Synergistic Antifungal Properties, Chemical Composition, and Frontier Molecular Orbital Analysis of Essential Oils from Lemongrass, Kaffir Lime, Lime, Dill, and Shatavari Against Malassezia furfur
Abstract
1. Introduction
2. Results
2.1. Extraction Yields of Essential Oils
2.2. Chemical Composition of Essential Oils
2.3. The Inhibitory Activity of Essential Oils Against Malassezia furfur
2.4. Synergistic Effects of Combined Essential Oils on Malassezia furfur
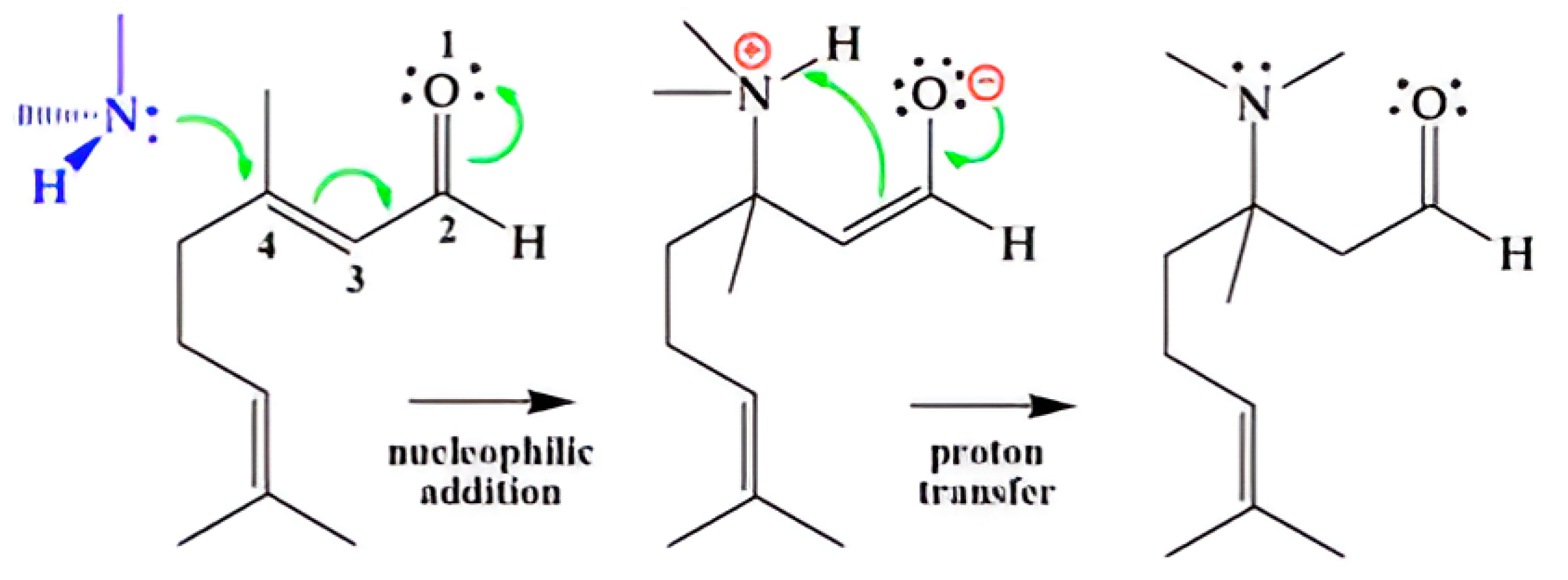
2.5. Frontier Molecular Orbitals and Conjugate Additions of Essential Oils Against Malassezia furfur
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Extraction of Essential Oil
4.3. Essential Oil Composition Analysis
4.4. Inhibition Assay of Malassesia furfur Using Agar Diffusion and Broth Microdilution Methods
4.4.1. Subculturing Malassesia furfur
4.4.2. Preparation of Modified Dixon’s Medium
4.4.3. Inhibition Assay of Malassesia furfur via Agar Diffusion Method
4.4.4. Inhibition Assay of Malassesia furfur via Broth Microdilution Method
4.4.5. Evaluation of the Synergistic Effects of Combined Essential Oils on Malassesia furfur Using the Broth Microdilution Method
- If ΣFIC > 1, it indicates an antagonistic effect of the mixed essential oils.
- If ΣFIC = 1, it signifies an additive effect of the mixed essential oils.
- If ΣFIC < 1, it demonstrates a synergistic effect of the mixed essential oils.
- MICA is the MIC of essential oil A (the first essential oil).
- MICB is the MIC of essential oil B (the second essential oil).
- {a} represents the proportion of essential oil A multiplied by the MIC of the mixed essential oils.
- {b} represents the proportion of essential oil B multiplied by the MIC of the mixed essential oils.
