Update on HDM Allergy: Principal Changes over the Years
Abstract
1. Introduction
2. Epidemiology
2.1. Geographic Distribution and Factors Affecting Epidemiology
2.2. Impact of Housing Conditions and Lifestyle
3. Microbiological Classification
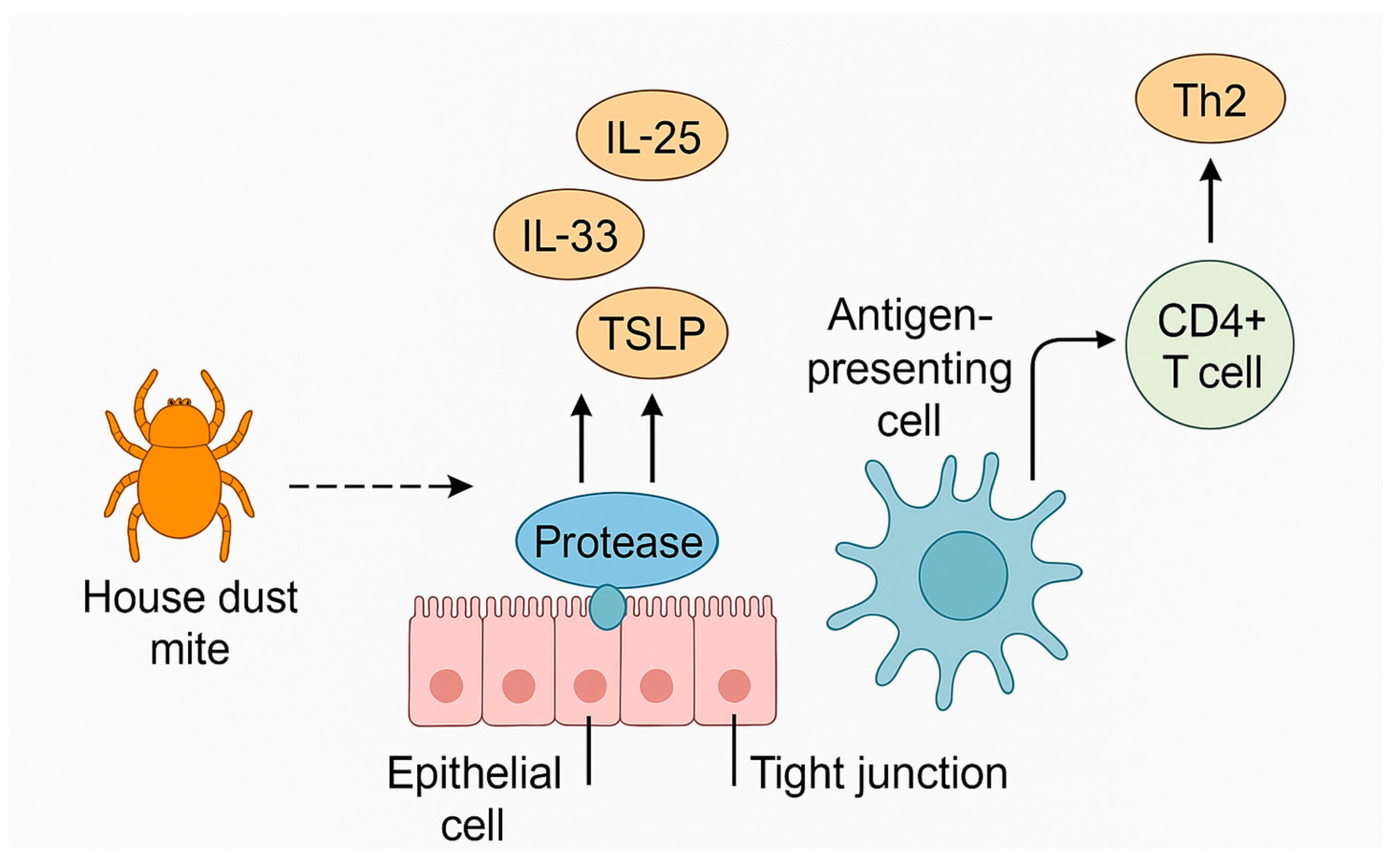
4. Allergens Relevant to Pathomechanism
5. Molecular and Cellular Basis of Allergic Response to House Dust Mite
5.1. The Lipid Properties of Allergens and Their Effect on Immunogenicity
5.2. Immunometabolic Reprogramming of Myeloid Cells
5.3. TRPC1 Calcium Channels and Airway Remodeling
5.4. Macrophages and Phenotype Polarization in Response to HDM
5.5. Differentiation of Monocytic and Macrophage Responses
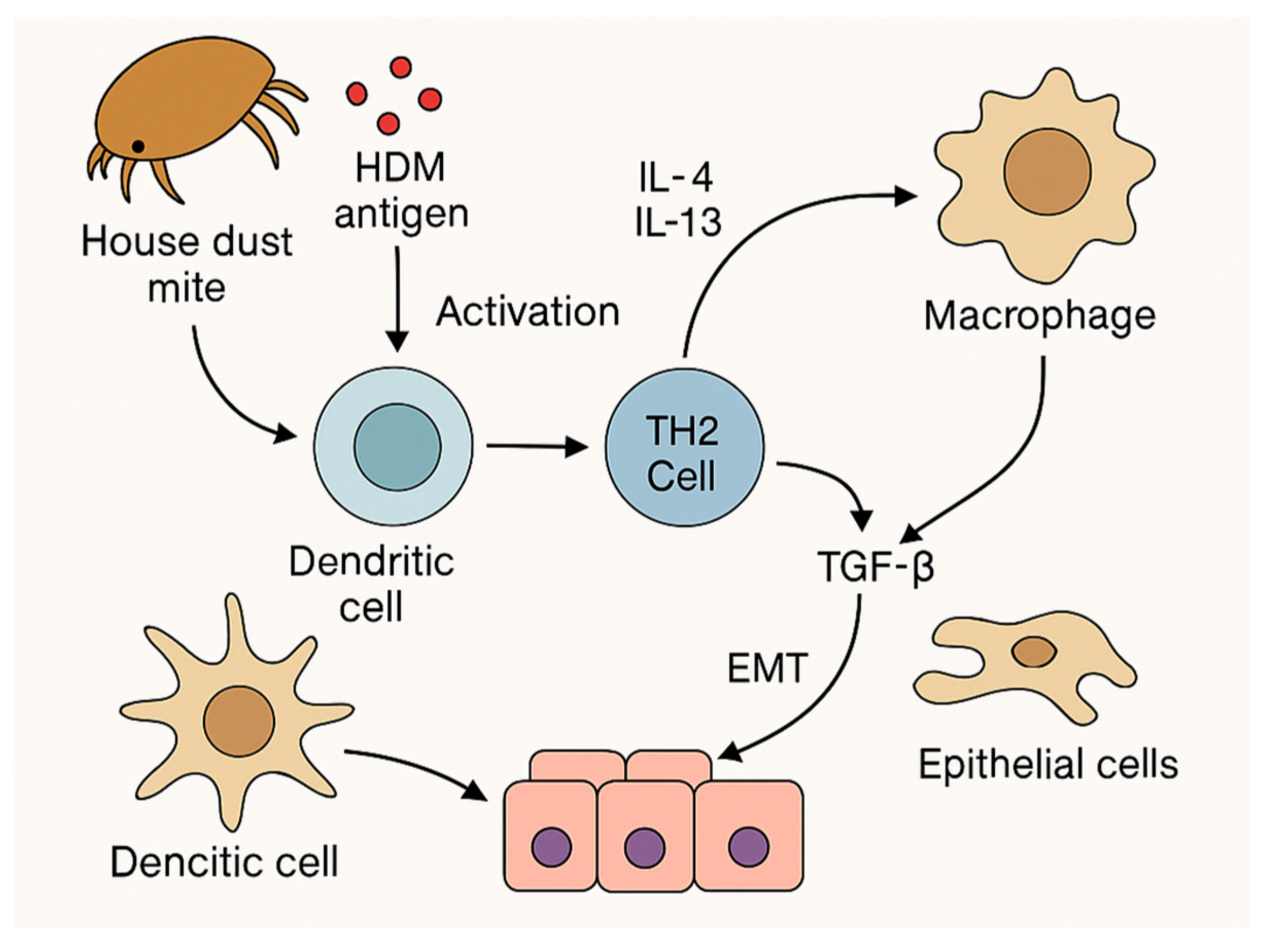
5.6. Epithelial–Mesenchymal Transition (EMT) and Epithelial Remodeling
5.7. Influence of Genetic and Epigenetic Determinants: Disruption of Epithelial Barrier Integrity and Activation of Structural Cells
5.8. Involvement of Toll-like Receptors (TLRs) in Modulation of Immune Response
5.9. Alternative Pathways for Initiating the Inflammatory Response
5.10. Therapeutic Implications and Prospects
- Inhibitors of TLR pathways (e.g., TLR4);
- Neutralization of IL-33, TSLP and PAR2;
- Use of mutant allergens lacking enzymatic activity in immunotherapy;
- Drugs modulating the activity of sensory neuron receptors (TRPs);
- Selective protease inhibitors (ADIs—allergen delivery inhibitors);
- Selective inhibition of MFI or its receptor;
- Modulation of innate immune training (e.g., by methyltransferase inhibitors);
- Blocking EMT (e.g., by inhibiting TGF-β1, the Wnt pathway);
- Targeting subtypes of immune cells that overreact to HDM.
6. New Developments in Allergen-Specific Immunotherapy
6.1. Biomarkers of AIT Efficacy
6.2. Neosensitization During AIT
6.3. Immunomodulation and Metabolism During AIT
6.4. The Role of Basophils and ILC Cells in the Mechanisms of AIT
6.5. Safety and New Approaches in Immunotherapy
6.6. Combination Therapy of SCIT and Dupilumab
7. Materials and Methods
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| HDM | House dust mites |
| AIT | Allergen-specific immunotherapy |
| OIT | Oral immunotherapy |
| SCIT | Subcutaneous immunotherapy |
| SLIT | Sublingual immunotherapy |
| ITIT | Intratonsillar immunotherapy |
| BMDM | Bone marrow-derived macrophages |
| BMDAM | Bone marrow-derived alveolar macrophages |
| BMDC | Bone marrow-derived dendritic cells |
| TRPC1 | Transient receptor potential canonical 1 |
| EMT | Epithelial–mesenchymal transition |
| TJs | Tight junctions |
| AD | Atopic dermatitis |
References
- Li, Z.; Zheng, N.; An, Q.; Li, X.; Sun, S.; Zhang, W.; Ji, Y.; Wang, S.; Li, P. Impact of environmental factors and bacterial interactions on dust mite allergens in different indoor dust. Sci. Total Environ. 2022, 844, 157177. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.H.; Wu, C.T.; Wu, T.S.; Yu, F.Y.; Ko, J.L.; Lue, K.H.; Liu, Y.F. Systematic Characterization of the Group 2 House Dust Mite Allergen in Dermatophagoides microceras. Front. Cell Infect. Microbiol. 2022, 11, 793559. [Google Scholar] [CrossRef] [PubMed]
- Imoto, Y.; Sakashita, M.; Tokunaga, T.; Kanno, M.; Saito, K.; Shimizu, A.; Maegawa, A.; Fujieda, S. Recent prevalence of allergic rhinitis caused by house dust mites among the pediatric population in Fukui, Japan. World Allergy Organ. J. 2024, 17, 100932. [Google Scholar] [CrossRef]
- Huang, H.J.; Sarzsinszky, E.; Vrtala, S. House dust mite allergy: The importance of house dust mite allergens for diagnosis and immunotherapy. Mol. Immunol. 2023, 158, 54–67. [Google Scholar] [CrossRef]
- Caraballo, L. Exploring the relationship between house dust mites and asthma. Expert Rev. Clin. Immunol. 2024, 20, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.H.; Kim, M.; Yong, T.S.; Kim, J.Y. Investigating the microbiome of house dust mites in South Korea. Front. Allergy 2023, 4, 1240727. [Google Scholar] [CrossRef]
- Erban, T.; Klimov, P.; Molva, V.; Hubert, J. Whole genomic sequencing and sex-dependent abundance estimation of Cardinium sp., a common and hyperabundant bacterial endosymbiont of the American house dust mite, Dermatophagoides farinae. Exp. Appl. Acarol. 2020, 80, 363–380. [Google Scholar] [CrossRef]
- Moingeon, P. Progress in the development of specific immunotherapies for house dust mite allergies. Expert Rev. Vaccines 2014, 13, 1463–1473. [Google Scholar] [CrossRef]
