Metabolic Reprogramming of Urothelial Carcinoma—A Theragnostic Target for Betulinic Acid
Abstract
1. Introduction
2. Results
2.1. Effect of 10% Glucose Increase on Doubling Time of Cell Lines
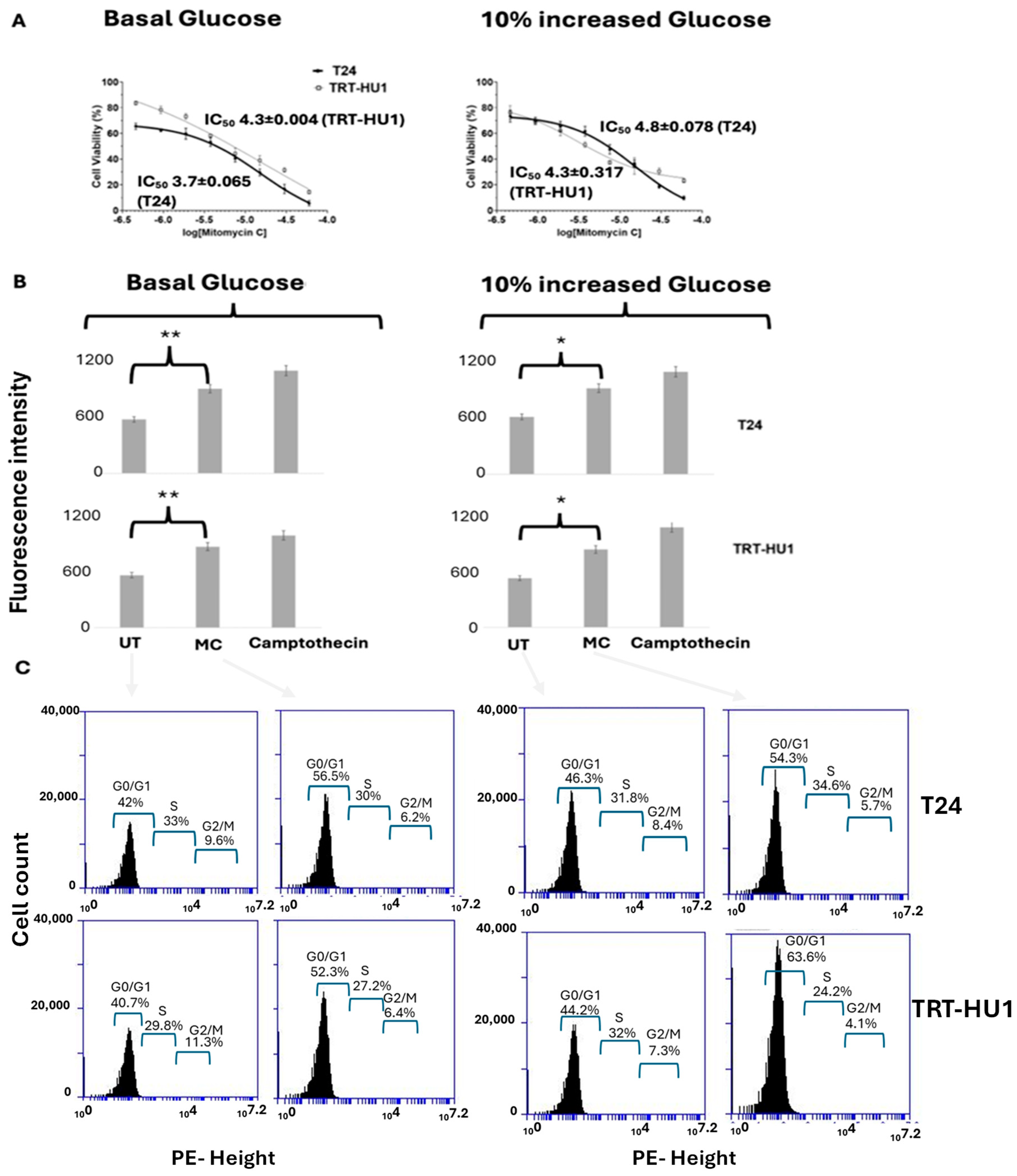
2.2. Effect of 10% Glucose Increase from Basal Levels on MC-Evoked Cytotoxicity, Cell Cycle Arrest, and Apoptosis
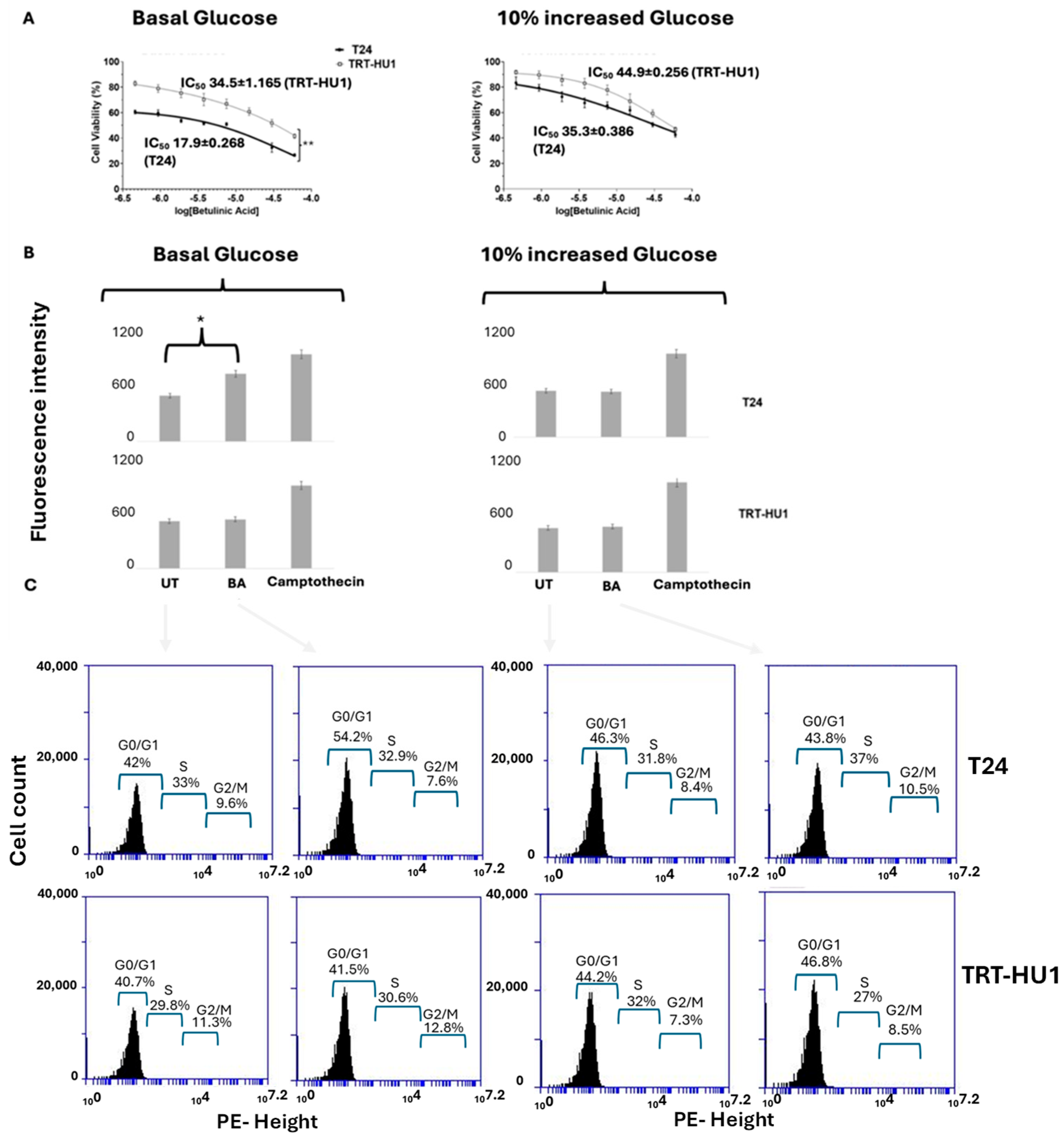
2.3. Effect of 10% Glucose Increase from Basal Levels on BA-Evoked Cytotoxicity, Cell Cycle Arrest, and Apoptosis
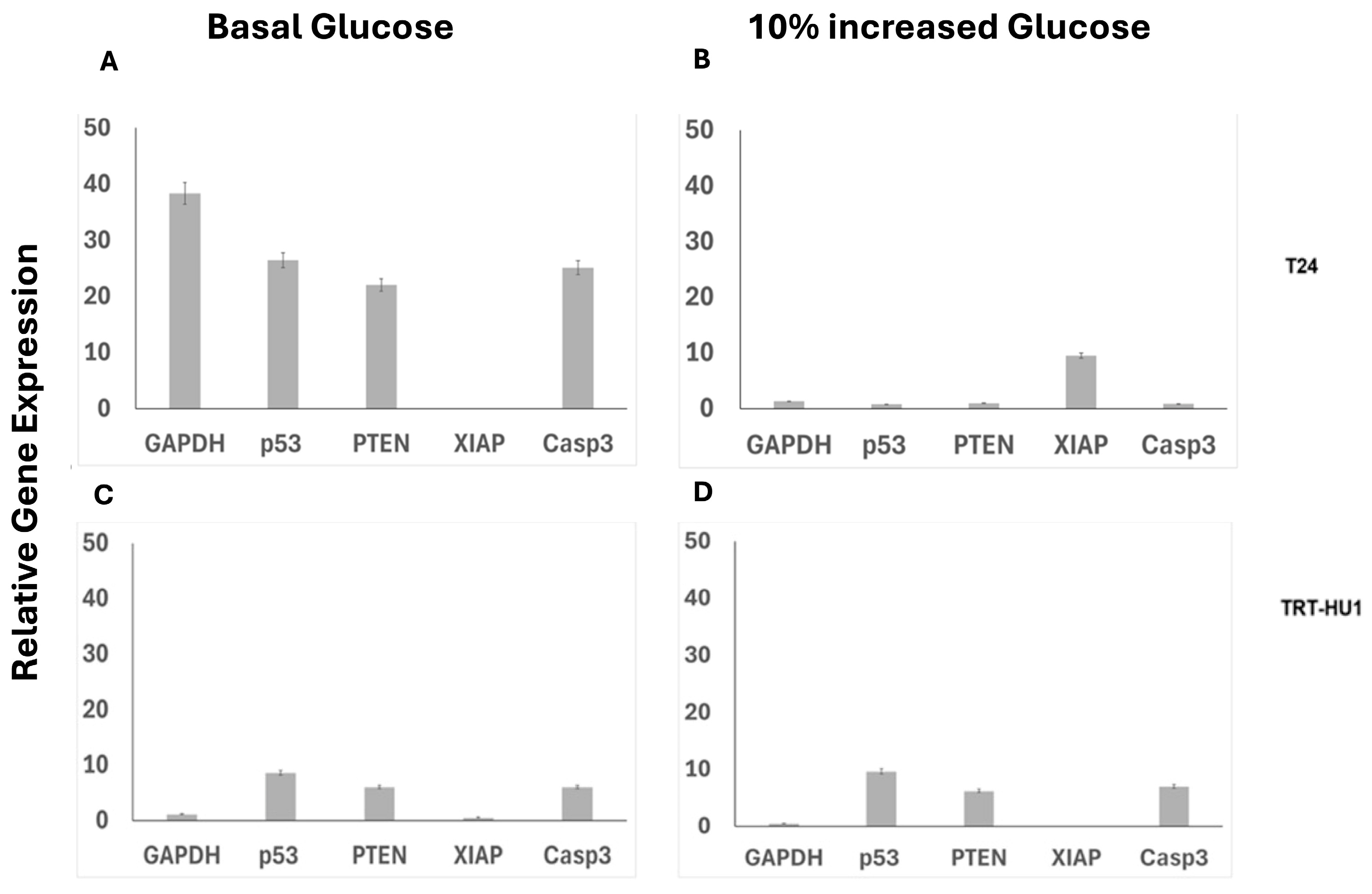
2.4. Combined and Isolated Effect of BA and 10% Glucose Increase on Gene Expression
2.5. Effect of 10% Glucose Increase on Mitochondrial Localization and Toxicity of Betulinic Acid Conjugated to FITC
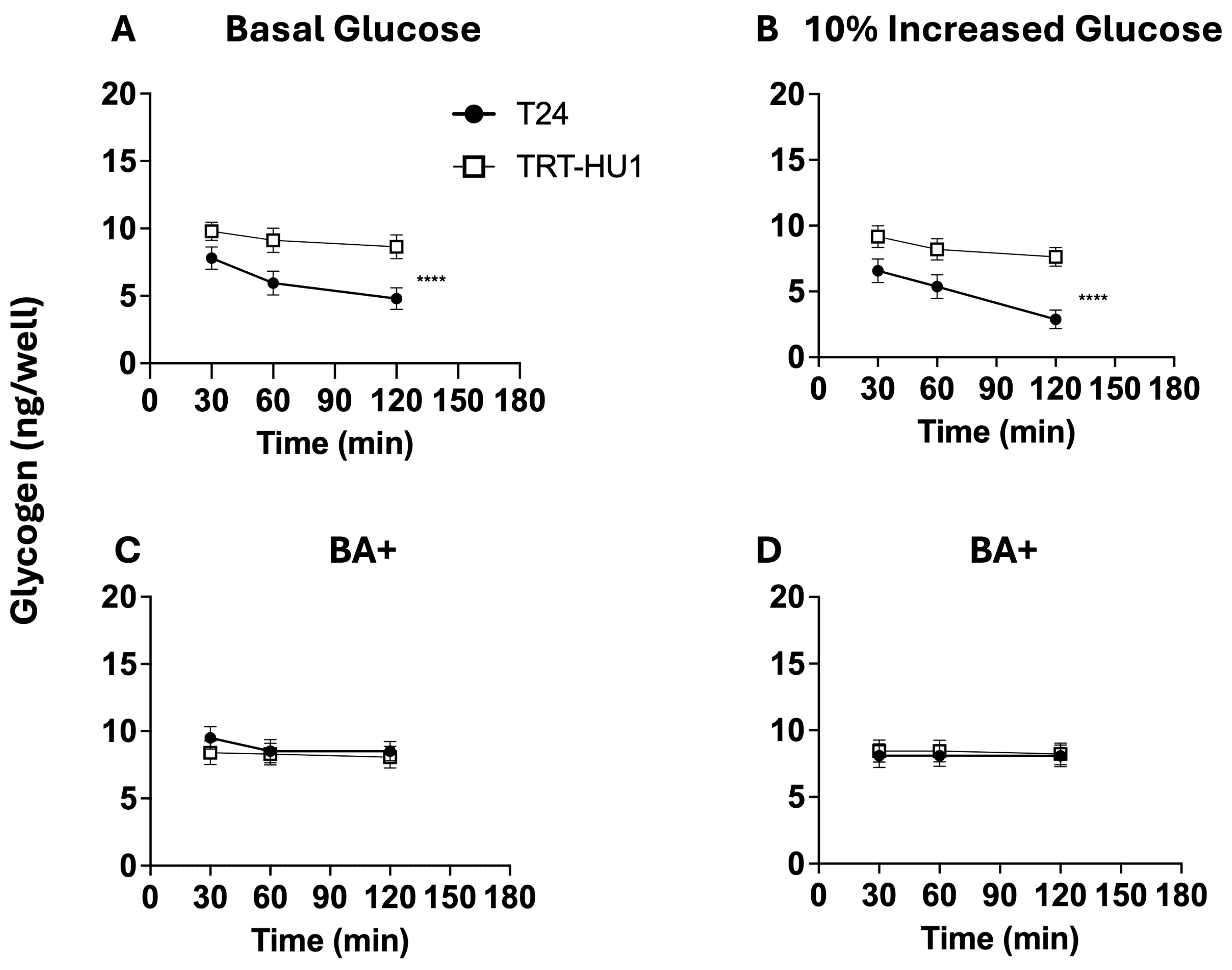
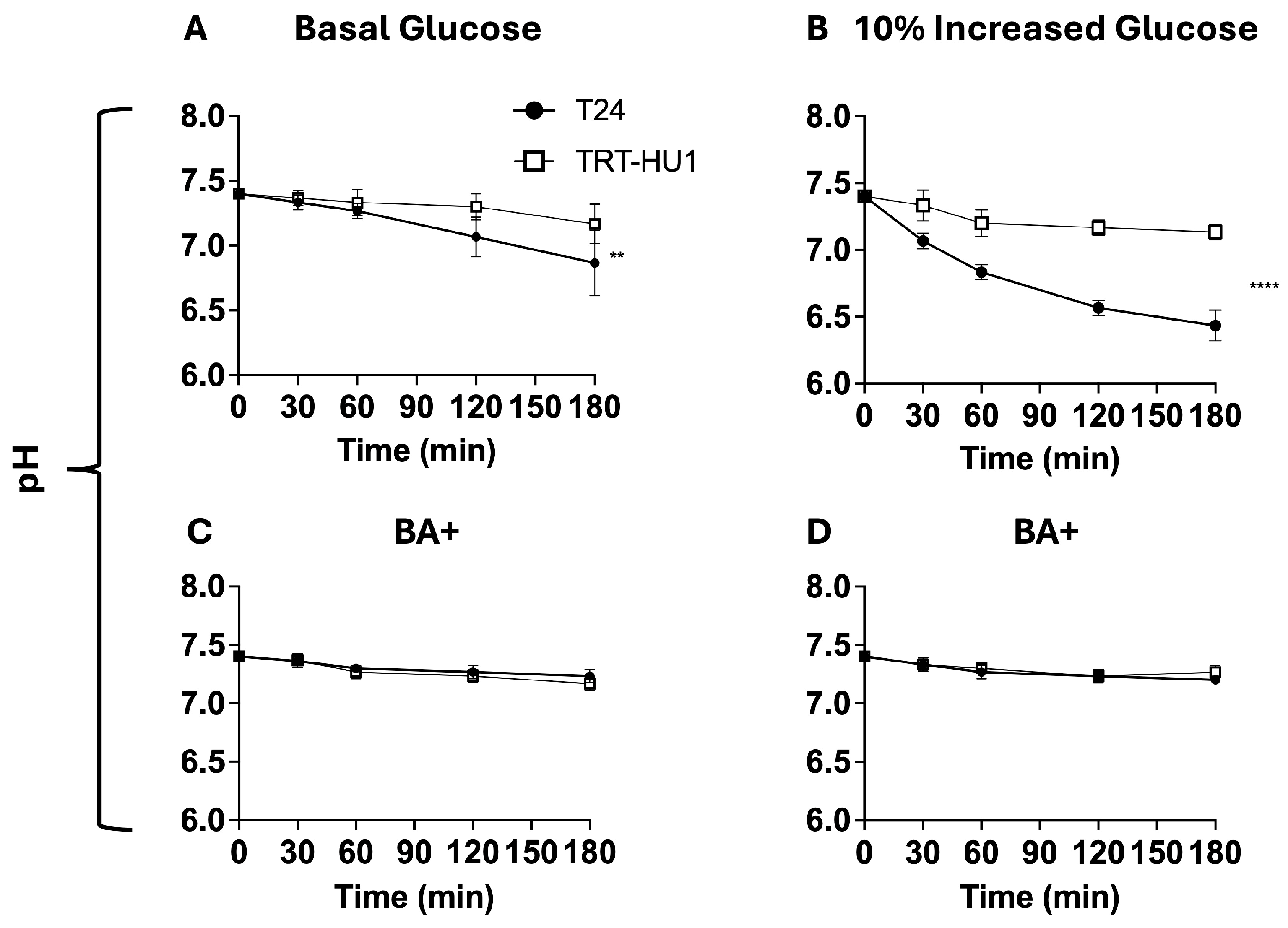
2.6. Effect of BA on Glycogen and pH Decline Amidst Glucose Scarcity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Cell Viability Assay (MTT)
4.4. Cell Cycle Analysis After Cell Synchronization by Double Thymidine Block
4.5. Caspase-3/7 Apoptotic Assay
4.6. Quantitative PCR
4.7. Confocal Microscopy
4.8. Glycogen and pH Assay
