Unique Structural Features Relate to Evolutionary Adaptation of Cytochrome P450 in the Abyssal Zone
Abstract
1. Introduction
2. Results
2.1. Sequence Analysis
2.2. Substrate Binding Profile and Catalytic Parameters
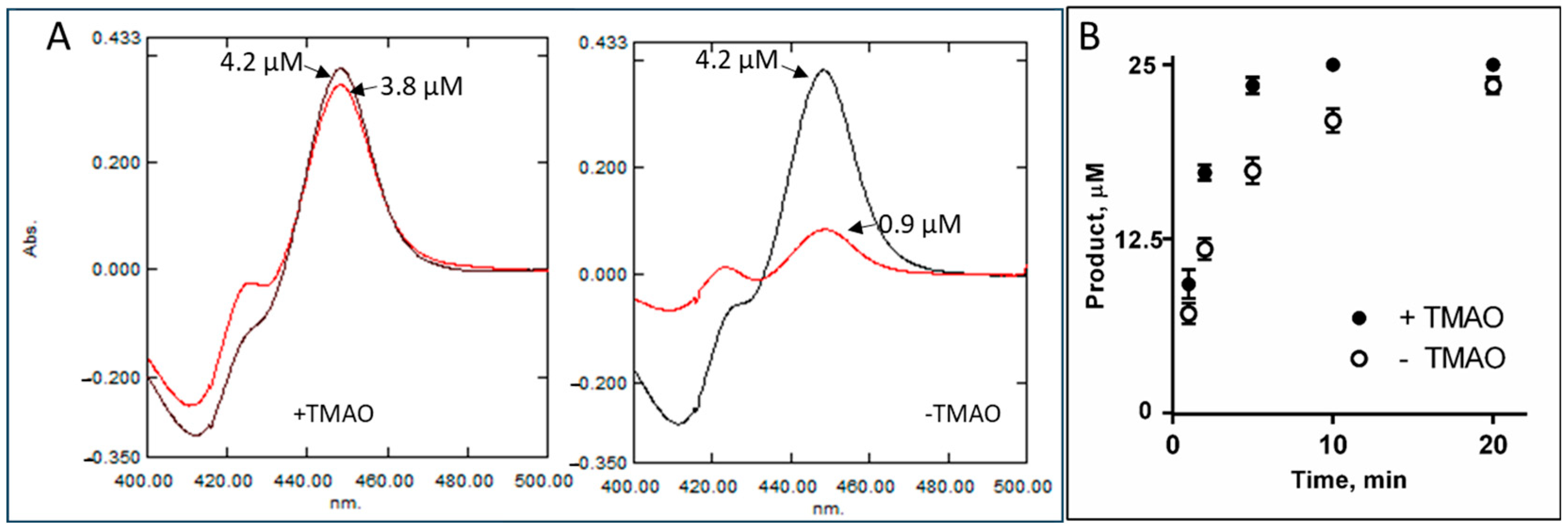
2.3. Intrinsic Resistance to Inhibition
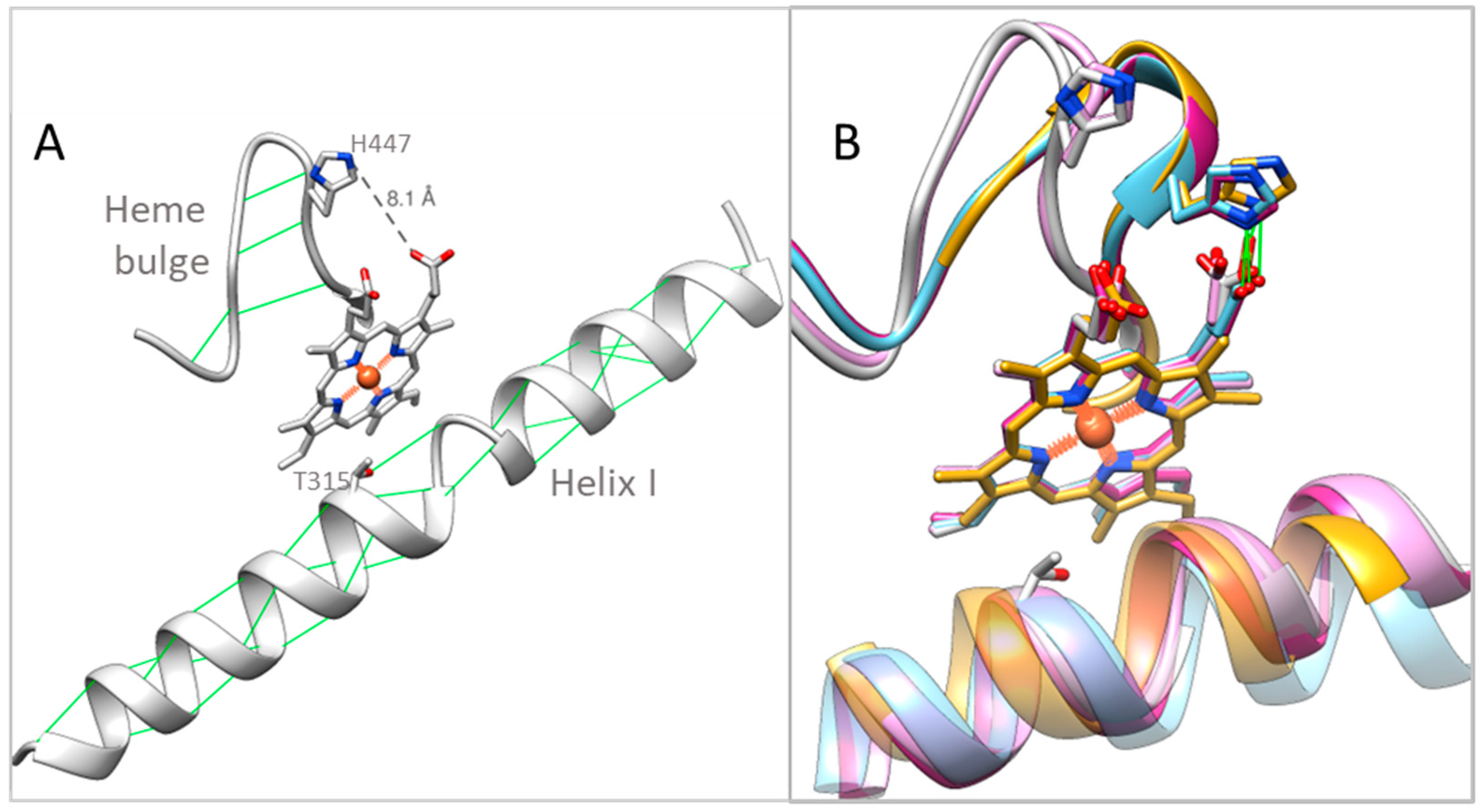
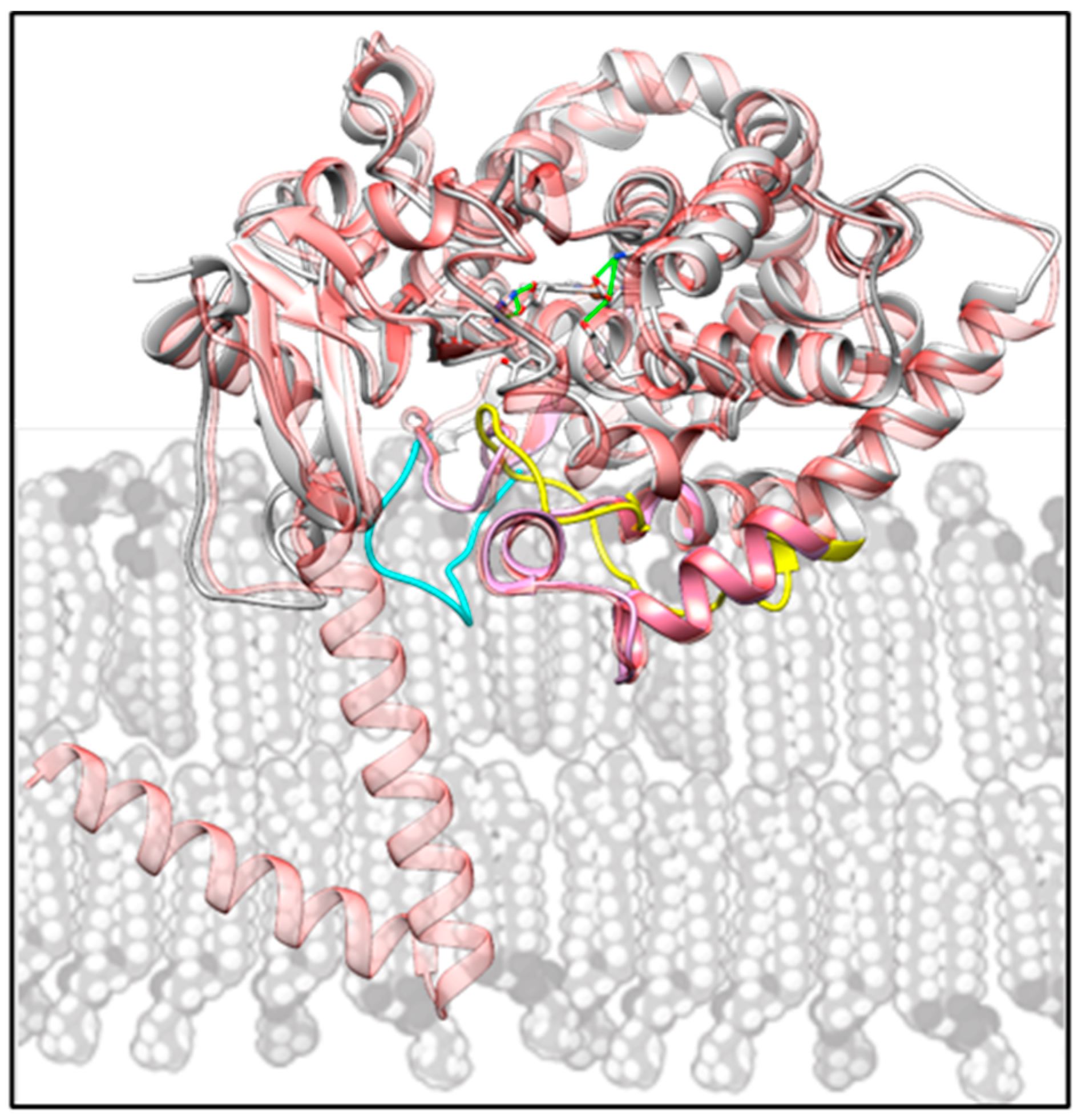
2.4. Structural Characterization
3. Discussion
4. Materials and Methods
4.1. Protein Expression and Purification
4.2. UV-Visible Spectroscopy
4.3. Spectral Titrations with Sterol Substrates and Azole-Based Ligands
4.4. Reconstitution of Catalytic Activity, Kinetic Analysis and Inhibition
4.5. X-Ray Crystallography
4.6. Spectral Characterization of the Dissolved C. Armatus CYP51 Crystals
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CYP | Cytochrome P450 |
| CYP51 | Sterol 14α-demethylase |
| C. armatus | Coryphaenoides armatus |
| TMAO | Trimethylamine oxide |
| RMSD | Root mean square deviation |
| PDB | Protein Data Bank |
References
- Ortiz de Montellano, P. Cytochrome P450: Structure, Mechanism, and Biochemistry, 3rd ed.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2005. [Google Scholar]
- Lepesheva, G.I.; Friggeri, L.; Waterman, M.R. CYP51 as drug targets for fungi and protozoan parasites: Past, present and future. Parasitology 2018, 145, 1820–1836. [Google Scholar] [CrossRef] [PubMed]
- Hargrove, T.Y.; Friggeri, L.; Wawrzak, Z.; Sivakumaran, S.; Yazlovitskaya, E.M.; Hiebert, S.W.; Guengerich, F.P.; Waterman, M.R.; Lepesheva, G.I. Human sterol 14α-demethylase as a target for anticancer chemotherapy: Towards structure-aided drug design. J. Lipid Res. 2016, 57, 1552–1563. [Google Scholar] [CrossRef]
- Hargrove, T.Y.; Wawrzak, Z.; Lamb, D.C.; Guengerich, F.P.; Lepesheva, G.I. Structure-functional characterization of cytochrome P450 sterol 14α-demethylase (CYP51B) from Aspergillus fumigatus and molecular basis for the development of antifungal drugs. J. Biol. Chem. 2015, 290, 23916–23934. [Google Scholar] [CrossRef] [PubMed]
- Hargrove, T.Y.; Friggeri, L.; Wawrzak, Z.; Qi, A.; Hoekstra, W.J.; Schotzinger, R.J.; York, J.D.; Guengerich, F.P.; Lepesheva, G.I. Structural analyses of Candida albicans sterol 14α-demethylase complexed with azole drugs address the molecular basis of azole-mediated inhibition of fungal sterol biosynthesis. J. Biol. Chem. 2017, 292, 6728–6743. [Google Scholar] [CrossRef]