- Vackova, T.; Pekar, S.; Klimov, P.B.; Hubert, J. Population growth and respiration in the dust mite Dermatophagoides farinae under different temperature and humidity regimes. Exp. Appl. Acarol. 2023, 89, 157–169. [Google Scholar] [CrossRef]
- Wise, S.K.; Damask, C.; Roland, L.T.; Ebert, C.; Levy, J.M.; Lin, S.; Luong, A.; Rodriguez, K.; Sedaghat, A.R.; Toskala, E.; et al. International consensus statement on allergy and rhinology: Allergic rhinitis—2023. Int. Forum Allergy Rhinol. 2023, 13, 293–859. [Google Scholar] [CrossRef]
- Wise, S.K.; Damask, C.; Greenhawt, M.; Oppenheimer, J.; Roland, L.T.; Shaker, M.S.; Wallace, D.V.; Lang, D.M. A Synopsis of Guidance for Allergic Rhinitis Diagnosis and Management From ICAR 2023. J. Allergy Clin. Immunol. Pract. 2023, 11, 773–796. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Xie, S.; Meng, L.; Zhong, W.; Zhang, H.; Wang, F.; Fan, R.; Jiang, W.; Xie, Z. Does skin prick test response intensity predict symptom severity and efficacy of subcutaneous immunotherapy in allergic rhinitis? Eur. Arch. Otorhinolaryngol. 2024, 281, 767–774. [Google Scholar] [CrossRef]
- Huang, H.H.; Xu, C.; Liu, L.; Chai, R.N. Efficacy comparison and safety analysis of subcutaneous specific immunotherapy with standardized house dust mite allergen in patients with single and multiple allergic rhinitis. Chin. J. Prev. Med. 2022, 56, 774–783. [Google Scholar] [CrossRef]
- Song, W.; Lin, X.; Chai, R. Evaluation of long-term effect for house dust mite subcutaneous immunotherapy for patients with allergic rhinitis. Chin. J. Otorhinolaryngol. Head Neck Surg. 2015, 50, 632–635. [Google Scholar]
- Marques, M.L.; Rezende, I.; Cunha, I.; Gouveia, J.; Rodrigues Dos Santos, F.; Falcão, I.; Cunha, L.; Falcão, H. Allergic sensitization to Storage Dust Mites: A prospective study of patients with respiratory allergy. Eur. Ann. Allergy Clin. Immunol. 2022, 54, 43–47. [Google Scholar] [CrossRef]
- Schoos, A.M. Atopic diseases—Diagnostics, mechanisms, and exposures. Pediatr. Allergy Immunol. 2024, 35, e14198. [Google Scholar] [CrossRef] [PubMed]
- van Boven, F.E.; Braunstahl, G.J.; Arends, L.R.; van Maaren, M.S.; Bramer, W.M.; van Wijk, R.G.; de Jong, N.W. House dust mite allergen avoidance strategies for the treatment of allergic asthma: A hypothesis-generating meta-analysis. World Allergy Organ. J. 2024, 17, 100919. [Google Scholar] [CrossRef]
- Kim, S.J.; Moon, J.W.; Cho, Y.; Lee, H.M. Clinical characteristics of local allergic rhinitis sensitized to house dust mites in Asia. Eur. Arch. Otorhinolaryngol. 2024, 281, 2413–2420. [Google Scholar] [CrossRef]
- Hamada, M.; Saeki, K.; Tanaka, I. Comparison of rush-subcutaneous and sublingual immunotherapy with house dust mite extract for pediatric allergic rhinitis: A prospective cohort study. Allergol. Int. 2023, 72, 573–579. [Google Scholar] [CrossRef]
- Calderon, M.A.; Casale, T.B.; Nelson, H.S.; Demoly, P. An evidence-based analysis of house dust mite allergen immunotherapy: A call for more rigorous clinical studies. J. Allergy Clin. Immunol. 2013, 132, 1322–1336. [Google Scholar] [CrossRef]
- Aljohani, A.; Clarke, D.; Byrne, M.; Fleming, G. The bacterial microbiome and resistome of house dust mites in Irish homes. Sci. Rep. 2024, 14, 19621. [Google Scholar] [CrossRef] [PubMed]
- Marko, M.; Pawliczak, R. Pharmacotherapy and immunotherapy of allergic rhinitis induced by house dust mite, grass, and birch pollen allergens: A meta-analysis of randomized clinical trials. Expert Rev. Respir. Med. 2023, 17, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Bogacz-Piaseczyńska, A.; Bożek, A.; Krupka-Olek, M.; Kawczyk-Krupka, A.; Zalejska-Fiolka, J.; Canonica, G.W. Dupilumab and House Dust Mite Immunotherapy in Patients with Atopic Dermatitis: A Preliminary Study. Vaccines 2024, 12, 1046. [Google Scholar] [CrossRef]
- Bożek, A. Clinical outcomes of AIT in the elderly population. Curr. Opin. Allergy Clin. Immunol. 2023, 23, 341–345. [Google Scholar] [CrossRef]
- Xue, Q.; Zou, M.; Guo, J.; Teng, Q.; Zhang, Q.; Sheng, L.; Xu, S.; Fang, C.; Yao, N.; Li, Y.; et al. Detection and assessment of dust mite allergens in an indoor environment in Anhui, China. Environ. Sci. Pollut. Res. Int. 2023, 30, 3045–3055. [Google Scholar] [CrossRef]
- Hubert, J.; Nesvorna, M.; Kopecky, J.; Erban, T.; Klimov, P. Population and Culture Age Influence the Microbiome Profiles of House Dust Mites. Microb. Ecol. 2019, 77, 1048–1066. [Google Scholar] [CrossRef] [PubMed]
