FMR1 Allelic Complexity and IVF Fertilization Success: Limitations and Future Perspectives
Abstract
1. Introduction
2. Results
2.1. Demographic and Clinical Characteristics of the Study Cohort
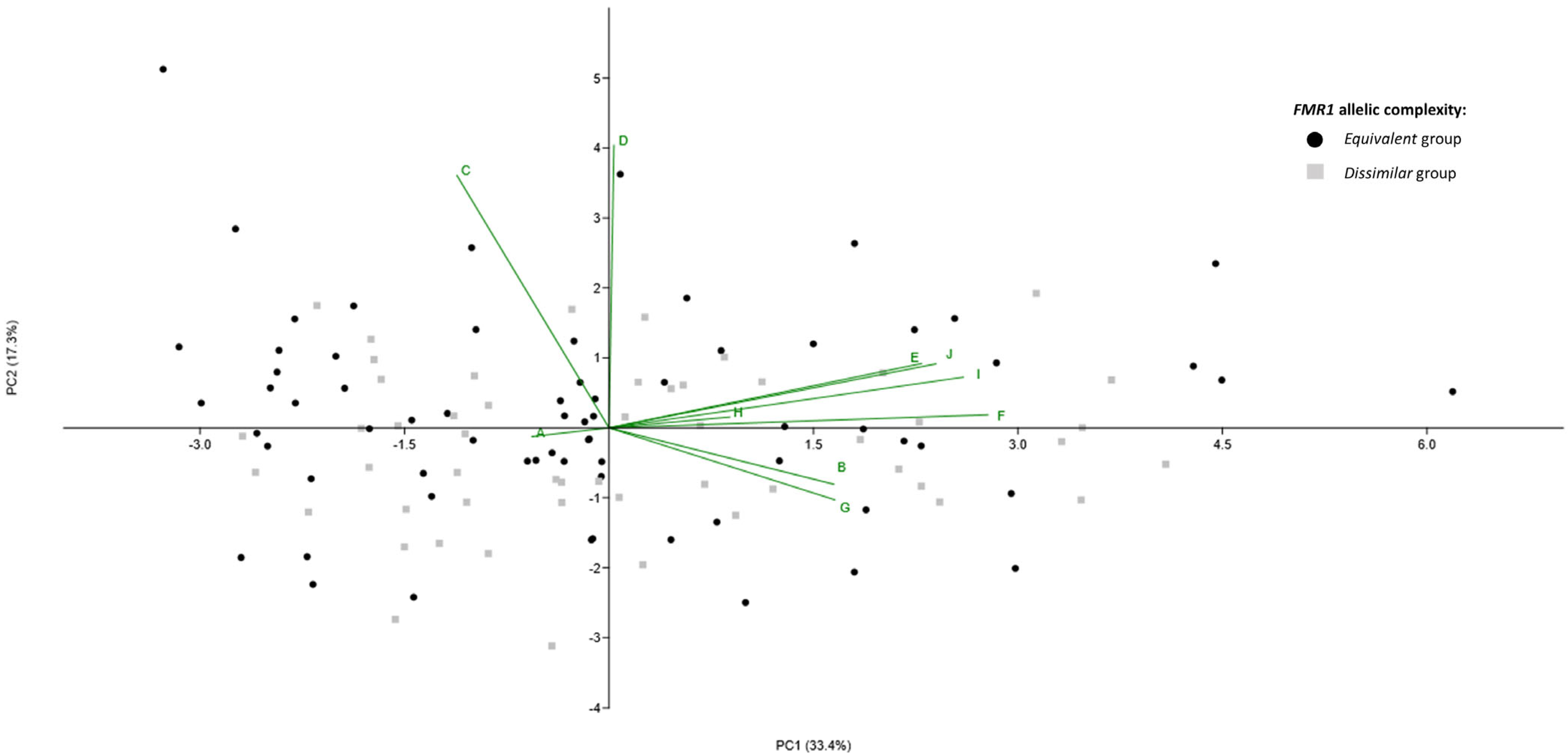
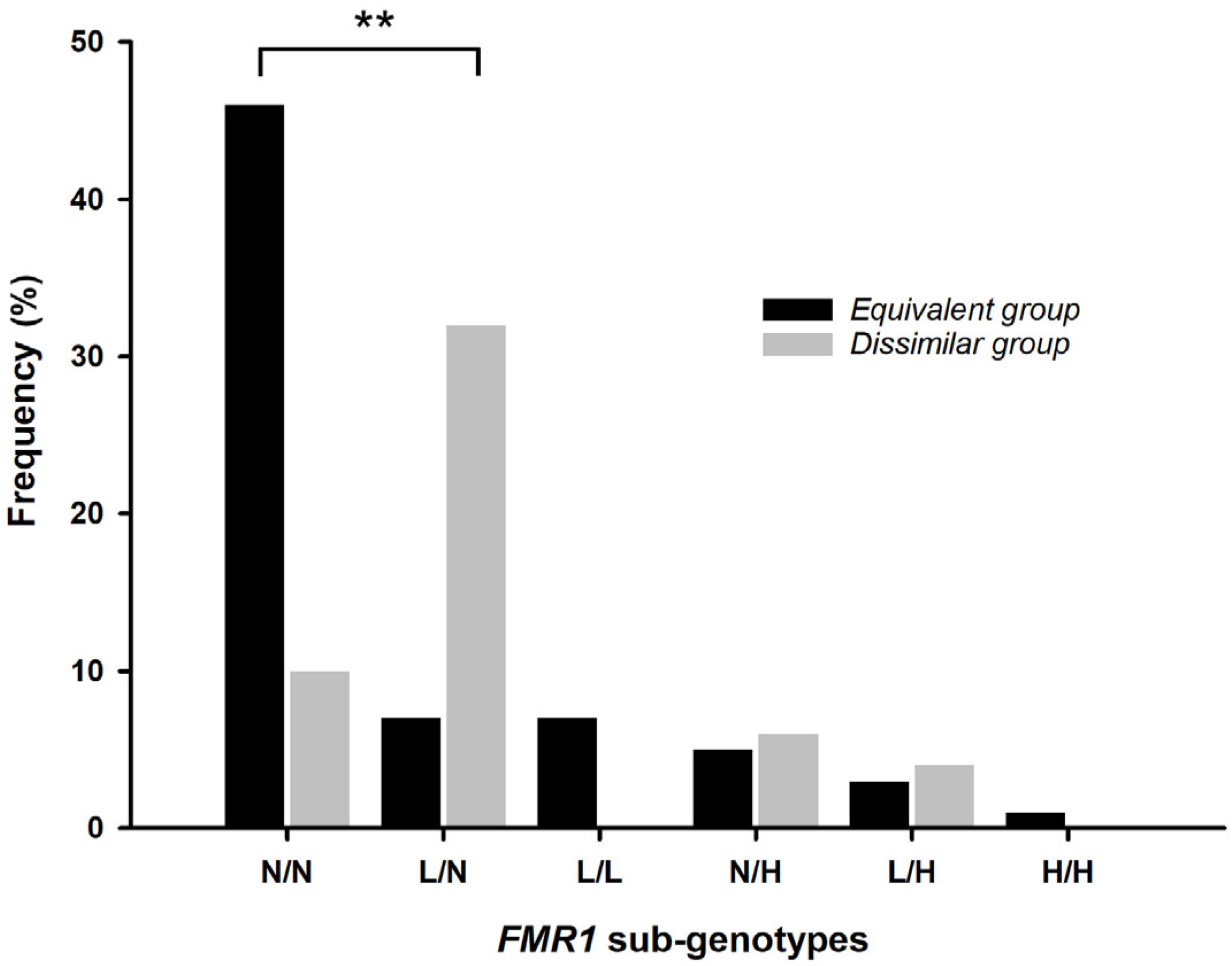
2.2. FMR1 Gene Repeat Region Characterization
2.3. FMR1 Allelic Scores Combination and Comparison of Mathematical Models
2.4. Markers of Ovarian Reserve and IVF Outcomes According to Stratification of FMR1 Allelic Complexities
2.5. Association of FMR1 Allelic Complexity with Markers of Ovarian Reserve and with IVF Outcomes
3. Discussion
4. Materials and Methods
4.1. Study Design and Participants
4.2. Demographic and Clinical Data
4.3. FMR1 CGG Repeat Region Analysis
4.3.1. Total CGG Repeat Length
4.3.2. AGG Interspersion Pattern
4.3.3. FMR1 Allelic Complexity
(Number of CGG repeats between the 1st and 2nd AGG × 42 − 1) +
(Number of CGG repeats after the last AGG × 42)
4.3.4. FMR1 Sub-Genotypes
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFC | Antral follicle count |
| AGG | Adenine-Guanine-Guanine |
| AMH | Anti-Müllerian hormone |
| ANCOVA | Analysis of covariance |
| CGG | Cytosine-Guanine-Guanine |
| DOR | Diminished ovarian reserve |
| FMR1 | Fragile X messenger ribonucleoprotein 1 |
| FSH | Follicle-stimulating hormone |
| FXPOI | Fragile X-associated primary ovarian insufficiency |
| IVF | In vitro fertilization |
| ICSI | Intracytoplasmic sperm injection |
| MII | Metaphase II |
| PC1 | First principal component |
| PC2 | Second principal component |
| PCR | Polymerase chain reaction |
| PM | Premutation |
| POI | Primary ovarian insufficiency |
| PCA | Principal component analysis |
| SD | Standard deviation |
| UTR | Untranslated region |
| 2PN | Two pronuclei |
References
- Ma, Y.; Wei, X.; Pan, H.; Wang, S.; Wang, X.; Liu, X.; Zou, L.; Wang, X.; Wang, X.; Yang, H.; et al. The prevalence of CGG repeat expansion mutation in FMR1 gene in the northern Chinese women of reproductive age. BMC Med. Genet. 2019, 20, 81. [Google Scholar] [CrossRef] [PubMed]
