Structure–Activity Relationship of Ionic Liquids for Acid Corrosion Inhibition
Abstract
1. Introduction
2. Results
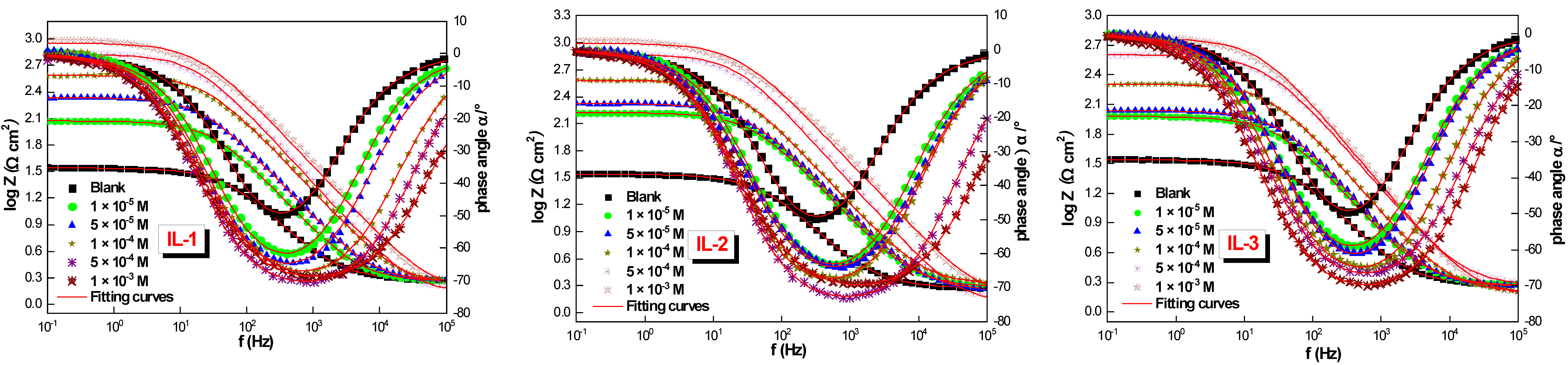
2.1. Electrochemical Impedance Spectroscopy (EIS)
2.2. Adsorption Isotherm Study
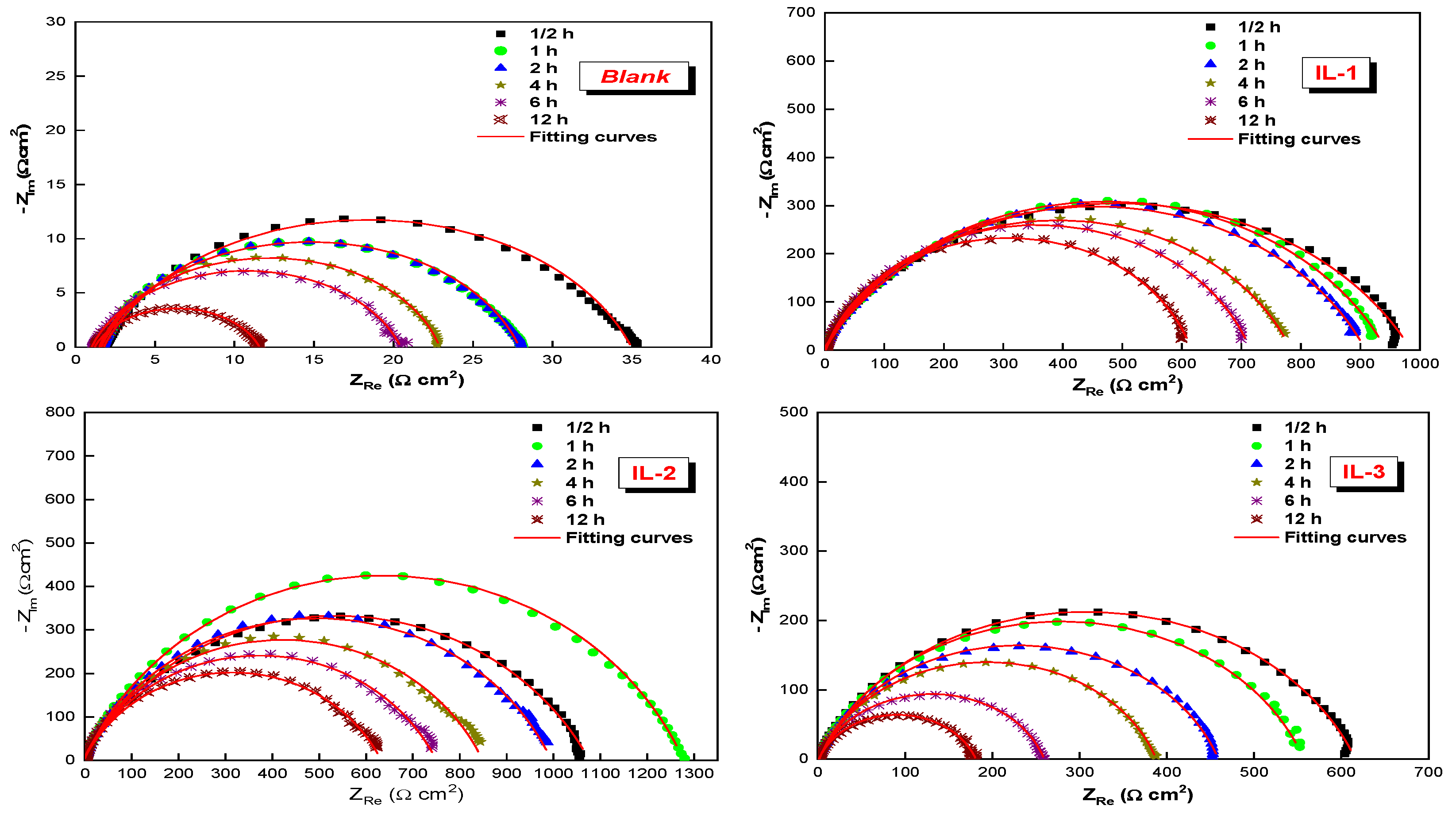
2.3. Effect of Immersion Time on the ILs Inhibition Efficiency
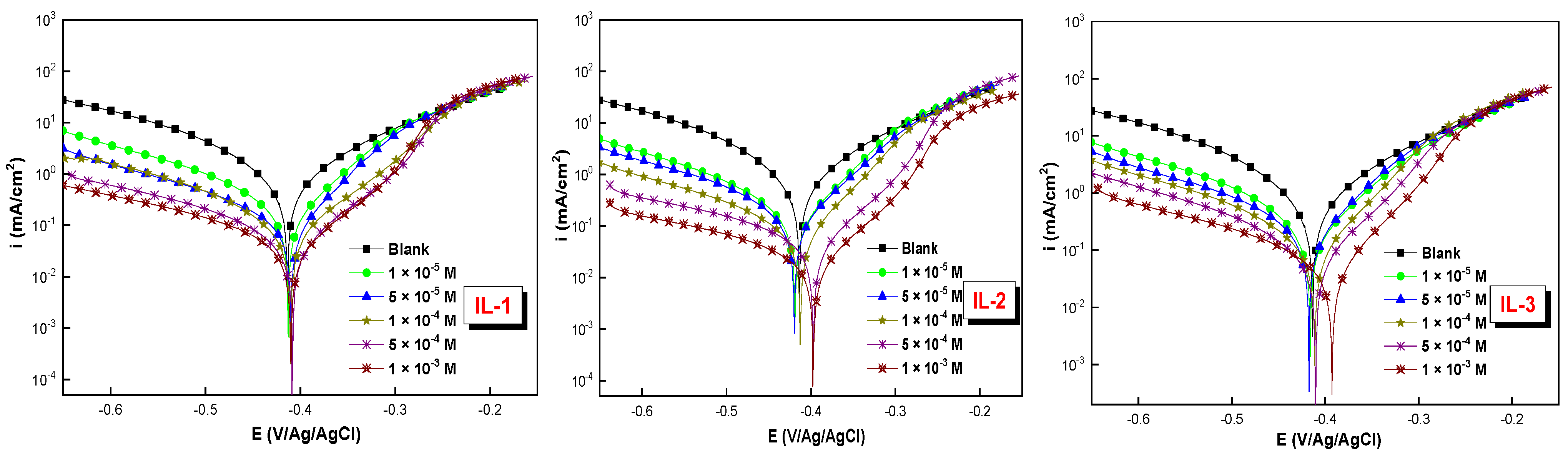
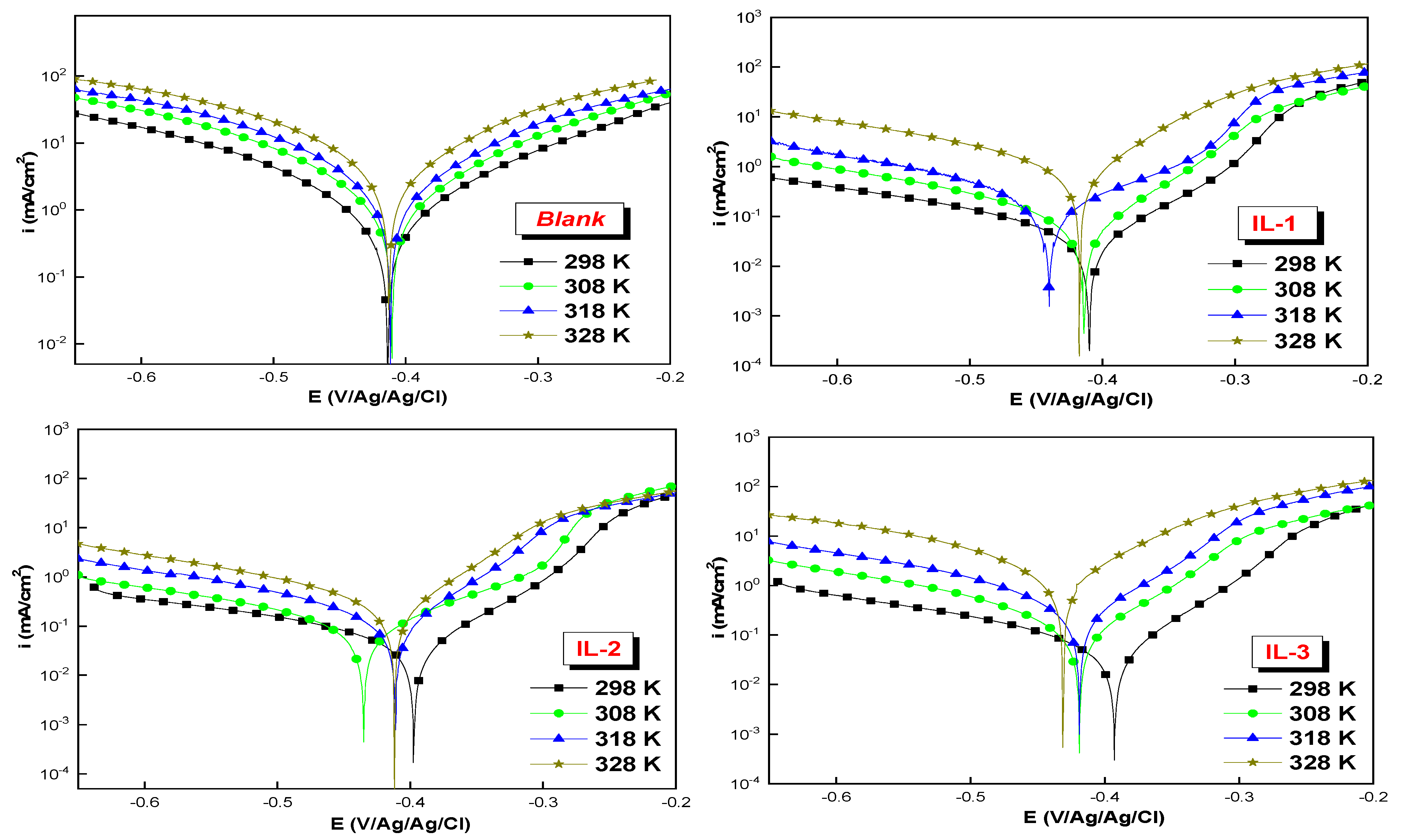
2.4. Potentiodynamic Polarization (PDP) Measurements
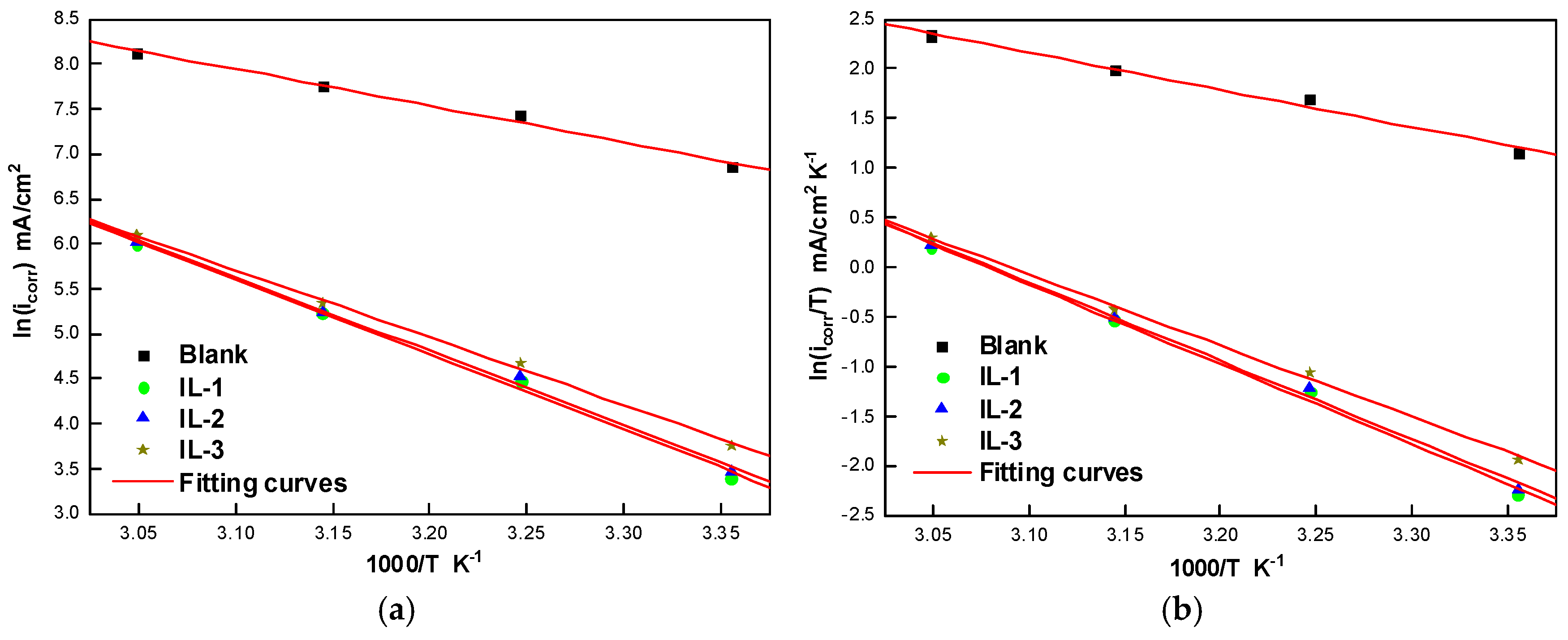
2.5. Effect of Temperature
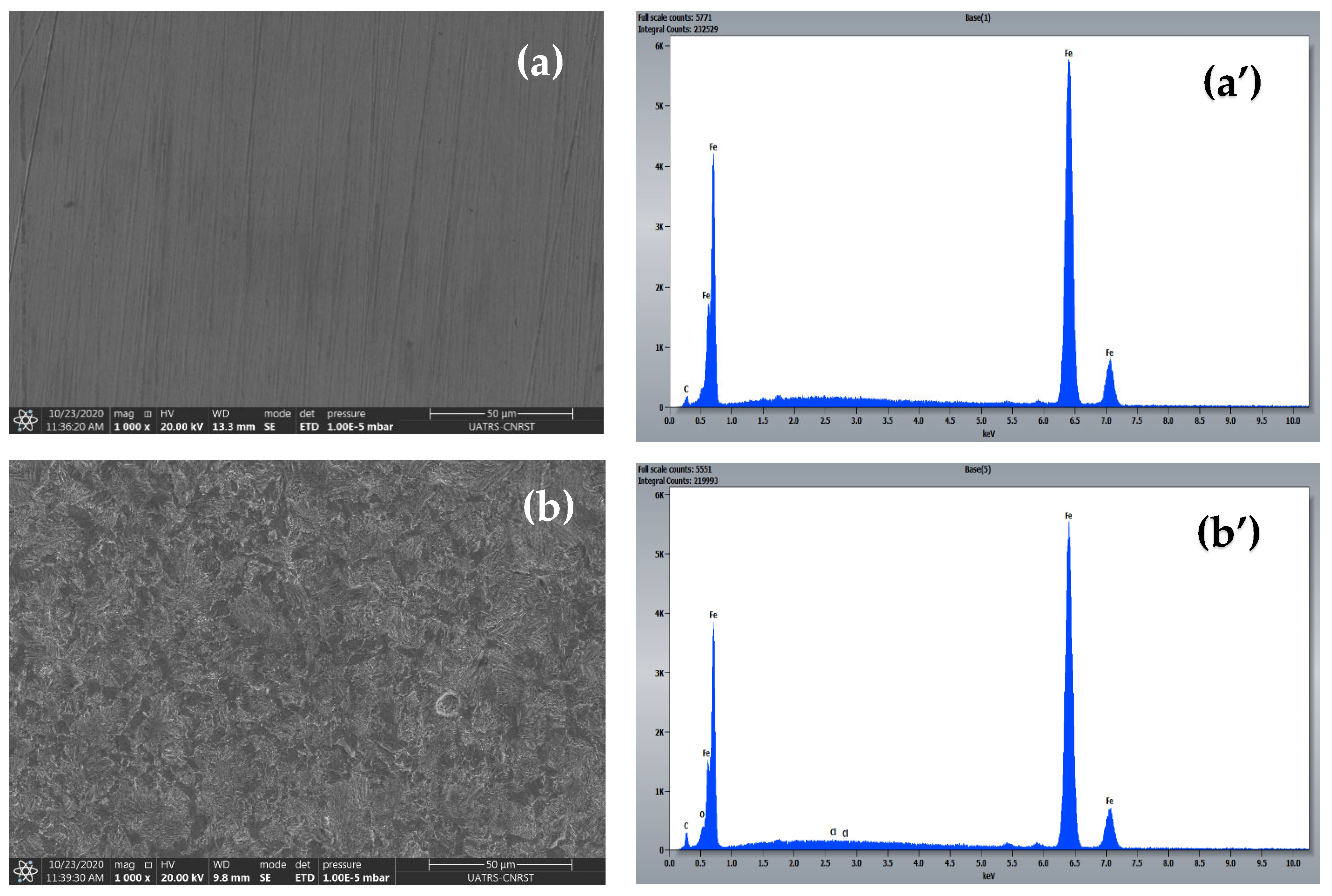
2.6. Surface Characterization (SEM-EDS)
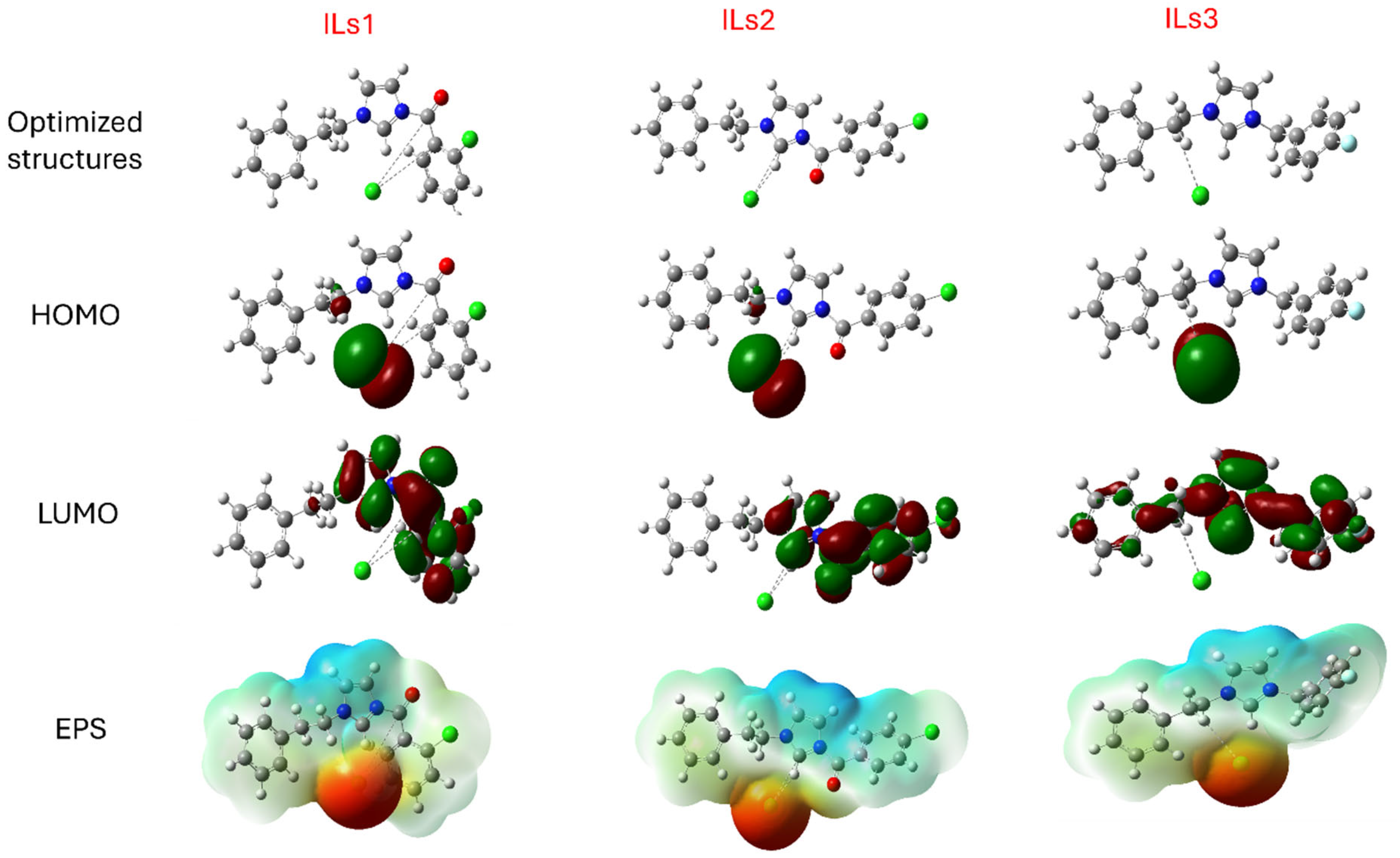
2.7. Theoretical Information
3. Materials and Methods
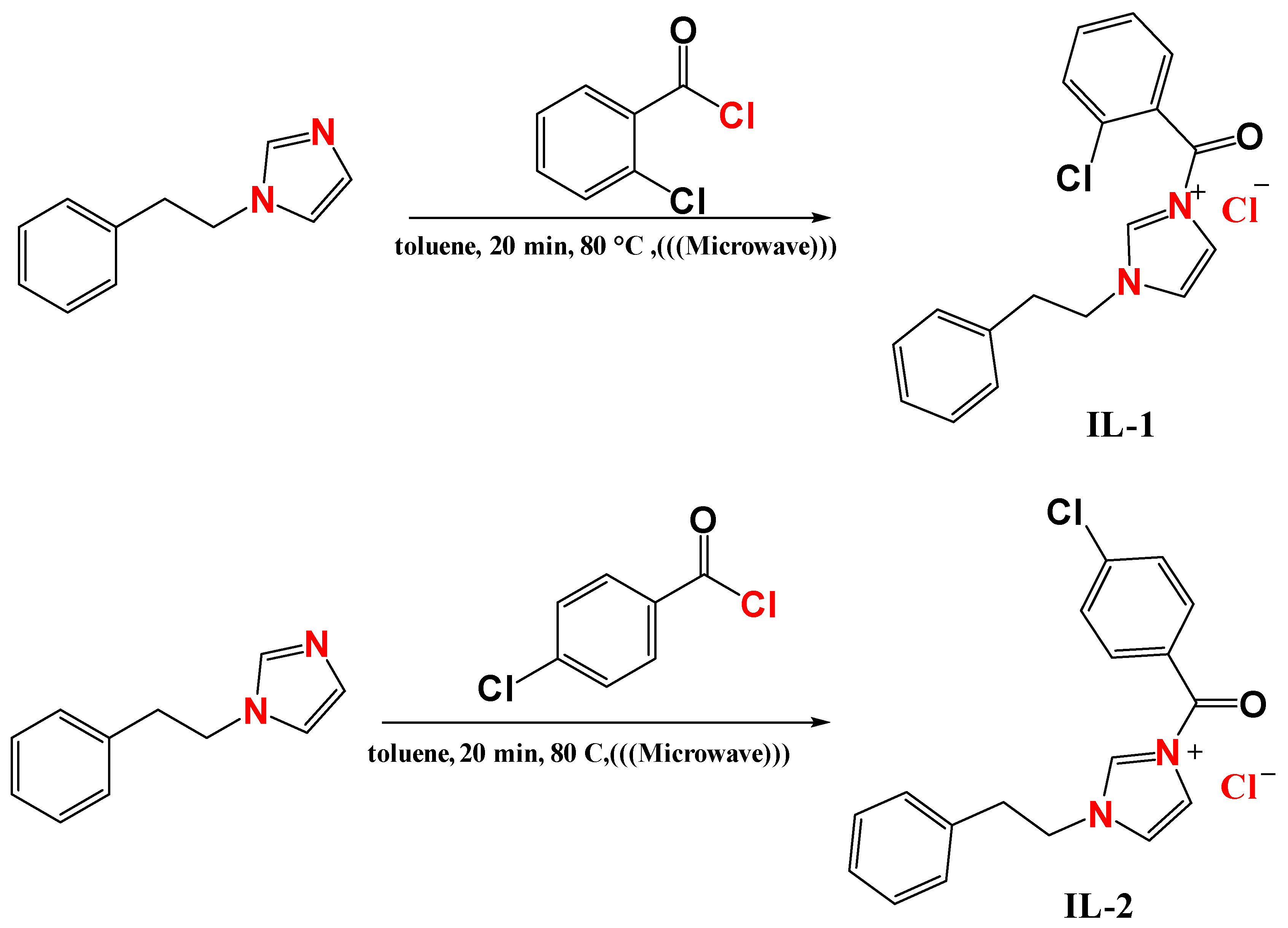
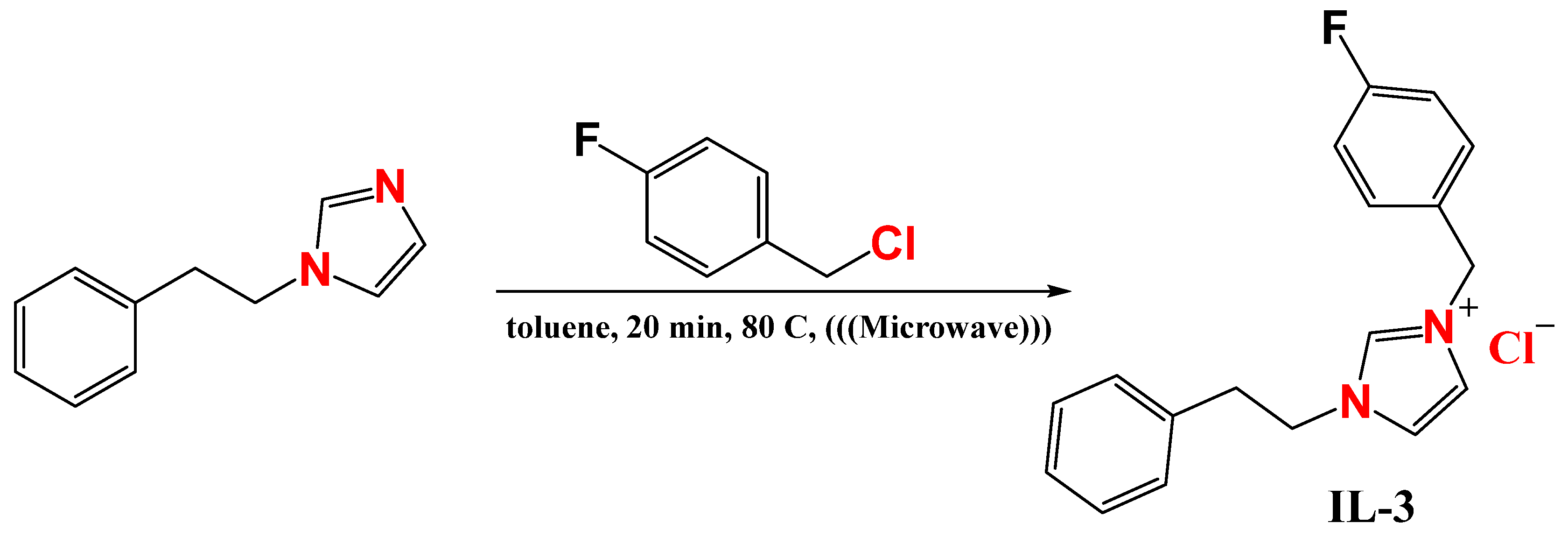
3.1. Synthesis of Imidazolium-Derived (IL-1, IL-2, and IL-3)
3.2. Mild Steel and Solutions Preparation
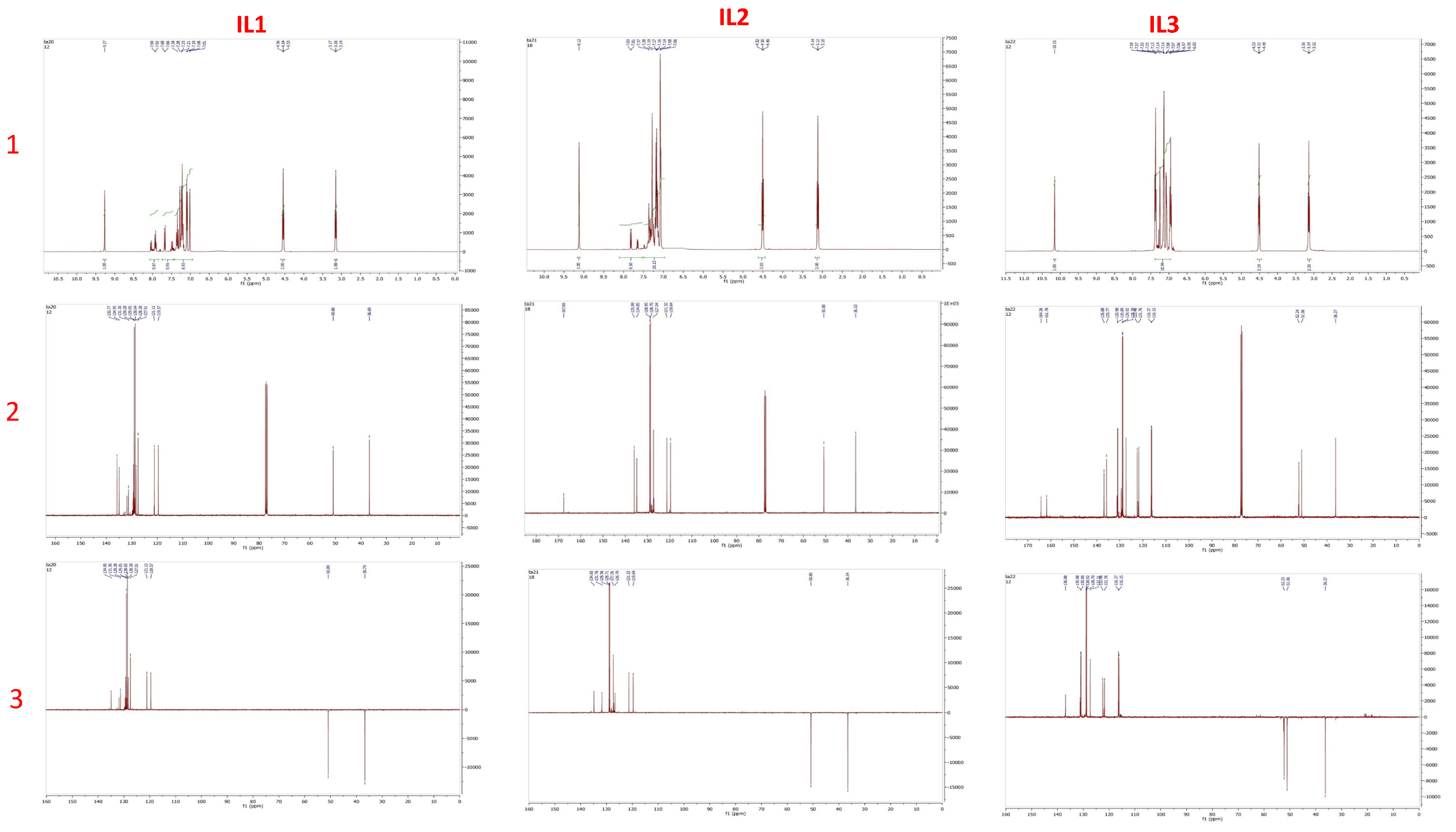
3.3. Characterization of IL-1, IL-2 and IL-3
3.3.1. The 3-(2-Chlorobenzoyl)-1-phenethyl-1H-imidazol-3-ium chloride (IL-1)
3.3.2. The 3-(4-Chlorobenzoyl)-1-phenethyl-1H-imidazol-3-ium chloride (IL-2)
3.3.3. The 3-(4-Fluorobenzyl)-1-phenethyl-1H-imidazol-3-ium chloride (IL-3)
3.4. Electrochemical Measurements
3.5. Surface Characterization
3.6. Theoretical Details
3.6.1. Density Functional Theory (DFT)
3.6.2. Monte Carlo Simulation (MC)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Verma, C.; Olasunkanmi, L.O.; Ebenso, E.E.; Quraishi, M.A.; Obot, I.B. Adsorption behavior of glucosamine-based, pyrimidine-fused heterocycles as green corrosion inhibitors for mild steel: Experimental and theoretical studies. J. Phys. Chem. C 2016, 120, 11598–11611. [Google Scholar] [CrossRef]
- Guo, L.; Zhu, S.; Zhang, S.; He, Q.; Li, W. Theoretical studies of three triazole derivatives as corrosion inhibitors for mild steel in acidic medium. Corros. Sci. 2014, 87, 366–375. [Google Scholar] [CrossRef]
- Srivastava, V.; Haque, J.; Verma, C.; Singh, P.; Lgaz, H.; Salghi, R.; Quraishi, M.A. Amino acid-based imidazolium zwitterions as novel and green corrosion inhibitors for mild steel: Experimental, DFT and MD studies. J. Mol. Liq. 2017, 244, 340–352. [Google Scholar] [CrossRef]
- Chauhan, D.S.; El-Hajjaji, F.; Quraishi, M.A. Chapter 18—Heterocyclic ionic liquids as environmentally benign corrosion inhibitors: Recent advances and future perspectives. In Ionic Liquid-Based Technologies for Environmental Sustainability; Elsevier: Amsterdam, The Netherlands, 2022; pp. 279–294. [Google Scholar]
