Lemon Verbena Extract Enhances Sleep Quality and Duration via Modulation of Adenosine A1 and GABAA Receptors in Pentobarbital-Induced and Polysomnography-Based Sleep Models
Abstract
1. Introduction
2. Results
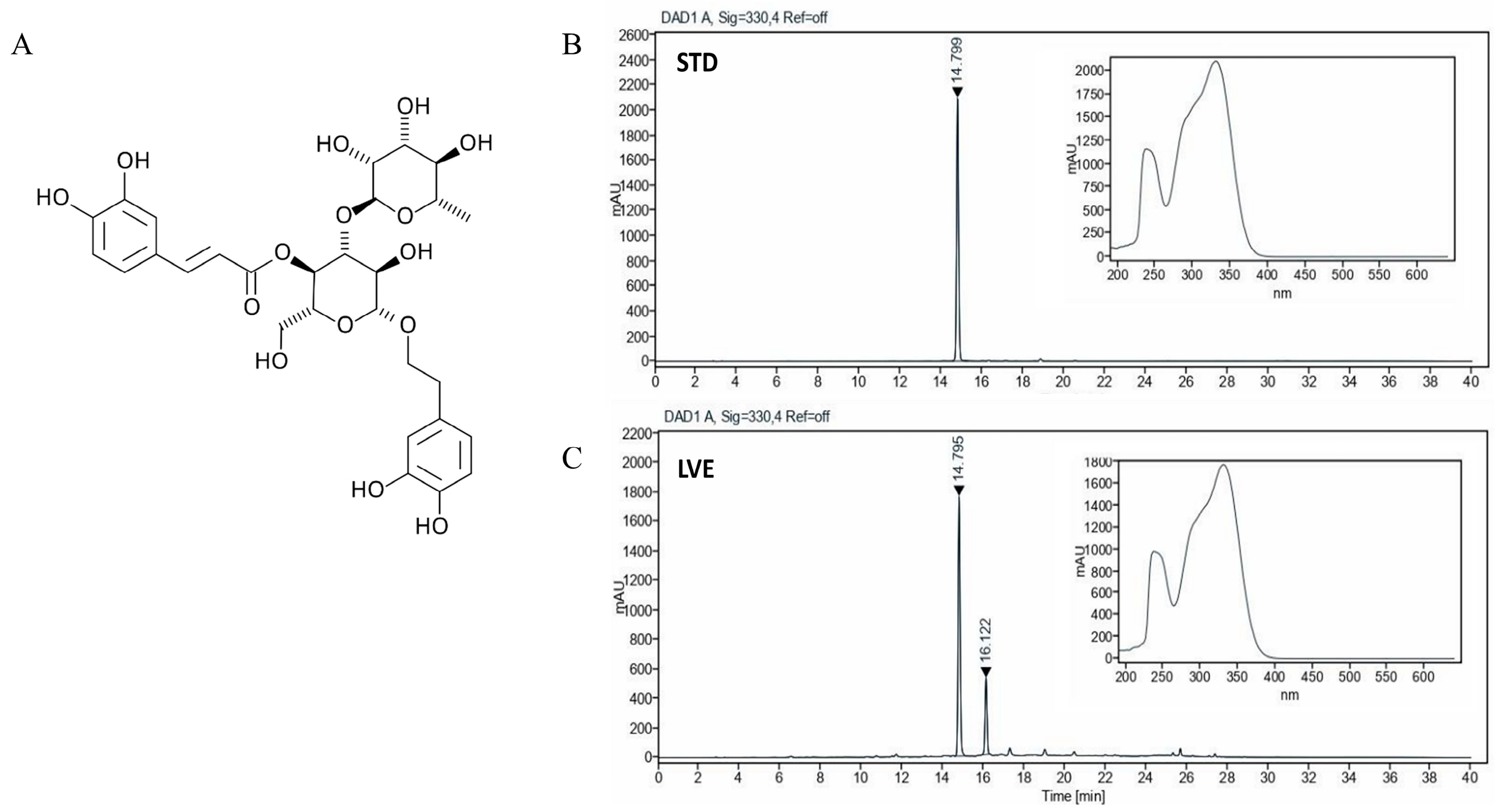
2.1. HPLC Analysis of Verbascoside in LVE
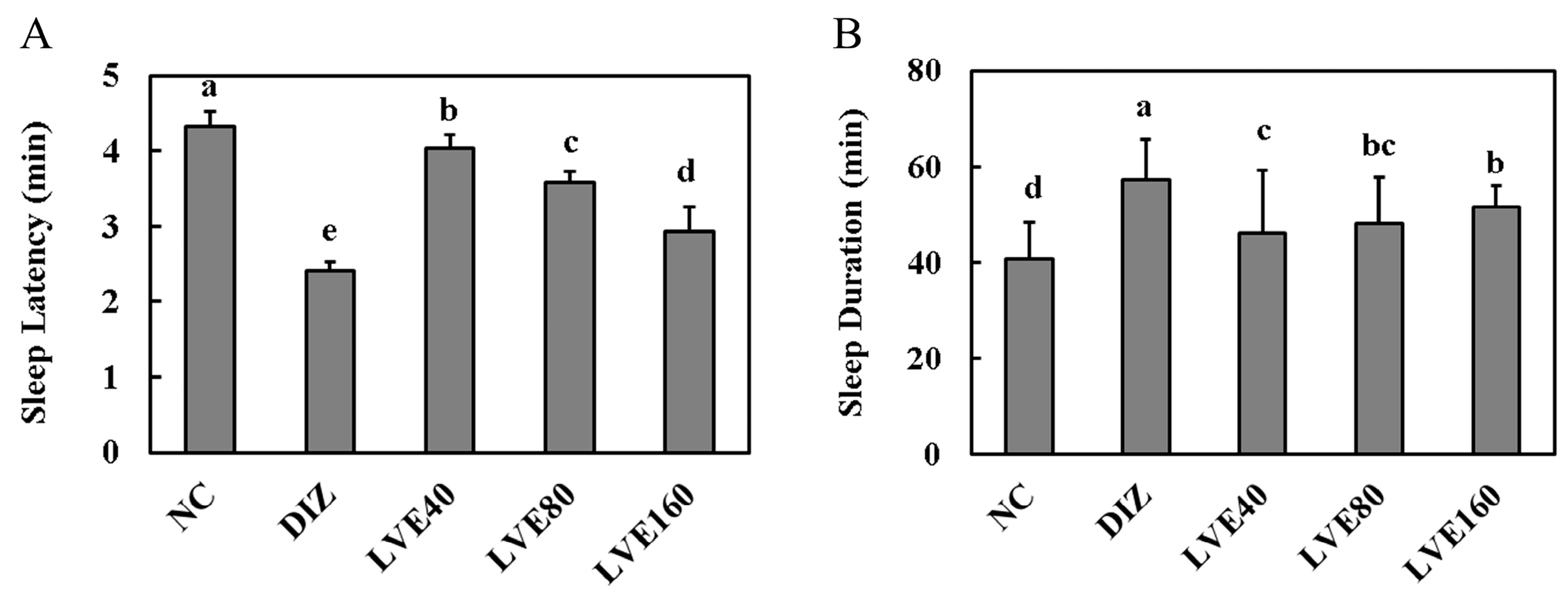
2.2. LVE Modulates Sleep Latency and Duration in Pentobarbital-Injected Mice
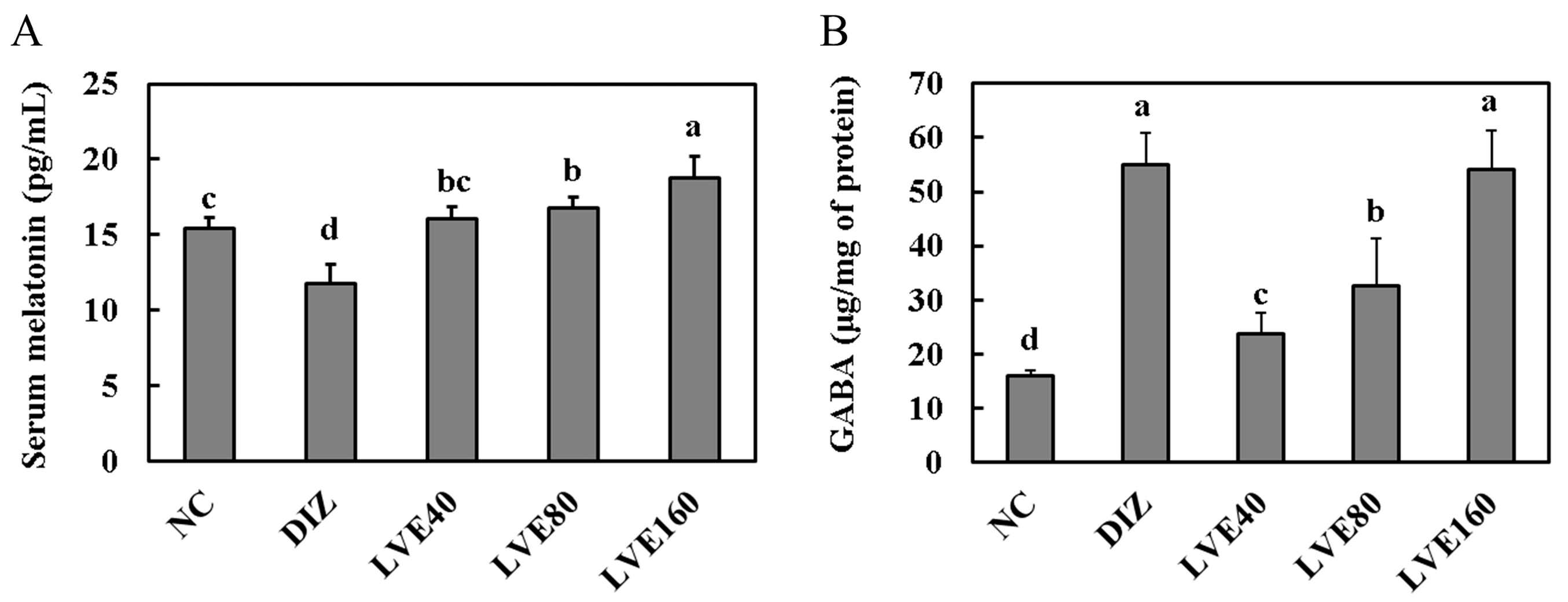
2.3. LVE Increases Serum Melatonin Levels and Brain GABA Concentrations in Pentobarbital-Injected Mice
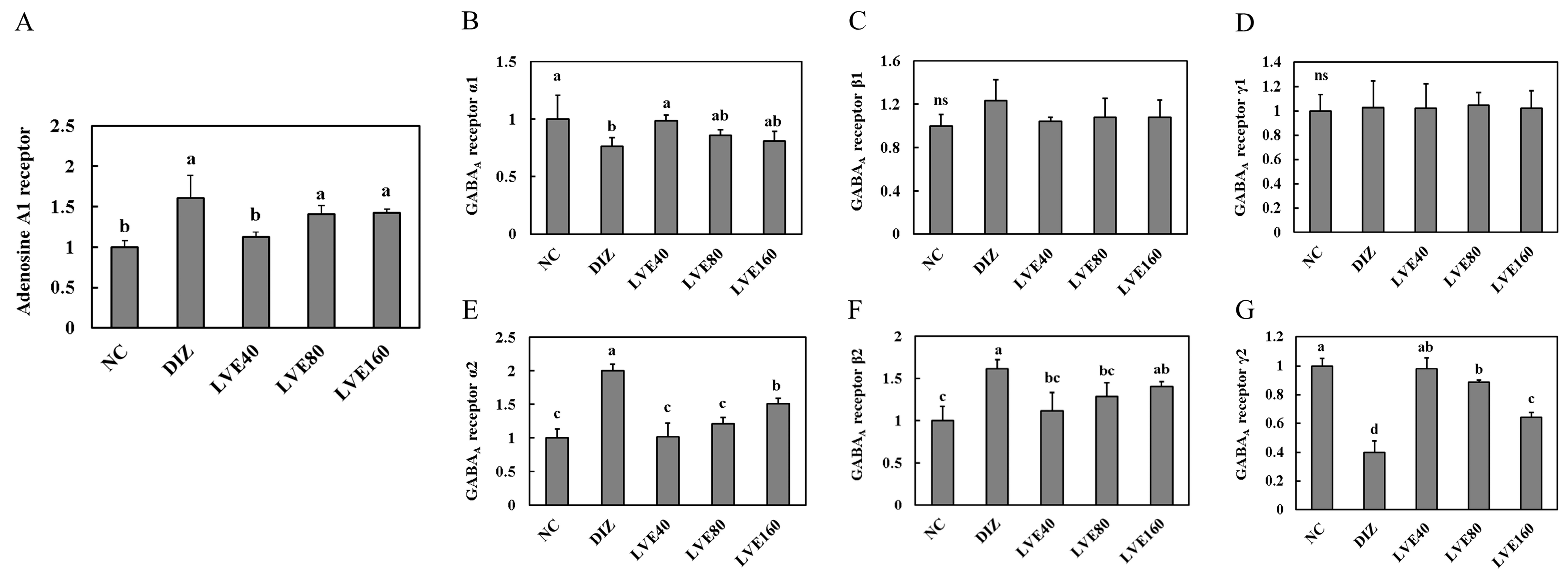
2.4. Effect of LVE on Adenosine A1 Receptor from Pentobarbital-Injected Mice
2.5. LVE Modulates GABAA Receptor Expression in the Brains of Pentobarbital-Injected Mice
2.6. LVE Modulates GABAA Receptor Protein Expression in the Brains of Pentobarbital-Injected Mice
2.7. Body Weight Changes in C57BL/6N Mice Related to EEG/EMG-Based Sleep Models
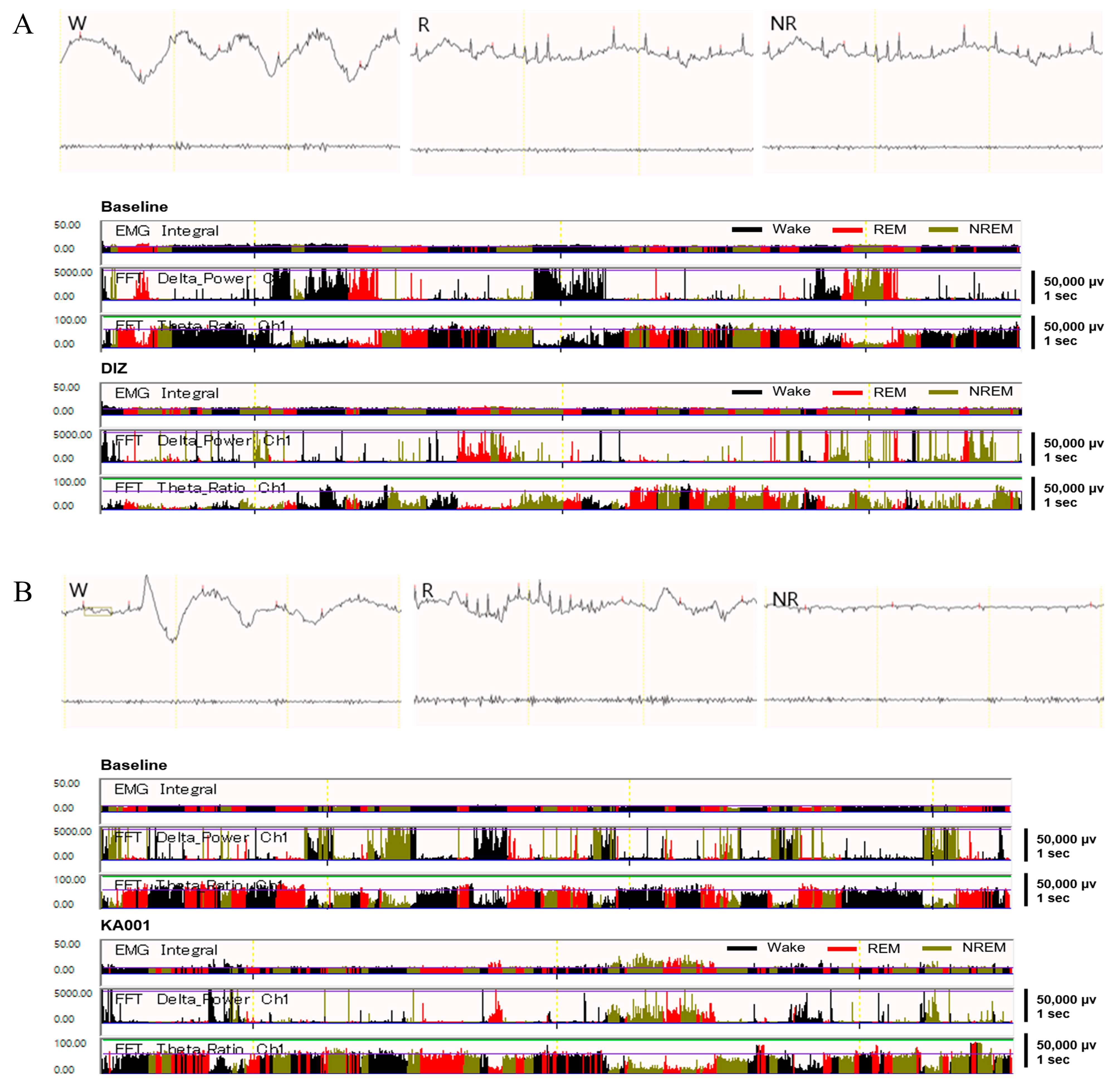
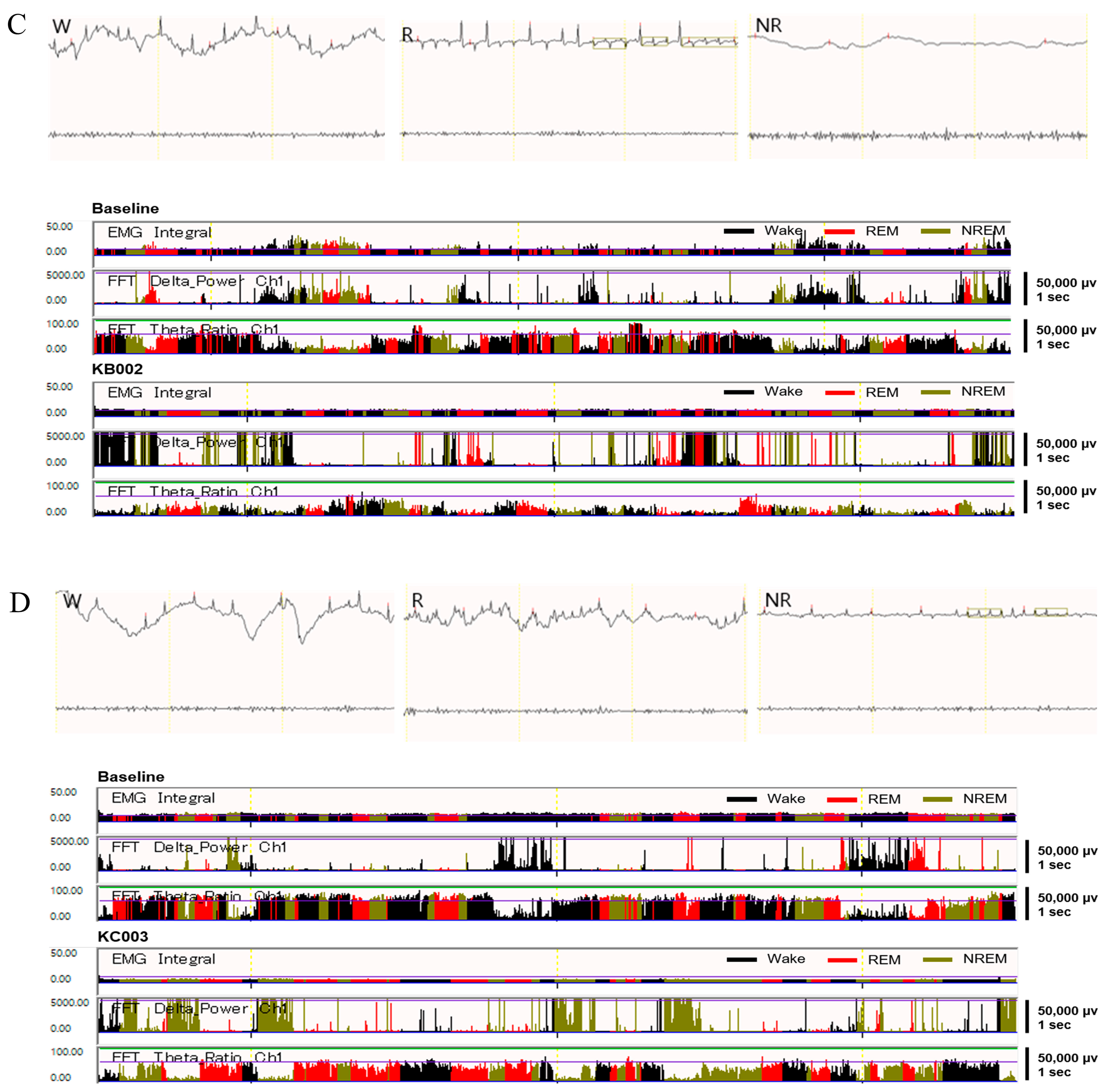
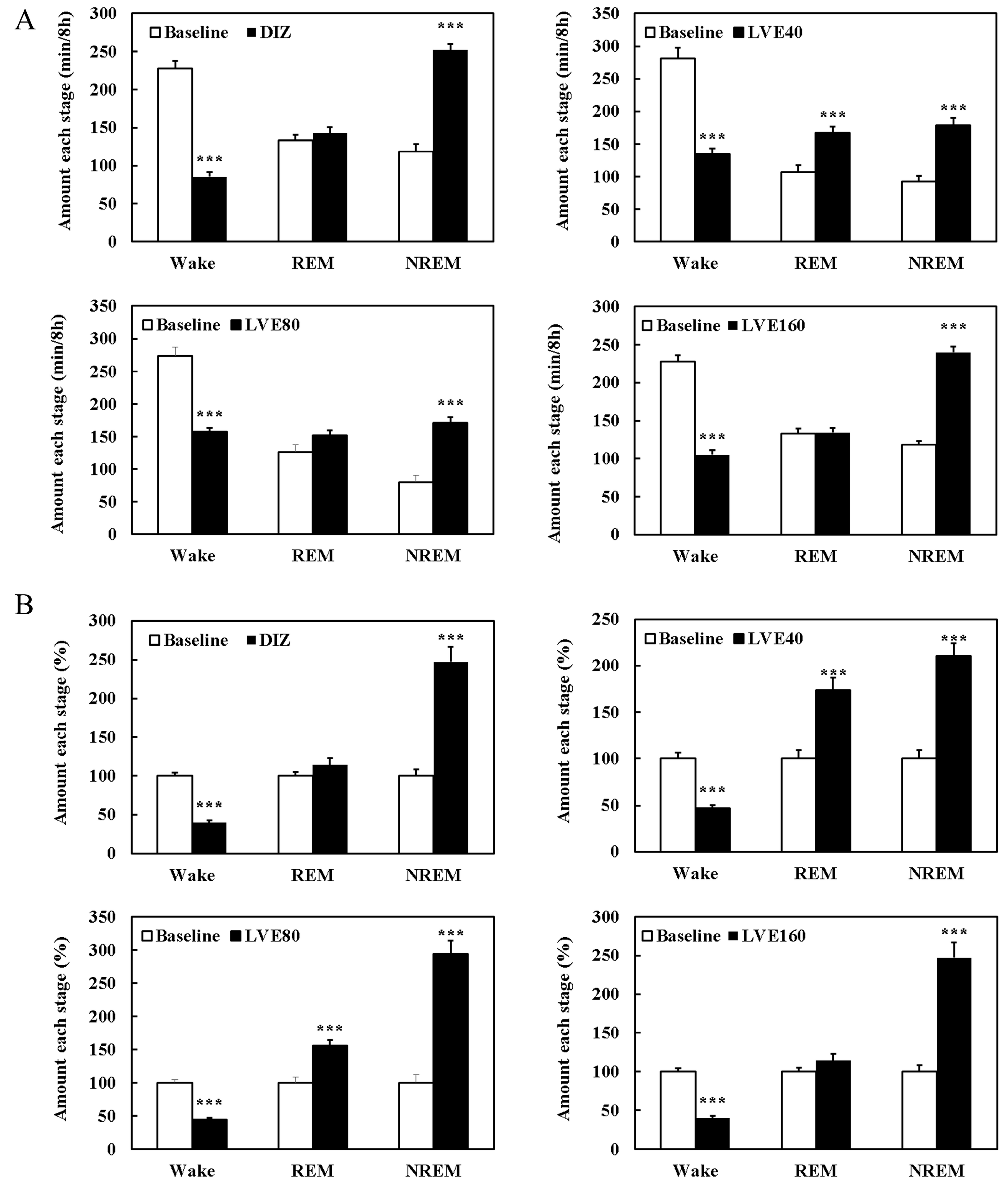
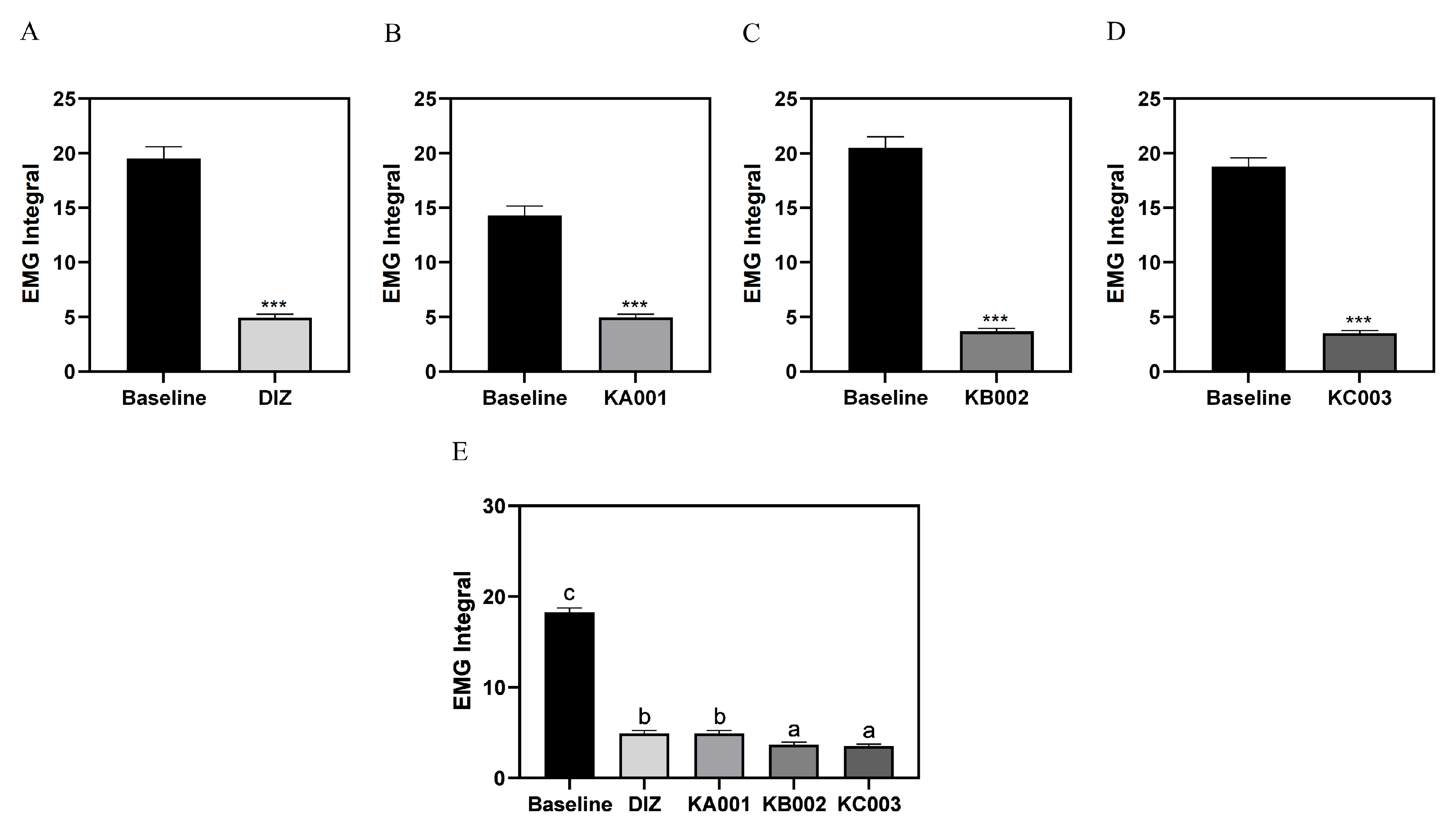
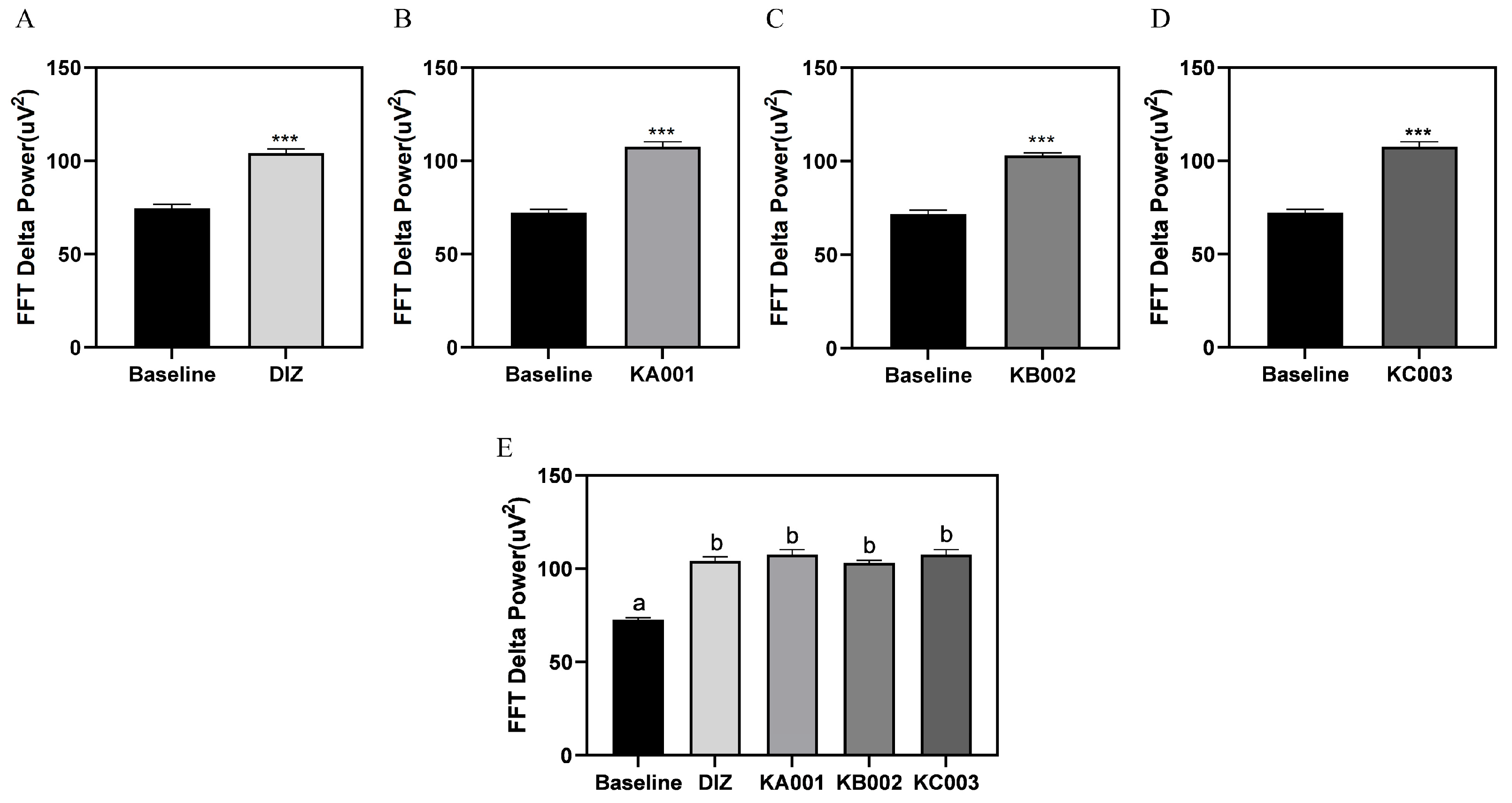
2.8. LVE Improves Sleep Quality and Duration in EEG/EMG-Based Sleep Models
3. Discussion
4. Materials and Methods
4.1. Preparation of Lemon Verbena Extract and High-Performance Liquid Chromatography (HPLC)
4.2. Pentobarbital-Induced Sleep in Mice
4.3. Sleep Latency and Sleep Duration
4.4. ELISA
4.5. Real-Time Polymerase Chain Reaction (RT-PCR)
4.6. Protein Extraction and Western Blot Analysis
4.7. Electroencephalography (EEG) and Electromyogram (EMG) Implantation Surgery Model
4.8. EEG Recording and Sleep–Wake State Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mukherjee, U.; Sehar, U.; Brownell, M.; Reddy, P.H. Mechanisms, consequences and role of interventions for sleep deprivation: Focus on mild cognitive impairment and Alzheimer’s disease in elderly. Ageing Res. Rev. 2024, 100, 102457. [Google Scholar] [CrossRef] [PubMed]