4.5. Calculation of Frontier Molecular Orbitals and Visualization
- Orca filename.inp > filename.out
- Orca_2mkl filename–molden
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CFU | Colony-forming unit |
| DMSO | Dimethyl sulfoxide |
| FMO | Frontier molecular orbital |
| GC-MS | Gas chromatography-mass spectrometry |
| HCl | Hydrochloric acid |
| HOMO | Highest occupied molecular orbital |
| IBO | Intrinsic bond orbital |
| LUMO | Lowest unoccupied molecular orbital |
| MIC | Minimum inhibitory concentration |
| MO | Molecular orbital |
| mL | Milliliter |
| NMGO | no microbial growth observed |
| i.d. | Internal diameter |
| ΣFIC | Sum of fractional inhibitory concentrations |
| °C | Degree Celsius |
| µg | Microgram |
| µL | Microliter |
| µm | Micrometer |
References
- Ranganathan, S.; Mukhopadhyay, T. Dandruff: The most commercially exploited skin disease. Indian J. Dermatol. 2010, 55, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Ahmad, K. Antimicrobial properties of some plant essential oils against two human pathogens. Int. J. Pharm. Chem. Anal. 2023, 9, 184–187. [Google Scholar] [CrossRef]
- Khusnul, K.; Anisa, P.P.; Virgianti, D.P. Effect of Clove essential oil (Syzygium aromaticum) against the growth of dandruff scalps-causing fungal pathogen using Kirby-Bauer method in vitro. J. Microb. Syst. Biotechnol. 2021, 3, 41–48. [Google Scholar] [CrossRef]
- Kanlayavattanakul, W.C.N.L.M. Anti-dandruff hair tonic containing lemongrass (Cymbopogon flexuosus) oil. Forsch. Komplementmed. 2015, 22, 226–229. [Google Scholar] [CrossRef]
- Sawarkar, S.; Deshmukh, V.; Jayaganesh, S.; Perumal, O. Clinical evaluation of herbal active enriched shampoo in anti-dandruff treatment. Theranostics Respir. Ski. Dis. 2018, 1, 31–34. [Google Scholar] [CrossRef]
- Wulandari, D.; Sopyan, I.; Ginaris, R.; Fathurrohim, M.; Maya, I. Potential of essential oil as anti-dandruff in scalp treatment preparations. FITOFARMAKA J. Ilm. Farm. 2022, 12, 156–168. [Google Scholar] [CrossRef]
- reza Naeini, A.; Nazeri, M.; Shokri, H. Inhibitory effect of plant essential oils on Malassezia strains from Iranian dermatitis patients. J. Herbmed Pharmacol. 2017, 7, 18–21. [Google Scholar] [CrossRef]
- Saxena, R.; Mittal, P.; Clavaud, C.; Dhakan, D.B.; Roy, N.; Breton, L.; Misra, N.; Sharma, V.K. Longitudinal study of the scalp microbiome suggests coconut oil to enrich healthy scalp commensals. Sci. Rep. 2021, 11, 7220. [Google Scholar] [CrossRef]
- Fozia Anjum, F.A.; Bukhari, S.; Muhammad Shahid, M.S.; Bokhari, T.; Talpur, M. Exploration of nutraceutical potential of herbal oil formulated from parasitic plant. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 78–86. [Google Scholar] [CrossRef]
- Alam, P.; Imran, M.; Ali, A.; Majid, H. Cananga odorata (Ylang-Ylang) essential oil containing nanoemulgel for the topical treatment of scalp psoriasis and dandruff. Gels 2024, 10, 303. [Google Scholar] [CrossRef]
- Al-Ammari, A.M.; Al-Attraqhchi, A.A.; Al-Ahmer, S.D. Molecular Characterization of Malassezia furfur isolated from patients with pityriasis versicolor compared to healthy control in Baghdad, Iraq. J. Fac. Med. Baghdad 2016, 58, 85–89. [Google Scholar] [CrossRef]
- Chou, S.-T.; Lai, C.-C.; Lai, C.-P.; Chao, W.-W. Chemical composition, antioxidant, anti-melanogenic and anti-inflammatory activities of Glechoma hederacea (Lamiaceae) essential oil. Ind. Crops Prod. 2018, 122, 675–685. [Google Scholar] [CrossRef]
- Soares, B.V.; Morais, S.M.; dos Santos Fontenelle, R.O.; Queiroz, V.A.; Vila-Nova, N.S.; Pereira, C.M.; Brito, E.S.; Neto, M.A.; Brito, E.H.; Cavalcante, C.S. Anti-fungal activity, toxicity and chemical composition of the essential oil of Coriandrum sativum L. fruits. Molecules 2012, 17, 8439–8448. [Google Scholar] [CrossRef]
