Protein Kinase C Isozyme Immaturity/Deficiency in Cord Blood Monocytes and Neutrophils
Abstract
1. Introduction
2. Results
2.1. PKC Isozyme Expression in CB Monocytes and Neutrophils
2.2. Correlation Assessment of PKC Isozyme Levels Between CB Leukocyte Subsets
2.3. Correlation Assessment Between CB Monocyte PKCζ Expression and T-Cell Cytokines
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Ethics Statement
4.3. Collection of Blood Samples and Isolation of Mononuclear Cells
4.4. Maturation of Cord Blood T Cells in Culture
4.5. Measurement of PKC Isozyme Expression by Flow Cytometry
4.6. Measurement of Intracellular Cytokines
4.7. Statistical Analysis
5. Conclusions
6. Study Limitation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lawrence, S.M.; Corriden, R.; Nizet, V. Age-Appropriate Functions and Dysfunctions of the Neonatal Neutrophil. Front. Pediatr. 2017, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Carr, R. Neutrophil Production and Function in Newborn Infants. Br. J. Haematol. 2000, 110, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Yost, C.C.; Cody, M.J.; Harris, E.S.; Thornton, N.L.; McInturff, A.M.; Martinez, M.L.; Chandler, N.B.; Rodesch, C.K.; Albertine, K.H.; Petti, C.A.; et al. Impaired neutrophil extracellular trap (NET) formation: A novel innate immune deficiency of human neonates. Blood 2009, 113, 6419–6427. [Google Scholar] [CrossRef] [PubMed]
- Lipp, P.; Ruhnau, J.; Lange, A.; Vogelgesang, A.; Dressel, A.; Heckmann, M. Less Neutrophil Extracellular Trap Formation in Term Newborns than in Adults. Neonatology 2017, 111, 182–188. [Google Scholar] [CrossRef]
