Shear Stress Regulates Osteogenic Differentiation of Human Dental Pulp Stem Cells via the p38 Pathway
Abstract
1. Introduction
2. Results
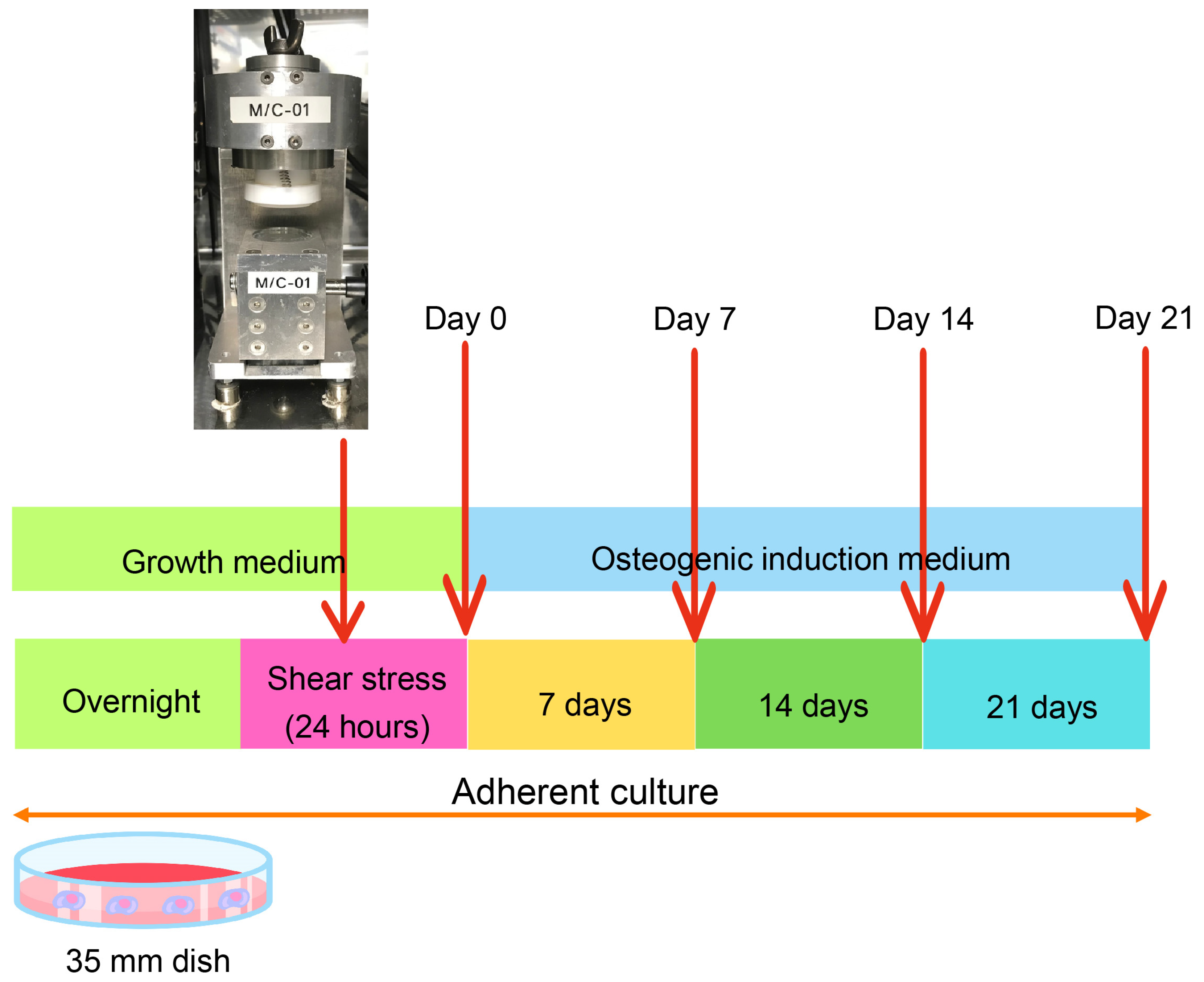
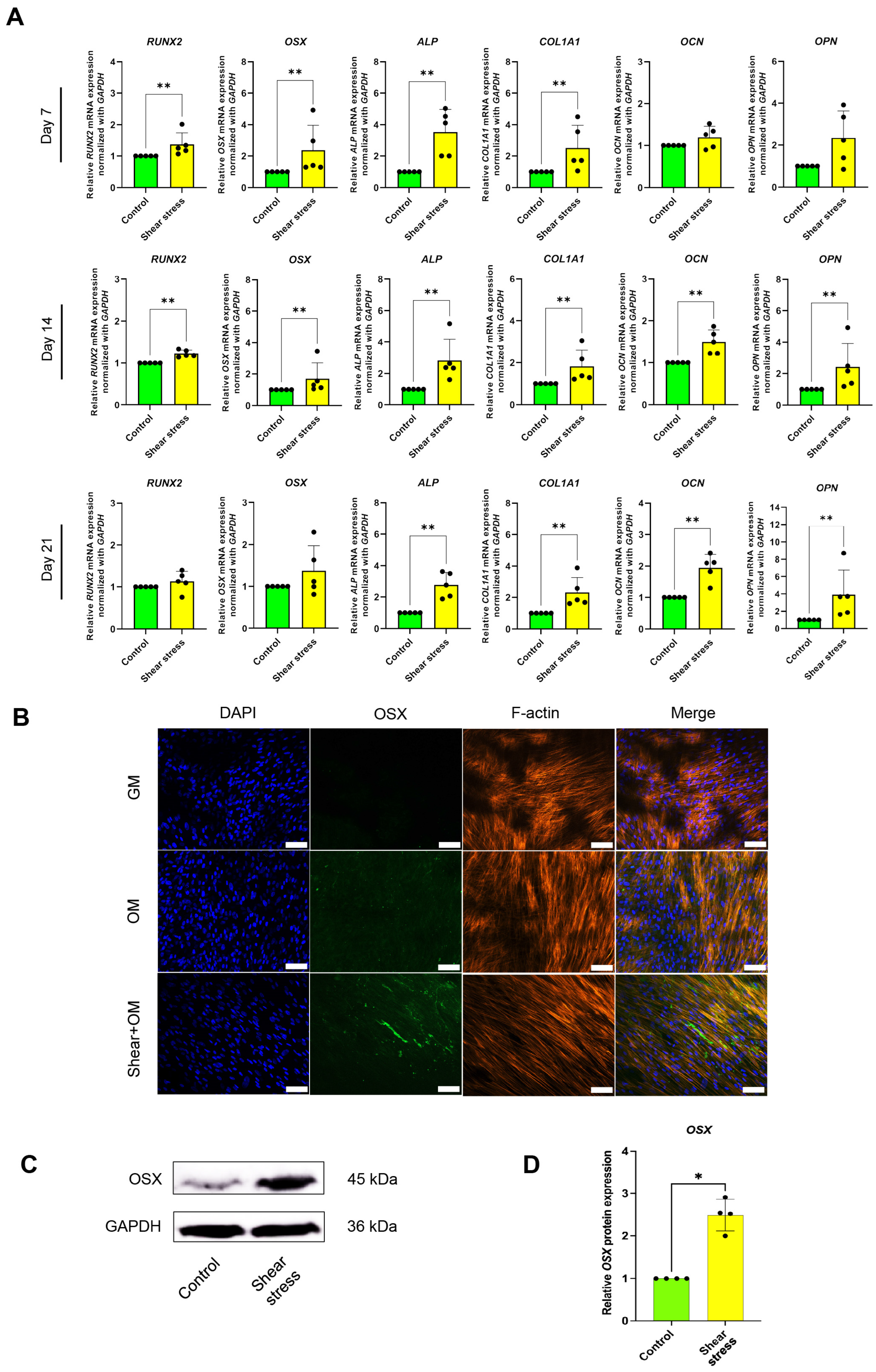
2.1. Shear Stress Induced Osteogenic Differentiation of hDPSCs
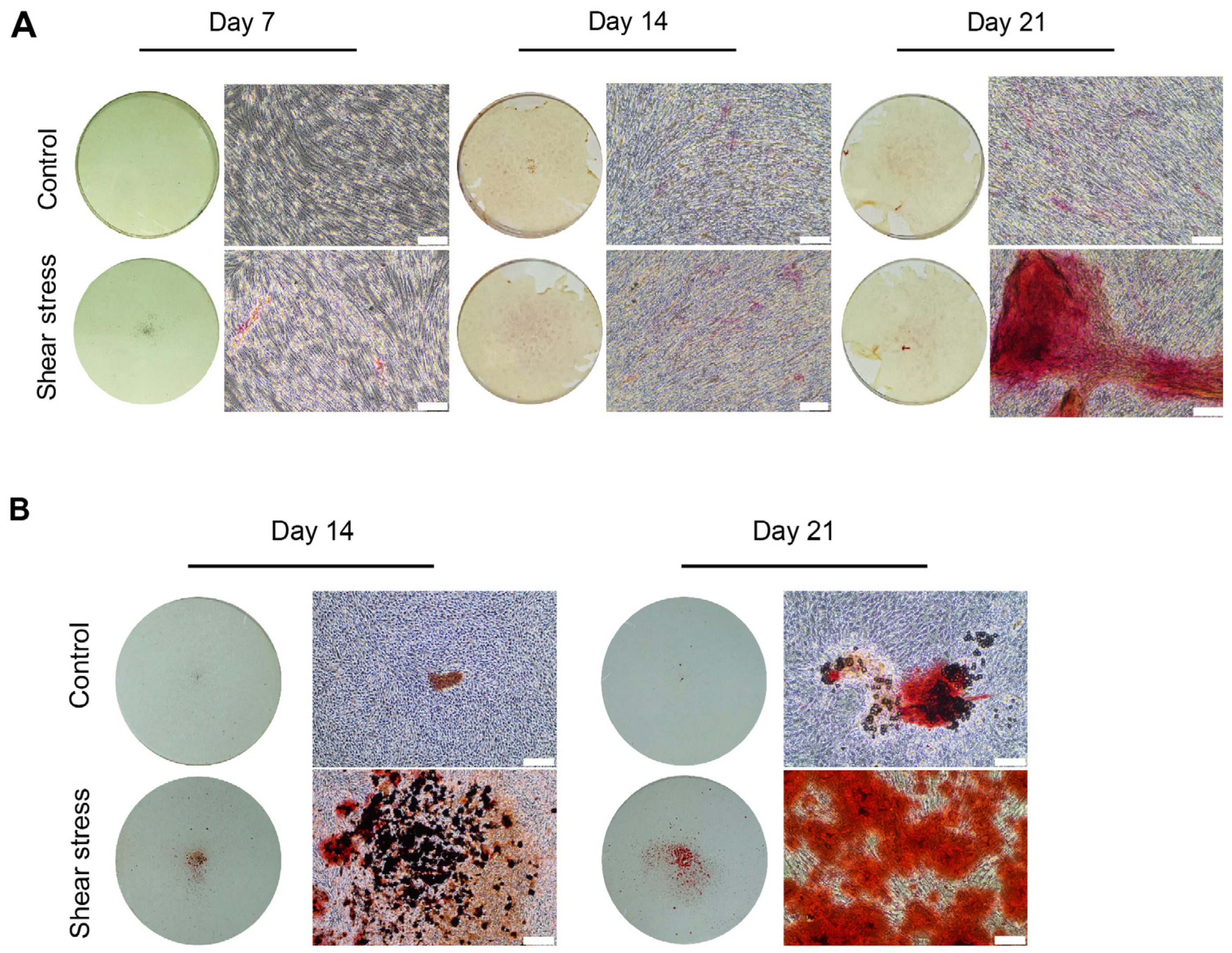
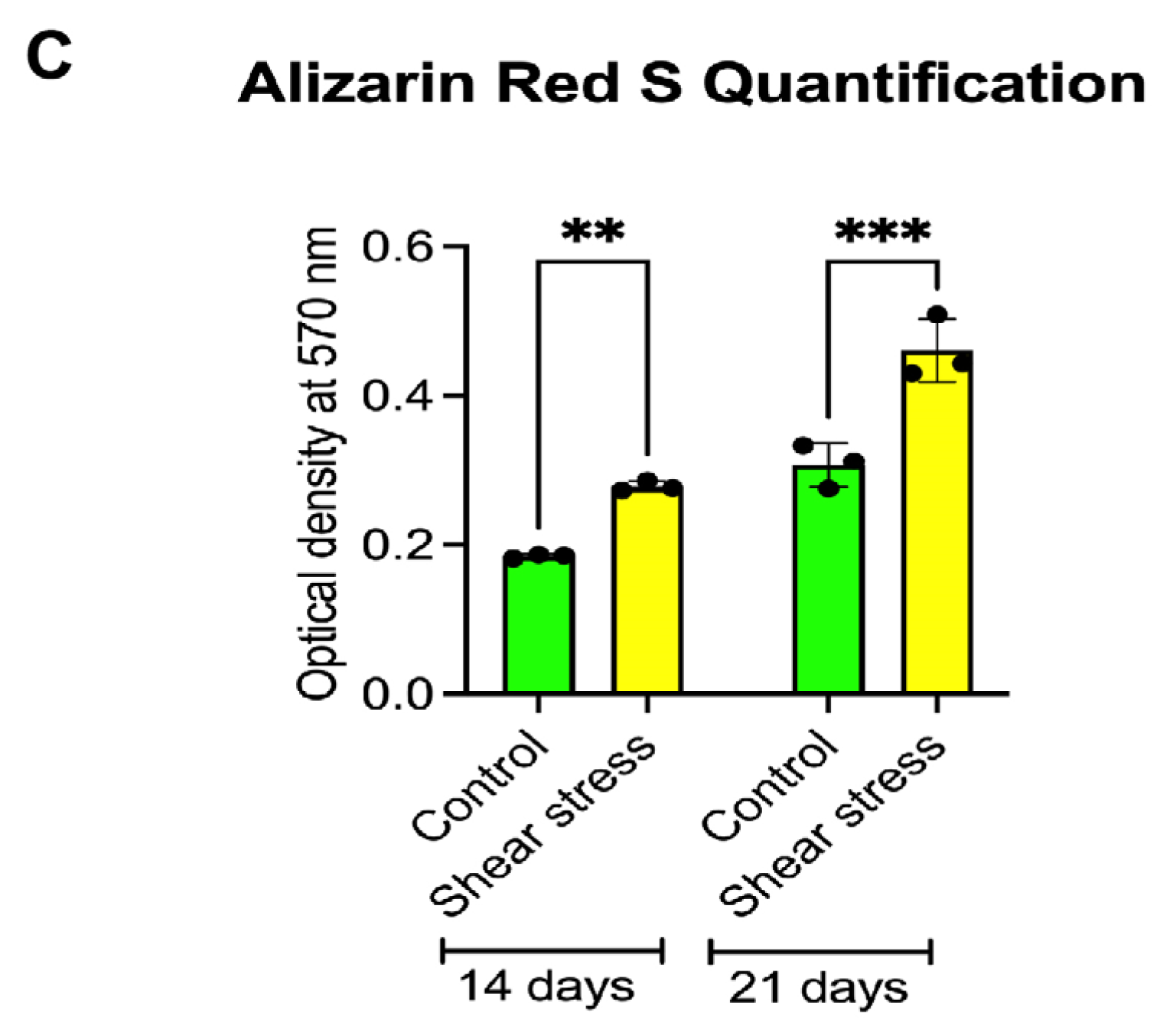
2.2. Shear Stress Promoted Mineralization of Osteogenically Induced hDPSCs
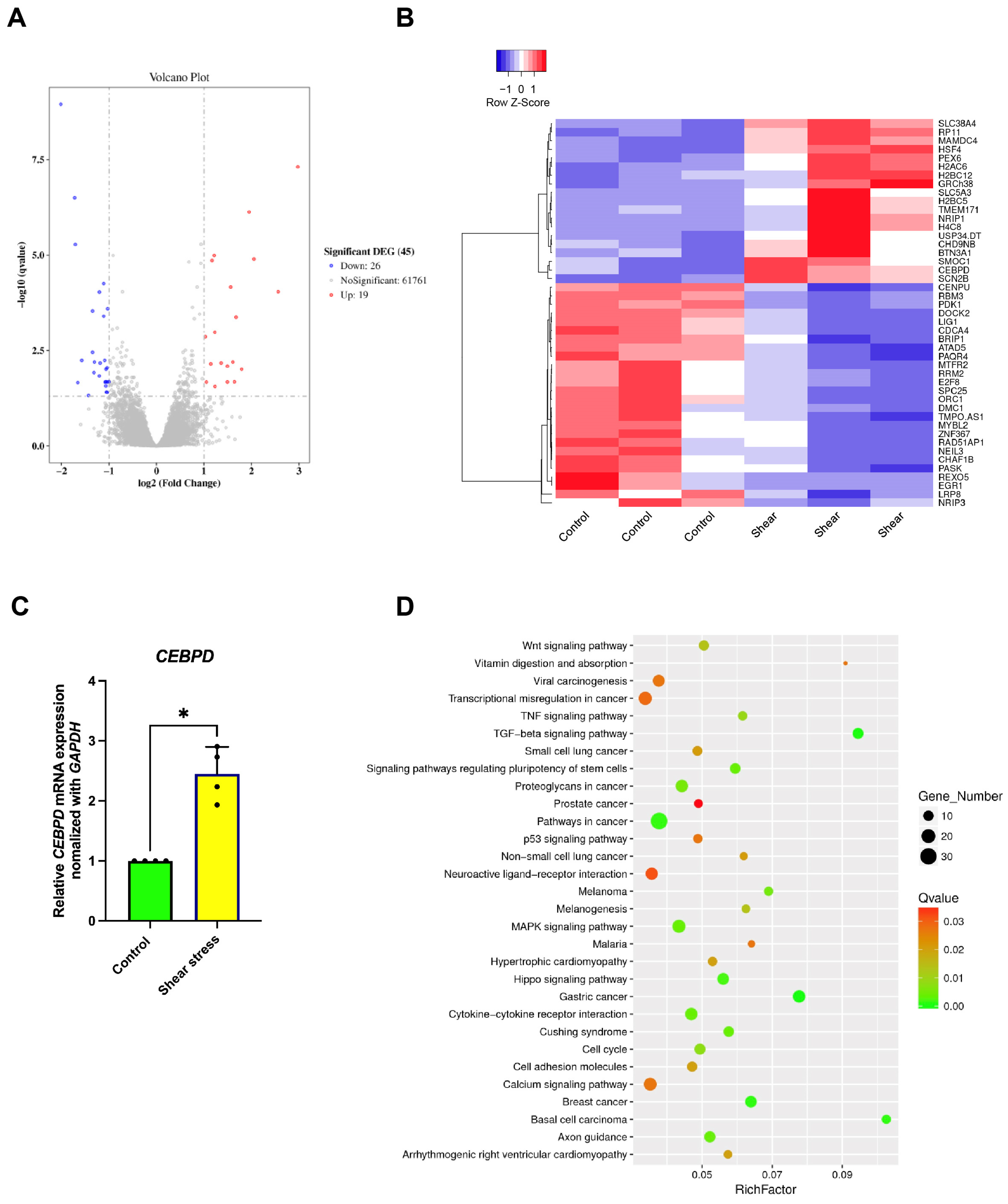
2.3. Identification of the Signaling Pathways Involved in Osteogenic Differentiation of hDPSCs After Shear-Stress Application
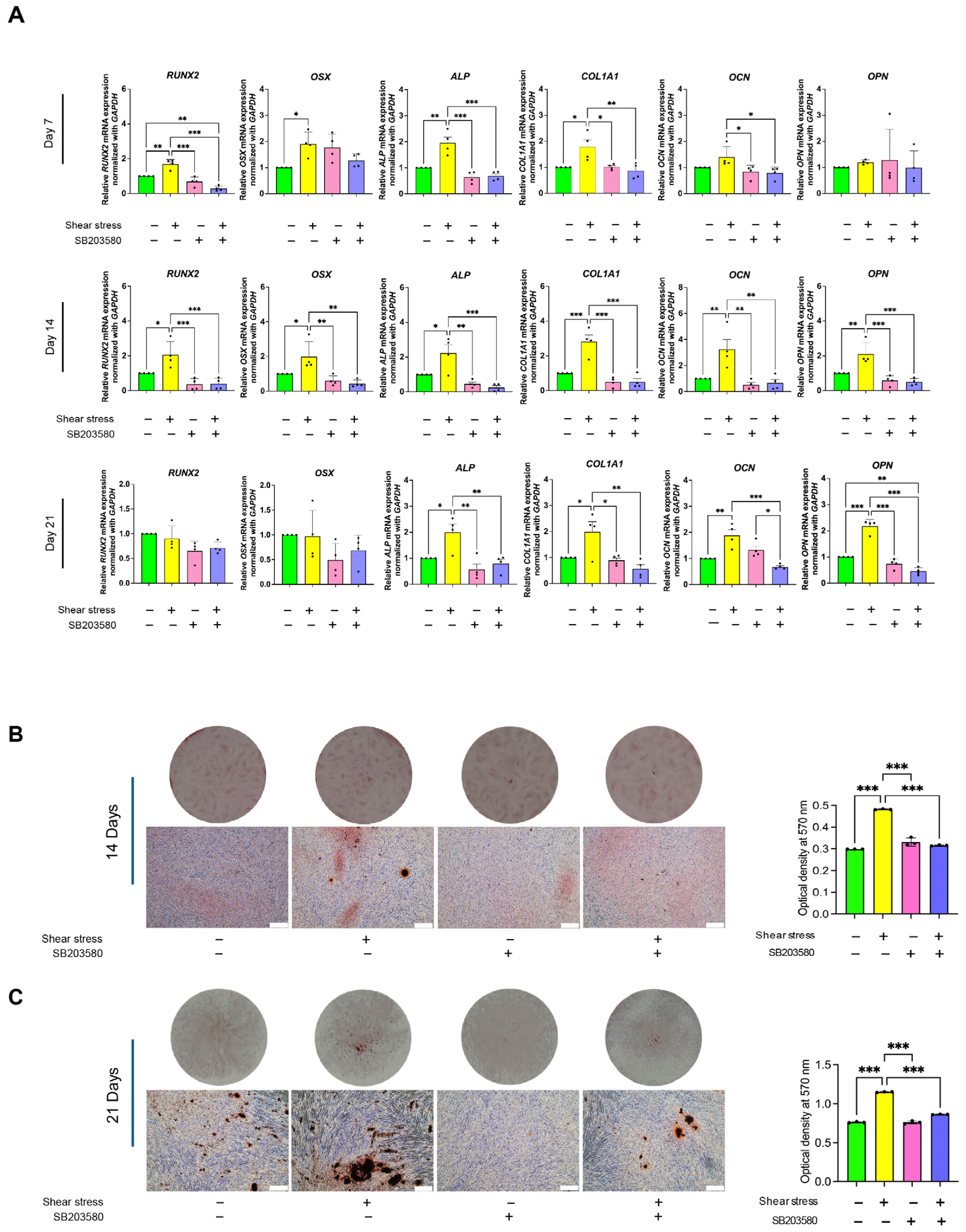
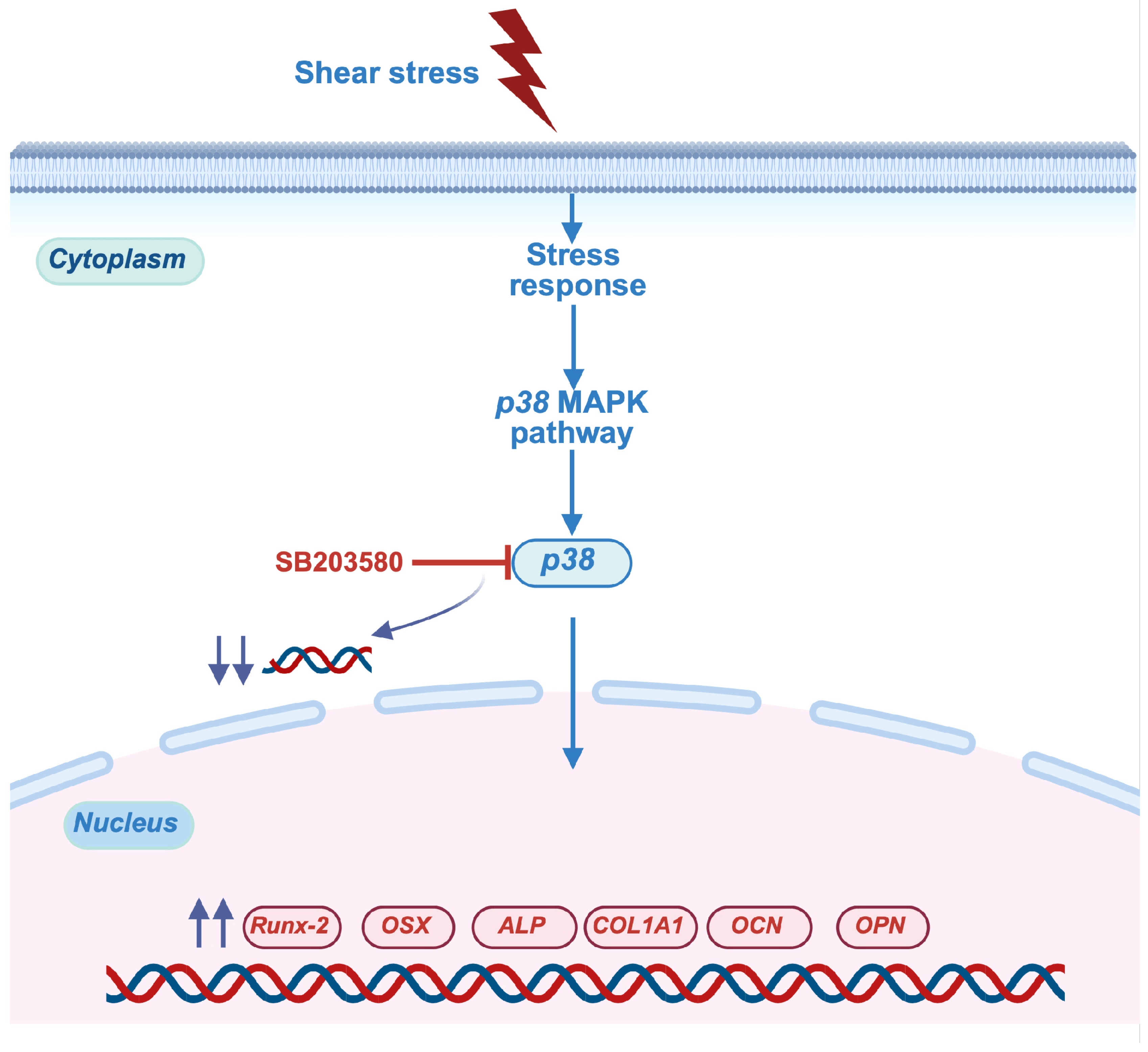
2.4. Shear Stress Stimulated Osteogenic Differentiation of hDPSCs Through p38 Mitogen-Activated Protein Kinase (MAPK) Pathway
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Shear-Stress Application
4.3. Real-Time Polymerase Chain Reaction (PCR) Analysis
4.4. Alkaline Phosphatase (ALP) Staining
4.5. Alizarin Red S (ARS) Staining and Quantification
4.6. Immunofluorescence Staining
4.7. Western Blotting Analysis
4.8. RNA Sequencing
4.9. Inhibition of p38 MAPK Pathway
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quirynen, M.; Lahoud, P.; Teughels, W.; Cortellini, S.; Dhondt, R.; Jacobs, R.; Temmerman, A. Individual “alveolar phenotype” limits dimensions of lateral bone augmentation. J. Clin. Periodontol. 2023, 50, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Mabuchi, Y.; Okawara, C.; Mendez-Ferrer, S.; Akazawa, C. Cellular Heterogeneity of Mesenchymal Stem/Stromal Cells in the Bone Marrow. Front. Cell Dev. Biol. 2021, 9, 689366. [Google Scholar] [CrossRef]
- Geng, Y.; Yang, J.; Li, S.; Chen, M. Chyloid Fat Carried Adipose-Derived Mesenchymal Stem Cells Accelerate Wound Healing Via Promoting Angiogenesis. Ann. Plast. Surg. 2021, 87, 472–477. [Google Scholar] [CrossRef]
- Zha, K.; Li, X.; Yang, Z.; Tian, G.; Sun, Z.; Sui, X.; Dai, Y.; Liu, S.; Guo, Q. Heterogeneity of mesenchymal stem cells in cartilage regeneration: From characterization to application. NPJ Regen. Med. 2021, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.R.; Park, G.Y.; Lee, S.C. Regenerative effects of mesenchymal stem cells by dosage in a chronic rotator cuff tendon tear in a rabbit model. Regen. Med. 2019, 14, 1001–1012. [Google Scholar] [CrossRef]
- Shin, H.A.; Park, M.; Banga, J.P.; Lew, H. TGFbeta-Treated Placenta-Derived Mesenchymal Stem Cells Selectively Promote Anti-Adipogenesis in Thyroid-Associated Ophthalmopathy. Int. J. Mol. Sci. 2022, 23, 5603. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K.; Han, X.; Kato, H.; Sato, H.; Zhang, Y.; Sun, X.; Hirofuji, Y.; Yamaza, H.; Yamada, A.; Fukumoto, S. Dental Pulp-Derived Mesenchymal Stem Cells for Modeling Genetic Disorders. Int. J. Mol. Sci. 2021, 22, 2269. [Google Scholar] [CrossRef]
