Integrated Plasma and Tumor Proteomics of Nasopharyngeal Carcinoma in a Moroccan Cohort
Abstract
1. Introduction
2. Results
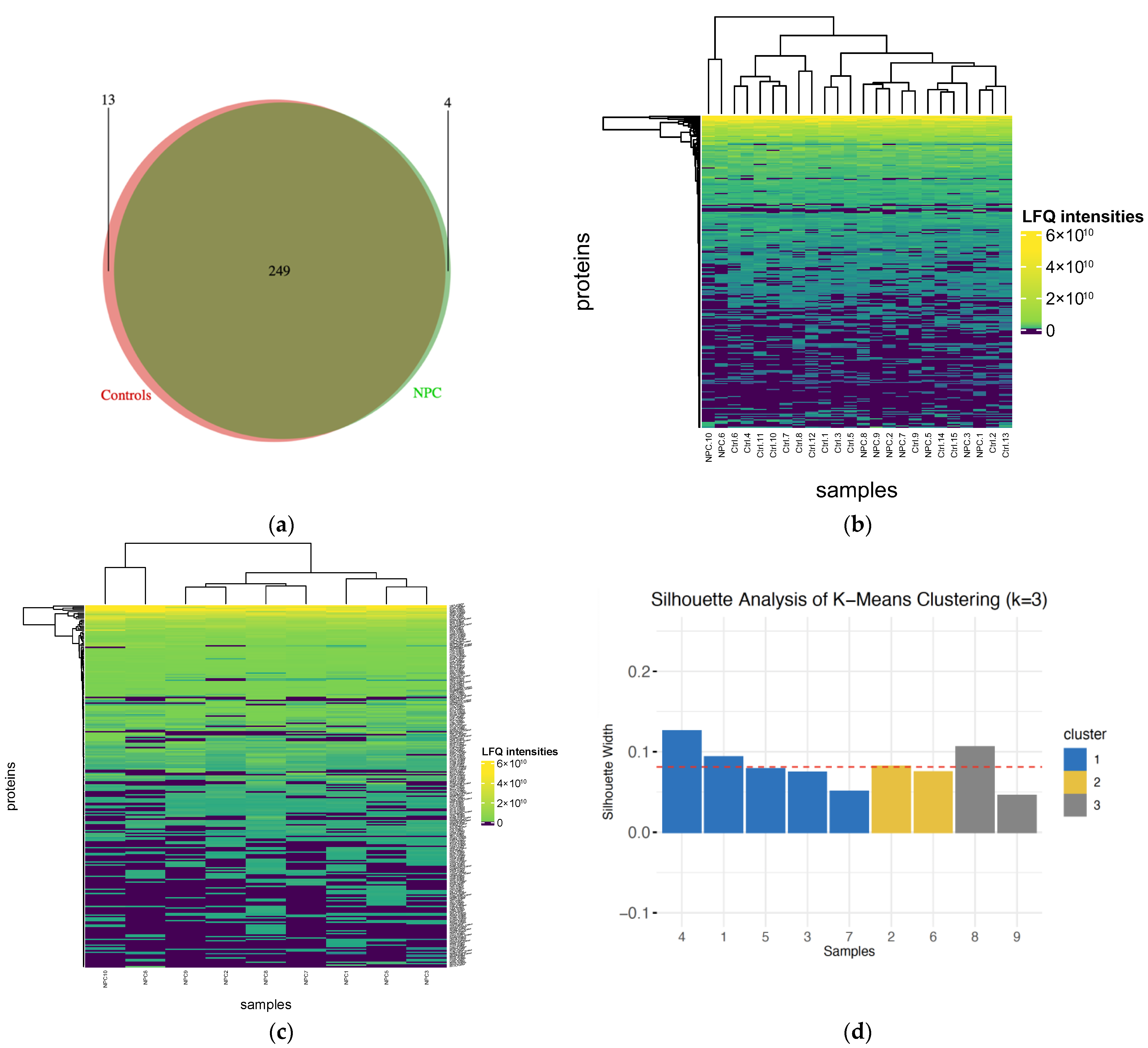
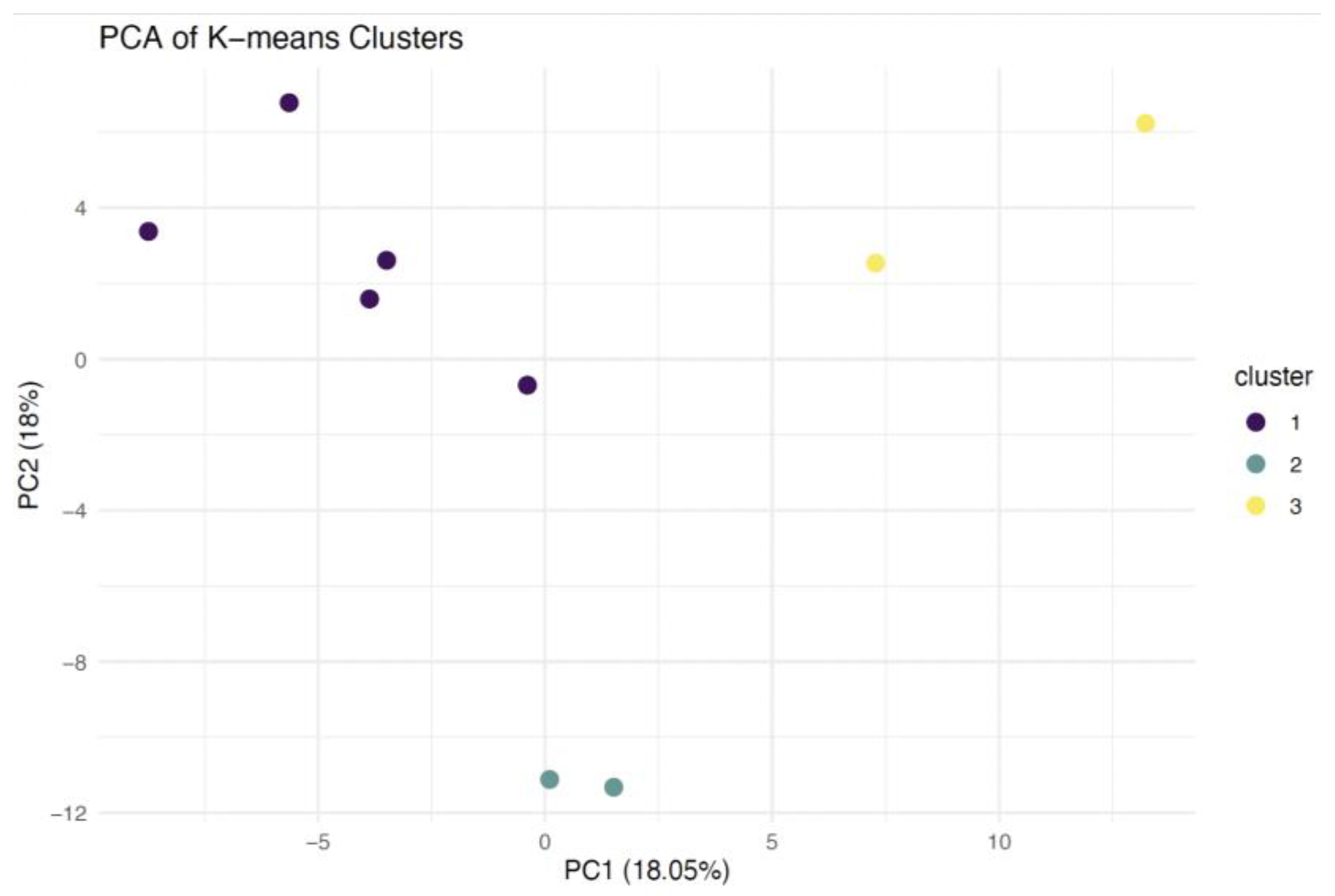
2.1. Protein Identification and Clustering
2.2. Differential Expression Analysis of NPC
2.3. Pathway Analysis of NPC
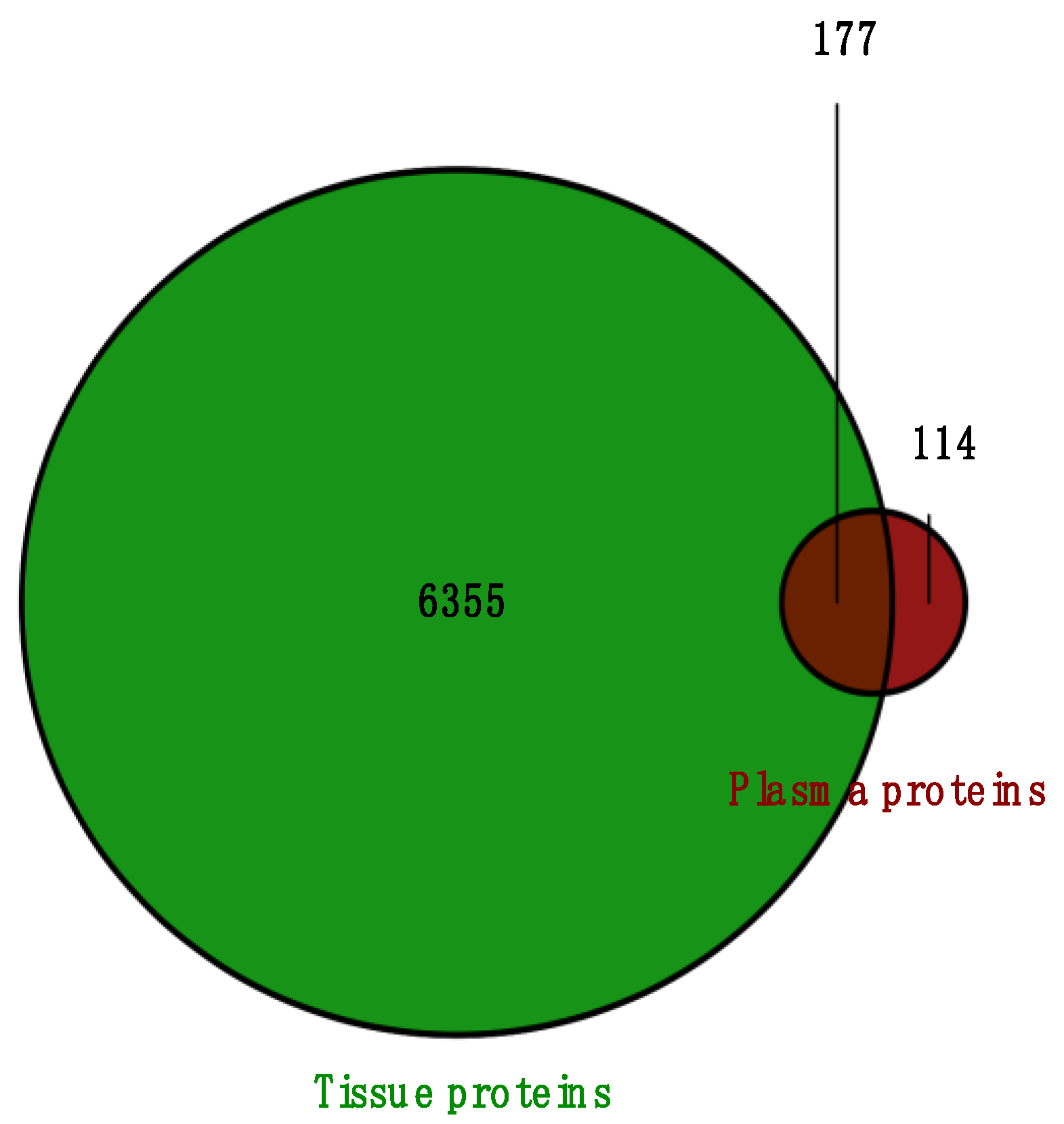
2.4. Overlapped DEPs Between Tissue and Plasma Samples
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Sample Preparation
4.3. LC-MS/MS Analysis
4.4. Bioinformatics and Data Analysis
4.4.1. Protein Identification and Quantification
4.4.2. Differential Expression Analysis
4.4.3. Pathway Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Pineros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Cancer Today. Available online: https://gco.iarc.who.int/today/ (accessed on 3 April 2024).
