Alteration of Lipid Metabolism in Patients with IPF and Its Association with Disease Severity and Prognosis: A Case–Control Study
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Fatty Acid, Sterol, and Oxysterol Quantification by Gas Chromatography–Mass Spectrometry
4.2. Statistical Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COPD | chronic obstructive pulmonary disease |
| ER | endothelial reticulum |
| FVC | forced vital capacity |
| GAP | Gender-Age-Physiology |
| HRCT | high-resolution computed tomography |
| IPF | idiopathic pulmonary fibrosis |
| VLFA | very-long-chain fatty acids |
References
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef] [PubMed]
- Wolters, P.J.; Collard, H.R.; Jones, K.D. Pathogenesis of idiopathic pulmonary fibrosis. Annu. Rev. Pathol. 2014, 9, 157–179. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, M.; Tarling, E.J.; Bridges, J.P.; Redente, E.F.; Byrne, A.J.; Keane, M.P.; McCarthy, C. Reexamining the Role of Pulmonary Lipids in the Pathogenesis of Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2024, 71, 407–419. [Google Scholar] [CrossRef]
- Chen, R.; Dai, J. Lipid Metabolism in Idiopathic Pulmonary Fibrosis: From Pathogenesis to Therapy. J. Mol. Med. 2023, 101, 905–915. [Google Scholar] [CrossRef]
- Oruqaj, G.; Karnati, S.; Vijayan, V.; Kotarkonda, L.K.; Boateng, E.; Zhang, W.; Ruppert, C.; Günther, A.; Shi, W.; Baumgart-Vogt, E. Compromised Peroxisomes in Idiopathic Pulmonary Fibrosis, a Vicious Cycle Inducing a Higher Fibrotic Response via TGF-β Signaling. Proc. Natl. Acad. Sci. USA 2015, 112, E2048–E2057. [Google Scholar] [CrossRef]
- Schuliga, M.; Pechkovsky, D.V.; Read, J.; Waters, D.W.; Blokland, K.E.C.; Reid, A.T.; Hogaboam, C.M.; Khalil, N.; Burgess, J.K.; Prêle, C.M.; et al. Mitochondrial Dysfunction Contributes to the Senescent Phenotype of IPF Lung Fibroblasts. J. Cell Mol. Med. 2018, 22, 5847–5861. [Google Scholar] [CrossRef]
