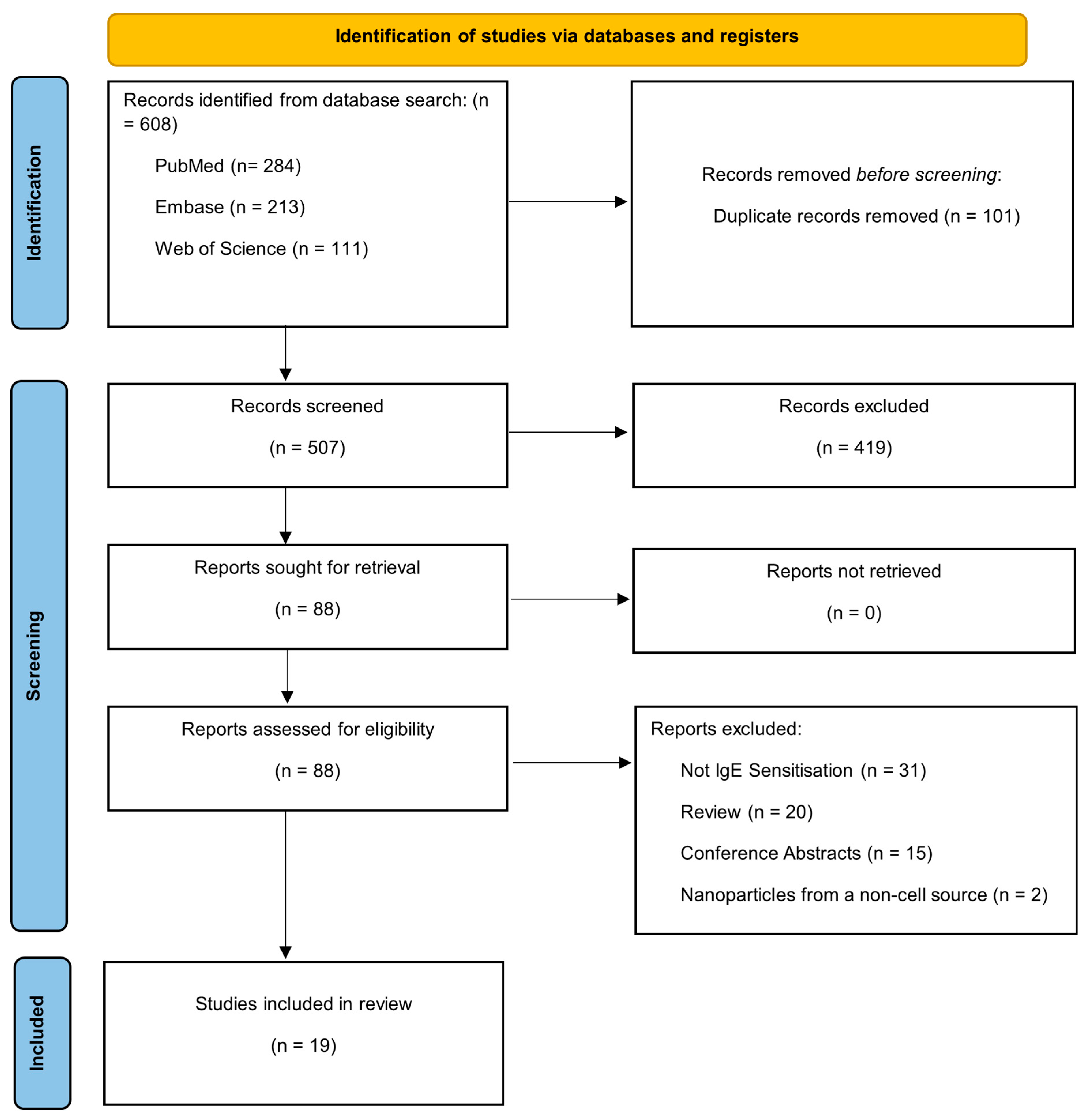
| Inclusion | Exclusion |
|---|---|
| IgE-mediated allergy | Non-original research paper, e.g., reviews, commentary, case report, etc. |
| Epithelial-derived EVs | Non-IgE-mediated allergies |
| Allergic sensitisation | Non-English-language publications |
| Clinical data | Conference abstract |
| Experimental data | Research involving nanoparticles but not extracellular vesicles from a cell source |
| Healthy subjects | Pre-prints |
| Allergic subjects | Correspondence |
| Human studies | |
| Animal models | |
| Research involving the extraction, identification or production of EVs or their contents, such as DNA, miRNA or protein | |
| Isolation method of EVs included |
| Category | Scoring Criteria |
|---|---|
| Model: If multiple models are used, a combined score is given |
|
| Material Used for Sensitisation: Summative score of all points |
|
| Robustness of Model: If multiple models used a combined score is given | Human Studies (Ex Vivo):
|
| Sample Size: If multiple models used a combined score is given: | Human Studies (Ex Vivo):
|
| EV Isolation |
No Isolation—Precipitation-only techniques. Studies looking directly at a liquid with no isolation applied (usually performed using samples with very low volumes) (0);
|
| EV Characterisation | No Characterisation—No attempt made to profile or characterise EVs or exosomes (0);
|
| First Author and Year | Title | Allergen | Cells Responding | Model | Isolation | Characterisation | Reported Findings |
|---|---|---|---|---|---|---|---|
| Qiu et al., 2011 and 2012 [35,36] | 2011 Cytotoxic T lymphocytes mediate chronic inflammation of the nasal mucosa of patients with atypical allergic rhinitis [35] 2012 Antigen-specific activities of CD8+T cells in the nasal mucosa of patients with nasal allergy [36] | Der P 1 | Dendritic cells and CD8+ T cells | Cell line (RPMI2650) and human patients | Serial Ultra-centrifugation: 300× g 10 min, 1200× g 20 min, 10,000× g 30 min, 100,000× g 1 h. | EM, Western blot and Bradford assay | Staphylococcal enterotoxin B (SEB) and Der p 1 containing EVs induced dendritic cell maturation, and generation of allergen specific granzyme B and perforin secreting CD8+ cytotoxic T Cells [35]. A higher frequency of CD8+ T cells in patient samples compared to controls [36]. |
| Luo et al., 2015 [37] | Epithelial cell-derived microRNA-146a generates interleukin-10-producing monocytes to inhibit nasal allergy | N/A | Monocytes | Human patients, cell line (RPMI 2650) and mouse model (BALB/c) | Serial Ultra-centrifugation: 300× g 10 min, 1200× g 20 min, 10,000× g 30 min, 100,000× g 1 h. | Western blot and RTq-PCR | Reduced expression of mRNA-146α in EVs of patients with AR compared to healthy controls prevents the induction of IL-10+ monocytes and was shown to have a suppressive effect on CD4+ effector T cells and Th2 polarisation. |
| Wu et al., 2015 [38] | Altered microRNA Expression Profiles of Extracellular Vesicles in Nasal Mucus from Patients with Allergic Rhinitis | N/A | N/A | Human patients | Serial Ultra-centrifugation: 3000× g 15 min, 10,000× g 30 min, 50,000× g 1 h, 100,000× g 1 h. | FACS and RTq-PCR | Cargo analysis of EVs from patients with AR compared to healthy controls showed changes in expression of a wide variety of mRNAs involved in key pathways associated with allergic development, such as the c-fos, Lyn and MUC7 |
