Modulation of the Immune Response by Nematode Derived Molecules
Abstract
1. Introduction
2. Results
- Prostaglandin E2 (PGE2)
- 2.
- Chitinase
- 3.
- Triosephosphate isomerase (TPI) and Nucleoside diphosphate kinase (NDK)
- 4.
- Interferon gamma mimic
- 5.
- The protein p43
- 6.
- Serine Protease Inhibitor (Serpin)
- 7.
- Abundant larval transcripts 1 and 2 in B. malayi
- 8.
- Cystatins
- 9.
- Tgh-2
- 10.
- Asparaginyl t-RNA synthetase
- 11.
- ES-62
- 12.
- Nematode-derived Migration inhibitory factor (nMIF)
- PAS-1
- Mucins
- Astacin
- Peptides that block the voltage-gated potassium channel 1.3 (Kv1.3)
- Tissue inhibitor of metalloprotease-1 and -2
- Neutrophil Inhibitory Factor (NIF)
- Metalloprotease
- Activation-associated protein 2 (ASP-2)
- Alarmin release inhibitor (HpARI)
- H. polygyrus Binds Alarmin receptor and Inhibits (HpBARI)
- Small RNA molecules that suppress immunity
- TGF-β mimic (TGM)
- Glutamate dehydrogenase (p66)
- Acetylcholinesterase from N. brasiliensis
- Apyrases
- Galectin
3. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Stear, M.; Preston, S.; Piedrafita, D.; Donskow-Łysoniewska, K. The Immune Response to Nematode Infection. Int. J. Mol. Sci. 2023, 24, 2283. [Google Scholar] [CrossRef] [PubMed]
- McNeilly, T.N.; Nisbet, A.J. Immune modulation by helminth parasites of ruminants: Implications for vaccine development and host immune competence. Parasite 2014, 21, 51. [Google Scholar] [CrossRef] [PubMed]
- Maizels, R.M. Regulation of immunity and allergy by helminth parasites. Allergy 2020, 75, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Maizels, R.M.; Smits, H.H.; McSorley, H.J. Modulation of Host Immunity by Helminths: The Expanding Repertoire of Parasite Effector Molecules. Immunity 2018, 49, 801–818. [Google Scholar] [CrossRef]
- Stear, M.J.; Doligalska, M.; Donskow-Schmelter, K. Alternatives to anthelmintics for the control of nematodes in livestock. Parasitology 2007, 134, 139–151. [Google Scholar] [CrossRef]
- Helmby, H. Human helminth therapy to treat inflammatory disorders-where do we stand? BMC Immunol. 2015, 16, 12. [Google Scholar] [CrossRef]
- Chakraborty, P.; Aravindhan, V.; Mukherjee, S. Helminth-derived biomacromolecules as therapeutic agents for treating inflammatory and infectious diseases: What lessons do we get from recent findings? Int. J. Biol. Macromol. 2023, 241, 124649. [Google Scholar] [CrossRef]
- Hewitson, J.P.; Grainger, J.R.; Maizels, R.M. Helminth immunoregulation: The role of parasite secreted proteins in modulating host immunity. Mol. Biochem. Parasitol. 2009, 167, 1–11. [Google Scholar] [CrossRef]
- Maizels, R.M.; McSorley, H.J. Regulation of the host immune system by helminth parasites. J. Allergy Clin. Immunol. 2016, 138, 666–675. [Google Scholar] [CrossRef]
- McManus, C.M.; Maizels, R.M. Regulatory T cells in parasite infections: Susceptibility, specificity and specialisation. Trends Parasitol. 2023, 39, 547–562. [Google Scholar] [CrossRef]
- Karabowicz, J.; Długosz, E.; Bąska, P.; Wiśniewski, M. Nematode Orthologs of Macrophage Migration Inhibitory Factor (MIF) as Modulators of the Host Immune Response and Potential Therapeutic Targets. Pathogens 2022, 11, 258. [Google Scholar] [CrossRef] [PubMed]
- Donskow-Łysoniewska, K.; Maruszewska-Cheruiyot, M.; Stear, M. The interaction of host and nematode galectins influences the outcome of gastrointestinal nematode infections. Parasitology 2021, 148, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Maruszewska-Cheruiyot, M.; Stear, M.; Donskow-Łysoniewska, K. Galectins-Important players of the immune response to CNS parasitic infection. Brain Behav. Immun.-Health 2021, 13, 100221. [Google Scholar] [CrossRef] [PubMed]
