Ballota hirsuta Benth Arrests the Cell Cycle, Induces Apoptosis and Inhibits the Invasion of MCF-7 and MDA-MB-231 Cell Lines in 2D and 3D Models
Abstract
1. Introduction
2. Results
2.1. Compounds Identification of Ballota hirsuta Benth Extract (EAB) by Ultra-Performance Liquid Chromatography with Time-of-Flight Mass Spectrometry (UPLC-Q-TOF/MSE) and the Quantification of Compounds in Crude Extract by HPLC
2.2. Two-Dimensional Cell Model
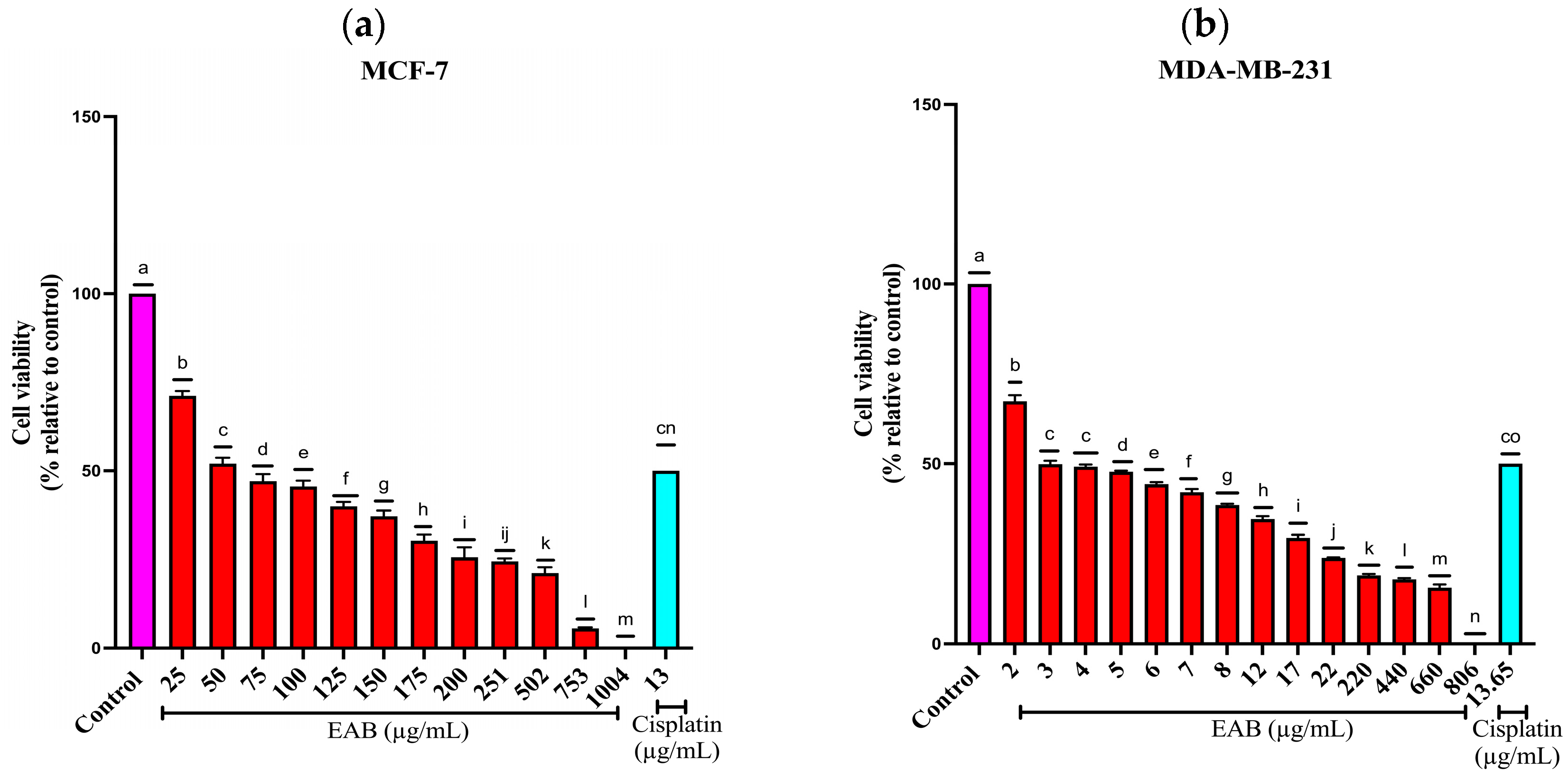
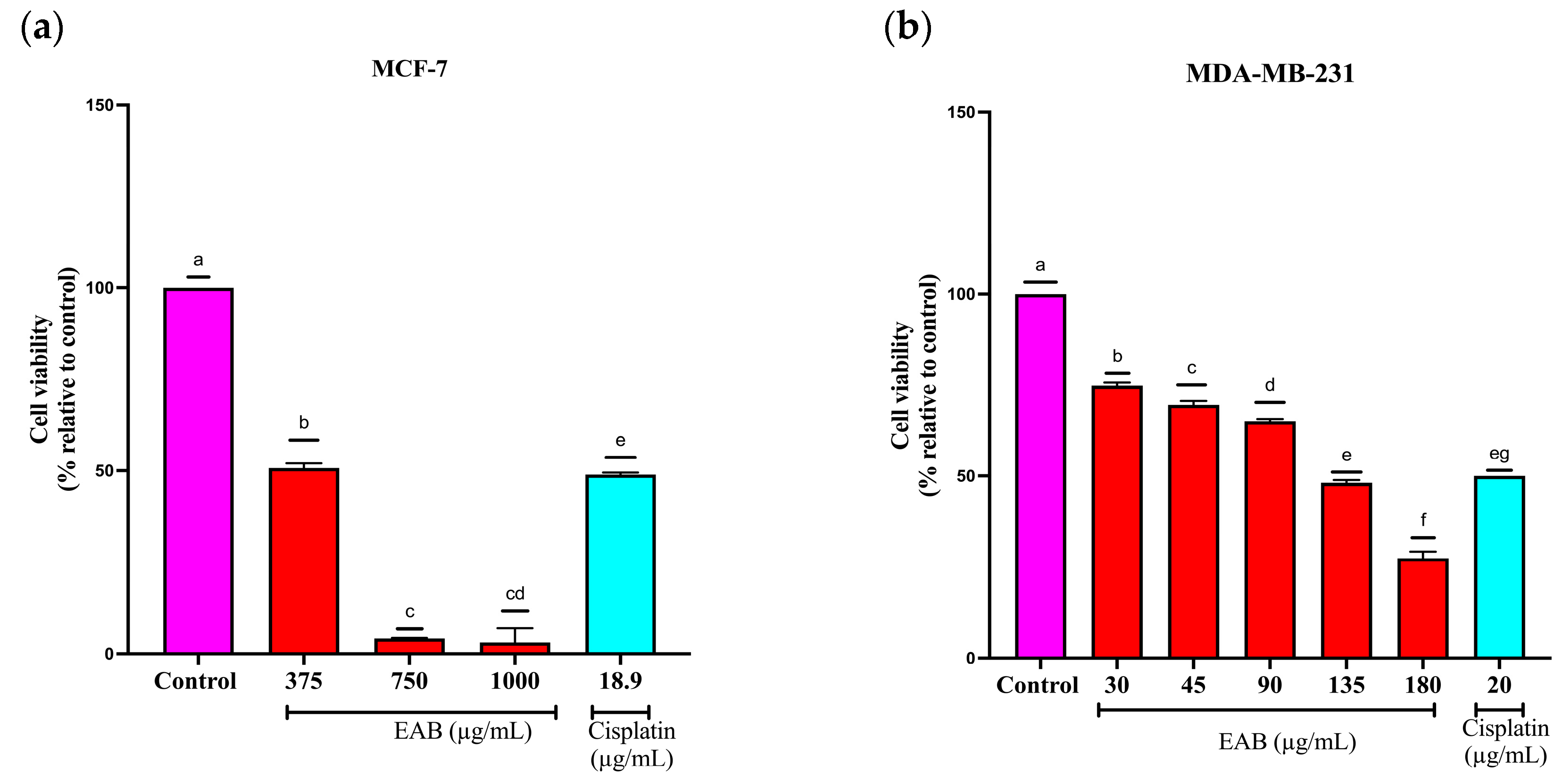
2.2.1. Effect of EAB on the Viability of MCF-7 y MDA-MB-231 Cells by the Sulforhodamine B (SRB) Method
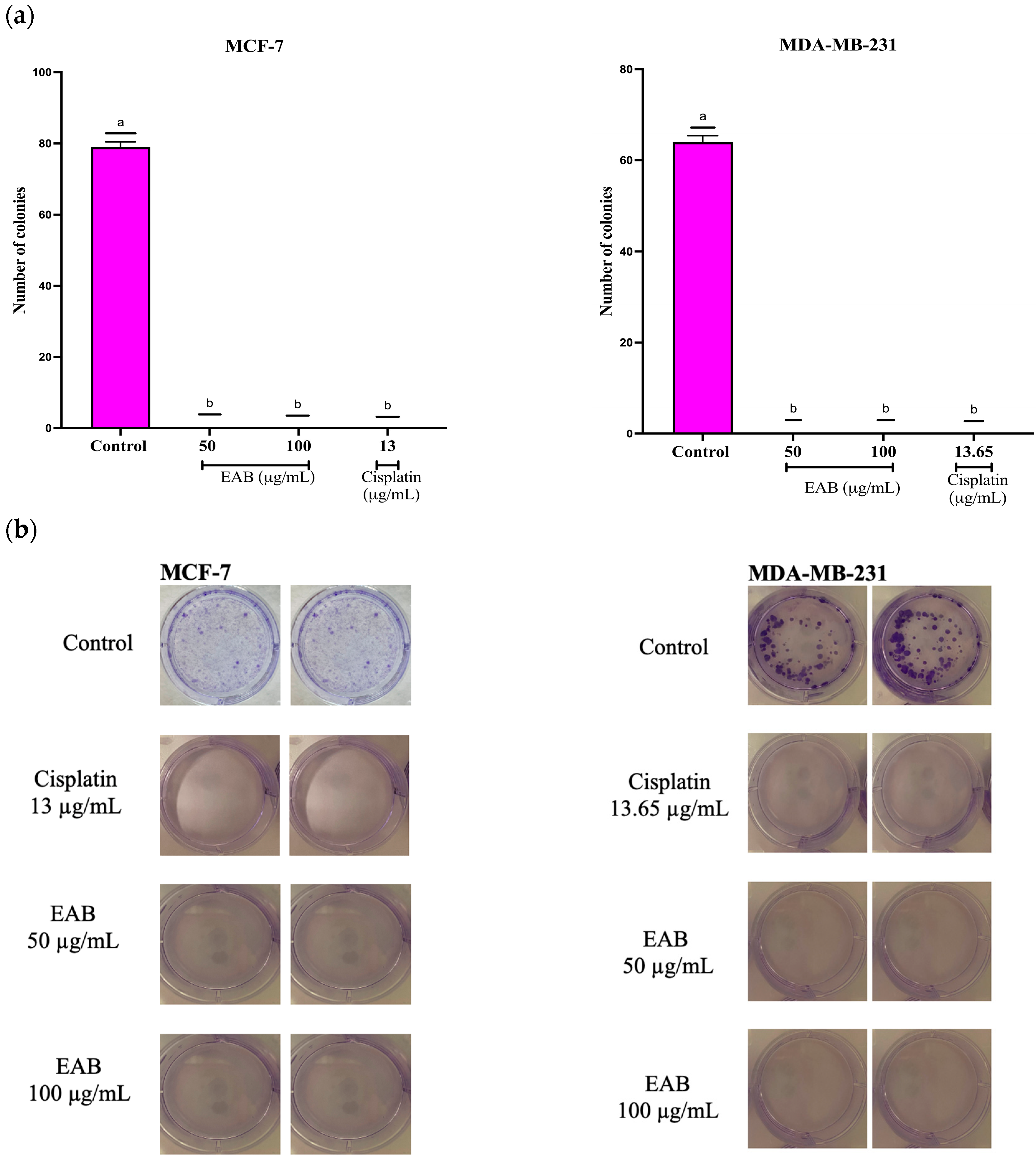
2.2.2. Antiproliferative Effect of EAB by Clonogenic Assay in a 2D Model of MCF-7 and MDA-MB-231 Cells
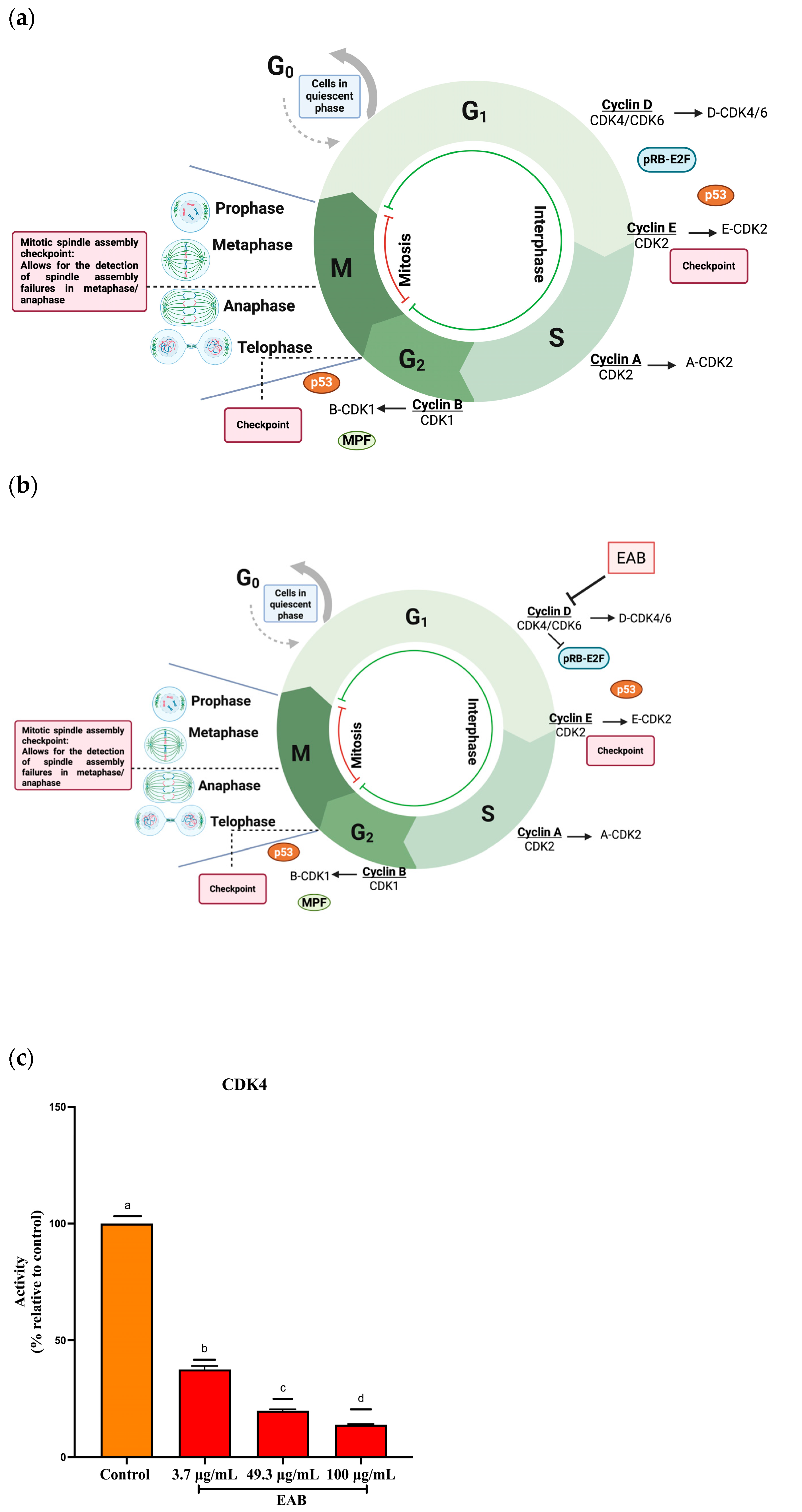
2.2.3. Cell Cycle Arrest in G1 Phase by EAB Treatment in a 2D Model of MCF-7 and MDA-MB-231 Cells
2.2.4. Inhibition of Cyclin-Dependent Kinase 4 (CDK4) Activity by EAB Treatment
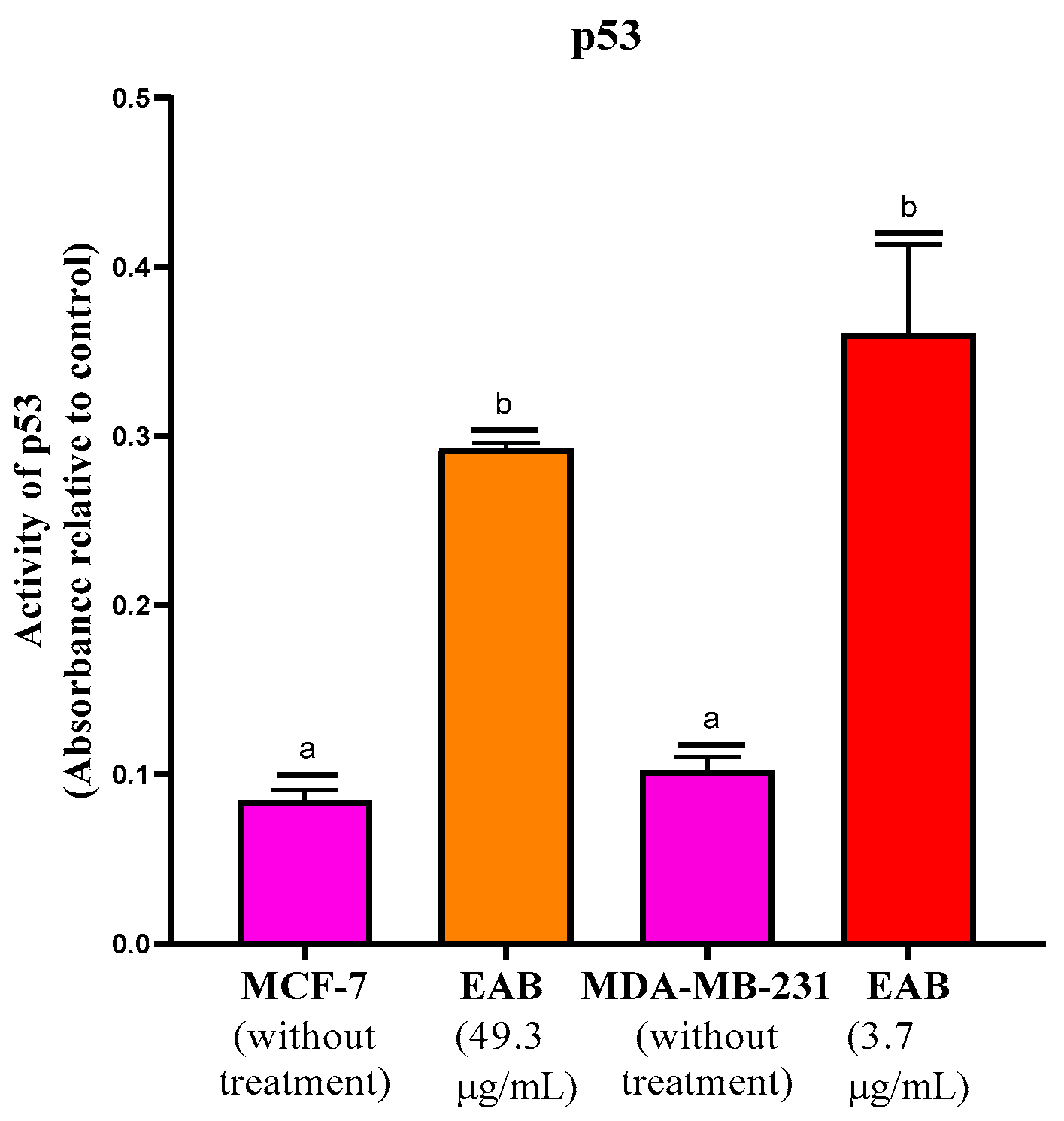
2.2.5. Increased p53 Protein Activity by EAB Treatment
2.3. Molecular Docking of the Interaction of the Compounds with Proteins Involved in Necroptosis, Cell Invasion and the Cell Cycle
2.4. Three-Dimensional Cell Model Assays
2.4.1. EAB Decreases Cell Viability in the 3D Model by Assessing Plasma Membrane Integrity in MCF-7 and MDA-MB-231 Cells
