An Updated Review on Essential Oils from Lauraceae Plants: Chemical Composition and Genetic Characteristics of Biosynthesis
Abstract
1. Introduction
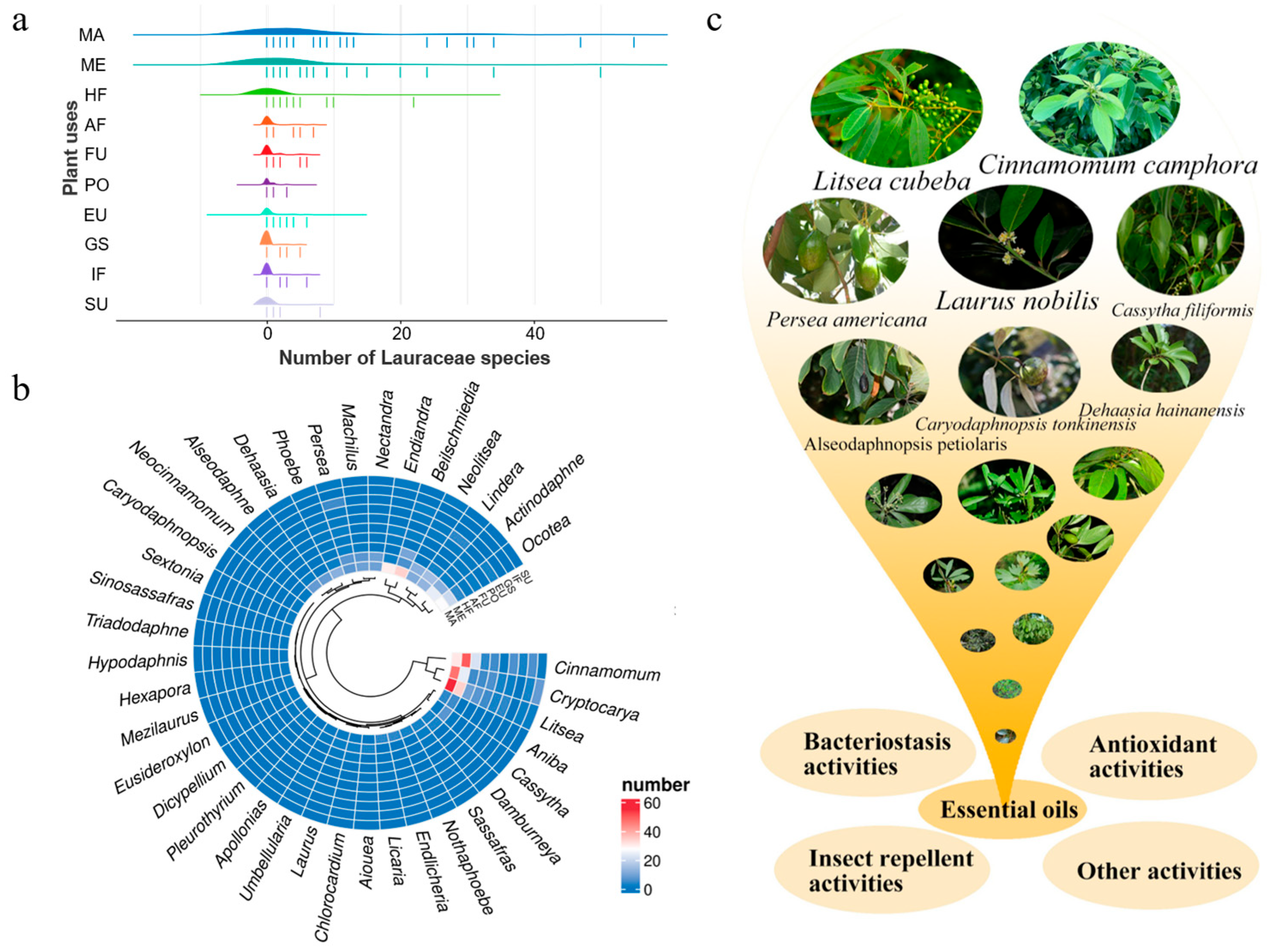
2. Lauraceae Essential Oils and Their Use by Humans
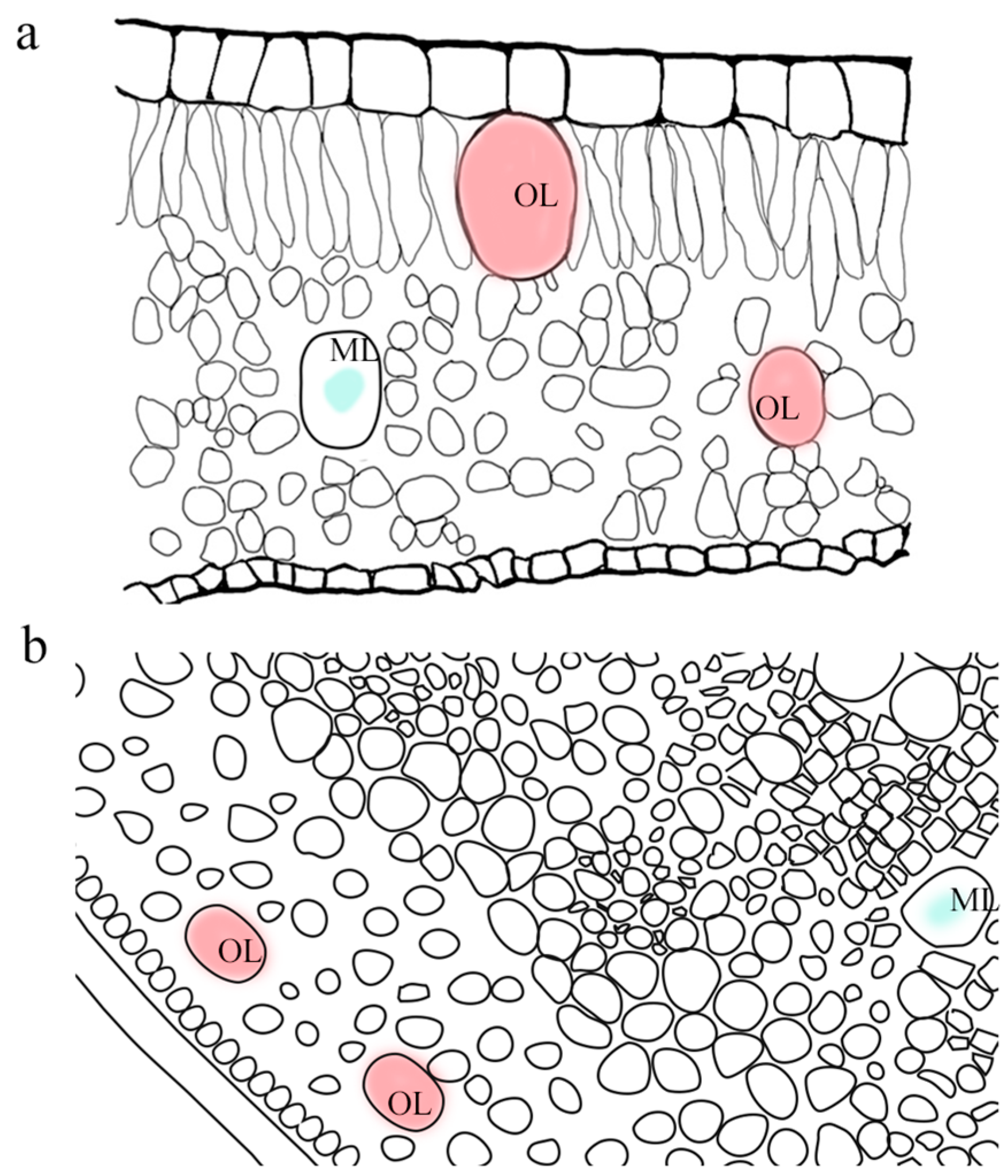
3. Cellular Structures of Lauraceae Producing or Secreting Essential Oils
4. The Diversity of Essential Oils in Lauraceae
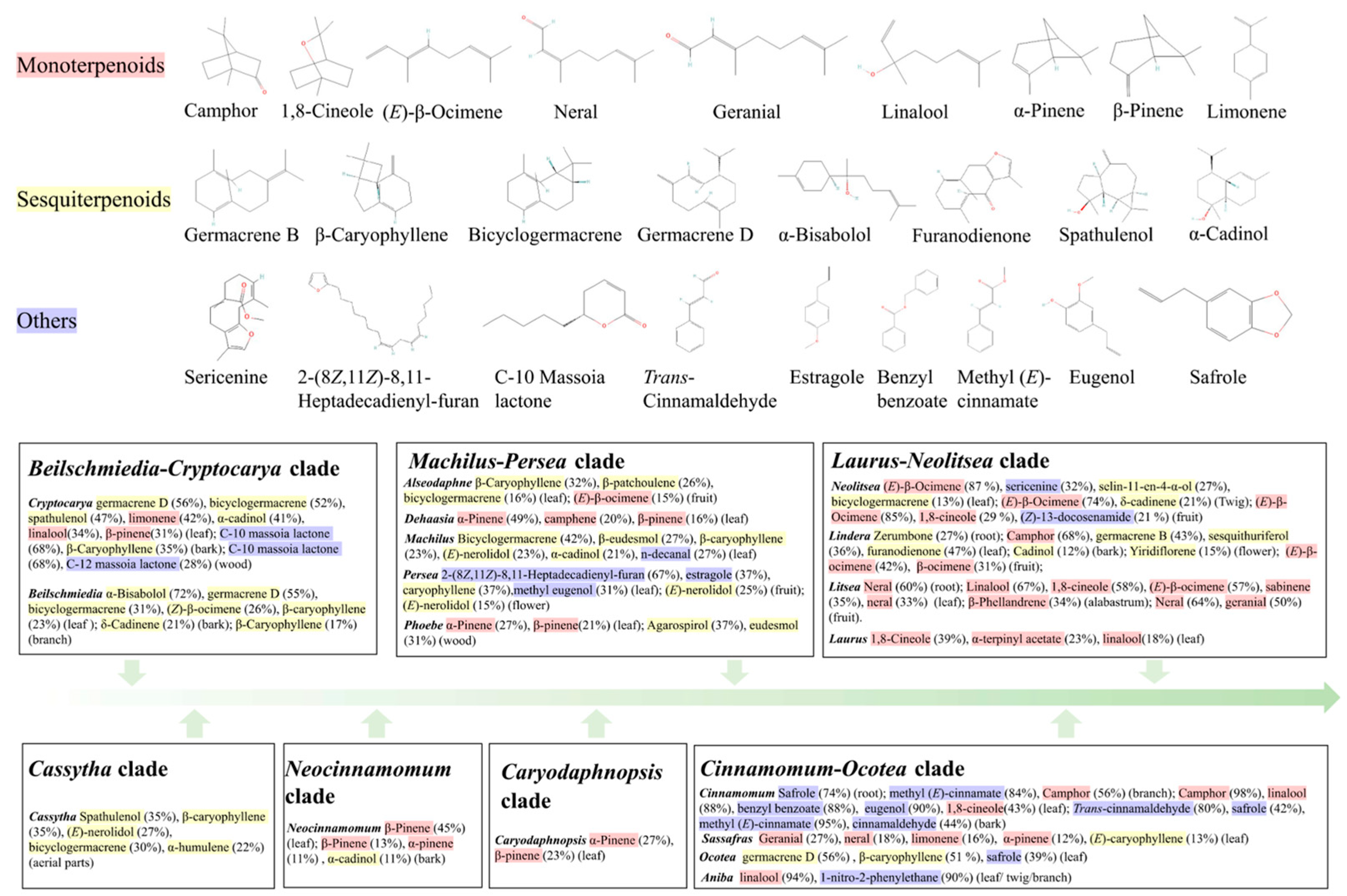
4.1. Monoterpenoids and Sesquiterpenoids in Lauraceae Essential Oils
4.2. Other Compounds in Lauraceae Essential Oils
4.3. The Evolutionary History of Essential Oil Composition and Content in Lauraceae Plants
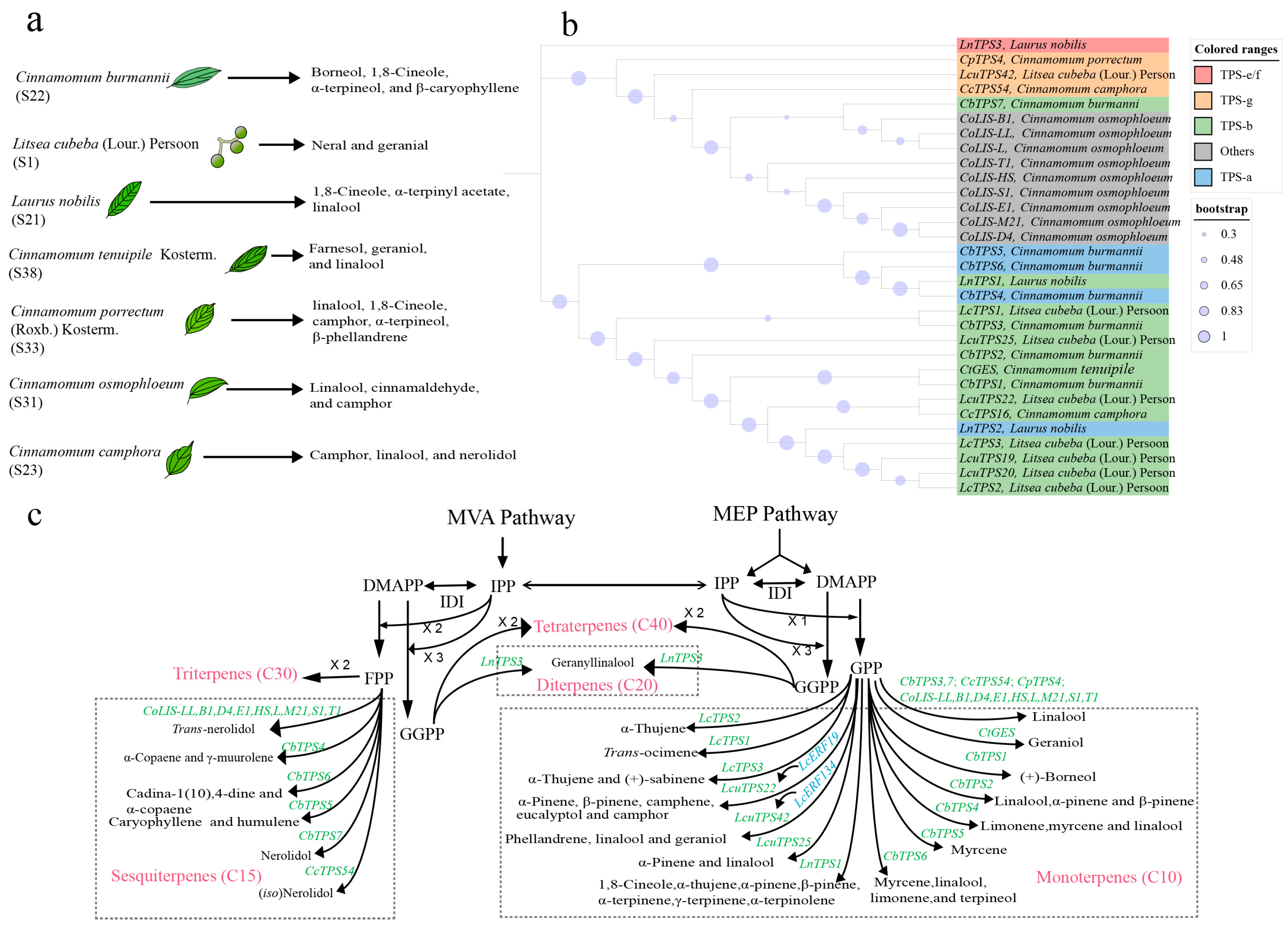
5. Genetic and Biochemical Bases of Essential Oil Terpenoids in Lauraceae
5.1. Terpenoids Biosynthesis Pathway
5.2. Terpene Synthase (TPS) Gene Family Size in Lauraceae Occurs Significantly Expansion
5.3. Gene Duplication Offers Dynamic for Diversity of Terpenoids in Lauraceae
5.4. Function and Regulatory Mechanism of TPSs in Lauraceae
6. Discussion
7. Prospect
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors Affecting Secondary Metabolite Production in Plants: Volatile Components and Essential Oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Ninkuu, V.; Zhang, L.; Yan, J.; Fu, Z.; Yang, T.; Zeng, H. Biochemistry of Terpenes and Recent Advances in Plant Protection. Int. J. Mol. Sci. 2021, 22, 5710. [Google Scholar] [CrossRef] [PubMed]
- Almeida, K.C.R.; Silva, B.B.; Alves, C.C.F.; Vieira, T.M.; Crotti, A.E.M.; Souza, J.M.; Martins, C.H.G.; Ribeiro, A.B.; Squarisi, I.S.; Tavares, D.C.; et al. Biological Properties and Chemical Composition of Essential Oil from Nectandra megapotamica (Spreng.) Mez. Leaves (Lauraceae). Nat. Prod. Res. 2020, 34, 3149–3153. [Google Scholar] [CrossRef]
- Du, Y.; Wang, Y.; Xue, W.; Fan, Y.; Jiang, Z. Research progress of essential oils from Lauraceae plants. Food Sci. 2023, 44, 346–359. [Google Scholar] [CrossRef]
- Camargo, M.J.D.; Miranda, M.L.D.; Kagamida, C.M.; Rodrigues, E.D.; Garcez, F.R.; Garcez, W.S. Sesquiterpenos de Ocotea Lancifolia (Lauraceae). Quím. Nova 2013, 36, 1008–1013. [Google Scholar] [CrossRef]
- Chanderbali, A.S.; van der Werff, H.; Renner, S.S. Phylogeny and Historical Biogeography of Lauraceae: Evidence from the Chloroplast and Nuclear Genomes. Ann. Mo. Bot. Gard. 2001, 88, 104. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, J.; Zhang, Y.; Wang, S.; Wang, Y.; Liu, H.; Wang, Z. Research Progress in Plant Molecular Systematics of Lauraceae. Biology 2021, 10, 391. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Rohwer, J.G.; van der Werff, H.; Wang, Z.; Li, H. Molecular Phylogenetic Analysis of the Persea Group (Lauraceae) and Its Biogeographic Implications on the Evolution of Tropical and Subtropical Amphi-Pacific Disjunctions. Am. J. Bot. 2011, 98, 1520–1536. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Conran, J.G.; Li, X.W.; Li, H.W. Phylogeny of Neolitsea (Lauraceae) Inferred from Bayesian Analysis of nrDNA ITS and ETS Sequences. Plant Syst. Evol. 2007, 269, 203–221. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Conran, J.G.; Li, H. Phylogeny of the Southeast Asian Endemic Genus Neocinnamomum H. Liu (Lauraceae). Plant Syst. Evol. 2010, 290, 173–184. [Google Scholar] [CrossRef]
- Diazgranados, M.; Allkin, B.; Black, N.; Cámara-Leret, R.; Canteiro, C.; Carretero, J.; Eastwood, R.; Hargreaves, S. World Checklist of Useful Plant Species; Royal Botanic Gardens: Kew, UK, 2020. [Google Scholar]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential Oils as Antimicrobials in Food Systems—A Review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Gogoi, R. A Comparative Study on Antioxidant, Anti-Inflammatory, Genotoxicity, Anti-Microbial Activities and Chemical Composition of Fruit and Leaf Essential Oils of <Litsea Cubeba> Pers from North-East India. Ind. Crops Prod. 2018, 125, 131–139. [Google Scholar] [CrossRef]
- Haouel-Hamdi, S.; Hamedou, M.B.; Bachrouch, O.; Boushih, E.; Zarroug, Y.; Sriti, J.; Messaoud, C.; Hammami, M.; Abderraba, M.; Limam, F.; et al. Susceptibility of Tribolium castaneum to Laurus nobilis Essential Oil and Assessment on Semolina Quality. Int. J. Trop. Insect Sci. 2020, 40, 667–675. [Google Scholar] [CrossRef]
- Ovidi, E.; Laghezza Masci, V.; Zambelli, M.; Tiezzi, A.; Vitalini, S.; Garzoli, S. Laurus nobilis, Salvia sclarea and Salvia officinalis Essential Oils and Hydrolates: Evaluation of Liquid and Vapor Phase Chemical Composition and Biological Activities. Plants 2021, 10, 707. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Miao, X.; Lin, Z.; Xiu, Y.; Shi, L.; Zhang, Q.; Liang, D.; Lin, S.; He, B. Disruption of Metabolic Function and Redox Homeostasis as Antibacterial Mechanism of Lindera glauca Fruit Essential Oil against Shigella Flexneri. Food Control 2021, 130, 108282. [Google Scholar] [CrossRef]