- Blaxter, M.L.; De Ley, P.; Garey, J.R.; Liu, L.X.; Scheldeman, P.; Vierstraete, A.; Vanfleteren, J.R.; Mackey, L.Y.; Dorris, M.; Frisse, L.M. A molecular evolutionary framework for the phylum Nematoda. Nature 1998, 392, 71–75. [Google Scholar] [CrossRef]
- Shears, R.K.; Grencis, R.K. Whipworm secretions and their roles in host-parasite interactions. Parasites Vectors 2022, 15, 348. [Google Scholar] [CrossRef]
- Leroux, L.-P.; Nasr, M.; Valanparambil, R.; Tam, M.; Rosa, B.A.; Siciliani, E.; Hill, D.E.; Zarlenga, D.S.; Jaramillo, M.; Weinstock, J.V.; et al. Analysis of the Trichuris suis excretory/secretory proteins as a function of life cycle stage and their immunomodulatory properties. Sci. Rep. 2018, 8, 15921. [Google Scholar] [CrossRef]
- Bancroft, A.J.; Levy, C.W.; Jowitt, T.A.; Hayes, K.S.; Thompson, S.; McKenzie, E.A.; Ball, M.D.; Dubaissi, E.; France, A.P.; Bellina, B.; et al. The major secreted protein of the whipworm parasite tethers to matrix and inhibits interleukin-13 function. Nat. Commun. 2019, 10, 2344. [Google Scholar] [CrossRef]
- Laan, L.C.; Williams, A.R.; Stavenhagen, K.; Giera, M.; Kooij, G.; Vlasakov, I.; Kalay, H.; Kringel, H.; Nejsum, P.; Thamsborg, S.M.; et al. The whipworm (Trichuris suis) secretes prostaglandin E2 to suppress proinflammatory properties in human dendritic cells. FASEB J. 2017, 31, 719–731. [Google Scholar] [CrossRef]
- Liu, L.X.; Buhlmann, J.E.; Weller, P.F. Release of prostaglandin E2 by microfilariae of Wuchereria bancrofti and Brugia malayi. Am. J. Trop. Med. Hyg. 1992, 46, 520–523. [Google Scholar] [CrossRef]
- Ebner, F.; Lindner, K.; Janek, K.; Niewienda, A.; Malecki, P.H.; Weiss, M.S.; Sutherland, T.E.; Heuser, A.; Kühl, A.A.; Zentek, J.; et al. A Helminth-Derived Chitinase Structurally Similar to Mammalian Chitinase Displays Immunomodulatory Properties in Inflammatory Lung Disease. J. Immunol. Res. 2021, 2021, 6234836. [Google Scholar] [CrossRef]
- Grencis, R.K.; Entwistle, G.M. Production of an interferon-gamma homologue by an intestinal nematode: Functionally significant or interesting artefact? Parasitology 1997, 115, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, A.J.; McKenzie, A.N.J.; Grencis, R.K. A Critical Role for IL-13 in Resistance to Intestinal Nematode Infection. J. Immunol. 1998, 160, 3453–3461. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.W.; Bancroft, A.J.; Grencis, R.K. The immunomodulatory p43 secreted protein of Trichuris whipworm parasites is a lipid carrier that binds signalling lipids and precursors. bioRxiv 2025. [Google Scholar] [CrossRef]
- Xu, N.; Liu, X.; Tang, B.; Wang, L.; Shi, H.N.; Boireau, P.; Liu, M.; Bai, X. Recombinant Trichinella pseudospiralis Serine Protease Inhibitors Alter Macrophage Polarization In Vitro. Front. Microbiol. 2017, 8, 1834. [Google Scholar] [CrossRef]
- Scudamore, C.L.; Thornton, E.M.; McMillan, L.; Newlands, G.F.J.; Miller, H.R.P. Release of the mucosal mast cell granule chymase, rat mast cell protease II during anaphylaxis is associated with the rapid development of paracellular permeability to macromolecules in rat jejunum. J. Exp. Med. 1995, 182, 1871–1881. [Google Scholar] [CrossRef]
- McDermott, J.R.; Bartram, R.E.; Knight, P.A.; Miller, H.R.P.; Garrod, D.R.; Grencis, R.K. Mast cells disrupt epithelial barrier function during enteric nematode infection. Proc. Natl. Acad. Sci. USA 2003, 100, 7761–7766. [Google Scholar] [CrossRef]