- Suryadevara, V.; Ramchandran, R.; Kamp, D.W.; Natarajan, V. Lipid Mediators Regulate Pulmonary Fibrosis: Potential Mechanisms and Signaling Pathways. Int. J. Mol. Sci. 2020, 21, 4257. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.G.; Villalba, J.A.; Liang, X.; Xiong, K.; Tsoyi, K.; Ith, B.; Ayaub, E.A.; Tatituri, R.V.; Byers, D.E.; Hsu, F.-F.; et al. Palmitic Acid-Rich High-Fat Diet Exacerbates Experimental Pulmonary Fibrosis by Modulating Endoplasmic Reticulum Stress. Am. J. Respir. Cell Mol. Biol. 2019, 61, 737–746. [Google Scholar] [CrossRef]
- Shi, X.; Chen, Y.; Shi, M.; Gao, F.; Huang, L.; Wang, W.; Wei, D.; Shi, C.; Yu, Y.; Xia, X.; et al. The novel molecular mechanism of pulmonary fibrosis: Insight into lipid metabolism from reanalysis of single-cell RNA-seq databases. Lipids Health Dis. 2024, 23, 98. [Google Scholar] [CrossRef]
- Kosmidis, I. Bias in Parametric Estimation: Reduction and Useful Side-Effects. WIREs Comput. Stat. 2014, 6, 185–196. [Google Scholar] [CrossRef]
- Marcello, A.; Civra, A.; Milan Bonotto, R.; Nascimento Alves, L.; Rajasekharan, S.; Giacobone, C.; Caccia, C.; Cavalli, R.; Adami, M.; Brambilla, P.; et al. The Cholesterol Metabolite 27-Hydroxycholesterol Inhibits SARS-CoV-2 and Is Markedly Decreased in COVID-19 Patients. Redox Biol. 2020, 36, 101682. [Google Scholar] [CrossRef]
- Ydreborg, M.; Lisovskaja, V.; Lagging, M.; Brehm Christensen, P.; Langeland, N.; Buhl, M.R.; Pedersen, C.; Mørch, K.; Wejstål, R.; Norkrans, G.; et al. A Novel Fibrosis Index Comprising a Non-Cholesterol Sterol Accurately Predicts HCV-Related Liver Cirrhosis. PLoS ONE 2014, 9, e93601. [Google Scholar] [CrossRef] [PubMed]
- Bockus, L.B.; Biggs, M.L.; Lai, H.T.M.; de Olivera Otto, M.C.; Fretts, A.M.; McKnight, B.; Sotoodehnia, N.; King, I.B.; Song, X.; Siscovick, D.S.; et al. Assessment of Plasma Phospholipid Very-Long-Chain Saturated Fatty Acid Levels and Healthy Aging. JAMA Netw. Open 2021, 4, e2120616. [Google Scholar] [CrossRef] [PubMed]
