The Role of the Tumor Microenvironment in Gastroenteropancreatic Neuroendocrine Tumors
Abstract
1. Introduction
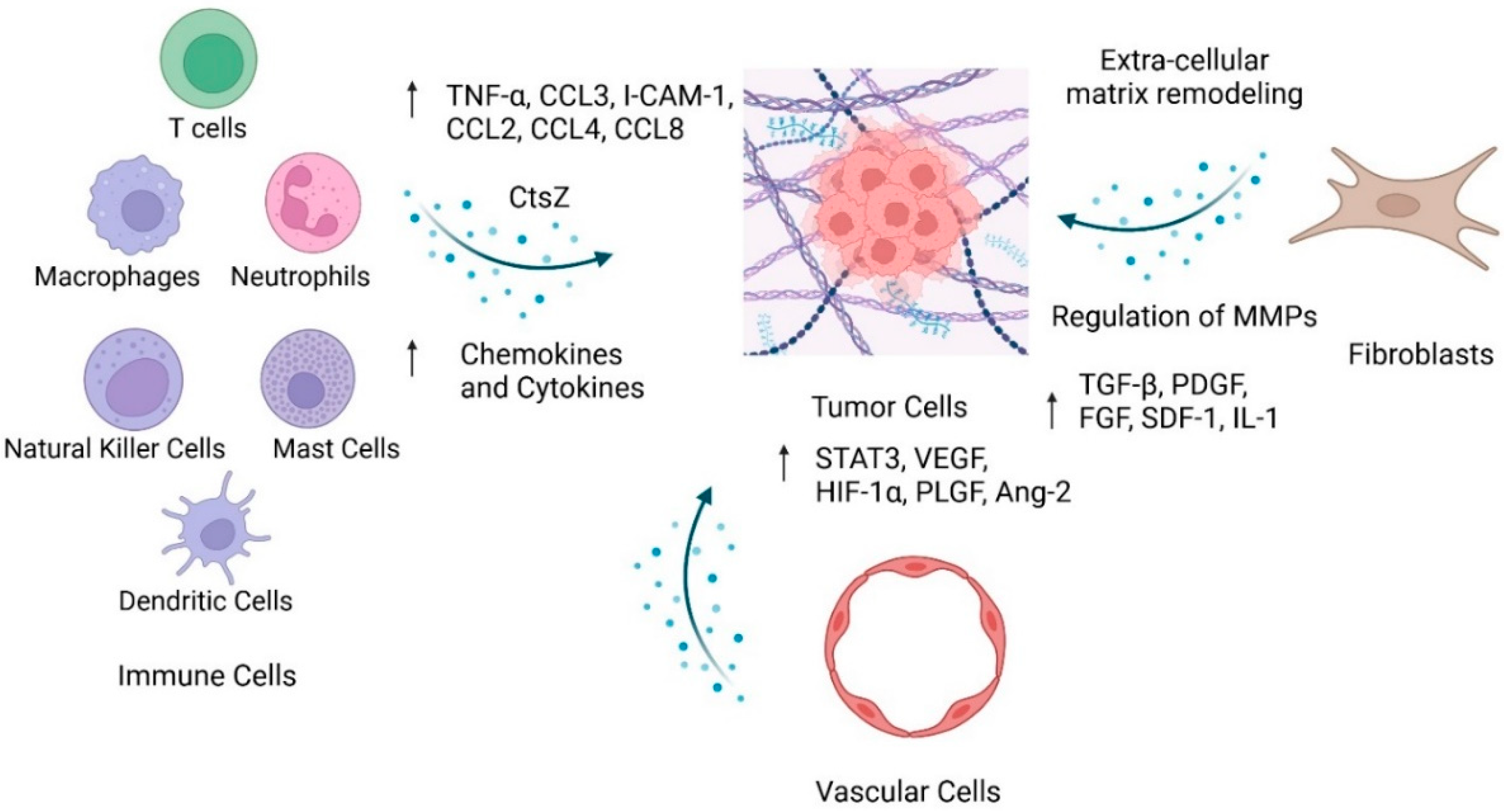
2. Components of the Tumor Microenvironment
2.1. Immune Cells
2.1.1. Macrophages
| Cell Population | Tumor-Supportive | Tumor-Suppressive |
|---|---|---|
| Tumor-Associated Macrophages (TAMs) | Colony stimulating factor (CSF-1) [20] Cathepsin Z (CtsZ) [21] CEACAM5 [21] Tie-2 [23] | |
| Tumor-Associated Neutrophils | Cyp46a1 [24] Hypoxia inducible factor 1-α (HIF 1-α) [24] | |
| Mast Cells | Stem cell factor (SCF) [25] Myc [26] Bruton tyrosine kinase (Btk) [26] | |
| Dendritic Cells | Toll-like receptor 3 (TLR3) [27] FMS-like tyrosine kinase 3 ligand (FLT3LG) [28] | |
| T Lymphocytes | PD-1/PD-L1 pathway [24] HHLA2 [29] H4 (B7x) [29] | CD47 [30] |
| Vascular Endothelial Cells (VECs) | Heparanase [31] VEGF/VEGFR-2 [32] CYR61 [32] VEGFC [33] VEGFR-2 [33] VEGF-B [34] RSUME [35] Cut homeobox 1 (CUX 1) [36] PDGFRα and PDGFRβ [37,38] | Neutropilin-2 (NRP-2) [34] |
| Cancer-Associated Fibroblasts (CAFs) | mTOR [39] STAT3 [40] TGF-β (β-1, β-2, β-3) [41] PDGF [42] bFGF [43,44] Thrombin, bombesin, bradykinin, and vasopressin [45] 5-HT’s [46] Stromal cell-derived factor-1 (SDF-1) [47] |
2.1.2. Neutrophils
2.1.3. Natural Killer Cells
2.1.4. Mast Cells
2.1.5. Myeloid-Derived Suppressor Cells
2.1.6. Dendritic Cells
2.1.7. T Lymphocytes
2.1.8. B Lymphocytes
2.1.9. Clinical Trials on the TIME
| Therapy Type | Patient Population | Therapy | Therapy Class and Target | Primary Endpoint |
|---|---|---|---|---|
| Immunotherapy | Well-differentiated or moderately differentiated PNETs GEP-NETs | Pembrozilumab (Keytruda®) | Monoclonal antibody for PD-1 | Phase 1: 12% ORR [79] Phase 2: 3.7% ORR and 39.3% 6-month PFS [80] |
| Advanced GEP-NETs | Spartalizumab | Monoclonal antibody for PD-1 | Phase 2: 7.4% partial response and 55.8% stable disease [81] | |
| All PNETs Low/intermediate PNETs | Ipilimumab (Yervoy®) + Nivolumab (Opdivo®) | Monoclonal antibody for CTLA-4 (Ipi) and PD-1 (Nivo) | Phase 2: 43% ORR [83] Phase 2: 0% ORR [82] | |
| Advanced GEP-NETs | Regorafenib + Avelumab | Multikinase Inhibitor and PD-1 | Phase 2: 18% ORR 5.5-month PFS [84] | |
| Anti-angiogenic therapy | GEP-NETs | Sunitinib (Sutent®) | Receptor tyrosine kinase inhibitor for VEGFR, PDGFR, and RET. | Phase 2: 81.1% 1-year survival rate [85] Phase 3: 9.3% ORR [86] |
| Surufatinib | Small-molecule tyrosine kinase inhibitor for VEGFR, FGFR1 | Phase 1b/2: 19% ORR and 92% PFS [87] | ||
| Axitinib (Inlyta®) | Small-molecule kinase inhibitor for VEGFR, c-Kit, PDGFR | Phase 2: 70% Stable Disease [88] | ||
| GEP-NETs Lower-grade PNETs | Pazopanib (Votrient®) | Receptor tyrosine kinase inhibitor for VEGFR, PDGFR, c-KIT, FGFR | Phase 2: 59.5% 6-month PFS [89] Phase 2: 21.9% ORR [90] | |
| GEP-NETs | Cabozantinib (Cabometyx®) | Small-molecule tyrosine kinase inhibitor for HGFR, VEGFR2, AXL, RET, FLT3 | Phase 3: 5% ORR extrapancreatic NET, 19% pancreatic NET [91] | |
| PNETs (VHL mutation) | Belzutifan (Welireg®) | Small-molecule inhibitor for HIF-2α | Phase 2: 91% ORR [92,93] | |
| Low-grade PNETs Advanced extra-PNETs | Bevacizumab (Avastin®) + Temozolomide (Temodar®) + Temsirolimus (Torisel®) | Monoclonal antibody for VEGF | Phase 2: 15% ORR, 14.3 months PFS [94] Phase 2: 41% ORR, 13.2 PFS [95] Phase 2: 2% ORR, 7.1 months PFS [96] | |
| Anti-angiogenic + Immunotherapy | Bevacizumab (Avastin®) + Atezolizumab (Tecentriq®) | Monoclonal antibody for VEGF (Bev) and PD-L1 (Atez) | Phase 2: 20% ORR [97] |
2.2. Vascular Cells
Clinical Trials Targeting VECs
2.3. Fibroblasts
Therapeutic Targeting of Cancer-Associated Fibroblasts
2.4. Extracellular Matrix (ECM)
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Sultana, Q.; Kar, J.; Verma, A.; Sanghvi, S.; Kaka, N.; Patel, N.; Sethi, Y.; Chopra, H.; Kamal, M.A.; Greig, N.H. A Comprehensive Review on Neuroendocrine Neoplasms: Presentation, Pathophysiology and Management. J. Clin. Med. 2023, 12, 5138. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Wu, Z.H.; Lai, J.P. New insights in diagnosis and treatment of gastroenteropancreatic neuroendocrine neoplasms. World J. Gastroenterol. 2022, 28, 1751–1767. [Google Scholar] [CrossRef] [PubMed]
- Mafficini, A.; Scarpa, A. Genetics and Epigenetics of Gastroenteropancreatic Neuroendocrine Neoplasms. Endocr. Rev. 2019, 40, 506–536. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, W.; Lu, J.; Pan, G.; Pan, Z.; Chen, Q.; Liu, W.; Zhao, Y. Gastric Neuroendocrine Tumors (G-Nets): Incidence, Prognosis and Recent Trend Toward Improved Survival. Cell Physiol. Biochem. 2018, 45, 389–396. [Google Scholar] [CrossRef]
- Lamberti, G.; Panzuto, F.; Pavel, M.; O’Toole, D.; Ambrosini, V.; Falconi, M.; Garcia-Carbonero, R.; Riechelmann, R.P.; Rindi, G.; Campana, D. Gastric neuroendocrine neoplasms. Nat. Rev. Dis. Primers 2024, 10, 25. [Google Scholar] [CrossRef]
- Scott, A.T.; Howe, J.R. Management of Small Bowel Neuroendocrine Tumors. Surg. Oncol. Clin. 2020, 29, 223–241. [Google Scholar] [CrossRef]
- Sonbol, M.B.; Mazza, G.L.; Mi, L.; Oliver, T.; Starr, J.; Gudmundsdottir, H.; Cleary, S.P.; Hobday, T.; Halfdanarson, T.R. Survival and Incidence Patterns of Pancreatic Neuroendocrine Tumors Over the Last 2 Decades: A SEER Database Analysis. Oncologist 2022, 27, 573–578. [Google Scholar] [CrossRef]
- Mpilla, G.B.; Philip, P.A.; El-Rayes, B.; Azmi, A.S. Pancreatic neuroendocrine tumors: Therapeutic challenges and research limitations. World J. Gastroenterol. 2020, 26, 4036–4054. [Google Scholar] [CrossRef]