- Gomez-Escobar, N.; Bennett, C.; Prieto-Lafuente, L.; Aebischer, T.; Blackburn, C.C.; Maizels, R.M. Heterologous expression of the filarial nematode alt gene products reveals their potential to inhibit immune function. BMC Biol. 2005, 3, 8. [Google Scholar] [CrossRef]
- Turk, V.; Stoka, V.; Turk, D. Cystatins: Biochemical and structural properties, and medical relevance. Front. Biosci. 2008, 13, 5406–5420. [Google Scholar] [CrossRef]
- Manoury, B.; Gregory, W.F.; Maizels, R.M.; Watts, C. Bm-CPI-2, a cystatin homolog secreted by the filarial parasite Brugia malayi, inhibits class II MHC-restricted antigen processing. Curr. Biol. 2001, 11, 447–451. [Google Scholar] [CrossRef]
- Hartmann, S.; Kyewski, B.; Sonnenburg, B.; Lucius, R. A filarial cysteine protease inhibitor down-regulates T cell proliferation and enhances interleukin-10 production. Eur. J. Immunol. 1997, 27, 2253–2260. [Google Scholar] [CrossRef]
- Schonemeyer, A.; Lucius, R.; Sonnenburg, B.; Brattig, N.; Sabat, R.; Schilling, K.; Bradley, J.; Hartmann, S. Modulation of human T cell responses and macrophage functions by onchocystatin, a secreted protein of the filarial nematode Onchocerca volvulus. J. Immunol. 2001, 167, 3207–3215. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, A.W.; Schulz-Key, H.; Soboslay, P.T.; Taylor, D.W.; MacLennan, K.; Hoffmann, W.H. Litomosoides sigmodontis cystatin acts as an immunomodulator during experimental filariasis. Int. J. Parasitol. 2002, 32, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, G.; Li, Z.; Chen, Y.; Liu, Y.; Liu, B.; Su, Z. Modulation of dendritic cell function and immune response by cysteine protease inhibitor from murine nematode parasite Heligmosomoides polygyrus. Immunology 2013, 138, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Dainichi, T.; Maekawa, Y.; Ishii, K.; Zhang, T.; Nashed, B.F.; Sakai, T.; Takashima, M.; Himeno, K. Nippocystatin, a cysteine protease inhibitor from Nippostrongylus brasiliensis, inhibits antigen processing and modulates antigen-specific immune response. Infect. Immun. 2001, 69, 7380–7386. [Google Scholar] [CrossRef]
- Mei, G.; Dong, J.; Li, Z.; Liu, S.; Liu, Y.; Sun, M.; Liu, G.; Su, Z.; Liu, J. Structural Basis for the Immunomodulatory Function of Cysteine Protease Inhibitor from Human Roundworm Ascaris lumbricoides. PLoS ONE 2014, 9, e96069. [Google Scholar] [CrossRef]
- Coronado, S.; Barrios, L.; Zakzuk, J.; Regino, R.; Ahumada, V.; Franco, L.; Ocampo, Y.; Caraballo, L. A recombinant cystatin from Ascaris lumbricoides attenuates inflammation of DSS-induced colitis. Parasite Immunol. 2017, 39, e12425. [Google Scholar] [CrossRef]
- Acevedo, N.; Lozano, A.; Zakzuk, J.; Llinás-Caballero, K.; Brodin, D.; Nejsum, P.; Williams, A.R.; Caraballo, L. Cystatin from the helminth Ascaris lumbricoides upregulates mevalonate and cholesterol biosynthesis pathways and immunomodulatory genes in human monocyte-derived dendritic cells. Front. Immunol. 2024, 15, 1328401. [Google Scholar] [CrossRef]
- Gomez-Escobar, N.; Gregory, W.F.; Maizels, R.M. Identification of tgh-2, a filarial nematode homolog of Caenorhabditis elegans daf-7 and human transforming growth factor β, expressed in microfilarial and adult stages of Brugia malayi. Infect. Immun. 2000, 68, 6402–6410. [Google Scholar] [CrossRef]
- Chen, W. TGF-beta Regulation of T Cells. Annu. Rev. Immunol. 2023, 41, 483–512. [Google Scholar] [CrossRef]
- Kron, M.A.; Metwali, A.; Vodanovic-Jankovic, S.; Elliott, D. Nematode asparaginyl-tRNA synthetase resolves intestinal inflammation in mice with T-cell transfer colitis. Clin. Vaccine Immunol. 2013, 20, 276–281. [Google Scholar] [CrossRef]
