Cherry Juice Improves Memory and Anxiety by Modulating Cell Number in the Hippocampus of Male Rat Pups Infected with Lipopolysaccharides During Gestation and Gestation-Lactation
Abstract
1. Introduction
2. Results
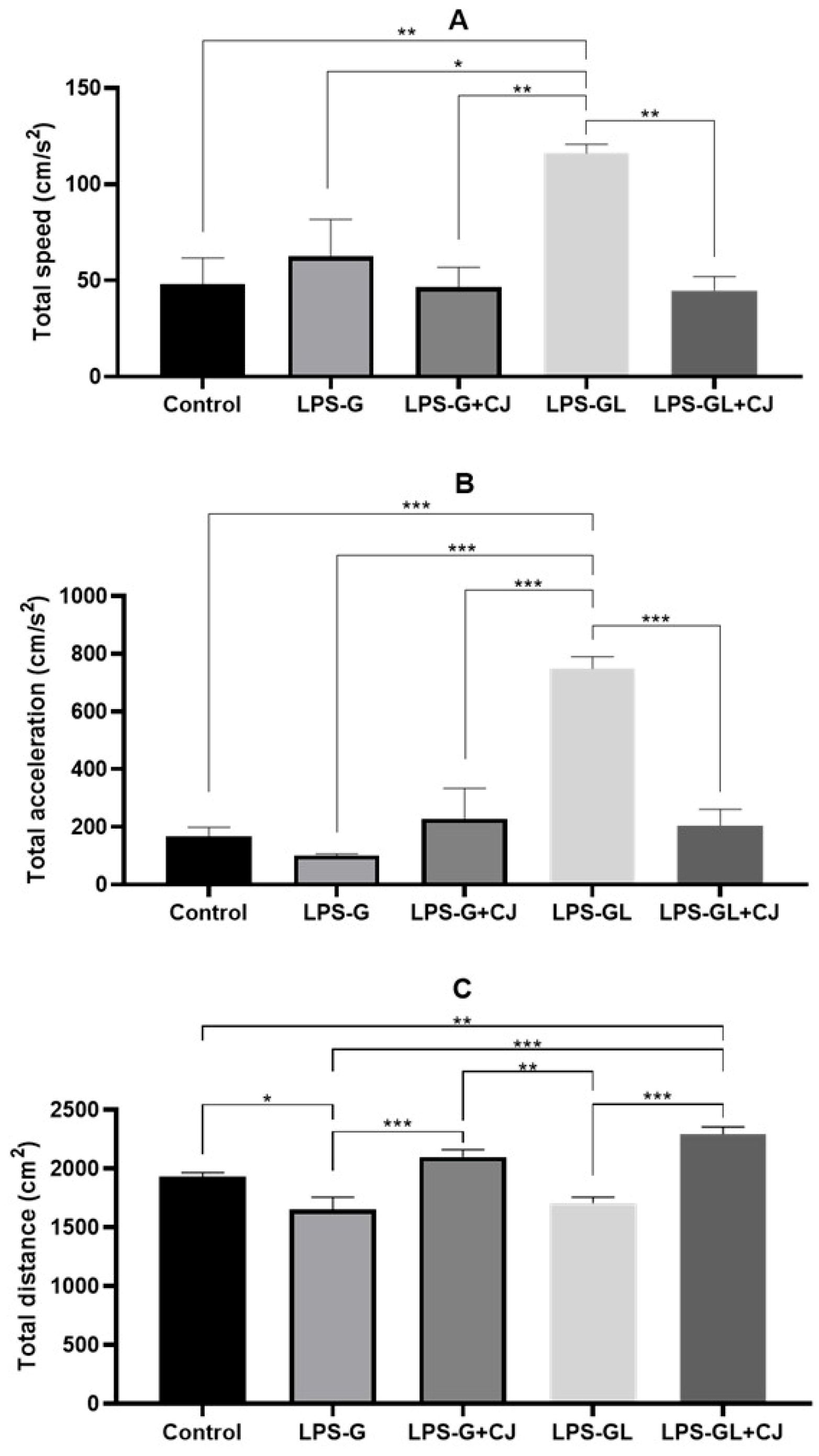
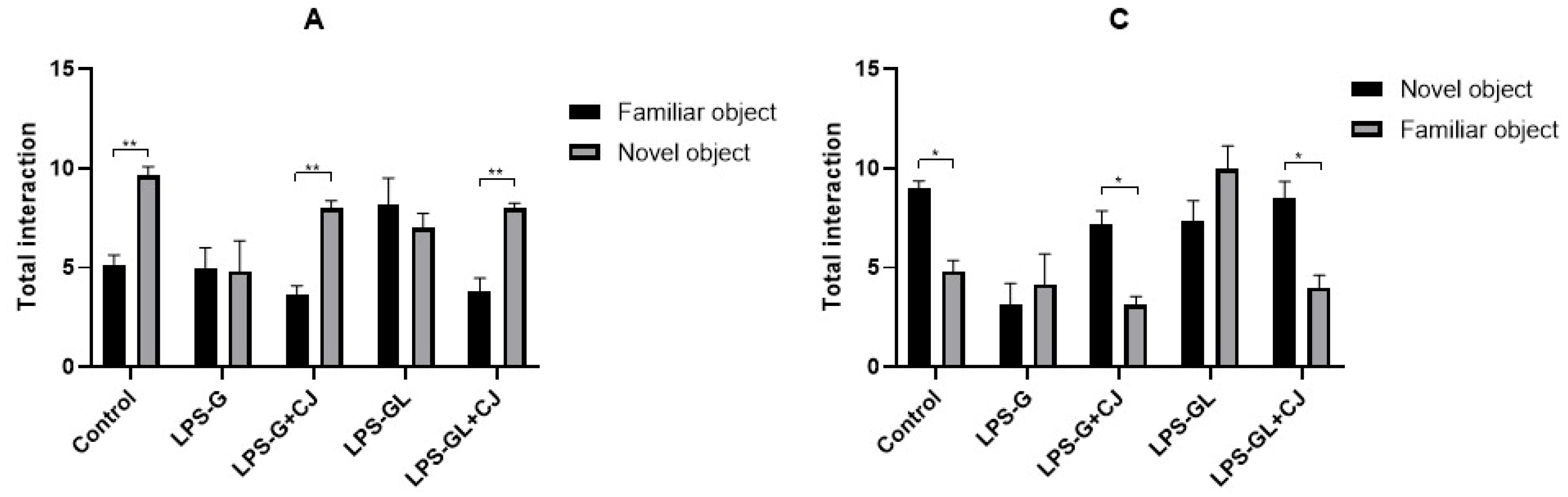
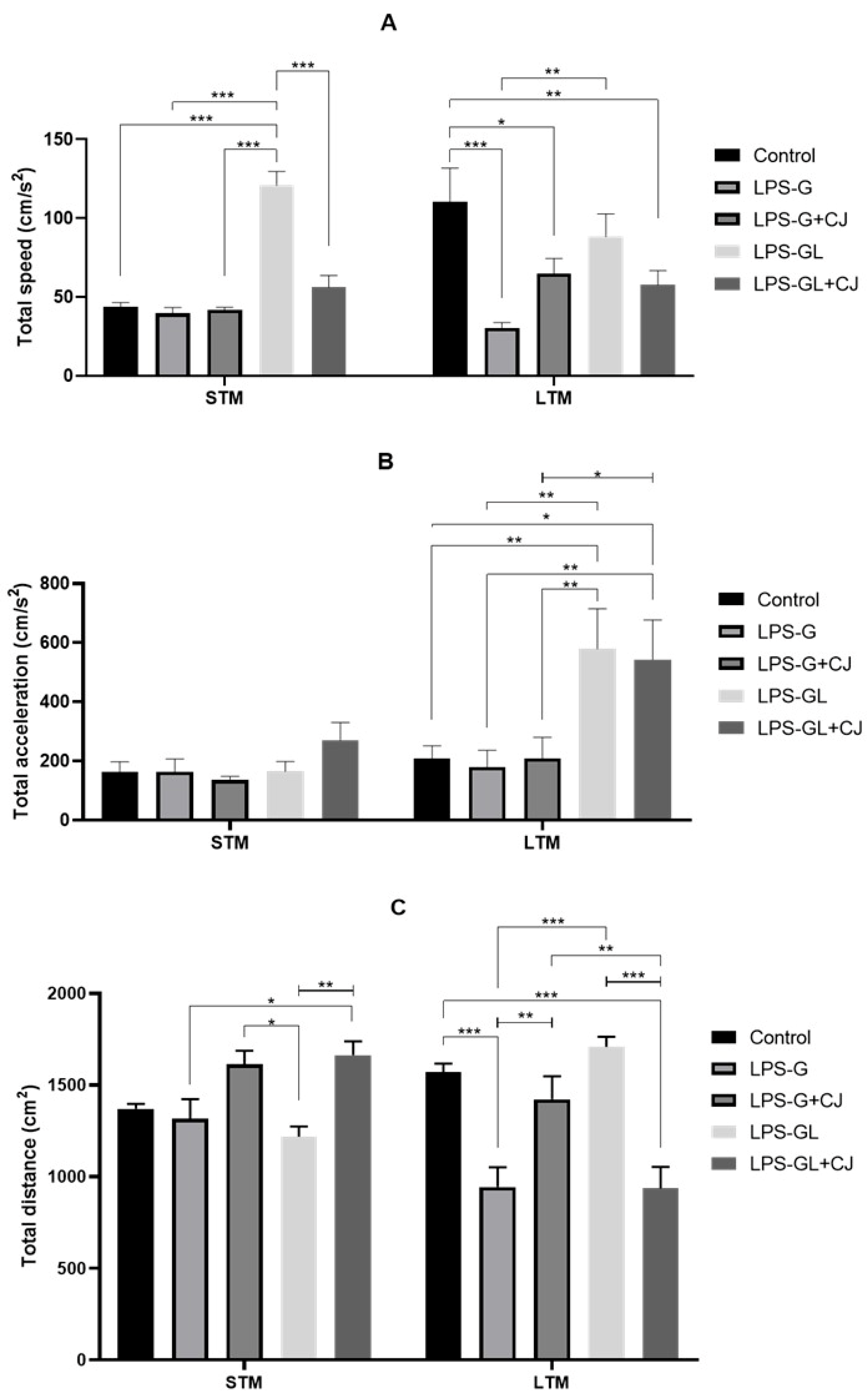
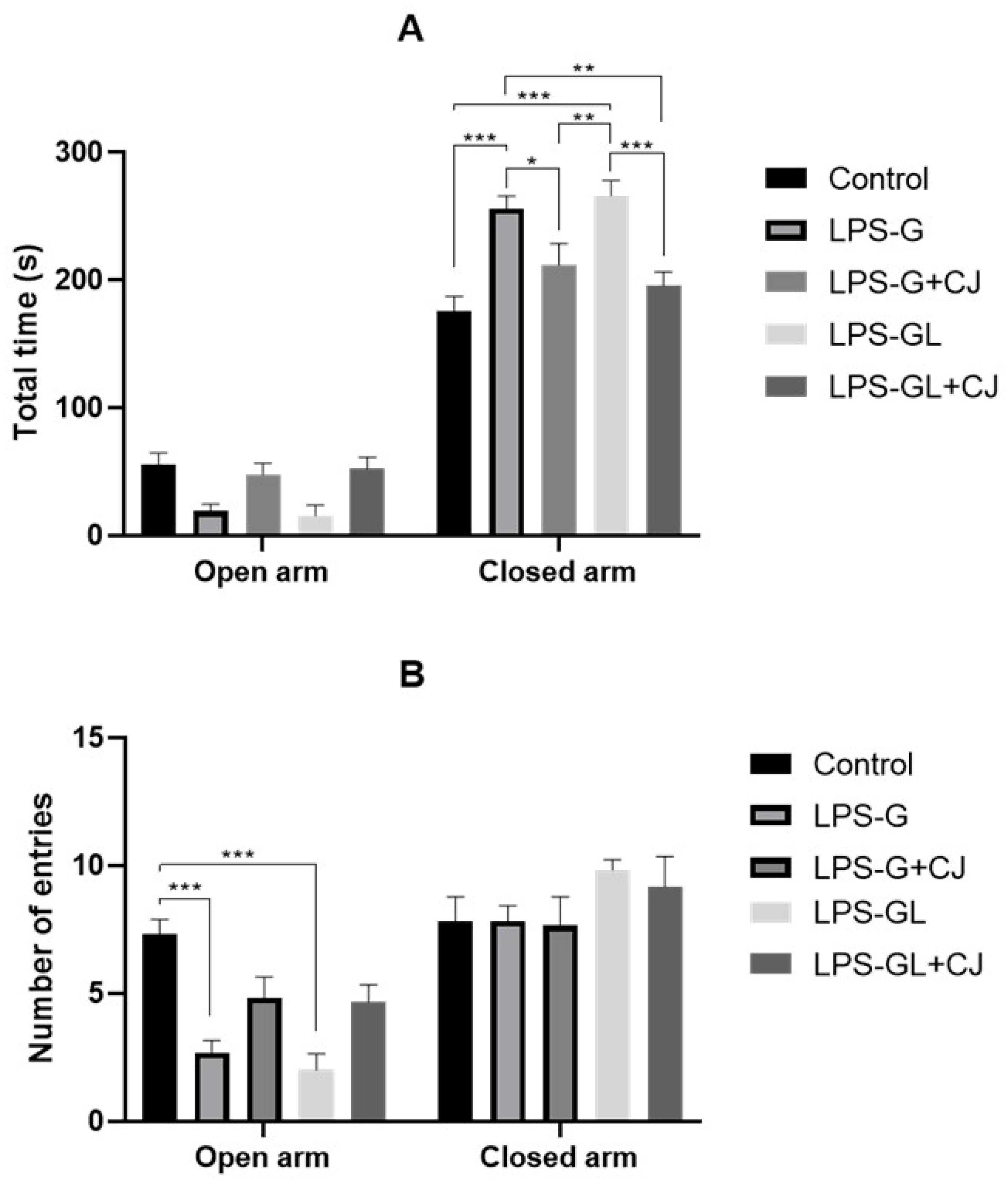
2.1. Effects of Cherry Juice in Maternal Infection by LPS on Behavioral Response
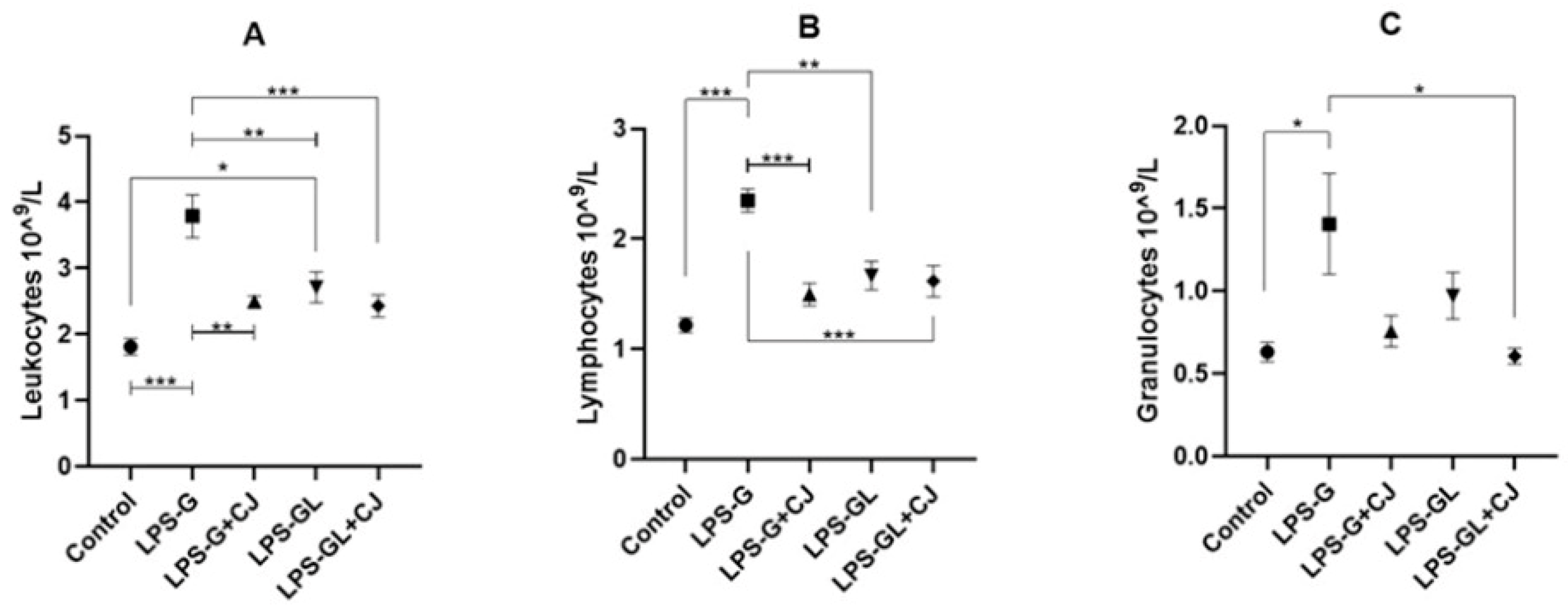
2.2. Leukocyte Analysis
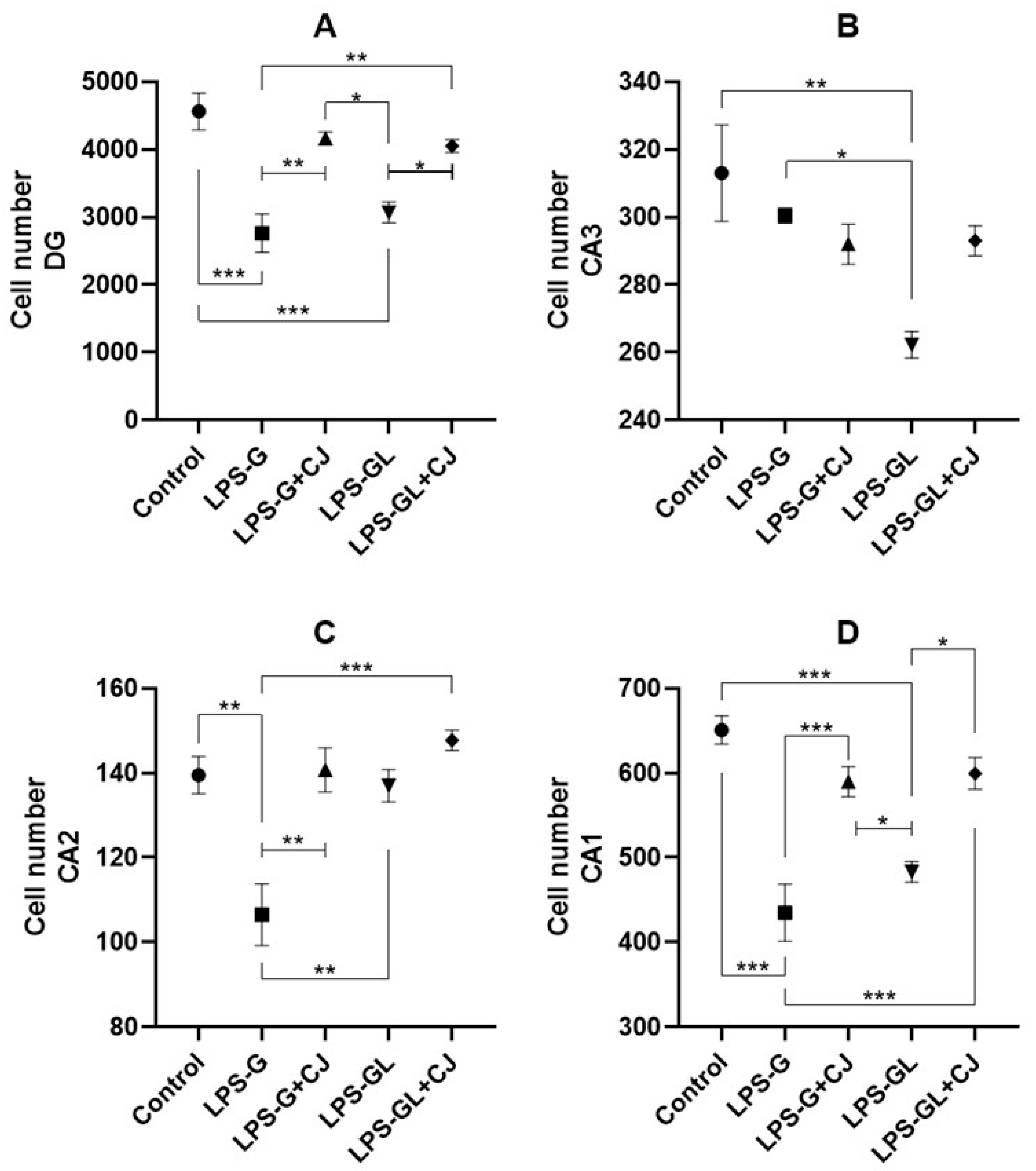
2.3. Cell Counts of Hippocampal Regions
3. Discussion
4. Materials and Methods
4.1. Preparation of Cherry Juice
4.2. Animal Study
4.3. Behavioral Studies
4.4. Leukocyte Analysis
4.5. Hippocampal Cell Count
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leclercq, S.O.; Branger, M.; Smith, D.G.E.; Germon, P. Lipopolysaccharide core type diversity in the Escherichia coli species in association with phylogeny, virulence gene repertoire and distribution of type vi secretion systems. Microb. Genom. 2021, 7, 000652. [Google Scholar] [CrossRef] [PubMed]
- Rosadini, C.V.; Kagan, J.C. Early innate immune responses to bacterial LPS. Curr. Opin. Immunol. 2017, 44, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Mouihate, A.; Mehdawi, H. Toll-like receptor 4-mediated immune stress in pregnant rats activates STAT3 in the fetal brain: Role of interleukin-6. Pediatr. Res. 2016, 79, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Kell, D.B.; Pretorius, E. The Role of lipopolysaccharide-induced cell signalling in chronic inflammation. Chronic Stress. 2022, 6, 24705470221076390. [Google Scholar] [CrossRef]
- Urakubo, A.; Jarskog, L.F.; Lieberman, J.A.; Gilmore, J.H. Prenatal exposure to maternal infection alters cytokine expression in the placenta, amniotic fluid, and fetal brain. Schizophr. Res. 2001, 47, 27–36. [Google Scholar] [CrossRef]