- Forsythe, S.D.; Pu, T.; Andrews, S.G.; Madigan, J.P.; Sadowski, S.M. Models in Pancreatic Neuroendocrine Neoplasms: Current Perspectives and Future Directions. Cancers 2023, 15, 3756. [Google Scholar] [CrossRef]
- Andrews, S.G.; Forsythe, S.D.; Madigan, J.P.; Sadowski, S.M. Multifaceted modeling of small intestinal neuroendocrine tumors. Endocr. Oncol. 2024, 4, e240038. [Google Scholar] [CrossRef] [PubMed]
- Bilotta, M.T.; Antignani, A.; Fitzgerald, D.J. Managing the TME to improve the efficacy of cancer therapy. Front. Immunol. 2022, 13, 954992. [Google Scholar] [CrossRef]
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018, 14, 49. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Kerneur, C.; Cano, C.E.; Olive, D. Major pathways involved in macrophage polarization in cancer. Front. Immunol. 2022, 13, 1026954. [Google Scholar] [CrossRef]
- Bied, M.; Ho, W.W.; Ginhoux, F.; Blériot, C. Roles of macrophages in tumor development: A spatiotemporal perspective. Cell. Mol. Immunol. 2023, 20, 983–992. [Google Scholar] [CrossRef]
- Wei, I.H.; Harmon, C.M.; Arcerito, M.; Cheng, D.F.; Minter, R.M.; Simeone, D.M. Tumor-associated macrophages are a useful biomarker to predict recurrence after surgical resection of nonfunctional pancreatic neuroendocrine tumors. Ann. Surg. 2014, 260, 1088–1094. [Google Scholar] [CrossRef]
- Harimoto, N.; Hoshino, K.; Muranushi, R.; Hagiwara, K.; Yamanaka, T.; Ishii, N.; Tsukagoshi, M.; Igarashi, T.; Tanaka, H.; Watanabe, A.; et al. Prognostic significance of neutrophil-lymphocyte ratio in resectable pancreatic neuroendocrine tumors with special reference to tumor-associated macrophages. Pancreatology 2019, 19, 897–902. [Google Scholar] [CrossRef]
- Ferrata, M.; Schad, A.; Zimmer, S.; Musholt, T.J.; Bahr, K.; Kuenzel, J.; Becker, S.; Springer, E.; Roth, W.; Weber, M.M.; et al. PD-L1 Expression and Immune Cell Infiltration in Gastroenteropancreatic (GEP) and Non-GEP Neuroendocrine Neoplasms with High Proliferative Activity. Front. Oncol. 2019, 9, 343. [Google Scholar] [CrossRef]
- Pyonteck, S.M.; Gadea, B.B.; Wang, H.W.; Gocheva, V.; Hunter, K.E.; Tang, L.H.; Joyce, J.A. Deficiency of the macrophage growth factor CSF-1 disrupts pancreatic neuroendocrine tumor development. Oncogene 2012, 31, 1459–1467. [Google Scholar] [CrossRef]
- Akkari, L.; Gocheva, V.; Kester, J.C.; Hunter, K.E.; Quick, M.L.; Sevenich, L.; Wang, H.W.; Peters, C.; Tang, L.H.; Klimstra, D.S.; et al. Distinct functions of macrophage-derived and cancer cell-derived cathepsin Z combine to promote tumor malignancy via interactions with the extracellular matrix. Genes Dev. 2014, 28, 2134–2150. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Lu, F.; Gu, D.; Xue, B.; Xu, L.; Hu, C.; Chen, J.; Yu, P.; Zheng, H.; Gao, Y.; et al. Hypoxia exosome derived CEACAM5 promotes tumor-associated macrophages M2 polarization to accelerate pancreatic neuroendocrine tumors metastasis via MMP9. FASEB J. 2024, 38, e23762. [Google Scholar] [CrossRef] [PubMed]
- Harney, A.S.; Karagiannis, G.S.; Pignatelli, J.; Smith, B.D.; Kadioglu, E.; Wise, S.C.; Hood, M.M.; Kaufman, M.D.; Leary, C.B.; Lu, W.P.; et al. The Selective Tie2 Inhibitor Rebastinib Blocks Recruitment and Function of Tie2(Hi) Macrophages in Breast Cancer and Pancreatic Neuroendocrine Tumors. Mol. Cancer Ther. 2017, 16, 2486–2501. [Google Scholar] [CrossRef] [PubMed]
- Bösch, F.; Brüwer, K.; Altendorf-Hofmann, A.; Auernhammer, C.J.; Spitzweg, C.; Westphalen, C.B.; Boeck, S.; Schubert-Fritschle, G.; Werner, J.; Heinemann, V.; et al. Immune checkpoint markers in gastroenteropancreatic neuroendocrine neoplasia. Endocr. Relat. Cancer 2019, 26, 293–301. [Google Scholar] [CrossRef]
- Tanno, L.; Naheed, S.; Dunbar, J.; Tod, J.; Lopez, M.A.; Taylor, J.; Machado, M.; Green, B.; Ashton-Key, M.; Chee, S.J.; et al. Analysis of Immune Landscape in Pancreatic and Ileal Neuroendocrine Tumours Demonstrates an Immune Cold Tumour Microenvironment. Neuroendocrinology 2022, 112, 370–383. [Google Scholar] [CrossRef]
- Zhang, W.H.; Wang, W.Q.; Han, X.; Gao, H.L.; Xu, S.S.; Li, S.; Li, T.J.; Xu, H.X.; Li, H.; Ye, L.Y.; et al. Infiltrating pattern and prognostic value of tertiary lymphoid structures in resected non-functional pancreatic neuroendocrine tumors. J. Immunother. Cancer 2020, 8, e001188. [Google Scholar] [CrossRef]
- Young, K.; Lawlor, R.T.; Ragulan, C.; Patil, Y.; Mafficini, A.; Bersani, S.; Antonello, D.; Mansfield, D.; Cingarlini, S.; Landoni, L.; et al. Immune landscape, evolution, hypoxia-mediated viral mimicry pathways and therapeutic potential in molecular subtypes of pancreatic neuroendocrine tumours. Gut 2021, 70, 1904–1913. [Google Scholar] [CrossRef]
- Zitzmann, K.; De Toni, E.N.; Brand, S.; Goke, B.; Meinecke, J.; Spottl, G.; Meyer, H.H.; Auernhammer, C.J. The novel mTOR inhibitor RAD001 (everolimus) induces antiproliferative effects in human pancreatic neuroendocrine tumor cells. Neuroendocrinology 2007, 85, 54–60. [Google Scholar] [CrossRef]
- Yuan, Z.; Gardiner, J.C.; Maggi, E.C.; Huang, S.; Adem, A.; Bagdasarov, S.; Li, G.; Lee, S.; Slegowski, D.; Exarchakis, A.; et al. B7 immune-checkpoints as targets for the treatment of neuroendocrine tumors. Endocr. Relat. Cancer 2021, 28, 135–149. [Google Scholar] [CrossRef]