- Mills, E.L.; Thompson, T.; Björkstén, B.; Filipovich, D.; Quie, P.G. The chemiluminescence response and bactericidal activity of polymorphonuclear neutrophils from newborns and their mothers. Pediatrics 1979, 63, 429–434. [Google Scholar] [CrossRef]
- Xanthou, M.; Valassi-Adam, E.; Kintsonidou, E.; Matsaniotis, N. Phagocytosis and killing ability of Candida albicans by blood leucocytes of healthy term and preterm babies. Arch. Dis. Child. 1975, 50, 72–75. [Google Scholar] [CrossRef][Green Version]
- Yegin, O. Chemotaxis in Childhood. Pediatr. Res. 1983, 17, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Bortolussi, R.; Howlett, S.; Rajaraman, K.; Halperin, S. Deficient priming activity of newborn cord blood-derived polymorphonuclear neutrophilic granulocytes with lipopolysaccharide and tumor necrosis factor-alpha triggered with formyl-methionyl-leucyl-phenylalanine. Pediatr. Res. 1993, 34, 243–248. [Google Scholar] [CrossRef][Green Version]
- Sanchez-Schmitz, G.; Morrocchi, E.; Cooney, M.; Soni, D.; Khatun, R.; Palma, P.; Dowling, D.J.; Levy, O. Neonatal monocytes demonstrate impaired homeostatic extravasation into a microphysiological human vascular model. Sci. Rep. 2020, 10, 17836. [Google Scholar] [CrossRef]
- Perveen, K.; Quach, A.; McPhee, A.; Prescott, S.L.; Barry, S.C.; Hii, C.S.; Ferrante, A. Cord Blood T Cells Expressing High and Low PKCζ Levels Develop into Cells with a Propensity to Display Th1 and Th9 Cytokine Profiles, Respectively. Int. J. Mol. Sci. 2021, 22, 4907. [Google Scholar] [CrossRef]