- Tanjung, I.K. Uji Aktivitas Antifungi Ekstrak Herba Floss Flower terhadap Pertumbuhan Malassezia furfur dan Tricophyton mentagrophytes. In Bandung Conference Series: Pharmacy; Universitas Islam Bandung: Bandung, Indonesia, 2024. [Google Scholar]
- Sim, J.X.F.; Khazandi, M.; Chan, W.Y.; Trott, D.J.; Deo, P. Antimicrobial activity of thyme oil, oregano oil, thymol and carvacrol against sensitive and resistant microbial isolates from dogs with otitis externa. Vet. Dermatol. 2019, 30, 524-e159. [Google Scholar] [CrossRef]
- Bounab, S.; Lograda, T.; Ramdani, M.; Chalard, P. Chemical composition and antibacterial activity of essential oils of Thymelaea hirsuta from Algeria. Biodiv. J. Biol. Divers. 2019, 20, 2868–2876. [Google Scholar] [CrossRef]
- Achar, P.N.; Quyen, P.; Adukwu, E.C.; Sharma, A.; Msimanga, H.Z.; Nagaraja, H.; Sreenivasa, M.Y. Investigation of the anti-fungal and anti-aflatoxigenic potential of plant-based essential oils against Aspergillus flavus in peanuts. J. Fungi 2020, 6, 383. [Google Scholar] [CrossRef]
- Savarirajan, D.; Ramesh, V.; Muthaiyan, A. In vitro antidermatophytic activity of bioactive compounds from selected medicinal plants. J. Anal. Sci. Technol. 2021, 12, 53. [Google Scholar] [CrossRef]
- Mansour, M.M.; Salem, M.Z.; Hassan, R.R.A.; Ali, H.M.; Al Farraj, D.A.; Elshikh, M.S. Anti-fungal potential of three natural oils and their effects on the thermogravimetric and chromatic behaviors when applied to historical paper and various commercial paper sheets. BioResources 2021, 16, 492–514. [Google Scholar] [CrossRef]
- Rahayu, M.S.; Mellaratna, W.P.; Najah, N. The preliminary study on the anti-fungal effect of Kaffir lime (Citrus hystrix DC) peels extract against Malassezia furfur. Int. J. Second. Metab. 2024, 11, 486–493. [Google Scholar] [CrossRef]
- Rhimi, W.; Mohammed, M.A.; Zarea, A.A.K.; Greco, G.; Tempesta, M.; Otranto, D.; Cafarchia, C. Anti-fungal, antioxidant and antibiofilm activities of essential oils of Cymbopogon spp. Antibiotics 2022, 11, 829. [Google Scholar] [CrossRef]
- Li, M.; Ren, Y.; Han, Y.; Dong, Y.-M.; Wu, S.-J.; Zhang, W.; Le, Q.; Lu, Y.-M.; Ma, H. Novel pyrimidine-triazole schiff bases: Synthesis, anti-fungal activities, DFT and molecular docking. Rev. Chim. 2021, 72, 65–74. [Google Scholar] [CrossRef]
- Dongare, R.K.; Inamdar, S.N.; Tigote, R.M. Dft calculations of thiourea derivatives containing a thiazole moiety for the evaluation of anti-fungal activity. J. Adv. Sci. Res. 2022, 13, 380–383. [Google Scholar] [CrossRef]
- Monika; Chander; Sharma, D.; Sharma, P.K.; Ram, S. Synthesis and biological evaluation of novel benzenesulfonamide incorporated thiazole–triazole hybrids as antimicrobial and antioxidant agents. Arch. Pharm. 2024, 357, 2300650. [Google Scholar] [CrossRef]
- Wu, W.N.; Jiang, Y.M.; Fei, Q.; Du, H.T.; Yang, M.F. Synthesis and anti-fungal activity of novel 1, 2, 4-triazole derivatives containing an amide moiety. J. Heterocycl. Chem. 2020, 57, 1379–1386. [Google Scholar] [CrossRef]
- Emami, L.; Jamshidzadeh, A.; Khabnadideh, S.; Pakshir, K.; Jamshidi, M.; Rahat, S.; Arya, S.; Rezaei, Z. In vivo anti-fungal activity and computational studies of some azole derivatives against Candida albicans. J. Chem. 2023, 2023, 7834474. [Google Scholar] [CrossRef]
- Morad, R.; Akbari, M.; Maaza, M. Theoretical study of chemical reactivity descriptors of some repurposed drugs for COVID-19. MRS Adv. 2023, 8, 656–660. [Google Scholar] [CrossRef]
- de Menezes, C.P.; Medeiros, C.I.S.; de Lima Perez, A.L.A.; de Sousa, J.P.; Pinheiro, L.S.; de Oliveira Filho, A.A.; de Oliveira Lima, E. Citral: Anti-fungal activity and mode of action, against Cladosporium oxysporum. Ciência Nat. 2020, 42, e54. [Google Scholar] [CrossRef]
- Mizukami, Y. Character of frontier orbitals of antiviral drugs: Candidate drugs against COVID-19. Open J. Phys. Chem. 2020, 10, 158–165. [Google Scholar] [CrossRef]