- Bottoni, M.; Milani, F.; Mozzo, M.; Radice Kolloffel, D.A.; Papini, A.; Fratini, F.; Maggi, F.; Santagostini, L. Sub-Tissue Localization of Phytochemicals in Cinnamomum camphora (L.) J. Presl. Growing in Northern Italy. Plants 2021, 10, 1008. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.-L.; Cui, Z.-X.; Shu, B.; Zhao, S.; Yu, X.-H. Histochemistry and Cell Wall Specialization of Oil Cells Related to the Essential Oil Accumulation in the Bark of Cinnamomum cassia Presl. (Lauraceae). Plant Prod. Sci. 2012, 15, 1–9. [Google Scholar] [CrossRef]
- Xueqin, L.; Youming, X.; Han, L.; Kunxi, W.; Jiaying, Z.; Min, D.; Lianhua, Z. Development and Distribution of the Oil Cells and Mucilage Cell in Cinnamomum camphora. Sci. Silvae Sin. 2014, 50, 154–158. [Google Scholar]
- Chu, Q.; Hu, Z. Comparative Anatomy of Oil Cells and Mucilage Cells in the Leaves of the Lauraceae in China. Acta Phytotaxon. Sin. 1999, 37, 529–540. [Google Scholar]
- Chu, Q.; Hu, Z. Developmental Ultrastructure of Oil and Mucilage Cells in Cinnamomum longepaniculatum. Sci. Silvae Sin. 2001, 37, 19–25. [Google Scholar]
- Wang, H.; Liu, Y. Chemical Composition and Antibacterial Activity of Essential Oils from Different Parts of Litsea cubeba. Chem. Biodivers. 2010, 7, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Y.; Li, B.-T.; Wen, Z.-Q. Volatile Constituents of the Leaf and Fruit Essential Oils of Litsea cubeba (Lour.) Pers. Growing Wild in Baoshan Region, China. Nat. Prod. Res. 2025, 39, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.; Ou, O.; Liu, Y.; Hung, C.; Tsai, M.; Liao, P.; Wang, E.I.C.; Chen, Y.; Su, Y. Compositions and in Vitro Anticancer Activities of the Leaf and Fruit Oils of Litsea cubeba from Taiwan. Nat. Prod. Commun. 2010, 5, 1934578X1000500. [Google Scholar] [CrossRef]
- Si, L.; Chen, Y.; Han, X.; Zhan, Z.; Tian, S.; Cui, Q.; Wang, Y. Chemical Composition of Essential Oils of Litsea Cubeba Harvested from Its Distribution Areas in China. Molecules 2012, 17, 7057–7066. [Google Scholar] [CrossRef]
- Guzmán-Gutiérrez, S.L.; Gómez-Cansino, R.; García-Zebadúa, J.C.; Jiménez-Pérez, N.C.; Reyes-Chilpa, R. Antidepressant Activity of Litsea glaucescens Essential Oil: Identification of β-Pinene and Linalool as Active Principles. J. Ethnopharmacol. 2012, 143, 673–679. [Google Scholar] [CrossRef]
- Ran, H.; Feng, L.; Mao, Y.; Zhou, L.; Liu, S. Identification and analysis of volatile components in essential oil from four Lauraceae wild species leaves in Chongqing by GC-MS. Sci. Silvae Sin. 2018, 54, 91–103. [Google Scholar]
- Son, L.C.; Dai, D.N.; Thang, T.D.; Huyen, D.D.; Ogunwande, I.A. Analysis of the Essential Oils from Five Vietnamese Litsea Species (Lauraceae). J. Essent. Oil Bear. Plants 2014, 17, 960–971. [Google Scholar] [CrossRef]
- Chau, D.T.M.; Chung, N.T.; Huong, L.T.; Hung, N.H.; Ogunwande, I.A.; Dai, D.N.; Setzer, W.N. Chemical Compositions, Mosquito Larvicidal and Antimicrobial Activities of Leaf Essential Oils of Eleven Species of Lauraceae from Vietnam. Plants 2020, 9, 606. [Google Scholar] [CrossRef]
- Hu, W.; Jiang, X.; Yang, H.; Zhang, T.; Zhao, L.; Xiao, F. Components and their contents in essential oils from different parts of camphor type. J. Northwest AF Univ. (Nat. Sci. Ed.) 2014, 42, 126–132. [Google Scholar] [CrossRef]
- Poudel, D.K.; Rokaya, A.; Ojha, P.K.; Timsina, S.; Satyal, R.; Dosoky, N.S.; Satyal, P.; Setzer, W.N. The Chemical Profiling of Essential Oils from Different Tissues of Cinnamomum camphora L. and Their Antimicrobial Activities. Molecules 2021, 26, 5132. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, T.; Zhang, J.; Shi, Z.; He, Z. Chemical Composition of Leaf Essential Oils of Four Cinnamomum Species and Their Larvicidal Activity against Anophelus sinensis (Diptera: Culicidae). J. Essent. Oil Bear. Plants 2018, 21, 1284–1294. [Google Scholar] [CrossRef]
- Qiu, F.; Wang, X.; Zheng, Y.; Wang, H.; Liu, X.; Su, X. Full-Length Transcriptome Sequencing and Different Chemotype Expression Profile Analysis of Genes Related to Monoterpenoid Biosynthesis in Cinnamomum porrectum. Int. J. Mol. Sci. 2019, 20, 6230. [Google Scholar] [CrossRef]
- Shyu, J.-G.; Hsu, C.-K.; Hsu, K.-P.; Yang, M.-L.; Wei, L.Y.; Ho, H.-T.; Ho, C.-L. Chemical Composition, in Vitro Antibacterial and Antifungal Activities of Different Parts Essential Oils of Neolitsea sericea Var. Aurata From Taiwan. Nat. Prod. Commun. 2023, 18, 1934578X231166290. [Google Scholar] [CrossRef]
- Jani, N.A.; Sirat, H.M.; Ahmad, F.; Ali, N.A.M.; Zainal, M.H. Chemical Composition, Antibacterial and α-Glucosidase Inhibitory Activities of the Essential Oils of Neolitsea coccinea (Lauraceae). Nat. Prod. Commun. 2016, 11, 1934578X1601101231. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Wei, S.; Cai, X. Characterisation of the Essential Oil from Different Aerial Parts of Lindera chunii Merr. (Lauraceae). Nat. Prod. Res. 2013, 27, 1804–1807. [Google Scholar] [CrossRef]