- Potdar, P.D.; Jethmalani, Y.D. Human dental pulp stem cells: Applications in future regenerative medicine. World J. Stem Cells 2015, 7, 839–851. [Google Scholar] [CrossRef]
- Kawashima, N. Characterisation of dental pulp stem cells: A new horizon for tissue regeneration? Arch. Oral Biol. 2012, 57, 1439–1458. [Google Scholar] [CrossRef]
- Lee, W.; Eo, S.-R.; Choi, J.-H.; Kim, Y.-M.; Nam, M.-H.; Seo, Y.-K. The Osteogenic Differentiation of Human Dental Pulp Stem Cells through G0/G1 Arrest and the p-ERK/Runx-2 Pathway by Sonic Vibration. Int. J. Mol. Sci. 2021, 22, 10167. [Google Scholar] [CrossRef]
- Manaspon, C.; Jongwannasiri, C.; Chumprasert, S.; Sa-Ard-Iam, N.; Mahanonda, R.; Pavasant, P.; Porntaveetus, T.; Osathanon, T. Human dental pulp stem cell responses to different dental pulp capping materials. BMC Oral Health 2021, 21, 209. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yuan, B.; Yin, R.; Zhang, H. Inflammation Responses to Bone Scaffolds under Mechanical Stimuli in Bone Regeneration. J. Funct. Biomater. 2023, 14, 169. [Google Scholar] [CrossRef]
- Steward, A.J.; Cole, J.H.; Ligler, F.S.; Loboa, E.G. Mechanical and Vascular Cues Synergistically Enhance Osteogenesis in Human Mesenchymal Stem Cells. Tissue Eng. Part A 2016, 22, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Kraft, D.C.; Bindslev, D.A.; Melsen, B.; Klein-Nulend, J. Human dental pulp cells exhibit bone cell-like responsiveness to fluid shear stress. Cytotherapy 2011, 13, 214–226. [Google Scholar] [CrossRef]
- Gao, Q.; Walmsley, A.D.; Cooper, P.R.; Scheven, B.A. Ultrasound Stimulation of Different Dental Stem Cell Populations: Role of Mitogen-activated Protein Kinase Signaling. J. Endod. 2016, 42, 425–431. [Google Scholar] [CrossRef]
- Han, M.-J.; Seo, Y.-K.; Yoon, H.-H.; Song, K.-Y.; Park, J.-K. Effect of mechanical tension on the human dental pulp cells. Biotechnol. Bioprocess Eng. 2008, 13, 410–417. [Google Scholar] [CrossRef]
- Kraft, D.C.; Bindslev, D.A.; Melsen, B.; Abdallah, B.M.; Kassem, M.; Klein-Nulend, J. Mechanosensitivity of dental pulp stem cells is related to their osteogenic maturity. Eur. J. Oral Sci. 2010, 118, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Kolind, K.; Kraft, D.; Bøggild, T.; Duch, M.; Lovmand, J.; Pedersen, F.S.; Bindslev, D.A.; Bünger, C.; Foss, M.; Besenbacher, F. Control of proliferation and osteogenic differentiation of human dental-pulp-derived stem cells by distinct surface structures. Acta Biomater. 2014, 10, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, F.S.; Jazayeri, M.; Ghahari, P.; Haghighipour, N. Effects of equiaxial strain on the differentiation of dental pulp stem cells without using biochemical reagents. Mol. Cell. Biomech. 2014, 11, 209–220. [Google Scholar]