- Wipper, B.; Mayer-Suess, L.; Cesari, M.; Ibrahim, A.; Winkelman, J.; Kiechl, S. Relationship of Suboptimal and Disordered Sleep with Cardiovascular Disease and Its Risk Factors—A Narrative Review. Neuroepidemiology 2024, 59, 176–192. [Google Scholar] [CrossRef]
- Dai, D.; Eng, K.J.; Alessi, C.A. Sleep and Sleep Disorders. In Geriatric Medicine: A Person Centered Evidence Based Approach; Wasserman, M.R., Bakerjian, D., Linnebur, S., Brangman, S., Cesari, M., Rosen, S., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 1251–1265. [Google Scholar]
- Yeom, J.W.; Cho, C.H. Herbal and Natural Supplements for Improving Sleep: A Literature Review. Psychiatry Investig. 2024, 21, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Rashid, H.M.; Mahmod, A.I.; Afifi, F.U.; Talib, W.H. Antioxidant and Antiproliferation Activities of Lemon Verbena (Aloysia citrodora): An in vitro and in vivo study. Plants 2022, 11, 785. [Google Scholar] [CrossRef]
- Gomes, D.B.; Serpa, P.Z.; Miorando, D.; Zanatta, M.E.D.; Carteri, C.S.; Somensi, L.B.; Venzon, L.; Santos, A.C.; França, T.C.; Silva, L.M. Involvement of anti-inflammatory and stress oxidative markers in the antidepressant-like activity of Aloysia citriodora and Verbascoside on mice with bacterial lipopolysaccharide-(LPS-) induced depression. Evid.-Based Complement. Altern. Med. 2022, 2022, 1041656. [Google Scholar] [CrossRef]
- Olivares-Vicente, M.; Sánchez-Marzo, N.; Herranz-López, M.; Micol, V. Analysis of Lemon Verbena Polyphenol Metabolome and Its Correlation with Oxidative Stress under Glucotoxic Conditions in Adipocyte. J. Agric. Food Chem. 2024, 72, 9768–9781. [Google Scholar] [CrossRef] [PubMed]
- Athanasiadis, V.; Chatzimitakos, T.; Makrygiannis, I.; Kalompatsios, D.; Bozinou, E.; Lalas, S.I. Antioxidant-Rich Extracts from Lemon Verbena (Aloysia citrodora L.) Leaves through Response Surface Methodology. Oxygen 2024, 4, 1–19. [Google Scholar] [CrossRef]