- Sun, Z.; Su, X.; Lin, Y.; Long, C.; Zhang, Y.; Zhao, T. Chemical Composition, and Antioxidant and Cholinesterase Inhibitory Activities of Lindera glauca Fruit Essential Oil and Molecular Docking Studies of Six Selected Compounds. Horticulturae 2023, 9, 289. [Google Scholar] [CrossRef]
- Dai, D.N.; Thang, T.D.; Pino, J.A. Essential Oil of Lindera rufa Hook. f. Leaves from Vietnam. J. Essent. Oil Bear. Plants 2013, 16, 832–834. [Google Scholar] [CrossRef]
- Wei, G.; Chen, H.; Kong, L.; Li, X.; Ma, C.; Jiang, H. Composition and Bioactivity of the Essential Oil from the Leaves of Lindera setchuenensis. Chem. Nat. Compd. 2016, 52, 520–522. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, H. GC-MS Analysis of Essential Oil from the Bark of Lindera obtusiloba. Chem. Nat. Compd. 2012, 48, 696–697. [Google Scholar] [CrossRef]
- Yan, R.; Yang, Y.; Zou, G. Cytotoxic and Apoptotic Effects of Lindera strychnifolia Leaf Essential Oil. J. Essent. Oil Res. 2014, 26, 308–314. [Google Scholar] [CrossRef]
- Yan, R.; Yang, Y.; Zeng, Y.; Zou, G. Cytotoxicity and Antibacterial Activity of Lindera Strychnifolia Essential Oils and Extracts. J. Ethnopharmacol. 2009, 121, 451–455. [Google Scholar] [CrossRef]
- Joshi, S.C.; Verma, A.R.; Mathela, C.S. Antioxidant and Antibacterial Activities of the Leaf Essential Oils of Himalayan Lauraceae Species. Food Chem. Toxicol. 2010, 48, 37–40. [Google Scholar] [CrossRef]
- Salleh, W.M.N.H.W.; Ahmad, F.; Yen, K.H. Chemical Compositions and Biological Activities of the Essential Oils of Beilschmiedia madang Blume (Lauraceae). Arch. Pharm. Res. 2015, 38, 485–493. [Google Scholar] [CrossRef]
- Chaverri, C.; Cicció, J.F. Essential Oils From Beilschmiedia pendula (Sw.) Hemsl. (Lauraceae) From Costa Rica. J. Essent. Oil Res. 2010, 22, 259–262. [Google Scholar] [CrossRef]
- Setzer, W.N.; Haber, W.A. Leaf Essential Oil Composition of Five Species of Beilschmiedia from Monteverde, Costa Rica. Nat. Prod. Commun. 2007, 2, 1934578X0700200116. [Google Scholar] [CrossRef]
- Brophy, J.J.; Goldsack, R.J.; Forster, P.I. The Essential Oils of Some Australian Cassytha Species (Lauraceae). J. Essent. Oil Res. 2009, 21, 543–546. [Google Scholar] [CrossRef]
- Ouattara, Z.A.; Sangaré, N.; Mamyrbekova-Bekro, A.J.; Békro, Y.-A.; Tomi, P.; Paoli, M.; Bighelli, A.; Tomi, F. Composition and Chemical Variability of Essential Oils Isolated from Aerial Parts of Cassytha filiformis from Côte d’Ivoire. Nat. Prod. Commun. 2018, 13, 1934578X1801300225. [Google Scholar] [CrossRef]
- Brophy, J.J.; Forster, P.I.; Goldsack, R.J. Coconut Laurels: The Leaf Essential Oils from Four Endemic Australian Cryptocarya Species: C. Bellendenkerana, C. Cocosoides, C. Cunninghamii and C. Lividula (Lauraceae). Nat. Prod. Commun. 2016, 11, 1934578X1601100. [Google Scholar] [CrossRef]
- Ray, A.; Jena, S.; Sahoo, A.; Kamila, P.K.; Das, P.K.; Mohanty, S.; Nayak, S.; Panda, P.C. Chemical Composition, Antioxidant, Anti-Inflammatory and Anticancer Activities of Bark Essential Oil of Cryptocarya amygdalina from India. J. Essent. Oil Bear. Plants 2021, 24, 617–631. [Google Scholar] [CrossRef]
- Azhar, M.A.M.; Salleh, W.M.N.H.W.; Khamis, S. Essential Oil Composition of Three Cryptocarya Species from Malaysia. Z. Naturforschung C 2020, 75, 297–301. [Google Scholar] [CrossRef]
- Andrade, P.M.D.; Melo, D.C.D.; Alcoba, A.E.T.; Ferreira Júnior, W.G.; Pagotti, M.C.; Magalhães, L.G.; Santos, T.C.L.D.; Crotti, A.E.M.; Alves, C.C.F.; Miranda, M.L.D. Chemical Composition and Evaluation of Antileishmanial and Cytotoxic Activities of the Essential Oil from Leaves of Cryptocarya aschersoniana Mez. (Lauraceae Juss.). An. Acad. Bras. Ciênc. 2018, 90, 2671–2678. [Google Scholar] [CrossRef]
- Rali, T.; Wossa, S.W.; Leach, D.N. Comparative Chemical Analysis of the Essential Oil Constituents in the Bark, Heartwood and Fruits of Cryptocarya massoy (Oken) Kosterm. (Lauraceae) from Papua New Guinea. Molecules 2007, 12, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Telascrea, M.; de Araújo, C.C.; Marques, M.O.M.; Facanali, R.; de Moraes, P.L.R.; Cavalheiro, A.J. Essential Oil from Leaves of Cryptocarya mandioccana Meisner (Lauraceae): Composition and Intraspecific Chemical Variability. Biochem. Syst. Ecol. 2007, 35, 222–232. [Google Scholar] [CrossRef]
- Touma, J.; Navarro, M.; Sepúlveda, B.; Pavon, A.; Corsini, G.; Fernández, K.; Quezada, C.; Torres, A.; Larrazabal-Fuentes, M.J.; Paredes, A.; et al. The Chemical Compositions of Essential Oils Derived from Cryptocarya alba and Laurelia sempervirens Possess Antioxidant, Antibacterial and Antitumoral Activity Potential. Molecules 2020, 25, 5600. [Google Scholar] [CrossRef]