- Lepesheva, G.I.; Park, H.W.; Hargrove, T.Y.; Vanhollebeke, B.; Wawrzak, Z.; Harp, J.M.; Sundaramoorthy, M.; Nes, W.D.; Pays, E.; Chaudhuri, M.; et al. Crystal structures of Trypanosoma brucei sterol 14α-demethylase and implications for selective treatment of human infections. J. Biol. Chem. 2010, 285, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Lepesheva, G.I.; Hargrove, T.Y.; Anderson, S.; Kleshchenko, Y.; Furtak, V.; Wawrzak, Z.; Villalta, F.; Waterman, M.R. Structural insights into inhibition of sterol 14α-demethylase in the human pathogen Trypanosoma cruzi. J. Biol. Chem. 2010, 285, 25582–25590. [Google Scholar] [CrossRef]
- Hargrove, T.Y.; Wawrzak, Z.; Rachakonda, G.; Nes, W.D.; Villalta, F.; Guengerich, F.P.; Lepesheva, G.I. Relaxed substrate requirements of sterol 14α-demethylase from Naegleria fowleri are accompanied by resistance to inhibition. J. Med. Chem. 2021, 64, 17511–17522. [Google Scholar] [CrossRef]
- Hargrove, T.Y.; Lamb, D.C.; Wawrzak, Z.; Hull, M.; Kelly, S.L.; Guengerich, F.P.; Lepesheva, G.I. Identification of potent and selective inhibitors of Acanthamoeba: Structural insights into sterol 14α-demethylase as a key drug target. J. Med. Chem. 2024, 67, 7443–7457. [Google Scholar] [CrossRef]
- Lamb, D.C.; Hargrove, T.Y.; Zhao, B.; Wawrzak, Z.; Goldstone, J.V.; Nes, W.D.; Kelly, S.L.; Waterman, M.R.; Stegeman, J.J.; Lepesheva, G.I. Concerning P450 evolution: Structural analyses support bacterial origin of sterol 14α-demethylases. Mol. Biol. Evol. 2021, 38, 952–967. [Google Scholar] [CrossRef]
- Strushkevich, N.; Usanov, S.A.; Park, H.W. Structural basis of human CYP51 inhibition by antifungal azoles. J. Mol. Biol. 2010, 397, 1067–1078. [Google Scholar] [CrossRef]
- Keniya, M.V.; Sabherwal, M.; Wilson, R.K.; Woods, M.A.; Sagatova, A.A.; Tyndall, J.D.A.; Monk, B.C. Crystal structures of full-length lanosterol 14α-demethylases of prominent fungal pathogens Candida albicans and Candida glabrata provide tools for antifungal discovery. Antimicrob. Agents Chemother. 2018, 62, e01134-18. [Google Scholar] [CrossRef] [PubMed]
- Podust, L.M.; Poulos, T.L.; Waterman, M.R. Crystal structure of cytochrome P450 14α-sterol demethylase (CYP51) from Mycobacterium tuberculosis in complex with azole inhibitors. Proc. Natl. Acad. Sci. USA 2001, 98, 3068–3073. [Google Scholar] [CrossRef] [PubMed]