- Harnett, W.; Rzepecka, J.; Houston, K.M. How do nematodes transfer phosphorylcholine to carbohydrates? Trends Parasitol. 2010, 26, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Rzepecka, J.; Harnett, W. Can the Study of Parasitic Helminths Be Fruitful for Human Diseases? In Helminth Infections and Their Impact on Global Public Health; Bruschi, F., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 607–640. [Google Scholar]
- Rzepecka, J.; Siebeke, I.; Coltherd, J.C.; Kean, D.E.; Steiger, C.N.; Al-Riyami, L.; McSharry, C.; Harnett, M.M.; Harnett, W. The helminth product, ES-62, protects against airway inflammation by resetting the Th cell phenotype. Int. J. Parasitol. 2013, 43, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, D.T.; McGrath, M.A.; Pineda, M.A.; Al-Riyami, L.; Rzepecka, J.; Lumb, F.; Harnett, W.; Harnett, M.M. The parasitic worm product ES-62 targets myeloid differentiation factor 88-dependent effector mechanisms to suppress antinuclear antibody production and proteinuria in MRL/lpr mice. Arthritis Rheumatol. 2015, 67, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Ball, D.H.; Al-Riyami, L.; Harnett, W.; Harnett, M.M. IL-33/ST2 signalling and crosstalk with FcepsilonRI and TLR4 is targeted by the parasitic worm product, ES-62. Sci. Rep. 2018, 8, 4497. [Google Scholar] [CrossRef]
- Lechner, A.; Bohnacker, S.; Esser-von Bieren, J. Macrophage regulation & function in helminth infection. Semin. Immunol. 2021, 53, 101526. [Google Scholar] [CrossRef]
- Coakley, G.; Harris, N.L. Interactions between macrophages and helminths. Parasite Immunol. 2020, 42, e12717. [Google Scholar] [CrossRef]
- Filbey, K.J.; Varyani, F.; Harcus, Y.; Hewitson, J.P.; Smyth, D.J.; McSorley, H.J.; Ivens, A.; Nylén, S.; Rottenberg, M.; Löser, S.; et al. Macrophage migration inhibitory factor (MIF) is essential for type 2 effector cell immunity to an intestinal helminth parasite. Front. Immunol. 2019, 10, 2375. [Google Scholar] [CrossRef]
- Damle, S.R.; Martin, R.K.; Cross, J.V.; Conrad, D.H. Macrophage migration inhibitory factor deficiency enhances immune response to Nippostrongylus brasiliensis. Mucosal Immunol. 2017, 10, 205–214. [Google Scholar] [CrossRef]
- Grieb, G.; Merk, M.; Bernhagen, J.; Bucala, R. Macrophage migration inhibitory factor (MIF): A promising biomarker. Drug News Perspect. 2010, 23, 257–264. [Google Scholar] [CrossRef]
- Pastrana, D.V.; Raghavan, N.; FitzGerald, P.; Eisinger, S.W.; Metz, C.; Bucala, R.; Schleimer, R.P.; Bickel, C.; Scott, A.L. Filarial nematode parasites secrete a homologue of the human cytokine macrophage migration inhibitory factor. Infect. Immun. 1998, 66, 5955–5963. [Google Scholar] [CrossRef]
- Antunes, M.F.P.; Titz, T.O.; Batista, I.F.C.; Marques-Porto, R.; Oliveira, C.F.; Alves de Araujo, C.A.; Macedo-Soares, M.F. Immunosuppressive PAS-1 is an excretory/secretory protein released by larval and adult worms of the ascarid nematode Ascaris suum. J. Helminthol. 2015, 89, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Spence, H.J.; Moore, J.; Heaney, N.; McDermott, L.; Cooper, A.; Watson, D.G.; Mei, B.; Komuniecki, R.; Kennedy, M.W. The ABA-1 allergen of Ascaris lumbricoides: Sequence polymorphism, stage and tissue-specific expression, lipid binding function, and protein biophysical properties. Parasitology 2000, 120, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Długosz, E.; Wasyl, K.; Klockiewicz, M.; Wisniewski, M. Toxocara canis mucins among other excretory-secretory antigens induce in vitro secretion of cytokines by mouse splenocytes. Parasitol. Res. 2015, 114, 3365–3371. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Allen, C.; Arora, M.; Barber, R.M.; Bhutta, Z.A.; Brown, A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef]