- Man, L.; Lekovich, J.; Rosenwaks, Z.; Gerhardt, J. Fragile X-Associated Diminished Ovarian Reserve and Primary Ovarian Insufficiency from Molecular Mechanisms to Clinical Manifestations. Front. Mol. Neurosci. 2017, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, A.C.; Bailey, D.B.; Berry-Kravis, E.; Greenberg, J.; Losh, M.; Mailick, M.; Milà, M.; Olichney, J.M.; Rodriguez-Revenga, L.; Sherman, S.; et al. Associated features in females with an FMR1 premutation. J. Neurodev. Disord. 2014, 6, 30. [Google Scholar] [CrossRef]
- Allen, E.G.; Glicksman, A.; Tortora, N.; Charen, K.; He, W.; Amin, A.; Hipp, H.; Shubeck, L.; Nolin, S.L.; Sherman, S.L. FXPOI: Pattern of AGG interruptions does not show an association with age at amenorrhea among women with a premutation. Front. Genet. 2018, 9, 292. [Google Scholar] [CrossRef]
- Hoyos, L.R.; Thakur, M. Fragile X premutation in women: Recognizing the health challenges beyond primary ovarian insufficiency. J. Assist. Reprod. Genet. 2017, 34, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.G.; Charen, K.; Hipp, H.S.; Shubeck, L.; Amin, A.; He, W.; Nolin, S.L.; Glicksman, A.; Tortora, N.; McKinnon, B.; et al. Refining the risk for fragile X–associated primary ovarian insufficiency (FXPOI) by FMR1 CGG repeat size. Genet. Med. 2021, 23, 1648–1655. [Google Scholar] [CrossRef]
- Fink, D.A.; Nelson, L.M.; Pyeritz, R.; Johnson, J.; Sherman, S.L.; Cohen, Y.; Elizur, S.E. Fragile X Associated Primary Ovarian Insufficiency (FXPOI): Case Report and Literature Review. Front. Genet. 2018, 9, 529. [Google Scholar] [CrossRef]
- Tassone, F.; Protic, D.; Allen, E.G.; Archibald, A.D.; Baud, A.; Brown, T.W.; Budimirovic, D.B.; Cohen, J.; Dufour, B.; Eiges, R.; et al. Insight and Recommendations for Fragile X-Premutation-Associated Conditions from the Fifth International Conference on FMR1 Premutation. Cells 2023, 12, 2330. [Google Scholar] [CrossRef]
- Biancalana, V.; Glaeser, D.; McQuaid, S.; Steinbach, P. EMQN best practice guidelines for the molecular genetic testing and reporting of fragile X syndrome and other fragile X-associated disorders. Eur. J. Hum. Genet. 2015, 23, 417–425. [Google Scholar] [CrossRef]
- Karimov, C.B.; Moragianni, V.A.; Cronister, A.; Srouji, S.; Petrozza, J.; Racowsky, C.; Ginsburg, E.; Thornton, K.L.; Welt, C.K. Increased frequency of occult fragile X-associated primary ovarian insufficiency in infertile women with evidence of impaired ovarian function. Hum. Reprod. 2011, 26, 2077–2083. [Google Scholar] [CrossRef]