- Nury, T.; Sghaier, R.; Zarrouk, A.; Ménétrier, F.; Uzun, T.; Leoni, V.; Caccia, C.; Meddeb, W.; Namsi, A.; Sassi, K.; et al. Induction of Peroxisomal Changes in Oligodendrocytes Treated with 7-Ketocholesterol: Attenuation by α-Tocopherol. Biochimie 2018, 153, 181–202. [Google Scholar] [CrossRef]
- Raas, Q.; Tawbeh, A.; Tahri-Joutey, M.; Gondcaille, C.; Keime, C.; Kaiser, R.; Trompier, D.; Nasser, B.; Leoni, V.; Bellanger, E.; et al. Peroxisomal Defects in Microglial Cells Induce a Disease-Associated Microglial Signature. Front. Mol. Neurosci. 2023, 16, 1170313. [Google Scholar] [CrossRef]
- Stradomska, T.J.; Syczewska, M.; Jamroz, E.; Pleskaczyńska, A.; Kruczek, P.; Ciara, E.; Tylki-Szymanska, A. Serum Very Long-Chain Fatty Acids (VLCFA) Levels as Predictive Biomarkers of Diseases Severity and Probability of Survival in Peroxisomal Disorders. PLoS ONE 2020, 15, e0238796. [Google Scholar] [CrossRef]
- Rattay, T.W.; Rautenberg, M.; Söhn, A.S.; Hengel, H.; Traschütz, A.; Röben, B.; Hayer, S.N.; Schüle, R.; Wiethoff, S.; Zeltner, L.; et al. Defining Diagnostic Cutoffs in Neurological Patients for Serum Very Long Chain Fatty Acids (VLCFA) in Genetically Confirmed X-Adrenoleukodystrophy. Sci. Rep. 2020, 10, 15093. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, Y.; Liu, C.; Li, Y.; Mi, S.; Yang, X.; Liu, T.; Tian, Y.; Zhang, Y.; Hu, P.; et al. Nervonic acid alleviates radiation-induced early phase lung inflammation by targeting macrophages activation in mice. Front Immunol. 2024, 15, 1405020. [Google Scholar] [CrossRef]
- Telenga, E.D.; Hoffmann, R.F.; t'Kindt, R.; Hoonhorst, S.J.M.; Willemse, B.W.M.; van Oosterhout, A.J.M.; Heijink, I.H.; van den Berge, M.; Jorge, L.; Sandra, P.; et al. Untargeted lipidomic analysis in chronic obstructive pulmonary disease: Uncovering sphingolipids. Am. J. Respir. Crit. Care. Med. 2014, 190, 155–164. [Google Scholar] [CrossRef]
- Cameli, P.; Carleo, A.; Bergantini, L.; Landi, C.; Prasse, A.; Bargagli, E. Oxidant/Antioxidant Disequilibrium in Idiopathic Pulmonary Fibrosis Pathogenesis. Inflammation 2020, 43, 1–7. [Google Scholar] [CrossRef]