- Hargrove, T.Y.; Lamb, D.C.; Smith, J.A.; Wawrzak, Z.; Kelly, S.L.; Lepesheva, G.I. Unravelling the role of transient redox partner complexes in P450 electron transfer mechanics. Sci. Rep. 2022, 12, 16232. [Google Scholar] [CrossRef] [PubMed]
- Hargrove, T.Y.; Wawrzak, Z.; Fisher, P.M.; Child, S.A.; Nes, W.D.; Guengerich, F.P.; Waterman, M.R.; Lepesheva, G.I. Binding of a physiological substrate causes large-scale conformational reorganization in cytochrome P450 51. J. Biol. Chem. 2018, 293, 19344–19353. [Google Scholar] [CrossRef]
- Hargrove, T.Y.; Wawrzak, Z.; Guengerich, F.P.; Lepesheva, G.I. A requirement for an active proton delivery network supports a compound I-mediated C–C bond cleavage in CYP51 catalysis. J. Biol. Chem. 2020, 295, 9998–10007. [Google Scholar] [CrossRef]
- Goldstone, J.V.; Lamb, D.C.; Kelly, S.L.; Lepesheva, G.I.; Stegeman, J.J. Structural modeling of cytochrome P450 51 from a deep-sea fish points to a novel structural feature in other CYP51s. J. Inorg. Biochem. 2023, 245, 112241. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Bhagat, J.; Singh, N.; Nishimura, N.; Shimada, Y. A comprehensive review on environmental toxicity of azole compounds to fish. Chemosphere 2021, 262, 128335. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Zhao, Y.; He, J.; Cheng, H.; Martyniuk, C.J. Endocrine disruption by azole fungicides in fish: A review of the evidence. Sci. Total Environ. 2022, 822, 153412. [Google Scholar] [CrossRef]
- Lepesheva, G.I.; Ott, R.D.; Hargrove, T.Y.; Kleshchenko, Y.Y.; Schuster, I.; Nes, W.D.; Hill, G.C.; Villalta, F.; Waterman, M.R. Sterol 14α-demethylase as a potential target for antitrypanosomal therapy: Enzyme inhibition and parasite cell growth. Chem. Biol. 2007, 14, 1283–1293. [Google Scholar] [CrossRef]
- Friggeri, L.; Hargrove, T.Y.; Wawrzak, Z.; Guengerich, F.P.; Lepesheva, G.I. Validation of human sterol 14α-demethylase (CYP51) druggability: Structure-guided design, synthesis, and evaluation of stoichiometric, functionally irreversible inhibitors. J. Med. Chem. 2019, 62, 10391–10401. [Google Scholar] [CrossRef]