- Gayle, D.A.; Beloosesky, R.; Desai, M.; Amidi, F.; Nuñez, S.E.; Ross, M.G. Maternal LPS induces cytokines in the amniotic fluid and corticotropin releasing hormone in the fetal rat brain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R1024-9. [Google Scholar] [CrossRef]
- Xu, M.; Sulkowski, Z.L.; Parekh, P.; Khan, A.; Chen, T.; Midha, S.; Iwasaki, T.; Shimokawa, N.; Koibuchi, N.; Zavacki, A.M.; et al. Effects of perinatal lipopolysaccharide (lps) exposure on the developing rat brain; modeling the effect of maternal infection on the developing human CNS. Cerebellum 2013, 12, 572–586. [Google Scholar] [CrossRef]
- Hudalla, H.; Karenberg, K.; Kuon, R.J.; Pöschl, J.; Tschada, R.; Frommhold, D. LPS-induced maternal inflammation promotes fetal leukocyte recruitment and prenatal organ infiltration in mice. J. Pediatr. Res. 2018, 84, 757–764. [Google Scholar] [CrossRef]
- Truong, V.L.; Jeong, W.S. Antioxidant and anti-inflammatory roles of tea polyphenols in inflammatory bowel diseases. Food Sci. Hum. Well 2022, 11, 502–511. [Google Scholar] [CrossRef]
- Singh, A.; Yau, Y.F.; Leung, K.S.; El-Nezami, H.; Lee, J.C.Y. Interaction of polyphenols as antioxidant and anti-inflammatory compounds in brain–liver–gut axis. Antioxidants 2020, 9, 669. [Google Scholar] [CrossRef]
- Nani, A.; Murtaza, B.; Sayed Khan, A.; Khan, N.A.; Hichami, A. Antioxidant and Anti-Inflammatory Potential of polyphenols contained in mediterranean diet in obesity: Molecular mechanisms. Molecules 2021, 26, 985. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary polyphenols and their role in oxidative stress-induced human diseases: Insights into protective effects, antioxidant potentials and mechanism(s) of action. Front. Pharmacol. 2022, 13, 2022. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Ortíz, M.; Reza-Vargas, M.D.C.; Chew-Madinaveita, R.G.; Meza-Velázquez, J.A. Propiedades funcionales de las antocianinas. Biotecnia 2011, 13, 16–22. [Google Scholar] [CrossRef]