- Afrasiabian, F.; Mirabzadeh Ardakani, M.; Rahmani, K.; Azadi, N.A.; Alemohammad, Z.B.; Bidaki, R.; Karimi, M.; Emtiazy, M.; Hashempur, M.H. Aloysia citriodora Palau (lemon verbena) for insomnia patients: A randomized, double-blind, placebo-controlled clinical trial of efficacy and safety. Phytother. Res. 2019, 33, 350–359. [Google Scholar] [CrossRef]
- Huang, Z.-L.; Urade, Y.; Hayaishi, O. The role of adenosine in the regulation of sleep. Curr. Top. Med. Chem. 2011, 11, 1047–1057. [Google Scholar] [CrossRef]
- Zhu, W.; Huang, L.; Cheng, H.; Li, N.; Zhang, B.; Dai, W.; Wu, X.; Zhang, D.; Feng, W.; Li, S.; et al. GABA and its receptors’ mechanisms in the treatment of insomnia. Heliyon 2024, 10, e40665. [Google Scholar] [CrossRef]
- Rostami, S.F.; Roufegarinejad, L.; Karimidastjerd, A.; Habibzadeh Khiabani, A.; Toker, O.S.; Ghorbani, M. Employing Aloysia citrodora (lemon verbena) as a substrate to improve toxicological and antioxidative properties of kombucha beverage. Acta Aliment. 2024, 53, 410–418. [Google Scholar] [CrossRef]
- Talebi, M.; Obeid, M.; Mojab, F. Detection and Quantification of GABA and Melatonin Contents in Five Hypnotic Medicinal Plants using Chromatography-Based Techniques: Detection of GABA and Melatonin in Hypnotic Plants. Iran. J. Pharm. Sci. 2024, 20, 336–344. [Google Scholar] [CrossRef]
- Sobhanifar, M.-A.; Rashidi, R.; Rajabian, A.; Forouzanfar, F.; Hasanpour, M.; Iranshahi, M.; Rakhshandeh, H.; Hosseini, A. The possible mechanism of Datura stramonium on pentobarbital-induced sleep in mice. Int. J. Neurosci. 2023, 133, 879–887. [Google Scholar] [CrossRef]
- Shah, V.K.; Choi, J.J.; Han, J.-Y.; Lee, M.K.; Hong, J.T.; Oh, K.-W. Pachymic acid enhances pentobarbital-induced sleeping behaviors via GABAA-ergic systems in mice. Biomol. Ther. 2014, 22, 314. [Google Scholar] [CrossRef] [PubMed]
- Desai, D.; Momin, A.; Hirpara, P.; Jha, H.; Thaker, R.; Patel, J.; Momin, A.S. Exploring the Role of Circadian Rhythms in Sleep and Recovery: A Review Article. Cureus 2024, 16, e61568. [Google Scholar] [CrossRef]
- Mamelak, M. Sleep, narcolepsy, and sodium oxybate. Curr. Neuropharmacol. 2022, 20, 272–291. [Google Scholar] [CrossRef]