- Gotoh, O. Substrate recognition sites in cytochrome P450 family 2 (CYP2) proteins inferred from comparative analyses of amino acid and coding nucleotide sequences. J. Biol. Chem. 1992, 267, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Poulos, T.L. Cytochrome P450 flexibility. Proc. Natl. Acad. Sci. USA 2003, 100, 13121–13122. [Google Scholar] [CrossRef]
- Scott, E.E.; He, Y.A.; Wester, M.R.; White, M.A.; Chin, C.C.; Halpert, J.R.; Johnson, E.F.; Stout, C.D. An open conformation of mammalian cytochrome P450 2B4 at 1.6-A resolution. Proc. Natl. Acad. Sci. USA 2003, 100, 13196–13201. [Google Scholar] [CrossRef]
- Siebenaller, J.; Somero, G.N. Pressure-adaptive differences in lactate dehydrogenases of congeneric fishes living at different depths. Science 1978, 201, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Brindley, A.A.; Pickersgill, R.W.; Partridge, J.C.; Dunstan, D.J.; Hunt, D.M.; Warren, M.J. Enzyme sequence and its relationship to hyperbaric stability of artificial and natural fish lactate dehydrogenases. PLoS ONE 2008, 3, e2042. [Google Scholar] [CrossRef]
- Royer, C.A. Application of pressure to biochemical equilibria: The other thermodynamic variable. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1995; Volume 259, pp. 357–377. [Google Scholar]
- Cossins, A.R.; MacDonald, A.G. Homeoviscous theory under pressure: II. The molecular order of membranes from deep-sea fish. Biochim. Biophys. Acta 1984, 776, 144–150. [Google Scholar] [CrossRef]
- Cossins, A.R.; Macdonald, A.G. Homeoviscous adaptation under pressure. III. The fatty acid composition of liver mitochondrial phospholipids of deep-sea fish. Biochim. Biophys. Acta Biomembr. 1986, 860, 325–335. [Google Scholar] [CrossRef]
- Simonato, F.; Campanaro, S.; Lauro, F.M.; Vezzi, A.; D’Angelo, M.; Vitulo, N.; Valle, G.; Bartlett, D.H. Piezophilic adaptation: A genomic point of view. J. Biotechnol. 2006, 126, 11–25. [Google Scholar] [CrossRef]
- Cossins, A.R.; Macdonald, A.G. The adaptation of biological membranes to temperature and pressure: Fish from the deep and cold. J. Bioenerg. Biomembr. 1989, 21, 115–135. [Google Scholar] [CrossRef]
- Parzanini, C.; Parrish, C.C.; Hamel, J.F.; Mercier, A. Functional diversity and nutritional content in a deep-sea faunal assemblage through total lipid, lipid class, and fatty acid analyses. PLoS ONE 2018, 13, e0207395. [Google Scholar] [CrossRef] [PubMed]
- Tamby, A.; Sinninghe Damsté, J.S.; Villanueva, L. Microbial membrane lipid adaptations to high hydrostatic pressure in the marine environment. Front. Mol. Biosci. 2023, 9, 1058381. [Google Scholar] [CrossRef]