- Olasunkanmi, L.O.; Obot, I.B.; Kabanda, M.M.; Ebenso, E.E. Some quinoxalin-6-yl derivatives as corrosion inhibitors for mild steel in hydrochloric acid: Experimental and theoretical studies. J. Phys. Chem. C 2015, 119, 16004–16019. [Google Scholar] [CrossRef]
- Alibakhshi, E.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B.; Mahdavian, M.; Motamedi, M. Glycyrrhiza glabra leaves extract as a green corrosion inhibitor for mild steel in 1 M hydrochloric acid solution: Experimental, molecular dynamics, Monte Carlo and quantum mechanics study. J. Mol. Liq. 2018, 255, 185–198. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, B.; Yang, W.; Yin, X.; Liu, Y.; Chen, Y. Halogen-substituted imidazoline derivatives as corrosion inhibitors for mild steel in hydrochloric acid solution. Corros. Sci. 2015, 90, 284–295. [Google Scholar] [CrossRef]
- Asadi, N.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B. Utilizing Lemon Balm extract as an effective green corrosion inhibitor for mild steel in 1 M HCl solution: A detailed experimental, molecular dynamics, Monte Carlo and quantum mechanics study. J. Taiwan Inst. Chem. Eng. 2019, 95, 252–272. [Google Scholar] [CrossRef]
- Yıldız, R. An electrochemical and theoretical evaluation of 4, 6-diamino-2-pyrimidinethiol as a corrosion inhibitor for mild steel in HCl solutions. Corros. Sci. 2015, 90, 544–553. [Google Scholar] [CrossRef]
- Saha, S.K.; Dutta, A.; Ghosh, P.; Sukul, D.; Banerjee, P. Novel Schiff-base molecules as efficient corrosion inhibitors for mild steel surface in 1 M HCl medium: An experimental and theoretical approach. Phys. Chem. Chem. Phys. 2016, 18, 17898–17911. [Google Scholar] [CrossRef]
- Saha, S.K.; Ghosh, P.; Hens, A.; Murmu, N.C.; Banerjee, P. Density functional theory and molecular dynamics simulation study on corrosion inhibition performance of mild steel by mercapto-quinoline Schiff base corrosion inhibitor. Phys. E Low-Dimens. Syst. Nanostructures 2015, 66, 332–341. [Google Scholar] [CrossRef]
- Cao, Z.; Tang, Y.; Cang, H.; Xu, J.; Lu, G.; Jing, W. Novel benzimidazole derivatives as corrosion inhibitors of mild steel in the acidic media. Part II: Theoretical studies. Corros. Sci. 2014, 83, 292–298. [Google Scholar] [CrossRef]
- Qiang, Y.; Zhang, S.; Guo, L.; Zheng, X.; Xiang, B.; Chen, S. Experimental and theoretical studies of four allyl imidazolium-based ionic liquids as green inhibitors for copper corrosion in sulfuric acid. Corros. Sci. 2017, 119, 68–78. [Google Scholar] [CrossRef]
- El-Hajjaji, F.; Messali, M.; Aljuhani, A.; Aouad, M.R.; Hammouti, B.; Belghiti, M.E.; Chauhan, D.S.; Quraishi, M.A. Pyridazinium-based ionic liquids as novel and green corrosion inhibitors of carbon steel in acid medium: Electrochemical and molecular dynamics simulation studies. J. Mol. Liq. 2018, 249, 997–1008. [Google Scholar] [CrossRef]