- Mercadante, S.; Bellastella, A. Chrono-Endocrinology in Clinical Practice: A Journey from Pathophysiological to Therapeutic Aspects. Life 2024, 14, 546. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, A.; Martínez-Olcina, M.; Mora, J.; Navarro, P.; Caturla, N.; Jones, J. Anxiolytic effect and improved sleep quality in individuals taking Lippia citriodora extract. Nutrients 2022, 14, 218. [Google Scholar] [CrossRef]
- Haryalchi, K.; Kazemi Aski, S.; Mansour Ghanaie, M.; Fotouhi, M.; Mansoori, R.; Sadraei, S.M.; Yaghobi, Y.; Olangian-Tehrani, S. Effects of the aroma of lemone verbena (Aloysia citriodora Paláu) essential oil on anxiety and the hemodynamic profile before cesarean section: A randomized clinical trial. Health Sci. Rep. 2023, 6, e1282. [Google Scholar] [CrossRef]
- Feistel, B.; Suarez-Rizzo, C.; Appel, K. Extracts of Aloysia citrodora Paláu (Lemon verbena) shows CNS activities by inhibition of neurotransmitter re-uptake in vitro. Z. Phytother. 2023, 44, P02. [Google Scholar]
- Hamdan, L.T. Origanum syriacum and Aloysia citrodora Essential Oil: Chemical Profile, Antibacterial, Anti-oxidant And Anticancer Activity. Ph.D. Thesis, Faculty of Graduate Studies, An-Najah National University, Nablus, Palestine, 2022. [Google Scholar]
- Molla, A.; Solomou, A.D.; Tziouvalekas, M.; Lolas, A.; Skoufogianni, E. Dynamics of Agronomic Characteristics and Plant Diversity in Lemon Verbena (Aloysia citrodora Paláu) Cultivation in Greece. Agriculture 2024, 14, 97. [Google Scholar] [CrossRef]
- Mansour, A.; Bechlem, H.; Labed, A. Traditional herbal medicines and natural remedies used for prevention and treatment of COVID-19 in Algeria. J. Mol. Pharm. Sci. 2022, 1, 74–91. [Google Scholar]
- Borrás, S.; Martínez-Solís, I.; Ríos, J.L. Medicinal plants for insomnia related to anxiety: An updated review. Planta Medica 2021, 87, 738–753. [Google Scholar] [CrossRef] [PubMed]