- Daniil, Z.D.; Papageorgiou, E.; Koutsokera, A.; Kostikas, K.; Kiropoulos, T.; Papaioannou, A.I.; Gourgoulianis, K.I. Serum Levels of Oxidative Stress as a Marker of Disease Severity in Idiopathic Pulmonary Fibrosis. Pulm. Pharmacol. Ther. 2008, 21, 26–31. [Google Scholar] [CrossRef]
- Matsuzawa, Y.; Kawashima, T.; Kuwabara, R.; Hayakawa, S.; Irie, T.; Yoshida, T.; Rikitake, H.; Wakabayashi, T.; Okada, N.; Kawashima, K.; et al. Change in Serum Marker of Oxidative Stress in the Progression of Idiopathic Pulmonary Fibrosis. Pulm. Pharmacol. Ther. 2015, 32, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Faverio, P.; Maloberti, A.; Rebora, P.; Intravaia, R.C.M.; Tognola, C.; Toscani, G.; Amato, A.; Leoni, V.; Franco, G.; Vitarelli, F.; et al. Cardiovascular Structural and Functional Parameters in Idiopathic Pulmonary Fibrosis at Disease Diagnosis. High Blood Press Cardiovasc. Prev. 2024, 31, 289–297. [Google Scholar] [CrossRef]
- Tonkin, A. High-density lipoprotein cholesterol and treatment guidelines. Am. J. Cardiol. 2001, 12, 41N–44N. [Google Scholar] [CrossRef] [PubMed]
- Ley, B.; Ryerson, C.J.; Vittinghoff, E.; Ryu, J.H.; Tomassetti, S.; Lee, J.S.; Poletti, V.; Buccioli, M.; Elicker, B.M.; Jones, K.D.; et al. Multidimensional Index and Staging System for Idiopathic Pulmonary Fibrosis. Ann. Intern. Med. 2012, 156, 684–691. [Google Scholar] [CrossRef]
- Wells, A.U.; Flaherty, K.R.; Brown, K.K.; Inoue, Y.; Devaraj, A.; Richeldi, L.; Moua, T.; Crestani, B.; Wuyts, W.A.; Stowasser, S.; et al. Nintedanib in Patients with Progressive Fibrosing Interstitial Lung Diseases—Subgroup Analyses by Interstitial Lung Disease Diagnosis in the INBUILD Trial: A Randomised, Double-Blind, Placebo-Controlled, Parallel-Group Trial. Lancet Respir. Med. 2020, 8, 453–460. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef] [PubMed]
- Poli, G.; Leoni, V.; Biasi, F.; Canzoneri, F.; Risso, D.; Menta, R. Oxysterols: From Redox Bench to Industry. Redox Biol. 2022, 49, 102220. [Google Scholar] [CrossRef]