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Afonso, J.; Barbosa-Matos, C.; Silvestre, R.; Pereira-Vieira, J.; Goncalves, S.M.; Mendes-Alves, C.; Parpot, P.; Pinto, J.; Carapito, A.; Guedes de Pinho, P.; et al. Cisplatin-Resistant Urothelial Bladder Cancer Cells Undergo Metabolic Reprogramming beyond the Warburg Effect. Cancers 2024, 16, 1418. [Google Scholar] [CrossRef] [PubMed]
- Afonso, J.; Goncalves, C.; Costa, M.; Ferreira, D.; Santos, L.; Longatto-Filho, A.; Baltazar, F. Glucose Metabolism Reprogramming in Bladder Cancer: Hexokinase 2 (HK2) as Prognostic Biomarker and Target for Bladder Cancer Therapy. Cancers 2023, 15, 982. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.H.; Chan, T.C.; Lai, H.Y.; Chen, T.J.; Wu, L.C.; Hsing, C.H.; Li, C.F. Overexpression of Pyruvate Dehydrogenase Kinase-3 Predicts Poor Prognosis in Urothelial Carcinoma. Front. Oncol. 2021, 11, 749142. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Zhu, H.; Xu, H.; Jin, X.; Zheng, G.; Zhu, J.; Gu, C.; Wang, X. PGK1 can affect the prognosis and development of bladder cancer. Cancer Med. 2024, 13, e70242. [Google Scholar] [CrossRef]
- Massari, F.; Ciccarese, C.; Santoni, M.; Iacovelli, R.; Mazzucchelli, R.; Piva, F.; Scarpelli, M.; Berardi, R.; Tortora, G.; Lopez-Beltran, A.; et al. Metabolic phenotype of bladder cancer. Cancer Treat. Rev. 2016, 45, 46–57. [Google Scholar] [CrossRef]