| Zhu et al., 2020 [39] | Exosomal long non-coding RNA GAS5 suppresses Th1 differentiation and promotes Th2 differentiation via downregulating EZH2 and T-bet in allergic rhinitis | Ovalbumin | Naïve CD4+ T cells | Human patients and cell culture (RPMI 2650) | Serial Ultra-centrifugation: 12,000× g 45 min, 110,000× g 2 h, 110,000× g 70 min | TEM, Western blot and RTq-PCR | GAS5 can influence Th1/Th2 differentiation, downregulating T-bet and ultimately suppressing Th1 differentiation and promoting Th2 polarisation |
| Wang et al., 2021 [40] | Exosomal lncRNA Nuclear Paraspeckle Assembly Transcript 1 (NEAT1)contributes to the progression of allergic rhinitis via modulating microRNA-511/Nuclear Receptor Subfamily 4 Group A Member 2 (NR4A2) axis | N/A | N/A | Human patients and cell culture (primary cells) | Precipitation—EXOQuick Kit | TEM, Western blot and RTq-PCR | EVs containing LncRNA NEAT1 induce IL-13-mediated inflammatory responses and nasal epithelial cell apoptosis. |
| Li et al., 2023 [41] | ESP-B4 promotes nasal epithelial cell-derived extracellular vesicles containing miR-146a-5p to modulate Smad3/GATA-3 thus relieving allergic rhinitis ESP-B4/miR-146a-5p in AR | Ovalbumin | Naïve CD4+ T cells | Human patients, rat model (Wistar rats) and cell culture (RPMI2650) | Serial Ultra-centrifugation: 12,000× g 45 min, 110,000× g 2 h, 110,000× g 70 min | TEM, Western blot, NTA and RTq-PCR | Downregulation of miR-146α-5p in AR patients compared to healthy controls was shown to play an important role in Th1/Th2 differentiation |
| Author | Model (n/10) | Robustness of Model | Sample Size | Sensitisation Material (n/3) | EV Isolation (n/3) | EV Characterisation (n/4) | Total Score | Bias Score | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Murine Model (n/4) | Cell Culture (n/4) | Human Studies (n/4) | Model (n/3) | Cell Culture (n/3) | Human Studies (n/3) | |||||||
| Qiu et al., 2011 [35] | Human patient samples (4) Cell culture—human (3) | Immortalized cell line, partially defined exposure (1) | From clinical setting and positive skin prick test (2) | 3 repeats (2) | 11 or more participants per group (3) | Allergen defined (1) | Serial UC (1) | One method utilized (1) | 18/34 | 53% | ||
| Qiu et al., 2012 [36] | Human patient samples (4) Cell culture—human (3) | Immortalized cell line, partially defined exposure (1) | Allergic patients from clinical setting with serum IgE, IgG, skin prick test and non-allergic but chronic rhinitis controls (4) | 3 repeats (2) | 6–10 participants per group (2) | Allergen defined (1) | Serial UC (1) | One method utilized (1) | 19/34 | 56% | ||
| Luo et al., 2015 [37] | Human patient samples (4), Cell culture—human (3), Animal model (2) | Sensitisation fully defined (4) | Immortalized cell line, partially defined exposure (1) | Allergic patients from clinical setting with serum IgE, IgG, skin prick test and healthy controls (4) | 6–10 animals per group (2) | 3 repeats (2) | 6–10 participants per group (2) | Allergen defined (1) | Serial UC (1) | Multiple complimentary techniques (2) | 28/41 | 66% |