- Chu, T.-S.; Mai, W.-J.; Li, H.-Z.; Wei, B.-X.; Xu, Y.-Q.; Liao, B.-K. Insights into the Corrosion Inhibition Performance of Plant Extracts of Different Genera in the Asteraceae Family for Q235 Steel in H2SO4 Medium. Int. J. Mol. Sci. 2025, 26, 561. [Google Scholar] [CrossRef]
- Palumbo, G.; Święch, D.; Górny, M. Guar Gum as an Eco-Friendly Corrosion Inhibitor for N80 Carbon Steel under Sweet Environment in Saline Solution: Electrochemical, Surface, and Spectroscopic Studies. Int. J. Mol. Sci. 2023, 24, 12269. [Google Scholar] [CrossRef]
- El-Nagar, R.A.; Khalil, N.A.; Atef, Y.; Nessim, M.I.; Ghanem, A. Evaluation of ionic liquids based imidazolium salts as an environmentally friendly corrosion inhibitors for carbon steel in HCl solutions. Sci. Rep. 2024, 14, 1889. [Google Scholar] [CrossRef]
- Ansari, K.R.; Quraishi, M.A.; Singh, A. Schiff’s base of pyridyl substituted triazoles as new and effective corrosion inhibitors for mild steel in hydrochloric acid solution. Corros. Sci. 2014, 79, 5–15. [Google Scholar] [CrossRef]
- Xu, B.; Yang, W.; Liu, Y.; Yin, X.; Gong, W.; Chen, Y. Experimental and theoretical evaluation of two pyridinecarboxaldehyde thiosemicarbazone compounds as corrosion inhibitors for mild steel in hydrochloric acid solution. Corros. Sci. 2014, 78, 260–268. [Google Scholar] [CrossRef]
- Zeng, C.; Zhou, Z.; Mai, W.J.; Chen, Q.H.; He, J.; Liao, B.K. Exploration on the corrosion inhibition performance of Salvia miltiorrhiza extract as a green corrosion inhibitor for Q235 steel in HCl environment. J. Mater. Res. Technol. 2024, 32, 3857–3870. [Google Scholar] [CrossRef]
- Ansari, K.R.; Ramkumar, S.; Chauhan, D.S.; Salman, M.; Nalini, D.; Srivastava, V.; Quraishi, M.A. Macrocyclic compounds as green corrosion inhibitors for aluminium: Electrochemical, surface and quantum chemical studies. Int. J. Corros. Scale Inhib. 2018, 7, 443–459. [Google Scholar]
- Nam, N.D.; Bui, Q.V.; Mathesh, M.; Tan, M.Y.J.; Forsyth, M. A study of 4-carboxyphenylboronic acid as a corrosion chemical engineering communications 13 inhibitor for steel in carbon dioxide-containing environments. Corros. Sci. 2013, 76, 257–266. [Google Scholar] [CrossRef]
- Cui, F.; Ni, Y.; Jiang, J.; Ni, L.; Wang, Z. Experimental and theoretical studies of five imidazolium-based ionic liquids as corrosion inhibitors for mild steel in H2S and HCl solutions. Chem. Eng. Commun. 2021, 208, 1580–1593. [Google Scholar] [CrossRef]