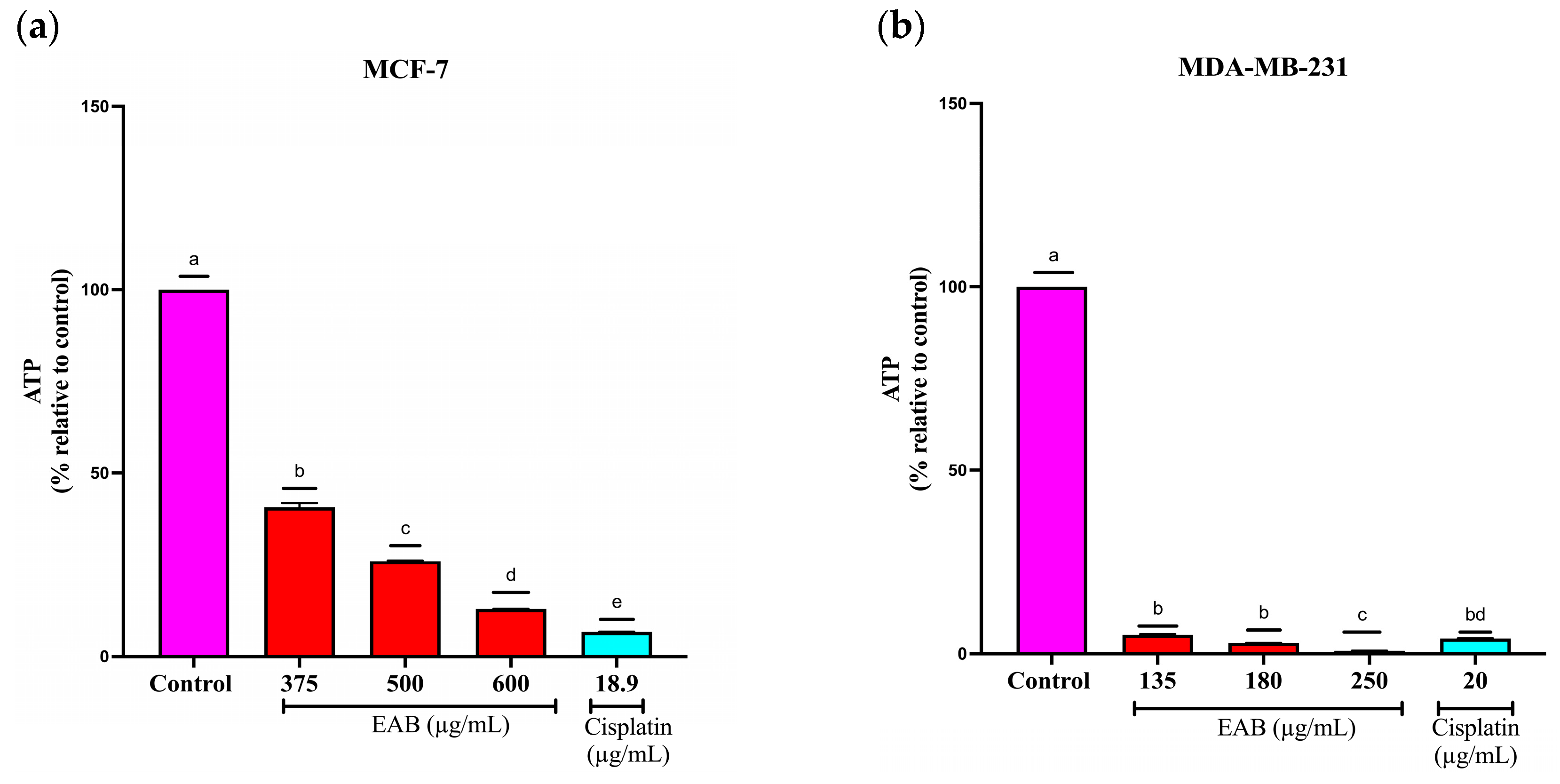
2.4.2. EAB Decreases ATP Levels of MCF-7 and MDA-MB-231 Cells in the 3D Model
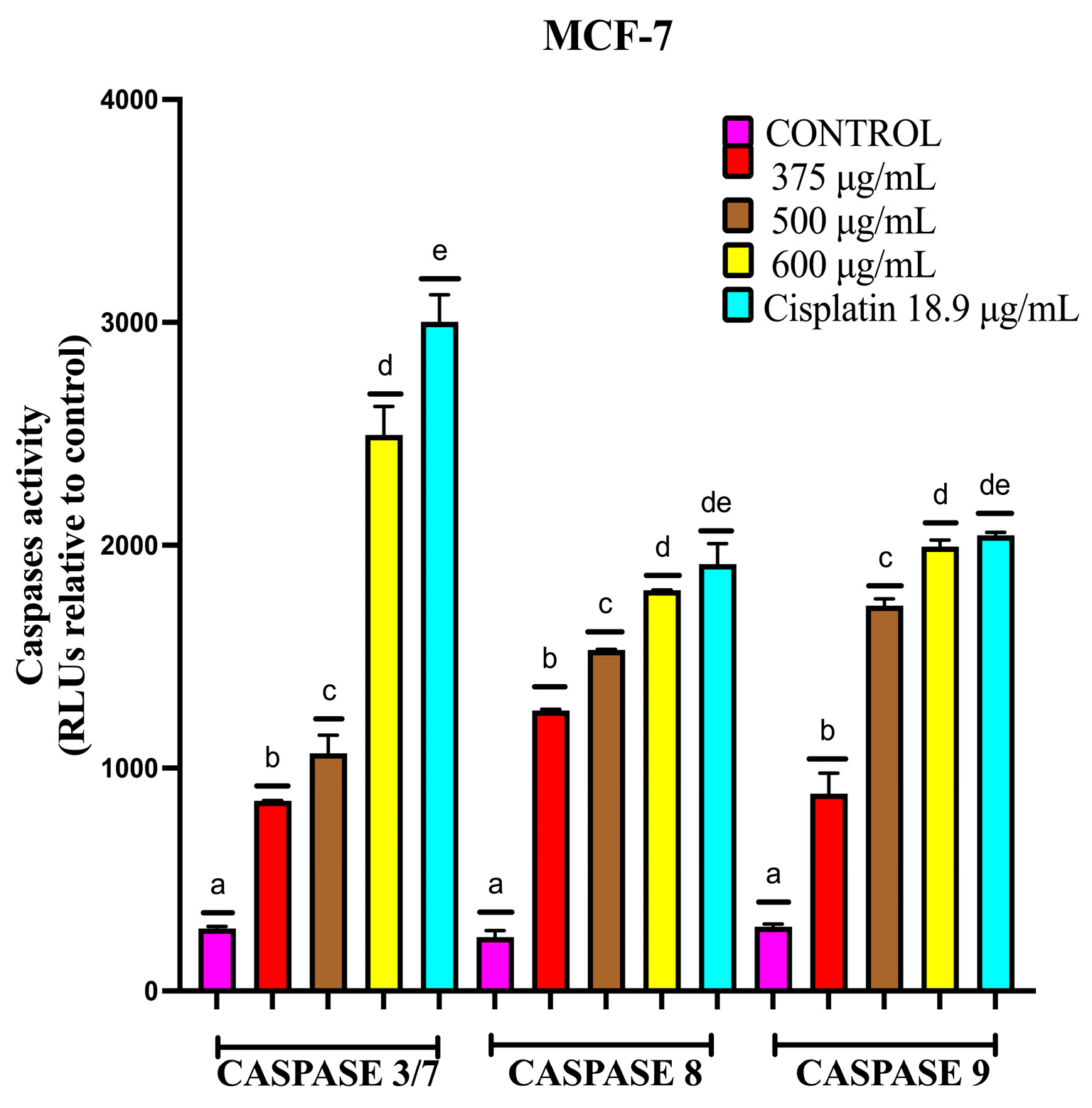
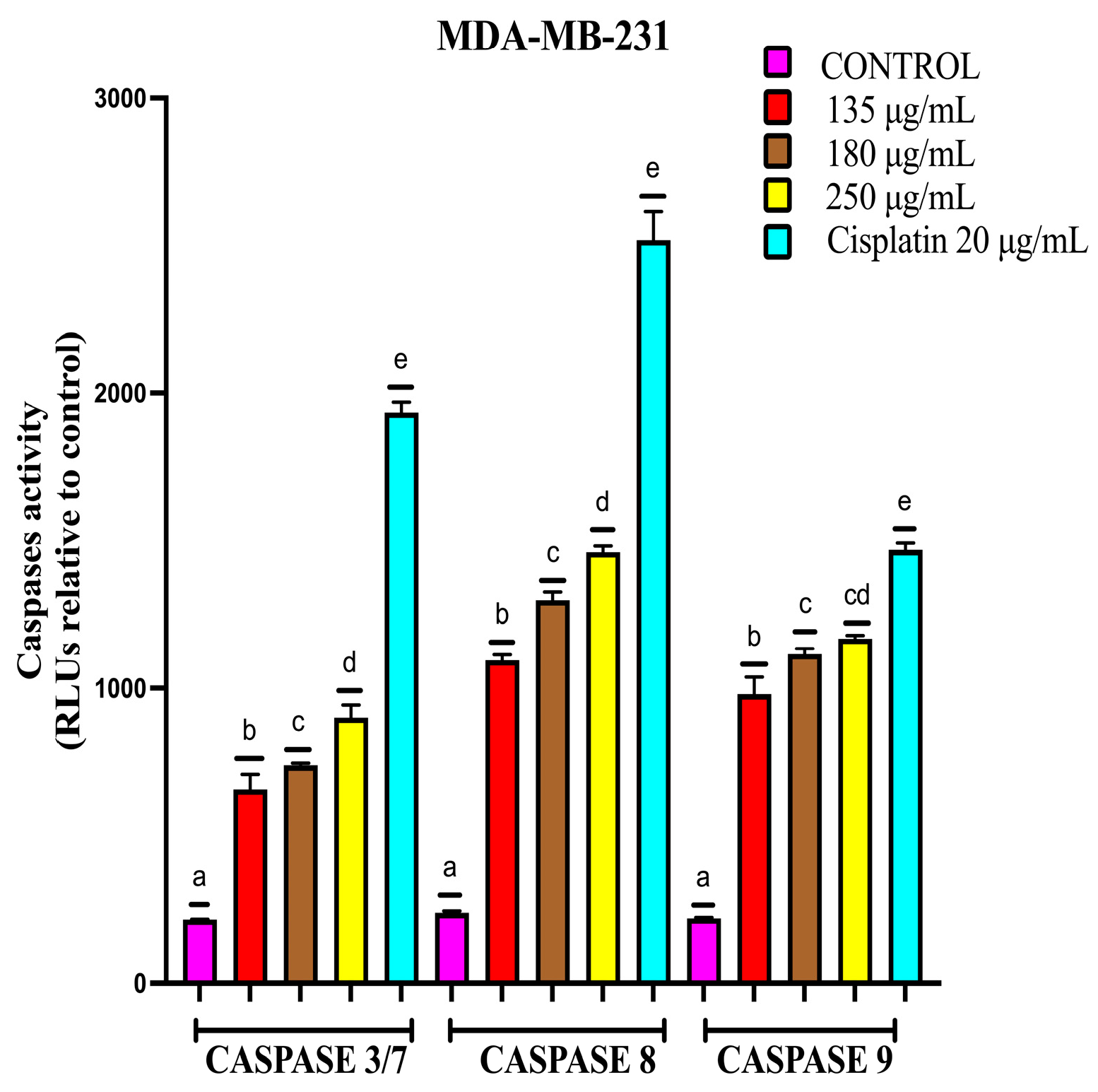
2.4.3. EAB Activates Caspases-3/7, -8 and -9 in MCF-7 and MDA-MB-231 Cell Lines in a 3D Model
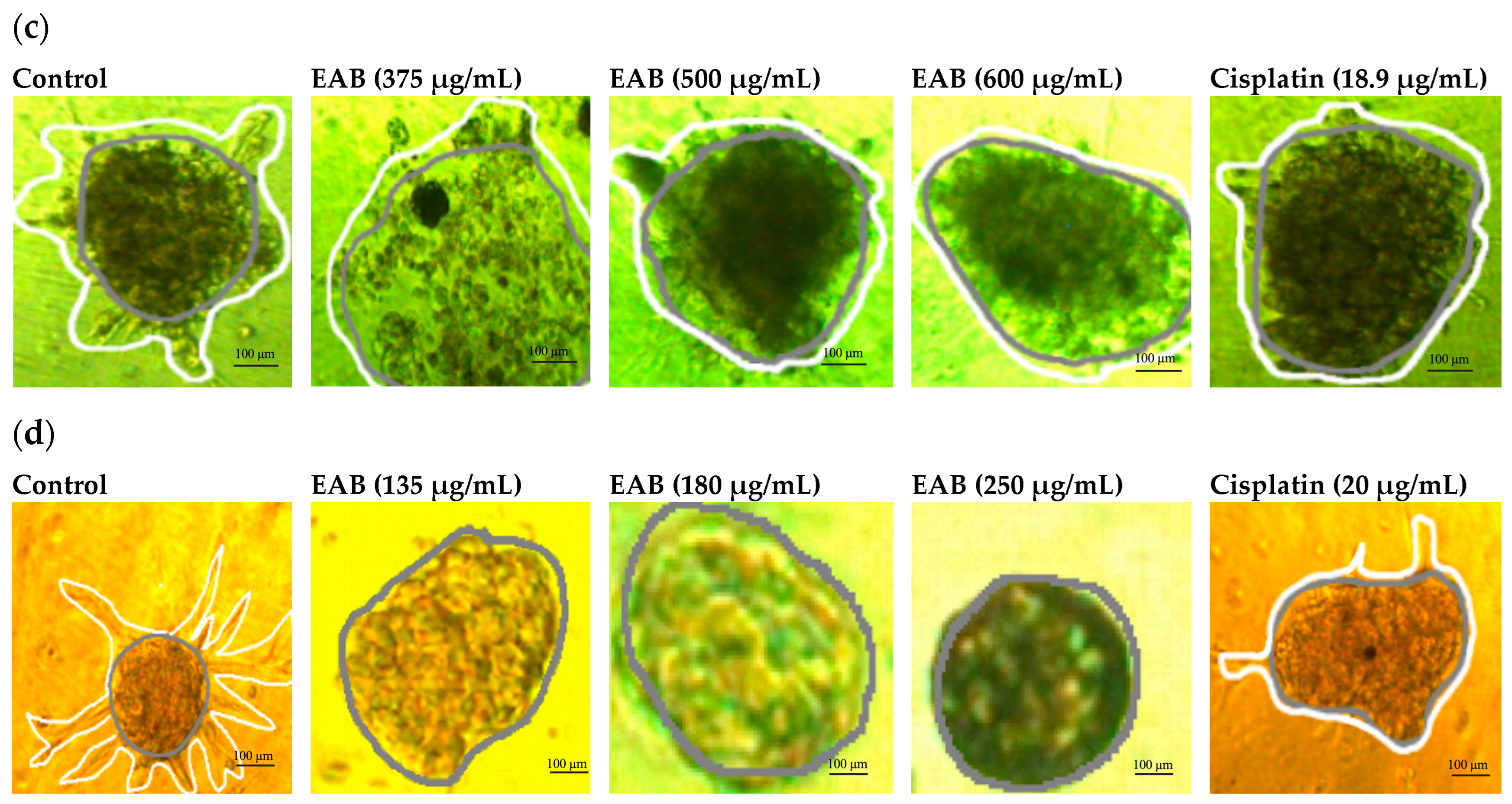
2.4.4. Inhibition of Cell Invasion by EAB in MCF-7 and MDA-MB-231 Cells in a 3D Model
2.4.5. EAB in Combination with Cisplatin and Paclitaxel Decreases Viability of MCF-7 and MDA-MB-231 in the 2D and 3D Models
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Preparation of Extracts
4.3. Ultra-Performance Liquid Chromatography with Time-of-Flight Mass Spectrometry (UPLC-Q-TOF/MSE) and Quantification of Compounds in Crude Extract by HPLC
4.4. Culture
4.5. Two-Dimensional Cell Model Assays
4.5.1. Two-Dimensional Cell Viability by SRB (Sulforhodamine B)
4.5.2. Clonogenic Assay in a 2D Culture of MCF-7 and MDA-MB-231 Cells
4.5.3. Cell Cycle Assay in a 2D Model in MCF-7 and MDA-MB-231 Cells by Flow Cytometry
4.5.4. Cyclin-Dependent Kinase 4 (CDK4) Activity Inhibition Assay
4.5.5. p53 Protein Transcription Assay
4.6. In Silico Analysis
4.7. Three-Dimensional Cell Model Assays
4.7.1. Cell Viability by Plasma Membrane Integrity in a 3D Culture of MCF-7 and MDA-MB-231 Cells
4.7.2. Quantification of ATP Levels in the 3D Model