- Telascrea, M.; de Araújo, C.C.; Cavalheiro, A.J.; Marques, M.O.M.; Facanali, R.; de Moraes, P.L.R. Essential Oils from Leaves of Cryptocarya Spp from the Atlantic Rain Forest. Quím. Nova 2008, 31, 503–507. [Google Scholar] [CrossRef]
- Ho, C.; Liao, P.; Hsu, K.; Wang, E.I.C.; Dong, W.; Su, Y. Composition and Antimicrobial and Anti-Wood-Decay Fungal Activities of the Leaf Essential Oils of Machilus pseudolongifolia from Taiwan. Nat. Prod. Commun. 2010, 5. [Google Scholar] [CrossRef]
- Su, Y.C.; Hsu, K.P.; Li, S.C.; Ho, C.L. Composition, in Vitro Cytotoxicity, and Anti-Mildew Activities of the Leaf Essential Oil of Machilus thunbergii from Taiwan. Nat. Prod. Commun. 2015, 10, 1934578X1501001. [Google Scholar] [CrossRef]
- Ho, C.; Su, Y. Composition, Antioxidant and Antimicrobial Activities of the Leaf Essential Oil of Machilus japonica from Taiwan. Nat. Prod. Commun. 2012, 7, 1934578X1200700. [Google Scholar] [CrossRef]
- Ho, C.; Hsu, K.; Tseng, Y.; Wang, E.I.C.; Liao, P.; Chou, J.-C.; Lin, C.-N.; Su, Y.-C. Composition and Antimicrobial Activities of the Leaf Essential Oil of Machilus kusanoi from Taiwan. Nat. Prod. Commun. 2011, 6, 1934578X1100600. [Google Scholar] [CrossRef]
- Ho, C.; Hsu, K.; Wang, E.I.C.; Lin, C.; Su, Y. Composition and Anti-Wood-Decay Fungal Activities of the Leaf Essential Oil of Machilus philippinensis from Taiwan. Nat. Prod. Commun. 2010, 5, 1934578X1000500233. [Google Scholar] [CrossRef]
- Larijani, K.; Rustaiyan, A.; Abroomand Azar, P.; Nematollahi, F.; Taban, S. Composition of Essential Oil of Leaves of Persea americana Cultivated in Iran. Chem. Nat. Compd. 2010, 46, 489–490. [Google Scholar] [CrossRef]
- Nasri, C.; Halabi, Y.; Aghzaf, S.; Nounah, I.; Brunel, M.; Oubihi, A.; El-Guorrami, O.; Harhar, H.; Costa, J.; Tabyaoui, M. Seven Persea americana Varieties Essential Oils Comparison: Chemical Composition, Toxicity, Antibacterial, and Antioxidant Activities. Biocatal. Agric. Biotechnol. 2022, 44, 102468. [Google Scholar] [CrossRef]
- Padalia, R.C.; Joshi, S.C.; Bisht, D.S.; Mathela, C.S. Essential Oil Composition of Persea duthiei. Chem. Nat. Compd. 2009, 45, 745–747. [Google Scholar] [CrossRef]
- Pino, J.A.; Fernandes, P.; Marbot, R.; Rosado, A.; Fontinha, S.S. Leaf Oils of Helichrysum melaleucum Rchb. Ex Holl., Oenanthe divaricata (R. Br.) Mabb. and Persea indica (L.) Spreng. from Madeira. J. Essent. Oil Res. 2004, 16, 487–489. [Google Scholar] [CrossRef]
- Verma, R.S.; Kumar, A.; Mishra, P.; Kuppusamy, B.; Padalia, R.C.; Sundaresan, V. Essential Oil Constituents of Alseodaphne semecarpifolia from Central Western Ghats, India. Chem. Nat. Compd. 2016, 52, 516–517. [Google Scholar] [CrossRef]
- Anuar, M.Z.A.; Salleh, W.M.N.H.W.; Khamis, S.; Nafiah, M.A.; Mat Said, Z. Essential Oil Composition of Alseodaphne perakensis (Gamble) Kosterm from Malaysia. Nat. Prod. Res. 2021, 35, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, S.; Gu, Y.; Peng, J.; Huang, X.; Guo, H.; Chen, L.; Jiang, Y.; Liu, M.; Luo, X.; et al. Identification and Variation Analysis of the Composition and Content of Essential Oil and Fragrance Compounds in Phoebe Zhennan Wood at Different Tree Ages. Front. Plant Sci. 2024, 15, 1368894. [Google Scholar] [CrossRef]
- Zhang, Q.; Ning, L.; Ding, W.; Xing, H.; Zhou, Q.; Wei, Z. Chemical Constituents of the Essential Oil Extracted from Phoebe Bournei. Chem. Nat. Compd. 2019, 55, 754–755. [Google Scholar] [CrossRef]
- Gil, E.; Cuca, L.E.; Delgado, W.A. Chemical Composition and Antimicrobial Activity of the Essential Oil of the Leaves of Ocotea Caudata (Nees) Mez (Lauraceae) from Colombia. Bol. Latinoam. Caribe Plantas Med. Aromát. 2016, 15, 258–263. [Google Scholar]
- Yamaguchi, K.K.D.L.; Alcantara, J.M.; Lima, E.S.; Veiga-Junior, V.F.D. Chemical Composition and Platelet Aggregation Activity of Essential Oils of Two Species of the Genus Ocotea (Lauraceae). J. Essent. Oil Bear. Plants 2013, 16, 518–523. [Google Scholar] [CrossRef]
- Barros Gomes, P.R.; Oliveira Cunha, M.J.; De Sousa, D.A.; Reis, J.B.; Melo, A.V.; De Freitas, A.C.; Lima Hunaldo, V.K.; Fontenele, M.A.; De Paula, M.D.L.; Louzeiro, H.C.; et al. Chemical Study and Antifungal Activity of the Essential Oil of the Branches of Aniba duckei Kostermans. J. Essent. Oil Bear. Plants 2019, 22, 1554–1561. [Google Scholar] [CrossRef]
- Krainovic, P.M.; Almeida, D.R.A.D.; Veiga Junior, V.F.D.; Sampaio, P.D.T.B. Changes in Rosewood (Aniba Rosaeodora Ducke) Essential Oil in Response to Management of Commercial Plantations in Central Amazonia. For. Ecol. Manag. 2018, 429, 143–157. [Google Scholar] [CrossRef]
- Da Silva, J.K.R.; Maia, J.G.S.; Dosoky, N.S.; Setzer, W.N. Antioxidant, Antimicrobial, and Cytotoxic Properties of Aniba parviflora Essential Oils from the Amazon. Nat. Prod. Commun. 2016, 11, 1934578X1601100738. [Google Scholar] [CrossRef]
