Increased O-GlcNAcylation in Leukocytes from Overweight Pediatric Subjects: A Pilot Study
Abstract
1. Introduction
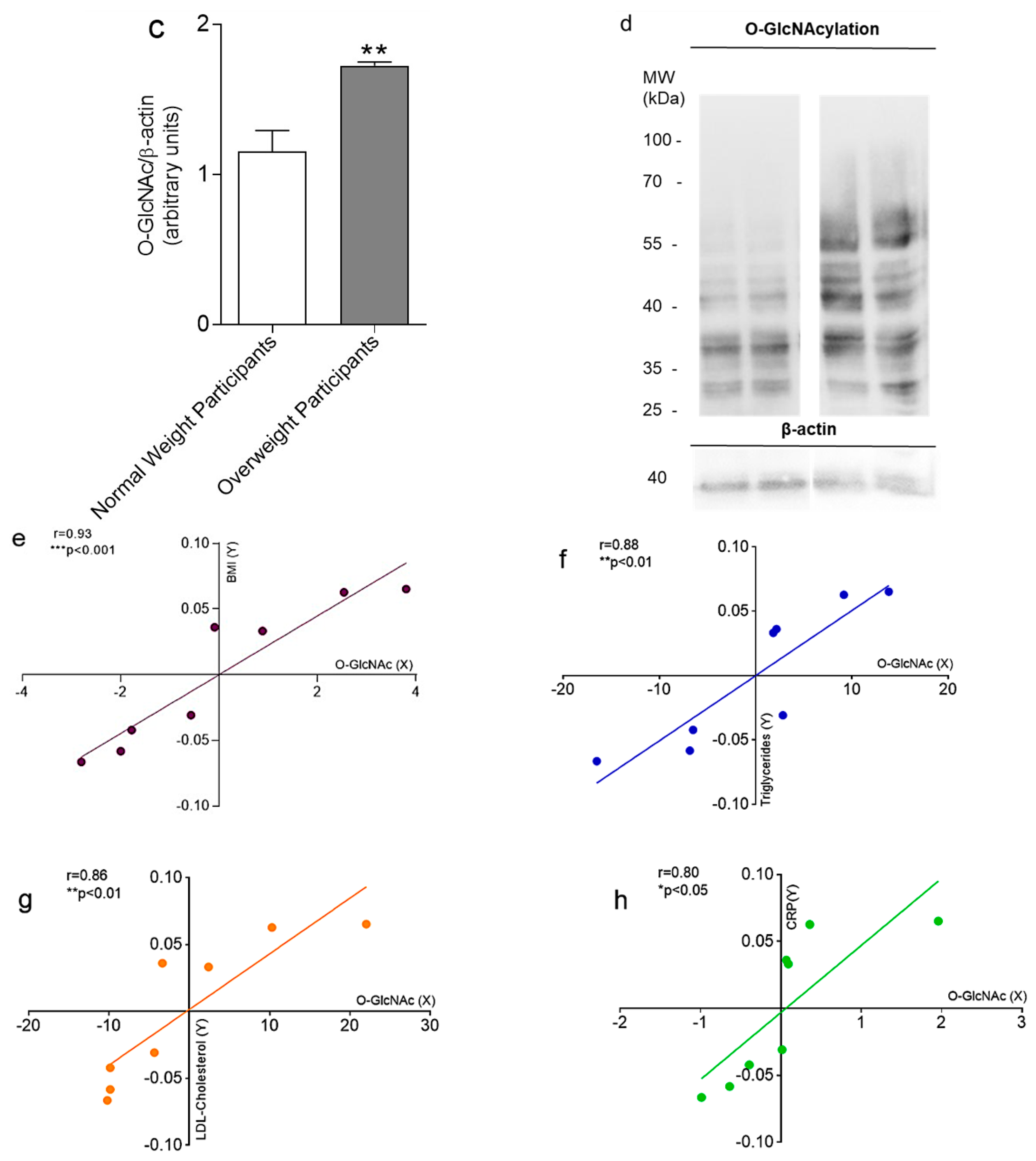
2. Results
3. Discussion
4. Materials and Methods
4.1. Enrollment of Pediatric Subjects
4.2. Determination of Protein O-GlcNAc Modification in Leukocytes by Western Blotting Analysis
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 10 January 2025).
- Italy: Over 20% of Children Are Overweight. Available online: https://www.who.int/europe/news-room/09-12-2020-italy-over-20-of-children-are-overweight-says-new-report (accessed on 10 January 2025).