- Tseng, D.; Volkmer, J.P.; Willingham, S.B.; Contreras-Trujillo, H.; Fathman, J.W.; Fernhoff, N.B.; Seita, J.; Inlay, M.A.; Weiskopf, K.; Miyanishi, M.; et al. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc. Natl. Acad. Sci. USA 2013, 110, 11103–11108. [Google Scholar] [CrossRef]
- Hunter, K.E.; Palermo, C.; Kester, J.C.; Simpson, K.; Li, J.P.; Tang, L.H.; Klimstra, D.S.; Vlodavsky, I.; Joyce, J.A. Heparanase promotes lymphangiogenesis and tumor invasion in pancreatic neuroendocrine tumors. Oncogene 2014, 33, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.T.; Lan, Q.; Ponsonnet, L.; Blanquet, M.; Christofori, G.; Zaric, J.; Rüegg, C. The matricellular protein CYR61 interferes with normal pancreatic islets architecture and promotes pancreatic neuroendocrine tumor progression. Oncotarget 2016, 7, 1663–1674. [Google Scholar] [CrossRef]
- Chang, T.M.; Chu, P.Y.; Hung, W.C.; Shan, Y.S.; Lin, H.Y.; Huang, K.W.; Chang, J.S.; Chen, L.T.; Tsai, H.J. c-Myc promotes lymphatic metastasis of pancreatic neuroendocrine tumor through VEGFC upregulation. Cancer Sci. 2021, 112, 243–253. [Google Scholar] [CrossRef]
- Bollard, J.; Patte, C.; Radkova, K.; Massoma, P.; Chardon, L.; Valantin, J.; Gadot, N.; Goddard, I.; Vercherat, C.; Hervieu, V.; et al. Neuropilin-2 contributes to tumor progression in preclinical models of small intestinal neuroendocrine tumors. J. Pathol. 2019, 249, 343–355. [Google Scholar] [CrossRef]
- Wu, Y.; Tedesco, L.; Lucia, K.; Schlitter, A.M.; Garcia, J.M.; Esposito, I.; Auernhammer, C.J.; Theodoropoulou, M.; Arzt, E.; Renner, U.; et al. RSUME is implicated in tumorigenesis and metastasis of pancreatic neuroendocrine tumors. Oncotarget 2016, 7, 57878–57893. [Google Scholar] [CrossRef]
- Krug, S.; Kühnemuth, B.; Griesmann, H.; Neesse, A.; Mühlberg, L.; Boch, M.; Kortenhaus, J.; Fendrich, V.; Wiese, D.; Sipos, B.; et al. CUX1: A modulator of tumour aggressiveness in pancreatic neuroendocrine neoplasms. Endocr. Relat. Cancer 2014, 21, 879–890. [Google Scholar] [CrossRef]
- Cavalcanti, E.; Ignazzi, A.; De Michele, F.; Caruso, M.L. PDGFRα expression as a novel therapeutic marker in well-differentiated neuroendocrine tumors. Cancer Biol. Ther. 2019, 20, 423–430. [Google Scholar] [CrossRef]
- Cortez, E.; Gladh, H.; Braun, S.; Bocci, M.; Cordero, E.; Bjorkstrom, N.K.; Miyazaki, H.; Michael, I.P.; Eriksson, U.; Folestad, E.; et al. Functional malignant cell heterogeneity in pancreatic neuroendocrine tumors revealed by targeting of PDGF-DD. Proc. Natl. Acad. Sci. USA 2016, 113, E864–E873. [Google Scholar] [CrossRef]
- Cuny, T.; van Koetsveld, P.M.; Mondielli, G.; Dogan, F.; de Herder, W.W.; Barlier, A.; Hofland, L.J. Reciprocal Interactions between Fibroblast and Pancreatic Neuroendocrine Tumor Cells: Putative Impact of the Tumor Microenvironment. Cancers 2022, 14, 3481. [Google Scholar] [CrossRef]
- Amin, T.; Viol, F.; Krause, J.; Fahl, M.; Eggers, C.; Awwad, F.; Schmidt, B.; Benten, D.; Ungefroren, H.; Fraune, C.; et al. Cancer-Associated Fibroblasts Induce Proliferation and Therapeutic Resistance to Everolimus in Neuroendocrine Tumors through STAT3 Activation. Neuroendocrinology 2023, 113, 501–518. [Google Scholar] [CrossRef]
- Chaudhry, A.; Oberg, K.; Gobl, A.; Heldin, C.H.; Funa, K. Expression of transforming growth factors beta 1, beta 2, beta 3 in neuroendocrine tumors of the digestive system. Anticancer. Res. 1994, 14, 2085–2091. [Google Scholar] [PubMed]
- Chaudhry, A.; Funa, K.; Oberg, K. Expression of growth factor peptides and their receptors in neuroendocrine tumors of the digestive system. Acta Oncol. 1993, 32, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Bordi, C.; Falchetti, A.; Buffa, R.; Azzoni, C.; D’Adda, T.; Caruana, P.; Rindi, G.; Brandi, M.L. Production of basic fibroblast growth factor by gastric carcinoid tumors and their putative cells of origin. Hum. Pathol. 1994, 25, 175–180. [Google Scholar] [CrossRef]
- Beauchamp, R.D.; Coffey, R.J., Jr.; Lyons, R.M.; Perkett, E.A.; Townsend, C.M., Jr.; Moses, H.L. Human carcinoid cell production of paracrine growth factors that can stimulate fibroblast and endothelial cell growth. Cancer Res. 1991, 51, 5253–5260. [Google Scholar]
- Seuwen, K.; Magnaldo, I.; Pouysségur, J. Serotonin stimulates DNA synthesis in fibroblasts acting through 5-HT1B receptors coupled to a Gi-protein. Nature 1988, 335, 254–256. [Google Scholar] [CrossRef]
- Svejda, B.; Kidd, M.; Giovinazzo, F.; Eltawil, K.; Gustafsson, B.I.; Pfragner, R.; Modlin, I.M. The 5-HT(2B) receptor plays a key regulatory role in both neuroendocrine tumor cell proliferation and the modulation of the fibroblast component of the neoplastic microenvironment. Cancer 2010, 116, 2902–2912. [Google Scholar] [CrossRef]
- Lai, T.Y.; Chiang, T.C.; Lee, C.Y.; Kuo, T.C.; Wu, C.H.; Chen, Y.I.; Hu, C.M.; Maskey, M.; Tang, S.C.; Jeng, Y.M.; et al. Unraveling the impact of cancer-associated fibroblasts on hypovascular pancreatic neuroendocrine tumors. Br. J. Cancer 2024, 130, 1096–1108. [Google Scholar] [CrossRef]
- Krug, S.; Abbassi, R.; Griesmann, H.; Sipos, B.; Wiese, D.; Rexin, P.; Blank, A.; Perren, A.; Haybaeck, J.; Hüttelmaier, S.; et al. Therapeutic targeting of tumor-associated macrophages in pancreatic neuroendocrine tumors. Int. J. Cancer 2018, 143, 1806–1816. [Google Scholar] [CrossRef]
- Masucci, M.T.; Minopoli, M.; Carriero, M.V. Tumor Associated Neutrophils. Their Role in Tumorigenesis, Metastasis, Prognosis and Therapy. Front. Oncol. 2019, 9, 1146. [Google Scholar] [CrossRef]