- Kreke, M.R.; Huckle, W.R.; Goldstein, A.S. Fluid flow stimulates expression of osteopontin and bone sialoprotein by bone marrow stromal cells in a temporally dependent manner. Bone 2005, 36, 1047–1055. [Google Scholar] [CrossRef]
- Yu, B.; Chang, J.; Liu, Y.; Li, J.; Kevork, K.; Al-Hezaimi, K.; Graves, D.T.; Park, N.H.; Wang, C.Y. Wnt4 signaling prevents skeletal aging and inflammation by inhibiting nuclear factor-kappaB. Nat. Med. 2014, 20, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Delaine-Smith, R.M.; MacNeil, S.; Reilly, G.C. Matrix production and collagen structure are enhanced in two types of osteogenic progenitor cells by a simple fluid shear stress stimulus. Eur. Cells Mater. 2012, 24, 162–174. [Google Scholar] [CrossRef]
- Rey, A.; Manen, D.; Rizzoli, R.; Ferrari, S.L.; Caverzasio, J. Evidences for a role of p38 MAP kinase in the stimulation of alkaline phosphatase and matrix mineralization induced by parathyroid hormone in osteoblastic cells. Bone 2007, 41, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T.; Gronthos, S.; Shi, S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef]
- Kornsuthisopon, C.; Nowwarote, N.; Chansaenroj, A.; Photichailert, S.; Rochanavibhata, S.; Klincumhom, N.; Petit, S.; Dingli, F.; Loew, D.; Fournier, B.P.J.; et al. Human dental pulp stem cells derived extracellular matrix promotes mineralization via Hippo and Wnt pathways. Sci. Rep. 2024, 14, 6777. [Google Scholar] [CrossRef]
- Phothichailert, S.; Nowwarote, N.; Fournier, B.P.J.; Trachoo, V.; Roytrakul, S.; Namangkalakul, W.; Osathanon, T. Effects of decellularized extracellular matrix derived from Jagged1-treated human dental pulp stem cells on biological responses of stem cells isolated from apical papilla. Front. Cell Dev. Biol. 2022, 10, 948812. [Google Scholar] [CrossRef]
- Sun, Y.; Wan, B.; Wang, R.; Zhang, B.; Luo, P.; Wang, D.; Nie, J.J.; Chen, D.; Wu, X. Mechanical Stimulation on Mesenchymal Stem Cells and Surrounding Microenvironments in Bone Regeneration: Regulations and Applications. Front. Cell Dev. Biol. 2022, 10, 808303. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.P.; Leleux, J.; Nerem, R.M.; Ahsan, T. Effects of shear stress on germ lineage specification of embryonic stem cells. Integr. Biol. 2012, 4, 1263–1273. [Google Scholar] [CrossRef]
- Wolfe, R.P.; Ahsan, T. Shear stress during early embryonic stem cell differentiation promotes hematopoietic and endothelial phenotypes. Biotechnol. Bioeng. 2013, 110, 1231–1242. [Google Scholar] [CrossRef]
- Ahsan, T.; Nerem, R.M. Fluid shear stress promotes an endothelial-like phenotype during the early differentiation of embryonic stem cells. Tissue Eng. Part A 2010, 16, 3547–3553. [Google Scholar] [CrossRef]