4.7.3. Caspases-3/7, -8 and -9 Activity of MCF-7 and MDA-MB-231 Cells in the 3D Model
4.7.4. Tumor Invasion Assay in a 3D Model of MCF-7 and MDA-MB-231 Cells
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Globocan. New Global Cancer Data. 2022. Available online: https://gco.iarc.fr/today/en/dataviz/pie?mode=cancer&group_populations=1 (accessed on 11 August 2024).
- Inegi. Statistics on the Occasion of the International Day of the Fight Against Breast Cancer. 2023. Available online: https://www.inegi.org.mx/contenidos/saladeprensa/aproposito/2024/EAP_CANCER24.pdf (accessed on 19 October 2024).
- Instituto Mexicano del Seguro Social. Cáncer de Mama. 15 July 2015. Available online: https://www.imss.gob.mx/salud-en-linea/cancer-mama (accessed on 9 August 2024).
- Comsa, S.; Campean, A.M.; Raica, M. The Story of MCF-7 Breast Cancer Cell Line: 40 years of Experience in Research. Anticancer. Res. 2015, 35, 3147–3154. [Google Scholar] [PubMed]
- Horvath, E. Subtipos moleculares del cáncer mamario-lo que el radiólogo dedicado a imágenes mamarias debe saber. Rev. Chil. Radiol. 2021, 27, 17–26. [Google Scholar] [CrossRef]
- Xiong, X.; Zheng, L.-W.; Ding, Y.; Chen, Y.-F.; Cai, Y.-W.; Wang, L.-P.; Huang, L.; Liu, C.-C.; Shao, Z.-M.; Yu, K.-D. Breast cancer: Pathogenesis and treatments. Signal Transduct. Target. Ther. 2025, 10, 49. [Google Scholar] [CrossRef]