- Bennett, C.E.; Conway, G.S.; MacPherson, J.N.; Jacobs, P.A.; Murray, A. Intermediate sized CGG repeats are not a common cause of idiopathic premature ovarian failure. Hum. Reprod. 2010, 25, 1335–1338. [Google Scholar] [CrossRef] [PubMed]
- Gleicher, N.; Weghofer, A.; Oktay, K.; Barad, D.H. Can the FMR1 (Fragile X) Gene Serve As Predictor of Response to Ovarian Stimulation? Reprod. Sci. 2009, 16, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Kushnir, V.A.; Yu, Y.; Barad, D.H.; Weghofer, A.; Himaya, E.; Lee, H.J.; Wu, Y.G.; Shohat-Tal, A.; Lazzaroni-Tealdi, E.; Gleicher, N. Utilizing FMR1 gene mutations as predictors of treatment success in human in vitro fertilization. PLoS ONE 2014, 9, e102274. [Google Scholar] [CrossRef] [PubMed]
- Gleicher, N.; Yu, Y.; Himaya, E.; Barad, D.H.; Weghofer, A.; Wu, Y.; Albertini, D.F.; Wang, V.Q.; Kushnir, V.A. Early decline in functional ovarian reserve in young women with low (CGGn<26) FMR1 gene alleles. Transl. Res. 2015, 166, 502–507. [Google Scholar]
- Lu, C.L.; Li, R.; Chen, X.N.; Xu, Y.Y.; Yan, L.Y.; Yan, J.; Zhang, Y.Y.; Jin, H.Y.; Zhang, W.X.; Qiao, J.; et al. The ‘normal’ range of FMR1 triple CGG repeats may be associated with primary ovarian insufficiency in China. Reprod. Biomed. Online 2017, 34, 175–180. [Google Scholar] [CrossRef]
- Rehnitz, J.; Alcoba, D.D.; Brum, I.S.; Dietrich, J.E.; Youness, B.; Hinderhofer, K.; Messmer, B.; Freis, A.; Strowitzki, T.; Germeyer, A. FMR1 expression in human granulosa cells increases with exon 1 CGG repeat length depending on ovarian reserve. Reprod. Biol. Endocrinol. 2018, 16, 65. [Google Scholar] [CrossRef]
- Banks, N.; Patounakis, G.; Devine, K.; DeCherney, A.H.; Widra, E.; Levens, E.D.; Whitcomb, B.W.; Hill, M.J. Is FMR1 CGG repeat length a predictor of in vitro fertilization stimulation response or outcome? Fertil. Steril. 2016, 105, 1537–1546.e8. [Google Scholar] [CrossRef]
- Gleicher, N.; Kim, A.; Weghofer, A.; Shohat-Tal, A.; Lazzaroni, E.; Lee, H.J.; Barad, D.H. Starting and resulting testosterone levels after androgen supplementation determine at all ages in vitro fertilization (IVF) pregnancy rates in women with diminished ovarian reserve (DOR). J. Assist. Reprod. Genet. 2013, 30, 49–62. [Google Scholar] [CrossRef]