- Martin-Galiano, A.J.; Sotillo, J. Insights into the functional expansion of the astacin peptidase family in parasitic helminths. Int. J. Parasitol. 2022, 52, 243–251. [Google Scholar] [CrossRef]
- Bąska, P.; Wiśniewski, M.; Krzyżowska, M.; Długosz, E.; Zygner, W.; Górski, P.; Wędrychowicz, H. Molecular cloning and characterisation of in vitro immune response against astacin-like metalloprotease Ace-MTP-2 from Ancylostoma ceylanicum. Exp. Parasitol. 2013, 133, 472–482. [Google Scholar] [CrossRef]
- Chhabra, S.; Chang, S.C.; Nguyen, H.M.; Huq, R.; Tanner, M.R.; Londono, L.M.; Estrada, R.; Dhawan, V.; Chauhan, S.; Upadhyay, S.K.; et al. Kv1.3 channel-blocking immunomodulatory peptides from parasitic worms: Implications for autoimmune diseases. FASEB J. 2014, 28, 3952–3964. [Google Scholar] [CrossRef]
- McNeilly, T.N.; Frew, D.; Burgess, S.T.G.; Wright, H.; Bartley, D.J.; Bartley, Y.; Nisbet, A.J. Niche-specific gene expression in a parasitic nematode; increased expression of immunomodulators in Teladorsagia circumcincta larvae derived from host mucosa. Sci. Rep. 2017, 7, 7214. [Google Scholar] [CrossRef]
- Ferreira, I.B.; Pickering, D.A.; Troy, S.; Croese, J.; Loukas, A.; Navarro, S. Suppression of inflammation and tissue damage by a hookworm recombinant protein in experimental colitis. Clin. Transl. Immunol. 2017, 6, e157. [Google Scholar] [CrossRef]
- Navarro, S.; Pickering, D.A.; Ferreira, I.B.; Jones, L.; Ryan, S.; Troy, S.; Leech, A.; Hotez, P.J.; Zhan, B.; Laha, T. Hookworm recombinant protein promotes regulatory T cell responses that suppress experimental asthma. Sci. Transl. Med. 2016, 8, 362ra143. [Google Scholar] [CrossRef]
- Moyle, M.; Foster, D.L.; McGrath, D.E.; Brown, S.M.; Laroche, Y.; De Meutter, J.; Stanssens, P.; Bogowitz, C.A.; Fried, V.A.; Ely, J.A. A hookworm glycoprotein that inhibits neutrophil function is a ligand of the integrin CD11b/CD18. J. Biol. Chem. 1994, 269, 10008–10015. [Google Scholar] [CrossRef] [PubMed]
- Anbu, K.A.; Joshi, P. Identification of a 55 kDa Haemonchus contortus excretory/secretory glycoprotein as a neutrophil inhibitory factor. Parasite Immunol. 2008, 30, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Culley, F.J.; Brown, A.; Conroy, D.M.; Sabroe, I.; Pritchard, D.I.; Williams, T.J. Eotaxin is specifically cleaved by hookworm metalloproteases preventing its action in vitro and in vivo. J. Immunol. 2000, 165, 6447–6453. [Google Scholar] [CrossRef] [PubMed]
- Bower, M.A.; Constant, S.L.; Mendez, S. Necator americanus: The Na-ASP-2 protein secreted by the infective larvae induces neutrophil recruitment in vivo and in vitro. Exp. Parasitol. 2008, 118, 569–575. [Google Scholar] [CrossRef]
- Tribolet, L.; Cantacessi, C.; Pickering, D.A.; Navarro, S.; Doolan, D.L.; Trieu, A.; Fei, H.; Chao, Y.; Hofmann, A.; Gasser, R.B.; et al. Probing of a human proteome microarray with a recombinant pathogen protein reveals a novel mechanism by which hookworms suppress B-cell receptor signaling. J. Infect. Dis. 2015, 211, 416–425. [Google Scholar] [CrossRef]
- Liew, F.Y.; Girard, J.P.; Turnquist, H.R. Interleukin-33 in health and disease. Nat. Rev. Immunol. 2016, 16, 676–689. [Google Scholar] [CrossRef]