- Mediouni Ben Jemâa, J.; Tersim, N.; Toudert, K.T.; Khouja, M.L. Insecticidal Activities of Essential Oils from Leaves of Laurus nobilis L. from Tunisia, Algeria and Morocco, and Comparative Chemical Composition. J. Stored Prod. Res. 2012, 48, 97–104. [Google Scholar] [CrossRef]
- Caputo, L.; Nazzaro, F.; Souza, L.F.; Aliberti, L.; De Martino, L.; Fratianni, F.; Coppola, R.; De Feo, V. Laurus nobilis: Composition of Essential Oil and Its Biological Activities. Molecules 2017, 22, 930. [Google Scholar] [CrossRef] [PubMed]
- Stefanova, G.; Girova, T.; Gochev, V.; Stoyanova, M.; Petkova, Z.; Stoyanova, A.; Zheljazkov, V.D. Comparative Study on the Chemical Composition of Laurel (Laurus nobilis L.) Leaves from Greece and Georgia and the Antibacterial Activity of Their Essential Oil. Heliyon 2020, 6, e05491. [Google Scholar] [CrossRef]
- Kaler, K.M.; Setzer, W.N. Seasonal Variation in the Leaf Essential Oil Composition of Sassafras albidum. Nat. Prod. Commun. 2008, 3, 1934578X0800300. [Google Scholar] [CrossRef]
- Ogunwande, I.A.; Huong, L.T.; Chau, D.T.M.; Dai, D.N. Essential Oils of Lauraceae: Constituents and Antimicrobial Activity of Dehaasia cuneata (Blume) Blume and Caryodaphnopsis tonkinensis (Lecomte) Airy-Shaw from Vietnam. Rec. Nat. Prod. 2022, 16, 477–482. [Google Scholar] [CrossRef]
- Jena, S.; Ray, A.; Mohanta, O.; Das, P.K.; Sahoo, A.; Nayak, S.; Panda, P.C. Neocinnamomum caudatum Essential Oil Ameliorates Lipopolysaccharide-Induced Inflammation and Oxidative Stress in RAW 264.7 Cells by Inhibiting NF-κB Activation and ROS Production. Molecules 2022, 27, 8193. [Google Scholar] [CrossRef]
- Ding, J.; Yu, X.; Ding, Z.; Cheng, B.; Yi, Y.; Yu, W.; Hayashi, N.; Komae, H. Essential Oils of Some Lauraceae Species from the Southwestern Parts of China. J. Essent. Oil Res. 1994, 6, 577–585. [Google Scholar] [CrossRef]
- Jantan, I.B.; Karim Moharam, B.A.; Santhanam, J.; Jamal, J.A. Correlation between Chemical Composition and Antifungal Activity of the Essential Oils of Eight Cinnamomum. Species. Pharm. Biol. 2008, 46, 406–412. [Google Scholar] [CrossRef]
- Unlu, M.; Ergene, E.; Unlu, G.V.; Zeytinoglu, H.S.; Vural, N. Composition, Antimicrobial Activity and in Vitro Cytotoxicity of Essential Oil from Cinnamomum Zeylanicum Blume (Lauraceae). Food Chem. Toxicol. 2010, 48, 3274–3280. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Long, H.; Wu, X.; Hou, J.; Gao, L.; Yao, S.; Lei, M.; Zhang, Z.; Guo, D.; Wu, W. Quantitative and Fingerprint Analysis of Proanthocyanidins and Phenylpropanoids in Cinnamomum Verum Bark, Cinnamomum Cassia Bark, and Cassia Twig by UPLC Combined with Chemometrics. Eur. Food Res. Technol. 2021, 247, 2687–2698. [Google Scholar] [CrossRef]
- Leporatti, M.L.; Pintore, G.; Foddai, M.; Chessa, M.; Piana, A.; Petretto, G.L.; Masia, M.D.; Mangano, G.; Nicoletti, M. Chemical, Biological, Morphoanatomical and Antimicrobial Study of Ocotea Puchury-Major Mart. Nat. Prod. Res. 2014, 28, 294–300. [Google Scholar] [CrossRef]
- Cruz, E.D.N.S.D.; Barros, L.D.S.P.; Guimarães, B.D.A.; Mourão, R.H.V.; Maia, J.G.S.; Setzer, W.N.; Da Silva, J.K.D.R.; Figueiredo, P.L.B. Seasonal Variation in Essential Oil Composition and Antioxidant Capacity of Aniba canelilla (Lauraceae): A Reliable Source of 1-Nitro-2-Phenylethane. Molecules 2023, 28, 7573. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, J. Flower fossils of Lauraceae in the geological time and its phylogenetic evolutionary significance. Guihaia 2018, 38, 210–219. [Google Scholar] [CrossRef]
- Moreau, J.D.; Gomez, B.; Daviero Gomez, V.; Néraudeau, D.; Tafforeau, P. Inflorescences of Mauldinia Sp. (Lauraceae) and Associated Fruits from the Cenomanian of Languedoc Roussillon, France. Cretac. Res. 2016, 59, 18–29. [Google Scholar] [CrossRef]
- Raven, P.H.; Axelrod, D.I. Angiosperm Biogeography and Past Continental Movements. Ann. Mo. Bot. Gard. 1974, 61, 539. [Google Scholar] [CrossRef]
- van der Werff, H.; Richter, H.G. Toward an Improved Classification of Lauraceae. Ann. Mo. Bot. Gard. 1996, 83, 409. [Google Scholar] [CrossRef]
- Rohwer, J.G. Toward a Phylogenetic Classification of the Lauraceae: Evidence from matK Sequences. Syst. Bot. 2000, 25, 60. [Google Scholar] [CrossRef]
- Rohwer, J.G.; Rudolph, B. Jumping Genera: The Phylogenetic Positions of Cassytha, Hypodaphnis, and Neocinnamomum( Lauraceae) Based on Different Analyses of trnK Intron Sequences. Ann. Mo. Bot. Gard. 2005, 92, 153–178. [Google Scholar]
- Song, Y.; Yu, W.; Tan, Y.; Jin, J.; Wang, B.; Yang, J.; Liu, B.; Corlett, R.T. Plastid Phylogenomics Improve Phylogenetic Resolution in the Lauraceae. J. Syst. Evol. 2020, 58, 423–439. [Google Scholar] [CrossRef]