- Lledo, B.; Guerrero, J.; Ortiz, J.A.; Morales, R.; Ten, J.; Llacer, J.; Gimenez, J.; Bernabeu, R. Intermediate and normal sized CGG repeat on the FMR1 gene does not negatively affect donor ovarian response. Hum. Reprod. 2012, 27, 609–614. [Google Scholar] [CrossRef][Green Version]
- Morin, S.J.; Tiegs, A.W.; Franasiak, J.M.; Juneau, C.R.; Hong, K.H.; Werner, M.D.; Zhan, Y.; Landis, J.; Scott, R.T. FMR1 gene CGG repeat variation within the normal range is not predictive of ovarian response in IVF cycles. Reprod. Biomed. Online 2016, 32, 496–502. [Google Scholar] [CrossRef]
- Rodrigues, B.; Vale-Fernandes, E.; Maia, N.; Santos, F.; Marques, I.; Santos, R.; Nogueira, A.J.A.; Jorge, P. Development and Validation of a Mathematical Model to Predict the Complexity of FMR1 Allele Combinations. Front. Genet. 2020, 11, 557147. [Google Scholar] [CrossRef] [PubMed]
- Tabolacci, E.; Nobile, V.; Pucci, C.; Chiurazzi, P. Mechanisms of the FMR1 Repeat Instability: How Does the CGG Sequence Expand? Int. J. Mol. Sci. 2022, 23, 5425. [Google Scholar] [CrossRef] [PubMed]
- Eichler, E.E.; Holden, J.J.A.; Popovich, B.W.; Reiss, A.L.; Snow, K.; Thibodeu, S.N.; Richards, C.S.; Ward, P.A.; Nelson, D.L. Length of uninterrupted CGG repeats determines instability in the FMR1 gene. Nat. Genet. 1994, 8, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Lekovich, J.; Man, L.; Xu, K.; Canon, C.; Lilienthal, D.; Stewart, J.D.; Pereira, N.; Rosenwaks, Z.; Gerhardt, J. CGG repeat length and AGG interruptions as indicators of fragile X–associated diminished ovarian reserve. Genet. Med. 2018, 20, 957–964. [Google Scholar] [CrossRef]
- Gleicher, N.; Weghofer, A.; Lee, I.H.; Barad, D.H. FMR1 genotype with autoimmunity-associated polycystic ovary-like phenotype and decreased pregnancy chance. PLoS ONE 2010, 5, e15303. [Google Scholar] [CrossRef]
- Eppig, J.J.; O’Brien, M.; Wigglesworth, K. Mammalian oocyte growth and development in vitro. Mol. Reprod. Dev. 1996, 44, 260–273. [Google Scholar] [CrossRef]
- Trebichalská, Z.; Kyjovská, D.; Kloudová, S.; Otevřel, P.; Hampl, A.; Holubcová, Z. Cytoplasmic maturation in human oocytes: An ultrastructural study. Biol. Reprod. 2021, 104, 106–116. [Google Scholar] [CrossRef]