- Roy, P.; Karfa, P.; Adhikari, U.; Sukul, D. Corrosion inhibition of mild steel in acidic medium by polyacrylamide grafted Guar gum with various grafting percentage: Effect of intramolecular synergism. Corros Sci. 2014, 88, 246–253. [Google Scholar] [CrossRef]
- Lopez, D.A.; Simison, S.N.; de Sanchez, S.R. The influence of steel microstructure on CO2 corrosion. EIS studies on the inhibition efficiency of benzimidazole. Electrochim. Acta 2003, 48, 845–854. [Google Scholar] [CrossRef]
- Murulana, L.C.; Singh, A.K.; Shukla, S.K.; Kabanda, M.M.; Ebenso, E.E. Experimental and quantum chemical studies of some bis (trifluoromethyl-sulfonyl) imide imidazolium-based ionic liquids as corrosion inhibitors for mild steel in hydrochloric acid solution. Ind. Eng. Chem. Res. 2012, 51, 13282–13299. [Google Scholar] [CrossRef]
- Zhang, S.; Tao, Z.; Liao, S.; Wu, F. Substitutional adsorption isotherms and corrosion inhibitive properties of some oxadiazol-triazole derivative in acidic solution. Corros. Sci. 2010, 52, 3126–3132. [Google Scholar] [CrossRef]
- Omotosho, O.A.; Okeniyi, J.O.; Oni, A.B.; Makinwa, T.O.; Ajibola, O.B.; Fademi, E.O.J.; Obi, C.E.; Loto, C.A.; Popoola, A.P.I. Inhibition and mechanism of Terminalia catappa on mild-steel corrosion in sulphuric-acid environment. Prog. Ind. Ecol. Int. J. 2016, 10, 398–413. [Google Scholar]
- Hassan, H.H.; Abdelghani, E.; Amin, M.A. Inhibition of mild steel corrosion in hydrochloric acid solution by triazole derivatives: Part I. Polarization and EIS studies. Electrochim. Acta 2007, 52, 6359–6366. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, M.; Feng, J.; Yin, C.; Li, D.; Fan, L.; Chen, Q.; Liu, H. Dimeric imidazolium ionic liquids connected by bipyridiyl as a corrosion inhibitor for N80 carbon steel in HCl. J. Mol. Liq. 2021, 344, 117962. [Google Scholar] [CrossRef]
- El Hajjaji, F.; Salim, R.; Messali, M.; Hammouti, B.; Chauhan, D.; Almutairi, S.; Quraishi, M. Electrochemical Studies on New Pyridazinium Derivatives as Corrosion Inhibitors of Carbon Steel in Acidic Medium. J. Bio-Tribo-Corros. 2019, 5, 4. [Google Scholar] [CrossRef]
- Go, L.C.; Depan, D.; Holmes, W.E.; Gallo, A.; Knierim, K.; Bertrand, T.; Hernandez, R. Kinetic and thermodynamic analyses of the corrosion inhibition of synthetic extracellular polymeric substances. PeerJ Mater. Sci. 2020, 2, e4. [Google Scholar] [CrossRef]
- Alaoui, A.O.; Elfalleh, W.; Hammouti, B.; Titi, A.; Messali, M.; Kaya, S.; EL IBrahimi, B.; El-Hajjaji, F. Theoretical prediction of corrosion inhibition by ionic liquid derivatives: A DFT and molecular dynamics approach. RSC Adv. 2025, 15, 2645–12652. [Google Scholar]