- Coronado, H.M.; Vega, S.; Rey, G.L.; Vázquez, F.M.; Radilla, V.C. Antioxidantes: Perspectiva actual para la salud humana. Rev. Chil. Nutr. 2015, 42, 206–212. [Google Scholar] [CrossRef]
- Chala, D.; Sabadashka, M.; Morozovych, A.; Krychowiak-Maśnicka, M.; Królicka, A.; Sybirna, N. Immunomodulatory and antibacterial effect of red wine concentrate rich in a natural complex of polyphenols under diabetes mellitus. Biomed. Pharmacother. 2024, 170, 116023. [Google Scholar] [CrossRef]
- Pontes, P.B.; Toscano, A.E.; Lacerda, D.C.; da Silva Araújo, E.R.; Costa, P.C.T.; da Alves, S.M.; Manhães de Castro, R. Effectiveness of polyphenols on perinatal brain damage: A systematic review of preclinical studies. Foods 2023, 12, 2278. [Google Scholar] [CrossRef]
- González-Nieto, P.F.; Alvarado-Olivarez, M.; Guzmán-Gerónimo, R.I.; Rodríguez-Landa, J.F.; Hernández-Salazar, L.T. Effect of traditional and non-traditionally processed blue corn tortilla consumption during the gestation of rats in the dentate gyrus of pups. Foods 2024, 13, 3639. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Guo, Z.; Lei, J.; Wang, Y. Pomegranate polyphenol punicalin ameliorates lipopolysaccharide-induced memory impairment, behavioral disorders, oxidative stress, and neuroinflammation via inhibition of TLR4-NF-κB pathway. Phytother. Res. 2024, 38, 3489–3508. [Google Scholar] [CrossRef]