- Cao, L.L.; Lu, J.; Lin, J.X.; Zheng, C.H.; Li, P.; Xie, J.W.; Wang, J.B.; Chen, Q.Y.; Lin, M.; Tu, R.H.; et al. Nomogram based on tumor-associated neutrophil-to-lymphocyte ratio to predict survival of patients with gastric neuroendocrine neoplasms. World J. Gastroenterol. 2017, 23, 8376–8386. [Google Scholar] [CrossRef]
- Guglietta, S.; Chiavelli, A.; Zagato, E.; Krieg, C.; Gandini, S.; Ravenda, P.S.; Bazolli, B.; Lu, B.; Penna, G.; Rescigno, M. Coagulation induced by C3aR-dependent NETosis drives protumorigenic neutrophils during small intestinal tumorigenesis. Nat. Commun. 2016, 7, 11037. [Google Scholar] [CrossRef] [PubMed]
- Soncini, M.; Corna, G.; Moresco, M.; Coltella, N.; Restuccia, U.; Maggioni, D.; Raccosta, L.; Lin, C.-Y.; Invernizzi, F.; Crocchiolo, R.; et al. 24-Hydroxycholesterol participates in pancreatic neuroendocrine tumor development. Proc. Natl. Acad. Sci. USA 2016, 113, E6219–E6227. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.S.; Li, H.; Li, T.J.; Li, S.; Xia, H.Y.; Long, J.; Wu, C.T.; Wang, W.Q.; Zhang, W.H.; Gao, H.L.; et al. Neutrophil Extracellular Traps and Macrophage Extracellular Traps Predict Postoperative Recurrence in Resectable Nonfunctional Pancreatic Neuroendocrine Tumors. Front. Immunol. 2021, 12, 577517. [Google Scholar] [CrossRef]
- Chan, I.S.; Ewald, A.J. The changing role of natural killer cells in cancer metastasis. J. Clin. Investig. 2022, 132, e143762. [Google Scholar] [CrossRef]
- Aparicio-Pagés, M.N.; Verspaget, H.W.; Peña, A.S.; Jansen, J.B.; Lamers, C.B. Natural killer cell activity in patients with neuroendocrine tumours of the gastrointestinal tract; relation with circulating gastrointestinal hormones. Neuropeptides 1991, 20, 1–7. [Google Scholar] [CrossRef]
- Inoue, M.; Kim, M.; Inoue, T.; Tait, M.; Byrne, T.; Nitschké, M.; Murer, P.; Cha, H.; Subramanian, A.; De Silva, N.; et al. Oncolytic vaccinia virus injected intravenously sensitizes pancreatic neuroendocrine tumors and metastases to immune checkpoint blockade. Mol. Ther. Oncolytics 2022, 24, 299–318. [Google Scholar] [CrossRef]
- Ribatti, D. New insights into the role of mast cells as a therapeutic target in cancer through the blockade of immune checkpoint inhibitors. Front. Med. 2024, 11, 1373230. [Google Scholar] [CrossRef]
- Soucek, L.; Buggy, J.J.; Kortlever, R.; Adimoolam, S.; Monclús, H.A.; Allende, M.T.; Swigart, L.B.; Evan, G.I. Modeling pharmacological inhibition of mast cell degranulation as a therapy for insulinoma. Neoplasia 2011, 13, 1093–1100. [Google Scholar] [CrossRef]
- Umansky, V.; Blattner, C.; Gebhardt, C.; Utikal, J. The Role of Myeloid-Derived Suppressor Cells (MDSC) in Cancer Progression. Vaccines 2016, 4, 36. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Y.; Chen, L.; Lin, Y.; He, Q.; Zeng, Y.; Chen, M.; Chen, J. Myeloid-derived suppressor cells in gastroenteropancreatic neuroendocrine neoplasms. Endocrine 2021, 71, 242–252. [Google Scholar] [CrossRef]
- Centonze, G.; Lagano, V.; Sabella, G.; Mangogna, A.; Garzone, G.; Filugelli, M.; Belmonte, B.; Cattaneo, L.; Crisafulli, V.; Pellegrinelli, A.; et al. Myeloid and T-Cell Microenvironment Immune Features Identify Two Prognostic Sub-Groups in High-Grade Gastroenteropancreatic Neuroendocrine Neoplasms. J. Clin. Med. 2021, 10, 1741. [Google Scholar] [CrossRef] [PubMed]
- Busse, A.; Mochmann, L.H.; Spenke, C.; Arsenic, R.; Briest, F.; Jöhrens, K.; Lammert, H.; Sipos, B.; Kühl, A.A.; Wirtz, R.; et al. Immunoprofiling in Neuroendocrine Neoplasms Unveil Immunosuppressive Microenvironment. Cancers 2020, 12, 3448. [Google Scholar] [CrossRef] [PubMed]
- Marciscano, A.E.; Anandasabapathy, N. The role of dendritic cells in cancer and anti-tumor immunity. Semin. Immunol. 2021, 52, 101481. [Google Scholar] [CrossRef] [PubMed]
- Werner, W.; Detjen, K.; Bruneau, A.; Lurje, I.; Nestel, N.; Jann, H.; Tacke, F.; Wiedenmann, B.; Roderburg, C.; Hammerich, L. Intratumoral dendritic cells and T cells predict survival in gastroenteropancreatic neuroendocrine neoplasms. Endocr. Relat. Cancer 2023, 30, e220357. [Google Scholar] [CrossRef]
- Meng, D.; Zhao, L.; Liu, J.; Ge, C.; Zhang, C. Identification of the Immune Subtypes for the Prediction of Metastasis in Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2023, 113, 719–735. [Google Scholar] [CrossRef]
- Schott, M. Immunesurveillance by dendritic cells: Potential implication for immunotherapy of endocrine cancers. Endocr. Relat. Cancer 2006, 13, 779–795. [Google Scholar] [CrossRef]
- Andoh, Y.; Makino, N.; Yamakawa, M. Dendritic cells fused with different pancreatic carcinoma cells induce different T-cell responses. Onco Targets Ther. 2013, 6, 29–40. [Google Scholar] [CrossRef]
- Schott, M.; Feldkamp, J.; Lettmann, M.; Simon, D.; Scherbaum, W.A.; Seissler, J. Dendritic cell immunotherapy in a neuroendocrine pancreas carcinoma. Clin. Endocrinol. 2001, 55, 271–277. [Google Scholar] [CrossRef]
- Xie, Q.; Ding, J.; Chen, Y. Role of CD8(+) T lymphocyte cells: Interplay with stromal cells in tumor microenvironment. Acta Pharm. Sin. 2021, 11, 1365–1378. [Google Scholar] [CrossRef]
- Gong, Y.; Fan, Z.; Luo, G.; Huang, Q.; Qian, Y.; Cheng, H.; Jin, K.; Ni, Q.; Yu, X.; Liu, C. Absolute Counts of Peripheral Lymphocyte Subsets Correlate with the Progression-Free Survival and Metastatic Status of Pancreatic Neuroendocrine Tumour Patients. Cancer Manag. Res. 2020, 12, 6727–6737. [Google Scholar] [CrossRef]
- Greenberg, J.; Limberg, J.; Verma, A.; Kim, D.; Chen, X.; Lee, Y.J.; Moore, M.D.; Ullmann, T.M.; Thiesmeyer, J.W.; Loewenstein, Z.; et al. Metastatic pancreatic neuroendocrine tumors feature elevated T cell infiltration. JCI Insight 2022, 7, e160130. [Google Scholar] [CrossRef] [PubMed]