- Badoual, C. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Oropharynx and Nasopharynx. Head. Neck Pathol. 2022, 16, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.P.; Raslan, W.F.; Gooneratne, S.; Kathuria, S.; Marks, J.E. Prognostic Significance of Keratinization in Nasopharyngeal Carcinoma. Am. J. Otolaryngol. 1995, 16, 103–108. [Google Scholar] [CrossRef]
- Tsang, C.M.; Lui, V.W.Y.; Bruce, J.P.; Pugh, T.J.; Lo, K.W. Translational Genomics of Nasopharyngeal Cancer. Semin. Cancer Biol. 2020, 61, 84–100. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.C.W.; Hui, E.P.; Lo, K.-W.; Lam, W.K.J.; Johnson, D.; Li, L.; Tao, Q.; Chan, K.C.A.; To, K.-F.; King, A.D.; et al. Nasopharyngeal Carcinoma: An Evolving Paradigm. Nat. Rev. Clin. Oncol. 2021, 18, 679–695. [Google Scholar] [CrossRef]
- Omenn, G.S.; Lane, L.; Overall, C.M.; Pineau, C.; Packer, N.H.; Cristea, I.M.; Lindskog, C.; Weintraub, S.T.; Orchard, S.; Roehrl, M.H.A.; et al. The 2022 Report on the Human Proteome from the HUPO Human Proteome Project. J. Proteome Res. 2023, 22, 1024–1042. [Google Scholar] [CrossRef]
- Reffai, A.; Mesmoudi, M.; Derkaoui, T.; Ghailani Nourouti, N.; Barakat, A.; Sellal, N.; Mallick, P.; Bennani Mechita, M. Epidemiological Profile and Clinicopathological, Therapeutic, and Prognostic Characteristics of Nasopharyngeal Carcinoma in Northern Morocco. Cancer Control 2021, 28, 10732748211050587. [Google Scholar] [CrossRef]
- Peng, M.; Zhou, Y.; Zhang, Y.; Cong, Y.; Zhao, M.; Wang, F.; Ding, T.; Liu, C.; Ni, C.; Ding, J.; et al. Small Extracellular Vesicle CA1 as a Promising Diagnostic Biomarker for Nasopharyngeal Carcinoma. Int. J. Biol. Macromol. 2024, 275, 133403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-Q.; Pan, S.-M.; Lai, S.-Z.; Situ, H.-J.; Liu, J.; Dai, W.-J.; Liang, S.-X.; Zhou, L.-Q.; Lu, Q.-Q.; Ke, P.-F.; et al. Novel Plasma Proteomic Biomarkers for Early Identification of Induction Chemotherapy Beneficiaries in Locoregionally Advanced Nasopharyngeal Carcinoma. Front. Oncol. 2022, 12, 889516. [Google Scholar] [CrossRef]
- Qian, S.; Golubnitschaja, O.; Zhan, X. Chronic Inflammation: Key Player and Biomarker-Set to Predict and Prevent Cancer Development and Progression Based on Individualized Patient Profiles. EPMA J. 2019, 10, 365–381. [Google Scholar] [CrossRef]
- Matsuda, T.; Kishimoto, T. Interleukin 6. In Encyclopedia of Immunology, 2nd ed.; Delves, P.J., Ed.; Elsevier: Oxford, UK, 1998; pp. 1458–1461. ISBN 978-0-12-226765-9. [Google Scholar]
- Malik, A.; Kanneganti, T.-D. Function and Regulation of IL-1α in Inflammatory Diseases and Cancer. Immunol. Rev. 2018, 281, 124–137. [Google Scholar] [CrossRef]
- Zhang, W.; Borcherding, N.; Kolb, R. IL-1 Signaling in Tumor Microenvironment. In Tumor Microenvironment: The Role of Interleukins—Part A; Birbrair, A., Ed.; Springer: Cham, Switzerland, 2020; pp. 1–23. ISBN 978-3-030-38315-2. [Google Scholar]
- Yang, Z.-H.; Dai, Q.; Zhong, L.; Zhang, X.; Guo, Q.-X.; Li, S.-N. Association of IL-1 Polymorphisms and IL-1 Serum Levels with Susceptibility to Nasopharyngeal Carcinoma. Mol. Carcinog. 2011, 50, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-T.; Liu, M.-Y.; Tsai, C.-H.; Yeh, T.-H. Upregulation of Interleukin-1 by Epstein-Barr Virus Latent Membrane Protein 1 and Its Possible Role in Nasopharyngeal Carcinoma Cell Growth. Head Neck 2010, 32, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Haemmerle, M.; Stone, R.L.; Menter, D.G.; Afshar-Kharghan, V.; Sood, A.K. The platelet lifeline to cancer: Challenges and opportunities. Cancer Cell 2018, 33, 965–983. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.E.R.; Ukoumunne, O.C.; Shephard, E.; Hamilton, W. How Useful Is Thrombocytosis in Predicting an Underlying Cancer in Primary Care? A Systematic Review. Fam. Pract. 2017, 34, 4–10. [Google Scholar] [CrossRef]