- Es-Safi, I.; Mechchate, H.; Amaghnouje, A.; Kamaly, O.M.; Jawhari, F.Z.; Imtara, H.; Grafov, A.; Bousta, D. The potential of parsley polyphenols and their antioxidant capacity to help in the treatment of depression and anxiety: An in vivo subacute study. Molecules 2021, 26, 2009. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Vauzour, D. Dietary polyphenols as modulators of brain functions: Biological actions and molecular mechanisms underpinning their beneficial effects. Oxid. Med. Cell Longev. 2012, 2012, 914273. [Google Scholar] [CrossRef] [PubMed]
- Ahles, S.; Joris, P.J.; Plat, J. Effects of berry anthocyanins on cognitive performance, vascular function and cardiometabolic risk markers: A systematic review of randomized placebo-controlled intervention studies in humans. Int. J. Mol. Sci. 2021, 22, 6482. [Google Scholar] [CrossRef] [PubMed]
- Kimble, R.; Keane, K.M.; Lodge, J.K.; Cheung, W.; Haskell-Ramsay, C.F.; Howatson, G. Polyphenol-rich tart cherries (Prunus Cerasus, cv Montmorency) improve sustained attention, feelings of alertness and mental fatigue and influence the plasma metabolome in middle-aged adults: A randomised, placebo-controlled trial. Br. J. Nutr. 2022, 128, 2409–2420. [Google Scholar] [CrossRef]
- Vírgen Gen, J.J.; Guzmán-Gerónimo, R.I.; Martínez-Flores, K.; Martínez-Nava, G.A.; Fernández-Torres, J.; Zamudio-Cuevas, Y. Cherry extracts attenuate inflammation and oxidative stress triggered by monosodium urate crystals in THP-1 cells. J. Food Biochem. 2020, 44, e13403. [Google Scholar] [CrossRef]
- Ruiz-Martínez, S.M.; Guzmán-Gerónimo, R.I.; Alvarado-Olivarez, M.; Santiago-Roque, I.; Palma-Jacinto, J.A. Effect of blackberry juice consumption by pregnant rats on brain length and cell density of dentate gyrus in male Wistar pups. J. Med. Food 2023, 27, 901–904. [Google Scholar] [CrossRef]
- Yates, D.T.; Löest, C.A.; Ross, T.T.; Hallford, D.M.; Carter, B.H.; Limesand, S.W. Effects of bacterial lipopolysaccharide injection on white blood cell counts, hematological variables, and serum glucose, insulin, and cortisol concentrations in ewes fed low- or high-protein diets. J. Anim. Sci. 2011, 89, 4286–4293. [Google Scholar] [CrossRef] [PubMed]
