Tryptophan Metabolite ITE Attenuates LPS-Induced MMP-9 via NF-κB/AP-1 in Monocytes
Abstract
1. Introduction
2. Results
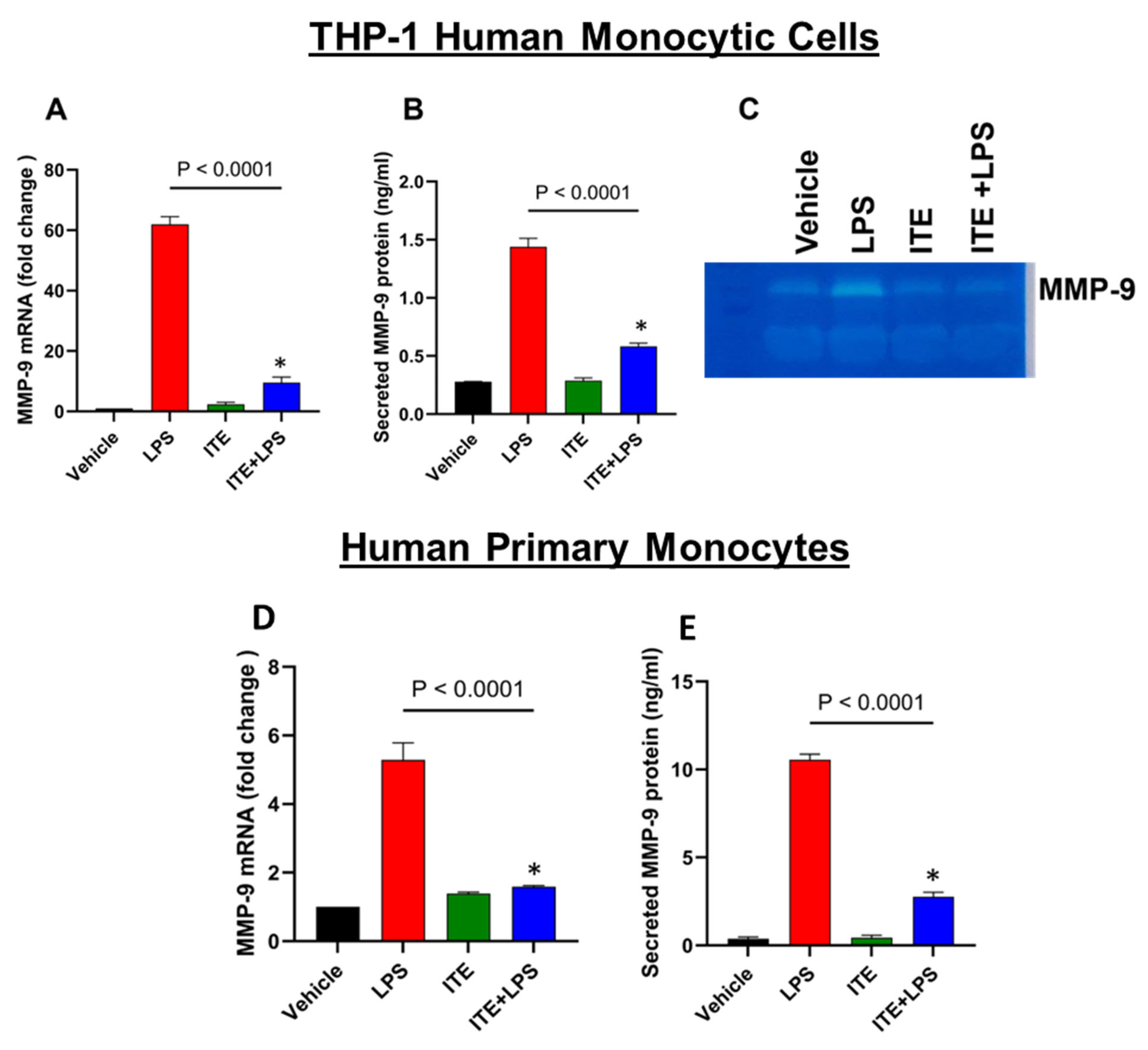
2.1. ITE Inhibits LPS-Induced MMP-9 Expression
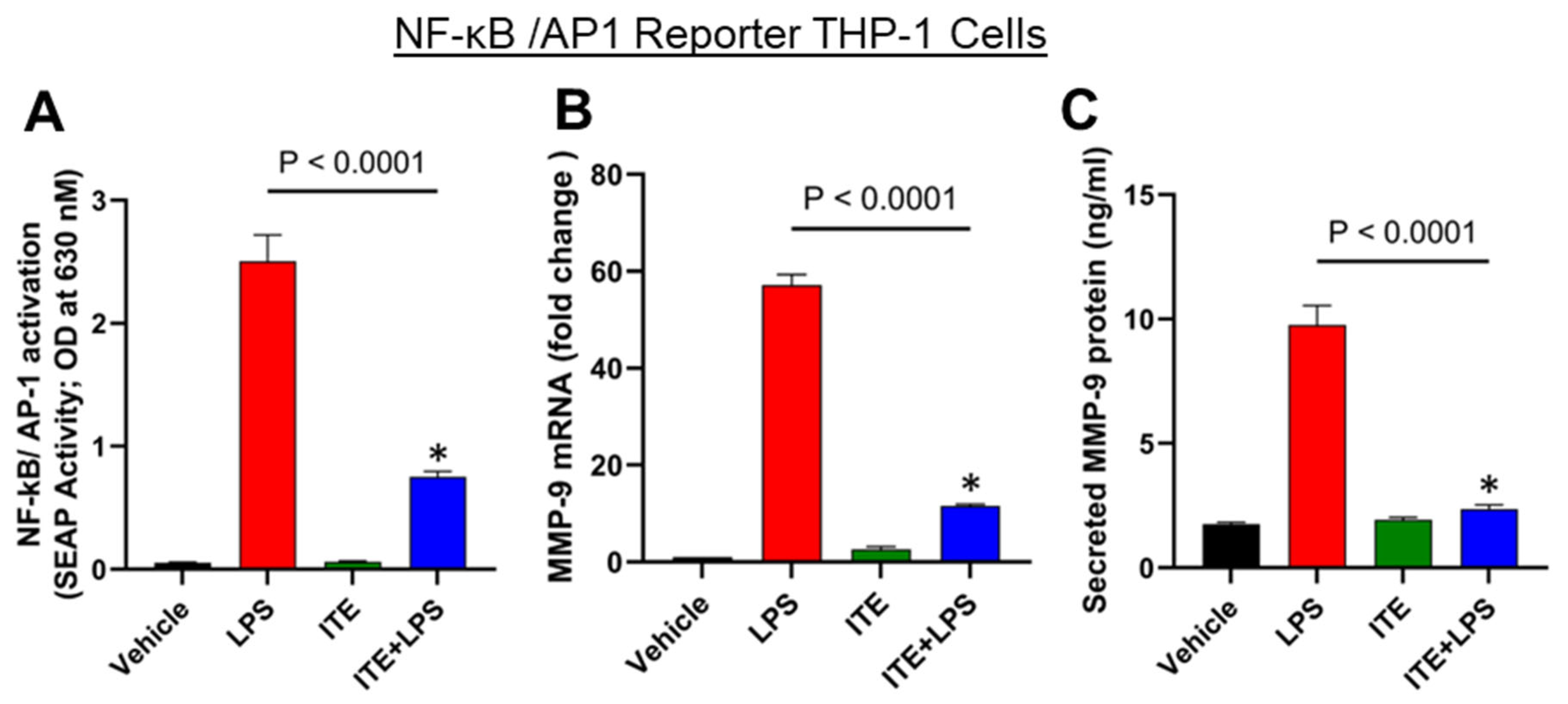
2.2. ITE Reduces LPS-Induced NF-κB/AP-1 Activity in Monocytic Cells
2.3. ITE Reduces the NF-κB/AP-1 Binding to the MMP-9 Promoter
3. Discussion
3.1. Study Limitations
3.2. Future Directions
4. Materials and Methods
4.1. Cell Culture
4.2. Measurement of NF-κB/AP-1 Activity
4.3. Real-Time Quantitative PCR
4.4. Quantification of MMP-9 Secretion
4.5. Gelatin Zymography
4.6. Chromatin Immunoprecipitation (ChIP) Assay
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Creely, S.J.; McTernan, P.G.; Kusminski, C.M.; Fisher, f.M.; Da Silva, N.F.; Khanolkar, M.; Evans, M.; Harte, A.L.; Kumar, S. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E740–E747. [Google Scholar] [CrossRef] [PubMed]
- Sylus, A.M.; Nandeesha, H.; Chitra, T. Matrix metalloproteinase-9 increases and Interleukin-10 reduces with increase in body mass index in polycystic ovary syndrome: A cross-sectional study. Int. J. Reprod. Biomed. 2020, 18, 605–610. [Google Scholar] [CrossRef]
- Sindhu, S.; Al-Roub, A.; Koshy, M.; Thomas, R.; Ahmad, R. Palmitate-Induced MMP-9 Expression in the Human Monocytic Cells is Mediated through the TLR4-MyD88 Dependent Mechanism. Cell. Physiol. Biochem. 2016, 39, 889–900. [Google Scholar] [CrossRef]
- Boumiza, S.; Chahed, K.; Tabka, Z.; Jacob, M.P.; Norel, X.; Ozen, G. MMPs and TIMPs levels are correlated with anthropometric parameters, blood pressure, and endothelial function in obesity. Sci. Rep. 2021, 11, 20052. [Google Scholar] [CrossRef]