- Eddin, L.B.; Jha, N.K.; Meeran, M.N.; Kesari, K.K.; Beiram, R.; Ojha, S. Neuroprotective potential of limonene and limonene containing natural products. Molecules 2021, 26, 4535. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Chen, J.; Chen, X.; Tang, M.; Song, N.; Zhang, L.; He, T. Comparing Effects of Aromatherapy with Five Herbs Essential Oils on PCPA-induced Insomnia Mice. J. Microbiol. Biotechnol. 2024, 35, e2409021. [Google Scholar] [CrossRef]
- Weng, X.; Jiang, J.; Li, H.; Tan, Y. Network pharmacology strategy to investigate the pharmacological effects of Suanzaoren Decoction on insomnia. Braz. J. Pharm. Sci. 2023, 59, e21182. [Google Scholar] [CrossRef]
- Bao, Y.; Zhou, H.; Fu, Y.; Wang, C.; Huang, Q. Zhumian Granules improves PCPA-induced insomnia by regulating the expression level of neurotransmitters and reducing neuronal apoptosis. J. Ethnopharmacol. 2024, 327, 118048. [Google Scholar] [CrossRef]
- Jia, Q.; Tan, H.; Li, T.; Duan, X. Role of adenosine in the pathophysiology and treatment of attention deficit hyperactivity disorder. Purinergic Signal. 2024, 1–9. [Google Scholar] [CrossRef]
- Reichert, C.F.; Deboer, T.; Landolt, H.P. Adenosine, caffeine, and sleep–wake regulation: State of the science and perspectives. J. Sleep Res. 2022, 31, e13597. [Google Scholar] [CrossRef]
- Bozorgi, H.; Rashidy-Pour, A.; Moradikor, N.; Zamani, M.; Motaghi, E. Neurobehavioral protective effects of Japanese sake yeast supplement against chronic stress-induced anxiety and depression-like symptoms in mice: Possible role of central adenosine receptors. Psychopharmacology 2024, 241, 401–416. [Google Scholar] [CrossRef]
- Oishi, Y.; Saito, Y.C.; Sakurai, T. GABAergic modulation of sleep-wake states. Pharmacol. Ther. 2023, 249, 108505. [Google Scholar] [CrossRef]
- Wisden, W.; Yu, X.; Franks, N. GABA receptors and the pharmacology of sleep. In Sleep-Wake Neurobiology and Pharmacology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 279–304. [Google Scholar]
- Kim, J.J.; Hibbs, R.E. Direct structural insights into GABAA receptor pharmacology. Trends Biochem. Sci. 2021, 46, 502–517. [Google Scholar] [CrossRef]