- Pichersky, E.; Raguso, R.A. Why Do Plants Produce so Many Terpenoid Compounds? New Phytol. 2018, 220, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Farias, K.S.; Alves, F.M.; Santos-Zanuncio, V.S.; De Sousa Jr, P.T.; Silva, D.B.; Carollo, C.A. Global Distribution of the Chemical Constituents and Antibacterial Activity of Essential Oils in Lauraceae Family: A Review. S. Afr. J. Bot. 2023, 155, 214–222. [Google Scholar] [CrossRef]
- Liu, J.; Lin, M.; Han, P.; Yao, G.; Jiang, H. Biosynthesis Progress of High-Energy-Density Liquid Fuels Derived from Terpenes. Microorganisms 2024, 12, 706. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Chen, X.; Liao, X.; Peng, D.; Han, X.; Zhu, C.; Wang, P.; Hufnagel, D.E.; Wang, L.; Li, K.; et al. A Chromosome-Level Genome of the Camphor Tree and the Underlying Genetic and Climatic Factors for Its Top-Geoherbalism. Front. Plant Sci. 2022, 13, 827890. [Google Scholar] [CrossRef]
- Li, D.; Lin, H.-Y.; Wang, X.; Bi, B.; Gao, Y.; Shao, L.; Zhang, R.; Liang, Y.; Xia, Y.; Zhao, Y.-P.; et al. Genome and Whole-Genome Resequencing of Cinnamomum camphora Elucidate Its Dominance in Subtropical Urban Landscapes. BMC Biol. 2023, 21, 192. [Google Scholar] [CrossRef]
- Shen, T.; Qi, H.; Luan, X.; Xu, W.; Yu, F.; Zhong, Y.; Xu, M. The Chromosome-level Genome Sequence of the Camphor Tree Provides Insights into Lauraceae Evolution and Terpene Biosynthesis. Plant Biotechnol. J. 2022, 20, 244–246. [Google Scholar] [CrossRef]
- Sun, W.; Xiang, S.; Zhang, Q.; Xiao, L.; Zhang, D.; Zhang, P.; Chen, D.; Hao, Y.; Liu, D.; Ding, L.; et al. The Camphor Tree Genome Enhances the Understanding of Magnoliid Evolution. J. Genet. Genom. 2022, 49, 249–253. [Google Scholar] [CrossRef]
- Wang, X.; Xu, C.; Zheng, Y.; Wu, Y.; Zhang, Y.; Zhang, T.; Xiong, Z.Y.; Yang, H.-K.; Li, J.; Fu, C.; et al. Chromosome-Level Genome Assembly and Resequencing of Camphor Tree (Cinnamomum camphora) Provides Insight into Phylogeny and Diversification of Terpenoid and Triglyceride Biosynthesis of Cinnamomum. Hortic. Res. 2022, 9, uhac216. [Google Scholar] [CrossRef]
- Tao, L.; Guo, S.; Xiong, Z.; Zhang, R.; Sun, W. Chromosome-Level Genome Assembly of the Threatened Resource Plant Cinnamomum chago. Sci. Data 2024, 11, 447. [Google Scholar] [CrossRef] [PubMed]
- Chaw, S.; Liu, Y.; Wu, Y.; Wang, H.; Lin, C.I.; Wu, C.; Ke, H.; Chang, L.; Hsu, C.; Yang, H.; et al. Stout Camphor Tree Genome Fills Gaps in Understanding of Flowering Plant Genome Evolution. Nat. Plants 2019, 5, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, Z.; Zhao, Y.; Gao, M.; Wang, J.; Liu, K.; Wang, X.; Wu, L.; Jiao, Y.; Xu, Z.; et al. The Litsea Genome and the Evolution of the Laurel Family. Nat. Commun. 2020, 11, 1675. [Google Scholar] [CrossRef]
- Tian, X.; Guo, J.; Yan, X.; Shi, T.; Nie, S.; Zhao, S.; Bao, Y.; Li, Z.; Kong, L.; Su, G.; et al. Unique Gene Duplications and Conserved Microsynteny Potentially Associated with Resistance to Wood Decay in the Lauraceae. Front. Plant Sci. 2023, 14, 1122549. [Google Scholar] [CrossRef]
- Xiong, B.; Zhang, L.; Xie, L.; Li, L.; He, X.; Niu, Y.; Zhang, T.; Liao, S.; Dong, S.; Zhang, Z. Genome of Lindera glauca Provides Insights into the Evolution of Biosynthesis Genes for Aromatic Compounds. iScience 2022, 25, 104761. [Google Scholar] [CrossRef]
- Han, X.; Zhang, J.; Han, S.; Chong, S.L.; Meng, G.; Song, M.; Wang, Y.; Zhou, S.; Liu, C.; Lou, L.; et al. The Chromosome-Scale Genome of Phoebe bournei Reveals Contrasting Fates of Terpene Synthase (TPS)-a and TPS-b Subfamilies. Plant Commun. 2022, 3, 100410. [Google Scholar] [CrossRef] [PubMed]
- Rendón Anaya, M.; Ibarra Laclette, E.; Méndez Bravo, A.; Lan, T.; Zheng, C.; Carretero-Paulet, L.; Perez-Torres, C.A.; Chacón-López, A.; Hernandez-Guzmán, G.; Chang, T.-H.; et al. The Avocado Genome Informs Deep Angiosperm Phylogeny, Highlights Introgressive Hybridization, and Reveals Pathogen-Influenced Gene Space Adaptation. Proc. Natl. Acad. Sci. USA 2019, 116, 17081–17089. [Google Scholar] [CrossRef]
- Yang, T.; Cai, Y.; Huang, T.; Yang, D.; Yang, X.; Yin, X.; Zhang, C.; Yang, Y.; Yang, Y. A Telomere-to-Telomere Gap-Free Reference Genome Assembly of Avocado Provides Useful Resources for Identifying Genes Related to Fatty Acid Biosynthesis and Disease Resistance. Hortic. Res. 2024, 11, uhae119. [Google Scholar] [CrossRef]
- Huo, N.; Dong, L.; Zhang, S.; Wang, Y.; Zhu, T.; Mohr, T.; Altenbach, S.; Liu, Z.; Dvorak, J.; Anderson, O.D.; et al. New Insights into Structural Organization and Gene Duplication in a 1.75-Mb Genomic Region Harboring the A-gliadin Gene Family in Aegilops tauschii, the Source of Wheat D Genome. Plant J. 2017, 92, 571–583. [Google Scholar] [CrossRef]
- Ren, L.; Liu, Y.; Liu, H.; Qian, T.; Qi, L.; Wang, X.; Zeng, Q. Subcellular Relocalization and Positive Selection Play Key Roles in the Retention of Duplicate Genes of Populus Class III Peroxidase Family. Plant Cell 2014, 26, 2404–2419. [Google Scholar] [CrossRef]