- Winnikoff, J.R.; Haddock, S.H.D.; Budin, I. Depth- and temperature-specific fatty acid adaptations in ctenophores from extreme habitats. J. Exp. Biol. 2021, 224, jeb242800. [Google Scholar] [CrossRef] [PubMed]
- Mostofian, B.; Zhuang, T.; Cheng, X.; Nickels, J.D. Branched-chain fatty acid content modulates structure, fluidity, and phase in model microbial cell membranes. J. Phys. Chem. B 2019, 123, 5814–5821. [Google Scholar] [CrossRef]
- Voronin, V.P.; Nemova, N.N.; Ruokolainen, T.R.; Artemenkov, D.V.; Rolskii, A.Y.; Orlov, A.M.; Murzina, S.A. Into the deep: New data on the lipid and fatty acid profile of redfish Sebastes mentella inhabiting different depths in the Irminger Sea. Biomolecules 2021, 11, 704. [Google Scholar] [CrossRef]
- Arts, M.T.; Kohler, C.C. Health and condition in fish: The influence of lipids on membrane competency and immune response. In Lipids in Aquatic Ecosystems; Kainz, M., Brett, M.T., Arts, M.T., Eds.; Springer: New York, NY, USA, 2009; pp. 237–256. [Google Scholar]
- Kijewska, M.; Radziszewska, K.; Kielmas, M.; Stefanowicz, P.; Szewczuk, Z. Nonenzymatic modification of Ubiquitin under high-pressure and -temperature treatment: Mass spectrometric studies. J. Agric. Food Chem. 2015, 63, 614–619. [Google Scholar] [CrossRef]
- Beckham, G.T.; Dai, Z.; Matthews, J.F.; Momany, M.; Payne, C.M.; Adney, W.S.; Baker, S.E.; Himmel, M.E. Harnessing glycosylation to improve cellulase activity. Curr. Opin. Biotechnol. 2012, 23, 338–345. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, H.; Zhou, Y.; Lu, Z.; Pu, Y.; Zhang, H. Cloning and functional characterization of the oxidative squalene cyclase gene in the deep-sea holothurian Chiridota sp. Gene 2024, 894, 147971. [Google Scholar] [CrossRef]
- Zielinska, D.F.; Gnad, F.; Schropp, K.; Wiśniewski, J.R.; Mann, M. Mapping N-glycosylation sites across seven evolutionarily distant species reveals a divergent substrate proteome despite a common core machinery. Mol. Cell 2012, 46, 542–548. [Google Scholar] [CrossRef]
- Shental-Bechor, D.; Levy, Y. Folding of glycoproteins: Toward understanding the biophysics of the glycosylation code. Curr. Opin. Struct. Biol. 2009, 19, 524–533. [Google Scholar] [CrossRef]