- Calzada, D.; Martín-López, L.; Carnés, J. Growth, allergen profile and microbiome studies in Dermatophagoides pteronyssinus cultures. Sci. Rep. 2023, 13, 10633. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Q.; Du, J.; Huang, C.; Li, D.; Dai, X.; Liang, R.; Li, B.; Shi, G. Comparative analysis of global transcriptome, proteome and acetylome in house dust mite-induced murine allergic asthma model. Clin. Transl. Med. 2021, 11, e590. [Google Scholar] [CrossRef]
- Sun, J.L.; Shen, L.; Chen, J.; Yu, J.M.; Yin, J. Species diversity of house dust mites in Beijing, China. J. Med. Entomol. 2013, 50, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Seow, I.; Siew, Z.Y.; Wong, S.T.; Wong, S.F.; Fang, C.M.; Kan, M.S.; Voon, K. House dust mites eradication treatments: Current updates emphasizing on tropical countries. Trop. Biomed. 2024, 41, 450–460. [Google Scholar] [CrossRef]
- Akdemir, C.; Karaman, Ü.; Cebeci Güler, N.; Direkel, Ş.; Uzunoğlu, E.; Şentürk, H.; Ayhan, U. Prevalence of House Dust Mites and Presence of Der p 1 and Der f 1 in Ordu, Giresun, Trabzon and Rize Provinces. Turk. Parazitol. Derg. 2023, 47, 179–183. [Google Scholar] [CrossRef]
- Xu, Q.; Shang, Y.; Li, X.; Ran, S.; Lu, M.; Cheng, L. Exploring the Role of Allergenic Components in Children with House Dust Mite-Induced Allergic Diseases. J. Asthma Allergy 2025, 18, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Colas, L.; Cheminant, M.A.; Brosseau, C.; Sauzeau, V.; Magnan, A.; Bouchaud, G. Der p 2.1 Peptide Abrogates House Dust Mites-Induced Asthma Features in Mice and Humanized Mice by Inhibiting DC-Mediated T Cell Polarization. Front. Immunol. 2020, 11, 565431. [Google Scholar] [CrossRef] [PubMed]
- Yuriev, S.; Rodinkova, V.; Mokin, V.; Varchuk, I.; Sharikadze, O.; Marushko, Y.; Halushko, B.; Kurchenko, A. Molecular sensitization pattern to house dust mites is formed from the first years of life and includes group 1, 2, Der p 23, Der p 5, Der p 7 and Der p 21 allergens. Clin. Mol. Allergy 2023, 21, 1. [Google Scholar] [CrossRef] [PubMed]
- Bouaziz, A.; Walgraffe, D.; Bouillot, C.; Herman, J.; Foguenne, J.; Gothot, A.; Louis, R.; Hentges, F.; Jacquet, A.; Mailleux, A.C.; et al. Development of recombinant stable house dust mite allergen Der p 3 molecules for component-resolved diagnosis and specific immunotherapy. Clin. Exp. Allergy 2015, 45, 823–834. [Google Scholar] [CrossRef]
- Adam, E.; Hansen, K.K.; Astudillo Fernandez, O.; Coulon, L.; Bex, F.; Duhant, X.; Jaumotte, E.; Hollenberg, M.D.; Jacquet, A. The house dust mite allergen Der p 1, unlike Der p 3, stimulates the expression of interleukin-8 in human airway epithelial cells via a proteinase-activated receptor-2-independent mechanism. J. Biol. Chem. 2006, 281, 6910–6923. [Google Scholar] [CrossRef]
- Bessot, J.C.; Pauli, G. Les acariens domestiques et leurs allergènes [House dust mites allergens]. Rev. Mal. Respir. 2011, 28, 475–495. [Google Scholar] [CrossRef]
- Cui, Y.B.; Yu, L.L.; Teng, F.X.; Wang, N.; Zhou, Y.; Yang, L.; Zhang, C.B. Dust mite allergen Der f 4: Expression, characterization, and IgE binding in pediatric asthma. Pediatr. Allergy Immunol. 2016, 27, 391–397. [Google Scholar] [CrossRef]
- Walsemann, T.; Böttger, M.; Traidl, S.; Schwager, C.; Gülsen, A.; Freimooser, S.; Roesner, L.M.; Werfel, T.; Jappe, U. Specific IgE against the house dust mite allergens Der p 5, 20 and 21 influences the phenotype and severity of atopic diseases. Allergy 2023, 78, 731–742. [Google Scholar] [CrossRef]
- Pulsawat, P.; Soongrung, T.; Satitsuksanoa, P.; Le Mignon, M.; Khemili, S.; Gilis, D.; Nony, E.; Kennedy, M.W.; Jacquet, A. The house dust mite allergen Der p 5 binds lipid ligands and stimulates airway epithelial cells through a TLR2-dependent pathway. Clin. Exp. Allergy 2019, 49, 378–390. [Google Scholar] [CrossRef]
- Vidal-Quist, J.C.; Ortego, F.; Hernández-Crespo, P. Contribution of cysteine and serine proteases to proteolytic digestion in an allergy-eliciting house dust mite. J. Insect Physiol. 2021, 133, 104285. [Google Scholar] [CrossRef]
- Resch, Y.; Weghofer, M.; Seiberler, S.; Horak, F.; Scheiblhofer, S.; Linhart, B.; Swoboda, I.; Thomas, W.R.; Thalhamer, J.; Valenta, R.; et al. Molecular characterization of Der p 10: A diagnostic marker for broad sensitization in house dust mite allergy. Clin. Exp. Allergy 2011, 41, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.Y.; Lee, J.Y.; Son, M.; Yi, M.H.; Yong, T.S.; Shin, J.U.; Lee, K.H.; Kim, Y.J.; Park, K.H.; Park, H.J.; et al. Profiles of IgE Sensitization to Der f 1, Der f 2, Der f 6, Der f 8, Der f 10, and Der f 20 in Korean House Dust Mite Allergy Patients. Allergy Asthma Immunol. Res. 2015, 7, 483–488. [Google Scholar] [CrossRef]