- Wu, C.; Wei, X.; Huang, Z.; Zheng, Z.; Zhang, W.; Chen, J.; Hong, H.; Li, W. Urinary microbiome dysbiosis is associated with an inflammatory environment and perturbed fatty acids metabolism in the pathogenesis of bladder cancer. J. Transl. Med. 2024, 22, 628. [Google Scholar] [CrossRef]
- Wang, R.; Kang, H.; Zhang, X.; Nie, Q.; Wang, H.; Wang, C.; Zhou, S. Urinary metabolomics for discovering metabolic biomarkers of bladder cancer by UPLC-MS. BMC Cancer 2022, 22, 214. [Google Scholar] [CrossRef]
- Yosef, H.K.; Krauss, S.D.; Lechtonen, T.; Jutte, H.; Tannapfel, A.; Kafferlein, H.U.; Bruning, T.; Roghmann, F.; Noldus, J.; Mosig, A.; et al. Noninvasive Diagnosis of High-Grade Urothelial Carcinoma in Urine by Raman Spectral Imaging. Anal. Chem. 2017, 89, 6893–6899. [Google Scholar] [CrossRef]
- Sasaki, K.; Takahashi, M.; Ogino, T.; Yasui, H. In vitro estimation of cell kinetic parameters in human urinary bladder cancers. Gan 1981, 72, 795–797. [Google Scholar]
- Li, S.; Zhu, H.; Chen, H.; Xia, J.; Zhang, F.; Xu, R.; Lin, Q. Glucose promotes epithelial-mesenchymal transitions in bladder cancer by regulating the functions of YAP1 and TAZ. J. Cell Mol. Med. 2020, 24, 10391–10401. [Google Scholar] [CrossRef]