- Peñailillo, A.K.; Sepulveda, M.A.; Palma, C.J.; Espinoza, A.; Aguilera, M.; Burgos, R.A.; Carretta, D.; Islas, A.; Pérez, R. Haematological and blood biochemical changes induced by the administration of low doses of Escherichia coli lipopolysaccharide in rabbits. Arch. Med. Vet. 2016, 48, 315–320. [Google Scholar] [CrossRef]
- Faas, M.M.; Moes, H.; van der Schaaf, G.; de Leij, L.F.M.H.; Heineman, M.J. Total white blood cell counts and LPS-induced TNFα production by monocytes of pregnant, pseudopregnant and cyclic rats. J. Reprod. Immunol. 2003, 59, 39–52. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhang, X.; Hu, X.; Li, Y. Effects of repeated lipopolysaccharide treatment on growth performance, immune organ index, and blood parameters of Sprague-Dawley rats. J. Vet. Res. 2018, 62, 341–346. [Google Scholar] [CrossRef]
- Kirsten, T.B.; Lippi, L.L.; Bevilacqua, E.; Bernardi, M.M. LPS exposure increases maternal corticosterone levels, causes placental injury and increases IL-1β levels in adult rat offspring: Relevance to autism. PLoS ONE 2013, 8, e82244. [Google Scholar] [CrossRef]
- Kasawara, K.T.; Cotechini, T.; Macdonald-Goodfellow, S.K.; Surita, F.G.; Pinto e Silva, J.L.; Tayade, C.; Othman, M.; Ozolins, T.R.S.; Graham, C.H. Moderate exercise attenuates lipopolysaccharide-induced inflammation and associated maternal and fetal morbidities in pregnant rats. PLoS ONE 2016, 11, e0154405. [Google Scholar] [CrossRef] [PubMed]