- Banerjee, S.; Resch, Y.; Chen, K.W.; Swoboda, I.; Focke-Tejkl, M.; Blatt, K.; Novak, N.; Wickman, M.; van Hage, M.; Ferrara, R.; et al. Der p 11 is a major allergen for house dust mite-allergic patients suffering from atopic dermatitis. J. Investig. Dermatol. 2015, 135, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Lee, J.; Jeong, K.Y.; Cheon, D.S.; Park, J.W. Comparison of sensitization patterns to dust mite allergens between atopic dermatitis patients and dogs, and non-specific reactivity of canine IgE to the storage mite Tyrophagus putrescentiae. Exp. Appl. Acarol. 2022, 88, 41–55. [Google Scholar] [CrossRef]
- Conti, A.; Burastero, G.J.; Hales, B.J.; Breda, D.; Alessio, M.; Burastero, S.E. IgE reactivity to house dust mite allergen components in sensitized asymptomatic subjects: A role for Der p 20. J. Biol. Regul. Homeost. Agents 2021, 35, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Yu, L.; Sun, J.; Wang, N.; Cui, Y. Homology modeling and prediction of B-cell and T-cell epitopes of the house dust mite allergen Der f 20. Mol. Med. Rep. 2018, 17, 1807–1812. [Google Scholar] [CrossRef]
- Gao, Y.; Li, J.; Xu, X.; Wang, C.; Zhang, Y.; Zhang, L. Sensitisation to House Dust Mite Component Der p 23 Is Associated with Severe Symptoms and Asthma in Allergic Rhinitis Patients. Int. Arch. Allergy Immunol. 2023, 184, 906–913. [Google Scholar] [CrossRef]
- Celi, G.; Brusca, I.; Scala, E.; Villalta, D.; Pastorello, E.; Farioli, L.; Cortellini, G.; Deleonardi, G.; Galati, P.; Losappio, L.; et al. House dust mite allergy in Italy—Diagnostic and clinical relevance of Der p 23 (and of minor allergens): A real-life, multicenter study. Allergy 2019, 74, 1787–1789. [Google Scholar] [CrossRef]
- Thomas, W.R. Hierarchy and molecular properties of house dust mite allergens. Allergol. Int. 2015, 64, 304–311. [Google Scholar] [CrossRef]
- Erban, T.; Klimov, P.; Talacko, P.; Harant, K.; Hubert, J. Proteogenomics of the house dust mite, Dermatophagoides farinae: Allergen repertoire, accurate allergen identification, isoforms, and sex-biased proteome differences. J. Proteom. 2020, 210, 103535. [Google Scholar] [CrossRef] [PubMed]
- Zemelka-Wiacek, M.; Jutel, M. AIT 2023: Current innovation and future outlook. Allergol. Select 2023, 7, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Villalta, D.; Scala, E.; Asero, R.; Da Re, M.; Conte, M.; Buzzulini, F. Evaluation and predictive value of IgE responses toward a comprehensive panel of house dust mite allergens using a new multiplex assay: A real-life experience on an Italian population. Eur. Ann. Allergy Clin. Immunol. 2022, 54, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Jappe, U.; Schwager, C.; Schromm, A.B.; González Roldán, N.; Stein, K.; Heine, H.; Duda, K.A. Lipophilic allergens, different modes of allergen-lipid interaction and their impact on asthma and allergy. Front. Immunol. 2019, 10, 122. [Google Scholar] [CrossRef]
- Pu, Q.; Zhao, Y.; Sun, Y.; Huang, T.; Lin, P.; Zhou, C.; Qin, S.; Singh, B.B.; Wu, M. TRPC1 intensifies house dust mite–induced airway remodeling by facilitating epithelial-to-mesenchymal transition and STAT3/NF-κB signaling. FASEB J. 2019, 33, 1074–1085. [Google Scholar] [CrossRef]
- Dunbar, M.L.; Gomez, A.C.; Richardson, B.D.; Wozniak, J.E.; Manlove, L.R.; Dulin, J.A.; Elgamal, O.A.; Meliopoulos, V.A.; Thomas, P.G.; Piiparinen, H.; et al. The human MIF polymorphism CATT7 enhances pro-inflammatory macrophage polarization in a model of house dust mite–induced allergic inflammation. FASEB J. 2024, 38, e23456. [Google Scholar] [CrossRef]
- Fransen, N.L.; Leonard, W.J. Single-cell transcriptomics reveals divergent myeloid responses to house dust mite and diesel exhaust particles in allergic airways. Sci. Rep. 2024, 14, 6483. [Google Scholar] [CrossRef]
- Yoshie, S.; Sato, K.; Fujii, Y.; Suzuki, Y.; Saito, K. The role of chloride channels in house dust mite-induced epithelial-mesenchymal transition. Int. J. Mol. Sci. 2024, 25, 289. [Google Scholar] [CrossRef]
- Soh, W.T.; Zhang, J.; Hollenberg, M.D.; Vliagoftis, H.; Rothenberg, M.E.; Sokol, C.L.; Robinson, C.; Jacquet, A. Protease allergens as initiators–regulators of allergic inflammation. Allergy 2023, 78, 1148–1168. [Google Scholar] [CrossRef]
- Wenger, M.; Grosse-Kathoefer, S.; Kraiem, A.; Pelamatti, E.; Nunes, N.; Pointner, L.; Aglas, L. When the allergy alarm bells toll: The role of Toll-like receptors in allergic diseases and treatment. Front. Mol. Biosci. 2023, 10, 1204025. [Google Scholar] [CrossRef]