- Perveen, K.; Quach, A.; Stark, M.J.; Prescott, S.L.; Barry, S.C.; Hii, C.S.; Ferrante, A. Characterization of the Transient Deficiency of PKC Isozyme Levels in Immature Cord Blood T Cells and Its Connection to Anti-Allergic Cytokine Profiles of the Matured Cells. Int. J. Mol. Sci. 2021, 22, 12650. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, N.; Alashkar Alhamwe, B.; Caraballo, L.; Ding, M.; Ferrante, A.; Garn, H.; Garssen, J.; Hii, C.S.; Irvine, J.; Llinás-Caballero, K.; et al. Perinatal and Early-Life Nutrition, Epigenetics, and Allergy. Nutrients 2021, 13, 724. [Google Scholar] [CrossRef] [PubMed]
- Perveen, K.; Quach, A.; McPhee, A.; Prescott, S.L.; Barry, S.C.; Hii, C.S.; Ferrante, A. Validation of monoclonal anti-PKC isozyme antibodies for flow cytometry analyses in human T cell subsets and expression in cord blood T cells. Sci. Rep. 2019, 9, 9263. [Google Scholar] [CrossRef]
- D’Vaz, N.; Ma, Y.; Dunstan, J.A.; Lee-Pullen, T.F.; Hii, C.; Meldrum, S.; Zhang, G.; Metcalfe, J.; Ferrante, A.; Prescott, S.L. Neonatal protein kinase C zeta expression determines the neonatal T-Cell cytokine phenotype and predicts the development and severity of infant allergic disease. Allergy 2012, 67, 1511–1518. [Google Scholar] [CrossRef]
- Perveen, K.; Quach, A.; Stark, M.J.; Prescott, S.; Barry, S.C.; Hii, C.S.; Ferrante, A. PKCζ activation promotes maturation of cord blood T cells towards a Th1 IFN-γ propensity. Immunology 2023, 170, 359–373. [Google Scholar] [CrossRef]