- Junaedi, S.; Al-Amiery, A.A.; Kadihum, A.; Kadhum, A.A.H.; Mohamad, A.B. Inhibition Effects of a Synthesized Novel 4-Aminoantipyrine Derivative on the Corrosion of Mild Steel in Hydrochloric Acid Solution together with Quantum Chemical Studies. Int. J. Mol. Sci. 2013, 14, 11915–11928. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Sánchez, G.; Olivares-Xometl, O.; Arellanes-Lozada, P.; Likhanova, N.V.; Lijanova, I.V.; Arriola-Morales, J.; Díaz-Jiménez, V.; López-Rodríguez, J. Temperature Effect on the Corrosion Inhibition of Carbon Steel by Polymeric Ionic Liquids in Acid Medium. Int. J. Mol. Sci. 2023, 24, 6291. [Google Scholar] [CrossRef]
- Kanzouai, Y.; Ech-chihbi, E.; Al Houari, G.; Arrousse, N.; Salim, R.; El-Hajjaji, F.; Rais, Z.; Taleb, M. Adsorption and Corrosion Inhibitive Properties of Some Aldehyde Derivatives on Mild Steel in 1 M HCl Solution: Electrochemical and Computational Investigations. J. Bio-Tribo-Corros. 2021, 7, 101. [Google Scholar] [CrossRef]
- EL-Hajjaji, F.; Salim, R.; Ech-chihbi, E.; Titi, A.; Messali, M.; Kaya, S.; El Ibrahimi, B.; Taleb, M. New imidazolium ionic liquids as eco-friendly corrosion inhibitors for mild steel in hydrochloric acid (1 M): Experimental and theoretical approach. J. Taiwan Inst. Chem. Eng. 2021, 123, 346–362. [Google Scholar] [CrossRef]
- El-Hajjaji, F.; Messali, M.; de Yuso, M.V.M.; Rodríguez-Castellón, E.; Almutairi, S.; Bandosz, T.J.; Algarra, M. Effect of 1-(3-phenoxypropyl) pyridazin-1-ium bromide on steel corrosion inhibition in acidic medium. J. Colloid Interface Sci. 2019, 541, 418–424. [Google Scholar] [CrossRef]
- Liu, F.G.; Du, M.; Zhang, J.; Qiu, M. Electrochemical behavior of Q235 steel in saltwater saturated with carbon dioxide based on new imidazoline derivative inhibitor. Corros. Sci. 2009, 51, 102–109. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Hada GAUSSIAN 09 R’evision; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Adardour, M.; Lasri, M.; Ait Lahcen, M.; Maatallah, M.; Idouhli, R.; Alanazi, M.M.; Lahmidi, S.; Abouelfida, A.; Mague, J.T.; Baouid, A. Exploring the efficacy of benzimidazolone derivative as corrosion inhibitors for copper in a 3.5 Wt.% NaCl solution: A comprehensive experimental and theoretical investigation. Molecules 2023, 28, 6948. [Google Scholar] [CrossRef]
- Verma, D.K.; Aslam, R.; Aslam, J.; Quraishi, M.A.; Ebenso, E.E.; Verma, C. Computational modeling: Theoretical predictive tools for designing potential organic corrosion inhibitors. J. Mol. Struct. 2021, 1236, 130294. [Google Scholar] [CrossRef]