- International Obesity Task Force-IOTF. Available online: https://www.epicentro.iss.it/okkioallasalute/indagine-2023-dati (accessed on 10 January 2025).
- Twig, G.; Zucker, I.; Afek, A.; Cukierman-Yaffe, T.; Bendor, C.D.; Derazne, E.; Lutski, M.; Shohat, T.; Mosenzon, O.; Tzur, D.; et al. Adolescent Obesity and Early-Onset Type 2 Diabetes. Diabetes Care 2020, 43, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Juszczyk, D.; van Jaarsveld, C.H.M.; Gulliford, M.C. Body Mass Index and Incident Type 1 and Type 2 Diabetes in Children and Young Adults: A Retrospective Cohort Study. J. Endocr. Soc. 2017, 1, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Pulgaron, E.R.; Delamater, A.M. Obesity and type 2 diabetes in children: Epidemiology and treatment. Curr. Diab. Rep. 2014, 14, 508. [Google Scholar] [CrossRef] [PubMed]
- Gedebjerg, A.; Almdal, T.P.; Berencsi, K.; Rungby, J.; Nielsen, J.S.; Witte, D.R.; Friborg, S.; Brandslund, I.; Vaag, A.; Beck-Nielsen, H.; et al. Prevalence of micro- and macrovascular diabetes complications at time of type 2 diabetes diagnosis and associated clinical characteristics: A cross-sectional baseline study of 6958 patients in the Danish DD2 cohort. J. Diabetes Complicat. 2018, 32, 34–40. [Google Scholar] [CrossRef]
- Spurr, S.; Bally, J.; Hill, P.; Gray, K.; Newman, P.; Hutton, A. Exploring the Prevalence of Undiagnosed Prediabetes, Type 2 Diabetes Mellitus, and Risk Factors in Adolescents: A Systematic Review. J. Pediatr. Nurs. 2020, 50, 94–104. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, J.W.; Li, Y.; Huang, K.; Chen, R.M.; Maimaiti, M.; Luo, J.S.; Chen, S.K.; Wu, D.; Zhu, M.; et al. Population-based prevalence of self-reported pediatric diabetes and screening for undiagnosed type 2 diabetes in Chinese children in years 2017-2019, a cross-sectional study. Lancet Reg. Health-West. Pac. 2024, 52, 101206. [Google Scholar] [CrossRef]
- Barrett, T.; Jalaludin, M.Y.; Turan, S.; Hafez, M.; Shehadeh, N.; Novo Nordisk Pediatric Type 2 Diabetes Global Expert Panel. Rapid progression of type 2 diabetes and related complications in children and young people-A literature review. Pediatr. Diabetes 2020, 21, 158–172. [Google Scholar] [CrossRef]
- Chiaradonna, F.; Ricciardiello, F.; Palorini, R. The Nutrient-Sensing Hexosamine Biosynthetic Pathway as the Hub of Cancer Metabolic Rewiring. Cells 2018, 7, 53. [Google Scholar] [CrossRef]
- Morales, M.M.; Pratt, M.R. The post-translational modification O-GlcNAc is a sensor and regulator of metabolism. Open Biol. 2024, 14, 240209. [Google Scholar] [CrossRef]
- Vosseller, K.; Wells, L.; Lane, M.D.; Hart, G.W. Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3-L1 adipocytes. Proc. Natl. Acad. Sci. USA 2002, 99, 5313–5318. [Google Scholar] [CrossRef]