- Osbourn, M.; Soares, D.C.; Vacca, F.; Cohen, E.S.; Scott, I.C.; Gregory, W.F.; Smyth, D.J.; Toivakka, M.; Kemter, A.M.; le Bihan, T.; et al. HpARI Protein Secreted by a Helminth Parasite Suppresses Interleukin-33. Immunity 2017, 47, 739–751.e735. [Google Scholar] [CrossRef]
- Shimokawa, C.; Kanaya, T.; Hachisuka, M.; Ishiwata, K.; Hisaeda, H.; Kurashima, Y.; Kiyono, H.; Yoshimoto, T.; Kaisho, T.; Ohno, H. Mast Cells Are Crucial for Induction of Group 2 Innate Lymphoid Cells and Clearance of Helminth Infections. Immunity 2017, 46, 863–874. [Google Scholar] [CrossRef]
- Cox, F.E.G.; Liew, E.Y. T-cell subsets and cytokines in parasitic infections. Parasitol. Today 1992, 8, 371–374. [Google Scholar] [CrossRef]
- Colomb, F.; Ogunkanbi, A.; Jamwal, A.; Dong, B.; Maizels, R.M.; Finney, C.A.; Wasmuth, J.D.; Higgins, M.K.; McSorley, H.J. IL-33-binding HpARI family homologues with divergent effects in suppressing or enhancing type 2 immune responses. Infect. Immun. 2024, 92, e0039523. [Google Scholar] [CrossRef]
- Jamwal, A.; Colomb, F.; McSorley, H.J.; Higgins, M.K. Structural basis for IL-33 recognition and its antagonism by the helminth effector protein HpARI2. Nat. Commun. 2024, 15, 5226. [Google Scholar] [CrossRef] [PubMed]
- Vacca, F.; Chauche, C.; Jamwal, A.; Hinchy, E.C.; Heieis, G.; Webster, H.; Ogunkanbi, A.; Sekne, Z.; Gregory, W.F.; Wear, M.; et al. A helminth-derived suppressor of ST2 blocks allergic responses. Elife 2020, 9, e54017. [Google Scholar] [CrossRef] [PubMed]
- Buck, A.H.; Coakley, G.; Simbari, F.; McSorley, H.J.; Quintana, J.F.; Le Bihan, T.; Kumar, S.; Abreu-Goodger, C.; Lear, M.; Harcus, Y.; et al. Exosomes secreted by nematode parasites transfer small RNAs to mammalian cells and modulate innate immunity. Nat. Commun. 2014, 5, 5488. [Google Scholar] [CrossRef]
- Chow, F.W.; Koutsovoulos, G.; Ovando-Vazquez, C.; Neophytou, K.; Bermudez-Barrientos, J.R.; Laetsch, D.R.; Robertson, E.; Kumar, S.; Claycomb, J.M.; Blaxter, M.; et al. Secretion of an Argonaute protein by a parasitic nematode and the evolution of its siRNA guides. Nucleic Acids Res. 2019, 47, 3594–3606. [Google Scholar] [CrossRef]
- Johnston, C.J.C.; Smyth, D.J.; Kodali, R.B.; White, M.P.J.; Harcus, Y.; Filbey, K.J.; Hewitson, J.P.; Hinck, C.S.; Ivens, A.; Kemter, A.M.; et al. A structurally distinct TGF-beta mimic from an intestinal helminth parasite potently induces regulatory T cells. Nat. Commun. 2017, 8, 1741. [Google Scholar] [CrossRef]
- Maizels, R.M.; McSorley, H.J.; Smits, H.H.; Ten Dijke, P.; Hinck, A.P. Cytokines from parasites: Manipulating host responses by molecular mimicry. Biochem. J. 2025, 482, 433–449. [Google Scholar] [CrossRef]
- Mukundan, A.; Byeon, C.H.; Hinck, C.S.; Cunningham, K.; Campion, T.; Smyth, D.J.; Maizels, R.M.; Hinck, A.P. Convergent evolution of a parasite-encoded complement control protein-scaffold to mimic binding of mammalian TGF-beta to its receptors, TbetaRI and TbetaRII. J. Biol. Chem. 2022, 298, 101994. [Google Scholar] [CrossRef]
- Rathore, D.K.; Suchitra, S.; Saini, M.; Singh, B.P.; Joshi, P. Identification of a 66kDa Haemonchus contortus excretory/secretory antigen that inhibits host monocytes. Vet. Parasitol. 2006, 138, 291–300. [Google Scholar] [CrossRef]
- Sokkalingam, M.; Samal, A.K.; Thangavelu, L.P.; Joshi, P. Characterization of a 66 kDa Secretory Protein of Haemonchus contortus and Its Effect on Host Mononuclear Cells. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020, 90, 895–902. [Google Scholar] [CrossRef]