- Limraksasin, P.; Nattasit, P.; Manokawinchoke, J.; Tiskratok, W.; Vinaikosol, N.; Okawa, H.; Limjeerajarus, C.N.; Limjeerajarus, N.; Pavasant, P.; Osathanon, T.; et al. Application of shear stress for enhanced osteogenic differentiation of mouse induced pluripotent stem cells. Sci. Rep. 2022, 12, 19021. [Google Scholar] [CrossRef] [PubMed]
- Suwittayarak, R.; Klincumhom, N.; Phrueksotsai, C.; Limjeerajarus, N.; Limjeerajarus, C.N.; Egusa, H.; Osathanon, T. Shear stress preconditioning enhances periodontal ligament stem cell survival. Arch. Oral Biol. 2025, 173, 106232. [Google Scholar] [CrossRef] [PubMed]
- Suwittayarak, R.; Klincumhom, N.; Ngaokrajang, U.; Namangkalakul, W.; Ferreira, J.N.; Pavasant, P.; Osathanon, T. Shear Stress Enhances the Paracrine-Mediated Immunoregulatory Function of Human Periodontal Ligament Stem Cells via the ERK Signalling Pathway. Int. J. Mol. Sci. 2022, 23, 7119. [Google Scholar] [CrossRef] [PubMed]
- Wittkowske, C.; Reilly, G.C.; Lacroix, D.; Perrault, C.M. In Vitro Bone Cell Models: Impact of Fluid Shear Stress on Bone Formation. Front. Bioeng. Biotechnol. 2016, 4, 87. [Google Scholar] [CrossRef]
- Verbruggen, S.W.; Vaughan, T.J.; McNamara, L.M. Fluid flow in the osteocyte mechanical environment: A fluid-structure interaction approach. Biomech. Model. Mechanobiol. 2014, 13, 85–97. [Google Scholar] [CrossRef]
- Grotheer, V.; Skrynecki, N.; Oezel, L.; Windolf, J.; Grassmann, J. Osteogenic differentiation of human mesenchymal stromal cells and fibroblasts differs depending on tissue origin and replicative senescence. Sci. Rep. 2021, 11, 11968. [Google Scholar] [CrossRef]
- Chan, W.C.W.; Tan, Z.; To, M.K.T.; Chan, D. Regulation and Role of Transcription Factors in Osteogenesis. Int. J. Mol. Sci. 2021, 22, 5445. [Google Scholar] [CrossRef]
- Limraksasin, P.; Okawa, H.; Zhang, M.; Kondo, T.; Osathanon, T.; Pavasant, P.; Egusa, H. Size-Optimized Microspace Culture Facilitates Differentiation of Mouse Induced Pluripotent Stem Cells into Osteoid-Rich Bone Constructs. Stem Cells Int. 2020, 2020, 7082679. [Google Scholar] [CrossRef]
- Manokawinchoke, J.; Limraksasin, P.; Okawa, H.; Pavasant, P.; Egusa, H.; Osathanon, T. Intermittent compressive force induces cell cycling and reduces apoptosis in embryoid bodies of mouse induced pluripotent stem cells. Int. J. Oral Sci. 2022, 14, 1. [Google Scholar] [CrossRef]
- Siddappa, R.; Licht, R.; van Blitterswijk, C.; de Boer, J. Donor variation and loss of multipotency during in vitro expansion of human mesenchymal stem cells for bone tissue engineering. J. Orthop. Res. 2007, 25, 1029–1041. [Google Scholar] [CrossRef]