- Shen, Y.; Schmidt, B.U.S.; Kubitschke, H.; Morawetz, E.W.; Wolf, B.; Käs, J.A.; Losert, W. Detecting heterogeneity in and between breast cancer cell lines. Cancer Converg. 2020, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Guo, P.; McCarthy, C.; Wang, B.; Tao, Y.; Auguste, D. Peptide density targets and impedes triple negative breast cancer metastasis. Nat. Commun. 2018, 9, 2612. [Google Scholar] [CrossRef]
- Ma, W.; Zhao, F.; Zhou, C.; Zhang, Y.; Zhao, Y.; Li, N.; Xie, P. Targeted neoadjuvant therapy in the HER-2-positive breast cancer patients: A systematic review and meta-analysis. OncoTargets Ther. 2019, 12, 379–390. [Google Scholar] [CrossRef]
- Schoos, A.; Knab, V.M.; Gabriel, C.; Tripolt, S.; Wagner, D.A.; Bauder, B.; Url, A.; Fux, D.A. In vitro study to assess the efficacy of CDK4/6 inhibitor Palbociclib (PD-0332991) for treating canine mammary tumours. Vet. Comp. Oncol. 2019, 17, 507–521. [Google Scholar] [CrossRef]
- González-Vélez, A.; Pino-Villareal, L.E.; Barrera-Barinas, A.; Castillo-Niuman, A.; Tolosa-Pérez, E.N.; Castelblanco, D.I.; Pinzón-Florez, C.E.; Yomayusa, N. Efficacy and safety of PD-1/PD-L1 in metastatic non-small cell lung cancer (NSCLC). Gac. Mex. Oncol. 2021, 20, 111–123. [Google Scholar] [CrossRef]
- American Cancer Society. Breast Cancer Treatment. 2019. Available online: https://www.cancer.org/es/cancer/tipos/cancer-de-seno/tratamiento.html (accessed on 9 August 2024).