- Obot, I.B. Recent Advances in Computational Design of Organic Materials for Corrosion Protection of Steel in Aqueous. In Developments in Corrosion Protection; IntechOpen: London, UK, 2014; p. 123. [Google Scholar]
- Toukal, L.; Foudia, M.; Haffar, D.; Aliouane, N.; Al-Noaimi, M.; Bellal, Y.; Elmsellem, H.; Abdel-Rahman, I. Monte Carlo simulation and electrochemical performance corrosion inhibition whid benzimidazole derivative for XC48 steel in 0.5 M H2SO4 and 1.0 M HCl solutions. J. Indian Chem. Soc. 2022, 99, 100634. [Google Scholar] [CrossRef]
- Chen, L.; Lu, D.; Zhang, Y. Organic compounds as corrosion inhibitors for carbon steel in HCl solution: A comprehensive review. Materials 2022, 15, 2023. [Google Scholar] [CrossRef] [PubMed]
- Hsissou, R.; Dahmani, K.; El Magri, A.; Hmada, A.; Safi, Z.; Dkhireche, N.; Galai, M.; Wazzan, N.; Berisha, A. A combined experimental and computational (DFT, RDF, MC and MD) investigation of epoxy resin as a potential corrosion inhibitor for mild steel in a 0.5 M H2SO4 environment. Polymers 2023, 15, 1967. [Google Scholar] [CrossRef]
- Kasprzhitskii, A.; Lazorenko, G.; Nazdracheva, T.; Yavna, V. Comparative computational study of l-amino acids as green corrosion inhibitors for mild steel. Computation 2020, 9, 1. [Google Scholar] [CrossRef]
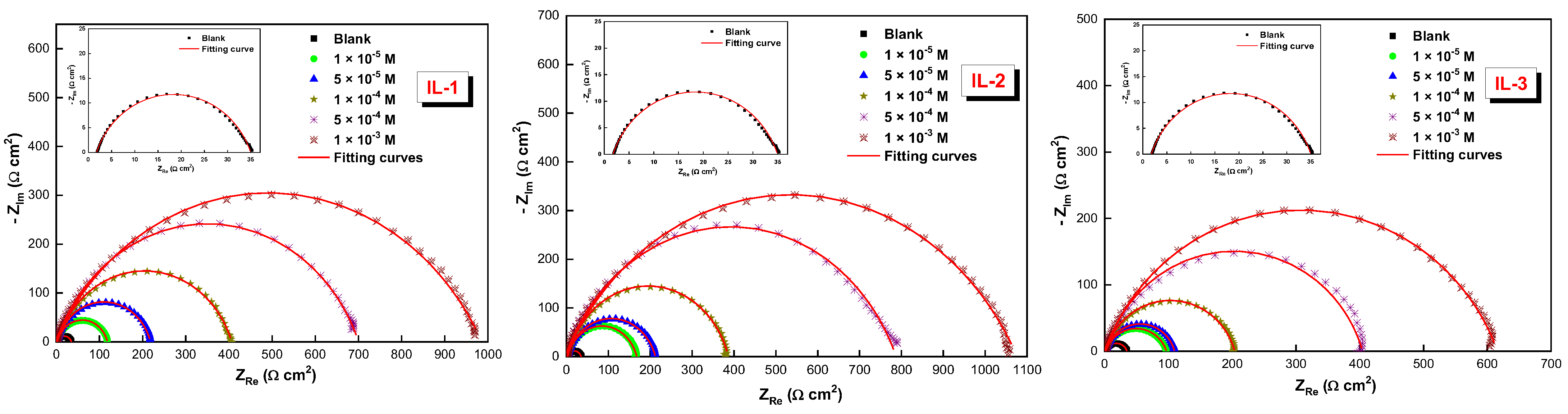



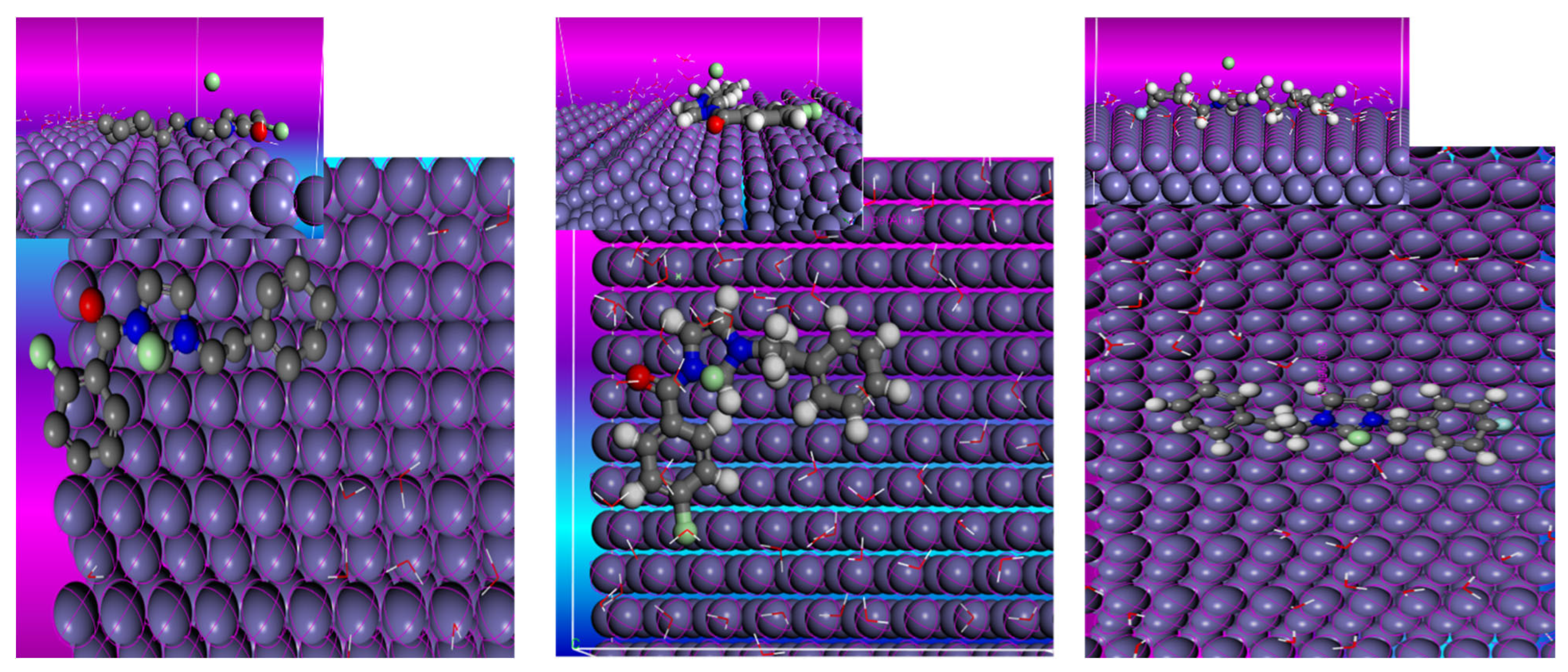
| Medium | Conc. (M) | Rs (Ω cm2) | Rct (Ω cm2) | CPE | Q (µF Sn−1) | Ɵ | ƞimp % | |
|---|---|---|---|---|---|---|---|---|
| Cdl (µFcm−2) | ndl | |||||||
| Blank | --- | 1.7 | 33 | 89.1 | 0.784 | 312.70 | --- | --- |
| IL-1 | 10−5 | 1.8 | 116.7 | 44.2 | 0.830 | 107.7 | 0.717 | 71.7 |
| 5 × 10−5 | 1.8 | 216.4 | 33.2 | 0.825 | 78.7 | 0.847 | 84.7 | |
| 10−4 | 1.9 | 404.5 | 18.4 | 0.793 | 50.5 | 0.918 | 91.8 | |
| 5 × 10−4 | 1.3 | 700.9 | 15.7 | 0.769 | 47.4 | 0.953 | 95.3 | |
| 10−3 | 1..0 | 989.0 | 12.2 | 0.703 | 45.3 | 0.966 | 96.6 | |
| IL-2 | 10−5 | 1.9 | 165.8 | 26.2 | 0.827 | 66.8 | 0.801 | 80.1 |
| 5 × 10−5 | 1.7 | 210.6 | 24.5 | 0.815 | 64.7 | 0.843 | 84.3 | |
| 10−4 | 1.5 | 384 | 19.6 | 0.825 | 46.2 | 0.914 | 91.4 | |
| 5 × 10−4 | 1.1 | 790 | 11.9 | 0.756 | 37.3 | 0.958 | 95.8 | |
| 10−3 | 0.8 | 1081 | 8.8 | 0.702 | 35.2 | 0.969 | 96.9 | |
| IL-3 | 10−5 | 1.6 | 95.3 | 52.8 | 0.791 | 159.0 | 0.654 | 65.4 |
| 5 × 10−5 | 1.8 | 107.8 | 43.3 | 0.828 | 108.4 | 0.694 | 69.4 | |
| 10−4 | 1.6 | 202.3 | 30.5 | 0.824 | 74.5 | 0.837 | 83.7 | |
| 5 × 10−4 | 1.8 | 402.4 | 18.5 | 0.818 | 45.1 | 0.918 | 91.8 | |
| 10−3 | 1.3 | 616.5 | 17.0 | 0.769 | 43.2 | 0.946 | 94.6 | |
| Compound. | K (L/mol) | ΔGads (KJ/mol) | R2 | Slopes |
|---|---|---|---|---|