- Wang, Z.; Park, K.; Comer, F.; Hsieh-Wilson, L.C.; Saudek, C.D.; Hart, G.W. Site-specific GlcNAcylation of human erythrocyte proteins: Potential biomarker(s) for diabetes. Diabetes 2009, 58, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Saudek, C.D.; Hart, G.W. Increased expression of beta-N-acetylglucosaminidase in erythrocytes from individuals with pre-diabetes and diabetes. Diabetes 2010, 59, 1845–1850. [Google Scholar] [CrossRef] [PubMed]
- Springhorn, C.; Matsha, T.E.; Erasmus, R.T.; Essop, M.F. Exploring leukocyte O-GlcNAcylation as a novel diagnostic tool for the earlier detection of type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2012, 97, 4640–4649. [Google Scholar] [CrossRef] [PubMed]
- Pagesy, P.; Tachet, C.; Mostefa-Kara, A.; Larger, E.; Issad, T. Increased OGA Expression and Activity in Leukocytes from Patients with Diabetes: Correlation with Inflammation Markers. Exp. Clin. Endocrinol. Diabetes 2019, 127, 517–523. [Google Scholar] [CrossRef]
- Ducheix, S.; Magre, J.; Cariou, B.; Prieur, X. Chronic O-GlcNAcylation and Diabetic Cardiomyopathy: The Bitterness of Glucose. Front. Endocrinol. 2018, 9, 642. [Google Scholar] [CrossRef]
- Soliman Wadan, A.H.; Abdelsattar Ahmed, M.; Hussein Ahmed, A.; El-Sayed Ellakwa, D.; Hamed Elmoghazy, N.; Gawish, A. The Interplay of Mitochondrial Dysfunction in Oral Diseases: Recent Updates in Pathogenesis and Therapeutic Implications. Mitochondrion 2024, 78, 101942. [Google Scholar] [CrossRef]
- Slawson, C.; Copeland, R.J.; Hart, G.W. O-GlcNAc signaling: A metabolic link between diabetes and cancer? Trends Biochem. Sci. 2010, 35, 547–555. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhou, S.; Lou, W.; Qian, H.; Xu, Z. Crosstalk between O-GlcNAcylation and phosphorylation in metabolism: Regulation and mechanism. Cell Death Differ. 2025. [Google Scholar] [CrossRef]
- Derr, R.; Garrett, E.; Stacy, G.A.; Saudek, C.D. Is HbA(1c) affected by glycemic instability? Diabetes Care 2003, 26, 2728–2733. [Google Scholar] [CrossRef]
- Lorenzo, C.; Wagenknecht, L.E.; Hanley, A.J.; Rewers, M.J.; Karter, A.J.; Haffner, S.M. A1C between 5.7 and 6.4% as a marker for identifying pre-diabetes, insulin sensitivity and secretion, and cardiovascular risk factors: The Insulin Resistance Atherosclerosis Study (IRAS). Diabetes Care 2010, 33, 2104–2109. [Google Scholar] [CrossRef]
- Calle, M.C.; Fernandez, M.L. Inflammation and type 2 diabetes. Diabetes Metab. 2012, 38, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Freedman, D.S.; Sherry, B. The validity of BMI as an indicator of body fatness and risk among children. Pediatrics 2009, 124 (Suppl. 1), S23–S34. [Google Scholar] [CrossRef] [PubMed]