- Borda, M.G.; Sanchis, P.L.; Baldera, J.P.; Tarazona-Santabalbina, F.J.; Chavarro-Carvajal, D.A.; Salazar-Londoño, S.; Bocharova, M.; Aarsland, D.; Martín-Marco, A. Assessing neutrophil-to-lymphocyte ratio as a nutritional indicator in community-dwelling older adults. Arch. Med. Res. 2024, 55, 103003. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, E.K.; Reham, R.I.; Ahmed, A.A.; Khattab, M.A.; Chen, L.Y.; Lai, K.H.; El Shaarawy, F.S.; Tawfik, N.F.; Moharram, F.A. Quercus coccinea Münchh leaves polyphenols: Appraisal acute lung injury induced by lipopolysaccharide in mice. Biomed. Pharmacother. 2023, 164, 114765. [Google Scholar] [CrossRef]
- Wischhof, L.; Irrsack, E.; Osorio, C.; Koch, M. Prenatal LPS-exposure—A neurodevelopmental rat model of schizophrenia--differentially affects cognitive functions, myelination and parvalbumin expression in male and female offspring. Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 3, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Romero, E.; Guaza, C.; Castellano, B.; Borrel, J. Ontogeny of sensorimotor gating and immune impairment induced by prenatal immune challenge in rats: Implications for the etiopathology of schizophrenia. Mol. Psychiatry 2010, 15, 372–383. [Google Scholar] [CrossRef]
- Watanobe, H.; Yoneda, M. A mechanism underlying the sexually dimorphic ACTH response to lipopolysaccharide in rats: Sex steroid modulation of cytokine binding sites in the hypothalamus. J. Physiol. 2003, 547, 221–232. [Google Scholar] [CrossRef]
- Spencer, S.J.; Boissé, L.; Mouihate, A.; Pittman, Q.J. Long term alterations in neuroimmune responses of female rats after neonatal exposure to lipopolysaccharide. Brain Behav. Immun. 2006, 20, 325–330. [Google Scholar] [CrossRef]
- Thangthaeng, N.; Poulose, S.M.; Gomes, S.M.; Miller, M.G.; Bielinski, D.F.; Shukitt-Hale, B. Tart cherry supplementation improves working memory, hippocampal inflammation, and autophagy in aged rats. Age 2016, 38, 393–404. [Google Scholar] [CrossRef]