- Leoni, V.; Nury, T.; Vejux, A.; Zarrouk, A.; Caccia, C.; Debbabi, M.; Fromont, A.; Sghaier, R.; Moreau, T.; Lizard, G. Mitochondrial Dysfunctions in 7-Ketocholesterol-Treated 158N Oligodendrocytes without or with α-Tocopherol: Impacts on the Cellular Profil of Tricarboxylic Cycle-Associated Organic Acids, Long Chain Saturated and Unsaturated Fatty Acids, Oxysterols, Cholesterol and Cholesterol Precursors. J. Steroid. Biochem. Mol. Biol. 2017, 169, 96–110. [Google Scholar] [CrossRef]
| Controls (n = 50) | Patients—GAP Index I (n = 27) | Patients—GAP Index II–III (n = 23) | |
|---|---|---|---|
| Demographics | |||
| Age (years), median [I–III quartiles] | 72 [68, 76] | 72 [69, 75] | 75 [72, 77] |
| N (%) | N (%) | N (%) | |
| Males (%) | 39 (78) | 18 (67) | 21 (91) |
| Never smoker * | 22 (45) | 8 (30) | 6 (26) |
| Comorbidities | |||
| Arterial hypertension | 25 (50) | 11 (41) | 13 (57) |
| Dyslipidemia | 13 (26) | 10 (37) | 11 (48) |
| Diabetes type 2 | 7 (14) | 5 (19) | 7 (30) |
| Obesity ** | 1 (2) | 2 (8) | 1 (5) |
| Valvular heart disease * | 2 (4) | 0 (0) | 2 (9) |
| Coronary artery disease * | 4 (8) | 1 (4) | 9 (39) |
| Prior myocardial infarction * | 3 (6) | 1 (4) | 5 (22) |
| Peripheral arterial disease | 0 (0) | 1 (4) | 3 (13) |
| Pharmacological Therapy | |||
| Statins *** | 8 (16) | 10 (37) | 11 (48) |
| Ezetimibe | 3 (6) | 1 (4) | 0 (0) |
| Pulmonary function tests at baseline | |||
| FVC%, median [I–III quartiles]. ** | - | 97 [88, 107] | 70 [65, 77] |
| DLCO%, median [I–III quartiles]. **** | - | 5.0 [4.4, 5.8] | 3.1 [2.7, 3.7] |
| Characteristic | IPF pts vs. Controls (N = 86) | GAP II, III vs. I (N = 47) | ||||
|---|---|---|---|---|---|---|
| OR 1 | 95% CI 1 | p-Value | OR 1 | 95% CI 1 | p-Value | |
| Cholesterol synthesis precursors | ||||||
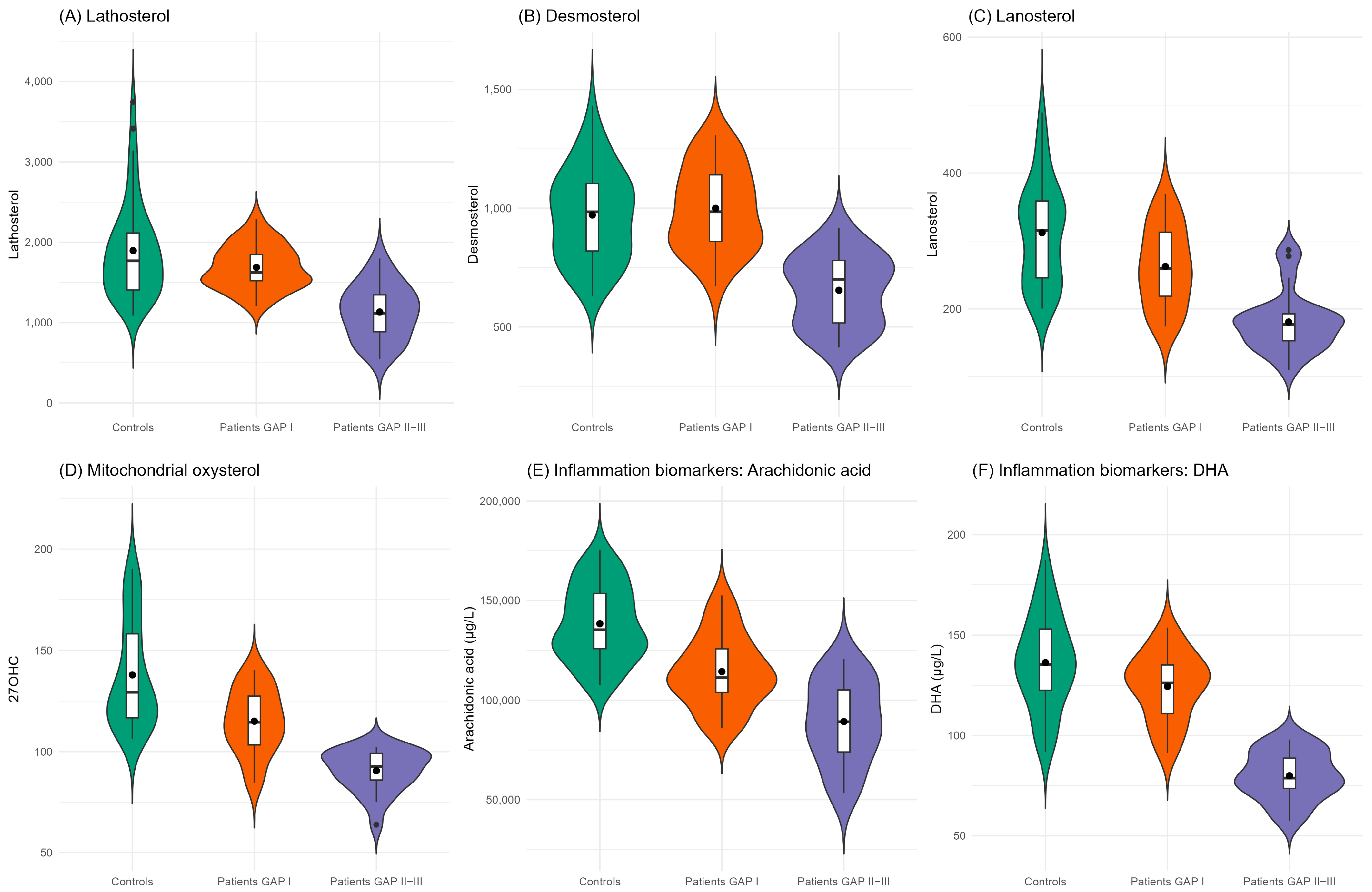
| Lathosterol | 0.336 | 0.152, 0.655 | 0.003 | 0.047 | 0.004, 0.218 | 0.002 |
| Desmosterol | 0.653 | 0.388, 1.065 | 0.095 | 0.017 | 0.000, 0.132 | 0.003 |