- Javed, A.; Jumean, M.; Murad, M.H.; Okorodudu, D.; Kumar, S.; Somers, V.K.; Sochor, O.; Lopez-Jimenez, F. Diagnostic performance of body mass index to identify obesity as defined by body adiposity in children and adolescents: A systematic review and meta-analysis. Pediatr. Obes. 2015, 10, 234–244. [Google Scholar] [CrossRef]
- Scazzina, F.; Del Rio, D.; Benini, L.; Melegari, C.; Pellegrini, N.; Marcazzan, E.; Brighenti, F. The effect of breakfasts varying in glycemic index and glycemic load on dietary induced thermogenesis and respiratory quotient. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 121–125. [Google Scholar] [CrossRef]
- Schulz, C.; Lemaire, Q.; Berthier, A.; Descat, A.; Kouach, M.; Vercoutter-Edouart, A.S.; Yazidi-Belkoura, I.E.; Hardivillé, S.; Goossens, J.F.; Lefebvre, P. Control of lipid metabolism by the dynamic and nutrient-dependent post-translational modification O-GlcNAcylation. Nat. Sci. 2023, 3, e20220006. [Google Scholar] [CrossRef]
- Lockridge, A.; Hanover, J.A. A nexus of lipid and O-Glcnac metabolism in physiology and disease. Front. Endocrinol. 2022, 13, 943576. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fu, M.; Li, M.D.; Zhang, K.; Zhang, B.; Wang, S.; Liu, Y.; Ni, W.; Ong, Q.; Mi, J.; et al. O-GlcNAc transferase inhibits visceral fat lipolysis and promotes diet-induced obesity. Nat. Commun. 2020, 11, 181. [Google Scholar] [CrossRef]
- Stefan, N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol. 2020, 8, 616–627. [Google Scholar] [CrossRef]
- Myslicki, J.P.; Shearer, J.; Hittel, D.S.; Hughey, C.C.; Belke, D.D. O-GlcNAc modification is associated with insulin sensitivity in the whole blood of healthy young adult males. Diabetol. Metab. Syndr. 2014, 6, 96. [Google Scholar] [CrossRef]
- Costa, R.; Remigante, A.; Civello, D.A.; Bernardinelli, E.; Szabo, Z.; Morabito, R.; Marino, A.; Sarikas, A.; Patsch, W.; Paulmichl, M.; et al. O-GlcNAcylation Suppresses the Ion Current IClswell by Preventing the Binding of the Protein ICln to alpha-Integrin. Front. Cell Dev. Biol. 2020, 8, 607080. [Google Scholar] [CrossRef]
- Yeung, Y.G.; Stanley, E.R. A solution for stripping antibodies from polyvinylidene fluoride immunoblots for multiple reprobing. Anal. Biochem. 2009, 389, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Morabito, R.; Costa, R.; Rizzo, V.; Remigante, A.; Nofziger, C.; La Spada, G.; Marino, A.; Paulmichl, M.; Dossena, S. Crude venom from nematocysts of Pelagia noctiluca (Cnidaria: Scyphozoa) elicits a sodium conductance in the plasma membrane of mammalian cells. Sci. Rep. 2017, 7, 41065. [Google Scholar] [CrossRef] [PubMed]
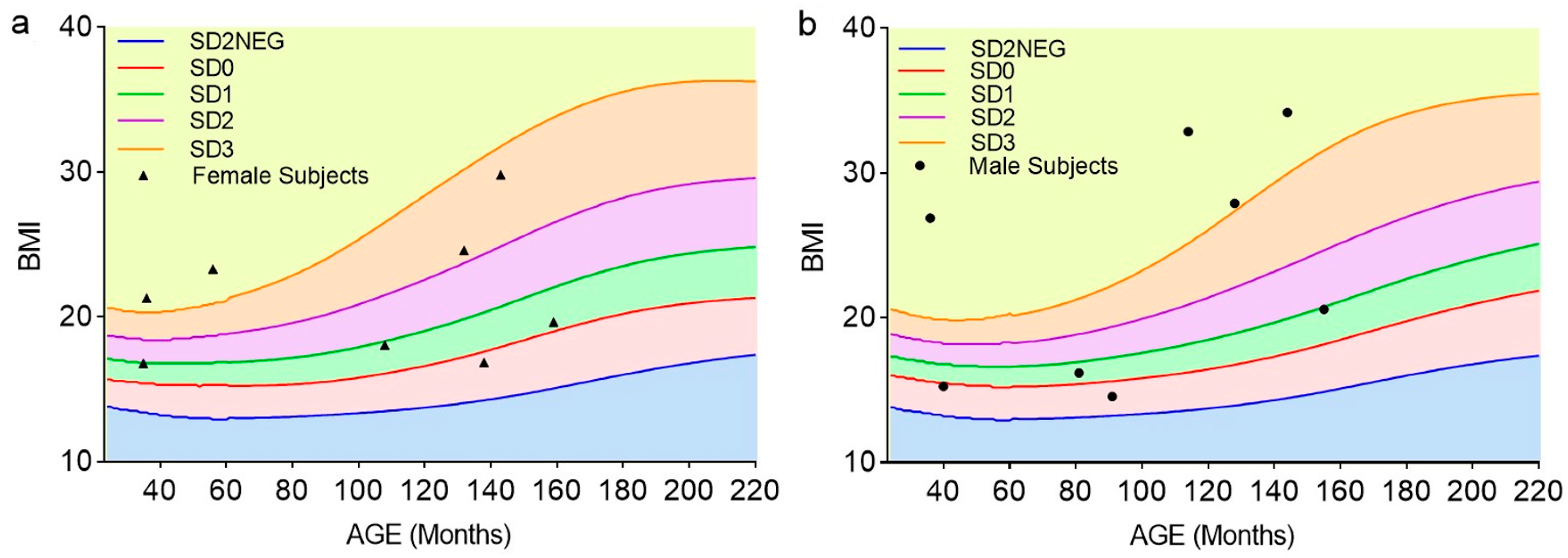
| Parameters | Normal-Weight Female Subjects (n = 4) | Normal-Weight Male Subjects (n = 4) | Overweight Female Subjects (n = 4) | Overweight Male Subjects (n = 4) | Physiological Reference Values |
|---|---|---|---|---|---|
| Age, months; average ± SD (range) | 110 ± 4.61 (35–159) | 91.75 ± 5.29 (40–155) | 81.25 ± 6.21 (37–143) | 105.5 ± 4.49 (36–144) | |