- Nwosu, G.; Reddy, S.B.; Riordan, H.R.M.; Kang, J.-Q. Variable expression of GABAA receptor subunit gamma 2 mutation in a nuclear family displaying developmental and encephalopathic phenotype. Int. J. Mol. Sci. 2022, 23, 9683. [Google Scholar] [CrossRef]
- Shi, Y.-W.; Zhang, Q.; Cai, K.; Poliquin, S.; Shen, W.; Winters, N.; Yi, Y.-H.; Wang, J.; Hu, N.; Macdonald, R.L. Synaptic clustering differences due to different GABRB3 mutations cause variable epilepsy syndromes. Brain 2019, 142, 3028–3044. [Google Scholar] [CrossRef]
- Kun, X.; Cai Hong, H.; Subramanian, P. Melatonin and sleep. Biol. Rhythm Res. 2019, 50, 490–493. [Google Scholar] [CrossRef]
- Descamps, A.; Rousset, C.; Millan, M.J.; Spedding, M.; Delagrange, P.; Cespuglio, R. Influence of the novel antidepressant and melatonin agonist/serotonin2C receptor antagonist, agomelatine, on the rat sleep-wake cycle architecture. Psychopharmacology 2009, 205, 93–106. [Google Scholar] [CrossRef]
- Korf, H.W.; von Gall, C. Mouse models in circadian rhythm and melatonin research. J. Pineal Res. 2024, 76, e12986. [Google Scholar] [CrossRef]
- Gao, T.; Wang, Z.; Dong, Y.; Cao, J.; Lin, R.; Wang, X.; Yu, Z.; Chen, Y. Role of melatonin in sleep deprivation-induced intestinal barrier dysfunction in mice. J. Pineal Res. 2019, 67, e12574. [Google Scholar] [CrossRef]
- Pongkitwitoon, B.; Putalun, W.; Triwitayakorn, K.; Kitisripanya, T.; Kanchanapoom, T.; Boonsnongcheep, P. Anti-inflammatory activity of verbascoside-and isoverbascoside-rich Lamiales medicinal plants. Heliyon 2024, 10, e23644. [Google Scholar] [CrossRef]
- Anfuso, C.D.; Giurdanella, G.; Longo, A.; Cosentino, A.; Agafonova, A.; Rusciano, D.; Lupo, G. Antioxidant activity of cyanidin-3-O-glucoside and verbascoside in an in vitro model of diabetic retinopathy. Front. Biosci.-Landmark 2022, 27, 308. [Google Scholar] [CrossRef]
- Wang, C.; Cai, X.; Wang, R.; Zhai, S.; Zhang, Y.; Hu, W.; Zhang, Y.; Wang, D. Neuroprotective effects of verbascoside against Alzheimer’s disease via the relief of endoplasmic reticulum stress in Aβ-exposed U251 cells and APP/PS1 mice. J. Neuroinflamm. 2020, 17, 309. [Google Scholar] [CrossRef]