- Chen, H.; Köllner, T.G.; Li, G.; Wei, G.; Chen, X.; Zeng, D.; Qian, Q.; Chen, F. Combinatorial Evolution of a Terpene Synthase Gene Cluster Explains Terpene Variations in Oryza. Plant Physiol. 2020, 182, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.; Shen, S.; Yang, C.; Liu, Z.; Fernie, A.R.; Graham, I.A.; Luo, J. Plant Metabolic Gene Clusters in the Multi-Omics Era. Trends Plant Sci. 2022, 27, 981–1001. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Fang, X.; Yang, C.; Li, J.; Chen, X. Biosynthesis and regulation of secondary terpenoid metabolism in plants. Sci. Sin. Vitae 2013, 43, 1030–1046. [Google Scholar] [CrossRef]
- Kamthan, A.; Chaudhuri, A.; Kamthan, M.; Datta, A. Genetically Modified (GM) Crops: Milestones and New Advances in Crop Improvement. Theor. Appl. Genet. 2016, 129, 1639–1655. [Google Scholar] [CrossRef]
- Spitz, F.; Furlong, E.E.M. Transcription Factors: From Enhancer Binding to Developmental Control. Nat. Rev. Genet. 2012, 13, 613–626. [Google Scholar] [CrossRef]
- Wang, M.; Gao, M.; Zhao, Y.; Chen, Y.; Wu, L.; Yin, H.; Yang, J.; Xiong, S.; Wang, S.; Wang, J.; et al. LcERF19, an AP2/ERF Transcription Factor from Litsea Cubeba, Positively Regulates Geranial and Neral Biosynthesis. Hortic. Res. 2022, 9, uhac093. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, M.; Chen, Y.; Gao, M.; Wu, L.; Wang, Y. LcERF134 Increases the Production of Monoterpenes by Activating the Terpene Biosynthesis Pathway in Litsea cubeba. Int. J. Biol. Macromol. 2023, 232, 123378. [Google Scholar] [CrossRef]
- Chen, N.; Liu, X.; Wei, Q.; Zhao, X. Influence of Different Light Intensities on 1,8-Cineole Content in Camphora longepaniculata Leaves. J. Sichuan For. Sci. Technol. 2024, 45, 49–57. [Google Scholar]
- Ni, Z.-J.; Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.-G.; Hu, F.; Wei, Z.-J. Recent Updates on the Chemistry, Bioactivities, Mode of Action, and Industrial Applications of Plant Essential Oils. Trends Food Sci. Technol. 2021, 110, 78–89. [Google Scholar] [CrossRef]
- Mo, C.; Liu, Q.; Gu, Z.; Gong, J.; Yang, R.; Yang, L.; Ji, Y.; Huang, P. Effect of Fertilization on Essential Oil Content and Its Main Components in Litsea cubeba Fruit. Non-Wood For. Res. 2023, 41, 162–170. [Google Scholar] [CrossRef]
- Jadhav, H.; Jadhav, A.; Morabiya, Y.; Takkalkar, P.; Qureshi, S.S.; Baloch, A.G.; Nizamuddin, S.; Mazari, S.A.; Abro, R.; Mubarak, N.M. Combined Impact of Ultrasound Pre-Treatment and Hydrodistillation on Bioactive Compounds and GC–MS Analysis of Cinnamomum cassia Bark Extract. Waste Biomass Valorization 2021, 12, 807–821. [Google Scholar] [CrossRef]
- Wang, F.; Lin, J.; Shan, S.; Pan, J.; Wang, M.; Deng, G. Extraction of the Essential Oil from Cinnamomum camphora Leaves by CO2-expanded Ethanol. J. Chin. Cereals Oils Assoc. 2020, 35, 125–130. [Google Scholar]
- Yang, Z.; Yang, Y. Research Advances on Nuclear Genomes of Economically Important Trees of Lauraceae. Chin. Bull. Bot. 2024, 59, 302–318. [Google Scholar]
- Zhao, Y.; Chen, Y.; Gao, M.; Wang, Y. Alcohol Dehydrogenases Regulated by a MYB44 Transcription Factor Underlie Lauraceae citral Biosynthesis. Plant Physiol. 2024, 194, 1674–1691. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Huang, S.; Mei, Y.; Wu, B.; Hou, Z.; Zhan, P.; Hou, Z.; Huang, W.; Zhao, J.; Wang, J. Genome Assembly Provided New Insights into the Cinnamomum burmannii Evolution and D-Borneol Biosynthesis Differences between Chemotypes. Ind. Crops Prod. 2022, 186, 115181. [Google Scholar] [CrossRef]
- Chen, S.; Sun, W.; Xiong, Y.; Jiang, Y.; Liu, X.; Liao, X.; Zhang, D.; Jiang, S.; Li, Y.; Liu, B.; et al. The Phoebe Genome Sheds Light on the Evolution of Magnoliids. Hortic. Res. 2020, 7, 146. [Google Scholar] [CrossRef]
- Linh, L.D.; Ban, P.H.; Hoi, T.M.; Huong, L.T.; Ogunwande, I.A. Compositions of Essential Oils from the Leaf, Stem and Fruit of Neolitsea Buisanensis (Lauraceae) from Vietnam. J. Essent. Oil Bear. Plants 2018, 21, 1257–1265. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, S.; Huang, Y.; Chen, M.; Wang, W.; Wang, J.; Hao, E.; Wu, H.; Li, Y. Chemical Composition, Antioxidant Activity, and Anti-Bacterial Activity of Essential Oils from Different Organs of Cinnamomum Burmanni. J. Essent. Oil Bear. Plants 2023, 26, 787–801. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.-T.; Feng, Y.-X.; Zhang, D.; Guo, S.-S.; Pang, X.; Geng, Z.-F.; Xi, C.; Du, S.-S. Comparative Evaluation of the Chemical Composition and Bioactivities of Essential Oils from Four Spice Plants (Lauraceae) against Stored-Product Insects. Ind. Crops Prod. 2019, 140, 111640. [Google Scholar] [CrossRef]
- Jeyaratnam, N.; Nour, A.H.; Kanthasamy, R.; Nour, A.H.; Yuvaraj, A.R.; Akindoyo, J.O. Essential Oil from Cinnamomum Cassia Bark through Hydrodistillation and Advanced Microwave Assisted Hydrodistillation. Ind. Crops Prod. 2016, 92, 57–66. [Google Scholar] [CrossRef]