- Ammar, A.H.; Meniai, A.H.; Zagrouba, F. Experimental study and modeling of essential oil extraction from plants by hydrodistillation. Chem. Eng. Technol. 2014, 37, 1235–1242. [Google Scholar] [CrossRef]
- Oliveira, E.C.d.; Fontes Silva, M.F.; Souza Ramos, C. Chemical composition and antimicrobial activity of essential oil from Piper marginatum leaves obtained by hydrodistillation in pH4, pH7 and pH10. Rev. Acad. Colomb. de Cienc. Exactas Físicas Nat. 2022, 46, 1002–1009. [Google Scholar] [CrossRef]
- Kabotso, D.E.; Neglo, D.; Kwashie, P.; Agbo, I.A.; Abaye, D.A. GC/MS composition and resistance modulatory inhibitory activities of three extracts of lemongrass: Citral modulates the activities of five antibiotics at sub-inhibitory concentrations on methicillin-Resistant Staphylococcus aureus. Chem. Biodivers. 2022, 19, e202200296. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, S. Development of essential oil-containing antimicrobial and deodorizing nanofibrous membranes for sanitary napkin applications. J. Ind. Text. 2024, 54, 15280837241248838. [Google Scholar] [CrossRef]
- Nofia, N.; Martosudiro, M.; Muhibuddin, A. Growth Inhibition of Botrytis cinerea Fungus on Strawberry (Fragaria sp.) Using Kaffir Lime (Citrus Hystrix) Leaf Essential Oil Emulsion. Agro Bali Agric. J. 2024, 7, 118–127. [Google Scholar] [CrossRef]
- Warsito, W.; Palungan, M.H.; Utomo, E.P. Profiling study of the major and minor components of kaffir lime oil (Citrus hystrix DC.) in the fractional distillation process. Pan Afr. Med. J. 2017, 27, 282. [Google Scholar] [CrossRef]
- Zaki, N.A.M.; Jai, J.; Shahrizan, I.S.K.; Fardhyanti, D.S.; Megawati, M.; Imani, N.A.C. An overview of the potential of Citrus hystrix (kaffir lime) essential oil as mosquito repellent. Int. J. Adv. Appl. Sci. 2022, 11, 360–366. [Google Scholar] [CrossRef]
- Thonglem, S.; Khumweera, P.; Lahpun, N. GC-MS analysis, antioxidant activity and antimicrobial activity of kaffir lime (Citrus hystrix DC.) and key lime (Citrus aurantifolia (Christm.) Swingle.) peels essential oils. J. Curr. Sci. Technol. 2023, 13, 620–629. [Google Scholar] [CrossRef]
- El-Zaeddi, H.; Martínez-Tomé, J.; Calín-Sánchez, Á.; Burló, F.; Carbonell-Barrachina, Á.A. Irrigation dose and plant density affect the volatile composition and sensory quality of dill (Anethum graveolens L.). J. Sci. Food Agric. 2017, 97, 427–433. [Google Scholar] [CrossRef]
- Tutulescu, F.; Maria, D.; Corbu, A.; Ionita, E. Antimicrobial effects of several essential oil from aromatic plants. Not. Sci. Biol. 2016, 8, 477. [Google Scholar] [CrossRef][Green Version]
- Kaur, V.; Kaur, R.; Bhardwaj, U. A review on dill essential oil and its chief compounds as natural biocide. Flavour. Fragr. J. 2021, 36, 412–431. [Google Scholar] [CrossRef]
- Tian, J.; Ban, X.; Zeng, H.; He, J.; Chen, Y.; Wang, Y. The mechanism of anti-fungal action of essential oil from dill (Anethum graveolens L.) on Aspergillus flavus. PLoS ONE 2012, 7, e30147. [Google Scholar] [CrossRef]
- Tian, F.; Woo, S.Y.; Lee, S.Y.; Chun, H.S. p-Cymene and its derivatives exhibit antiaflatoxigenic activities against Aspergillus flavus through multiple modes of action. Appl. Biol. Chem. 2018, 61, 489–497. [Google Scholar] [CrossRef]
- Peirovy, Y.; Asle-Rousta, M. Thymol and p-Cymene protect the liver by mitigating oxidative stress, suppressing TNF-α/NF-κ B, and enhancing Nrf2/HO-1 expression in immobilized rats. Chem. Biol. Drug Des. 2024, 104, e14618. [Google Scholar] [CrossRef] [PubMed]
- Baishya, D.; Kadir, A.; Ahmed, A.; Kumar, A.; Srivastav, Y.; Kumar, V.; Krishna, K.; Jain, S.; Sri Venkatesh, U. Taxonomy, phytochemistry and pharmacological significance of Asparagus Racemosus plant: A Comprehensive Review. Int. J. Pharm. Res. 2024, 8, 118–126. [Google Scholar]
- Liu, Y.-T.; Lee, M.-H.; Lin, Y.-S.; Lai, W.-L. The Inhibitory Activity of Citral against Malassezia furfur. Processes 2022, 10, 802. [Google Scholar] [CrossRef]