- Ye, M.; Rheu, K.-m.; Lee, B.-j.; Shim, I. GABALAGEN Facilitates Pentobarbital-Induced Sleep by Modulating the Serotonergic System in Rats. Curr. Issues Mol. Biol. 2024, 46, 11176–11189. [Google Scholar] [CrossRef]
- Rakhshandeh, H.; Heidari, A.; Pourbagher-Shahri, A.M.; Rashidi, R.; Forouzanfar, F. Hypnotic effect of a. absinthium hydroalcoholic extract in pentobarbital-treated mice. Neurol. Res. Int. 2021, 2021, 5521019. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, H.; Ma, K.; Deng, Y.; Li, M.; Ma, J. A novel alcohol steamed preparation from Gastrodia elata Blume: Pharmacological assessment of a functional food. Front. Pharmacol. 2023, 14, 1092693. [Google Scholar] [CrossRef]
- Nisar, M.A.; Saleem, M.Z. Mechanism of action of sedatives, hypnotics, and antianxiety drugs. In How Synthetic Drugs Work; Elsevier: Amsterdam, The Netherlands, 2023; pp. 161–176. [Google Scholar]
- Becker, M.; Pinhasov, A.; Ornoy, A. Animal models of depression: What can they teach us about the human disease? Diagnostics 2021, 11, 123. [Google Scholar] [CrossRef]
- Sun, S.; Lu, Z.; Shi, Y.; Zhao, L.; Guo, X. Study on elevating sleep efficacy of γ-Aminobutyric acid. IOP Conf. Ser. Earth Environ. Sci. 2020, 440, 022010. [Google Scholar] [CrossRef]
- Lee, M.; Park, J.; Cho, W.; Jun, Y.; Lee, H.; Jeon, G.; Jun, W.; Kim, O.-K. Lactuca sativa L. Extract Enhances Sleep Duration Through Upregulation of Adenosine A1 Receptor and GABAA Receptors Subunits in Pentobarbital-Injected Mice. J. Med. Food 2024, 27, 661–668. [Google Scholar] [CrossRef]
- Abdollahi Nejat, M.; Stiedl, O.; Smit, A.B.; van Kesteren, R.E. Continuous locomotor activity monitoring to assess animal welfare following intracranial surgery in mice. Front. Behav. Neurosci. 2024, 18, 1457894. [Google Scholar] [CrossRef]