- Hernández Guiance, S.; Marino, L.; Isern, D.; Coria, I.; Irurzun, I. Flavonoids: Medicinal and industrial applications. Invenio 2019, 22, 11–27. [Google Scholar]
- Morteza-Semnani, K.; Ghanbarimasir, Z. A review on traditional uses phytochemistry and pharmacological activities of the genus Ballota. J. Ethnopharmacol. 2019, 233, 197–217. [Google Scholar] [CrossRef]
- Ou-Ani, O.; Oucheikh, L.; Dabbous, A.; Znini, M.; Costa, J.; Majidi, L. Optimization of hydrodistillation extraction using response surface methodology and chemical composition of essential oil from Moroccan endemic medicinal plant Ballota hirsuta. Res. Sq. 2020, 65, 1–22. [Google Scholar] [CrossRef]
- Través, P.G.; López-Fontal, R.; Cuadrado, I.; Luque, A.; Boscá, L.; de las Heras, B.; Hortelano, S. Critical role of the death receptor pathway in the antitumoral effects induced by hispanolone derivatives. Oncogene 2012, 32, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Martín, V. Bases Moleculares de la Actividad Antitumoral de Nuevos Agentes Terapéuticos Derivados de la Hispanolona. Ph.D. Thesis, Universidad Complutense de Madrid, Madrid, Spain, 2020. [Google Scholar]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures–a comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Cosme, F.; Aires, A.; Pinto, T.; Oliveira, I.; Vilela, A.; Gonçalves, B. A Comprehensive Review of Bioactive Tannins in Foods and Beverages: Functional Properties, Health Benefits, and Sensory Qualities. Molecules 2025, 30, 800. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Jabit, M.L.; Wahyuni, F.S.; Khalid, R.; Israf, D.A.; Shaari, K.; Lajis, N.H.; Stanslas, J. Cytotoxic and nitric oxide inhibitory activities of methanol extracts ofGarciniaspecies. Pharm. Biol. 2009, 47, 1019–1026. [Google Scholar] [CrossRef]
- Diaz, G.A.; Rodriguez, S.H.; Scull, L.R. Cytotoxicity of medicinal plant extracts on human lung carcinoma cell line A549. Cuba. J. Pharm. 2011, 45, 101–108. [Google Scholar]
- del Barrio Alonso, G.D.C.; Morier Díaz, L.F.; Marrero, R.J. In vitro cytotoxic and antiproliferative activity of Phyllanthus comosus aqueous extract. Cuba. J. Trop. Med. 2021, 73, e674. [Google Scholar]
- Jiang, H.; Cui, J.; Chu, H.; Xu, T.; Xie, M.; Jing, X.; Xu, J.; Zhou, J.; Shu, Y. Targeting IL8 as a sequential therapy strategy to overcome chemotherapy resistance in advanced gastric cancer. Cell Death Discov. 2022, 8, 235. [Google Scholar] [CrossRef]
- Nikolaou, M.; Pavlopoulou, A.; Georgakilas, A.G.; Kyrodimos, E. The challenge of drug resistance in cancer treatment: A current overview. Clin. Exp. Metastasis 2018, 35, 309–318. [Google Scholar] [CrossRef]
- Eslami, M.; Memarsadeghi, O.; Davarpanah, A.; Arti, A.; Nayernia, K.; Behnam, B. Overcoming Chemotherapy Resistance in Metastatic Cancer: A Comprehensive Review. Biomedicines 2024, 12, 183. [Google Scholar] [CrossRef] [PubMed]
- Colomeu, T.C.; de Figueiredo, D.; de Matos da Silva, P.; Fernandes, L.G.; Zollner, R.D. Antiproliferative and Pro-Oxidant Effect of Polyphenols in Aqueous Leaf Extract of Passiflora alata Curtis on Activated T Lymphocytes from Non-Obese Diabetic (NOD SHILT/J) Mice. Antioxidants 2022, 11, 1503. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Zhang, Z.; He, X.; Pan, G.; Li, G.; Lv, J.; Xu, Y.; Xie, M.; Feng, J.; Wang, W.; et al. A novel ribociclib derivative WXJ-103 exerts anti-breast cancer effect through CDK4/6. Anti-Cancer Drugs Publ. Ahead Print 2023, 34, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Pinazo, R.; Pinazo-Durán, M.D.; Serrano, M. The cell cycle and p53 gene. An approach to molecular ophthalmology. Arch. Soc. Española Oftalmol. Span. Soc. Ophthalmol. 2010, 85, 229–231. [Google Scholar] [CrossRef][Green Version]
- Castellví, A.; Pequerul, R.; Barracco, V.; Juanhuix, J.; Parés, X.; Farrés, J. Structural and biochemical evidence that ATP inhibits the cancer biomarker human aldehyde dehydrogenase 1A3. Commun. Biol. 2022, 5, 354. [Google Scholar] [CrossRef]
- Gelsinger, D.R.; Reddy, R.; Whittington, K.; Debic, S.; DiRuggiero, J. Post-transcriptional regulation of redox homeostasis by the small RNA SHOxi in haloarchaea. RNA Biol. 2021, 18, 1867–1881. [Google Scholar] [CrossRef]
- Yang, T.; Wang, Y.; Liao, W.; Zhang, S.; Wang, S.; Xu, N.; Xie, W.; Luo, C.; Wang, Y.; Wang, Z.; et al. Down-regulation of EPB41L4A-AS1 mediated brain aging and neurodegenerative diseases via damaging synthesis of NAD+ and ATP. Cell Biosci. 2021, 11, 192. [Google Scholar] [CrossRef]
- Wang, Y.; Wen, X.; Zhang, N.; Wang, L.; Hao, D.; Jiang, X.; He, G. Small-molecule compounds target paraptosis to improve cancer therapy. Biomed. Pharmacother. 2019, 118, 109203. [Google Scholar] [CrossRef]
- Ahearn, T.U.; Peisch, S.; Pettersson, A.; Ebot, E.M.; Zhou, C.K.; Graff, R.E.; Sinnott, J.A.; Fazli, L.; Judson, G.L.; Bismar, T.A.; et al. Expression of IGF/insulin receptor in prostate cancer tissue and progression to lethal disease. Carcinogenesis 2018, 39, 1431–1437. [Google Scholar] [CrossRef]
- Xie, M.; Sun, M.; Ji, X.; Li, D.; Chen, X.; Zhang, B.; Huang, W.; Zhang, T.; Wang, Y.; Tian, D.; et al. Overexpression of BACH1 mediated by IGF2 facilitates hepatocellular carcinoma growth and metastasis via IGF1R and PTK2. Theranostics 2022, 12, 1097–1116. [Google Scholar] [CrossRef]
- Muoio, M.G.; Talia, M.; Lappano, R.; Sims, A.H.; Vella, V.; Cirillo, F.; Manzella, L.; Giuliano, M.; Maggiolini, M.; Belfiore, A.; et al. Activation of the S100A7/RAGE Pathway by IGF-1 Contributes to Angiogenesis in Breast Cancer. Cancers 2021, 13, 621. [Google Scholar] [CrossRef]
- Yamamura, M.; Sato, Y.; Takahashi, K.; Sasaki, M.; Harada, K. The cyclin-dependent kinase pathway involving CDK1 is a potential therapeutic target for cholangiocarcinoma. Oncol. Rep. 2020, 43, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Shamanna, R.A.; Kulikowicz, T.; Borhan Fakouri, N.; Kim, E.W.; Christiansen, L.S.; Croteau, D.L.; Bohr, V.A. CDK2 phosphorylation of Werner protein (WRN) contributes to WRN’s DNA double-strand break repair pathway choice. Aging Cell 2021, 20, e13484. [Google Scholar] [CrossRef]
- Liu, H.; Wang, H.; Zhang, H.; Yu, M.; Tang, Y. TEX19 increases the levels of CDK4 and promotes breast cancer by disrupting SKP2-mediated CDK4 ubiquitination. Cancer Cell Int. 2024, 24, 207. [Google Scholar] [CrossRef]
- Antonia, R.J.; Karelehto, E.; Toriguchi, K.; Matli, M.; Warren, R.S.; Pfeffer, L.M.; Donner, D.B. STAT3 regulates inflammatory cytokine production downstream of TNFR1 by inducing expression of TNFAIP3/A20. J. Cell. Mol. Med. 2022, 26, 4591–4601. [Google Scholar] [CrossRef]
- Chadwick, J.W.; Macdonald, R.; Ali, A.A.; Glogauer, M.; Magalhaes, M.A. TNFα Signaling Is Increased in Progressing Oral Potentially Malignant Disorders and Regulates Malignant Transformation in an Oral Carcinogenesis Model. Front. Oncol. 2021, 11, 741013. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Jaffer, T.; Eguchi, S.; Wang, Z.; Linkermann, A.; Ma, D. Role of necroptosis in the pathogenesis of solid organ injury. Cell Death Dis. 2015, 6, e1975. [Google Scholar] [CrossRef]
- Song, Z.; Wang, J.; Su, Q.; Luan, M.; Chen, X.; Xu, X. The role of MMP-2 and MMP-9 in the metastasis and development of hypopharyngeal carcinoma. Braz. J. Otorhinolaryngol. 2021, 87, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Augoff, K.; Hryniewicz-Jankowska, A.; Tabola, R.; Stach, K. MMP9: A Tough Target for Targeted Therapy for Cancer. Cancers 2022, 14, 1847. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, J.; Min, L.; Sun, X.; Guo, Q.; Li, H.; Zhang, Z.; Zhao, Y.; Gu, J.; Zhang, S. Cadherin Expression Shift Could Well Distinguish Esophageal Squamous Cell Carcinoma from Non- Cancerous Esophageal Tissues. Oncol. Res. Treat. 2018, 41, 380–385. [Google Scholar] [CrossRef]
