IL-1b-Bearing NETs: Bridging Inflammation to Early Cirrhosis in Hepatitis B
Abstract
1. Introduction
2. Results
2.1. Inflammatory Proteins and NETs Are Detected in the Circulation of HBV Patients
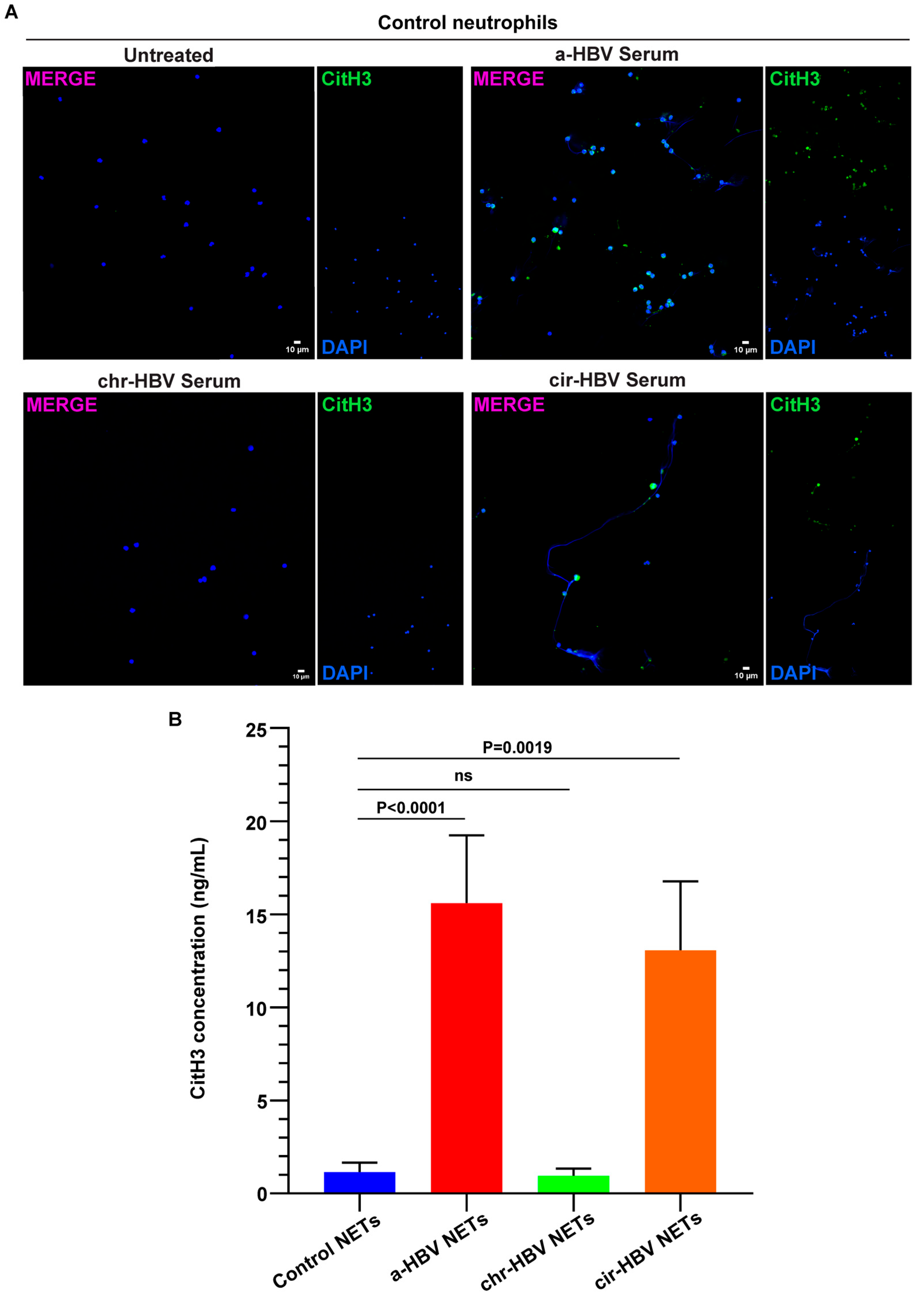
2.2. The Inflammatory Milieu of HBV Infection Triggers the Formation of NETs In Vitro
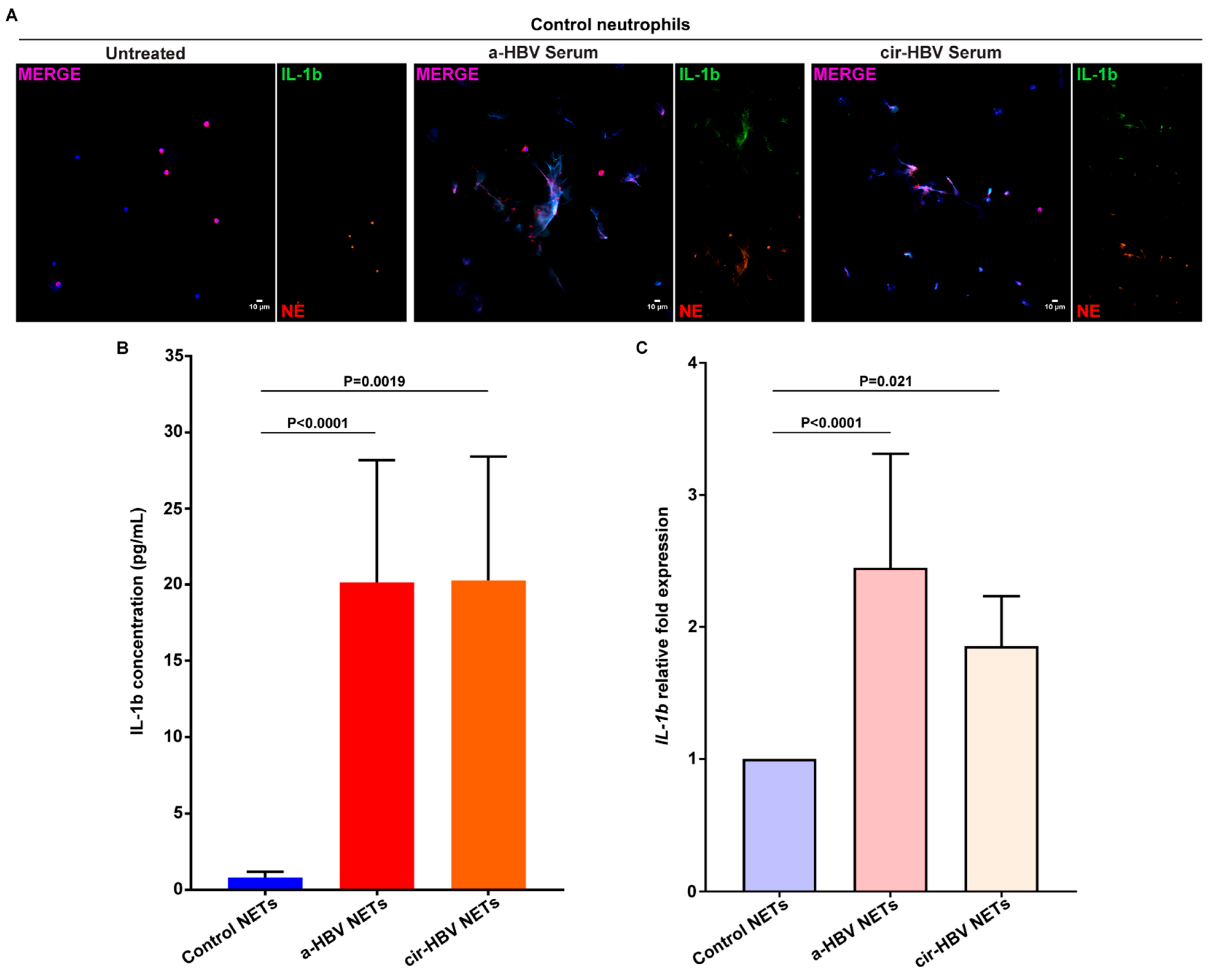
2.3. NETs, Formed in Response to the Inflammatory Environment of HBV, Are Coated with IL-1b
2.4. Inflammatory NETs Enhance the Fibrotic Dynamic of Mesenchymal Cells In Vitro
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Serum Collection
4.3. Neutrophil Isolation
4.4. Fibroblast Cell Culture
4.5. Stimulation and Inhibition Studies
4.5.1. Neutrophils
4.5.2. Fbs
4.6. NET Structures Generation and Isolation
4.7. Immunofluorescence
4.8. Bead-Based Multiplex Immunoassay
4.9. Citrullinated Histone H3 ELISA
4.10. Interleukin-1b ELISA
4.11. RNA Isolation, cDNA Synthesis, and Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
4.12. Migration/Wound Healing Assay
4.13. May–Grünwald Giemsa (MGG) Stain
4.14. Collagen Assay
4.15. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gutierrez-Reyes, G.; Gutierrez-Ruiz, M.C.; Kershenobich, D. Liver Fibrosis and Chronic Viral Hepatitis. Arch. Med. Res. 2007, 38, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Bedossa, P. Reversibility of Hepatitis B Virus Cirrhosis after Therapy: Who and Why? Liver Int. 2015, 35 (Suppl. 1), 78–81. [Google Scholar] [CrossRef] [PubMed]
- Rockey, D.C. Liver Fibrosis Reversion after Suppression of Hepatitis B Virus. Clin. Liver disease 2016, 20, 667. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wu, J.-F.; Zhang, Q.; Zhang, H.-W.; Cao, G.-W. Virus-Related Liver Cirrhosis: Molecular Basis and Therapeutic Options. World J. Gastroenterol. WJG 2014, 20, 6457. [Google Scholar] [CrossRef] [PubMed]
- Ringehan, M.; McKeating, J.A.; Protzer, U. Viral Hepatitis and Liver Cancer. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160274. [Google Scholar] [CrossRef]
- Nguyen, M.H.; Wong, G.; Gane, E.; Kao, J.-H.; Dusheiko, G. Hepatitis B Virus: Advances in Prevention, Diagnosis, and Therapy. Clin. Microbiol. Rev. 2020, 33, e00046-19. [Google Scholar] [CrossRef]
- Axiaris, G.; Zampeli, E.; Michopoulos, S.; Bamias, G. Management of Hepatitis B Virus Infection in Patients with Inflammatory Bowel Disease under Immunosuppressive Treatment. World J. Gastroenterol. 2021, 27, 3762–3779. [Google Scholar] [CrossRef]
- Zhao, J.; Bian, D.; Liao, H.; Wang, Y.; Ren, Y.; Jiang, Y.; Liu, S.; Chen, X.; Hu, Z.; Duan, Z.; et al. Serum HBsAg and HBcrAg Is Associated with Inflammation in HBeAg-Positive Chronic Hepatitis B Patients. Front. Cell. Infect. Microbiol. 2023, 13, 1083912. [Google Scholar] [CrossRef]
- Revill, P.A.; Chisari, F.V.; Block, J.M.; Dandri, M.; Gehring, A.J.; Guo, H.; Hu, J.; Kramvis, A.; Lampertico, P.; Janssen, H.L.A.; et al. A Global Scientific Strategy to Cure Hepatitis B. Lancet Gastroenterol. Hepatol. 2019, 4, 545–558. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Zhang, X.-Y. Innate Immune Recognition of Hepatitis B Virus. World J. Hepatol. 2015, 7, 2319. [Google Scholar] [CrossRef]