- Hiltunen, N.; Väyrynen, J.P.; Böhm, J.; Helminen, O. CD3(+), CD8(+), CD4(+) and FOXP3(+) T Cells in the Immune Microenvironment of Small Bowel Neuroendocrine Tumors. Diseases 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Hofving, T.; Liang, F.; Karlsson, J.; Yrlid, U.; Nilsson, J.A.; Nilsson, O.; Nilsson, L.M. The Microenvironment of Small Intestinal Neuroendocrine Tumours Contains Lymphocytes Capable of Recognition and Activation after Expansion. Cancers 2021, 13, 4305. [Google Scholar] [CrossRef] [PubMed]
- Someach, E.; Halder, D.; Spitzer, A.; Barbolin, C.; Tyler, M.; Halperin, R.; Biton, M.; Tirosh, A.; Tirosh, I. Subtypes and proliferation patterns of small intestine neuroendocrine tumors revealed by single cell RNA sequencing. bioRxiv 2024. [Google Scholar] [CrossRef]
- Oberg, K. Interferon in the management of neuroendocrine GEP-tumors: A review. Digestion 2000, 62 (Suppl. 1), 92–97. [Google Scholar] [CrossRef]
- Mirvis, E.; Mandair, D.; Garcia-Hernandez, J.; Mohmaduvesh, M.; Toumpanakis, C.; Caplin, M. Role of interferon-alpha in patients with neuroendocrine tumors: A retrospective study. Anticancer Res. 2014, 34, 6601–6607. [Google Scholar]
- Papantoniou, D.; Grönberg, M.; Thiis-Evensen, E.; Sorbye, H.; Landerholm, K.; Welin, S.; Tiensuu Janson, E. Treatment efficacy in a metastatic small intestinal neuroendocrine tumour grade 2 cohort. Endocr. Relat. Cancer 2023, 30, e220316. [Google Scholar] [CrossRef]
- Yao, J.C.; Guthrie, K.A.; Moran, C.; Strosberg, J.R.; Kulke, M.H.; Chan, J.A.; LoConte, N.; McWilliams, R.R.; Wolin, E.M.; Mattar, B.; et al. Phase III Prospective Randomized Comparison Trial of Depot Octreotide Plus Interferon Alfa-2b Versus Depot Octreotide Plus Bevacizumab in Patients with Advanced Carcinoid Tumors: SWOG S0518. J. Clin. Oncol. 2017, 35, 1695–1703. [Google Scholar] [CrossRef]
- Mehnert, J.M.; Bergsland, E.; O’Neil, B.H.; Santoro, A.; Schellens, J.H.M.; Cohen, R.B.; Doi, T.; Ott, P.A.; Pishvaian, M.J.; Puzanov, I.; et al. Pembrolizumab for the treatment of programmed death-ligand 1-positive advanced carcinoid or pancreatic neuroendocrine tumors: Results from the KEYNOTE-028 study. Cancer 2020, 126, 3021–3030. [Google Scholar] [CrossRef]
- Strosberg, J.; Mizuno, N.; Doi, T.; Grande, E.; Delord, J.P.; Shapira-Frommer, R.; Bergsland, E.; Shah, M.; Fakih, M.; Takahashi, S.; et al. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Neuroendocrine Tumors: Results From the Phase II KEYNOTE-158 Study. Clin. Cancer Res. 2020, 26, 2124–2130. [Google Scholar] [CrossRef]
- Yao, J.C.; Strosberg, J.; Fazio, N.; Pavel, M.E.; Ruszniewski, P.; Bergsland, E.; Li, D.; Tafuto, S.; Raj, N.; Campana, D.; et al. Activity & safety of spartalizumab (PDR001) in patients (pts) with advanced neuroendocrine tumors (NET) of pancreatic (Pan), gastrointestinal (GI), or thoracic (T) origin, & gastroenteropancreatic neuroendocrine carcinoma (GEP NEC) who have progressed on prior treatment (Tx). Ann. Oncol. 2018, 29, viii467–viii468. [Google Scholar] [CrossRef]
- Patel, S.P.; Othus, M.; Chae, Y.K.; Giles, F.J.; Hansel, D.E.; Singh, P.P.; Fontaine, A.; Shah, M.H.; Kasi, A.; Baghdadi, T.A.; et al. A Phase II Basket Trial of Dual Anti-CTLA-4 and Anti-PD-1 Blockade in Rare Tumors (DART SWOG 1609) in Patients with Nonpancreatic Neuroendocrine Tumors. Clin. Cancer Res. 2020, 26, 2290–2296. [Google Scholar] [CrossRef]
- Klein, O.; Kee, D.; Markman, B.; Michael, M.; Underhill, C.; Carlino, M.S.; Jackett, L.; Lum, C.; Scott, C.; Nagrial, A.; et al. Immunotherapy of Ipilimumab and Nivolumab in Patients with Advanced Neuroendocrine Tumors: A Subgroup Analysis of the CA209-538 Clinical Trial for Rare Cancers. Clin. Cancer Res. 2020, 26, 4454–4459. [Google Scholar] [CrossRef]
- Cousin, S.; Guégan, J.P.; Palmieri, L.J.; Metges, J.P.; Pernot, S.; Bellera, C.A.; Assenat, E.; Korakis, I.; Cassier, P.A.; Hollebecque, A.; et al. Regorafenib plus avelumab in advanced gastroenteropancreatic neuroendocrine neoplasms: A phase 2 trial and correlative analysis. Nat. Cancer 2025, 6, 584–594. [Google Scholar] [CrossRef]
- Kulke, M.H.; Lenz, H.-J.; Meropol, N.J.; Posey, J.; Ryan, D.P.; Picus, J.; Bergsland, E.; Stuart, K.; Tye, L.; Huang, X.; et al. Activity of sunitinib in patients with advanced neuroendocrine tumors. J. Clin. Oncol. 2008, 26, 3403–3410. [Google Scholar] [CrossRef]
- Raymond, E.; Dahan, L.; Raoul, J.-L.; Bang, Y.-J.; Borbath, I.; Lombard-Bohas, C.; Valle, J.; Metrakos, P.; Smith, D.; Vinik, A.; et al. Sunitinib Malate for the Treatment of Pancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2011, 364, 501–513. [Google Scholar] [CrossRef]
- Xu, J.; Li, J.; Bai, C.; Xu, N.; Zhou, Z.; Li, Z.; Zhou, C.; Jia, R.; Lu, M.; Cheng, Y.; et al. Surufatinib in Advanced Well-Differentiated Neuroendocrine Tumors: A Multicenter, Single-Arm, Open-Label, Phase Ib/II Trial. Clin. Cancer Res. 2019, 25, 3486–3494. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Cives, M.; Hwang, J.; Weber, T.; Nickerson, M.; Atreya, C.E.; Venook, A.; Kelley, R.K.; Valone, T.; Morse, B.; et al. A phase II study of axitinib in advanced neuroendocrine tumors. Endocr. Relat. Cancer 2016, 23, 411–418. [Google Scholar] [CrossRef]