- Lai, P.M.; Chan, K.M. Roles of Histone H2A Variants in Cancer Development, Prognosis, and Treatment. Int. J. Mol. Sci. 2024, 25, 3144. [Google Scholar] [CrossRef]
- Valdés-Mora, F.; Song, J.Z.; Statham, A.L.; Strbenac, D.; Robinson, M.D.; Nair, S.S.; Patterson, K.I.; Tremethick, D.J.; Stirzaker, C.; Clark, S.J. Acetylation of H2A.Z Is a Key Epigenetic Modification Associated with Gene Deregulation and Epigenetic Remodeling in Cancer. Genome Res. 2012, 22, 307–321. [Google Scholar] [CrossRef]
- Rasmussen, L.; Christensen, I.J.; Herzog, M.; Micallef, J.; Nielsen, H.J.; Danish Collaborative Group on Early Detection of Colorectal Cancer. Circulating Cell-Free Nucleosomes as Biomarkers for Early Detection of Colorectal Cancer. Oncotarget 2018, 9, 10247–10258. [Google Scholar] [CrossRef]
- Chen, Q.; Chu, L.; Li, X.; Li, H.; Zhang, Y.; Cao, Q.; Zhuang, Q. Investigation of an FGFR-Signaling-Related Prognostic Model and Immune Landscape in Head and Neck Squamous Cell Carcinoma. Front. Cell Dev. Biol. 2022, 9, 801715. [Google Scholar] [CrossRef]
- Wong, Y.-L.; Ramanathan, A.; Yuen, K.M.; Mustafa, W.M.W.; Abraham, M.T.; Tay, K.K.; Rahman, Z.A.A.; Chen, Y. Comparative Sera Proteomics Analysis of Differentially Expressed Proteins in Oral Squamous Cell Carcinoma. PeerJ 2021, 9, e11548. [Google Scholar] [CrossRef]
- Expression of IGHG2 in Cancer-Summary-The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000211893-IGHG2/cancer (accessed on 17 May 2025).
- Wu, Y.; Liu, L.; Li, Z.; Zhang, T.; Wang, Q.; Cheng, M. A Risk Model Based on Ferroptosis-Related Genes OSMR, G0S2, IGFBP6, IGHG2, and FMOD Predicts Prognosis in Glioblastoma Multiforme. CNS Neurosci. Ther. 2025, 31, e70161. [Google Scholar] [CrossRef]
- Ma, R.; Ye, X.; Cheng, H.; Ma, Y.; Cui, H.; Chang, X. PRSS3 Expression Is Associated with Tumor Progression and Poor Prognosis in Epithelial Ovarian Cancer. Gynecol. Oncol. 2015, 137, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Hu, Y.-L.; Feng, Y.; Guo, Y.-B.; Liu, Y.-F.; Mao, Q.-S.; Xue, W.-J. High-Level Expression of PRSS3 Correlates with Metastasis and Poor Prognosis in Patients with Gastric Cancer. J. Surg. Oncol. 2019, 119, 1108–1121. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Gao, X.; Huang, H.; Lu, S.; Cai, Y.; Hua, Y.; Liu, Y.; Zhang, J. PRSS3 Is a Prognostic Marker in Invasive Ductal Carcinoma of the Breast. Oncotarget 2017, 8, 21444–21453. [Google Scholar] [CrossRef]
- Jiang, G.; Cao, F.; Ren, G.; Gao, D.; Bhakta, V.; Zhang, Y.; Cao, H.; Dong, Z.; Zang, W.; Zhang, S.; et al. PRSS3 Promotes Tumour Growth and Metastasis of Human Pancreatic Cancer. Gut 2010, 59, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Sanjurjo, L.; Aran, G.; Roher, N.; Valledor, A.F.; Sarrias, M.-R. AIM/CD5L: A Key Protein in the Control of Immune Homeostasis and Inflammatory Disease. J. Leukoc. Biol. 2015, 98, 173–184. [Google Scholar] [CrossRef]
- Choi, E.-S.; Faruque, H.A.; Kim, J.-H.; Kim, K.J.; Choi, J.E.; Kim, B.A.; Kim, B.; Kim, Y.J.; Woo, M.H.; Park, J.Y.; et al. CD5L as an Extracellular Vesicle-Derived Biomarker for Liquid Biopsy of Lung Cancer. Diagnostics 2021, 11, 620. [Google Scholar] [CrossRef]
- Reffai, A.; Hori, M.; Adusumilli, R.; Bermudez, A.; Bouzoubaa, A.; Pitteri, S.; Bennani Mechita, M.; Mallick, P. A Proteomic Analysis of Nasopharyngeal Carcinoma in a Moroccan Subpopulation. Cancers 2024, 16, 3282. [Google Scholar] [CrossRef] [PubMed]