- Yancey, P.H.; Siebenaller, J.F. Co-evolution of proteins and solutions: Protein adaptation versus cytoprotective micromolecules and their roles in marine organisms. J. Exp. Biol. 2015, 218, 1880–1896. [Google Scholar] [CrossRef] [PubMed]
- Laurent, H.; Youngs, T.G.A.; Headen, T.F.; Soper, A.K.; Dougan, L. The ability of trimethylamine N-oxide to resist pressure induced perturbations to water structure. Commun. Chem. 2022, 5, 116. [Google Scholar] [CrossRef]
- Kelly, R.H.; Yancey, P.H. High contents of trimethylamine oxide correlating with depth in deep-sea teleost fishes, skates, and decapod crustaceans. Biol. Bull. 1999, 196, 18–25. [Google Scholar] [CrossRef]
- Laxson, C.J.; Condon, N.E.; Drazen, J.C.; Yancey, P.H. Decreasing urea: Trimethylamine N-oxide ratios with depth in chondrichthyes: A physiological depth limit? Physiol. Biochem. Zool. 2011, 84, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Yancey, P.H.; Speers-Roesch, B.; Atchinson, S.; Reist, J.D.; Majewski, A.R.; Treberg, J.R. Osmolyte adjustments as a pressure adaptation in deep-sea chondrichthyan fishes: An intraspecific test in Arctic skates (Amblyraja hyperborea) along a depth gradient. Physiol. Biochem. Zool. 2018, 91, 788–796. [Google Scholar] [CrossRef]
- Yancey, P.H.; Gerringer, M.E.; Drazen, J.C.; Rowden, A.A.; Jamieson, A. Marine fish may be biochemically constrained from inhabiting the deepest ocean depths. Proc. Natl. Acad. Sci. USA 2014, 111, 4461–4465. [Google Scholar] [CrossRef]
- Xu, H.; Fang, C.; Xu, W.; Wang, C.; Song, Y.; Zhu, C.; Fang, W.; Fan, G.; Lv, W.; Bo, J.; et al. Evolution and genetic adaptation of fishes to the deep sea. Cell 2025, 188, 1393–1408.e13. [Google Scholar] [CrossRef]
- Brooks, N.J. Pressure effects on lipids and bio-membrane assemblies. IUCrJ 2014, 1 Pt 6, 470–477. [Google Scholar] [CrossRef]
- Pozza, A.; Giraud, F.; Cece, Q.; Casiraghi, M.; Point, E.; Damian, M.; Le Bon, C.; Moncoq, K.; Banères, J.L.; Lescop, E.; et al. Exploration of the dynamic interplay between lipids and membrane proteins by hydrostatic pressure. Nat. Commun. 2022, 13, 1780. [Google Scholar] [CrossRef]
- Corradi, V.; Sejdiu, B.I.; Mesa-Galloso, H.; Abdizadeh, H.; Noskov, S.Y.; Marrink, S.J.; Tieleman, D.P. Emerging diversity in lipid–protein interactions. Chem. Rev. 2019, 119, 5775–5848. [Google Scholar] [CrossRef]