- Yang, G.; Wan, P.; Zhang, Y.; Tan, Q.; Qudus, M.S.; Yue, Z.; Luo, W.; Zhang, W.; Ouyang, J.; Li, Y.; et al. Innate Immunity, Inflammation, and Intervention in HBV Infection. Viruses 2022, 14, 2275. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Zhang, T.; Tang, L.; Li, Y. Cytokines and Chemokines in HBV Infection. Front. Mol. Biosci. 2021, 8, 805625. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Chen, J.; Zhang, J.; An, Y.; Liao, Y.; Wu, X.; Tao, C.; Wang, L.; Cai, B. Circulating IL-1β, IL-17, and IP-10 as Potential Predictors of Hepatitis B Virus Infection Prognosis. J. Immunol. Res. 2022, 2022, 5202898. [Google Scholar] [CrossRef]
- Jia, Y.; Jiao, X.; Shi, W.; Luo, Y.; Xiang, H.; Liang, J.; Gao, Y. Expression of 10 Circulating Cytokines/Chemokines in HBV-Related Liver Disease. Infect. Agents Cancer 2024, 19, 20. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, Y.; Feng, S.; Ishida, Y.; Chiu, T.-P.; Saito, T.; Wang, S.; Ann, D.K.; Ou, J.-H.J. Macrophages Activated by Hepatitis B Virus Have Distinct Metabolic Profiles and Suppress the Virus via IL-1β to Downregulate PPARα and FOXO3. Cell Rep. 2022, 38, 110284. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, K.; Katelani, S.; Pappa, M.; Fragkoulis, G.E.; Androutsakos, T. The Role of Interleukins in HBV Infection: A Narrative Review. J. Pers. Med. 2023, 13, 1675. [Google Scholar] [CrossRef]
- Ribeiro, C.R.d.A.; Beghini, D.G.; Lemos, A.S.; Martinelli, K.G.; de Mello, V.d.M.; de Almeida, N.A.A.; Lewis-Ximenez, L.L.; de Paula, V.S. Cytokines Profile in Patients with Acute and Chronic Hepatitis B Infection. Microbiol. Immunol. 2022, 66, 31–39. [Google Scholar] [CrossRef]
- Burn, G.L.; Foti, A.; Marsman, G.; Patel, D.F.; Zychlinsky, A. The Neutrophil. Immunity 2021, 54, 1377–1391. [Google Scholar] [CrossRef]
- Wang, J.P.; Bowen, G.N.; Padden, C.; Cerny, A.; Finberg, R.W.; Newburger, P.E.; Kurt-Jones, E.A. Toll-like Receptor–Mediated Activation of Neutrophils by Influenza A Virus. Blood 2008, 112, 2028. [Google Scholar] [CrossRef]
- Prince, L.R.; Whyte, M.K.; Sabroe, I.; Parker, L.C. The Role of TLRs in Neutrophil Activation. Curr. Opin. Pharmacol. 2011, 11, 397–403. [Google Scholar] [CrossRef]
- Johansson, C.; Kirsebom, F.C.M. Neutrophils in Respiratory Viral Infections. Mucosal Immunol. 2021, 14, 815–827. [Google Scholar] [CrossRef]
- Moles, A.; Murphy, L.; Wilson, C.L.; Chakraborty, J.B.; Fox, C.; Park, E.J.; Mann, J.; Oakley, F.; Howarth, R.; Brain, J.; et al. A TLR2/S100A9/CXCL-2 Signaling Network Is Necessary for Neutrophil Recruitment in Acute and Chronic Liver Injury in the Mouse. J. Hepatol. 2014, 60, 782–791. [Google Scholar] [CrossRef]