- Ockermann, P.; Headley, L.; Lizio, R.; Hansmann, J. A review of the properties of anthocyanins and their influence on factors affecting cardiometabolic and cognitive health. Nutrients 2021, 13, 2831. [Google Scholar] [CrossRef]
- Kent, K.; Charlton, K.; Roodenrys, S.; Batterham, M.; Potter, J.; Traynor, V.; Gilbert, H.; Morgan, O.; Richards, R. Consumption of anthocyanin-rich cherry juice for 12 weeks improves memory and cognition in older adults with mild-to-moderate dementia. Eur. J. Nut. 2017, 56, 333–334. [Google Scholar] [CrossRef]
- Carvalho, F.B.; Gutierres, J.M.; Bueno, A.; Agostinho, P.; Zago, A.M.; Vieira, J.; Cechella, J.L.; Nogueira, C.W.; Oliveira, S.M.; Rizzi, C.; et al. Anthocyanins control neuroinflammation and consequent memory dysfunction in mice exposed to lipopolysaccharide. Mol. Neurobiol. 2017, 54, 3350–3367. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.A.; Harte, M.K.; Stenson, G.; Reynold, G.P. Neonatal lipopolysaccharide induces pathological changes in parvalbumin immunoreactivity in the hippocampus of the rat. Behav. Brain Res. 2009, 28, 355–359. [Google Scholar] [CrossRef]
- Vargas-Caraveo, A.; Sayd, A.; Maus, S.R.; Caso, J.R.; Madrigal, J.L.M.; García-Bueno, B.; Leza, J.C. Lipopolysaccharide enters the rat brain by a lipoprotein-mediated transport mechanism in physiological conditions. Sci. Rep. 2017, 7, 13113. [Google Scholar] [CrossRef]
- Buffler, R.R.C. Power measurement test procedures. In Micro-Wave Cooking and Processing; Engineering Fundamentals for the Food Scientist, 1st ed.; AVI Books: New York, NY, USA, 1992; pp. 157–158. [Google Scholar]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- NOM-062-ZOO-1999; Especificaciones Técnicas para la Reprodución Cuidado y Uso de Animales de Laboratorio D.O.F. 22-VIII-2001. Norma Oficial Mexicana: Ciudad de México, México, 2001; pp. 107–163.