- Sohlberg, E.; Saghafian-Hedengren, S.; Bremme, K.; Sverremark-Ekström, E. Cord blood monocyte subsets are similar to adult and show potent peptidoglycan-stimulated cytokine responses. Immunology 2011, 133, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.R.; Qing, G.; Byers, D.M.; Stadnyk, A.W.; Al-Hertani, W.; Bortolussi, R. Role of MyD88 in diminished tumor necrosis factor alpha production by newborn mononuclear cells in response to lipopolysaccharide. Infect. Immun. 2004, 72, 1223–1229. [Google Scholar] [CrossRef]
- Yan, S.R.; Byers, D.M.; Bortolussi, R. Role of protein tyrosine kinase p53/56lyn in diminished lipopolysaccharide priming of formylmethionylleucyl- phenylalanine-induced superoxide production in human newborn neutrophils. Infect. Immun. 2004, 72, 6455–6462. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, X.; Jin, T. The Novel Functions of the PLC/PKC/PKD Signaling Axis in G Protein-Coupled Receptor-Mediated Chemotaxis of Neutrophils. J. Immunol. Res. 2015, 2015, 817604. [Google Scholar] [CrossRef]
- Damascena, H.L.; Silveira, W.A.A.; Castro, M.S.; Fontes, W. Neutrophil Activated by the Famous and Potent PMA (Phorbol Myristate Acetate). Cells 2022, 11, 2889. [Google Scholar] [CrossRef]
- Gray, R.D.; Lucas, C.D.; MacKellar, A.; Li, F.; Hiersemenzel, K.; Haslett, C.; Davidson, D.J.; Rossi, A.G. Activation of conventional protein kinase C (PKC) is critical in the generation of human neutrophil extracellular traps. J. Inflamm. 2013, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Dang, P.M.; Fontayne, A.; Hakim, J.; El Benna, J.; Périanin, A. Protein kinase C zeta phosphorylates a subset of selective sites of the NADPH oxidase component p47phox and participates in formyl peptide-mediated neutrophil respiratory burst. J. Immunol. 