- Rosales, C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front. Physiol. 2018, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Silvestre-Roig, C.; Fridlender, Z.G.; Glogauer, M.; Scapini, P. Neutrophil Diversity in Health and Disease. Trends Immunol. 2019, 40, 565–583. [Google Scholar] [CrossRef] [PubMed]
- Venetsanopoulou, A.I.; Ntinopoulou, M.; Papagianni, E.; Koletsos, N.; Voulgari, P.V.; Chrysanthopoulou, A. Neutrophil Extracellular Traps as Immunofibrotic Mediators in RA-ILD; Pilot Evaluation of the Nintedanib Therapy. Front. Immunol. 2024, 15, 1480594. [Google Scholar] [CrossRef] [PubMed]
- Skendros, P.; Mitsios, A.; Chrysanthopoulou, A.; Mastellos, D.C.; Metallidis, S.; Rafailidis, P.; Ntinopoulou, M.; Sertaridou, E.; Tsironidou, V.; Tsigalou, C.; et al. Complement and Tissue Factor-Enriched Neutrophil Extracellular Traps Are Key Drivers in COVID-19 Immunothrombosis. J. Clin. Investig. 2020, 130, 6151–6157. [Google Scholar] [CrossRef]
- Gierlikowska, B.; Stachura, A.; Gierlikowski, W.; Demkow, U. Phagocytosis, Degranulation and Extracellular Traps Release by Neutrophils-The Current Knowledge, Pharmacological Modulation and Future Prospects. Front. Pharmacol. 2021, 12, 666732. [Google Scholar] [CrossRef]
- Rosales, C. Neutrophils at the Crossroads of Innate and Adaptive Immunity. J. Leukoc. Biol. 2020, 108, 377–396. [Google Scholar] [CrossRef]
- Frangou, E.; Chrysanthopoulou, A.; Mitsios, A.; Kambas, K.; Arelaki, S.; Angelidou, I.; Arampatzioglou, A.; Gakiopoulou, H.; Bertsias, G.K.; Verginis, P.; et al. REDD1/Autophagy Pathway Promotes Thromboinflammation and Fibrosis in Human Systemic Lupus Erythematosus (SLE) through NETs Decorated with Tissue Factor (TF) and Interleukin-17A (IL-17A). Ann. Rheum. Dis. 2019, 78, 238–248. [Google Scholar] [CrossRef]
- Ntinopoulou, M.; Cassimos, D.; Roupakia, E.; Kolettas, E.; Panopoulou, M.; Mantadakis, E.; Konstantinidis, T.; Chrysanthopoulou, A. Ιnterleukin-17A-Enriched Neutrophil Extracellular Traps Promote Immunofibrotic Aspects of Childhood Asthma Exacerbation. Biomedicines 2023, 11, 2104. [Google Scholar] [CrossRef]
- Chrysanthopoulou, A.; Mitroulis, I.; Apostolidou, E.; Arelaki, S.; Mikroulis, D.; Konstantinidis, T.; Sivridis, E.; Koffa, M.; Giatromanolaki, A.; Boumpas, D.T.; et al. Neutrophil Extracellular Traps Promote Differentiation and Function of Fibroblasts. J. Pathol. 2014, 233, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Hoar, D.I.; Bowen, T.; Matheson, D.; Poon, M.C. Hepatitis B Virus DNA Is Enriched in Polymorphonuclear Leukocytes. Blood 1985, 66, 1251–1253. [Google Scholar] [CrossRef]
- Leu, C.-M.; Lu, Y.-C.; Peng, W.-L.; Chu, H.-T.; Hu, C. The Hepatitis B Virus e Antigen Suppresses the Respiratory Burst and Mobility of Human Monocytes and Neutrophils. Immunobiology 2014, 219, 880–887. [Google Scholar] [CrossRef]
- Feng, Z.; Fu, J.; Tang, L.; Bao, C.; Liu, H.; Liu, K.; Yang, T.; Yuan, J.-H.; Zhou, C.-B.; Zhang, C.; et al. HBeAg Induces Neutrophils Activation Impairing NK Cells Function in Patients with Chronic Hepatitis B. Hepatol. Int. 2024, 18, 1122–1134. [Google Scholar] [CrossRef]