- Davydov, D.R. Merging thermodynamics and evolution: How the studies of high pressure adaptation may help to understand enzymatic mechanisms. J. Thermodyn. Catal. 2012, 3, e110. [Google Scholar] [CrossRef]
- Hanna, I.H.; Teiber, J.F.; Kokones, K.L.; Hollenberg, P.F. Role of the alanine at position 363 of cytochrome P450 2B2 in influencing the NADPH- and hydroperoxide-supported activities. Arch. Biochem. Biophys. 1998, 350, 324–332. [Google Scholar] [CrossRef]
- Omura, T.; Sato, R. The carbon monoxide-binding pigment of liver microsomes. II. Solubilization, purification, and properties. J. Biol. Chem. 1964, 239, 2379–2385. [Google Scholar] [CrossRef]
- Vonrhein, C.; Flensburg, C.; Keller, P.; Sharff, A.; Smart, O.; Paciorek, W.; Womack, T.; Bricogne, G. Data processing and analysis with the autoPROC toolbox. Acta Crystallogr. D Biol. Crystallogr. 2011, 67, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.R.; Murshudov, G.N. How good are my data and what is the resolution? Acta Crystallogr. D Biol. Crystallogr. 2013, 69, 1204–1214. [Google Scholar] [CrossRef]
- Winn, M.D.; Ballard, C.C.; Cowtan, K.D.; Dodson, E.J.; Emsley, P.; Evans, P.R.; Keegan, R.M.; Krissinel, E.B.; Leslie, A.G.; McCoy, A.; et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 2011, 67, 235–242. [Google Scholar] [CrossRef]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 486–501. [Google Scholar] [CrossRef]
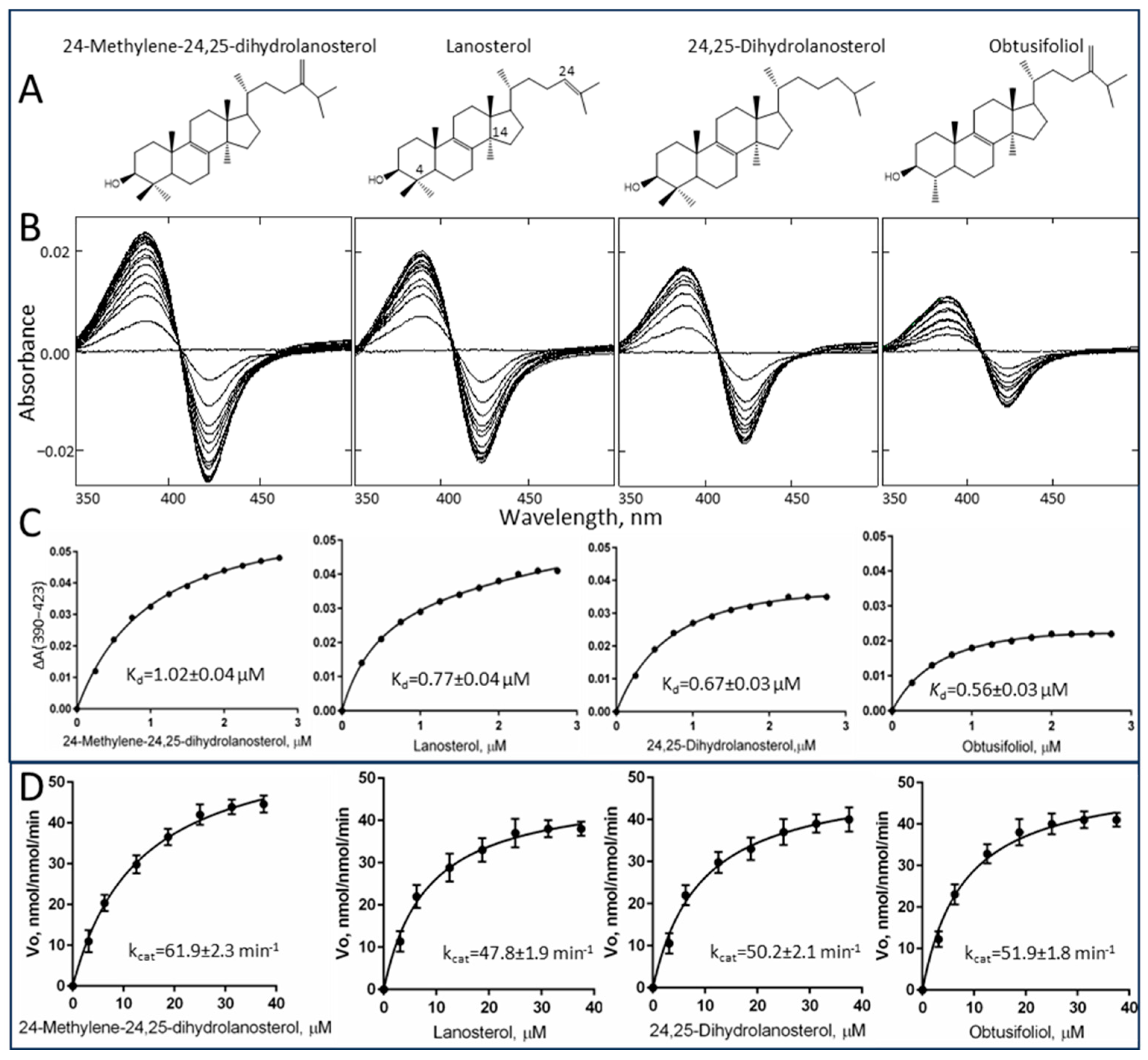



| Sterol Substrate | Spin State Transition in the Heme Iron, % | Change in Absorbance, ΔAmax per µM P450 | Kd, µM | Binding Efficiency, ΔAmax/Kd | kcat, min−1 | Km, µM | Specificity Constant, kcat/Km |
|---|---|---|---|---|---|---|---|
| Lanosterol | 22 | 24 | 0.77 ± 0.04 | 31 | 47.8 ± 1.9 | 8.1 ± 1.1 | 5.9 |
| 24,25-Dihydrolanosterol | 18 | 20 | 0.67 ± 0.03 | 30 | 50.2 ± 2.1 | 9.1 ± 1.2 | 5.5 |
| 24-Methylene-24,25-dihydrolanosterol | 27 | 30 | 1.02 ± 0.04 | 29 | 61.9 ± 2.3 | 13.0 ± 0.9 | 4.8 |
| Obtusifoliol | 13 | 14 | 0.56 ± 0.03 | 25 | 51.9 ± 1.8 | 7.9 ± 0.6 | 6.6 |
| Organism [PDB ID] | C. armatus [9BAT] | Human [8SBI] |
|---|---|---|
| Data collection Beamline | ID-23-2, ESRF | 21-ID-F, LS-cat |
| Wavelength, Å | 0.9677 | 0.97872 |
| Space group | P1211 | P21212 |
| Cell dimensions | ||
| a, b, c, Å | 69.596, 63.135, 104.860 | 143.810, 55.640, 103.070 |
| α, β, γ, ° | 90.0, 97.59, 90.0 | 90.00, 90.00, 90.00 |
| Molecules per asymmetric unit | 2 | 2 |