- Grande, E.; Capdevila, J.; Castellano, D.; Teulé, A.; Durán, I.; Fuster, J.; Sevilla, I.; Escudero, P.; Sastre, J.; García-Donas, J.; et al. Pazopanib in pretreated advanced neuroendocrine tumors: A phase II, open-label trial of the Spanish Task Force Group for Neuroendocrine Tumors (GETNE). Ann. Oncol. 2015, 26, 1987–1993. [Google Scholar] [CrossRef]
- Phan, A.T.; Halperin, D.M.; Chan, J.A.; Fogelman, D.R.; Hess, K.R.; Malinowski, P.; Regan, E.; Ng, C.S.; Yao, J.C.; Kulke, M.H. Pazopanib and depot octreotide in advanced, well-differentiated neuroendocrine tumours: A multicentre, single-group, phase 2 study. Lancet Oncol. 2015, 16, 695–703. [Google Scholar] [CrossRef]
- Chan, J.A.; Geyer, S.; Zemla, T.; Knopp, M.V.; Behr, S.; Pulsipher, S.; Ou, F.-S.; Dueck, A.C.; Acoba, J.; Shergill, A.; et al. Phase 3 Trial of Cabozantinib to Treat Advanced Neuroendocrine Tumors. N. Engl. J. Med. 2024, 392, 653. [Google Scholar] [CrossRef] [PubMed]
- Jonasch, E.; Donskov, F.; Iliopoulos, O.; Rathmell, W.K.; Narayan, V.K.; Maughan, B.L.; Oudard, S.; Else, T.; Maranchie, J.K.; Welsh, S.J.; et al. Belzutifan for Renal Cell Carcinoma in von Hippel–Lindau Disease. N. Engl. J. Med. 2021, 385, 2036–2046. [Google Scholar] [CrossRef] [PubMed]
- Else, T.; Jonasch, E.; Iliopoulos, O.; Beckermann, K.E.; Narayan, V.; Maughan, B.L.; Oudard, S.; Maranchie, J.K.; Iversen, A.B.; Goldberg, C.M.; et al. Belzutifan for von Hippel-Lindau Disease: Pancreatic Lesion Population of the Phase 2 LITESPARK-004 Study. Clin. Cancer Res. 2024, 30, 1750–1757. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.A.; Stuart, K.; Earle, C.C.; Clark, J.W.; Bhargava, P.; Miksad, R.; Blaszkowsky, L.; Enzinger, P.C.; Meyerhardt, J.A.; Zheng, H.; et al. Prospective study of bevacizumab plus temozolomide in patients with advanced neuroendocrine tumors. J. Clin. Oncol. 2012, 30, 2963–2968. [Google Scholar] [CrossRef]
- Hobday, T.J.; Qin, R.; Reidy-Lagunes, D.; Moore, M.J.; Strosberg, J.; Kaubisch, A.; Shah, M.; Kindler, H.L.; Lenz, H.-J.; Chen, H.; et al. Multicenter Phase II Trial of Temsirolimus and Bevacizumab in Pancreatic Neuroendocrine Tumors. J. Clin. Oncol. 2015, 33, 1551–1556. [Google Scholar] [CrossRef]
- Abuzakhm, S.M.; Sukrithan, V.; Fruth, B.; Qin, R.; Strosberg, J.; Hobday, T.J.; Semrad, T.; Reidy-Lagunes, D.; Kindler, H.L.; Kim, G.P.; et al. A phase II study of bevacizumab and temsirolimus in advanced extra-pancreatic neuroendocrine tumors. Endocr. Relat. Cancer 2023, 30, e220301. [Google Scholar] [CrossRef]
- Halperin, D.M.; Liu, S.; Dasari, A.; Fogelman, D.; Bhosale, P.; Mahvash, A.; Estrella, J.S.; Rubin, L.; Morani, A.C.; Knafl, M.; et al. Assessment of Clinical Response Following Atezolizumab and Bevacizumab Treatment in Patients with Neuroendocrine Tumors: A Nonrandomized Clinical Trial. JAMA Oncol. 2022, 8, 904–909. [Google Scholar] [CrossRef]
- Feng, Y.; Luo, S.; Fan, D.; Guo, X.; Ma, S. The role of vascular endothelial cells in tumor metastasis. Acta Histochem. 2023, 125, 152070. [Google Scholar] [CrossRef]
- Ren, B.; Rose, J.B.; Liu, Y.; Jaskular-Sztul, R.; Contreras, C.; Beck, A.; Chen, H. Heterogeneity of Vascular Endothelial Cells, De Novo Arteriogenesis and Therapeutic Implications in Pancreatic Neuroendocrine Tumors. J. Clin. Med. 2019, 8, 1980. [Google Scholar] [CrossRef]
- Díaz-Flores, L.; Gutiérrez, R.; García-Suárez, M.P.; González-Gómez, M.; Carrasco, J.L.; Madrid, J.F.; Díaz-Flores, L., Jr. “Vascular tuft sign” in neuroendocrine tumors of the pancreas. Histol. Histopathol. 2024, 39, 1457–1472. [Google Scholar] [CrossRef]
- Chu, X.; Gao, X.; Jansson, L.; Quach, M.; Skogseid, B.; Barbu, A. Multiple microvascular alterations in pancreatic islets and neuroendocrine tumors of a Men1 mouse model. Am. J. Pathol. 2013, 182, 2355–2367. [Google Scholar] [CrossRef]
- Shen, H.C.; He, M.; Powell, A.; Adem, A.; Lorang, D.; Heller, C.; Grover, A.C.; Ylaya, K.; Hewitt, S.M.; Marx, S.J.; et al. Recapitulation of pancreatic neuroendocrine tumors in human multiple endocrine neoplasia type I syndrome via Pdx1-directed inactivation of Men1. Cancer Res. 2009, 69, 1858–1866. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Peng, L.; Song, Y.L.; Xu, S.; Hua, Z.; Fang, N.; Zhai, M.; Liu, H.; Fang, Q.; Deng, T.; et al. Pseudo-hemorrhagic region formation in pancreatic neuroendocrine tumors is a result of blood vessel dilation followed by endothelial cell detachment. Oncol. Lett. 2018, 15, 4255–4261. [Google Scholar] [CrossRef] [PubMed]
- Balan, M.; Trusohamn, M.; Ning, F.C.; Jacob, S.; Pietras, K.; Eriksson, U.; Berggren, P.O.; Nyqvist, D. Noninvasive intravital high-resolution imaging of pancreatic neuroendocrine tumours. Sci. Rep. 2019, 9, 14636. [Google Scholar] [CrossRef]
- Kasuya, K.; Nagakawa, Y.; Suzuki, M.; Tanaka, H.; Ohta, H.; Itoi, T.; Tsuchida, A. Anti-vascular endothelial growth factor antibody single therapy for pancreatic neuroendocrine carcinoma exhibits a marked tumor growth-inhibitory effect. Exp. Ther. Med. 2011, 2, 1047–1052. [Google Scholar] [CrossRef][Green Version]
- Silva, S.R.; Bowen, K.A.; Rychahou, P.G.; Jackson, L.N.; Weiss, H.L.; Lee, E.Y.; Townsend, C.M., Jr.; Evers, B.M. VEGFR-2 expression in carcinoid cancer cells and its role in tumor growth and metastasis. Int. J. Cancer 2011, 128, 1045–1056. [Google Scholar] [CrossRef]
- Pinato, D.J.; Tan, T.M.; Toussi, S.T.; Ramachandran, R.; Martin, N.; Meeran, K.; Ngo, N.; Dina, R.; Sharma, R. An expression signature of the angiogenic response in gastrointestinal neuroendocrine tumours: Correlation with tumour phenotype and survival outcomes. Br. J. Cancer 2014, 110, 115–122. [Google Scholar] [CrossRef][Green Version]