- Yurakova, T.R.; Gubernatorova, E.O.; Gorshkova, E.A.; Nosenko, M.A.; Nedospasov, S.A.; Drutskaya, M.S. HDM induces distinct immunometabolic phenotype in macrophages in TLR4-dependent manner. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166531. [Google Scholar] [CrossRef] [PubMed]
- Serhan, N.; Basso, L.; Sibilano, R.; Petitfils, C.; Meixiong, J.; Bonnart, C.; Reber, L.L.; Marichal, T.; Starkl, P.; Cenac, N.; et al. House dust mites activate nociceptor-mast cell clusters to drive type 2 skin inflammation. Nat. Immunol. 2019, 20, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Manti, S.; Gambadauro, A.; Galletta, F.; Ruggeri, P.; Piedimonte, G. Update on the Role of β2AR and TRPV1 in Respiratory Diseases. Int. J. Mol. Sci. 2024, 25, 10234. [Google Scholar] [CrossRef] [PubMed]
- Augustin, S.; Eichhorn, T.; Wald, M.; Fischer, F.; Jutel, M.; Pfaar, O.; Willers, C. Improved quality control of allergen products: Assessing the molecular allergen composition by mass spectrometry. Allergy 2024, 79, 3489–3500. [Google Scholar] [CrossRef]
- Zemelka-Wiacek, M.; Kosowska, A.; Winiarska, E.; Sobanska, E.; Jutel, M. Validated allergen exposure chamber is plausible tool for the assessment of house dust mite-triggered allergic rhinitis. Allergy 2023, 78, 168–177. [Google Scholar] [CrossRef]
- Jutel, M.; Vogelberg, C.; Duwensee, K.; Troyke, D.; Klimek, L. One-strength dose escalation of house dust mite depot product for subcutaneous immunotherapy is safe and tolerable. Allergy 2025, 80, 807–816. [Google Scholar] [CrossRef]
- Ansotegui, I.J.; Melioli, G.; Canonica, G.W.; Caraballo, L.; Villa, E.; Ebisawa, M.; Passalacqua, G.; Savi, E.; Ebo, D.; Gómez, R.M.; et al. IgE allergy diagnostics and other relevant tests in allergy, a World Allergy Organization position paper. World Allergy Organ. J. 2020, 13, 100080. [Google Scholar] [CrossRef]
- Haidar, L.; Bănărescu, C.F.; Uța, C.; Moldovan, S.I.; Zimbru, E.-L.; Zimbru, R.-I.; Ciurariu, E.; Georgescu, M.; Panaitescu, C. Pollen–Food Allergy Syndrome: Allergens, Clinical Insights, Diagnostic and Therapeutic Challenges. Appl. Sci. 2025, 15, 66. [Google Scholar] [CrossRef]
- Zimbru, R.-I.; Grijincu, M.; Tănasie, G.; Zimbru, E.-L.; Bojin, F.-M.; Buzan, R.-M.; Tamaș, T.-P.; Cotarcă, M.-D.; Harich, O.O.; Pătrașcu, R.; et al. Breaking Barriers: The Detrimental Effects of Combined Ragweed and House Dust Mite Allergen Extract Exposure on the Bronchial Epithelium. Appl. Sci. 2025, 15, 4113. [Google Scholar] [CrossRef]
- Hamed, A.; Todd, I.; Tighe, P.J.; Powell, R.J.; Harrison, T.; Fairclough, L.C. Array-based measurements of aeroallergen-specific IgE correlate with skin-prick test reactivity in asthma regardless of specific IgG4 or total IgE measurements. J. Immunol. Methods 2021, 492, 112999. [Google Scholar] [CrossRef]
- Yang, L.; Yang, Y.; Xu, Q.; Zhang, W.; Jiang, Q.; Li, W.; Wang, Y.; Ma, D.; Lin, X.; Sun, B.; et al. Specific IgE and IgG4 profiles of house dust mite components in allergen-specific immunotherapy. Front. Immunol. 2022, 12, 786738. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, C.; Li, H.; Wang, X. Exploring the role of basophil activation test in diagnosis of Dermatophagoides farinae sensitization and evaluation of therapeutic efficacy of subcutaneous immunotherapy in children. Scand. J. Immunol. 2022, 96, e13168. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, Y.; Wang, Y.; Zheng, Y.; Lai, X.; Westermann-Clark, E.; Cho, S.H.; Kong, W. Specific immunoglobulin E and immunoglobulin G4 toward major allergens of house-dust mite during allergen-specific immunotherapy. Am. J. Rhinol. Allergy 2017, 31, 156–160. [Google Scholar] [CrossRef]
- Feng, M.; Zeng, X.; Su, Q.; Shi, X.; Xian, M.; Qin, R.; Li, J. Allergen immunotherapy-induced immunoglobulin G4 reduces basophil activation in house dust mite-allergic asthma patients. Front. Cell Dev. Biol. 2020, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Qin, R.; Fu, W.; Huang, R.; Xian, M.; Guo, Y.; He, L.; Shi, X.; Li, J. Predicting allergen immunotherapy efficacy based on early maintenance phase response in routine clinical practice. World Allergy Organ. J. 2024, 17, 100986. [Google Scholar] [CrossRef]
- Aggarwal, P.; Senthilkumaran, S. Dust Mite Allergy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Gellrich, D.; Eder, K.; Högerle, C.; Becker, S.; Canis, M.; Gröger, M. De novo sensitization during subcutaneous allergen specific immunotherapy—An analysis of 51 cases of SCIT and 33 symptomatically treated controls. Sci. Rep. 2020, 10, 6048. [Google Scholar] [CrossRef]