- He, D.; Wu, X.Q.; Wu, K.; Chai, X.H.; Liang, Y.L.; Zhang, X.Y.; Cha, Q.; Xie, W. Synergistic activity of clove essential oil and thyme essential oil and their interaction against Malassezia furfur, Escherichia coli, Staphylococcus aureus. LWT 2024, 204, 116431. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system—Version 5.0. WIREs Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Knizia, G. IboView v.20211019-RevA.: A Program for Chemical Analysis. Available online: http://www.iboview.org/ (accessed on 20 March 2025).
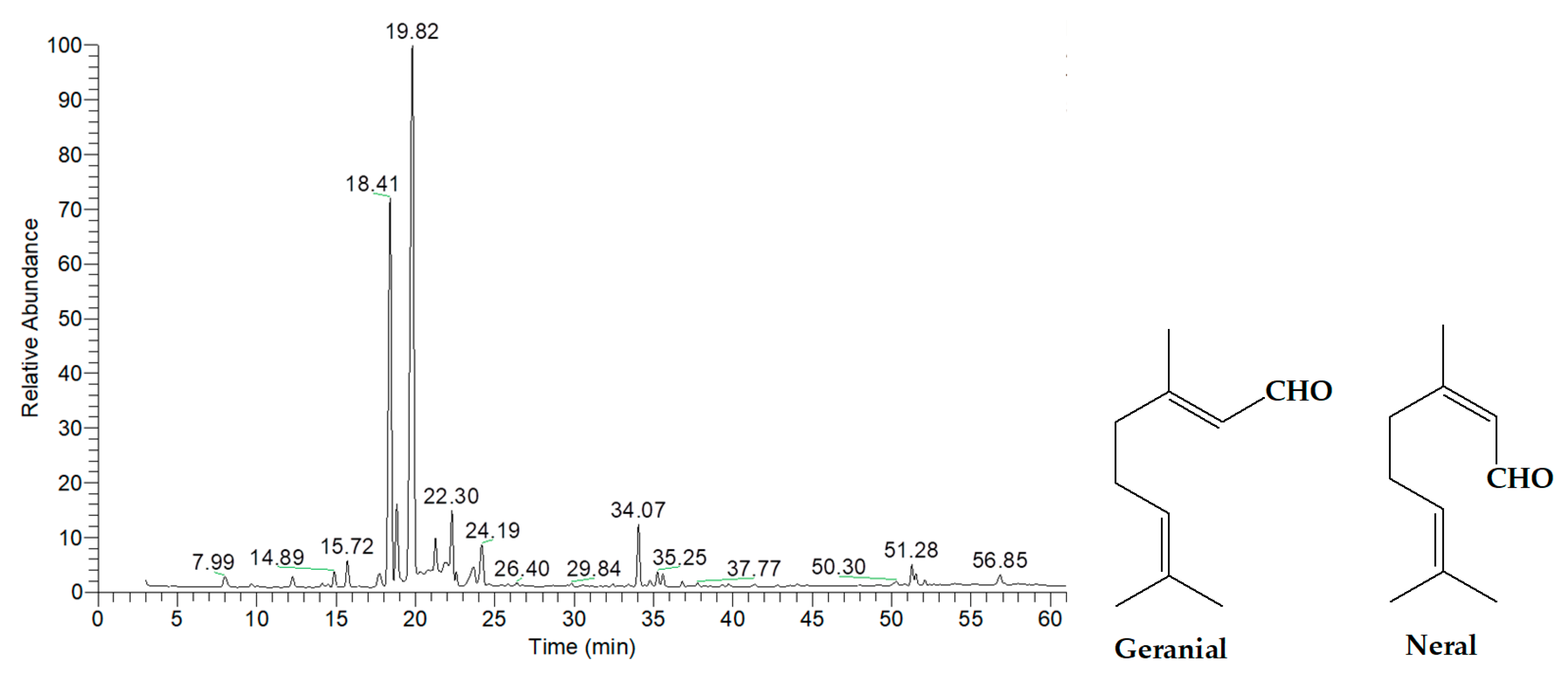










| Plant Samples | Dill Aerial Part | Kaffir Lime Peels | Lime Peels | Shatavari Areal Part | Lemongrass | Kaffir Lime Leaves |
|---|---|---|---|---|---|---|
| Yield (%w/w) | 1.08 ± 0.11 | 0.86 ± 0.12 | 0.68 ± 0.08 | 0.65 ± 0.09 | 0.61 ± 0.08 | 0.27 ± 0.05 |
| RT | Name Compound | Chemical Class | Kovats Index | Area% |
|---|---|---|---|---|
| 19.82 | Geranial | Oxygenated Monoterpenes | 1267 | 45.03 |
| 18.41 | Neral | Oxygenated Monoterpenes | 1238 | 27.07 |
| 18.82 | Geraniol | Oxygenated Monoterpenes | 1252 | 4.52 |
| 22.3 | Citral<dimethoxy-(E)-> | Oxygenated Monoterpenes | 1341 | 3.50 |
| 34.07 | Selina-6-en-4-ol | Oxygenated Sesquiterpenes | 1624 | 3.38 |
| 24.19 | Geranyl acetate | Oxygenated Monoterpenes | 1381 | 3.28 |
| 23.67 | Neric acid | Oxygenated Monoterpenes | 1368 | 2.44 |
| 21.27 | Citral<dimethoxy-(Z)-> | Oxygenated Monoterpenes | 1318 | 1.75 |
| 15.72 | Isocitral<E-> | Oxygenated Monoterpenes | 1180 | 1.49 |
| 17.73 | Citronellol | Oxygenated Monoterpenes | 1225 | 1.16 |
| 51.28 | Unidentified | Other | 1.71 | |
| 14.89 | Isocitral<Z-> | Oxygenated Monoterpenes | 1164 | 0.85 |
| 7.99 | Myrcene | Monoterpene Hydrocarbons | 990 | 0.83 |
| 35.25 | Cadinol<alpha-> | Oxygenated Sesquiterpenes | 1654 | 0.74 |
| 12.26 | Linalool | Oxygenated Monoterpenes | 1096 | 0.71 |
| 56.85 | Unidentified | Other | 0.64 | |
| 35.6 | Juniper camphor | Oxygenated Monoterpenes | 1663 | 0.61 |
| 34.79 | Murrolol<epi-alpha-> (=tau-muurolol) | Oxygenated Sesquiterpenes | 1642 | 0.39 |
| 36.82 | Eudesm-7(11)-en-4-ol | Oxygenated Sesquiterpenes | 1700 | 0.25 |
| 33.4 | Eudesmol<5-epi-7-epi-alpha-> | Oxygenated Sesquiterpenes | 1607 | 0.16 |
| 32.43 | Caryophyllene oxide | Oxygenated Sesquiterpenes | 1583 | 0.14 |
| Sums of percentage of oxygenated monoterpenes | 92.41 | |||
| Sums of percentage oxygenated sesquiterpenes | 5.06 | |||
| Sums of percentage of monoterpene hydrocarbons | 0.83 | |||
| Sums of percentage of other compounds | 2.35 | |||
| Total percentage of all identified compounds | 97.65 | |||
| RT | Name Compound | Chemical Class | Kovats Index | Area% |
|---|---|---|---|---|
| 14.74 | Citronellal | Oxygenated Monoterpenes | 1153 | 83.76 |
| 17.75 | Citronellol | Oxygenated Monoterpenes | 1225 | 5.20 |
| 22.99 | Citronellyl acetate | Oxygenated Monoterpenes | 1352 | 4.61 |
| 12.26 | Linalool | Oxygenated Monoterpenes | 1096 | 2.04 |
| 7.54 | Sabinene | Monoterpene Hydrocarbons | 975 | 0.85 |
| 31.58 | Nerolidol<E-> | Oxygenated Sesquiterpenes | 1563 | 0.55 |
| 11.14 | Linalool oxide <cis-> (furanoid) | Oxygenated Monoterpenes | 1072 | 0.52 |
| 7.99 | Myrcene | Monoterpene Hydrocarbons | 990 | 0.51 |
| 24.21 | Geranyl acetate | Oxygenated Monoterpenes | 1381 | 0.42 |
| 11.78 | Linalool oxide<trans-> (furanoid) | Oxygenated Monoterpenes | 1086 | 0.3 |
| 31.11 | Elemol | Oxygenated Sesquiterpenes | 1549 | 0.25 |
| 25.83 | Caryophyllene(E-) | Oxygenated Sesquiterpenes | 1419 | 0.24 |
| 15.78 | Terpinen-4-ol | Oxygenated Monoterpenes | 1177 | 0.22 |
| 10.06 | Ocimene<(E)-beta-> | Monoterpene Hydrocarbons | 1050 | 0.21 |
| 29.82 | Amorphene<delta-> | Sesquiterpene Hydrocarbons | 1512 | 0.14 |
| 28.95 | Bicyclogermacrene | Sesquiterpene Hydrocarbons | 1500 | 0.13 |
| 27.32 | Humulene<alpha-> | Sesquiterpene Hydrocarbons | 1454 | 0.04 |