- Huang, W.L.; Huang, K.H.; Huang, C.Y.; Pu, Y.S.; Chang, H.C.; Chow, P.M. Effect of diabetes mellitus and glycemic control on the prognosis of non-muscle invasive bladder cancer: A retrospective study. BMC Urol. 2020, 20, 117. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.A.; Willett, W.C.; Yusuf, S.; Hu, F.B.; Glenn, A.J.; Liu, S.; Mente, A.; Miller, V.; Bangdiwala, S.I.; Gerstein, H.C.; et al. Association of glycaemic index and glycaemic load with type 2 diabetes, cardiovascular disease, cancer, and all-cause mortality: A meta-analysis of mega cohorts of more than 100 000 participants. Lancet Diabetes Endocrinol. 2024, 12, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Yao, B.; Yan, Y.; Xu, H.; Liu, Y.; Tang, H.; Zhou, J.; Cao, L.; Wang, W.; Zhang, J.; et al. Diabetes mellitus increases the risk of bladder cancer: An updated meta-analysis of observational studies. Diabetes Technol. Ther. 2013, 15, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Sprinz, C.; Altmayer, S.; Zanon, M.; Watte, G.; Irion, K.; Marchiori, E.; Hochhegger, B. Effects of blood glucose level on 18F-FDG uptake for PET/CT in normal organs: A systematic review. PLoS ONE 2018, 13, e0193140. [Google Scholar] [CrossRef]