2001, 166, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Neeli, I.; Radic, M. Opposition between PKC isoforms regulates histone deimination and neutrophil extracellular chromatin release. Front. Immunol. 2013, 4, 38. [Google Scholar] [CrossRef]
- Laudanna, C.; Mochly-Rosen, D.; Liron, T.; Constantin, G.; Butcher, E.C. Evidence of zeta protein kinase C involvement in polymorphonuclear neutrophil integrin-dependent adhesion and chemotaxis. J. Biol. Chem. 1998, 273, 30306–30315. [Google Scholar] [CrossRef] [PubMed]
- Kollmann, T.R.; Crabtree, J.; Rein-Weston, A.; Blimkie, D.; Thommai, F.; Wang, X.Y.; Lavoie, P.M.; Furlong, J.; Fortuno, E.S., 3rd; Hajjar, A.M.; et al. Neonatal innate TLR-mediated responses are distinct from those of adults. J. Immunol. 2009, 183, 7150–7160. [Google Scholar] [CrossRef]
- Weinberger, B.; Laskin, D.L.; Mariano, T.M.; Sunil, V.R.; DeCoste, C.J.; Heck, D.E.; Gardner, C.R.; Laskin, J.D. Mechanisms underlying reduced responsiveness of neonatal neutrophils to distinct chemoattractants. J. Leukoc. Biol. 2001, 70, 969–976. [Google Scholar] [CrossRef]
- Brichard, B.; Varis, I.; Latinne, D.; Deneys, V.; de Bruyere, M.; Leveugle, P.; Cornu, G. Intracellular cytokine profile of cord and adult blood monocytes. Bone Marrow Transplant. 2001, 27, 1081–1086. [Google Scholar] [CrossRef][Green Version]
- Shigeoka, A.O.; Santos, J.I.; Hill, H.R. Functional analysis of neutrophil granulocytes from healthy, infected, and stressed neonates. J. Pediatr. 1979, 95, 454–460. [Google Scholar] [CrossRef]