| Height, cm; average ± SD (range) | 133.25 ± 2.61 (88–158) | 131 ± 2.73 (104–170) | 124.5 ± 1.65 (102–151) | 131.75 ± 2.29 (91–150) | |
| Weight, Kg; average ± SD (range) | 33.5 ± 2.42 (13–49) | 31 ± 3.91 (16.5–59.5) | 38.5 ± 2.51 (21–68) | 64.62 ± 1.37 (55–83) | |
| FPG, mg/dL; average ± SD (range) | 90.66 ± 0.94 (80–96) | 85 ± 0.15 (84–86) | 85.75 ± 1.10 (70–92) | 82.4 ± 0.75 (70–86) | 60–110 mg/dL |
| A1c, mmol/mol; average ± SD (range) | 31.66 ± 0.10 (31–32) | 34 ± 0.17 (33–35) | 36.5 ± 0.93 (32–42) | 37 ± 0.64 (32–42) | 20–38 mmol/mol |
| Insulin, mlU/L; average ± SD (range) | 7.03 ± 1.49 (3.7–13.3) | 11.56 ± 5.20 (4.2–23.6) | 8.85 ± 3.75 (2–22) | 5.5 ± 1.02 (3–9) | 2–25 mlU/L |
| Triglycerides, mg/dL; average ± SD (range) | 85.75 ± 1.76 (66–102) | 78.66 ± 2.14 (55–94) | 137 ± 1.06 (120–150) | 105.5 ± 0.79 (98–115) | 30–180 mg/dL |
| Total Cholesterol, mg/dL; average ± SD (range) | 141.25 ± 2.93 (122–190) | 134.66 ± 1.29 (124–153) | 182.5 ± 1.38 (156–196) | 154 ± 2.74 (107–199) | 0–190 mg/dL |
| HDL-Cholesterol, mg/dL; average ± SD (range) | 53.5 ± 2.25 (33–74) | 46.33 ± 1.15 (37–52) | 46.75 ± 1.24 (38–55) | 46 ± 0.95 (36–49) | >43 mg/dL |
| LDL-Cholesterol, mg/dL; average ± SD (range) | 80.25 ± 2.36 (66–108) | 78.33 ± 1.68 (61–93) | 105.25 ± 1.44 (94–124) | 81.25 ± 1.33 (64–92) | 0–115 mg/dL |
| CRP, mg/dL; average ± SD (range) | 0.28 ± 0.02 (0.27–0.29) | 0.28 ± 0.02 (0.27–0.29) | 3.60 ± 1.44 (1.84–6.43) | 1.32 ± 0.03 (1.29–1.36) | 0–0.5 mg/dL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Remigante, A.; Spinelli, S.; Rizzo, G.; Caruso, D.; Marino, A.; Straface, E.; Dossena, S.; Morabito, R. Increased O-GlcNAcylation in Leukocytes from Overweight Pediatric Subjects: A Pilot Study. Int. J. Mol. Sci. 2025, 26, 5665. https://doi.org/10.3390/ijms26125665
Remigante A, Spinelli S, Rizzo G, Caruso D, Marino A, Straface E, Dossena S, Morabito R. Increased O-GlcNAcylation in Leukocytes from Overweight Pediatric Subjects: A Pilot Study. International Journal of Molecular Sciences. 2025; 26(12):5665. https://doi.org/10.3390/ijms26125665
Chicago/Turabian StyleRemigante, Alessia, Sara Spinelli, Gianluca Rizzo, Daniele Caruso, Angela Marino, Elisabetta Straface, Silvia Dossena, and Rossana Morabito. 2025. "Increased O-GlcNAcylation in Leukocytes from Overweight Pediatric Subjects: A Pilot Study" International Journal of Molecular Sciences 26, no. 12: 5665. https://doi.org/10.3390/ijms26125665
APA StyleRemigante, A., Spinelli, S., Rizzo, G., Caruso, D., Marino, A., Straface, E., Dossena, S., & Morabito, R. (2025). Increased O-GlcNAcylation in Leukocytes from Overweight Pediatric Subjects: A Pilot Study. International Journal of Molecular Sciences, 26(12), 5665. https://doi.org/10.3390/ijms26125665