- Yabluchanskiy, A.; Ma, Y.; Iyer, R.P.; Hall, M.E.; Lindsey, M.L. Matrix metalloproteinase-9: Many shades of function in cardiovascular disease. Physiology 2013, 28, 391–403. [Google Scholar] [CrossRef]
- Xue, M.; McKelvey, K.; Shen, K.; Minhas, N.; March, L.; Park, S.Y.; Jackson, C.J. Endogenous MMP-9 and not MMP-2 promotes rheumatoid synovial fibroblast survival, inflammation and cartilage degradation. Rheumatology 2014, 53, 2270–2279. [Google Scholar] [CrossRef] [PubMed]
- Ganaie, I.A.; Malik, M.Z.; Naqvi, S.H.; Jain, S.K.; Wajid, S. Differential levels of Alpha-1-inhibitor III, Immunoglobulin heavy chain variable region, and Hypertrophied skeletal muscle protein GTF3 in rat mammary tumorigenesis. Biochimie 2020, 174, 57–68. [Google Scholar] [CrossRef]
- Sahu, D.; Bishwal, S.C.; Malik, M.Z.; Sahu, S.; Kaushik, S.R.; Sharma, S.; Saini, E.; Arya, R.; Rastogi, A.; Sharma, S.; et al. Troxerutin-Mediated Complement Pathway Inhibition is a Disease-Modifying Treatment for Inflammatory Arthritis. Front. Cell Dev. Biol. 2022, 10, 845457. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Xie, G.; Ding, F.; Zhou, X. LPS-induced MMP-9 expression is mediated through the MAPKs-AP-1 dependent mechanism in BEAS-2B and U937 cells. Exp. Lung Res. 2018, 44, 217–225. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Monte, S.V.; Caruana, J.A.; Ghanim, H.; Sia, C.L.; Korzeniewski, K.; Schentag, J.J.; Dandona, P. Reduction in endotoxemia, oxidative and inflammatory stress, and insulin resistance after Roux-en-Y gastric bypass surgery in patients with morbid obesity and type 2 diabetes