- Baba, S.M.; Rasool, R.; Gull, A.; Qureshi, T.A.; Beigh, A.H.; Qadri, Q.; Shah, Z.A. Effectiveness of sublingual immunotherapy in the treatment of HDM-induced nasobronchial allergies: A 3-year randomized case-control study from Kashmir. Front. Immunol. 2021, 12, 723814. [Google Scholar] [CrossRef]
- Nittner-Marszalska, M.; Kopeć, A.; Foks-Ciekalska, A.; Lata, A.; Bogacz-Piaseczyńska, A.; Rosiek-Biegus, M.; Zajac, M.; Bożek, A. Monitoring of molecular profiling of allergen-antibody responses in HDM-immunotherapy patients. Hum. Vaccines Immunother. 2022, 18, 2148815. [Google Scholar] [CrossRef]
- Figo, D.D.; Cordeiro Macedo, P.R.; Gadermaier, G.; Remuzgo, C.; Castro, F.F.M.; Kalil, J.; Galvão, C.E.S.; Santos, K.S. IgE and IgG4 epitopes of Dermatophagoides and Blomia allergens before and after sublingual immunotherapy. Int. J. Mol. Sci. 2023, 24, 4173. [Google Scholar] [CrossRef]
- Aydogan, M.; Eifan, A.O.; Keles, S.; Akkoc, T.; Nursoy, M.A.; Bahceciler, N.N.; Barlan, I.B. Sublingual immunotherapy in children with allergic rhinoconjunctivitis mono-sensitized to house-dust-mites: A double-blind placebo-controlled randomised trial. Respir. Med. 2013, 107, 1322–1329. [Google Scholar] [CrossRef]
- Im, Y.H.; Kim, D.H.; Jeon, E.J.; Nam, I.C.; Lee, H.J.; Yu, K.J.; Kim, D.Y. Effect of house dust mite allergen on sleep parameters and sleep quality. Sleep Breath. 2023, 27, 2231–2239. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Quist, J.C.; Declercq, J.; Vanhee, S.; Lambrecht, B.N.; Gómez-Rial, J.; Vidal, C.; Aydogdu, E.; Rombauts, S.; Hernández-Crespo, P. RNA viruses alter house dust mite physiology and allergen production with no detected consequences for allergenicity. Insect Mol. Biol. 2023, 32, 173–186. [Google Scholar] [CrossRef] [PubMed]
- van der Borght, K.; Brimnes, J.; Haspeslagh, E.; Brand, S.; Neyt, K.; Gupta, S.; Knudsen, N.P.H.; Hammad, H.; Andersen, P.S.; Lambrecht, B.N. Sublingual allergen immunotherapy prevents house dust mite inhalant type 2 immunity through dendritic cell-mediated induction of Foxp3+ regulatory T cells. Mucosal Immunol. 2024, 17, 618–632. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhong, J.; Hu, J.; Cao, C.; Qi, S.; Ma, R.; Fu, W.; Zhang, X.; Akdis, C.A.; Gao, Y. IL-37 protects against house dust mite-induced airway inflammation and airway epithelial barrier dysfunction via inhibiting store-operated calcium entry. Int. Immunopharmacol. 2024, 138, 112525. [Google Scholar] [CrossRef]
- Liang, G.; Zhou, J.; Jiang, L.; Wang, W.; Wu, Q.; Gao, C.; Liu, W.; Li, S.; Feng, S.; Song, Z. Higher house dust mite-specific IgE levels among chronic spontaneous urticaria patients may implicate higher basophil reactivity. Int. Arch. Allergy Immunol. 2023, 184, 1126–1134. [Google Scholar] [CrossRef]
- Zolkipli, Z.; Roberts, G.; Cornelius, V.; Clayton, B.; Pearson, S.; Michaelis, L.; Djukanovic, R.; Kurukulaaratchy, R.; Arshad, S.H. Randomized controlled trial of primary prevention of atopy using house dust mite allergen oral immunotherapy in early childhood. J. Allergy Clin. Immunol. 2015, 136, 1541–1547.e11. [Google Scholar] [CrossRef]
- Jutel, M.; Brüggenjürgen, B.; Richter, H.; Vogelberg, C. Real-world evidence of subcutaneous allergoid immunotherapy in house dust mite-induced allergic rhinitis and asthma. Allergy 2020, 75, 2050–2058. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, C.L.; Yu, D.; Liu, Z. Roles of follicular helper and regulatory T cells in allergic diseases and allergen immunotherapy. Allergy 2021, 76, 456–470. [Google Scholar] [CrossRef]
- Agache, I.; Zemelka-Wiącek, M.; Shamji, M.H.; Jutel, M. Immunotherapy: State-of-the-art review of therapies and theratypes. J. Allergy Clin. Immunol. 2022, 150, 1279–1288. [Google Scholar] [CrossRef]
- Radzikowska, U.; Baerenfaller, K.; Cornejo-Garcia, J.A.; Karaaslan, C.; Barletta, E.; Sarac, B.E.; Zhakparov, D.; Villaseñor, A.; Eguiluz-Gracia, I.; Mayorga, C.; et al. Omics technologies in allergy and asthma research: An EAACI position paper. Allergy 2022, 77, 2888–2908. [Google Scholar] [CrossRef]
- Shamji, M.H.; Ollert, M.; Adcock, I.M.; Bennett, O.; Favaro, A.; Sarama, R.; Riggioni, C.; Annesi-Maesano, I.; Custovic, A.; Fontanella, S.; et al. EAACI guidelines on environmental science in allergic diseases and asthma—Leveraging artificial intelligence and machine learning to develop a causality model in exposomics. Allergy 2023, 78, 1742–1757. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qu, Z.; Wang, X.; Wang, Q.; Lv, Z.; Wang, W.; Ying, S.; Zhang, L.; Lan, F. House dust mite allergen directly activates ILC2 cells via the TLR4 signaling pathway in allergic airway diseases. Cell. Immunol. 2024, 405–406, 104884. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guo, L.; Zeng, Z.; Huang, Y.; Tang, H.; Hu, H.; Yuan, X.; Deng, J.; Qin, G.; Wang, X.; et al. Neferine attenuates HDM-induced allergic inflammation by inhibiting the activation of dendritic cell. Inflammation 2023, 46, 2433–2448. [Google Scholar] [CrossRef]