- Liang, H.; Dong, J.; Cheng, Z.; Li, Q.; Feng, D.; Ling, B. B-cell receptor-associated protein 31 promotes migration and invasion in ovarian cancer cells. Exp. Ther. Med. 2021, 22, 858. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Zhang, H.; Gong, X.; Wei, W.; Lv, Y.; Chen, Z.; Wang, R.; Yi, J.; Shen, Y.; Jin, S. The Role of the Tumor Microenvironment in Neuropilin 1-Induced Radiation Resistance in Lung Cancer Cells. J. Cancer 2019, 10, 4017–4030. [Google Scholar] [CrossRef] [PubMed]
- Gallardo Pérez, J.C.; Espinosa Castilla, M.; Meléndez Zajgla, J.; Maldonado Lagunas, V. Multicellular tumor spheroids in the evaluation of anticancer therapeutic strategies. J. Biochem. Educ. 2006, 25, 101–107. [Google Scholar]
- Singh, H.; Sreedharan, S.; Tiwari, R.; Walther, C.; Smythe, C.; Pramanik, S.K.; Thomas, J.A.; Das, A. A Fluorescent Chemodosimeter for Organelle-Specific Imaging of Nucleoside Polyphosphate Dynamics in Living Cells. Cryst. Growth Des. 2018, 18, 7199–7206. [Google Scholar] [CrossRef]
- Borquez, J.C.; Montes, N.; Diaz, E. Combating cancer cell metabolism through SIRT3 activation and physical exercise. Rev. Médica Chile 2018, 146, 762–769. [Google Scholar]
- Mustafa, M.; Ahmad, R.; Tantry, I.Q.; Ahmad, W.; Siddiqui, S.; Alam, M.; Abbas, K.; Moinuddin Hassan, M.I.; Habib, S.; Islam, S. Apoptosis: A Comprehensive Overview of Signaling Pathways, Morphological Changes, and Physiological Significance and Therapeutic Implications. Cells 2024, 13, 1838. [Google Scholar] [CrossRef]
- García, M.; Vecino, E. Intracellular signaling pathways leading to retinal cell apoptosis. Arch. Soc. Española Oftalmol. 2003, 78, 351–364. [Google Scholar] [CrossRef]
- Maelfait, J.; Beyaert, R. Non-apoptotic functions of caspase-8. Biochem. Pharmacol. 2008, 76, 1365–1373. [Google Scholar] [CrossRef]
- Zhao, C.; He, C.; Lu, J.; Huang, X.; Chen, C.; Lin, X. Progression Patterns and Post-Progression Survival in Recurred Intrahepatic Cholangiocarcinoma Patients: A Novel Prognostic Nomogram Based on Multicenter Cohorts. Front. Oncol. 2022, 12, 832038. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Muñoz Cendales, D.R.; Cuca Suárez, L.E. Plant-derived cytotoxic compounds and their relationship with apoptosis inhibitor proteins (AIPs). Rev. Colomb. Cancerología 2016, 20, 124–134. [Google Scholar] [CrossRef]
- Callando- Riva, D.; Quispe- Mauricio, A.; Lindo-Gamarra, S.; Vaisberg, A.J. Cytotoxic activity of the ethanolic extract of gnaphalium spicatum “keto keto” in cultures of human tumor cell lines. Rev. Peru. Med. Exp. Salud Pública 2008, 25, 380–385. [Google Scholar]
- Staff, N.P.; Fehrenbacher, J.C.; Caillaud, M.; Damaj, M.I.; Segal, R.A.; Rieger, S. Pathogenesis of paclitaxel-induced peripheral neuropathy: A current review of in vitro and in vivo findings using rodent and human model systems. Exp. Neurol. 2020, 324, 113121. [Google Scholar] [CrossRef] [PubMed]
- Klein, I.; Lehmann, H.C. Pathomechanisms of Paclitaxel-Induced Peripheral Neuropathy. Toxics 2021, 9, 229. [Google Scholar] [CrossRef] [PubMed]
- Kuban-Jankowska, A.; Kostrzewa, T.; Musial, C.; Barone, G.; Lo-Bosco, G.; Lo-Celso, F.; Gorska-Ponikowska, M. Green Tea Catechins Induce Inhibition of PTP1B Phosphatase in Breast Cancer Cells with Potent Anti-Cancer Properties: In Vitro Assay Molecular Docking, and Dynamics Studies. Antioxidants 2020, 9, 1208. [Google Scholar] [CrossRef]
- Oh, S.-S.; Lee, K.W.; Madhi, H.; Jeong, J.-W.; Park, S.; Kim, M.; Lee, Y.; Han, H.-T.; Hwangbo, C.; Yoo, J.; et al. Cordycepin Resensitizes T24R2 Cisplatin-Resistant Human Bladder Cancer Cells to Cisplatin by Inactivating Ets-1 Dependent MDR1 Transcription. Int. J. Mol. Sci. 2020, 21, 1710. [Google Scholar] [CrossRef]
- Goka, E.T.; Chaturvedi, P.; Lopez, D.T.M.; Garza, A.D.L.; Lippman, M.E. RAC1b Overexpression Confers Resistance to Chemotherapy Treatment in Colorectal Cancer. Mol. Cancer Ther. 2019, 18, 957–968. [Google Scholar] [CrossRef]
- Emran, T.B.; Shahriar, A.; Mahmud, A.R.; Rahman, T.; Abir, M.H.; Siddiquee, M.F.R.; Ahmed, H.; Rahman, N.; Nainu, F.; Wahyudin, E.; et al. Multidrug Resistance in Cancer: Understanding Molecular Mechanisms Immunoprevention and Therapeutic Approaches. Front. Oncol. 2022, 12, 891652. [Google Scholar] [CrossRef]
- Faria, M.; Shepherd, P.; Pan, Y.; Chatterjee, S.S.; Navone, N.; Gustafsson, J.-Å.; Strom, A. The estrogen receptor variants β2 and β5 induce stem cell characteristics and chemotherapy resistance in prostate cancer through activation of hypoxic signaling. Oncotarget 2018, 9, 36273–36288. [Google Scholar] [CrossRef]
- He, M.; Wu, H.; Jiang, Q.; Liu, Y.; Han, L.; Yan, Y.; Wei, B.; Liu, F.; Deng, X.; Chen, H.; et al. Hypoxia-inducible factor-2α directly promotes BCRP expression and mediates the resistance of ovarian cancer stem cells to adriamycin. Mol. Oncol. 2019, 13, 403–421. [Google Scholar] [CrossRef]
- Morales-Pérez, M.; García-Milian, A.J. Role of the ABC superfamily in drug resistance. Horiz. Sanit. 2017, 16, 93–101. [Google Scholar] [CrossRef][Green Version]
- Poon, R.Y. Cell cycle control. Ref. Modul. Biomed. Sci. 2015, 1–13. [Google Scholar] [CrossRef]
- Malumbres, M. Control of the Cell Cycle. Abeloff’s Clin. Oncol. 2020, 56–73. [Google Scholar] [CrossRef]
- Ramírez García, M.Á.; Márquez González, H.; Barranco Lampón, G.; López Aguilar, J.E. Bcl-2: Its role in cell cycle, apoptosis and cancer. Resid. 2014, 9, 84–94. [Google Scholar]
- Chen, S.-J.; Lu, J.-H.; Lin, C.-C.; Zeng, S.-W.; Chang, J.-F.; Chung, Y.-C.; Chang, H.; Hsu, C.-P. Synergistic Chemopreventive Effects of a Novel Combined Plant Extract Comprising Gallic Acid and Hesperidin on Colorectal Cancer. Curr. Issues Mol. Biol. 2023, 45, 4908–4922. [Google Scholar] [CrossRef] [PubMed]
- Matthews, H.K.; Bertoli, C.; de Bruin, R.A. Cell cycle control in cancer. Nat. Rev. Mol. Cell Biol. 2022, 23, 74–88. [Google Scholar] [CrossRef]
- Pandey, K.; Katuwal, N.B.; Park, N.; Hur, J.; Cho, Y.B.; Kim, S.K.; Lee, S.A.; Kim, I.; Lee, S.-R.; Moon, Y.W. Combination of Abemaciclib following Eribulin Overcomes Palbociclib-Resistant Breast Cancer by Inhibiting the G2/M Cell Cycle Phase. Cancers 2022, 14, 210. [Google Scholar] [CrossRef]
- Tian, J.-M.; Ran, B.; Zhang, C.-L.; Yan, D.-M.; Li, X.-H. Estrogen and progesterone promote breast cancer cell proliferation by inducing cyclin G1 expression. Braz. J. Med. Biol. Res. 2018, 51, e5612. [Google Scholar] [CrossRef]
- Menna, P.L.; Cardama, G.A.; Comin, M.J.; Alfonso, D.F.; Gómez, D.E. RHO GTAases as relevant therapeutic targets in cancer and other human diseases. Medicine 2010, 70, 555–564. [Google Scholar]
- Shendge, A.K.; Chaudhuri, D.; Basu, T.; Mandal, N. A natural flavonoid, apigenin isolated from Clerodendrum viscosum leaves, induces G2/M phase cell cycle arrest and apoptosis in MCF-7 cells through the regulation of p53 and caspase-cascade pathway. Clin. Transl. Oncol. 2021, 23, 718–730. [Google Scholar] [CrossRef]
- He, Q.; Chen, Y.-P.; Li, J.; Wu, H.; Chen, F.; Li, M.; Wu, C. Antioxidant and Photoprotective Activities of 3,4-Dihydroxybenzoic Acid and (+)-Catechin, Identified from Schima argentea Extract, in UVB-Irradiated HaCaT Cells. Antioxidants 2025, 14, 241. [Google Scholar] [CrossRef] [PubMed]