mellitus. Surgery 2012, 151, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, S.; Witta, J.; Zhong, J.; de Villiers, W.; Eckhardt, E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J. Lipid Res. 2009, 50, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Schäffler, A.; Schölmerich, J. Innate immunity and adipose tissue biology. Trends Immunol. 2010, 31, 228–235. [Google Scholar] [CrossRef]
- Sondermann, N.C.; Faßbender, S.; Hartung, F.; Hätälä, A.M.; Rolfes, K.M.; Vogel, C.F.; Haarmann-Stemmann, T. Functions of the aryl hydrocarbon receptor (AHR) beyond the canonical AHR/ARNT signaling pathway. Biochem. Pharmacol. 2022, 208, 115371. [Google Scholar] [CrossRef]
- Gutiérrez-Vázquez, C.; Quintana, F.J. Regulation of the immune response by the aryl hydrocarbon receptor. Immunity 2018, 48, 19–33. [Google Scholar] [CrossRef]
- Riaz, F.; Pan, F.; Wei, P. Aryl hydrocarbon receptor: The master regulator of immune responses in allergic diseases. Front. Immunol. 2022, 13, 1057555. [Google Scholar] [CrossRef]
- Rappaport, N.; Watanabe, K. Biological BMI uncovers hidden health risks and is more responsive to lifestyle shifts. Nat. Med. 2023, 29, 801–802. [Google Scholar]
- Bahman, F.; Choudhry, K.; Al-Rashed, F.; Al-Mulla, F.; Sindhu, S.; Ahmad, R. Aryl hydrocarbon receptor: Current perspectives on key signaling partners and immunoregulatory role in inflammatory diseases. Front. Immunol. 2024, 15, 1421346. [Google Scholar] [CrossRef]
- Manuel, R.S.; Rundquist, A.; Ambrogi, M.; Scharpf, B.R.; Peterson, N.T.; Sandhu, J.K.; Chandrashekar, S.; Ridlon, M.; Crawford, L.K.; Keil-Stietz, K.P.; et al. The aryl hydrocarbon receptor agonist ITE reduces inflammation and urinary dysfunction in a mouse model of autoimmune prostatitis. Am. J. Clin. Exp. Urol. 2024, 12, 149–161. [Google Scholar] [CrossRef]