- Tong, Y.; Wang, L.; Wang, L.; Song, J.; Fan, J.; Lai, C.; Bao, J.; Weng, C.; Wang, Y.; Shuai, J.; et al. Allergen immunotherapy combined with Notch pathway inhibitors improves HDM-induced allergic airway inflammation and inhibits ILC2 activation. Front. Immunol. 2023, 14, 1264071. [Google Scholar] [CrossRef]
- Ramchandani, R.; Hossenbaccus, L.; Ellis, A.K. Immunoregulatory T cell epitope peptides for the treatment of allergic disease. Immunotherapy 2021, 13, 1283–1291. [Google Scholar] [CrossRef]
- Heldner, A.; Alessandrini, F.; Russkamp, D.; Heine, S.; Schnautz, B.; Chaker, A.; Mwange, J.; Carreno Velazquez, T.L.; Heath, M.D.; Skinner, M.A.; et al. Immunological effects of adjuvanted low-dose allergoid allergen-specific immunotherapy in experimental murine house dust mite allergy. Allergy 2022, 77, 907–919. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Li, J.; Hu, L.; Hu, Y.; Li, J.; Li, Y. Impact of the COVID-19 pandemic and lockdown measures on clinical visits and subjective symptoms in childhood allergic rhinitis induced by house dust mites in Shanghai. BMC Public Health 2024, 24, 3088. [Google Scholar] [CrossRef]
- Li, J.; Hu, Y.; Li, H.; Lin, Y.; Tong, S.; Li, Y. Assessing the impact of air pollutants on clinical visits for childhood allergic respiratory disease induced by house dust mite in Shanghai, China. Respir. Res. 2022, 23, 48. [Google Scholar] [CrossRef]
- Tempels-Pavlica, Ž.; Aarts, M.C.J.; Welsing, P.M.J.; van der Meer, A.N.; van der Zwan, L.P.; Uss, E.; Knulst, A.C. House dust mite sublingual allergen immunotherapy tablet is safe and well-tolerated in Dutch clinical practice. Front. Allergy 2024, 5, 1355324. [Google Scholar] [CrossRef]
- Harintajinda, S.; Klangkalya, N.; Kanchongkittiphon, W.; Rerkpattanapipat, T.; Kerddonfak, S.; Manuyakorn, W. Allergic rhinitis in remission with house dust mite subcutaneous immunotherapy. Asian Pac. J. Allergy Immunol. 2024. advance online publication. [Google Scholar] [CrossRef]
- Kim, J.; Boo, J.; Jang, H.; Jung, Y.W.; Kim, J.; Zhang, K.; Park, C.O. Combined Dupilumab and Allergen-Specific Immunotherapy in Severe Refractory Atopic Dermatitis. Allergy Asthma Immunol. Res. 2024, 16, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Boguniewicz, M.; Sher, L.D.; Paller, A.S.; Arkwright, P.D.; Yoshihara, S.; Chen, Z.; Shah, P.; Marco, A.R. Dupilumab is efficacious in young children with atopic dermatitis regardless of type 2 comorbidities. Adv. Ther. 2024, 41, 4601–4616. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wu, H.; Ye, Y.; Xu, Q.; Shao, J.; Bai, Z.; Zhou, Y.; Li, Z.; Liu, J.; Li, Z. Efficacy, safety, and early relapse after cessation of upadacitinib versus dupilumab in adolescents with moderate-to-severe atopic dermatitis: A real-world study in China. Dermatitis 2024, 35, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.J.; Karner, C.; Jhita, T.; Barton, S.; Marceniuk, G.; Yiu, Z.Z.N.; Wittmann, M. Abrocitinib, tralokinumab and upadacitinib for treating moderate-to-severe atopic dermatitis. Health Technol. Assess. 2024, 28, 1–113. [Google Scholar] [CrossRef]
- Scott, G.; Asrat, S.; Allinne, J.; Keat Lim, W.; Nagashima, K.; Birchard, D.; Srivatsan, S.; Ajithdoss, D.K.; Oyejide, A.; Ben, L.H.; et al. IL-4 and IL-13, not eosinophils, drive type 2 airway inflammation, remodeling and lung function decline. Cytokine 2023, 162, 156091. [Google Scholar] [CrossRef]
| Taxonomic Level | Taxon | Description |
|---|---|---|
| Cluster | Arachnida (arachnids) | Arachnids are a cluster of arthropods that includes organisms such as spiders, scorpions and mites. They are characterized by the presence of four pairs of legs, the absence of feelers and the division of the body into two main parts: the cephalothorax and the abdomen. |
| Order | Acariformes (proper mites) | Proper mites are one of the main orders of mites, encompassing many species with diverse ecology, including saprophytes, parasitoids and predators. |
| Family | Pyroglyphidae | The Pyroglyphidae family includes mites commonly found in the domestic environment, especially in dust. They are the main allergens responsible for allergic reactions in humans. Species:
|
| Family | Glycyphagidae | Mites of this family are often found in food warehouses but can also be found in homes, especially where organic products are stored. Species:
|
| Family | Acaridae | Mites in this family are commonly known as pests of food products, but they can also be found in house dust. Species:
|
| Family | Echimyopodidae | Mites in this family are less well known but can occur in the home environment and affect human health. Species:
|
| Allergen Group No. | Abbreviation by Species | IgE Reactivity | Biological and Clinical Significance | |
|---|---|---|---|---|
| D. pteronyssinus | D. farinae | |||
| 1 | Der p 1 | Der f 1 | 90% | These are proteolytic enzymes belonging to the cysteine protease group. Their action degrades intercellular junctions in the airway epithelium, which facilitates the penetration of allergens and the initiation of the immune response. Significantly higher levels are observed in children with asthma than in children without asthma. |
| 2 | Der p 2 | Der f 2 | 90% | These proteins act as modulators of the immune response. They bind lipids to the ML domain. Structurally, they resemble TLR4 receptors, thus stimulating innate response mechanisms and enhancing the allergic response. Significantly higher levels are observed in children with asthma than in children without asthma. |
| 3 | Der p 3 | Der f 3 | 55% | These are proteolytic enzymes with trypsin-like function. They have the ability to break down epithelial cell membranes, which increases the permeability of other allergens. |
| 4 | Der p 4 | Der f 4 | 15% | This is an alpha-amylase that can cross-react with other food allergens, such as those of plant origin. It causes allergic reactions in patients allergic to mites. High titers of IgE-binding anti-alpha amylase antibodies are observed in patients infected with scabies or reporting previous exposure to scabies. |
| 5 | Der p 5 | Der f 5 | 35% | These are proteins of unknown function, but they have been shown to strongly stimulate the immune system, leading to the production of IgE antibodies. Significantly higher levels are observed in children with asthma than in children without asthma. |
| 6 | Der p 6 | Der f 6 | 53% | Chymotrypsin is a proteolytic enzyme that can destroy the epithelial barrier in the respiratory tract. |
| 7 | Der p 7 | Der f 7 | 40% | These are proteins with a structure that resembles bacterial membranes, making their mechanism of action likely to resemble endotoxin activity. |
| 10 | Der p 10 | Der f 10 | 42% | Tropomyosin is a protein with high homology to seafood tropomyosins, which means it can cause cross-reactions in patients allergic to shellfish. |
| 11 | Der p 11 | Der f 11 | 45% | Paramyosin is a muscle protein of mites that shows the ability to stimulate the immune system. It is used clinically as a serological marker for HDM-associated atopic dermatitis. |
| 14 | Der p 14 | Der f 14 | 43% | Apolipoprotein, a protein with a potential role in the immune response. |
| 15 | Der p 15 | Der f 15 | 43% | Chitinase, a chitin-degrading enzyme that may play a role in allergic reactions. |
| 20 | Der p 20 | Der f 20 | 25% | Arginine kinase, potentially associated with allergic reactions in people with asthma. It has been clinically correlated with HDM-related asthma, as well as active scabies infection. |
| 23 | Der p 23 | Der f 23 | 77% | Peritrophin-like protein plays a key role in stabilizing the intestinal barrier of mites. Significantly higher levels are observed in children with asthma than in children without asthma. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurkiewicz, K.; Jutel, M.; Smolinska, S. Update on HDM Allergy: Principal Changes over the Years. Int. J. Mol. Sci. 2025, 26, 5660. https://doi.org/10.3390/ijms26125660
Jurkiewicz K, Jutel M, Smolinska S. Update on HDM Allergy: Principal Changes over the Years. International Journal of Molecular Sciences. 2025; 26(12):5660. https://doi.org/10.3390/ijms26125660
Chicago/Turabian StyleJurkiewicz, Krzysztof, Marek Jutel, and Sylwia Smolinska. 2025. "Update on HDM Allergy: Principal Changes over the Years" International Journal of Molecular Sciences 26, no. 12: 5660. https://doi.org/10.3390/ijms26125660
APA StyleJurkiewicz, K., Jutel, M., & Smolinska, S. (2025). Update on HDM Allergy: Principal Changes over the Years. International Journal of Molecular Sciences, 26(12), 5660. https://doi.org/10.3390/ijms26125660