| IL-1 | 16.13 × 104 | −39.6 | 0.99999 | 1.03 |
| IL-2 | 16.80 × 104 | −39.7 | 0.99998 | 1.02 |
| IL-3 | 7.03 × 104 | −37.6 | 0.99988 | 1.04 |
| Medium | Time (h) | Rs (Ω cm2) | Rct (Ω cm2) | CPE | Q (µF Sn−1) | ηEIS % | |
|---|---|---|---|---|---|---|---|
| Cdl (µF cm−2) | ndl | ||||||
| Blank | ½ | 1.7 | 33.0 | 89.1 | 0.784 | 312.70 | -- |
| 1 | 1.6 | 26.4 | 122.7 | 0.810 | 364.90 | -- | |
| 2 | 1.6 | 26.3 | 122.8 | 0.810 | 364.80 | -- | |
| 4 | 1.5 | 21.4 | 267.0 | 0.834 | 627.00 | -- | |
| 6 | 1.0 | 19.4 | 349.4 | 0.796 | 963.80 | -- | |
| 12 | 1.2 | 10.4 | 419.5 | 0.764 | 949.2 | -- | |
| IL-1 | ½ | 1.0 | 989 | 12.2 | 0.703 | 45.3 | 96.6 |
| 1 | 1.2 | 943 | 15.4 | 0.737 | 48.9 | 97.2 | |
| 2 | 1.3 | 909 | 18.3 | 0.739 | 59.0 | 97.2 | |
| 4 | 1.8 | 784 | 22.6 | 0.767 | 66.8 | 97.3 | |
| 6 | 1.6 | 715 | 25.0 | 0.800 | 73.2 | 97.3 | |
| 12 | 1.3 | 615 | 30.8 | 0.826 | 80.9 | 98.3 | |
| IL-2 | ½ | 0.8 | 1081 | 8.8 | 0.702 | 35.2 | 96.9 |
| 1 | 0.5 | 1280 | 7.5 | 0.747 | 32.6 | 97.9 | |
| 2 | 0.8 | 998 | 11.8 | 0.737 | 37.8 | 97.4 | |
| 4 | 1.0 | 853 | 13.0 | 0.734 | 43.1 | 97.4 | |
| 6 | 0.7 | 754 | 14.9 | 0.724 | 51.6 | 97.4 | |
| 12 | 1.2 | 635 | 18.2 | 0.722 | 62.9 | 98.3 | |
| IL-3 | ½ | 1.3 | 616.5 | 17.0 | 0.769 | 43.2 | 94.6 |
| 1 | 1.6 | 555.2 | 20.2 | 0.791 | 57.6 | 95.2 | |
| 2 | 1.6 | 457.5 | 21.7 | 0.792 | 56.5 | 94.2 | |
| 4 | 1.4 | 386.4 | 26.0 | 0.798 | 65.9 | 94.4 | |
| 6 | 1.5 | 258.0 | 28.8 | 0.799 | 76.9 | 92.5 | |
| 12 | 1.2 | 179.8 | 34.6 | 0.786 | 102.6 | 94.2 | |
| Medium | Conc. M | −Ecorr mV/Ag/AgCl | icorr µA cm−2 | −βc mV dec−1 | βa mV dec−1 | ηPDP % |
|---|---|---|---|---|---|---|
| 1 M HCl | --- | 413 | 944 | 139 | 128 | ---- |
| IL-1 | 1 × 10−5 | 412 | 263 | 124 | 108 | 72.1 |
| 5 × 10−5 | 410 | 137 | 127 | 112 | 85.5 | |
| 1 × 10−4 | 411 | 77 | 129 | 114 | 91.8 | |
| 5 × 10−4 | 409 | 38 | 133 | 122 | 95.9 | |
| 1 × 10−3 | 410 | 30 | 135 | 125 | 96.8 | |
| IL-2 | 1 × 10−5 | 419 | 172 | 127 | 112 | 81.8 |
| 5 × 10−5 | 420 | 135 | 128 | 113 | 85.7 | |
| 1 × 10−4 | 413 | 83 | 132 | 122 | 91.2 | |
| 5 × 10−4 | 398 | 36 | 135 | 125 | 96.2 | |
| 1 × 10−3 | 397 | 32 | 138 | 126 | 96.6 | |
| IL-3 | 1 × 10−5 | 415 | 335 | 130 | 121 | 64.5 |
| 5 × 10−5 | 417 | 282 | 134 | 118 | 70.1 | |
| 1 × 10−4 | 411 | 152 | 136 | 119 | 83.8 | |
| 5 × 10−4 | 410 | 83 | 135 | 116 | 91.2 | |
| 1 × 10−3 | 393 | 43 | 132 | 120 | 95.4 |
| Medium | Temperature K | −Ecorr mV/ECS | icorr µA cm−2 | −βc mV dec−1 | βa mV dec−1 | ηPDP % |
|---|---|---|---|---|---|---|
| Blank | 298 | 413 | 944 | 139 | 128 | --- |
| 308 | 410 | 1690 | 137 | 129 | --- | |
| 318 | 411 | 2328 | 126 | 125 | --- | |