- Aborehab, N.M.; Elnagar, M.R.; Waly, N.E. Gallic acid potentiates the apoptotic effect of paclitaxel and carboplatin via overexpression of Bax and P53 on the MCF-7 human breast cancer cell line. J. Biochem. Mol. Toxicol. 2021, 35, e22638. [Google Scholar] [CrossRef]
- Lin, S.; Qin, H.-Z.; Li, Z.-Y.; Zhu, H.; Long, L.; Xu, L.-B. Gallic acid suppresses the progression of triple-negative breast cancer HCC1806 cells via modulating PI3K/AKT/EGFR and MAPK signaling pathways. Front. Pharmacol. 2022, 13, 1049117. [Google Scholar] [CrossRef] [PubMed]
- Meseguer, J.; Ángeles Esteban, M.; Mulero, V.; Cuesta, A.; Sepulcre, M.P. Spheroids and liquid spheres. 3D cell cultures to mimic the environment of cells in the organism. Eubacteria J. 2015, 34, 15–20. [Google Scholar]
- Llewellyn, S.V.; Niemeijer, M.; Nymark, P.; Moné, M.J.; Water, B.; Conway, G.E.; Jenkins, G.J.S.; Doak, S.H. In Vitro Three-Dimensional Liver Models for Nanomaterial DNA Damage Assessment. Small 2021, 17, 2006055. [Google Scholar] [CrossRef]
- Keeratichamroen, S.; Sornprachum, T.; Ngiwsara, L.; Ornnork, N.; Svasti, J. p-STAT3 influences doxorubicin and etoposide resistance of A549 cells grown in an in vitro 3D culture model. Oncol. Rep. 2023, 49, 71. [Google Scholar] [CrossRef]
- Nunes, A.S.; Costa, E.C.; Barros, A.S.; de Melo-Diogo, D.; Correia, I.J. Establishment of 2D Cell Cultures Derived From 3D MCF-7 Spheroids Displaying a Doxorubicin Resistant Profile. Biotechnol. J. 2018, 14, 1800268. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhu, X.; Zhang, K.; Zhu, L.; Zhou, F. Investigation of Gallic Acid Induced Anticancer Effect in Human Breast Carcinoma MCF-7 Cells. J. Biochem. Mol. Toxicol. 2014, 28, 387–393. [Google Scholar] [CrossRef]
- Yi, L.; Zongyuan, Y.; Cheng, G.; Lingyun, Z.; GuiLian, Y.; Wei, G. Quercetin enhances apoptotic effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in ovarian cancer cells through reactive oxygen species (ROS) mediated CCAAT enhancer-binding protein homologous protein (CHOP)-death receptor 5 pathway. Cancer Sci. 2014, 105, 520–527. [Google Scholar] [CrossRef]
- Psahoulia, F.H.; Drosopoulos, K.G.; Doubravska, L.; Andera, L.; Pintzas, A. Quercetin enhances TRAIL-mediated apoptosis in colon cancer cells by inducing the accumulation of death receptors in lipid rafts. Mol. Cancer Ther. 2007, 6, 2591–2599. [Google Scholar] [CrossRef] [PubMed]
- Kedhari Sundaram, M.; Raina, R.; Afroze, N.; Bajbouj, K.; Hamad, M.; Haque, S.; Hussain, A. Quercetin modulates signaling pathways and induces apoptosis in cervical cancer cells. Biosci. Rep. 2019, 39, BSR20190720. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Yang, L.; Zhang, W.; Shen, Y. Pyoluteorin induces cell cycle arrest and apoptosis in human triple-negative breast cancer cells MDA-MB-231. J. Pharm. Pharmacol. 2020, 72, 969–978. [Google Scholar] [CrossRef]
- Troncoso, D.; Madariaga Perpiñan, I.; Aldana Mancera, S.A.; Herreño Pachón, A.M.; Chaparro Ramírez, V.P.; Molina Camargo, M.L.; Rey Vargas, L.; Ramírez Rodríguez, A.C.; Montoya Estupiñan, C.F.; Valderrama Álvarez, A.; et al. Epithelial-mesenchymal transition: From molecular to physiological. Univ. Méd. 2017, 58, 1–10. [Google Scholar] [CrossRef][Green Version]
- Guerra-González, A.; Silva, E.; Montero, S.; Rodríguez, D.J.; Mansilla, R.; Nieto Villar, J.M. Metastasis: A milestone for knowledge, a challenge for science. Rev. Cuba. De Med. 2020, 59, 1–20. [Google Scholar]
- Koual, M.; Tomkiewicz, C.; Guerrera, I.C.; Sherr, D.; Barouki, R.; Coumoul, X. Aggressiveness and Metastatic Potential of Breast Cancer Cells Co-Cultured with Preadipocytes and Exposed to an Environmental Pollutant Dioxin: An in Vitro and in Vivo Zebrafish Study. Environ. Health Perspect. 2021, 129, 037002. [Google Scholar] [CrossRef] [PubMed]
- Román Curto, C. The metastatic process I: Local invasion of the extracellular matrix. Actas Dermo-Sifiliográficas 1999, 90, 143–155. [Google Scholar]
- Cao, Z.-Q.; Wang, Z.; Leng, P. Aberrant N-cadherin expression in cancer. Biomed. Pharmacother. 2019, 118, 109320. [Google Scholar] [CrossRef]
- Mantilla, C.; Suárez Mellado, I.; Duque Jaramillo, A.; Navas, M.C. Signaling mechanisms of β-catenin and its role in carcinogenesis. CES Med. 2015, 29, 109–127. [Google Scholar]
- Gil-Ugarteburu, R.; Rivas del Fresno, M.; González Rodríguez, I.; González Arriaga, P.; López Cima, F.; Fernandez Samoano, A.; Fernández García, I.; Benito García, P.; Muruamendiaraz Fernández, V.; Tardón, A. Matrix metalloprotease 9 (MMP-9) polymorphisms in the diagnosis of prostatic carcinoma: Preliminary experience. Arch. Españoles Urol. 2010, 63, 125–132. [Google Scholar] [CrossRef][Green Version]
- Xing, F.; Sun, C.; Luo, N.; He, Y.; Chen, M.; Ding, S.; Liu, C.; Feng, L.; Cheng, Z. Wogonin Increases Cisplatin Sensitivity in Ovarian Cancer Cells Through Inhibition of the Phosphatidylinositol 3-Kinase (PI3K)/Akt Pathway. Med. Sci. Monit. 2019, 25, 6007–6014. [Google Scholar] [CrossRef]
- Prasad, R.; Prasad, S. Histoprotective effect of rutin against cisplatin-induced toxicities in tumor-bearing mice: Rutin lessens cisplatin-induced toxicities. Hum. Exp. Toxicol. 2021, 40, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Wang, F.; Hu, J.; Duan, X.; Bai, W.; Wang, X.; Wang, B.; Su, Y.; Hu, J. Inhibitory interaction of flavonoids with organic cation transporter 2 and their structure-activity relationships for predicting nephroprotective effects. J. Appl. Toxicol. 2023, 43, 1421–1435. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, L.; Wu, P.; Li, W.; Li, T.; Gu, R.; Dan, X.; Li, Z.; Fan, X.; Xiao, Z. Gallic acid has anticancer activity and enhances the anticancer effects of cisplatin in non-small cell lung cancer A549 cells via the JAK/STAT3 signaling pathway. Oncol. Reports. 2019, 41, 1779–1788. [Google Scholar] [CrossRef]
- Asnaashari, S.; Amjad, E.; Sokouti, B. Synergistic effects of flavonoids and paclitaxel in cancer treatment: A systematic review. Cancer Cell Int. 2023, 23, 211. [Google Scholar] [CrossRef]
- Michel, O.; Przystupski, D.; Saczko, J.; Szewczyk, A.; Niedzielska, N.; Rossowska, J.; Kulbacka, J. The favourable effect of catechin in electrochemotherapy in human pancreatic cancer cells. Acta Biochim. Pol. 2018, 65, 173–184. [Google Scholar] [CrossRef]
- Luo, T.; Wang, J.; Yin, Y.; Hua, H.; Jing, J.; Sun, X.; Li, M.; Zhang, Y.; Jiang, Y. (-)-Epigallocatechin gallate sensitizes breast cancer cells to paclitaxel in a murine model of breast carcinoma. Breast Cancer Res. 2010, 12, R8. [Google Scholar] [CrossRef]
- Dhanasekaran, D.N.; Reddy, E.P. JNK signaling in apoptosis. Oncogene 2008, 27, 6245–6251. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.-F.; Bai, L.; Guo, S.; Hu, J.-B.; Zhang, S.-S.; Liu, S.-J.; Zhang, Y.; Li, S.; Ho, C.-T.; Bai, N.-S. Nontargeted metabolomic analysis of four different parts of Actinidia arguta by UPLC-Q-TOF-MSE. Food Res. Int. 2023, 163, 112228. [Google Scholar] [CrossRef]
- Liu, P.-P.; Shan, G.-S.; Zhang, F.; Chen, J.-N.; Jia, T.-Z. Metabolomics analysis and rapid identification of changes in chemical ingredients in crude and processed Astragali Radix by UPLC-QTOF-MS combined with novel informatics UNIFI platform. Chin. J. Nat. Med. 2018, 16, 714–720. [Google Scholar] [CrossRef]