- Freedman, A.H.; Gaspar, J.M.; Sackton, T.B. Short paired-end reads trump long single-end reads for expression analysis. BMC Bioinform. 2020, 21, 149. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Huang, M.F.; Xu, A.; Gao, X.; Huang, Y.W.; Phan, T.T.T.; Lu, L.; Chi, T.Y.; Dai, Y.; Pang, L.K.; et al. Systematic transcriptome profiling of hPSC-derived osteoblasts unveils CORIN’s mastery in governing osteogenesis through CEBPD modulation. J. Biol. Chem. 2024, 300, 107494. [Google Scholar] [CrossRef] [PubMed]
- Yodthong, T.; Kedjarune-Leggat, U.; Smythe, C.; Wititsuwannakul, R.; Pitakpornpreecha, T. l-Quebrachitol Promotes the Proliferation, Differentiation, and Mineralization of MC3T3-E1 Cells: Involvement of the BMP-2/Runx2/MAPK/Wnt/beta-Catenin Signaling Pathway. Molecules 2018, 23, 3086. [Google Scholar] [CrossRef]
- Wang, K.; Kong, X.; Du, M.; Yu, W.; Wang, Z.; Xu, B.; Yang, J.; Xu, J.; Liu, Z.; Cheng, Y.; et al. Novel Soy Peptide CBP: Stimulation of Osteoblast Differentiation via TβRI-p38-MAPK-Depending RUNX2 Activation. Nutrients 2022, 14, 1940. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Cen, L.; Zhou, H.; Yin, S.; Liu, G.; Liu, W.; Cao, Y.; Cui, L. The role of the extracellular signal-related kinase signaling pathway in osteogenic differentiation of human adipose-derived stem cells and in adipogenic transition initiated by dexamethasone. Tissue Eng. Part A 2009, 15, 3487–3497. [Google Scholar] [CrossRef]
- Higuchi, C.; Myoui, A.; Hashimoto, N.; Kuriyama, K.; Yoshioka, K.; Yoshikawa, H.; Itoh, K. Continuous inhibition of MAPK signaling promotes the early osteoblastic differentiation and mineralization of the extracellular matrix. J. Bone Miner. Res. 2002, 17, 1785–1794. [Google Scholar] [CrossRef]
- Ru, L.; Pan, B.; Zheng, J. Signalling pathways in the osteogenic differentiation of periodontal ligament stem cells. Open Life Sci. 2023, 18, 20220706. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, W.; Masson, A.; Li, Y.P. Cell signaling and transcriptional regulation of osteoblast lineage commitment, differentiation, bone formation, and homeostasis. Cell Discov. 2024, 10, 71. [Google Scholar] [CrossRef]
- Ballhause, T.M.; Jiang, S.; Baranowsky, A.; Brandt, S.; Mertens, P.R.; Frosch, K.H.; Yorgan, T.; Keller, J. Relevance of Notch Signaling for Bone Metabolism and Regeneration. Int. J. Mol. Sci. 2021, 22, 1325. [Google Scholar] [CrossRef]
- Lu, W.; Zheng, C.; Zhang, H.; Cheng, P.; Miao, S.; Wang, H.; He, T.; Fan, J.; Hu, Y.; Liu, H.; et al. Hedgehog signaling regulates bone homeostasis through orchestrating osteoclast differentiation and osteoclast-osteoblast coupling. Cell. Mol. Life Sci. 2023, 80, 171. [Google Scholar] [CrossRef]