- Hildersley, K.A.; McNeilly, T.N.; Gillan, V.; Otto, T.D.; Loser, S.; Gerbe, F.; Jay, P.; Maizels, R.M.; Devaney, E.; Britton, C. Tuft Cells Increase Following Ovine Intestinal Parasite Infections and Define Evolutionarily Conserved and Divergent Responses. Front. Immunol. 2021, 12, 781108. [Google Scholar] [CrossRef]
- Ndjim, M.; Gasmi, I.; Herbert, F.; Josephine, C.; Bas, J.; Lamrani, A.; Coutry, N.; Henry, S.; Zimmermann, V.S.; Dardalhon, V.; et al. Tuft cell acetylcholine is released into the gut lumen to promote anti-helminth immunity. Immunity 2024, 57, 1260–1273.e1267. [Google Scholar] [CrossRef] [PubMed]
- Vaux, R.; Schnoeller, C.; Berkachy, R.; Roberts, L.B.; Hagen, J.; Gounaris, K.; Selkirk, M.E. Modulation of the Immune Response by Nematode Secreted Acetylcholinesterase Revealed by Heterologous Expression in Trypanosoma musculi. PLoS Pathog. 2016, 12, e1005998. [Google Scholar] [CrossRef] [PubMed]
- Berkachy, R.; Smyth, D.J.; Schnoeller, C.; Harcus, Y.; Maizels, R.M.; Selkirk, M.E.; Gounaris, K. Characterisation of the secreted apyrase family of Heligmosomoides polygyrus. Int. J. Parasitol. 2021, 51, 39–48. [Google Scholar] [CrossRef]
- Donskow-Łysoniewska, K.; Maruszewska-Cheruiyot, M.; Krawczak-Wojcik, K.; Gonzalez, J.F.; Hernandez, J.N.; Stear, M.J. Nematode galectin binds IgE and modulates mast cell activity. Vet. Parasitol. 2022, 311, 109807. [Google Scholar] [CrossRef]
- Maruszewska-Cheruiyot, M.; Szewczak, L.; Krawczak-Wójcik, K.; Stear, M.J.; Donskow-Łysoniewska, K. Nematode Galectin Inhibits Basophilic Leukaemia RBL-2H3 Cells Apoptosis in IgE-Mediated Activation. Int. J. Mol. Sci. 2024, 25, 7419. [Google Scholar] [CrossRef]
- Shi, X.; Xiao, M.; Xie, Z.; Shi, Q.; Zhang, Y.; Leavenworth, J.W.; Yan, B.; Huang, H. Angiostrongylus cantonensis Galectin-1 interacts with Annexin A2 to impair the viability of macrophages via activating JNK pathway. Parasit. Vectors 2020, 13, 183. [Google Scholar] [CrossRef]
- Houzelstein, D.; Goncalves, I.R.; Fadden, A.J.; Sidhu, S.S.; Cooper, D.N.; Drickamer, K.; Leffler, H.; Poirier, F. Phylogenetic analysis of the vertebrate galectin family. Mol. Biol. Evol. 2004, 21, 1177–1187. [Google Scholar] [CrossRef]
- Katze, M.G.; Fornek, J.L.; Palermo, R.E.; Walters, K.A.; Korth, M.J. Innate immune modulation by RNA viruses: Emerging insights from functional genomics. Nat. Rev. Immunol. 2008, 8, 644–654. [Google Scholar] [CrossRef]
- Ryan, S.; Shiels, J.; Taggart, C.C.; Dalton, J.P.; Weldon, S. Fasciola hepatica-Derived Molecules as Regulators of the Host Immune Response. Front. Immunol. 2020, 11, 2182. [Google Scholar] [CrossRef]
- Sansonetti, P.J.; Di Santo, J.P. Debugging how bacteria manipulate the immune response. Immunity 2007, 26, 149–161. [Google Scholar] [CrossRef]
- Grencis, R.K. Immunity to helminths: Resistance, regulation, and susceptibility to gastrointestinal nematodes. Annu. Rev. Immunol. 2015, 33, 201–225. [Google Scholar] [CrossRef]
- Sloan, S.; Jenvey, C.; Cairns, C.; Stear, M. Cathepsin F of Teladorsagia circumcincta is a recently evolved cysteine protease. Evol. Bioinform. Online 2020, 16, 1176934320962521. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Munyard, K.; Gregg, K.; Wetherall, J.D.; Stear, M.J.; Groth, D.M. Immunoglobulins A and E as well as MHC influence host resistance to gastrointestinal parasites in sheep. J. Parasitol. Res. 2011, 2011, 101848. [Google Scholar] [CrossRef]
- Nisbet, A.J.; McNeilly, T.N.; Wildblood, L.A.; Morrison, A.A.; Bartley, D.J.; Bartley, Y.; Longhi, C.; McKendrick, I.J.; Palarea-Albaladejo, J.; Matthews, J.B. Successful immunization against a parasitic nematode by vaccination with recombinant proteins. Vaccine 2013, 31, 4017–4023. [Google Scholar] [CrossRef] [PubMed]