- De Vos, A.; Van De Velde, H.; Joris, H.; Van Steirteghem, A. In-vitro matured metaphase-I oocytes have a lower fertilization rate but similar embryo quality as mature metaphase-II oocytes after intracytoplasmic sperm injection. Hum. Reprod. 1999, 14, 1859–1863. [Google Scholar] [CrossRef]
- Pastore, L.M.; Young, S.L.; Baker, V.L.; Karns, L.B.; Williams, C.D.; Silverman, L.M. Elevated Prevalence of 35-44 FMR1 Trinucleotide Repeats in Women With Diminished Ovarian Reserve. Reprod. Sci. 2012, 19, 1226–1231. [Google Scholar] [CrossRef]
- Barasoain, M.; Barrenetxea, G.; Huerta, I.; Télez, M.; Carrillo, A.; Pérez, C.; Criado, B.; Arrieta, I. Study of FMR1 gene association with ovarian dysfunction in a sample from the Basque Country. Gene 2013, 521, 145–149. [Google Scholar] [CrossRef]
- Vale-fernandes, E.; Moreira, M.V.; Rodrigues, B.; Pereira, S.; Leal, C. Anti-Müllerian hormone a surrogate of follicular fl uid oxidative stress in polycystic ovary syndrome ? Front. Cell Dev. Biol. 2024, 12, 1408879. [Google Scholar] [CrossRef] [PubMed]
- Gleicher, N.; Kushnir, V.A.; Weghofer, A.; Barad, D.H. How the FMR1 gene became relevant to female fertility and reproductive medicine. Front. Genet. 2014, 5, 284. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gleicher, N.; Weghofer, A.; Oktay, K.; Barad, D.H. Revelance of triple CGG repeats in the FMR1 gene to ovarian reserve. Acta Obstet. Gynecol. Scand. 2009, 88, 1024–1030. [Google Scholar] [CrossRef]
- Quilichini, J.; Perol, S.; Cuisset, L.; Grotto, S.; Fouveaut, C.; Barbot, J.C.; Verebi, C.; Jordan, P.; Héron, D.; Molina-Gomes, D.; et al. Stratification of the risk of ovarian dysfunction by studying the complexity of intermediate and premutation alleles of the FMR1 gene. Am. J. Med. Genet. Part A 2024, 194, e63479. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.C.V.; Trevisan, C.M.; Peluso, C.; Loureiro, F.A.; Dias, A.T.; Rincon, D.; Fonseca, F.L.A.; Christofolini, D.M.; Laganà, A.S.; Montagna, E.; et al. Low and High-Normal FMR1 Triplet Cytosine, Guanine Guanine Repeats Affect Ovarian Reserve and Fertility in Women Who Underwent In Vitro Fertilization Treatment? Results from a Cross-Sectional Study. DNA Cell Biol. 2024, 43, 414–424. [Google Scholar] [CrossRef]