| Lanosterol | 0.281 | 0.139, 0.513 | <0.001 * | 0.085 | 0.014, 0.287 | <0.001 * |
| Mitochondrial oxysterol | ||||||
| 27-hydroxycholesterol | 0.103 | 0.030, 0.267 | <0.001 * | 0.03 | 0.002, 0.183 | 0.003 |
| Inflammation biomarkers | ||||||
| Arachidonic acid | 0.045 | 0.009, 0.142 | <0.001 * | 0.192 | 0.053, 0.506 | 0.003 |
| Docosahexaenoic acid ^ | 0.154 | 0.067, 0.354 | <0.001 * | 0.023 | 0.002, 0.265 | 0.003 |
| Very-long-chain fatty acids | ||||||
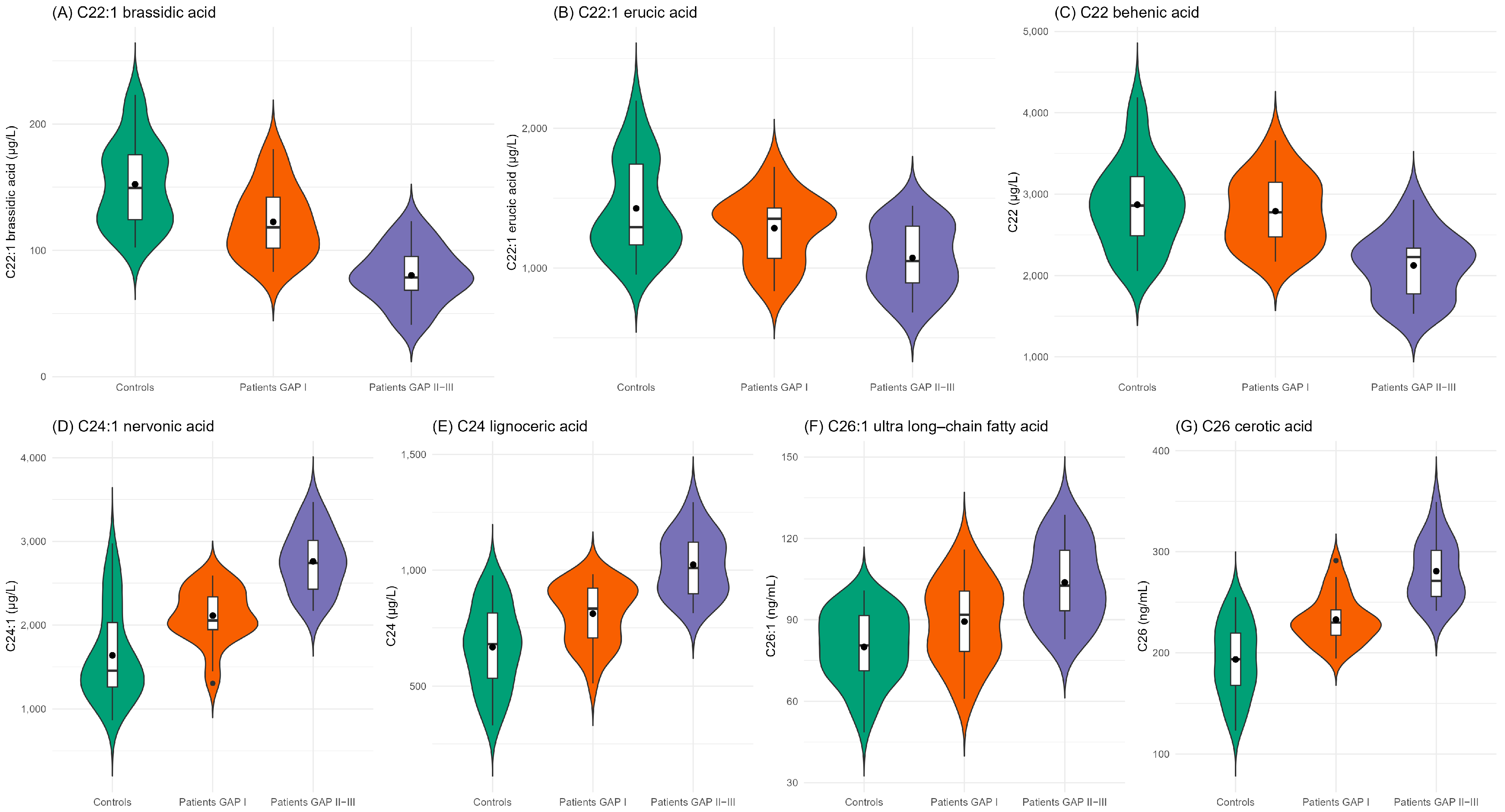
| C22:1 brassidic acid | 0.183 | 0.072, 0.375 | <0.001 * | 0.037 | 0.002, 0.200 | 0.002 |
| C22:1 erucic acid | 0.391 | 0.206, 0.678 | 0.002 | 0.396 | 0.166, 0.833 | 0.021 |
| C22 behenic acid | 0.576 | 0.330, 0.957 | 0.04 | 0.09 | 0.013, 0.308 | 0.002 |
| C24:1 nervonic acid | 4.525 | 2.380, 9.828 | <0.001 * | 35.686 | 6.217, 575.303 | 0.001 |
| C24 lignoceric acid | 5.253 | 2.649, 12.242 | <0.001 * | 13.599 | 3.557, 110.217 | 0.002 |
| C26:1 ultra-long-chain fatty acid | 12.228 | 4.584, 45.908 | <0.001 * | 12.284 | 3.528, 83.602 | 0.001 |
| C26 cerotic acid | 4.013 | 2.191, 8.364 | <0.001 * | 3.682 | 1.632, 10.398 | 0.005 |
| Slow Progressor (n = 33 *) | Rapid Progressor (n = 9) | |
|---|---|---|
| Median [I–III Quartiles] | Median [I–III Quartiles] | |
| Cholesterol synthesis precursors | ||
| Lathosterol (µg/L) | 1542.16 [1296.72, 1803.48] | 1050.96 [979.52, 1344.20] |
| Desmosterol (µg/L) | 858.72 [738.28, 985.84] | 779.96 [572.88, 834.28] |
| Lanosterol (µg/L) | 232.84 [177.04, 283.64] | 188.84 [164.76, 245.96] |
| Mitochondrial oxysterol | ||
| 27-hydroxycholesterol (µg/L) | 104.84 [88.64, 121.56] | 99.16 [97.00, 102.00] |
| Inflammation biomarkers | ||
| Arachidonic acid (µg/L) | 107,250 [89,925, 116,160] | 103,545 [89,175, 113,190] |
| Docosahexaenoic acid (µg/L) | 108.69 [91.44, 132.99] | 76.92 [73.65, 97.98] |
| Very-long-chain fatty acids | ||
| C22:1 brassidic acid (µg/L) | 107.04 [91.00, 124.36] | 83.44 [81.44, 95.16] |
| C22:1 erucic acid (µg/L) | 1183.52 [941.40, 1392.40] | 1276.08 [1011.12, 1344.68] |