- Feghali, C.A.; Wright, T.M. Cytokines in Acute and Chronic Inflammation. Front. Biosci. Landmark 1997, 2, 12–26. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, J.; Qu, C.; Peng, Y.; Lei, J.; Li, K.; Zong, B.; Sun, L.; Liu, S. Overexpression of SERPINA3 Promotes Tumor Invasion and Migration, Epithelial-Mesenchymal-Transition in Triple-Negative Breast Cancer Cells. Breast Cancer 2021, 28, 859–873. [Google Scholar] [CrossRef]
- Yang, G.-D.; Yang, X.-M.; Lu, H.; Ren, Y.; Ma, M.-Z.; Zhu, L.-Y.; Wang, J.-H.; Song, W.-W.; Zhang, W.-M.; Zhang, R.; et al. SERPINA3 Promotes Endometrial Cancer Cells Growth by Regulating G2/M Cell Cycle Checkpoint and Apoptosis. Int. J. Clin. Exp. Pathol. 2014, 7, 1348–1358. [Google Scholar]
- Liu, R.; Zhou, M.; Zhang, P.; Zhao, Y.; Zhang, Y. Cell Proliferation and Invasion Is Promoted by circSERPINA3 in Nasopharyngeal Carcinoma by Regulating miR-944/MDM2 Axis. J. Cancer 2020, 11, 3910–3918. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, D.; Roksandić-Milenković, M.; Kotur-Stevuljević, J.; Ćeriman, V.; Vukanić, I.; Samardžić, N.; Popević, S.; Ilić, B.; Gajić, M.; Simon, M.; et al. Soluble sPD-L1 and Serum Amyloid A1 as Potential Biomarkers for Lung Cancer. J. Med. Biochem. 2019, 38, 332–341. [Google Scholar] [CrossRef]
- Takehara, M.; Sato, Y.; Kimura, T.; Noda, K.; Miyamoto, H.; Fujino, Y.; Miyoshi, J.; Nakamura, F.; Wada, H.; Bando, Y.; et al. Cancer-Associated Adipocytes Promote Pancreatic Cancer Progression through SAA1 Expression. Cancer Sci. 2020, 111, 2883–2894. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, Y.; Wan, Q.; Xiao, C.; Guo, Z.; Du, X.; Hu, Y.; Zheng, A.; Cao, Z. Identification of SAA1 as a Novel Metastasis Marker in Ovarian Cancer and Development of a Graphene-Based Detection Platform for Early Assessment. J. Cancer Res. Clin. Oncol. 2023, 149, 16391–16406. [Google Scholar] [CrossRef]
- Lung, H.L.; Man, O.Y.; Yeung, M.C.; Ko, J.M.Y.; Cheung, A.K.L.; Law, E.W.L.; Yu, Z.; Shuen, W.H.; Tung, E.; Chan, S.H.K.; et al. SAA1 Polymorphisms Are Associated with Variation in Antiangiogenic and Tumor-Suppressive Activities in Nasopharyngeal Carcinoma. Oncogene 2015, 34, 878–889. [Google Scholar] [CrossRef]
- Li, J.; Lai, C.; Peng, S.; Chen, H.; Zhou, L.; Chen, Y.; Chen, S. The Prognostic Value of Integration of Pretreatment Serum Amyloid A (SAA)–EBV DNA (S-D) Grade in Patients with Nasopharyngeal Carcinoma. Clin. Transl. Med. 2020, 9, 2. [Google Scholar] [CrossRef]
- Hart, P.C.; Rajab, I.M.; Alebraheem, M.; Potempa, L.A. C-Reactive Protein and Cancer—Diagnostic and Therapeutic Insights. Front. Immunol. 2020, 11, 595835. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Ma, Z.; Zhang, X.; Hang, D.; Yin, R.; Feng, J.; Xu, L.; Shen, H. C-Reactive Protein and Cancer Risk: A Pan-Cancer Study of Prospective Cohort and Mendelian Randomization Analysis. BMC Med. 2022, 20, 301. [Google Scholar] [CrossRef]
- Fang, Y.; Xu, C.; Wu, P.; Zhang, L.-H.; Li, D.-W.; Sun, J.-H.; Li, W.-F.; Liao, Z.-S. Prognostic Role of C-Reactive Protein in Patients with Nasopharyngeal Carcinoma: A Meta-Analysis and Literature Review. Medicine 2017, 96, e8463. [Google Scholar] [CrossRef]
- Chen, R.; Zhou, Y.; Yuan, Y.; Zhang, Q.; He, S.; Chen, Y.; Ren, Y. Effect of CRP and Kinetics of CRP in Prognosis of Nasopharyngeal Carcinoma. Front. Oncol. 2019, 9, 89. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, G.-Q.; Liu, X.; Chen, L.; Li, W.-F.; Tang, L.-L.; Liu, Q.; Sun, Y.; Ma, J. Exploration and Validation of C-Reactive Protein/Albumin Ratio as a Novel Inflammation-Based Prognostic Marker in Nasopharyngeal Carcinoma. J. Cancer 2016, 7, 1406. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhao, K.; Ding, X.; Jiang, H.; Lu, H. Prognostic Significance of Hematological Markers for Patients with Nasopharyngeal Carcinoma: A Meta-Analysis. J. Cancer 2019, 10, 2568–2577. [Google Scholar] [CrossRef] [PubMed]