- Abron, J.D.; Singh, N.P.; Mishra, M.K.; Price, R.L.; Nagarkatti, M.; Nagarkatti, P.S.; Singh, U.P. An endogenous aryl hydrocarbon receptor ligand, ITE, induces regulatory T cells and ameliorates experimental colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G220–G230. [Google Scholar] [CrossRef]
- Wu, H.T.; Sie, S.S.; Kuan, T.C.; Lin, C.S. Identifying the regulative role of NF-κB binding sites within promoter region of human matrix metalloproteinase 9 (mmp-9) by TNF-α induction. Appl. Biochem. Biotechnol. 2013, 169, 438–449. [Google Scholar] [CrossRef]
- Domínguez-Acosta, O.; Vega, L.; Estrada-Muñiz, E.; Rodríguez, M.S.; Gonzalez, F.J.; Elizondo, G. Activation of aryl hydrocarbon receptor regulates the LPS/IFNγ-induced inflammatory response by inducing ubiquitin-proteosomal and lysosomal degradation of RelA/p65. Biochem. Pharmacol. 2018, 155, 141–149. [Google Scholar] [CrossRef]
- Wang, C.; Ye, Z.; Kijlstra, A.; Zhou, Y.; Yang, P. Activation of the aryl hydrocarbon receptor affects activation and function of human monocyte-derived dendritic cells. Clin. Exp. Immunol. 2014, 177, 521–530. [Google Scholar] [CrossRef]
- Al-Rashed, F.; Kochumon, S.; Usmani, S.; Sindhu, S.; Ahmad, R. Pam3CSK4 Induces MMP-9 Expression in Human Monocytic THP-1 Cells. Cell. Physiol. Biochem. 2017, 41, 1993–2003. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, N.; Xu, L.; Liu, Y.; Huang, L.; Gu, M.; Wu, Y.; Guo, W.; Sun, H. Aryl hydrocarbon receptor dependent anti-inflammation and neuroprotective effects of tryptophan metabolites on retinal ischemia/reperfusion injury. Cell Death Dis. 2023, 14, 92. [Google Scholar] [CrossRef]