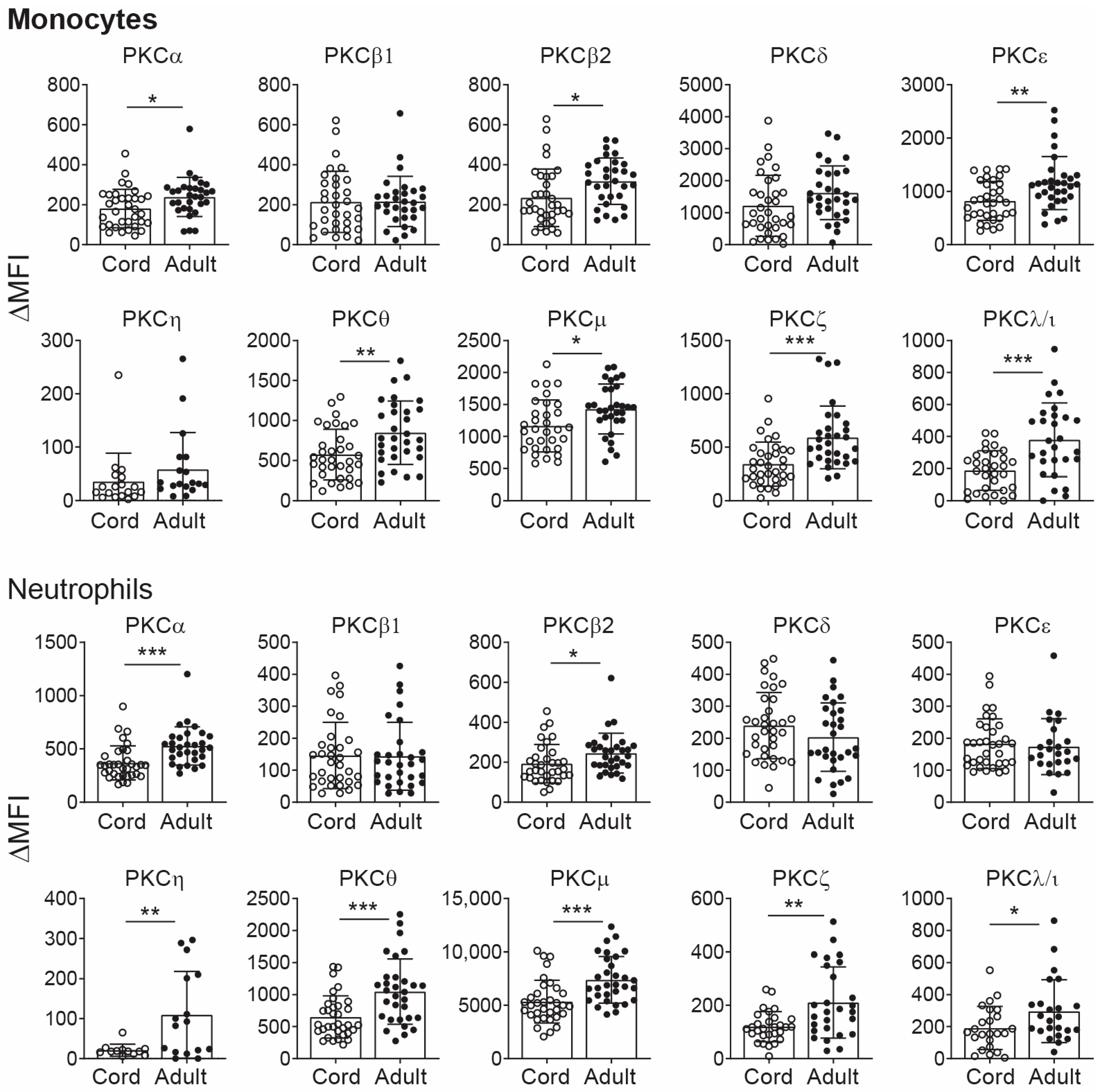
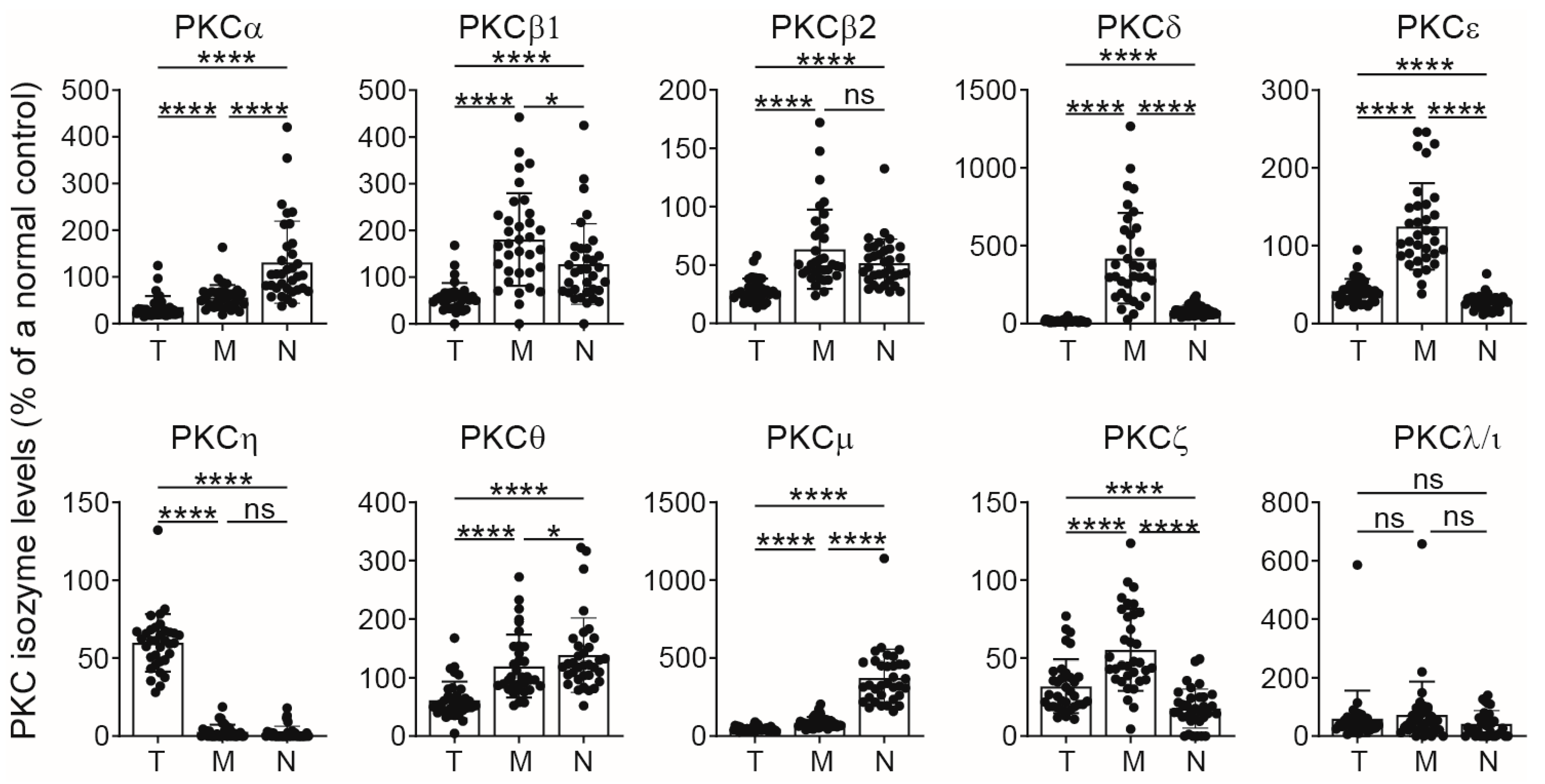
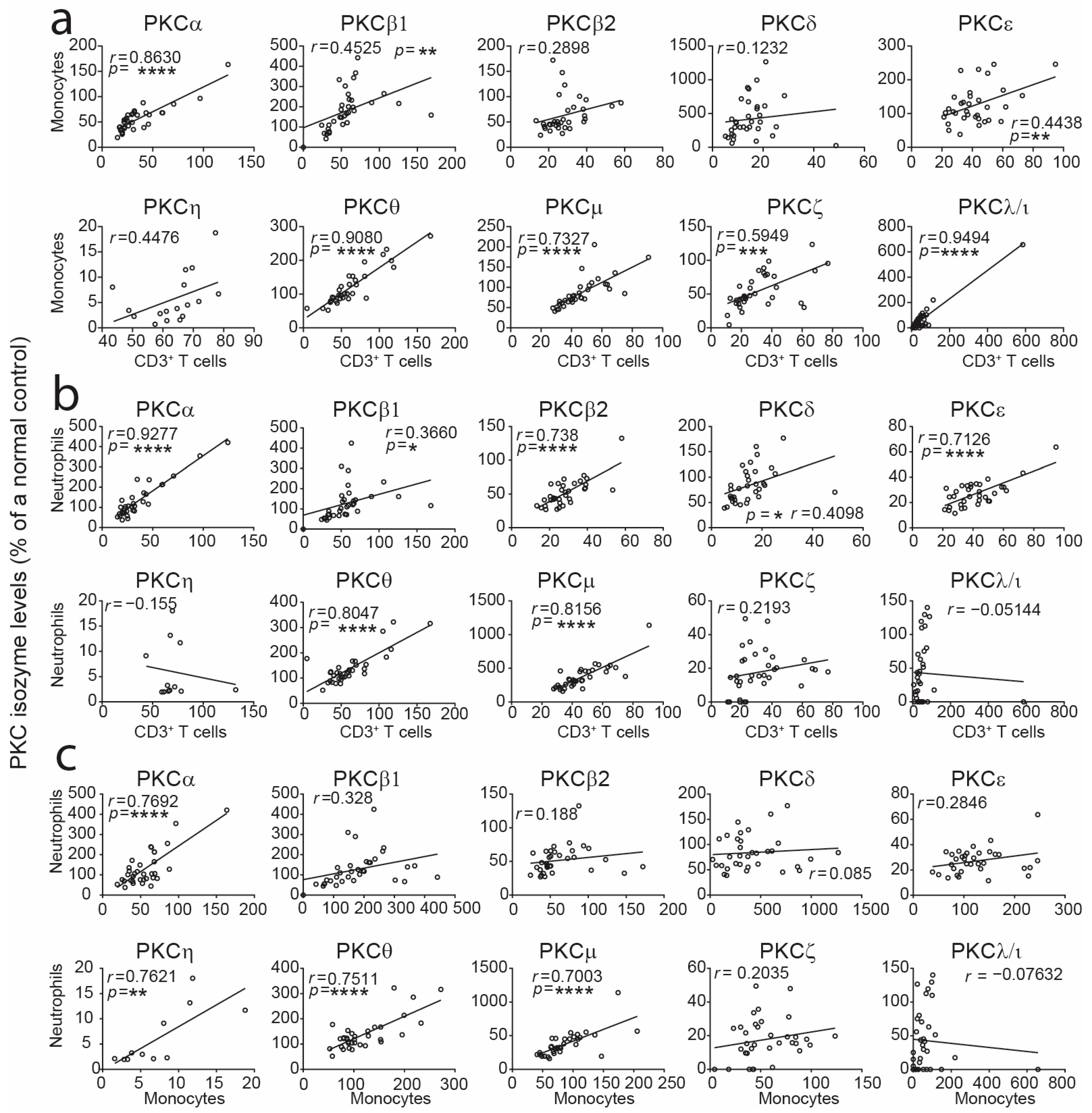
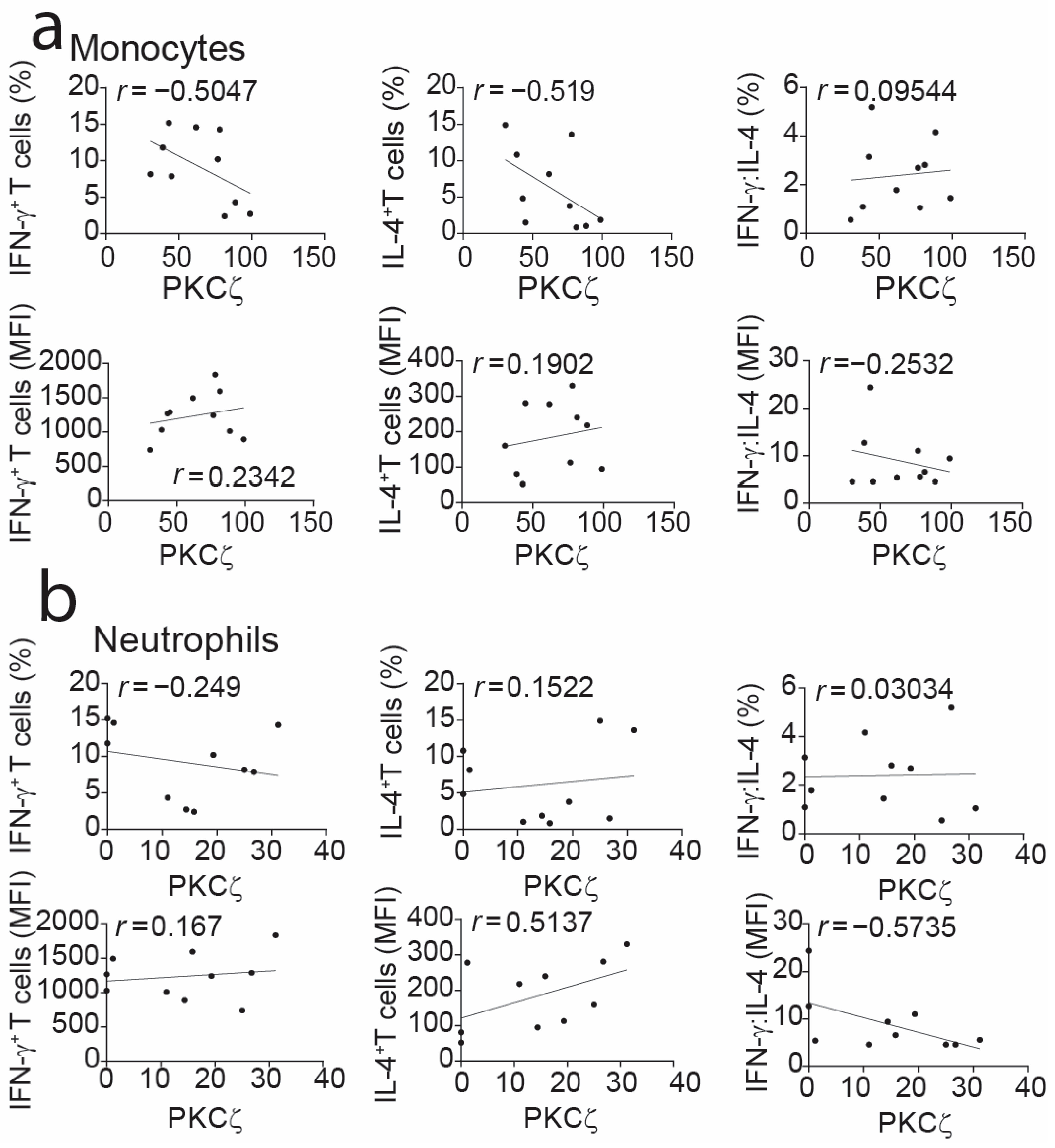
| Cell Type | PKCα | PKCβI | PKCβII | PKCδ | PKCε | PKCη | PKCθ | PKCμ | PKCζ | PKCλ/ι |
|---|---|---|---|---|---|---|---|---|---|---|
| CD4 | - | - | *** ↓ | **** ↓ | - | - | - | * ↓ | ** ↓ | *** ↓ |
| CD8 | - | - | **** ↓ | **** ↓ | ** ↓ | - | ** ↓ | * ↓ | **** ↓ | **** ↓ |
| CD3 | - | - | **** ↓ | **** ↓ | * ↓ | - | * ↓ | * ↓ | **** ↓ | **** ↓ |
| Monocytes | * ↓ | - | * ↓ | - | ** ↓ | - | ** ↓ | * ↓ | *** ↓ | *** ↓ |
| Neutrophils | *** ↓ | - | * ↓ | - | - | ** ↓ | *** ↓ | *** ↓ | ** ↓ | * ↓ |
| Monocytes | Neutrophils | |||||
|---|---|---|---|---|---|---|
| PKC | 5th % Cut-Off Adults (MFI) * | No. CB (<5th %) † | % of CB ‡ | 5th % Cut-Off Adults (MFI) * | No. CB (<5th %) † | % of CB ‡ |
| PKCα | 38 | 8 | 24 | 77 | 11 | 33 |
| PKCβ1 | 44 | 1 | 3 | 32 | 0 | 0 |
| PKCβ2 | 49 | 15 | 45 | 37 | 8 | 24 |
| PKCδ | 152 | 5 | 15 | 13 | 0 | 0 |
| PKCε | 53 | 2 | 6 | 10 | 0 | 0 |
| PKCη | 2 | 3 | 9 | 4 | 8 | 24 |
| PKCθ | 66 | 3 | 9 | 63 | 1 | 3 |
| PKCμ | 50 | 4 | 12 | 249 | 9 | 27 |
| PKCζ | 39 | 10 | 30 | 7 | 1 | 3 |
| PKCλ/ι | 31 | 7 | 21 | 25 | 5 | 15 |
| Antibody | Fluorochrome | Clone | Catalogue |
|---|---|---|---|
| Anti-PKCα | AF647 | H-7 1 | sc-8393 |
| Anti-PKCβ1 | AF647 | EPR18512 2 | ab223452 |
| Anti-PKCβII | AF647 | F-7 1 | sc-13149 |
| Anti-PKCθ | PE | E-7 1 | sc-1680 |
| Anti-PKCε | AF488 | EPR1482(2) 2 | ab217980 |
| Anti-PKCδ | AF488 | EPR17075 2 | ab206282 |
| Anti-PKCη | AF488 | EPR18513 2 | ab179524 |