- Vale-Fernandes, E.; Carrageta, D.F.; Moreira, M.V.; Guerra-Carvalho, B.; Rodrigues, B.; Sousa, D.; Brandão, R.; Leal, C.; Barreiro, M.; Tomé, A.; et al. Follicular fluid profiling unveils anti-Müllerian hormone alongside glycolytic and mitochondrial dysfunction as markers of polycystic ovary syndrome. Mol. Cell. Endocrinol. 2025, 602, 112536. [Google Scholar] [CrossRef]
- Tassone, F.; Iong, K.P.; Tong, T.H.; Lo, J.; Gane, L.W.; Berry-Kravis, E.; Nguyen, D.; Mu, L.Y.; Laffin, J.; Bailey, D.B.; et al. FMR1 CGG allele size and prevalence ascertained through newborn screening in the United States. Genome Med. 2012, 4, 100. [Google Scholar] [CrossRef]
- Seltzer, M.M.; Baker, M.W.; Hong, J.; Maenner, M.; Greenberg, J.; Mandel, D. Prevalence of CGG Expansions of the FMR1 Gene in a US Population-Based Sample. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2012, 159B, 589–597. [Google Scholar] [CrossRef]
- Pesso, R.; Berkenstadt, M.; Cuckle, H.; Gak, E.; Peleg, L.; Frydman, M.; Barkai, G. Screening for fragile X syndrome in women of reproductive age. Prenat. Diagn. 2000, 20, 611–614. [Google Scholar] [CrossRef]
- Bussani, C.; Papi, L.; Sestini, R.; Baldinotti, F.; Bucciantini, S.; Bruni, V.; Scarselli, G. Premature ovarian failure and fragile X premutation: A study on 45 women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 112, 189–191. [Google Scholar] [CrossRef]
- Rousseau, F.; Rouillard, P.; Morel, M.; Khandjian, E.W.; Morgan, K. Prevalence of Carriers of Premutation-Size Alleles of the FMRI Gene-and ImplicationsforthePopulationGeneticsofthe FragileX Syndrome. Am. J. Hum. Genet. 1995, 57, 1006–1018. [Google Scholar] [PubMed]
- Gleicher, N.; Weghofer, A.; Barad, D.H. Ovarian reserve determinations suggest new function of FMR1 (fragile X gene) in regulating ovarian ageing. Reprod. Biomed. Online 2010, 20, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Zar, J.H. Bioestatistical Analysis, 5th ed.; Prentice Hall. Inc.: Upper Saddle River, NJ, USA, 2010; Volume 5, ISBN 9781493906581. [Google Scholar]
- Hammer, Ø. PAleontological STatistics Version 4.16—Reference Manual. University of Oslo, Oslo, Norway. Available online: https://www.nhm.uio.no/english/research/resources/past/ (accessed on 5 April 2025).