- Gschwandtner, M.; Derler, R.; Midwood, K.S. More Than Just Attractive: How CCL2 Influences Myeloid Cell Behavior Beyond Chemotaxis. Front. Immunol. 2019, 10, 2759. [Google Scholar] [CrossRef] [PubMed]
- Balamayooran, G.; Batra, S.; Balamayooran, T.; Cai, S.; Jeyaseelan, S. Monocyte Chemoattractant Protein 1 Regulates Pulmonary Host Defense via Neutrophil Recruitment during Escherichia Coli Infection. Infect. Immun. 2011, 79, 2567. [Google Scholar] [CrossRef]
- Leung, B.P.; Culshaw, S.; Gracie, J.A.; Hunter, D.; Canetti, C.A.; Campbell, C.; Cunha, F.; Liew, F.Y.; McInnes, I.B. A Role for IL-18 in Neutrophil Activation. J. Immunol. 2001, 167, 2879–2886. [Google Scholar] [CrossRef] [PubMed]
- Artru, F.; Bou Saleh, M.; Maggiotto, F.; Lassailly, G.; Ningarhari, M.; Demaret, J.; Ntandja-Wandji, L.-C.; Pais de Barros, J.-P.; Labreuche, J.; Drumez, E.; et al. IL-33/ST2 Pathway Regulates Neutrophil Migration and Predicts Outcome in Patients with Severe Alcoholic Hepatitis. J. Hepatol. 2020, 72, 1052–1061. [Google Scholar] [CrossRef]
- Sun, B.; Zhu, L.; Tao, Y.; Sun, H.-X.; Li, Y.; Wang, P.; Hou, Y.; Zhao, Y.; Zhang, X.; Zhang, L.; et al. Characterization and Allergic Role of IL-33-Induced Neutrophil Polarization. Cell. Mol. Immunol. 2018, 15, 782–793. [Google Scholar] [CrossRef]
- Hofbauer, T.M.; Ondracek, A.S.; Mangold, A.; Scherz, T.; Nechvile, J.; Seidl, V.; Brostjan, C.; Lang, I.M. Neutrophil Extracellular Traps Induce MCP-1 at the Culprit Site in ST-Segment Elevation Myocardial Infarction. Front. Cell Dev. Biol. 2020, 8, 564169. [Google Scholar] [CrossRef]
- Kahlenberg, J.M.; Carmona-Rivera, C.; Smith, C.K.; Kaplan, M.J. Neutrophil Extracellular Trap-Associated Protein Activation of the NLRP3 Inflammasome Is Enhanced in Lupus Macrophages. J. Immunol. 2013, 190, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, H.O.; Chen, H.-W.; Tohme, S.; Tai, S.; van der Windt, D.J.; Loughran, P.; Rosborough, B.R.; Sud, V.; Beer-Stolz, D.; Turnquist, H.R.; et al. IL-33 Exacerbates Liver Sterile Inflammation by Amplifying Neutrophil Extracellular Trap Formation. J. Hepatol. 2018, 68, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Apostolidou, E.; Skendros, P.; Kambas, K.; Mitroulis, I.; Konstantinidis, T.; Chrysanthopoulou, A.; Nakos, K.; Tsironidou, V.; Koffa, M.; Boumpas, D.T.; et al. Neutrophil Extracellular Traps Regulate IL-1β-Mediated Inflammation in Familial Mediterranean Fever. Ann. Rheum. Dis. 2016, 75, 269–277. [Google Scholar] [CrossRef]
- Buckley, C.D.; Filer, A.; Haworth, O.; Parsonage, G.; Salmon, M. Defining a Role for Fibroblasts in the Persistence of Chronic Inflammatory Joint Disease. Ann. Rheum. Dis. 2004, 63 (Suppl. 2), ii92–ii95. [Google Scholar] [CrossRef]
- Wei, K.; Nguyen, H.N.; Brenner, M.B. Fibroblast Pathology in Inflammatory Diseases. J. Clin. Investig. 2021, 131, e149538. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.D.; Pilling, D.; Lord, J.M.; Akbar, A.N.; Scheel-Toellner, D.; Salmon, M. Fibroblasts Regulate the Switch from Acute Resolving to Chronic Persistent Inflammation. Trends Immunol. 2001, 22, 199–204. [Google Scholar] [CrossRef]