- Kaya, F.; Komek, H.; Dursun, I.H.; Senses, V.; Gundogan, C. Pseudoprogression Shown on (18)F-FDG PET/CT After Pembrolizumab Treatment in a Case of Metastatic Bladder Cancer. Mol. Imaging Radionucl. Ther. 2025, 34, 70–72. [Google Scholar] [CrossRef]
- Xing, Z.; Yang, T.; Li, X.; Xu, H.; Hong, Y.; Shao, S.; Li, T.; Ye, L.; Li, Y.; Jin, X.; et al. High-glucose-associated YTHDC1 lactylation reduces the sensitivity of bladder cancer to enfortumab vedotin therapy. Cell Rep. 2025, 44, 115545. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Powles, T.; Sonpavde, G.P.; Loriot, Y.; Duran, I.; Lee, J.L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Mamtani, R.; et al. EV-301 long-term outcomes: 24-month findings from the phase III trial of enfortumab vedotin versus chemotherapy in patients with previously treated advanced urothelial carcinoma. Ann. Oncol. 2023, 34, 1047–1054. [Google Scholar] [CrossRef]
- Pisha, E.; Chai, H.; Lee, I.S.; Chagwedera, T.E.; Farnsworth, N.R.; Cordell, G.A.; Beecher, C.W.; Fong, H.H.; Kinghorn, A.D.; Brown, D.M.; et al. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat. Med. 1995, 1, 1046–1051. [Google Scholar] [CrossRef]