| Study Cohort | ||
|---|---|---|
| Characteristics | Mean ± SD | Range |
| Age (years) | 34.7 ± 3.7 | 22–40 |
| Markers of ovarian reserve | ||
| Day 3 FSH (mUI/mL) | 8.4 ± 11.8 (n = 118) | 3.3–40.0 |
| AMH (ng/mL) | 3.1 ± 2.9 (n = 123) | 0.1–18.9 |
| AFC | 7.4 ± 3.5 (n = 76) | 1.0–16.0 |
| IVF outcomes | ||
| Response to ovarian stimulation | ||
| Total dose of gonadotrophins (IU/mL) | 26,582.0 ± 885.3 (n = 122) | 1025.0–7200.0 |
| Stimulation duration (days) | 10.2 ± 1.9 (n = 123) | 5.0–16.0 |
| Number of follicles on the trigger day | 7.2 ± 4.8 (0–21.0) (n = 122) | 0–21.0 |
| Number of retrieved oocytes | 11.7 ± 8.5 (n = 122) | 0–46.0 |
| Number of immature oocytes | 2.0 ± 2.2 (n = 120) | 0–10.0 |
| Number of aberrant oocytes | 0.8 ± 1.6 (n = 124) | 0–12.0 |
| Oocyte maturation | ||
| Number of injected MII oocytes | 8.1 ± 5.7 (n = 120) | 0–28.0 |
| Fertilization success | ||
| Number of 2PN oocytes | 5.3 ± 4.0 (n = 116) | 0–17.0 |
| Allele 1 (Shorter CGG Repeat Length) | Allele 2 (Longer CGG Repeat Length) | |
|---|---|---|
| Number of alleles | 121 | 121 |
| Allelic score | ||
| Mean ± SD | 125.5 ± 95.6 | 198.9 ± 135.4 |
| Median (range) | 61.0 (23–765) | 205.0 (23–829) |
| Most frequent (n, %) | 205 (34, 28.1) | 205 (46, 38.0) |
| 49 (26, 21.5) | 206 (11, 9.1) | |
| 189 (16, 13.2) | 201 (10, 8.3) |
| Equivalent Group (n = 69 a) | Dissimilar Group (n = 52 a) | p-Value b | |
|---|---|---|---|
| Age (years) | 34.7 ± 3.9 | 34.6 ± 3.3 | 0.550 |
| Markers of ovarian reserve | |||
| Day 3 FSH (mUI/mL) | 7.3 ± 2.3 (n = 65) | 9.8 ± 17.7 (n = 51) | 0.676 |
| AMH (ng/mL) | 2.9 ± 2.4 (n = 64) | 3.6 ± 3.4 (n = 46) | 0.238 |
| AFC c | 6.9 ± 3.3 (n = 40) | 7.9 ± 3.3 (n = 33) | 0.205 |
| IVF outcomes | |||
| Response to ovarian stimulation | |||
| Total dose of gonadotrophins (IU/mL) | 2811.8 ± 1032.9 (n = 68) | 2452.5 ± 620.5 (n = 51) | 0.075 |
| Stimulation duration (days) | 10.4 ± 2.0 (n = 68) | 9.9 ± 1.8 | 0.08 d |
| Number of follicles on the trigger day | 6.9 ± 4.7 (n = 68) | 7.6 ± 5.0 (n = 51) | 0.451 |
| Number of retrieved oocytes | 11.4 ± 9.0 (n = 68) | 12.0 ± 7.6 (n = 51) | 0.411 |
| Number of immature oocytes | 1.9 ± 2.0 (n = 68) | 2.4 ± 2.2 (n = 49) | 0.694 |
| Number of aberrant oocytes | 0.9 ± 1.8 (n = 68) | 0.7 ± 1.5 (n = 49) | 0.334 |
| Oocyte maturation | |||
| Number of injected MII oocytes | 8.0 ± 6.1 (n = 68) | 8.2 ± 5.0 (n = 49) | 0.578 |
| Fertilization success | |||