- Hartupee, J.; Mann, D.L. Role of Inflammatory Cells in Fibroblast Activation. J. Mol. Cell. Cardiol. 2015, 93, 143. [Google Scholar] [CrossRef]
- Van Linthout, S.; Miteva, K.; Tschöpe, C. Crosstalk between Fibroblasts and Inflammatory Cells. Cardiovasc. Res. 2014, 102, 258–269. [Google Scholar] [CrossRef]
- Setten, E.; Castagna, A.; Nava-Sedeño, J.M.; Weber, J.; Carriero, R.; Reppas, A.; Volk, V.; Schmitz, J.; Gwinner, W.; Hatzikirou, H.; et al. Understanding Fibrosis Pathogenesis via Modeling Macrophage-Fibroblast Interplay in Immune-Metabolic Context. Nat. Commun. 2022, 13, 6499. [Google Scholar] [CrossRef]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and Chemokines: At the Crossroads of Cell Signalling and Inflammatory Disease. Biochim. Biophys. Acta 2014, 1843, 2563–2582. [Google Scholar] [CrossRef]
- Sokol, C.L.; Luster, A.D. The Chemokine System in Innate Immunity. Cold Spring Harb. Perspect. Biol. 2015, 7, a016303. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, Regulation, and Involvement in Disease. Int. Immunopharmacol. 2021, 101, 107598. [Google Scholar] [CrossRef]
- Johnston, B.; Burns, A.R.; Suematsu, M.; Issekutz, T.B.; Woodman, R.C.; Kubes, P. Chronic Inflammation Upregulates Chemokine Receptors and Induces Neutrophil Migration to Monocyte Chemoattractant Protein-1. J. Clin. Investig. 1999, 103, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Sahin, H.; Wasmuth, H.E. Chemokines in Tissue Fibrosis. Biochim. Biophys. Acta 2013, 1832, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Ihim, S.A.; Abubakar, S.D.; Zian, Z.; Sasaki, T.; Saffarioun, M.; Maleknia, S.; Azizi, G. Interleukin-18 Cytokine in Immunity, Inflammation, and Autoimmunity: Biological Role in Induction, Regulation, and Treatment. Front. Immunol. 2022, 13, 919973. [Google Scholar] [CrossRef]
- Robertson, S.E.; Young, J.D.; Kitson, S.; Pitt, A.; Evans, J.; Roes, J.; Karaoglu, D.; Santora, L.; Ghayur, T.; Liew, F.Y.; et al. Expression and Alternative Processing of IL-18 in Human Neutrophils. Eur. J. Immunol. 2006, 36, 722–731. [Google Scholar] [CrossRef]
- Elbim, C.; Guichard, C.; Dang, P.M.C.; Fay, M.; Pedruzzi, E.; Demur, H.; Pouzet, C.; El Benna, J.; Gougerot-Pocidalo, M.-A. Interleukin-18 Primes the Oxidative Burst of Neutrophils in Response to Formyl-Peptides: Role of Cytochrome B558 Translocation and N-Formyl Peptide Receptor Endocytosis. Clin. Diagn. Lab. Immunol. 2005, 12, 436–446. [Google Scholar] [CrossRef]
- Yasuda, K.; Nakanishi, K.; Tsutsui, H. Interleukin-18 in Health and Disease. Int. J. Mol. Sci. 2019, 20, 649. [Google Scholar] [CrossRef]
- Baggio, C.; Bindoli, S.; Guidea, I.; Doria, A.; Oliviero, F.; Sfriso, P. IL-18 in Autoinflammatory Diseases: Focus on Adult Onset Still Disease and Macrophages Activation Syndrome. Int. J. Mol. Sci. 2023, 24, 11125. [Google Scholar] [CrossRef]