- Ricklin, D.; Hajishengallis, G.; Yang, K.; Lambris, J.D. Complement: A Key System for Immune Surveillance and Homeostasis. Nat. Immunol. 2010, 11, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Ajona, D.; Ortiz-Espinosa, S.; Pio, R. Complement Anaphylatoxins C3a and C5a: Emerging Roles in Cancer Progression and Treatment. Semin. Cell Dev. Biol. 2019, 85, 153–163. [Google Scholar] [CrossRef]
- Gann, P.H.; Hennekens, C.H.; Ma, J.; Longcope, C.; Stampfer, M.J. Prospective Study of Sex Hormone Levels and Risk of Prostate Cancer. JNCI J. Natl. Cancer Inst. 1996, 88, 1118–1126. [Google Scholar] [CrossRef]
- Fortunati, N.; Catalano, M.G.; Boccuzzi, G.; Frairia, R. Sex Hormone-Binding Globulin (SHBG), Estradiol and Breast Cancer. Mol. Cell Endocrinol. 2010, 316, 86–92. [Google Scholar] [CrossRef]
- Qi, X.; Li, X.; Sun, X. Reduced Expression of Polymeric Immunoglobulin Receptor (pIgR) in Nasopharyngeal Carcinoma and Its Correlation with Prognosis. Tumor Biol. 2016, 37, 11099–11104. [Google Scholar] [CrossRef]
- Chang, Y.; Lee, T.-C.; Li, J.-C.; Lai, T.-L.; Chua, H.-H.; Chen, C.-L.; Doong, S.-L.; Chou, C.-K.; Sheen, T.-S.; Tsai, C.-H. Differential Expression of Osteoblast-Specific Factor 2 and Polymeric Immunoglobulin Receptor Genes in Nasopharyngeal Carcinoma. Head. Neck 2005, 27, 873–882. [Google Scholar] [CrossRef]
- Ocak, S.; Pedchenko, T.V.; Chen, H.; Harris, F.T.; Qian, J.; Polosukhin, V.; Pilette, C.; Sibille, Y.; Gonzalez, A.L.; Massion, P.P. Loss of Polymeric Immunoglobulin Receptor Expression Is Associated with Lung Tumourigenesis. Eur. Respir. J. 2012, 39, 1171–1180. [Google Scholar] [CrossRef]
- Fristedt, R.; Elebro, J.; Gaber, A.; Jonsson, L.; Heby, M.; Yudina, Y.; Nodin, B.; Uhlén, M.; Eberhard, J.; Jirström, K. Reduced Expression of the Polymeric Immunoglobulin Receptor in Pancreatic and Periampullary Adenocarcinoma Signifies Tumour Progression and Poor Prognosis. PLoS ONE 2014, 9, e112728. [Google Scholar] [CrossRef]
- Chae, J.; Choi, J.; Chung, J. Polymeric Immunoglobulin Receptor (pIgR) in Cancer. J. Cancer Res. Clin. Oncol. 2023, 149, 17683–17690. [Google Scholar] [CrossRef] [PubMed]
- Avisha, M.; Pelupessy, N.U.; Rahman, A.; Rauf, S.; Rakhmah, N.; Hamid, F. Pre-Treatment Inflammatory and Immune System Parameters Predicting Cervical Cancer Metastasis. Turk. J. Obs. Gynecol. 2023, 20, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qi, G.; Liu, Y. Proteomics Analysis of Serum from Thymoma Patients. Sci. Rep. 2023, 13, 5117. [Google Scholar] [CrossRef]
- Xu, R.; Shen, J.; Song, Y.; Lu, J.; Liu, Y.; Cao, Y.; Wang, Z.; Zhang, J. Exploration of the Application Potential of Serum Multi-Biomarker Model in Colorectal Cancer Screening. Sci. Rep. 2024, 14, 10127. [Google Scholar] [CrossRef]
- Kang, K.N.; Koh, E.Y.; Jang, J.Y.; Kim, C.W. Multiple Biomarkers Are More Accurate than a Combination of Carbohydrate Antigen 125 and Human Epididymis Protein 4 for Ovarian Cancer Screening. Obs. Gynecol. Sci. 2022, 65, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Baichan, P.; Naicker, P.; Augustine, T.N.; Smith, M.; Candy, G.; Devar, J.; Nweke, E.E. Proteomic Analysis Identifies Dysregulated Proteins and Associated Molecular Pathways in a Cohort of Gallbladder Cancer Patients of African Ancestry. Clin. Proteom. 2023, 20, 8. [Google Scholar] [CrossRef]
- Pierce Albumin/IgG Removal Kit. Available online: https://assets.thermofisher.com/TFS-Assets/LSG/manuals/MAN0017001_2161499_AlbuminIgGRemoval_PI.pdf (accessed on 11 April 2024).