- Kim, J.H.; Liu, X.; Wang, J.; Chen, X.; Zhang, H.; Kim, S.H.; Cui, J.; Li, R.; Zhang, W.; Kong, Y.; et al. Wnt signaling in bone formation and its therapeutic potential for bone diseases. Ther. Adv. Musculoskelet. Dis. 2013, 5, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Jaganathan, B.G. Signaling network regulating osteogenesis in mesenchymal stem cells. J. Cell Commun. Signal 2022, 16, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Nantavisai, S.; Pisitkun, T.; Osathanon, T.; Pavasant, P.; Kalpravidh, C.; Dhitavat, S.; Makjaroen, J.; Sawangmake, C. Systems biology analysis of osteogenic differentiation behavior by canine mesenchymal stem cells derived from bone marrow and dental pulp. Sci. Rep. 2020, 10, 20703. [Google Scholar] [CrossRef]
- Kreke, M.R.; Goldstein, A.S. Hydrodynamic shear stimulates osteocalcin expression but not proliferation of bone marrow stromal cells. Tissue Eng. 2004, 10, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, S.; Wendt, D.; Miggino, S.; Papadimitropoulos, A.; Fato, M.; Quarto, R.; Martin, I. Effects of fluid flow and calcium phosphate coating on human bone marrow stromal cells cultured in a defined 2D model system. J. Biomed. Mater. Res. A 2008, 86, 411–419. [Google Scholar] [CrossRef]
- Genetos, D.C.; Geist, D.J.; Liu, D.; Donahue, H.J.; Duncan, R.L. Fluid shear-induced ATP secretion mediates prostaglandin release in MC3T3-E1 osteoblasts. J. Bone Miner. Res. 2005, 20, 41–49. [Google Scholar] [CrossRef]
- Genetos, D.C.; Kephart, C.J.; Zhang, Y.; Yellowley, C.E.; Donahue, H.J. Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes. J. Cell. Physiol. 2007, 212, 207–214. [Google Scholar] [CrossRef]
- Chang, M.C.; Wu, J.H.; Chen, S.Y.; Hsu, Y.T.; Yeung, S.Y.; Pan, Y.H.; Jeng, J.H. Inducing cyclooxygenase-2 expression, prostaglandin E(2) and prostaglandin F(2alpha) production of human dental pulp cells by activation of toll-like receptor-3, mitogen-activated protein kinase kinase/extracellular signal-regulated kinase and p38 signaling. J. Dent. Sci. 2024, 19, 1190–1199. [Google Scholar] [CrossRef]
- Ridnour, L.A.; Isenberg, J.S.; Espey, M.G.; Thomas, D.D.; Roberts, D.D.; Wink, D.A. Nitric oxide regulates angiogenesis through a functional switch involving thrombospondin-1. Proc. Natl. Acad. Sci. USA 2005, 102, 13147–13152. [Google Scholar] [CrossRef]
- Newman, H.; Shih, Y.V.; Varghese, S. Resolution of inflammation in bone regeneration: From understandings to therapeutic applications. Biomaterials 2021, 277, 121114. [Google Scholar] [CrossRef]