| C22 behenic acid (µg/L) | 2492.88 [2186.76, 2894.08] | 2336.96 [2273.60, 2565.36] |
| C24:1 nervonic acid (µg/L) | 2335.20 [2041.08, 2679.48] | 2424.44 [2297.80, 2428.36] |
| C24 lignoceric acid (µg/L) | 918.22 [800.82, 955.14] | 897.27 [848.14, 1058.16] |
| C26:1 ultra-long-chain fatty acid (µg/L) | 95.56 [81.16, 104.56] | 93.30 [83.25, 100.46] |
| C26 cerotic acid (µg/L) | 242.02 [225.78, 261.10] | 265.54 [255.65, 271.20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faverio, P.; Rebora, P.; Franco, G.; Amato, A.; Corti, N.; Cattaneo, K.; Spiti, S.; Zanini, U.; Maloberti, A.; Giannattasio, C.; et al. Alteration of Lipid Metabolism in Patients with IPF and Its Association with Disease Severity and Prognosis: A Case–Control Study. Int. J. Mol. Sci. 2025, 26, 5790. https://doi.org/10.3390/ijms26125790
Faverio P, Rebora P, Franco G, Amato A, Corti N, Cattaneo K, Spiti S, Zanini U, Maloberti A, Giannattasio C, et al. Alteration of Lipid Metabolism in Patients with IPF and Its Association with Disease Severity and Prognosis: A Case–Control Study. International Journal of Molecular Sciences. 2025; 26(12):5790. https://doi.org/10.3390/ijms26125790
Chicago/Turabian StyleFaverio, Paola, Paola Rebora, Giovanni Franco, Anna Amato, Nicole Corti, Katya Cattaneo, Simona Spiti, Umberto Zanini, Alessandro Maloberti, Cristina Giannattasio, and et al. 2025. "Alteration of Lipid Metabolism in Patients with IPF and Its Association with Disease Severity and Prognosis: A Case–Control Study" International Journal of Molecular Sciences 26, no. 12: 5790. https://doi.org/10.3390/ijms26125790
APA StyleFaverio, P., Rebora, P., Franco, G., Amato, A., Corti, N., Cattaneo, K., Spiti, S., Zanini, U., Maloberti, A., Giannattasio, C., Luppi, F., & Leoni, V. (2025). Alteration of Lipid Metabolism in Patients with IPF and Its Association with Disease Severity and Prognosis: A Case–Control Study. International Journal of Molecular Sciences, 26(12), 5790. https://doi.org/10.3390/ijms26125790