- Colafrancesco, S.; Priori, R.; Alessandri, C.; Perricone, C.; Pendolino, M.; Picarelli, G.; Valesini, G. IL-18 Serum Level in Adult Onset Still’s Disease: A Marker of Disease Activity. Int. J. Inflamm. 2012, 2012, 156890. [Google Scholar] [CrossRef]
- Knorr, J.; Kaufmann, B.; Inzaugarat, M.E.; Holtmann, T.M.; Geisler, L.; Hundertmark, J.; Kohlhepp, M.S.; Boosheri, L.M.; Chilin-Fuentes, D.R.; Birmingham, A.; et al. Interleukin-18 Signaling Promotes Activation of Hepatic Stellate Cells in Mouse Liver Fibrosis. Hepatology 2023, 77, 1968–1982. [Google Scholar] [CrossRef] [PubMed]
- Liew, F.Y.; Girard, J.-P.; Turnquist, H.R. Interleukin-33 in Health and Disease. Nat. Rev. Immunol. 2016, 16, 676–689. [Google Scholar] [CrossRef] [PubMed]
- McHedlidze, T.; Waldner, M.; Zopf, S.; Walker, J.; Rankin, A.L.; Schuchmann, M.; Voehringer, D.; McKenzie, A.N.J.; Neurath, M.F.; Pflanz, S.; et al. Interleukin-33-Dependent Innate Lymphoid Cells Mediate Hepatic Fibrosis. Immunity 2013, 39, 357–371. [Google Scholar] [CrossRef]
- Tan, Z.; Liu, Q.; Jiang, R.; Lv, L.; Shoto, S.S.; Maillet, I.; Quesniaux, V.; Tang, J.; Zhang, W.; Sun, B.; et al. Interleukin-33 Drives Hepatic Fibrosis through Activation of Hepatic Stellate Cells. Cell. Mol. Immunol. 2018, 15, 388–398. [Google Scholar] [CrossRef]
- Wang, H.; Kim, S.J.; Lei, Y.; Wang, S.; Wang, H.; Huang, H.; Zhang, H.; Tsung, A. Neutrophil Extracellular Traps in Homeostasis and Disease. Signal Transduct. Target. Ther. 2024, 9, 235. [Google Scholar] [CrossRef]
- Hu, S.; Liu, X.; Gao, Y.; Zhou, R.; Wei, M.; Dong, J.; Yan, H.; Zhao, Y. Hepatitis B Virus Inhibits Neutrophil Extracellular Trap Release by Modulating Reactive Oxygen Species Production and Autophagy. J. Immunol. 2019, 202, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Wu, R.; Kong, X.; You, Y.; He, K.; Sun, X.; Huang, Y.; Chen, W.; Duan, L. Elevated Neutrophil Extracellular Traps by HBV-mediated S100A9-TLR4/RAGE-ROS Cascade Facilitate the Growth and Metastasis of Hepatocellular Carcinoma. Cancer Commun. 2022, 43, 225–245. [Google Scholar] [CrossRef]
- Yuen, M.-F.; Chen, D.-S.; Dusheiko, G.M.; Janssen, H.L.A.; Lau, D.T.Y.; Locarnini, S.A.; Peters, M.G.; Lai, C.-L. Hepatitis B Virus Infection. Nat. Rev. Dis. Primers 2018, 4, 1–20. [Google Scholar] [CrossRef]
- Taylor, N.J.; Nishtala, A.; Manakkat Vijay, G.K.; Abeles, R.D.; Auzinger, G.; Bernal, W.; Ma, Y.; Wendon, J.A.; Shawcross, D.L. Circulating Neutrophil Dysfunction in Acute Liver Failure. Hepatology 2013, 57, 1142–1152. [Google Scholar] [CrossRef]
- Fabre, T.; Molina, M.F.; Soucy, G.; Goulet, J.-P.; Willems, B.; Villeneuve, J.-P.; Bilodeau, M.; Shoukry, N.H. Type 3 Cytokines IL-17A and IL-22 Drive TGF-β-Dependent Liver Fibrosis. Sci. Immunol. 2018, 3, eaar7754. [Google Scholar] [CrossRef]