- Tishkina, A.; Stepanichev, M.; Kudryashova, I.; Freiman, S.; Onufriev, M.; Lazareva, N.; Gulyaeva, N. Neonatal proinflammatory challenge in male Wistar rats: Effects on behavior, synaptic plasticity, and adrenocortical stress response. Behav. Brain Res. 2016, 304, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nair, A.B.; Morsy, M.A. Dose conversion between animals and humans: A practical solution. Indian. J. Pharm. Educ. Res. 2022, 56, 600–607. [Google Scholar] [CrossRef]
- Losso, J.N.; Finley, J.W.; Karki, N.; Liu, A.G.; Prudente, A.; Tipton, R.; Yu, Y.; Greenway, F.L. Pilot Study of the tart cherry juice for the treatment of insomnia and investigation of mechanisms. Am. J. Ther. 2018, 25, e194–e201. [Google Scholar] [CrossRef]
- Pérez-Escudero, A.; Vicente-Page, J.; Hinz, R.C.; Arganda, S.; de Polavieja, G.G. idTracker: Tracking individuals in a group by automatic identification of unmarked animals. Nat. Methods 2014, 11, 743–748. [Google Scholar] [CrossRef]
- León, A.; Tamayo Tamayo, J.; Hernández Eslava, V.; Toledo Hernández, P.; Avendaño Garrido, M.L.; Hernández Linares, C.A.; Escamilla Navarro, E. MOTUS: Software para el análisis conductual de patrones de desplazamiento. MJBA 2020, 46, 222–242. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain Stereotaxic Coordinates, 6th ed.; Academic Press: San Diego, CA, USA, 2006. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Virgen-Gen, J.J.; Alvarado-Olivarez, M.; Guzmán-Gerónimo, R.I.; González-Nieto, P.F.; Gutiérrez-García, A.G.; Oliart-Ros, R.M. Cherry Juice Improves Memory and Anxiety by Modulating Cell Number in the Hippocampus of Male Rat Pups Infected with Lipopolysaccharides During Gestation and Gestation-Lactation. Int. J. Mol. Sci. 2025, 26, 5642. https://doi.org/10.3390/ijms26125642
Virgen-Gen JJ, Alvarado-Olivarez M, Guzmán-Gerónimo RI, González-Nieto PF, Gutiérrez-García AG, Oliart-Ros RM. Cherry Juice Improves Memory and Anxiety by Modulating Cell Number in the Hippocampus of Male Rat Pups Infected with Lipopolysaccharides During Gestation and Gestation-Lactation. International Journal of Molecular Sciences. 2025; 26(12):5642. https://doi.org/10.3390/ijms26125642
Chicago/Turabian StyleVirgen-Gen, Juan J., Mayvi Alvarado-Olivarez, Rosa I. Guzmán-Gerónimo, Paola F. González-Nieto, Ana G. Gutiérrez-García, and Rosa M. Oliart-Ros. 2025. "Cherry Juice Improves Memory and Anxiety by Modulating Cell Number in the Hippocampus of Male Rat Pups Infected with Lipopolysaccharides During Gestation and Gestation-Lactation" International Journal of Molecular Sciences 26, no. 12: 5642. https://doi.org/10.3390/ijms26125642
APA StyleVirgen-Gen, J. J., Alvarado-Olivarez, M., Guzmán-Gerónimo, R. I., González-Nieto, P. F., Gutiérrez-García, A. G., & Oliart-Ros, R. M. (2025). Cherry Juice Improves Memory and Anxiety by Modulating Cell Number in the Hippocampus of Male Rat Pups Infected with Lipopolysaccharides During Gestation and Gestation-Lactation. International Journal of Molecular Sciences, 26(12), 5642. https://doi.org/10.3390/ijms26125642