- Haigh, J.L.; Adhikari, A.; Copping, N.A.; Stradleigh, T.; Wade, A.A.; Catta-Preta, R.; Su-Feher, L.; Zdilar, I.; Morse, S.; Fenton, T.A. Deletion of a non-canonical regulatory sequence causes loss of Scn1a expression and epileptic phenotypes in mice. Genome Med. 2021, 13, 69. [Google Scholar] [CrossRef]
- Kaushik, M.K.; Kaul, S.C.; Wadhwa, R.; Yanagisawa, M.; Urade, Y. Triethylene glycol, an active component of Ashwagandha (Withania somnifera) leaves, is responsible for sleep induction. PLoS ONE 2017, 12, e0172508. [Google Scholar] [CrossRef]


| DIZ | KA001 | KB002 | KC003 | |
|---|---|---|---|---|
| Body weight (g) | 24.0 ± 0.2 ns | 24.1 ± 0.2 | 23.9 ± 0.3 | 24.1 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, M.; Koo, Y.K.; Kim, N.; Lee, Y.; Yim, D.J.; Kim, S.; Park, E.; Park, S.-J. Lemon Verbena Extract Enhances Sleep Quality and Duration via Modulation of Adenosine A1 and GABAA Receptors in Pentobarbital-Induced and Polysomnography-Based Sleep Models. Int. J. Mol. Sci. 2025, 26, 5723. https://doi.org/10.3390/ijms26125723
Choi M, Koo YK, Kim N, Lee Y, Yim DJ, Kim S, Park E, Park S-J. Lemon Verbena Extract Enhances Sleep Quality and Duration via Modulation of Adenosine A1 and GABAA Receptors in Pentobarbital-Induced and Polysomnography-Based Sleep Models. International Journal of Molecular Sciences. 2025; 26(12):5723. https://doi.org/10.3390/ijms26125723
Chicago/Turabian StyleChoi, Mijoo, Yean Kyoung Koo, Nayoung Kim, Yunjung Lee, Dong Joon Yim, SukJin Kim, Eunju Park, and Soo-Jeung Park. 2025. "Lemon Verbena Extract Enhances Sleep Quality and Duration via Modulation of Adenosine A1 and GABAA Receptors in Pentobarbital-Induced and Polysomnography-Based Sleep Models" International Journal of Molecular Sciences 26, no. 12: 5723. https://doi.org/10.3390/ijms26125723
APA StyleChoi, M., Koo, Y. K., Kim, N., Lee, Y., Yim, D. J., Kim, S., Park, E., & Park, S.-J. (2025). Lemon Verbena Extract Enhances Sleep Quality and Duration via Modulation of Adenosine A1 and GABAA Receptors in Pentobarbital-Induced and Polysomnography-Based Sleep Models. International Journal of Molecular Sciences, 26(12), 5723. https://doi.org/10.3390/ijms26125723