- Zenlander, R.; Havervall, S.; Magnusson, M.; Engstrand, J.; Ågren, A.; Thålin, C.; Stål, P. Neutrophil Extracellular Traps in Patients with Liver Cirrhosis and Hepatocellular Carcinoma. Sci. Rep. 2021, 11, 18025. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, R.; Kaur, N.; Maiwall, R.; Ramakrishna, G.; Maras, J.S.; Trehanpati, N. Plasma Proteomic Analysis Identified Proteins Associated with Faulty Neutrophils Functionality in Decompensated Cirrhosis Patients with Sepsis. Cells 2022, 11, 1745. [Google Scholar] [CrossRef]
- Wu, W.; Sun, S.; Wang, Y.; Zhao, R.; Ren, H.; Li, Z.; Zhao, H.; Zhang, Y.; Sheng, J.; Chen, Z.; et al. Circulating Neutrophil Dysfunction in HBV-Related Acute-on-Chronic Liver Failure. Front. Immunol. 2021, 12, 620365. [Google Scholar] [CrossRef]
- Tilg, H.; Wilmer, A.; Vogel, W.; Herold, M.; Nölchen, B.; Judmaier, G.; Huber, C. Serum Levels of Cytokines in Chronic Liver Diseases. Gastroenterology 1992, 103, 264–274. [Google Scholar] [CrossRef]
- Ludwiczek, O.; Vannier, E.; Moschen, A.; Salazar-Montes, A.; Borggraefe, I.; Gabay, C.; Enrich, B.; Kaser, A.; Siegmund, B.; Dinarello, C.; et al. Impaired Counter-Regulation of Interleukin-1 by the Soluble IL-1 Receptor Type II in Patients with Chronic Liver Disease. Scand. J. Gastroenterol. 2008, 43, 1360–1365. [Google Scholar] [CrossRef]
- Gieling, R.G.; Wallace, K.; Han, Y.-P. Interleukin-1 Participates in the Progression from Liver Injury to Fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G1324–G1331. [Google Scholar] [CrossRef] [PubMed]
- Skendros, P.; Chrysanthopoulou, A.; Rousset, F.; Kambas, K.; Arampatzioglou, A.; Mitsios, A.; Bocly, V.; Konstantinidis, T.; Pellet, P.; Angelidou, I.; et al. Regulated in Development and DNA Damage Responses 1 (REDD1) Links Stress with IL-1β-Mediated Familial Mediterranean Fever Attack through Autophagy-Driven Neutrophil Extracellular Traps. J. Allergy Clin. Immunol. 2017, 140, 1378–1387.e13. [Google Scholar] [CrossRef] [PubMed]
- Mitroulis, I.; Kambas, K.; Chrysanthopoulou, A.; Skendros, P.; Apostolidou, E.; Kourtzelis, I.; Drosos, G.I.; Boumpas, D.T.; Ritis, K. Neutrophil Extracellular Trap Formation Is Associated with IL-1β and Autophagy-Related Signaling in Gout. PLoS ONE 2011, 6, e29318. [Google Scholar] [CrossRef]
- Fanning, G.C.; Zoulim, F.; Hou, J.; Bertoletti, A. Therapeutic Strategies for Hepatitis B Virus Infection: Towards a Cure. Nat. Rev. Drug Discov. 2019, 18, 827–844. [Google Scholar] [CrossRef]
- Tillmann, H.L. Antiviral Therapy and Resistance with Hepatitis B Virus Infection. World J. Gastroenterol. 2007, 13, 125–140. [Google Scholar] [CrossRef]
- Block, T.M.; Gish, R.; Guo, H.; Mehta, A.; Cuconati, A.; London, W.T.; Guo, J.-T. Chronic Hepatitis B: What Should Be the Goal for New Therapies? Antivir. Res. 2013, 98, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Angelidou, I.; Chrysanthopoulou, A.; Mitsios, A.; Arelaki, S.; Arampatzioglou, A.; Kambas, K.; Ritis, D.; Tsironidou, V.; Moschos, I.; Dalla, V.; et al. REDD1/Autophagy Pathway Is Associated with Neutrophil-Driven IL-1β Inflammatory Response in Active Ulcerative Colitis. J. Immunol. 2018, 200, 3950–3961. [Google Scholar] [CrossRef] [PubMed]