- Stear, M.J.; Bishop, S.C.; Henderson, N.G.; Scott, I. A Key Mechanism of Pathogenesis in Sheep Infected with the Nematode Teladorsagia circumcincta. Anim. Health Res. Rev. 2003, 4, 45–52. [Google Scholar] [CrossRef] [PubMed]
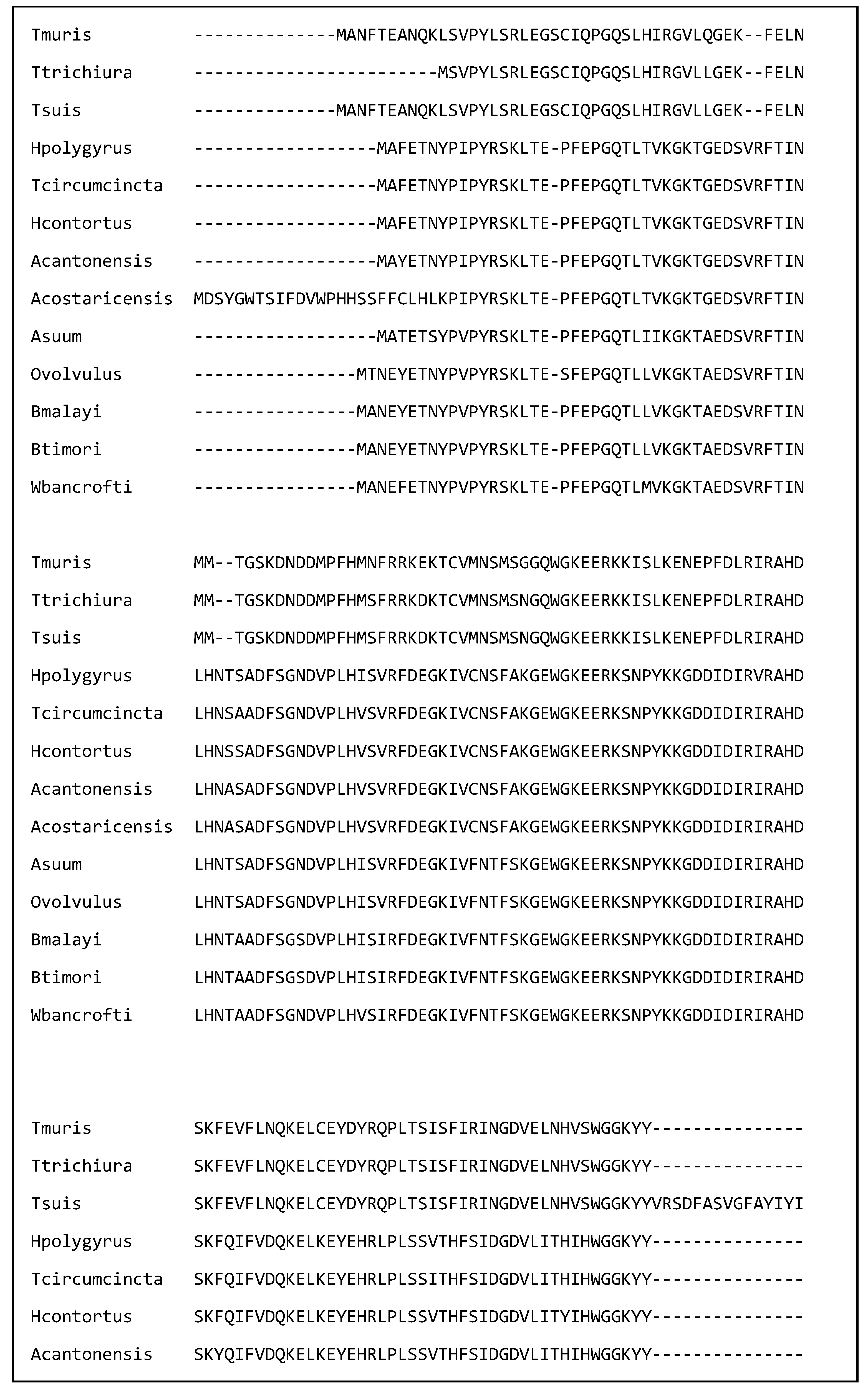
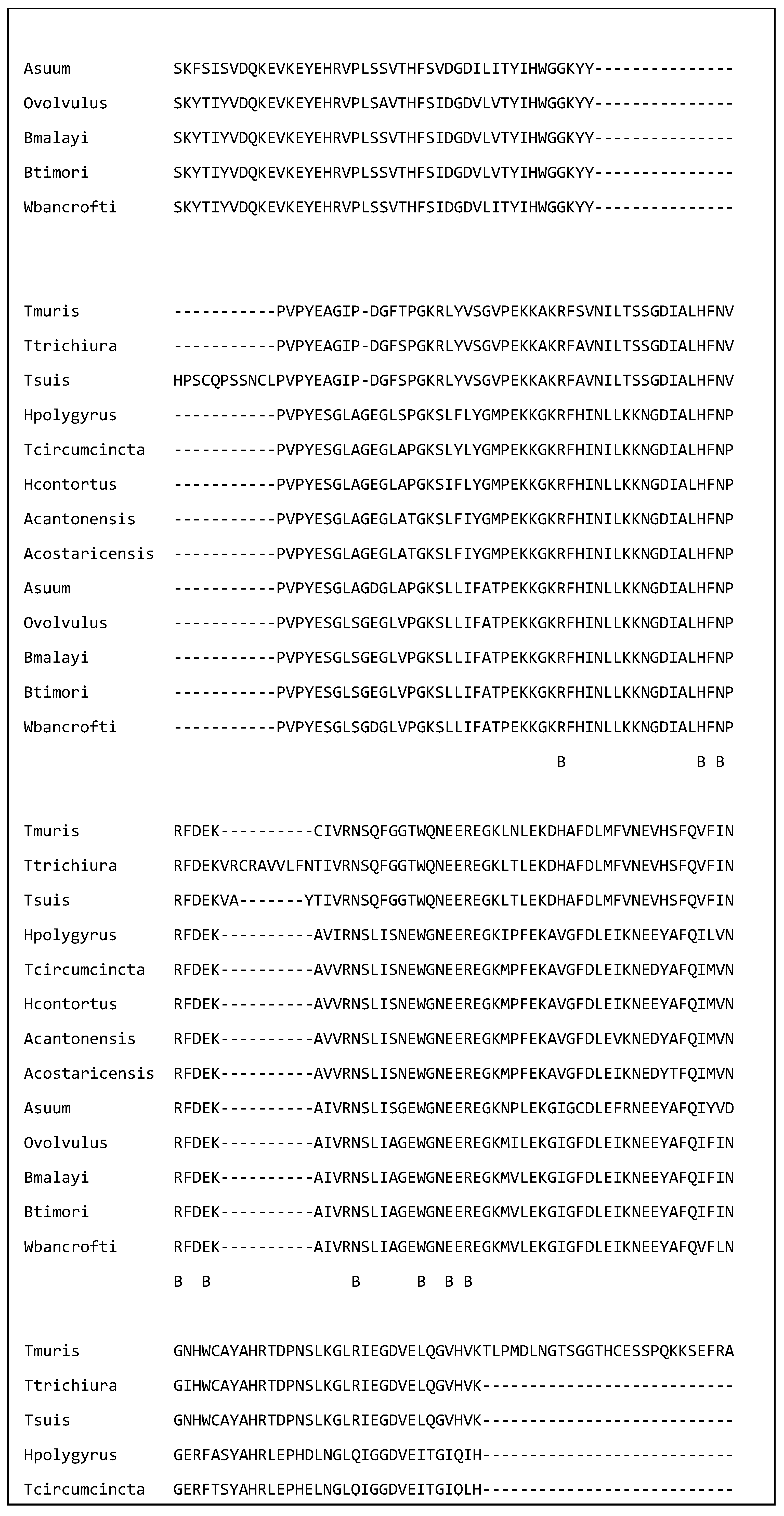
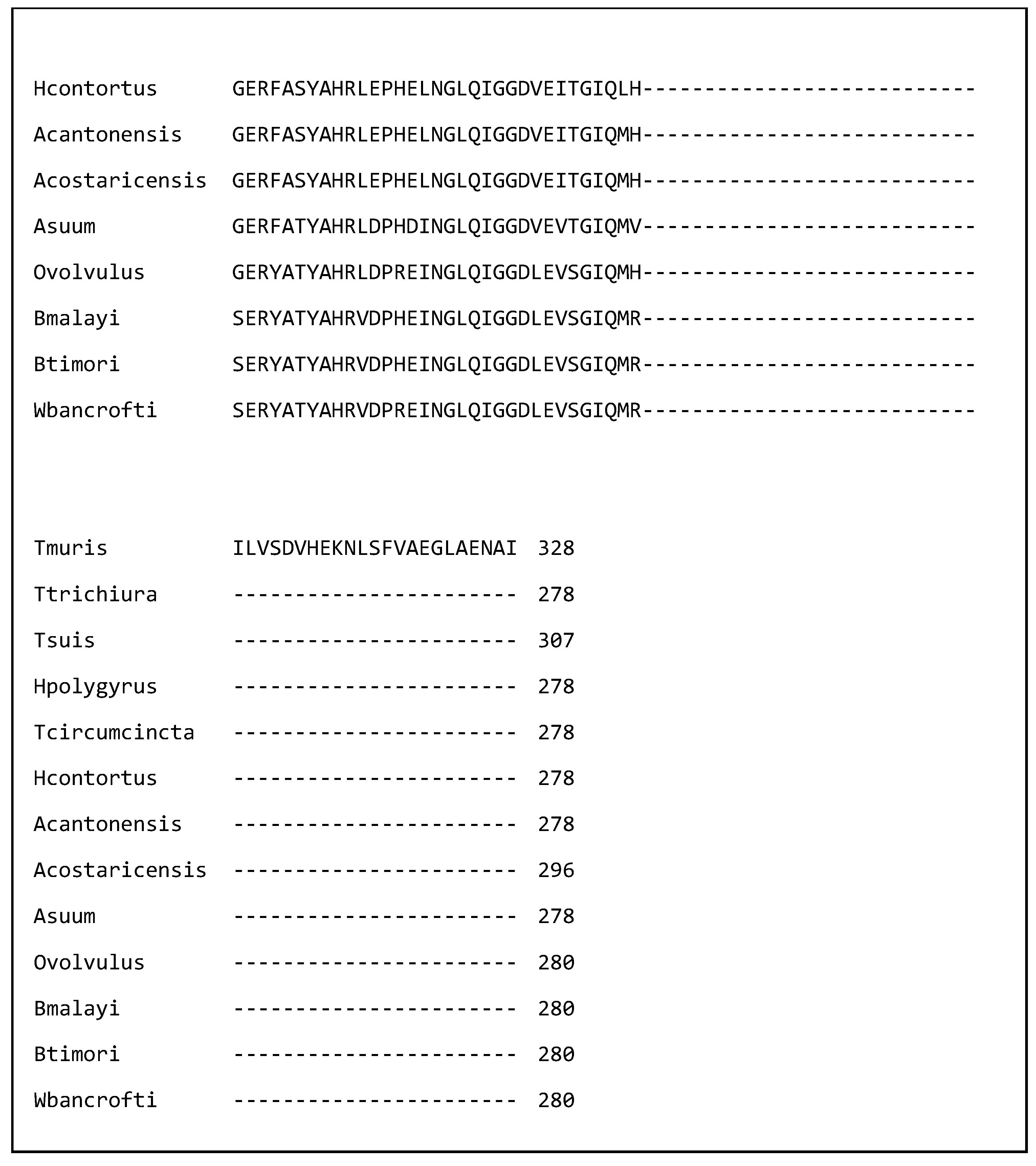
| Species | Name of Nematode Molecule | Target Cell or Molecule in the Host |
|---|---|---|
| Trichuris spp.; Brugia malayi; Wuchereria bancrofti. | Prostaglandin E2 (PGE2) | Dendritic cells |
| Trichuris suis | Chitinase | Eosinophils; Th2 cells. |
| Trichuris suis | Triosephosphate isomerase Nucleoside diphosphate kinase | Cytokine release |
| Trichuris muris | Interferon gamma mimic | Interferon gamma |
| Trichuris muris | P43 | IL-13 |
| Trichinella pseudospiralis | Serine protease inhibitor (Serpin) | Serine proteases |
| Brugia malayi | Abundant larval transcripts 1 and 2 (Bmalt-1; Bmalt-2) | Expression of GATA-3 and SOCS-1 |
| Brugia malayi | Tgh-2 | TGF-β |
| Brugia malayi | Asparaginyl t-RNA synthetase | Various |
| Acanthocheilonema viteae | ES-62 | TLR4 |
| Multiple species | Nematode-derived Migration inhibitory factor (nMIF) | Macrophages |
| Ascaris suum | Protein 1 from A. suum (PAS-1) | Regulatory T cells? |
| Toxocara canis | Mucins | Helper T cells |
| Ancylostoma ceylanicum | Astacin (AceMTP-2) | Macrophages |
| Ancylostoma caninum; Brugia malayi | Peptides that block the voltage-gated potassium channel 1.3 (Kv1.3) | T cells |
| Ancylostoma caninum | Tissue inhibitor of metalloprotease-1 and -2 | Dendritic cells |
| Ancylostoma ceylanicum | Neutrophil Inhibitory Factor (NIF) | Neutrophils |
| Necator americanus | Metalloprotease | Eosinophils |
| Necator americanus | Activation associated protein 2 (ASP-2) | Neutrophils; B cells |
| Multiple species | Cystatins | Antigen presenting cells |
| Heligmosomoides polygyrus | Alarmin release inhibitor (HpARI) | IL-33 |
| Heligmosomoides polygyrus | Binds Alarmin receptor and Inhibits (HpBARI) | IL-33 receptor |
| Heligmosomoides polygyrus | Small RNA molecules | Expression of IL-33 receptor; MAPK signalling |
| Heligmosomoides polygyrus | TGF-β mimic (TGM) | TGF-β |
| Haemonchus contortus | Glutamate dehydrogenase (p66) | Lymphocytes and monocytes |
| Nippostongylus brasiliensis | Acetylcholinesterase | Macrophages |
| Multiple | Apyrases | Mast cells |
| Multiple | Galectin | Mast cells, mucus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stear, M.; Maruszewska-Cheruiyot, M.; Donskow-Łysoniewska, K. Modulation of the Immune Response by Nematode Derived Molecules. Int. J. Mol. Sci. 2025, 26, 5600. https://doi.org/10.3390/ijms26125600
Stear M, Maruszewska-Cheruiyot M, Donskow-Łysoniewska K. Modulation of the Immune Response by Nematode Derived Molecules. International Journal of Molecular Sciences. 2025; 26(12):5600. https://doi.org/10.3390/ijms26125600
Chicago/Turabian StyleStear, Michael, Marta Maruszewska-Cheruiyot, and Katarzyna Donskow-Łysoniewska. 2025. "Modulation of the Immune Response by Nematode Derived Molecules" International Journal of Molecular Sciences 26, no. 12: 5600. https://doi.org/10.3390/ijms26125600
APA StyleStear, M., Maruszewska-Cheruiyot, M., & Donskow-Łysoniewska, K. (2025). Modulation of the Immune Response by Nematode Derived Molecules. International Journal of Molecular Sciences, 26(12), 5600. https://doi.org/10.3390/ijms26125600