| Wu et al., 2015 [38] | Human patient samples (4) | Allergic patients from clinical setting with serum IgE, IgG, skin prick test and healthy controls (4) | 11 or more participants per group (3) | Serial UC (1) | Multiple complimentary techniques (2) | 14/27 | 52% | |||||
| Zhu et al., 2020 [39] | Human patient samples (4) | Allergic patients from clinical setting with serum IgE, IgG, skin prick test and healthy controls (4) | 11 or more participants per group (3) | Serial UC (1) | Multiple complementary techniques and suitable controls (3) | 15/27 | 56% | |||||
| Wang et al., 2021 [40] | Human patient samples (4) Cell culture—human (3) | Primary cell line and fully defined exposures (4) | Allergic patients from a clinical setting and a control group (3) | 3 repeats (2) | 11 or more participants per group (3) | Precipitation—EXOQuick-TC (3) | Multiple complementary techniques and suitable controls (3) | 25/34 | 74% | |||
| Li et al., 2023 [41] | Human patient samples (4), Cell culture—human (3), Animal model (2) | Sensitisation fully defined (4) | Transformed cell line and fully defined exposure (2) | Allergic patients from clinical setting with serum IgE, IgG, skin prick test and healthy controls (4) | 6–10 animals per group (2) | 3 repeats (2) | 11 or more participants per group (3) | Allergen defined (1) | Serial UC (1) | Multiple complementary techniques, suitable controls and additional biomarkers (4) | 32/41 | 78% |
| First Author and Year | Title | Allergen | Cells Responding | Model | Isolation | Characterisation | Outcome |
|---|---|---|---|---|---|---|---|
| Prado et al., 2008 [42] | Exosomes from bronchoalveolar fluid of tolerized mice prevent allergic reaction | Ole e 1 | T Cells | Mouse model (BALB/c) and cell culture | Ultracentrifugation: 100,000× g for 18 h | EM, Western blot and FACS | EVs isolated from the BALF of mice tolerised to the allergen Ole e 1 were able to inhibit Th2 responses, suppressing IgE and IgG1 and upregulating TGF-β |
| Prado et al., 2010 [43] | Bystander suppression to unrelated allergen sensitisation through intranasal administration of tolerogenic exosomes in mouse | Ole e 1 and Bet v 1 | T Cells | Mouse model (BALB/c) and cell culture | Ultracentrifugation: 100,000× g for 18 h | EM, Western blot and FACS | Bystander suppression was observed after nasal administration of BALF-derived EVs from tolerised mice inhibiting sensitisation to other allergens, supressing IgE and IgG1 as well as the Th2 cytokines Il-5 and IL-13 |
| Shin et al., 2010 [44] | Extracellular vesicles are key intercellular mediators in the development of immune dysfunction to allergens in the airways | Ovalbumin | T Cells and dendritic cells | Mouse model (BALB/c) | Sucrose Cushion Serial Ultracentrifugation: 500× g 10 min, 3000× g 20 min, Cushioned: 100,000× g 2 h 100,000× g 2 h. | TEM, Western blot, FACS and Bradford assay | LPS exposure enhanced airway epithelial derived-EV (ae-EV) production. These LPS-induced EVs were shown to enhanced sensitisation to allergens and promote TNF-a and IL-6 secretion in macrophages |