- Vázquez-Gómez, G.; Karasová, M.; Tylichová, Z.; Kabátková, M.; Hampl, A.; Matthews, J.; Neča, J.; Ciganek, M.; Machala, M.; Vondráček, J. Aryl Hydrocarbon Receptor (AhR) Limits the Inflammatory Responses in Human Lung Adenocarcinoma A549 Cells via Interference with NF-κB Signaling. Cells 2022, 11, 707. [Google Scholar] [CrossRef]
- Whitmarsh, A.J.; Davis, R.J. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J. Mol. Med. 1996, 74, 589–607. [Google Scholar] [CrossRef]
- Kochumon, S.; Jacob, T.; Koshy, M.; Al-Rashed, F.; Sindhu, S.; Al-Ozairi, E.; Al-Mulla, F.; Rosen, E.D.; Ahmad, R. Palmitate Potentiates Lipopolysaccharide-Induced IL-6 Production via Coordinated Acetylation of H3K9/H3K18, p300, and RNA Polymerase II. J. Immunol. 2022, 209, 731–741. [Google Scholar] [CrossRef]
- Kochumon, S.; Al-Sayyar, A.; Jacob, T.; Bahman, F.; Akhter, N.; Wilson, A.; Sindhu, S.; Hannun, Y.A.; Ahmad, R.; Al-Mulla, F. TGF-β and TNF-α interaction promotes the expression of MMP-9 through H3K36 dimethylation: Implications in breast cancer metastasis. Front. Immunol. 2024, 15, 1430187. [Google Scholar] [CrossRef]
- Al Madhoun, A.; Haddad, D.; Kochumon, S.; Thomas, R.; Miranda, L.; George, P.; Abu-Khalaf, N.; Al-Mulla, F.; Ahmad, R. TNF-α/NF-κB mediated upregulation of Dectin-1 in hyperglycemic obesity: Implications for metabolic inflammation and diabetes. J. Transl. Med. 2025, 23, 462. [Google Scholar] [CrossRef] [PubMed]