| 328 | 412 | 3387 | 120 | 133 | --- | |
| IL-1 | 298 | 410 | 30 | 135 | 125 | 96.8 |
| 308 | 414 | 88 | 127 | 127 | 94.7 | |
| 318 | 440 | 187 | 135 | 119 | 91.9 | |
| 328 | 416 | 402 | 122 | 128 | 88.1 | |
| IL-2 | 298 | 397 | 32 | 138 | 126 | 96.6 |
| 308 | 436 | 92 | 135 | 124 | 94.5 | |
| 318 | 411 | 190 | 130 | 128 | 91.8 | |
| 328 | 412 | 413 | 132 | 123 | 87.8 | |
| IL-3 | 298 | 393 | 43 | 132 | 120 | 95.4 |
| 308 | 419 | 107 | 130 | 123 | 93.7 | |
| 318 | 418 | 208 | 131 | 122 | 91.0 | |
| 328 | 431 | 442 | 127 | 126 | 86.9 |
| Medium | Ea (KJ/mol) | ΔHa (KJ/mol) | ΔSa (J/mol K) |
|---|---|---|---|
| Blank | 33.8 | 31.2 | −82.7 |
| IL-1 | 69.5 | 66.9 | 8.5 |
| IL-2 | 68.5 | 65.7 | 5.0 |
| IL-3 | 62.2 | 59.6 | −13.2 |
| Parameters | Ils 1 | Ils 2 | Ils3 |
|---|---|---|---|
| EHOMO | −0.2343 | −0.2350 | −0.2336 |
| ELUMO | −0.0941 | −0.0913 | −0.0288 |
| ΔEgap (eV) | 0.1402 | 0.1437 | 0.2048 |
| η (eV) | 0.0701 | 0.0718 | 0.1024 |
| σ (eV−1) | 14.2653 | 13.9275 | 9.7656 |
| χ (eV) | −0.1642 | −0.1631 | −0.1312 |
| 0.1923 | 0.1852 | 0.0840 | |
| ε | 5.2000 | 5.3995 | 11.9047 |
| ΔN110 | 35.5506 | 34.7012 | 24.1757 |
| ΔEb–d (eV) | −0.0175 | −0.0179 | −0.0256 |
| Structures | Total Energy | Adsorption Energy | Rigid Adsorption Energy |
|---|---|---|---|
| Fe(1 1 0)@ILs 1@H2O | −2459.130 | −3145.745 | −2521.515 |
| Fe(1 1 0)@ILs 2@H2O | −2293.572 | −2980.188 | −2347.356 |
| Fe(1 1 0)@ILs 3@H2O | −2029.694 | −2099.160 | −2088.581 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omari Alaoui, A.; Messali, M.; Elfalleh, W.; Hammouti, B.; Titi, A.; El-Hajjaji, F. Structure–Activity Relationship of Ionic Liquids for Acid Corrosion Inhibition. Int. J. Mol. Sci. 2025, 26, 5750. https://doi.org/10.3390/ijms26125750
Omari Alaoui A, Messali M, Elfalleh W, Hammouti B, Titi A, El-Hajjaji F. Structure–Activity Relationship of Ionic Liquids for Acid Corrosion Inhibition. International Journal of Molecular Sciences. 2025; 26(12):5750. https://doi.org/10.3390/ijms26125750
Chicago/Turabian StyleOmari Alaoui, Aymane, Mouslim Messali, Walid Elfalleh, Belkheir Hammouti, Abderrahim Titi, and Fadoua El-Hajjaji. 2025. "Structure–Activity Relationship of Ionic Liquids for Acid Corrosion Inhibition" International Journal of Molecular Sciences 26, no. 12: 5750. https://doi.org/10.3390/ijms26125750
APA StyleOmari Alaoui, A., Messali, M., Elfalleh, W., Hammouti, B., Titi, A., & El-Hajjaji, F. (2025). Structure–Activity Relationship of Ionic Liquids for Acid Corrosion Inhibition. International Journal of Molecular Sciences, 26(12), 5750. https://doi.org/10.3390/ijms26125750