- Stegen, S.; van Gastel, N.; Carmeliet, G. Bringing new life to damaged bone: The importance of angiogenesis in bone repair and regeneration. Bone 2015, 70, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Longoni, A.; Utomo, L.; van Hooijdonk, I.E.; Bittermann, G.K.; Vetter, V.C.; Kruijt Spanjer, E.C.; Ross, J.; Rosenberg, A.J.; Gawlitta, D. The chondrogenic differentiation potential of dental pulp stem cells. Eur. Cell Mater. 2020, 39, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Urkia, N.; Luzuriaga, J.; Uribe-Etxebarria, V.; Irastorza, I.; Fernandez-San-Argimiro, F.J.; Olalde, B.; Briz, N.; Unda, F.; Ibarretxe, G.; Madarieta, I.; et al. Enhanced Adipogenic Differentiation of Human Dental Pulp Stem Cells in Enzymatically Decellularized Adipose Tissue Solid Foams. Biology 2022, 11, 1099. [Google Scholar] [CrossRef] [PubMed]
- Yourek, G.; McCormick, S.M.; Mao, J.J.; Reilly, G.C. Shear stress induces osteogenic differentiation of human mesenchymal stem cells. Regen. Med. 2010, 5, 713–724. [Google Scholar] [CrossRef]
- Choi, J.; Lee, S.Y.; Yoo, Y.M.; Kim, C.H. Maturation of Adipocytes is Suppressed by Fluid Shear Stress. Cell Biochem. Biophys. 2017, 75, 87–94. [Google Scholar] [CrossRef]
- Charoenpong, H.; Kamoltham, K.; Limsiriwong, S.; Insawak, R.; Veerawattanatigul, A.; Lertchatripong, S. Compressive Force Upregulates Notch Target Genes and NOTCH2 mRNA in Human Dental Pulp Cells. J. Curr. Sci. Technol. 2024, 14, 14. [Google Scholar] [CrossRef]
- Cai, X.; Zhang, Y.; Yang, X.; Grottkau, B.E.; Lin, Y. Uniaxial cyclic tensile stretch inhibits osteogenic and odontogenic differentiation of human dental pulp stem cells. J. Tissue Eng. Regen. Med. 2011, 5, 347–353. [Google Scholar] [CrossRef]
- Navas, T.; Zhou, L.; Estes, M.; Haghnazari, E.; Nguyen, A.N.; Mo, Y.; Pahanish, P.; Mohindru, M.; Cao, T.; Higgins, L.S. Inhibition of p38α MAPK disrupts the pathological loop of proinflammatory factor production in the myelodysplastic syndrome bone marrow microenvironment. Leuk. Lymphoma 2008, 49, 1963–1975. [Google Scholar] [CrossRef]
- He, J.; Liu, Z.; Zheng, Y.; Qian, J.; Li, H.; Lu, Y.; Xu, J.; Hong, B.; Zhang, M.; Lin, P. p38 MAPK in myeloma cells regulates osteoclast and osteoblast activity and induces bone destruction. Cancer Res. 2012, 72, 6393–6402. [Google Scholar] [CrossRef]
- Goldstein, R.H.; Reagan, M.R.; Anderson, K.; Kaplan, D.L.; Rosenblatt, M. Human bone marrow–derived MSCs can home to orthotopic breast cancer tumors and promote bone metastasis. Cancer Res. 2010, 70, 10044–10050. [Google Scholar] [CrossRef]
- Börgeling, Y.; Schmolke, M.; Viemann, D.; Nordhoff, C.; Roth, J.; Ludwig, S. Inhibition of p38 mitogen-activated protein kinase impairs influenza virus-induced primary and secondary host gene responses and protects mice from lethal H5N1 infection. J. Biol. Chem. 2014, 289, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Young, P.R.; McLaughlin, M.M.; Kumar, S.; Kassis, S.; Doyle, M.L.; McNulty, D.; Gallagher, T.F.; Fisher, S.; McDonnell, P.C.; Carr, S.A.; et al. Pyridinyl imidazole inhibitors of p38 mitogen-activated protein kinase bind in the ATP site. J. Biol. Chem. 1997, 272, 12116–12121. [Google Scholar] [CrossRef] [PubMed]