- Atallah, M.; Flory, M.R.; Mallick, P. A Robust Protocol for Protein Extraction and Digestion. In Proteomics; Humana Press: New York, NY, USA, 2017; pp. 1–10. ISBN 978-1-4939-6747-6. [Google Scholar]
- Pierce High pH Reversed-Phase Peptide Fractionation Kit. Available online: https://assets.thermofisher.com/TFS-Assets/LSG/manuals/84868_highph_rp_peptidefract_UG.pdf (accessed on 11 April 2024).
- Tyanova, S.; Temu, T.; Cox, J. The MaxQuant Computational Platform for Mass Spectrometry-Based Shotgun Proteomics. Nat. Protoc. 2016, 11, 2301–2319. [Google Scholar] [CrossRef]
- Kohler, D.; Staniak, M.; Tsai, T.-H.; Huang, T.; Shulman, N.; Bernhardt, O.M.; MacLean, B.X.; Nesvizhskii, A.I.; Reiter, L.; Sabido, E.; et al. MSstats Version 4.0: Statistical Analyses of Quantitative Mass Spectrometry-Based Proteomic Experiments with Chromatography-Based Quantification at Scale. J. Proteome Res. 2023, 22, 1466–1482. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A Web Server for Functional Enrichment Analysis and Functional Annotation of Gene Lists (2021 Update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]

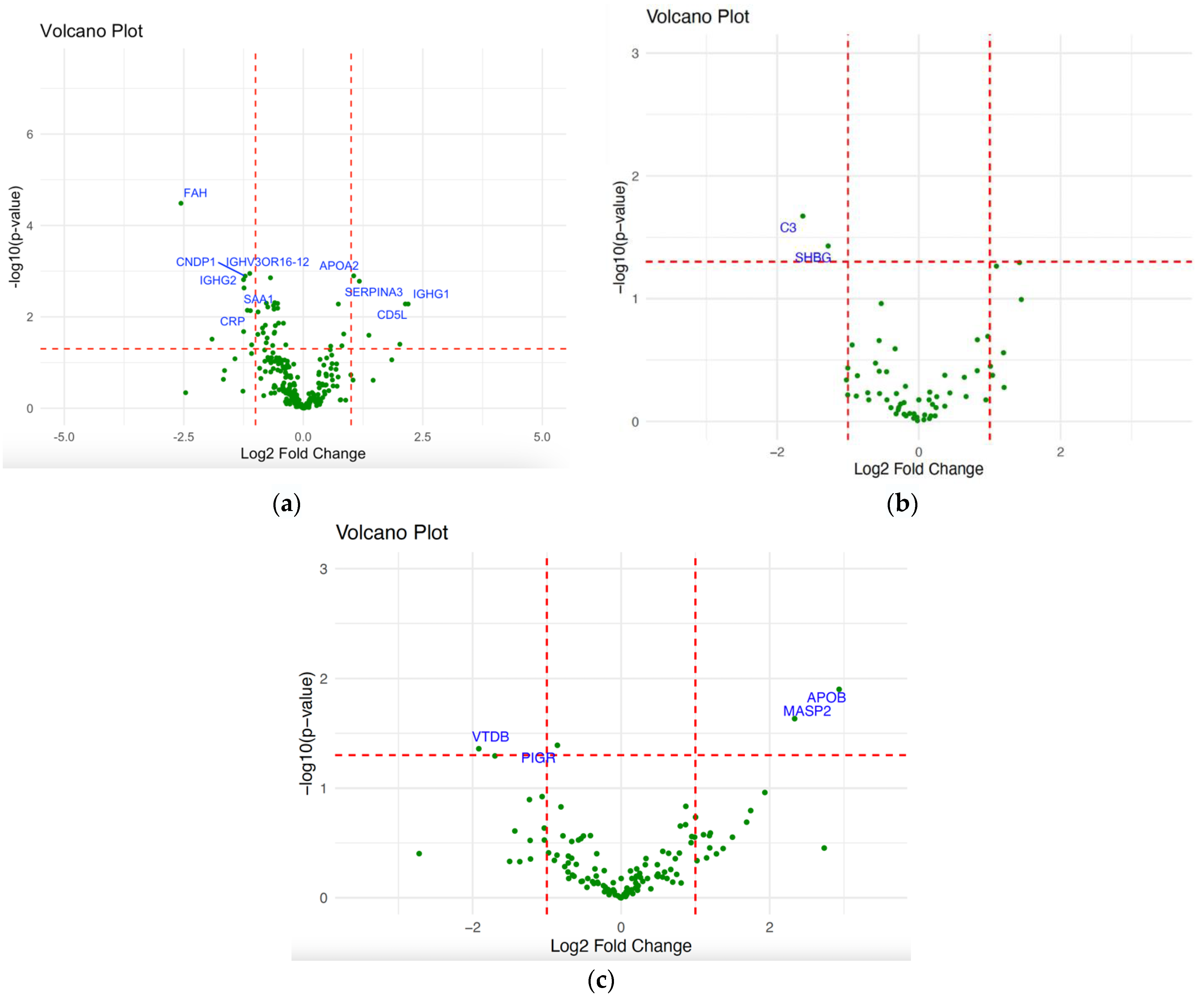
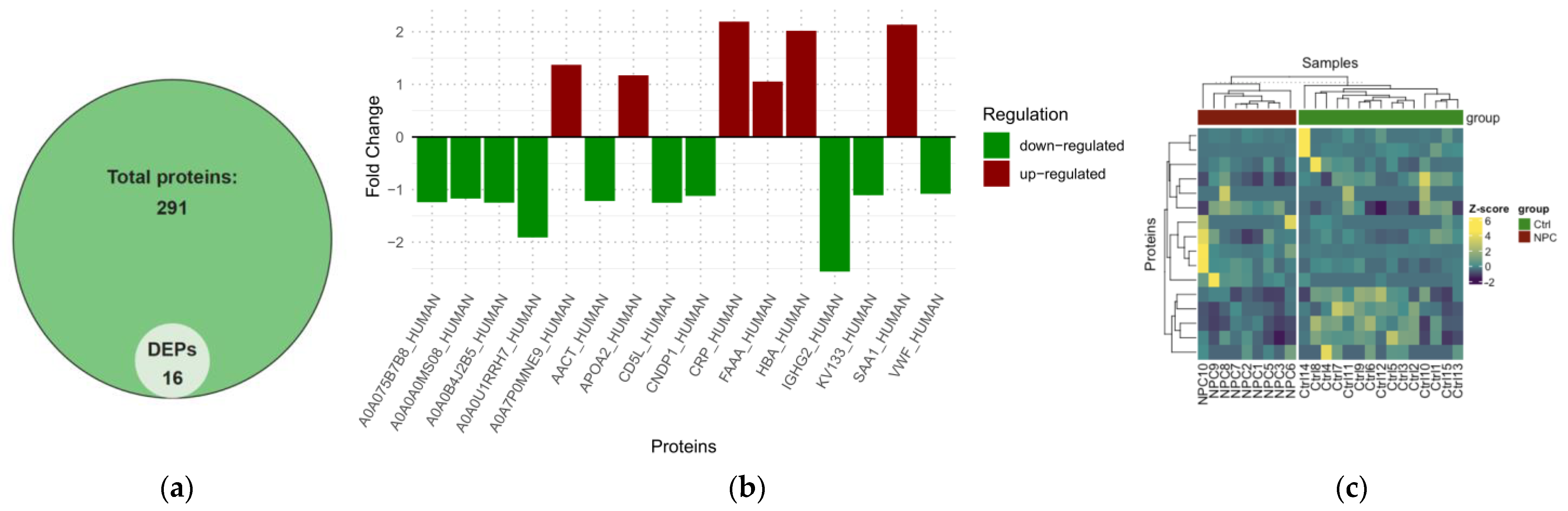

| DEPs | Log2FC | p-Value |
|---|---|---|
| IGHG2 | −2.5596411 | 3.27 × 10−5 |
| CNDP1 | −1.1208327 | 0.0011 |
| FAH | 1.05426614 | 0.0012 |
| SERPINA3 | −1.2171742 | 0.0013 |
| CD5L | −1.2504989 | 0.0015 |
| APOA2 | 1.17201774 | 0.0016 |
| IGHV3OR16-12 | −1.2401787 | 0.0023 |
| SAA1 | 2.13436008 | 0.0052 |
| CRP | 2.19295647 | 0.0053 |
| IGHG1 | −1.170474 | 0.0072 |
| DEPs | Plasma p-Value | Plasma Condition | Tissue Condition |
|---|---|---|---|