| Number of 2PN oocytes | 5.4 ± 4.0 (n = 64) | 5.1 ± 3.9 (n = 48) | 0.690 |
| Equivalent Group | Dissimilar Group | |||||
|---|---|---|---|---|---|---|
| Clinical Characteristics | Pearson’s Correlation Coefficient | p-Value | n | Pearson’s Correlation Coefficient | p-Value | n |
| Markers of ovarian reserve | ||||||
| Day 3 FSH (mUI/mL) | −0.068 | 0.588 | 65 | −0.053 | 0.713 | 51 |
| AMH (ng/mL) | −0.029 | 0.819 | 64 | −0.082 | 0.589 | 46 |
| AFC | 0.015 | 0.925 | 40 | −0.045 | 0.806 | 33 |
| IVF outcomes | ||||||
| Response to ovarian stimulation | ||||||
| Total dose of gonadotrophins (IU/mL) | 0.096 | 0.437 | 68 | 0.135 | 0.343 | 51 |
| Stimulation duration (days) | 0.010 | 0.937 | 68 | −0.078 | 0.581 | 52 |
| Number of follicles on the trigger day | 0.237 | 0.0512 | 68 | −0.050 | 0.726 | 51 |
| Number of retrieved oocytes | 0.060 | 0.627 | 68 | −0.153 | 0.283 | 51 |
| Number of immature oocytes | 0.139 | 0.257 | 67 | −0.168 | 0.249 | 49 |
| Number of aberrant oocytes | −0.019 | 0.876 | 68 | −0.094 | 0.523 | 49 |
| Oocyte maturation | ||||||
| Number of injected MII oocytes | 0.0794 | 0.520 | 68 | −0.289 | 0.044 | 49 |
| Fertilization success | ||||||
| Number of 2PN oocytes | 0.044 | 0.731 | 64 | −0.311 | 0.031 | 48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, B.; Vale-Fernandes, E.; Sousa, V.; Marques, I.; Santos, R.; Nogueira, A.J.A.; Jorge, P. FMR1 Allelic Complexity and IVF Fertilization Success: Limitations and Future Perspectives. Int. J. Mol. Sci. 2025, 26, 5752. https://doi.org/10.3390/ijms26125752
Rodrigues B, Vale-Fernandes E, Sousa V, Marques I, Santos R, Nogueira AJA, Jorge P. FMR1 Allelic Complexity and IVF Fertilization Success: Limitations and Future Perspectives. International Journal of Molecular Sciences. 2025; 26(12):5752. https://doi.org/10.3390/ijms26125752
Chicago/Turabian StyleRodrigues, Bárbara, Emídio Vale-Fernandes, Vanessa Sousa, Isabel Marques, Rosário Santos, António J. A. Nogueira, and Paula Jorge. 2025. "FMR1 Allelic Complexity and IVF Fertilization Success: Limitations and Future Perspectives" International Journal of Molecular Sciences 26, no. 12: 5752. https://doi.org/10.3390/ijms26125752
APA StyleRodrigues, B., Vale-Fernandes, E., Sousa, V., Marques, I., Santos, R., Nogueira, A. J. A., & Jorge, P. (2025). FMR1 Allelic Complexity and IVF Fertilization Success: Limitations and Future Perspectives. International Journal of Molecular Sciences, 26(12), 5752. https://doi.org/10.3390/ijms26125752