- Kern, J.S.; Sprecher, E.; Fernandez, M.F.; Schauer, F.; Bodemer, C.; Cunningham, T.; Lowe, S.; Davis, C.; Sumeray, M.; Bruckner, A.L.; et al. Efficacy and safety of Oleogel-S10 (birch triterpenes) for epidermolysis bullosa: Results from the phase III randomized double-blind phase of the EASE study. Br. J. Dermatol. 2023, 188, 12–21. [Google Scholar] [CrossRef]
- Weber, L.A.; Delarocque, J.; Feige, K.; Kietzmann, M.; Kalbitz, J.; Meissner, J.; Paschke, R.; Cavalleri, J.V. Effects of Topically Applied Betulinic Acid and NVX-207 on Melanocytic Tumors in 18 Horses. Animals 2021, 11, 3250. [Google Scholar] [CrossRef]
- Tyagi, P.; Hafron, J.; Kaufman, J.; Chancellor, M. Enhancing Therapeutic Efficacy and Safety of Immune Checkpoint Inhibition for Bladder Cancer: A Comparative Analysis of Injectable vs. Intravesical Administration. Int. J. Mol. Sci. 2024, 25, 4945. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Ganguly, A.; Chowdhuri, S.; Yousuf, M.; Ghosh, A.; Barui, A.K.; Kotcherlakota, R.; Adhikari, S.; Banerjee, R. Bis-arylidene oxindole-betulinic Acid conjugate: A fluorescent cancer cell detector with potent anticancer activity. ACS Med. Chem. Lett. 