| Kulshreshtha et al., 2013 [45] | Proinflammatory role of epithelial cell-derived exosomes in allergic airway inflammation | Ovalbumin | Monocytes | Mouse model (BALB/c) cell culture (BEAS-2B) | Serial Ultracentrifugation: 300× g 5 min, 800× g 5 min, 2000× g 10 min, 10,000× g 30 min, 70,000× g 60 min | TEM, Western blot and FACS | Th2 cytokine-stimulated epithelial cells had increased EV secretion and cargo changes. These EVs induce monocyte proliferation |
| Gon et al., 2017 [46] | Selective release of miRNAs via extracellular vesicles is associated with house-dust mite allergen-induced airway inflammation | House dust mite | CD4+ T helper cells | Mouse model (C57BL/6J) | Precipitation—EXOQuick Kit | TEM, Western blot and qNano counter | EVs used to remove Th2 inhibitory miRNAs that downregulate IL-5 and Il-13 receptors on epithelial cells |
| Bartel et al., 2020 [47] | Human airway epithelial extracellular vesicle miRNA signature is altered upon asthma development | N/A | N/A | Human patients and cell cell culture (primary cells) | Precipitation—EXOQuick Kit | TEM, Western blot, NTA and SeramiR miRNA | Changes in expression of miR-34a, miR-92b and miR-210 predicted by pathway analysis to promote DC-induced Th2 polarisation of CD4+ T cells, regulating Th2 polarisation and DC maturation |
| Yu et al., 2021 [48] | Increased airway epithelial cell-derived exosomes activate macrophage-mediated allergic inflammation via CD100 shedding | Ovalbumin | macrophages | Mouse models (C57BL/6J) and cell culture (primary cells and BEAS-2B) | Serial Ultracentrifugation: 300× g 10 min, 3000× g 15 min, 10,000× g 30 min 100,000× g 70 min, 100,000× g 70 min. | TEM, Western blot and NTA | OVA containing EVs promote infiltration of neutrophils, monocytes and DCs into the lung and induce macrophages to secrete IL-6, TNF-a and IL-1β |
| Zhang et al., 2021 [49] | Epithelial exosomal contactin-1 promotes monocyte-derived dendritic cell-dominant T-cell responses in asthma | House dust mite | Dendritic cells | Human patient, mouse model (C57BL/6N) and cell culture (primary cells) | Serial Ultracentrifugation: 2000× g 10 min, 10,000× g 30 min 100,000× g 70 min, 100,000× g 70 min. | TEM, Western blot and NTA | HDM stimulation released EVs that recruited DCs in the lung. These EVs can activate DC though the cargo CNTN1 and upregulate the expression of CD40 |
| Author | Model (n/10) | Robustness of Model | Sample Size | Sensitisation Material (n/3) | EV Isolation (n/3) | EV Characterisation (n/4) | Total Score | Bias Score | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Murine Model (n/4) | Cell Culture (n/4) | Human Studies (n/4) | Murine Model (n/3) | Cell Culture (n/3) | Human Studies (n/3) | |||||||
| Prado et al., 2008 [42] | Animal model (2), cell culture—murine (1) | Sensitisation fully defined (4) | Primary cell line and fully defined exposure (4) | 11 or more mice per group (3) | more than 3 repeats (3) | Allergen defined (1) | UC (1) | Multiple complementary techniques, suitable controls and additional biomarkers (4) | 23/34 | 68% | ||
| Prado et al., 2010 [43] | Animal model (2), cell culture—murine (1) | Sensitisation fully defined (4) | Primary cell line and fully defined exposure (4) | 5 or fewer mice per group (1) | more than 3 repeats (3) | Allergen defined (1) | Serial UC (1) | Multiple complementary techniques, suitable controls and additional biomarkers (4) | 21/34 | 62% | ||