| IGHG2 | 3.27 × 10−5 | NPC vs. controls | NPC vs. controls and NPC male vs. female |
| SERPINA3 | 0.0013 | NPC vs. controls | NPC vs. controls and NPC clusters |
| APOA2 | 0.0016 | NPC vs. controls | NPC vs. controls and NPC clusters |
| FAH | 0.0013 | NPC vs. controls | NPC vs. controls |
| HBA | 0.0397 | NPC vs. controls | NPC vs. controls |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reffai, A.; Hori, M.; Adusumilli, R.; Bermudez, A.; Haddad, H.; Tawfiq, N.; Pitteri, S.; Bennani Mechita, M.; Mallick, P. Integrated Plasma and Tumor Proteomics of Nasopharyngeal Carcinoma in a Moroccan Cohort. Int. J. Mol. Sci. 2025, 26, 5771. https://doi.org/10.3390/ijms26125771
Reffai A, Hori M, Adusumilli R, Bermudez A, Haddad H, Tawfiq N, Pitteri S, Bennani Mechita M, Mallick P. Integrated Plasma and Tumor Proteomics of Nasopharyngeal Carcinoma in a Moroccan Cohort. International Journal of Molecular Sciences. 2025; 26(12):5771. https://doi.org/10.3390/ijms26125771
Chicago/Turabian StyleReffai, Ayman, Michelle Hori, Ravali Adusumilli, Abel Bermudez, Houssam Haddad, Nezha Tawfiq, Sharon Pitteri, Mohcine Bennani Mechita, and Parag Mallick. 2025. "Integrated Plasma and Tumor Proteomics of Nasopharyngeal Carcinoma in a Moroccan Cohort" International Journal of Molecular Sciences 26, no. 12: 5771. https://doi.org/10.3390/ijms26125771
APA StyleReffai, A., Hori, M., Adusumilli, R., Bermudez, A., Haddad, H., Tawfiq, N., Pitteri, S., Bennani Mechita, M., & Mallick, P. (2025). Integrated Plasma and Tumor Proteomics of Nasopharyngeal Carcinoma in a Moroccan Cohort. International Journal of Molecular Sciences, 26(12), 5771. https://doi.org/10.3390/ijms26125771