- Lopez-Aguiar, A.G.; Postlewait, L.M.; Ethun, C.G.; Zaidi, M.Y.; Zhelnin, K.; Krasinskas, A.; Russell, M.C.; Kooby, D.A.; Cardona, K.; El-Rayes, B.F.; et al. STAT3 Inhibition for Gastroenteropancreatic Neuroendocrine Tumors: Potential for a New Therapeutic Target? J. Gastrointest. Surg. 2020, 24, 1138–1148. [Google Scholar] [CrossRef]
- Franco, M.; Pàez-Ribes, M.; Cortez, E.; Casanovas, O.; Pietras, K. Use of a mouse model of pancreatic neuroendocrine tumors to find pericyte biomarkers of resistance to anti-angiogenic therapy. Horm. Metab. Res. 2011, 43, 884–889. [Google Scholar] [CrossRef]
- Yazdani, S.; Kasajima, A.; Tamaki, K.; Nakamura, Y.; Fujishima, F.; Ohtsuka, H.; Motoi, F.; Unno, M.; Watanabe, M.; Sato, Y.; et al. Angiogenesis and vascular maturation in neuroendocrine tumors. Hum. Pathol. 2014, 45, 866–874. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, Z.; Li, Q.; Wang, L.; Rashid, A.; Zhu, Z.; Evans, D.B.; Vauthey, J.N.; Xie, K.; Yao, J.C. Elevated expression of vascular endothelial growth factor correlates with increased angiogenesis and decreased progression-free survival among patients with low-grade neuroendocrine tumors. Cancer 2007, 109, 1478–1486. [Google Scholar] [CrossRef] [PubMed]
- Cigrovski Berković, M.; Čačev, T.; Catela Ivković, T.; Marout, J.; Ulamec, M.; Zjačić-Rotkvić, V.; Kapitanović, S. High VEGF serum values are associated with locoregional spread of gastroenteropancreatic neuroendocrine tumors (GEP-NETs). Mol. Cell. Endocrinol. 2016, 425, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, P.; Hawinkels, L.J.; de Jonge-Muller, E.S.; Biemond, I.; Lamers, C.B.; Verspaget, H.W. Angiogenic markers endoglin and vascular endothelial growth factor in gastroenteropancreatic neuroendocrine tumors. World J. Gastroenterol. 2011, 17, 219–225. [Google Scholar] [CrossRef]
- Sandra, I.; Cazacu, I.M.; Croitoru, V.M.; Mihaila, M.; Herlea, V.; Diculescu, M.M.; Dima, S.O.; Croitoru, A.E. Circulating Angiogenic Markers in Gastroenteropancreatic Neuroendocrine Neoplasms: A Systematic Review. Curr. Issues Mol. Biol. 2022, 44, 4001–4014. [Google Scholar] [CrossRef]
- Detjen, K.M.; Rieke, S.; Deters, A.; Schulz, P.; Rexin, A.; Vollmer, S.; Hauff, P.; Wiedenmann, B.; Pavel, M.; Scholz, A. Angiopoietin-2 promotes disease progression of neuroendocrine tumors. Clin. Cancer Res. 2010, 16, 420–429. [Google Scholar] [CrossRef]
- Allen, E.; Walters, I.B.; Hanahan, D. Brivanib, a dual FGF/VEGF inhibitor, is active both first and second line against mouse pancreatic neuroendocrine tumors developing adaptive/evasive resistance to VEGF inhibition. Clin. Cancer Res. 2011, 17, 5299–5310. [Google Scholar] [CrossRef]
- Yao, J.C.; Phan, A.; Hoff, P.M.; Chen, H.X.; Charnsangavej, C.; Yeung, S.C.; Hess, K.; Ng, C.; Abbruzzese, J.L.; Ajani, J.A. Targeting vascular endothelial growth factor in advanced carcinoid tumor: A random assignment phase II study of depot octreotide with bevacizumab and pegylated interferon alpha-2b. J. Clin. Oncol. 2008, 26, 1316–1323. [Google Scholar] [CrossRef]
- Oxboel, J.; Binderup, T.; Knigge, U.; Kjaer, A. Quantitative gene-expression of the tumor angiogenesis markers vascular endothelial growth factor, integrin alphaV and integrin beta3 in human neuroendocrine tumors. Oncol. Rep. 2009, 21, 769–775. [Google Scholar]
- Plikus, M.V.; Wang, X.; Sinha, S.; Forte, E.; Thompson, S.M.; Herzog, E.L.; Driskell, R.R.; Rosenthal, N.; Biernaskie, J.; Horsley, V. Fibroblasts: Origins, definitions, and functions in health and disease. Cell 2021, 184, 3852–3872. [Google Scholar] [CrossRef]
- Joshi, R.S.; Kanugula, S.S.; Sudhir, S.; Pereira, M.P.; Jain, S.; Aghi, M.K. The Role of Cancer-Associated Fibroblasts in Tumor Progression. Cancers 2021, 13, 1399. [Google Scholar] [CrossRef]
- Niedra, H.; Peculis, R.; Saksis, R.; Mandrika, I.; Vilisova, S.; Nazarovs, J.; Breiksa, A.; Gerina, A.; Earl, J.; Ruz-Caracuel, I.; et al. Tumor and α-SMA-expressing stromal cells in pancreatic neuroendocrine tumors have a distinct RNA profile depending on tumor grade. Mol. Oncol. 2024, 19, 659–681. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Li, Q.; Hu, Y.; Hu, H.; Xu, J.; Guo, M.; Zhang, W.; Lou, X.; Wang, Y.; Gao, H.; et al. The stromal microenvironment endows pancreatic neuroendocrine tumors with spatially specific invasive and metastatic phenotypes. Cancer Lett. 2024, 588, 216769. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, S.; Liu, C.; Yang, J.; Lin, Q.; Zheng, S.; Chen, C.; Zhou, Q.; Chen, R. Single-cell RNA sequencing reveals spatiotemporal heterogeneity and malignant progression in pancreatic neuroendocrine tumor. Int. J. Biol. Sci. 2021, 17, 3760–3775. [Google Scholar] [CrossRef] [PubMed]
- Dumortier, J.; Ratineau, C.; Scoazec, J.Y.; Pourreyron, C.; Anderson, W.; Jacquier, M.F.; Blanc, M.; Bernard, C.; Bellaton, C.; Remy, L.; et al. Site-specific epithelial-mesenchymal interactions in digestive neuroendocrine tumors. An experimental in vivo and in vitro study. Am. J. Pathol. 2000, 156, 671–683. [Google Scholar] [CrossRef]
- Johansen, A.M.; Forsythe, S.D.; McGrath, C.T.; Barker, G.; Jimenez, H.; Paluri, R.K.; Pasche, B.C. TGFβ in Pancreas and Colorectal Cancer: Opportunities to Overcome Therapeutic Resistance. Clin. Cancer Res. 2024, 30, 3676–3687. [Google Scholar] [CrossRef]