| Sums of percentage of oxygenated monoterpenes | 97.62 | |||
| Sums of percentage oxygenated sesquiterpenes | 1.04 | |||
| Sums of percentage of monoterpene hydrocarbons | 1.57 | |||
| Sums of percentage of sesquiterpene hydrocarbons | 0.31 | |||
| Sums of percentage of other compounds | 0.00 | |||
| Total percentage of all identified compounds | 100.00 | |||
| RT | Name Compound | Chemical Class | Kovats Index | Area% |
|---|---|---|---|---|
| 7.72 | Pinene<beta-> | Monoterpene Hydrocarbons | 979 | 29.49 |
| 9.5 | Sylvestrene | Monoterpene Hydrocarbons | 1030 | 20.77 |
| 14.56 | Citronellal | Oxygenated Monoterpenes | 1153 | 14.02 |
| 7.58 | Sabinene | Monoterpene Hydrocarbons | 975 | 7.49 |
| 17.75 | Citronellol | Oxygenated Monoterpenes | 1225 | 5.80 |
| 15.78 | Terpinen-4-ol | Oxygenated Monoterpenes | 1177 | 5.27 |
| 16.44 | Terpineol<alpha-> | Oxygenated Monoterpenes | 1188 | 4.48 |
| 12.26 | Linalool | Oxygenated Monoterpenes | 1096 | 2.05 |
| 6.36 | Pinene<alpha-> | Monoterpene Hydrocarbons | 939 | 1.83 |
| 11.14 | Linalool oxide <cis-> (furanoid) | Oxygenated Monoterpenes | 1072 | 1.80 |
| 11.76 | Linalool oxide<trans-> (furanoid) | Oxygenated Monoterpenes | 1086 | 1.07 |
| 10.56 | Terpinene<gamma-> | Monoterpene Hydrocarbons | 1059 | 1.04 |
| 29.84 | Cadinene<delta-> | Sesquiterpene Hydrocarbons | 1523 | 0.89 |
| 23.96 | Copaene<alpha-> | Sesquiterpene Hydrocarbons | 1376 | 0.64 |
| 24.5 | Cubebene<beta-> | Sesquiterpene Hydrocarbons | 1388 | 0.46 |
| 18.74 | Geraniol | Oxygenated Monoterpenes | 1252 | 0.40 |
| 22.97 | Citronellyl acetate | Oxygenated Monoterpenes | 1352 | 0.40 |
| 25.83 | Caryophyllene(E-) | Sesquiterpene Hydrocarbons | 1419 | 0.32 |
| 13.38 | Menth-2-en-1ol<trans-para-> | Oxygenated Monoterpenes | 1140 | 0.30 |
| 24.21 | Geranyl acetate | Oxygenated Monoterpenes | 1381 | 0.23 |
| 28.35 | Germacrene D | Sesquiterpene Hydrocarbons | 1485 | 0.23 |
| 29.04 | Muurolene<alpha-> | Sesquiterpene Hydrocarbons | 1500 | 0.22 |
| 31.11 | Elemol | Oxygenated Sesquiterpenes | 1549 | 0.21 |
| 27.32 | Humulene<alpha-> | Sesquiterpene Hydrocarbons | 1454 | 0.18 |
| 47.78 | Unidentified | Other | 0.16 | |
| 35.23 | Cadinol<alpha-> | Oxygenated Sesquiterpenes | 1654 | 0.13 |
| 22.72 | Menthol<8-hydroxy-neo-> | Oxygenated Monoterpenes | 1330 | 0.12 |
| Sums of percentage of monoterpene hydrocarbons | 60.62 | |||
| Sums of percentage of oxygenated monoterpenes | 35.94 | |||
| Sums of percentage oxygenated sesquiterpenes | 0.34 | |||
| Sums of percentage of sesquiterpene hydrocarbons | 2.62 | |||
| Sums of percentage of other compounds | 0.16 | |||
| Total percentage of all identified compounds | 99.84 | |||
| RT | Name Compound | Chemical Class | Kovats Index | Area% |
|---|---|---|---|---|
| 9.55 | Sylvestrene | Monoterpene Hydrocarbons | 1030 | 62.29 |
| 7.72 | Pinene<beta-> | Monoterpene Hydrocarbons | 979 | 15.33 |
| 10.58 | Terpinene<gamma-> | Monoterpene Hydrocarbons | 1059 | 3.48 |
| 6.34 | Pinene<alpha-> | Monoterpene Hydrocarbons | 939 | 2.82 |
| 29.45 | Bisabolene<beta-> | Sesquiterpene Hydrocarbons | 1505 | 2.31 |
| 23.36 | Neryl acetate | Oxygenated Monoterpenes | 1361 | 1.91 |
| 19.57 | Geranial | Oxygenated Monoterpenes | 1267 | 1.82 |
| 26.36 | Bergamotene<alpha-trans-> | Sesquiterpene Hydrocarbons | 1434 | 1.55 |
| 16.42 | Terpineol<alpha-> | Oxygenated Monoterpenes | 1188 | 1.53 |
| 18.26 | Neral | Oxygenated Monoterpenes | 1238 | 1.52 |
| 17.62 | Nerol | Oxygenated Monoterpenes | 1229 | 1.31 |
| 18.74 | Geraniol | Oxygenated Monoterpenes | 1252 | 0.60 |
| 15.74 | Terpinen-4-ol | Oxygenated Monoterpenes | 1177 | 0.57 |
| 12.26 | Linalool | Oxygenated Monoterpenes | 1096 | 0.54 |
| 32.43 | Caryophyllene oxide | Oxygenated Sesquiterpenes | 1583 | 0.48 |
| 21.23 | Citral<dimethoxy-(Z)-> | Oxygenated Monoterpenes | 1318 | 0.45 |