- Vannabhum, M.; Ziangchin, N.; Thepnorarat, P.; Akarasereenont, P. Metabolomic analysis of Thai Herbal Analgesic Formula based on ultra-high-performance liquid chromatography-quadrupole time-of-flight mass spectrometry. Heliyon 2023, 9, e18296. [Google Scholar] [CrossRef] [PubMed]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Doello, K.; Mesas, C.; Quiñonero, F.; Perazzoli, G.; Cabeza, L.; Prados, J.; Melguizo, C.; Ortiz, R. The Antitumor Activity of Sodium Selenite Alone and in Combination with Gemcitabine in Pancreatic Cancer: An In Vitro and In Vivo Study. Cancers 2021, 13, 3169. [Google Scholar] [CrossRef]
- Gomes, D.; Telles, C.; Costa, M.; Almeida-Lima, J.; Costa, L.; Keesen, T.; Rocha, H. Methanolic Extracts from Brown Seaweeds Dictyota cilliolata and Dictyota menstrualis Induce Apoptosis in Human Cervical Adenocarcinoma HeLa Cells. Molecules 2015, 20, 6573–6591. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rodríguez, O.P.; González-Torres, A.; Álvarez-Salas, L.M.; Hernández-Sánchez, H.; García-Pérez, B.E.; Thompson-Bonilla, M.D.R.; Jaramillo-Flores, M.E. Effect of naringenin and its combination with cisplatin in cell death, proliferation and invasion of cervical cancer spheroids. RSC Adv. 2021, 11, 129–141. [Google Scholar] [CrossRef]



| Identification | Tr | Formula | Measured m/z | Mass Error (ppm) | Fragmentation |
|---|---|---|---|---|---|
| Gallic acid | 2.38 | C7H6O5 | 169.0000 | −6.6 | 125.02269, 109.0276, 125.0230, 137.023 |
| Epigallocatechin | 3.65 | C15H14O7 | 305.0667 | 1.6 | 139.0389, 165.0183, 167.033 |
| 3,4-Dihydroxybenzoic acid | 3.77 | C7H6O4 | 153.0230 | −4.0 | 109.0276, 108.0198 |
| Gallic acid 4-O-(6-galloylglucoside) | 4.00 | C20H20O14 | 483.0800 | 5.8 | 125.023, 169.0136, 211.0248, 313.0562 |
| Catechin | 4.06 | C15H14O6 | 289.0716 | 1.3 | 123.0437, 125.0231, 203.0710, 245.0823 |
| Epicatechin | 4.17 | C15H14O6 | 289.0721 | 1.4 | 109.0276, 123.0437, 161.0235 |
| Procyanidin B2 | 4.31 | C30H26O12 | 577.1357 | 1.0 | 161.0231, 245.0834, 289.0713, 407.0771 |
| Epigallocatechin gallate | 4.92 | C22H18O11 | 457.7900 | 3.5 | 125.023, 169.0136, 287.0567, 305.0671 |
| Gallocatechin 3-gallate | 5.02 | C22H18O11 | 457.0798 | 4.9 | 125.0231, 169.0136, 271.60, 289.0722 |
| Quercetin-3-O-arabinoglucoside | 5.12 | C26H28O16 | 595.1308 | 1.4 | 300.01261, 271.0249, 255.0291 |
| Quercetin-3-O-glucoside | 5.38 | C21H19O12 | 463.0876 | 0.4 | 300.0273, 285.0391 |
| Ellagic acid | 5.41 | C14H6O8 | 300.9999 | 3.4 | 145.0283, 283.995 |
| Epicatechin-3-O-gallate | 5.45 | C22H18O10 | 441.0912 | 2.7 | 125.023, 169.0136, 289.0722 |
| Quercitrin | 5.74 | C21H20O11 | 447.0935 | 2.4 | 271.0274, 300.0815, 301.0350 |
| Phloridzin | 5.96 | C21H24O10 | 435.1302 | 1.3 | 125.0228, 167.0340, 273.0767 |
| Treatment/ Phases | MCF-7 | MDA-MB-231 | ||||
|---|---|---|---|---|---|---|
| G1 | S | G2 | G1 | S | G2 | |
| Control | 51.7 ± 3.04 | 3.7 ± 0.21 | 44.6 ± 1.30 | 56.7 ± 0.28 | 1.51 ± 0.12 | 41.75 ± 0.07 |
| Starvation | 64 ± 4.38 | 0.15 ± 0.11 | 35.85 ± 3.17 | 58 ± 0.49 | 5.65 ± 0.15 | 36.35 ± 3.87 |
| Cisplatin 13/13.65 μg/mL | 19.85 ± 3.46 | 79.4 ± 3.53 | 0.75 ± 0.07 | 16.2 ± 0.14 | 82.8 ± 0.14 | 1 ± 0 |
| EAB 500 μg/mL | 84.05 ± 4.03 | 8.33 ± 0.73 | 7.35 ± 0.73 | 76.2 ± 2.26 | 8.3 ± 0.08 | 15.3 ± 0.06 |
| EAB 1000 μg/mL | 82.15 ± 7.61 | 0 ± 0 | 17.85 ± 0.56 | 82.85 ± 0.63 | 15.8 ± 1.13 | 1.36 ± 0.01 |
| Energy (Kcal/mol) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Protein | Gallic Acid | Epigallocatechin | 3,4-Dihydroxybenzoic Acid | Catechin | Epicatechin | Epigallocatechin Gallate | Gallocatechin 3-Gallate | Epicatechin-3-O-Gallate |
| ALDH1A3 | −14.03 | −6.96 | −13.77 | −6.76 | −8.01 | −0.27 | −1.77 | −1.85 |
| IGF-R1 | −13.79 | - | −14.2 | −2.37 | −3.44 | - | - | - |
| CDK1 | −14.84 | −3.00 | −12.02 | −3.52 | −5.35 | - | - | −0.35 |
| CDK2 | −10.43 | - | −11.59 | −3.21 | −0.96 | - | - | - |
| CDK4 | −11.98 | −0.31 | −11.21 | −7.51 | −1.51 | - | - | - |
| P53 | −15.29 | −0.93 | −14.98 | −6.79 | −3.16 | - | - | −1.2 |
| TNFR1 | −9.44 | - | −9.45 | - | - | - | - | - |
| MLKL | −9.51 | - | −10.82 | - | - | - | - | - |
| MMP2 | −17.47 | −3.41 | −18.72 | −3.34 | −3.73 | - | - | −3.57 |
| MMP9 | −18.26 | −3.53 | −16.25 | −4.67 | −5.95 | - | - | - |
| E-cadherin | −10.98 | - | −9.73 | −0.45 | −1.08 | - | - | - |
| N-cadherin | −15.56 | −1.94 | −15.92 | −2.29 | −5.38 | −0.25 | −2.07 | - |
| Extract or Cisplatin/Combination | MCF-7 | MDA-MB-231 | Extract or Paclitaxel/ Combination | MCF-7 | MDA-MB-231 |
|---|---|---|---|---|---|
| Cell Viability (%) | Cell Viability (%) | ||||
| Control | 100 | 100 | Control | 100 | 100 |
| ½ IC50 EAB | 72 | 73 | ½ IC50 EAB | 72 | 73 |
| IC50 EAB | 50 | 49 | IC50 EAB | 50 | 49 |
| ½ IC50 Cisplatin | 72 | 74 | ½ IC50 Paclitaxel | 73 | 75 |
| IC50 Cisplatin | 49 | 49 | IC50 Paclitaxel | 52 | 50 |
| ½ IC50 EAB + ½ IC50 Cisplatin | 68 | 67 | ½ IC50 EAB +½ IC50 Paclitaxel | 43 | 41 |
| ½ IC50 EAB + IC50 Cisplatin | 35 | 29 | ½ IC50 EAB + IC50 Paclitaxel | 33 | 35 |
| IC50 EAB + ½ IC50 Cisplatin | 43 | 41 | IC50 EAB + ½ IC50 Paclitaxel | 41 | 38 |
| IC50 EAB + IC50 Cisplatin | 25 | 31 | IC50 EAB + IC50 Paclitaxel | 14 | 25 |
| Extract or Cisplatin/Combination | MCF-7 | MDA-MB231 | Extract or Paclitaxel/ Combination | MCF-7 | MDA-MB231 |
|---|---|---|---|---|---|
| Cell Viability (%) | Cell Viability (%) | ||||
| Control | 100 | 100 | Control | 100 | 100 |
| ½ IC50 EAB | 73 | 72 | ½ IC50 EAB | 72 | 74 |
| IC50 EAB | 50 | 49 | IC50 EAB | 51 | 48 |
| ½ IC50 Cisplatin | 76 | 75 | ½ IC50 Paclitaxel | 74 | 74 |
| IC50 Cisplatin | 52 | 48 | IC50 Paclitaxel | 50 | 50 |
| ½ IC50 EAB + ½ IC50 Cisplatin | 66 | 63 | ½ IC50 EAB + ½ IC50 Paclitaxel | 65 | 60 |
| ½ IC50 EAB + IC50 Cisplatin | 35 | 45 | ½ IC50 EAB + IC50 Paclitaxel | 34 | 38 |
| IC50 EAB + ½ IC50 Cisplatin | 31 | 42 | IC50 EAB + ½ IC50 Paclitaxel | 31 | 43 |
| IC50 EAB + IC50 Cisplatin | 25 | 28 | IC50 EAB + IC50 Paclitaxel | 24 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Méndez, D.d.C.; Sánchez-Mundo, M.d.l.L.; Ortiz-León, L.A.; Álvarez-Salas, L.M.; Rosales-García, V.H.; Rodríguez-Campos, J.; Jaramillo-Flores, M.E. Ballota hirsuta Benth Arrests the Cell Cycle, Induces Apoptosis and Inhibits the Invasion of MCF-7 and MDA-MB-231 Cell Lines in 2D and 3D Models. Int. J. Mol. Sci. 2025, 26, 5672. https://doi.org/10.3390/ijms26125672
Martínez-Méndez DdC, Sánchez-Mundo MdlL, Ortiz-León LA, Álvarez-Salas LM, Rosales-García VH, Rodríguez-Campos J, Jaramillo-Flores ME. Ballota hirsuta Benth Arrests the Cell Cycle, Induces Apoptosis and Inhibits the Invasion of MCF-7 and MDA-MB-231 Cell Lines in 2D and 3D Models. International Journal of Molecular Sciences. 2025; 26(12):5672. https://doi.org/10.3390/ijms26125672
Chicago/Turabian StyleMartínez-Méndez, Diana del Carmen, María de la Luz Sánchez-Mundo, Laura Adriana Ortiz-León, Luis Marat Álvarez-Salas, Víctor Hugo Rosales-García, Jacobo Rodríguez-Campos, and María Eugenia Jaramillo-Flores. 2025. "Ballota hirsuta Benth Arrests the Cell Cycle, Induces Apoptosis and Inhibits the Invasion of MCF-7 and MDA-MB-231 Cell Lines in 2D and 3D Models" International Journal of Molecular Sciences 26, no. 12: 5672. https://doi.org/10.3390/ijms26125672
APA StyleMartínez-Méndez, D. d. C., Sánchez-Mundo, M. d. l. L., Ortiz-León, L. A., Álvarez-Salas, L. M., Rosales-García, V. H., Rodríguez-Campos, J., & Jaramillo-Flores, M. E. (2025). Ballota hirsuta Benth Arrests the Cell Cycle, Induces Apoptosis and Inhibits the Invasion of MCF-7 and MDA-MB-231 Cell Lines in 2D and 3D Models. International Journal of Molecular Sciences, 26(12), 5672. https://doi.org/10.3390/ijms26125672