- Zhang, Y.; Manouchehri Doulabi, E.; Herre, M.; Cedervall, J.; Qiao, Q.; Miao, Z.; Hamidi, A.; Hellman, L.; Kamali-Moghaddam, M.; Olsson, A.K. Platelet-Derived PDGFB Promotes Recruitment of Cancer-Associated Fibroblasts, Deposition of Extracellular Matrix and Tgfβ Signaling in the Tumor Microenvironment. Cancers 2022, 14, 1947. [Google Scholar] [CrossRef]
- Cao, Z.; Quazi, S.; Arora, S.; Osellame, L.D.; Burvenich, I.J.; Janes, P.W.; Scott, A.M. Cancer-associated fibroblasts as therapeutic targets for cancer: Advances, challenges, and future prospects. J. Biomed. Sci. 2025, 32, 7. [Google Scholar] [CrossRef]
- Yue, B. Biology of the extracellular matrix: An overview. J. Glaucoma 2014, 23, S20–S23. [Google Scholar] [CrossRef]
- Naba, A.; Clauser, K.R.; Mani, D.R.; Carr, S.A.; Hynes, R.O. Quantitative proteomic profiling of the extracellular matrix of pancreatic islets during the angiogenic switch and insulinoma progression. Sci. Rep. 2017, 7, 40495. [Google Scholar] [CrossRef]
- Duerr, E.M.; Mizukami, Y.; Ng, A.; Xavier, R.J.; Kikuchi, H.; Deshpande, V.; Warshaw, A.L.; Glickman, J.; Kulke, M.H.; Chung, D.C. Defining molecular classifications and targets in gastroenteropancreatic neuroendocrine tumors through DNA microarray analysis. Endocr. Relat. Cancer 2008, 15, 243–256. [Google Scholar] [CrossRef]
- Gurevich, L.E. Role of matrix metalloproteinases 2 and 9 in determination of invasive potential of pancreatic tumors. Bull. Exp. Biol. Med. 2003, 136, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.; Zeng, L.; Zhang, J.; Yang, S.; Lou, W. THBS2, a microRNA-744-5p target, modulates MMP9 expression through CUX1 in pancreatic neuroendocrine tumors. Oncol. Lett. 2020, 19, 1683–1692. [Google Scholar] [CrossRef] [PubMed]
- Blicharz-Dorniak, J.; Kos-Kudła, B.; Foltyn, W.; Kajdaniuk, D.; Marek, B.; Zemczak, A.; Strzelczyk, J. Is determination of matrix metalloproteinases and their tissue inhibitors serum concentrations useful in patients with gastroenteropancreatic and bronchopulmonary neuroendocrine neoplasms? Endokrynol. Pol. 2012, 63, 470–476. [Google Scholar]
- Zhang, W.; Erkan, M.; Abiatari, I.; Giese, N.A.; Felix, K.; Kayed, H.; Büchler, M.W.; Friess, H.; Kleeff, J. Expression of extracellular matrix metalloproteinase inducer (EMMPRIN/CD147) in pancreatic neoplasm and pancreatic stellate cells. Cancer Biol. Ther. 2007, 6, 218–227. [Google Scholar] [CrossRef]
- Bluyssen, H.A.; Lolkema, M.P.; van Beest, M.; Boone, M.; Snijckers, C.M.; Los, M.; Gebbink, M.F.; Braam, B.; Holstege, F.C.; Giles, R.H.; et al. Fibronectin is a hypoxia-independent target of the tumor suppressor VHL. FEBS Lett. 2004, 556, 137–142. [Google Scholar] [CrossRef]
- Skardal, A.; Devarasetty, M.; Forsythe, S.; Atala, A.; Soker, S. A reductionist metastasis-on-a-chip platform for in vitro tumor progression modeling and drug screening. Biotechnol. Bioeng. 2016, 113, 2020–2032. [Google Scholar] [CrossRef]
- Forsythe, S.D.; Erali, R.A.; Sasikumar, S.; Laney, P.; Shelkey, E.; D’Agostino, R., Jr.; Miller, L.D.; Shen, P.; Levine, E.A.; Soker, S.; et al. Organoid Platform in Preclinical Investigation of Personalized Immunotherapy Efficacy in Appendiceal Cancer: Feasibility Study. Clin. Cancer Res. 2021, 27, 5141–5150. [Google Scholar] [CrossRef]
- Forsythe, S.D.; Sivakumar, H.; Erali, R.A.; Wajih, N.; Li, W.; Shen, P.; Levine, E.A.; Miller, K.E.; Skardal, A.; Votanopoulos, K.I. Patient-Specific Sarcoma Organoids for Personalized Translational Research: Unification of the Operating Room with Rare Cancer Research and Clinical Implications. Ann. Surg. Oncol. 2022, 29, 7354–7367. [Google Scholar] [CrossRef]
- Forsythe, S.D.; Erali, R.A.; Laney, P.; Sivakumar, H.; Li, W.; Skardal, A.; Soker, S.; Votanopoulos, K.I. Application of immune enhanced organoids in modeling personalized Merkel cell carcinoma research. Sci. Rep. 2022, 12, 13865. [Google Scholar] [CrossRef]
- Lamberti, G.; La Salvia, A. Neuroendocrine Tumors: Challenges and Future Perspectives. J. Clin. Med. 2022, 11, 4351. [Google Scholar] [CrossRef]
- Yao, J.C.; Lagunes, D.R.; Kulke, M.H. Targeted therapies in neuroendocrine tumors (NET): Clinical trial challenges and lessons learned. Oncologist 2013, 18, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Du, L.; Lee, C.L.; Arhin, N.D.; Chan, J.A.; Kohn, E.C.; Halperin, D.M.; Berlin, J.; LaFerriere, H.; Singh, S.; et al. Comparison of Design, Eligibility, and Outcomes of Neuroendocrine Neoplasm Trials Initiated From 2000 to 2009 vs. 2010 to 2020. JAMA Netw. Open 2021, 4, e2131744. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yellapragada, S.V.; Forsythe, S.D.; Madigan, J.P.; Sadowski, S.M. The Role of the Tumor Microenvironment in Gastroenteropancreatic Neuroendocrine Tumors. Int. J. Mol. Sci. 2025, 26, 5635. https://doi.org/10.3390/ijms26125635
Yellapragada SV, Forsythe SD, Madigan JP, Sadowski SM. The Role of the Tumor Microenvironment in Gastroenteropancreatic Neuroendocrine Tumors. International Journal of Molecular Sciences. 2025; 26(12):5635. https://doi.org/10.3390/ijms26125635
Chicago/Turabian StyleYellapragada, Srujana V., Steven D. Forsythe, James P. Madigan, and Samira M. Sadowski. 2025. "The Role of the Tumor Microenvironment in Gastroenteropancreatic Neuroendocrine Tumors" International Journal of Molecular Sciences 26, no. 12: 5635. https://doi.org/10.3390/ijms26125635
APA StyleYellapragada, S. V., Forsythe, S. D., Madigan, J. P., & Sadowski, S. M. (2025). The Role of the Tumor Microenvironment in Gastroenteropancreatic Neuroendocrine Tumors. International Journal of Molecular Sciences, 26(12), 5635. https://doi.org/10.3390/ijms26125635