| 22.22 | Citral<dimethoxy-(E)-> | Oxygenated Monoterpenes | 1341 | 0.29 |
| 25.82 | Caryophyllene(E-) | Sesquiterpene Hydrocarbons | 1419 | 0.27 |
| 24.21 | Geranyl acetate | Oxygenated Monoterpenes | 1381 | 0.26 |
| 25.56 | Bergamotene<alpha-cis-> | Sesquiterpene Hydrocarbons | 1412 | 0.17 |
| 36.41 | Bisabolol<alpha-> | Oxygenated Sesquiterpenes | 1685 | 0.17 |
| 35.39 | Unidentified | Other | 0.14 | |
| 35.87 | Unidentified | Other | 0.13 | |
| 34.73 | Unidentified | Other | 0.06 | |
| Sums of percentage of monoterpene hydrocarbons | 83.92 | |||
| Sums of percentage of oxygenated monoterpenes | 11.45 | |||
| Sums of percentage oxygenated sesquiterpenes | 0.65 | |||
| Sums of percentage of sesquiterpene hydrocarbons | 4.30 | |||
| Sums of percentage of other compounds | 0.33 | |||
| Total percentage of all identified compounds | 99.67 | |||
| RT | Name Compound | Chemical Class | Kovat’s Index | Area% |
|---|---|---|---|---|
| 8.7 | Phellandrene<alpha-> | Monoterpene Hydrocarbons | 1002 | 43.54 |
| 16.09 | Dill ether | Oxygenated Monoterpenes | 1186 | 25.24 |
| 9.55 | Phellandrene<beta-> | Monoterpene Hydrocarbons | 1029 | 10.46 |
| 9.42 | Cymene<ortho-> | Monoterpene Hydrocarbons | 1026 | 5.05 |
| 18.8 | Limonene dioxide | Monoterpene Hydrocarbons | 1251 | 3.45 |
| 30.15 | Myristicin | Other | 1518 | 3.26 |
| 22.18 | Unidentified | Other | 2.91 | |
| 6.34 | Pinene<alpha-> | Monoterpene Hydrocarbons | 939 | 1.99 |
| 26.11 | Barosma camphor | Oxygenated Monoterpenes | 1427 | 0.92 |
| 25.06 | 2,3-Bornanediol | Other | 1410 | 0.87 |
| 21.93 | Pinanediol<cis-2,3-> | Oxygenated Monoterpenes | 1320 | 0.48 |
| 26.47 | 2-Cyclohexen-1-one, 4-hydroxy-3-methyl-6-(1-methylethyl)-, trans- | Oxygenated Monoterpenes | 1436 | 0.47 |
| 8.01 | Myrcene | Monoterpene Hydrocarbons | 990 | 0.46 |
| 20.96 | Unidentified | Other | 0.44 | |
| 20.61 | Unidentified | Other | 0.36 | |
| 7.54 | Sabinene | Monoterpene Hydrocarbons | 975 | 0.11 |
| Sums of percentage of monoterpene hydrocarbons | 65.06 | |||
| Sums of percentage of oxygenated monoterpenes | 27.11 | |||
| Sums of percentage of other compounds | 7.84 | |||
| Total percentage of all identified compounds | 96.29 | |||
| RT | Name Compound | Chemical Class | Kovats Index | Area% |
|---|---|---|---|---|
| 8.7 | Phellandrene<alpha-> | Monoterpene Hydrocarbons | 1002 | 26.14 |
| 17.83 | Thymol, methyl ether | Oxygenated Monoterpenes | 1235 | 18.07 |
| 9.36 | Cymene<ortho-> | Monoterpene Hydrocarbons | 1026 | 10.30 |
| 28.33 | Germacrene D | Sesquiterpene Hydrocarbons | 1485 | 10.03 |
| 25.66 | Cymene<2,5-dimethoxy-para-> | Oxygenated Monoterpenes | 1426 | 5.17 |
| 7.72 | Pinene<beta-> | Monoterpene Hydrocarbons | 979 | 4.00 |
| 7.97 | Myrcene | Monoterpene Hydrocarbons | 990 | 2.90 |
| 18.8 | Limonene dioxide | Oxygenated Monoterpenes | 1251 | 2.70 |
| 31.83 | Unidentified | Other | 2.08 | |
| 33.16 | Unidentified | Other | 1.62 | |
| 22.18 | Unidentified | Other | 1.60 | |
| 22.32 | Unidentified | Other | 1.56 | |
| 25.1 | 2,3-Bornanediol | Other | 1401 | 1.43 |
| 21.95 | Pinanediol<cis-2,3-> | Oxygenated Monoterpenes | 1320 | 1.42 |
| 10.06 | Ocimene<(E)-beta-> | Monoterpene Hydrocarbons | 1050 | 1.14 |
| 6.34 | Pinene<alpha-> | Monoterpene Hydrocarbons | 939 | 1.07 |
| 26.51 | Unidentified | Other | 1.05 | |
| 26.16 | Piperitone oxide | Oxygenated Monoterpenes | 1428 | 0.96 |
| 38.89 | Unidentified | Other | 0.79 | |
| 18.43 | Mesityl methyl ketone | Other | 1239 | 0.77 |
| 42.13 | Unidentified | Other | 0.72 | |
| 27.32 | Humulene<alpha-> | Sesquiterpene Hydrocarbons | 1454 | 0.67 |
| 35.23 | Cadinol<alpha-> | Oxygenated Sesquiterpenes | 1654 | 0.51 |
| 20.96 | Unidentified | Other | 0.47 | |
| 20.58 | Unidentified | Other | 0.45 | |
| 20.19 | Bornyl acetate | Oxygenated Monoterpenes | 1288 | 0.43 |
| 43.62 | Unidentified | Other | 0.43 | |
| 34.38 | Unidentified | Other | 0.42 | |