| Anti-PKCμ | AF647 | EP1493Y 2 | ab51246 |
| Anti-PKCζ | PE | H-1 1 | sc-17781 |
| Anti-PKCλ/ι | PE | H-12 1 | sc-17837 |
| Rabbit mAb IgG Isotype Control | AF647 | - 4 | 2985 |
| Rabbit mAb IgG Isotype Control | AF488 | - 4 | 2975 |
| Mouse IgG1k Isotype control | AF647 | MOPC-31C 3 | 566011 |
| Mouse mAb IgG2ak | PE | X39 3 | 340459 |
| Antibody | Fluorochrome | Clone | Catalogue |
|---|---|---|---|
| Anti-CD3 | PE-CY5 | HIT3a 1 | 555341 |
| Anti-CD45 | APC-H7 | 2D1 1 | 641399 |
| Anti-IFN-γ | FITC | 4S.B3 1 | 554551 |
| Anti-IL-4 | PE | 8D4-8 2 | 12-7049-42 |
| Mouse-IgG1k | FITC | MOPC-21 1 | 555748 |
| Mouse-IgG1k | PE | MOPC-21 1 | 556650 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perveen, K.; Ferrante, A. Protein Kinase C Isozyme Immaturity/Deficiency in Cord Blood Monocytes and Neutrophils. Int. J. Mol. Sci. 2024, 25, 11665. https://doi.org/10.3390/ijms252111665
Perveen K, Ferrante A. Protein Kinase C Isozyme Immaturity/Deficiency in Cord Blood Monocytes and Neutrophils. International Journal of Molecular Sciences. 2024; 25(21):11665. https://doi.org/10.3390/ijms252111665
Chicago/Turabian StylePerveen, Khalida, and Antonio Ferrante. 2024. "Protein Kinase C Isozyme Immaturity/Deficiency in Cord Blood Monocytes and Neutrophils" International Journal of Molecular Sciences 25, no. 21: 11665. https://doi.org/10.3390/ijms252111665
APA StylePerveen, K., & Ferrante, A. (2024). Protein Kinase C Isozyme Immaturity/Deficiency in Cord Blood Monocytes and Neutrophils. International Journal of Molecular Sciences, 25(21), 11665. https://doi.org/10.3390/ijms252111665