- Chen, G.; Sun, F.; Wang, S.; Wang, W.; Dong, J.; Gao, F. Enhanced Extraction of Essential Oil from Cinnamomum Cassia Bark by Ultrasound Assisted Hydrodistillation. Chin. J. Chem. Eng. 2021, 36, 38–46. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, T.; Wang, X.; Wen, S.; Guo, Y.; Jiang, X. A Study on the Chemical Components in Essential Oil from Leaves of Cinnamomum Kanehirae and Chemotype Divisions. Acta Agric. Univ. Jiangxiensis 2016, 38, 668–673. [Google Scholar] [CrossRef]
- Lee, S.-C.; Xu, W.-X.; Lin, L.-Y.; Yang, J.-J.; Liu, C.-T. Chemical Composition and Hypoglycemic and Pancreas-Protective Effect of Leaf Essential Oil from Indigenous Cinnamon (Cinnamomum Osmophloeum Kanehira). J. Agric. Food Chem. 2013, 61, 4905–4913. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Li, J.; Wang, H.; Zeng, Y. A Geraniol-Synthase Gene from Cinnamomum Tenuipilum. Phytochemistry 2005, 66, 285–293. [Google Scholar] [CrossRef]
- Damasceno, C.S.B.; Oliveira, L.F.D.; Szabo, E.M.; Souza, Â.M.; Dias, J.F.G.; Miguel, M.D.; Miguel, O.G. Chemical Composition, Antioxidant and Biological Activity of Ocotea Bicolor Vattimo-Gil (LAURACEAE) Essential Oil. Braz. J. Pharm. Sci. 2018, 53. [Google Scholar] [CrossRef][Green Version]
- Chaverri, C.; Cicció, J.F. Essential Oil of Trees of the Genus Ocotea (Lauraceae) in Costa Rica. I. Ocotea Brenesii. Rev. Biol. Trop. 2014, 53, 431. [Google Scholar] [CrossRef]
- Teles, A.M.; Silva-Silva, J.V.; Fernandes, J.M.P.; Calabrese, K.D.S.; Abreu-Silva, A.L.; Marinho, S.C.; Mouchrek, A.N.; Filho, V.E.M.; Almeida-Souza, F. Aniba Rosaeodora (Var. Amazonica Ducke) Essential Oil: Chemical Composition, Antibacterial, Antioxidant and Antitrypanosomal Activity. Antibiotics 2020, 10, 24. [Google Scholar] [CrossRef]
- Cuong, N.T.; Ban, P.H.; Chung, M.V. Chemical Composition and Antioxidant Activity of the Essential Oil of Alseodaphne Velutina Chev. from Viet Nam. Nat. Prod. Res. 2022, 36, 617–620. [Google Scholar] [CrossRef]
- Yahyaa, M.; Matsuba, Y.; Brandt, W.; Doron-Faigenboim, A.; Bar, E.; McClain, A.; Davidovich-Rikanati, R.; Lewinsohn, E.; Pichersky, E.; Ibdah, M. Identification, Functional Characterization, and Evolution of Terpene Synthases from a Basal Dicot. Plant Physiol. 2015, 169, 1683–1697. [Google Scholar] [CrossRef]
- Ma, Q.; Ma, R.; Su, P.; Jin, B.; Guo, J.; Tang, J.; Chen, T.; Zeng, W.; Lai, C.; Ling, F.; et al. Elucidation of the Essential Oil Biosynthetic Pathways in Cinnamomum Burmannii through Identification of Six Terpene Synthases. Plant Sci. 2022, 317, 111203. [Google Scholar] [CrossRef]
- Chang, Y.T.; Chu, F.H. Molecular Cloning and Characterization of Monoterpene Synthases from Litsea Cubeba (Lour.) Persoon. Tree Genet. Genomes 2011, 7, 835–844. [Google Scholar] [CrossRef]
- Lin, Y.L.; Lee, Y.R.; Huang, W.; Chang, S.T.; Chu, F.H. Characterization of S-(+)-Linalool Synthase from Several Provenances of Cinnamomum Osmophloeum. Tree Genet. Genomes 2014, 10, 75–86. [Google Scholar] [CrossRef]
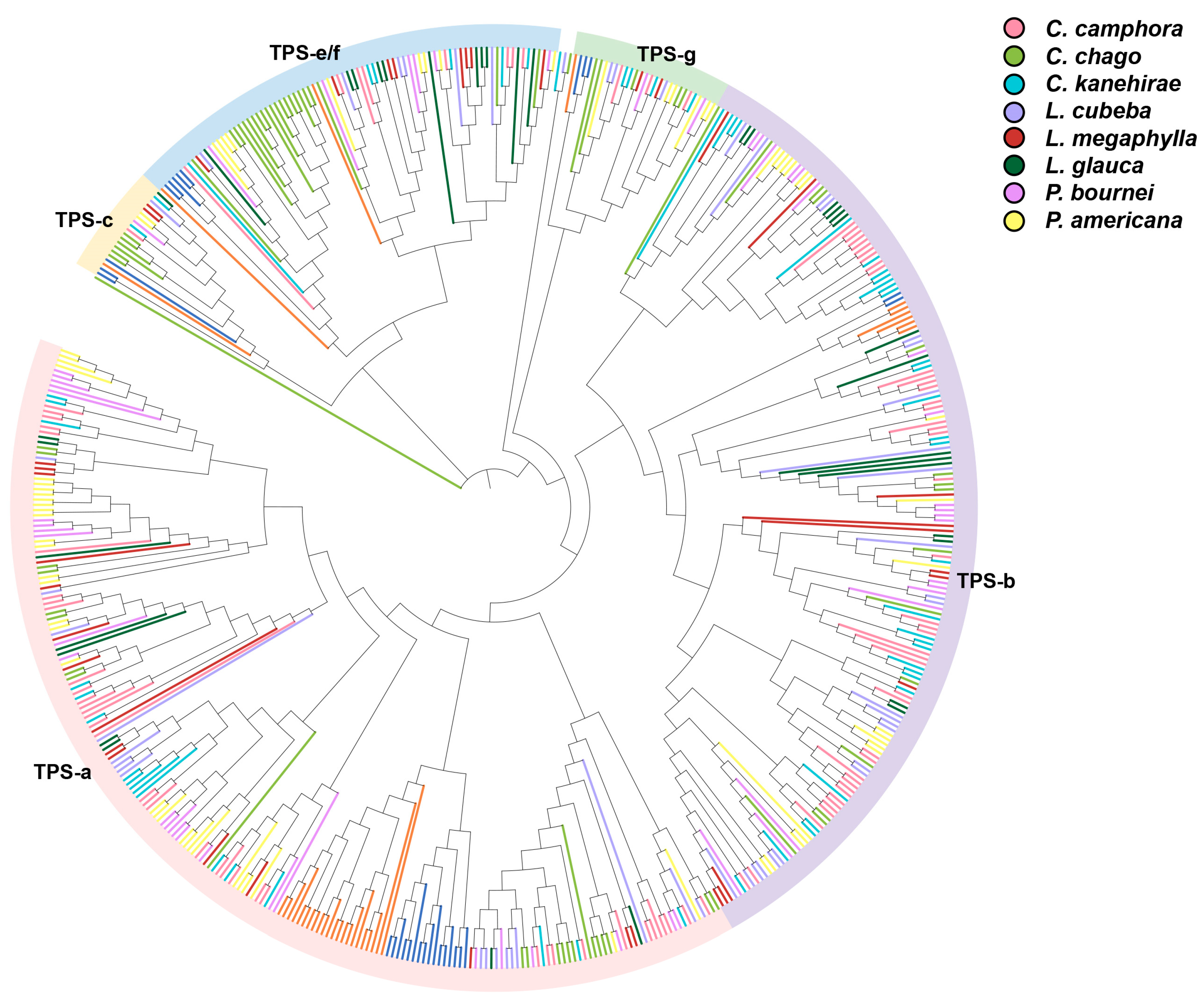
| Species | Genome Size | TPS Numbers | TPS Subfamily | |||||
|---|---|---|---|---|---|---|---|---|
| a | b | c | e/f | g | x | |||
| Cinnamomum camphora | 706.47 Mb | 93 | 38 | 43 | 1 | 8 | 3 | 0 |
| Cinnamomum chago | 1061.15 Mb | 72 | 22 | 15 | 7 | 22 | 5 | 1 |
| Cinnamomum kanehirae | 730.7 Mb | 60 | 17 | 30 | 2 | 6 | 4 | 1 |
| Litsea cubeba | 1325.69 Mb | 52 | 17 | 24 | 1 | 6 | 3 | 1 |
| Lindera megaphylla | 1268.6 Mb | 39 | 16 | 11 | 2 | 8 | 2 | 0 |
| Lindera glauca | 2092.2 Mb | 35 | 8 | 15 | 1 | 11 | 0 | 0 |
| Phoebe bournei | 1065.88 Mb | 53 | 25 | 19 | 1 | 6 | 2 | 0 |
| Persea americana | 841.6 Mb | 69 | 32 | 20 | 2 | 9 | 6 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, F.; Chen, Y.; Gao, M.; Li, W.; Zhao, Y.; Wang, Y. An Updated Review on Essential Oils from Lauraceae Plants: Chemical Composition and Genetic Characteristics of Biosynthesis. Int. J. Mol. Sci. 2025, 26, 5690. https://doi.org/10.3390/ijms26125690
Wu F, Chen Y, Gao M, Li W, Zhao Y, Wang Y. An Updated Review on Essential Oils from Lauraceae Plants: Chemical Composition and Genetic Characteristics of Biosynthesis. International Journal of Molecular Sciences. 2025; 26(12):5690. https://doi.org/10.3390/ijms26125690
Chicago/Turabian StyleWu, Fanglan, Yicun Chen, Ming Gao, Wei Li, Yunxiao Zhao, and Yangdong Wang. 2025. "An Updated Review on Essential Oils from Lauraceae Plants: Chemical Composition and Genetic Characteristics of Biosynthesis" International Journal of Molecular Sciences 26, no. 12: 5690. https://doi.org/10.3390/ijms26125690
APA StyleWu, F., Chen, Y., Gao, M., Li, W., Zhao, Y., & Wang, Y. (2025). An Updated Review on Essential Oils from Lauraceae Plants: Chemical Composition and Genetic Characteristics of Biosynthesis. International Journal of Molecular Sciences, 26(12), 5690. https://doi.org/10.3390/ijms26125690