- Manokawinchoke, J.; Limraksasin, P.; Limjeerajarus, C.N.; Limjeerajarus, N.; Samaranayake, L.P.; Egusa, H.; Osathanon, T. Mechanical Force Induces Osteogenic Differentiation of Murine Induced Pluripotent Stem Cells via TGF-β Signalling. Orthod. Craniofac. Res. 2025. [Google Scholar] [CrossRef] [PubMed]
| Gene | Sequence | |
|---|---|---|
| RUNX2 | Forward | ATGATGACACTGCCACCTCTGA |
| Reverse | GGCTGGATAGTGCATTCGTG | |
| OSX | Forward | GCCAGAAGCTGTGAAACCTC |
| Reverse | GCTGCAAGCTCTCCATAACC | |
| ALP | Forward | CGAGATACAAGCACTCCCACTTC |
| Reverse | CTGTTCAGCTCGTACTGCATCATGTC | |
| COL1A1 | Forward | GTGCTAAAGGTGCCAATGGT |
| Reverse | ACCAGGTTCACCGCTGTTAC | |
| OCN | Forward | CTTTGTGTCCAAGCAGGAGG |
| Reverse | CTGAAAGCCGATGTGGTCAG | |
| OPN | Forward | AGGAGGAGGCAGAGCACA |
| Reverse | CTGGTATGGCACAGGTGATG | |
| CEBPD | Forward | AGCGCAACAACATCGCCGTG |
| Reverse | GTCGGGTCTGAGGTATGGGTC | |
| GAPDH | Forward | CACTGCCAACGTGTCAGTGGTG |
| Reverse | GTAGCCCAGGATGCCCTTGAG | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lwin, H.Y.; Tiskratok, W.; Kyawsoewin, M.; Manokawinchoke, J.; Termkwanchareon, C.; Limjeerajarus, N.; Limjeerajarus, C.N.; Egusa, H.; Osathanon, T.; Limraksasin, P. Shear Stress Regulates Osteogenic Differentiation of Human Dental Pulp Stem Cells via the p38 Pathway. Int. J. Mol. Sci. 2025, 26, 5667. https://doi.org/10.3390/ijms26125667
Lwin HY, Tiskratok W, Kyawsoewin M, Manokawinchoke J, Termkwanchareon C, Limjeerajarus N, Limjeerajarus CN, Egusa H, Osathanon T, Limraksasin P. Shear Stress Regulates Osteogenic Differentiation of Human Dental Pulp Stem Cells via the p38 Pathway. International Journal of Molecular Sciences. 2025; 26(12):5667. https://doi.org/10.3390/ijms26125667
Chicago/Turabian StyleLwin, Hnin Yu, Watcharaphol Tiskratok, Maythwe Kyawsoewin, Jeeranan Manokawinchoke, Chutimon Termkwanchareon, Nuttapol Limjeerajarus, Chalida Nakalekha Limjeerajarus, Hiroshi Egusa, Thanaphum Osathanon, and Phoonsuk Limraksasin. 2025. "Shear Stress Regulates Osteogenic Differentiation of Human Dental Pulp Stem Cells via the p38 Pathway" International Journal of Molecular Sciences 26, no. 12: 5667. https://doi.org/10.3390/ijms26125667
APA StyleLwin, H. Y., Tiskratok, W., Kyawsoewin, M., Manokawinchoke, J., Termkwanchareon, C., Limjeerajarus, N., Limjeerajarus, C. N., Egusa, H., Osathanon, T., & Limraksasin, P. (2025). Shear Stress Regulates Osteogenic Differentiation of Human Dental Pulp Stem Cells via the p38 Pathway. International Journal of Molecular Sciences, 26(12), 5667. https://doi.org/10.3390/ijms26125667