- Al-Roub, A.; Akhter, N.; Al-Rashed, F.; Wilson, A.; Alzaid, F.; Al-Mulla, F.; Sindhu, S.; Ahmad, R. TNFα induces matrix metalloproteinase-9 expression in monocytic cells through ACSL1/JNK/ERK/NF-kB signaling pathways. Sci. Rep. 2023, 13, 14351. [Google Scholar] [CrossRef] [PubMed]
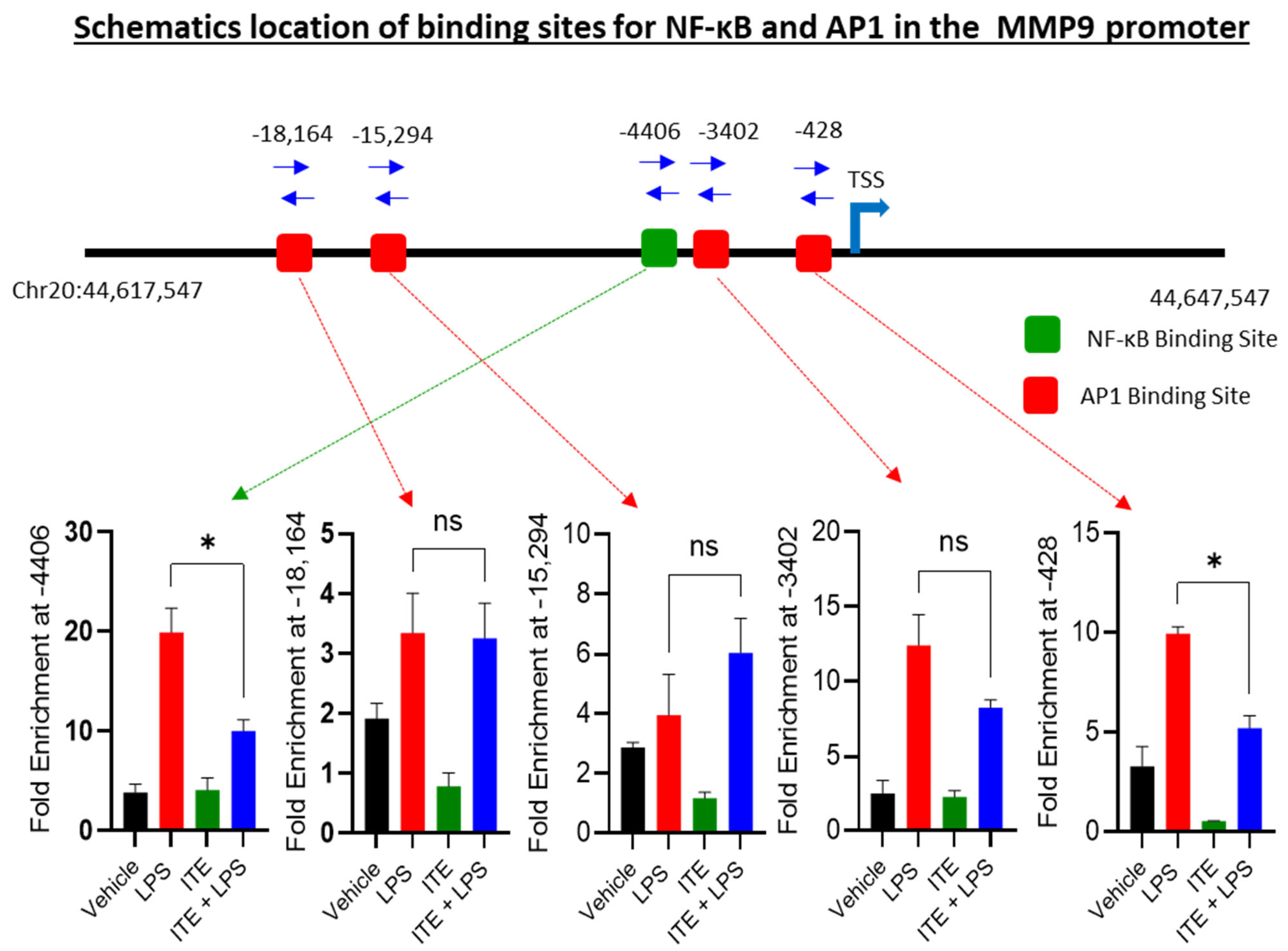
| ChIP-qPCR Assay Cat# | TF-BS Relative to MMP9 TSS | TF-BS Position (NC_) |
|---|---|---|
| GPH1008476(-)19A | AP-1 (−18,164) | 44619180 |
| GPH1008476(-)16A | AP-1 (−15,294) | 44622312 |
| GPH1008476(-)04A | AP-1 (−3402) | 44633814 |
| GPH1008476(-)01A | AP-1 (−428) | 44637547 |
| GPH1008476(-)05A | NF-kappaB (−4406) | 44633494 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahman, F.; Akhter, N.; Kochumon, S.; Al-Mulla, F.; Ahmad, R. Tryptophan Metabolite ITE Attenuates LPS-Induced MMP-9 via NF-κB/AP-1 in Monocytes. Int. J. Mol. Sci. 2025, 26, 5663. https://doi.org/10.3390/ijms26125663
Bahman F, Akhter N, Kochumon S, Al-Mulla F, Ahmad R. Tryptophan Metabolite ITE Attenuates LPS-Induced MMP-9 via NF-κB/AP-1 in Monocytes. International Journal of Molecular Sciences. 2025; 26(12):5663. https://doi.org/10.3390/ijms26125663
Chicago/Turabian StyleBahman, Fatemah, Nadeem Akhter, Shihab Kochumon, Fahd Al-Mulla, and Rasheed Ahmad. 2025. "Tryptophan Metabolite ITE Attenuates LPS-Induced MMP-9 via NF-κB/AP-1 in Monocytes" International Journal of Molecular Sciences 26, no. 12: 5663. https://doi.org/10.3390/ijms26125663
APA StyleBahman, F., Akhter, N., Kochumon, S., Al-Mulla, F., & Ahmad, R. (2025). Tryptophan Metabolite ITE Attenuates LPS-Induced MMP-9 via NF-κB/AP-1 in Monocytes. International Journal of Molecular Sciences, 26(12), 5663. https://doi.org/10.3390/ijms26125663