| Shin et al., 2010 [44] | Animal model (2) | Sensitisation fully defined (4 | 5 or fewer mice per group (1) | Allergen defined (1) | Sucrose Cushioned UC (2) | Multiple complementary techniques, suitable controls and additional biomarkers (4) | 14/27 | 52% | ||||
| Kulshreshtha et al., 2013 [45] | Cell culture—human (3), animal model (2) | Sensitisation fully defined (4) | Transformed cell line and partially defined exposure (1) | 6–10 mice per group (2) | 3 repeats (2) | Allergen defined (1) | Serial UC (1) | Multiple complementary techniques and suitable controls (3) | 19/34 | 56% | ||
| Gon et al., 2017 [46] | Animal model (2) | Sensitisation fully defined (4) | 11 or more mice per group (3) | Allergen defined (1) | Precipitation—EXOQuick-TC (3) | Multiple complementary techniques and suitable controls (3) | 16/27 | 59% | ||||
| Bartel et al., 2020 [47] | Human patient samples (4), cell culture—human (3) | Primary cell line and fully defined exposure (4) | Allergic patients from clinical setting with serum IgE, IgG, skin prick test and healthy controls (4) | 3 repeats (2) | 6–10 participants per group (3) | Precipitation—EXOQuick-TC (3) | Multiple complementary techniques, suitable controls and additional biomarkers (4) | 27/34 | 79% | |||
| Yu et al., 2021 [48] | cell culture—human (3), animal model (2) | Sensitisation fully defined (4) | Immortalised cell line and fully defined exposure (2) | 5 or fewer mice per group (1) | Not specified (0) | Allergen defined (1) | Serial UC (1) | Multiple complementary techniques and suitable controls (3) | 17/34 | 50% | ||
| Zhang et al., 2021 [49] | Human patient samples (4), animal model (2) cell culture—murine (1) | Sensitisation fully defined (4) | Primary cell line and partially defined exposures (3) | Allergic patients from a clinical setting and a control group (3) | 5 or fewer mice per group (1) | Not specified (0) | 6–10 participants per group (3) | Allergen defined (1) | Serial UC (1) | Multiple complementary techniques and suitable controls (3) | 26/41 | 63% |
| First Author | Title | Allergen | Cells Responding | Model | Isolation | Characterisation | Outcome |
|---|---|---|---|---|---|---|---|
| Chen et al., 2011 [50] | Intestinal epithelial cell-derived integrin αβ6 plays an important role in the induction of regulatory T cells and inhibits an antigen-specific Th2 response | Ovalbumin | Dendritic cells | Mouse model (Balb/c); cell culture (IEC4.1) | Serial Ultracentrifugation: 300× g 10 min, 1200× g 20 min, 10,000× g 30 min, 100,000× g 1 h | EM, Western blot and Bradford assay | Intestinal epithelial cells post-OVA uptake secrete EVs containing integrin αvβ6 and OVA. These EVs induced antigen-specific Tregs and TGF-β+ DCs |
| Zeng et al., 2020 [51] | Exosomes carry IL-10 and antigen/MHC II complexes to induce antigen-specific oral tolerance | Ovalbumin | Tregs and Tr1 Cells | Cell culture (mode K cells) and mouse models (VIPd and BALB/c) | Serial Ultracentrifugation: 300× g 10 min, 1200× g 20 min, 10,000× g 30 min, 100,000× g 1 h | Western blot and Bradford assay | VIPd mice fail to induce Tr1 cells in the intestine. EVs from OVA/VIP-primed IECs carry allergen-MHC II complexes and IL-10, which are able to induce Tr1 differentiation in OVA-specific CD4+ cells; the administration of these suppressed experimental food allergy. |