2015, 6, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Rzeski, W.; Stepulak, A.; Szymanski, M.; Sifringer, M.; Kaczor, J.; Wejksza, K.; Zdzisinska, B.; Kandefer-Szerszen, M. Betulinic acid decreases expression of bcl-2 and cyclin D1, inhibits proliferation, migration and induces apoptosis in cancer cells. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2006, 374, 11–20. [Google Scholar] [CrossRef]
- Zuco, V.; Supino, R.; Righetti, S.C.; Cleris, L.; Marchesi, E.; Gambacorti-Passerini, C.; Formelli, F. Selective cytotoxicity of betulinic acid on tumor cell lines, but not on normal cells. Cancer Lett. 2002, 175, 17–25. [Google Scholar] [CrossRef]
- Wang, S.; Wang, K.; Zhang, C.; Zhang, W.; Xu, Q.; Wang, Y.; Zhang, Y.; Li, Y.; Zhang, Y.; Zhu, H.; et al. Overaccumulation of p53-mediated autophagy protects against betulinic acid-induced apoptotic cell death in colorectal cancer cells. Cell Death Dis. 2017, 8, e3087. [Google Scholar] [CrossRef]
- Zhan, X.K.; Li, J.L.; Zhang, S.; Xing, P.Y.; Xia, M.F. Betulinic acid exerts potent antitumor effects on paclitaxel-resistant human lung carcinoma cells (H460) via G2/M phase cell cycle arrest and induction of mitochondrial apoptosis. Oncol. Lett. 2018, 16, 3628–3634. [Google Scholar] [CrossRef]