| Resolution (upper shell), Å | 29.09–2.90 (2.96–2.90) | 49.01–2.73 (2.80–2.73) |
| Solvent content, % | 45 | 51 |
| Rmerge (upper shell) | 0.075 (0.726) | 0.12(0.87) |
| CC (1/2) (upper shell) | 1.000 (0.786) | 0.996 (0.627) |
| I/σ(I) (upper shell) | 27 (2.5) | 9.1(1.3) |
| Completeness (upper shell), % | 96.9 (98.1) | 98.2 (96.2) |
| Redundancy (upper shell) | 5.9 (5.4) | 4.0 (3.9) |
| Refinement | ||
| No. of unique reflections | 18,679 | 22,239 |
| Rwork/Rfree | 0.232/0.248 | 0.230/0.246 |
| R.m.s deviations | ||
| Bond lengths, Å | 0.004 | 0.007 |
| Bond angles, ° | 1.06 | 1.1 |
| Ramachandran plot | ||
| Favourable/allowed, % | 93.5/99.8 | 97/100 |
| Outliers, % | 0.2 | 0 |
| Average B factor, Å2 | 98.0 | 51.2 |
| Model | ||
| No. of atoms | 7173 | 7299 |
| No. of residues per molecule | ||
| Protein (B factor, Å2, A/B/) | 445 (91.8/106.2) | 445 (51/55.5) |
| Heme (B factor, Å2) Water (B factor, Å2) | 1 (66.7/67.5) 22 (52.0) | 1 (50/52.0) 93 (44) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hargrove, T.Y.; Lamb, D.C.; Wawrzak, Z.; Minasov, G.; Goldstone, J.V.; Kelly, S.L.; Stegeman, J.J.; Lepesheva, G.I. Unique Structural Features Relate to Evolutionary Adaptation of Cytochrome P450 in the Abyssal Zone. Int. J. Mol. Sci. 2025, 26, 5689. https://doi.org/10.3390/ijms26125689
Hargrove TY, Lamb DC, Wawrzak Z, Minasov G, Goldstone JV, Kelly SL, Stegeman JJ, Lepesheva GI. Unique Structural Features Relate to Evolutionary Adaptation of Cytochrome P450 in the Abyssal Zone. International Journal of Molecular Sciences. 2025; 26(12):5689. https://doi.org/10.3390/ijms26125689
Chicago/Turabian StyleHargrove, Tatiana Y., David C. Lamb, Zdzislaw Wawrzak, George Minasov, Jared V. Goldstone, Steven L. Kelly, John J. Stegeman, and Galina I. Lepesheva. 2025. "Unique Structural Features Relate to Evolutionary Adaptation of Cytochrome P450 in the Abyssal Zone" International Journal of Molecular Sciences 26, no. 12: 5689. https://doi.org/10.3390/ijms26125689
APA StyleHargrove, T. Y., Lamb, D. C., Wawrzak, Z., Minasov, G., Goldstone, J. V., Kelly, S. L., Stegeman, J. J., & Lepesheva, G. I. (2025). Unique Structural Features Relate to Evolutionary Adaptation of Cytochrome P450 in the Abyssal Zone. International Journal of Molecular Sciences, 26(12), 5689. https://doi.org/10.3390/ijms26125689