- Saffarzadeh, M.; Juenemann, C.; Queisser, M.A.; Lochnit, G.; Barreto, G.; Galuska, S.P.; Lohmeyer, J.; Preissner, K.T. Neutrophil Extracellular Traps Directly Induce Epithelial and Endothelial Cell Death: A Predominant Role of Histones. PLoS ONE 2012, 7, e32366. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Mitroulis, I.; Chrysanthopoulou, A.; Divolis, G.; Ioannidis, C.; Ntinopoulou, M.; Tasis, A.; Konstantinidis, T.; Antoniadou, C.; Soteriou, N.; Lallas, G.; et al. A Gene Expression Map of Host Immune Response in Human Brucellosis. Front. Immunol. 2022, 13, 951232. [Google Scholar] [CrossRef]
- Chrysanthopoulou, A.; Mitroulis, I.; Kambas, K.; Skendros, P.; Kourtzelis, I.; Vradelis, S.; Kolios, G.; Aslanidis, S.; Doumas, M.; Ritis, K. Tissue Factor-Thrombin Signaling Enhances the Fibrotic Activity of Myofibroblasts in Systemic Sclerosis through up-Regulation of Endothelin Receptor A. Arthritis Rheum. 2011, 63, 3586–3597. [Google Scholar] [CrossRef]


| Acute HBV | Chronic HBV | Early Cirrhosis | Control | |
| Age | 55 ± 9.8 | 56.21 ± 9.4 | 69.1 ± 11.9 | 55 ± 9.8 |
| Male (%) | 7 (63.6%) | 8 (72.7%) | 8 (72.7%) | 9 (81.8%) |
| Laboratory findings | ||||
| WBC (109/L) | 5.1 ± 1.3 | 5.7 ± 2.6 | 6.6 ± 3.2 | 5.3 ± 5.6 |
| Hg (g/L) | 13.4 ± 1.3 | 13.8 ± 0.4 | 13.4 ± 2 | 14.3 ± 1.3 |
| PLT (109/L) | 162 ± 50.4 | 146 ± 48.1 | 142 ± 68.8 | 241 ± 43.7 |
| SGOT (Mean ± SD) | 409.5 ± 498.9 | 30 ± 4.2 | 29.3 ± 7.2 | 15.6 ± 9.5 |
| SGPT (Mean ± SD) | 271.8 ± 404.5 | 34.2 ± 4.4 | 30.3 ± 22.5 | 13.8 ± 9.5 |
| gGT (Mean ± SD) | 145.7 ± 83.5 | 98.2 ± 36.2 | 56.6 ± 102.2 | 12.2 ± 3.8 |
| HBsAg | 3161.4 ± 1618.3 | 1806.7 ± 1025.4 | 3850.5 ± 2080.3 | 0.03 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntinopoulou, M.; Konstantinidis, T.; Chalkidou, A.; Papagianni, E.; Skeva, A.; Panopoulou, M.; Chrysanthopoulou, A. IL-1b-Bearing NETs: Bridging Inflammation to Early Cirrhosis in Hepatitis B. Int. J. Mol. Sci. 2025, 26, 5733. https://doi.org/10.3390/ijms26125733
Ntinopoulou M, Konstantinidis T, Chalkidou A, Papagianni E, Skeva A, Panopoulou M, Chrysanthopoulou A. IL-1b-Bearing NETs: Bridging Inflammation to Early Cirrhosis in Hepatitis B. International Journal of Molecular Sciences. 2025; 26(12):5733. https://doi.org/10.3390/ijms26125733
Chicago/Turabian StyleNtinopoulou, Maria, Theocharis Konstantinidis, Anna Chalkidou, Eleni Papagianni, Aikaterini Skeva, Maria Panopoulou, and Akrivi Chrysanthopoulou. 2025. "IL-1b-Bearing NETs: Bridging Inflammation to Early Cirrhosis in Hepatitis B" International Journal of Molecular Sciences 26, no. 12: 5733. https://doi.org/10.3390/ijms26125733
APA StyleNtinopoulou, M., Konstantinidis, T., Chalkidou, A., Papagianni, E., Skeva, A., Panopoulou, M., & Chrysanthopoulou, A. (2025). IL-1b-Bearing NETs: Bridging Inflammation to Early Cirrhosis in Hepatitis B. International Journal of Molecular Sciences, 26(12), 5733. https://doi.org/10.3390/ijms26125733