| 39.91 | Unidentified | Other | 0.42 | |
| 16.75 | Pinocarveol<cis-> | Oxygenated Monoterpenes | 1184 | 0.35 |
| 34.73 | Cadinol<epi-alpha-> (=tau-cadinol) | Oxygenated Sesquiterpenes | 1640 | 0.31 |
| Sums of percentage of monoterpene hydrocarbons | 45.55 | |||
| Sums of percentage of oxygenated monoterpenes | 29.10 | |||
| Sums of percentage of sesquiterpene hydrocarbons | 10.70 | |||
| Sums of percentage oxygenated sesquiterpenes | 0.82 | |||
| Sums of percentage of other compounds | 13.81 | |||
| Total percentage of all identified compounds | 88.39 | |||
| Plant Samples | Lemongrass | Kaffir Lime Leaves | Kaffir Lime Peels | Lime Peels | Dill Aerial Parts | Shatavari Aerial Parts |
|---|---|---|---|---|---|---|
| Diameter (cm ± SD) | NMGO | NMGO | NMGO | NMGO | NMGO | 1.05 ± 0.12 |
| MIC (% v/v) | 0.125 | 0.25 | 0.50 | 1.00 | 0.25 | 0.25 |
| Combined Essential Oils | Ratios | MIC (% v/v) | ΣFIC | Interpretation |
|---|---|---|---|---|
| Lemongrass and Lime peels essential oils | 1:3 | 0.25 | 2.75 | Antagonism |
| 1:1 | 0.125 | 1.125 | Antagonism | |
| 3:1 | 0.125 | 3.125 | Antagonism | |
| Lemongrass and Kaffir lime leaves essential oils | 1:3 | 0.25 | 3.5 | Antagonism |
| 1:1 | 0.125 | 1.25 | Antagonism | |
| 3:1 | 0.0625 | 1.625 | Antagonism | |
| Lemongrass and Kaffir lime peels essential oils | 1:3 | 0.0625 | 1.25 | Antagonism |
| 1:1 | 0.0625 | 0.75 | Synergism | |
| 3:1 | 0.0625 | 1.75 | Antagonism | |
| Lemongrass and Dill essential oils | 1:3 | 0.0625 | 1.25 | Antagonism |
| 1:1 | 0.0625 | 0.75 | Synergism | |
| 3:1 | 0.0625 | 1.75 | Antagonism | |
| Lemongrass and Shatavari essential oils | 1:3 | 0.0625 | 1.25 | Antagonism |
| 1:1 | 0.0625 | 0.75 | Synergism | |
| 3:1 | 0.0625 | 1.75 | Antagonism |
| Combined Essential Oils | Ratios | MIC (% v/v) | ΣFIC | Interpretation |
|---|---|---|---|---|
| Citral | – | 0.03125 | – | – |
| Citronellal | – | 0.125 | – | – |
| Citral and Citronellal | 1:3 | 0.0625 | 0.875 | Synergism |
| 1:1 | 0.0625 | 1.25 | Antagonism | |
| 3:1 | 0.03125 | 0.8125 | Synergism |
| Essential Oils | HOMO (eV) | LUMO (eV) | Energy Gap (eV) |
|---|---|---|---|
| Citral | −7.5407 | 1.6642 | 9.2049 |
| Citronellal | −6.7305 | 1.1048 | 7.8353 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tadtong, S.; Chantavacharakorn, R.; Khayankan, S.; Akachaipaibul, P.; Eiamart, W.; Samee, W. Synergistic Antifungal Properties, Chemical Composition, and Frontier Molecular Orbital Analysis of Essential Oils from Lemongrass, Kaffir Lime, Lime, Dill, and Shatavari Against Malassezia furfur. Int. J. Mol. Sci. 2025, 26, 5601. https://doi.org/10.3390/ijms26125601
Tadtong S, Chantavacharakorn R, Khayankan S, Akachaipaibul P, Eiamart W, Samee W. Synergistic Antifungal Properties, Chemical Composition, and Frontier Molecular Orbital Analysis of Essential Oils from Lemongrass, Kaffir Lime, Lime, Dill, and Shatavari Against Malassezia furfur. International Journal of Molecular Sciences. 2025; 26(12):5601. https://doi.org/10.3390/ijms26125601
Chicago/Turabian StyleTadtong, Sarin, Rada Chantavacharakorn, Sarocha Khayankan, Puriputt Akachaipaibul, Wanna Eiamart, and Weerasak Samee. 2025. "Synergistic Antifungal Properties, Chemical Composition, and Frontier Molecular Orbital Analysis of Essential Oils from Lemongrass, Kaffir Lime, Lime, Dill, and Shatavari Against Malassezia furfur" International Journal of Molecular Sciences 26, no. 12: 5601. https://doi.org/10.3390/ijms26125601
APA StyleTadtong, S., Chantavacharakorn, R., Khayankan, S., Akachaipaibul, P., Eiamart, W., & Samee, W. (2025). Synergistic Antifungal Properties, Chemical Composition, and Frontier Molecular Orbital Analysis of Essential Oils from Lemongrass, Kaffir Lime, Lime, Dill, and Shatavari Against Malassezia furfur. International Journal of Molecular Sciences, 26(12), 5601. https://doi.org/10.3390/ijms26125601