| Shin et al., 2022 [52] | Extracellular vesicles derived from small intestinal lamina propria reduce antigen-specific immune response | Ovalbumin | Tregs | Mouse model (C57BL/6) | Sucrose Cushion Serial Ultracentrifugation: 500× g 10 min, 3000× g 20 min, Cushioned: 100,000× g 2 h 100,000× g 2 h. | TEM, Western blot, Bradford assay and dynamic light scattering (sizing) | EVs containing OVA and MHCII induce CD4+Foxp3+ T cell differentiation and promote the secretion of Treg-promoting cytokines IL-10 and TGF-β in macrophages |
| Author | Model (n/10) | Robustness of Model | Sample Size | Sensitisation Material (n/3) | EV Isolation (n/3) | EV Characterisation (n/4) | Total Score | Bias Score | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Murine Model (n/4) | Cell Culture (n/4) | Human Studies (n/4) | Murine Model (n/3) | Cell Culture (n/3) | Human Studies (n/3) | |||||||
| Chen et al., 2011 [50] | Animal model (2), cell culture—murine (1) | Sensitisation fully defined (4) | Transformed cell line and fully defined exposure (2) | 6–10 mice per group (2) | 3 repeats (2) | Allergen defined (1) | Serial UC (1) | Multiple complimentary techniques (2) | 17/34 | 50% | ||
| Zeng et al., 2020 [51] | Animal model (2), cell culture—murine (1) | Sensitisation fully defined (4) | Transformed cell line and fully defined exposure (2) | 6–10 mice per group (2) | more than 3 repeats (3) | Allergen defined (1) | Serial UC (1) | Multiple complimentary techniques (2) | 18/34 | 53% | ||
| Shin et al., 2022 [52] | Animal model (2) | Sensitisation of mice partially defined (3) | Number not defined (0) | Allergen defined (1) | Sucrose Cushioned UC (2) | Multiple complimentary techniques (2) | 10/27 | 37% | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Browne, W.; Hopkins, G.; Cochrane, S.; James, V.; Onion, D.; Fairclough, L.C. The Role of Epithelial-Derived Extracellular Vesicles in Allergic Sensitisation: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 5791. https://doi.org/10.3390/ijms26125791
Browne W, Hopkins G, Cochrane S, James V, Onion D, Fairclough LC. The Role of Epithelial-Derived Extracellular Vesicles in Allergic Sensitisation: A Systematic Review. International Journal of Molecular Sciences. 2025; 26(12):5791. https://doi.org/10.3390/ijms26125791
Chicago/Turabian StyleBrowne, William, Georgina Hopkins, Stella Cochrane, Victoria James, David Onion, and Lucy C. Fairclough. 2025. "The Role of Epithelial-Derived Extracellular Vesicles in Allergic Sensitisation: A Systematic Review" International Journal of Molecular Sciences 26, no. 12: 5791. https://doi.org/10.3390/ijms26125791
APA StyleBrowne, W., Hopkins, G., Cochrane, S., James, V., Onion, D., & Fairclough, L. C. (2025). The Role of Epithelial-Derived Extracellular Vesicles in Allergic Sensitisation: A Systematic Review. International Journal of Molecular Sciences, 26(12), 5791. https://doi.org/10.3390/ijms26125791