- Kim, S.Y.; Hwangbo, H.; Kim, M.Y.; Ji, S.Y.; Kim, D.H.; Lee, H.; Kim, G.Y.; Moon, S.K.; Leem, S.H.; Yun, S.J.; et al. Betulinic Acid Restricts Human Bladder Cancer Cell Proliferation In Vitro by Inducing Caspase-Dependent Cell Death and Cell Cycle Arrest, and Decreasing Metastatic Potential. Molecules 2021, 26, 1381. [Google Scholar] [CrossRef]
- Coricovac, D.; Dehelean, C.A.; Pinzaru, I.; Mioc, A.; Aburel, O.M.; Macasoi, I.; Draghici, G.A.; Petean, C.; Soica, C.; Boruga, M.; et al. Assessment of Betulinic Acid Cytotoxicity and Mitochondrial Metabolism Impairment in a Human Melanoma Cell Line. Int. J. Mol. Sci. 2021, 22, 4870. [Google Scholar] [CrossRef]
- Zeng, A.Q.; Yu, Y.; Yao, Y.Q.; Yang, F.F.; Liao, M.; Song, L.J.; Li, Y.L.; Yu, Y.; Li, Y.J.; Deng, Y.L.; et al. Betulinic acid impairs metastasis and reduces immunosuppressive cells in breast cancer models. Oncotarget 2018, 9, 3794–3804. [Google Scholar] [CrossRef]
- Malkovsky, M.; Bubenik. Human urinary bladder carcinoma cell line (T24) in long-term culture: Chromosomal studies on a wild population and derived sublines. Neoplasma 1977, 24, 319–326. [Google Scholar]
- Nagao, A.; Kobayashi, M.; Koyasu, S.; Chow, C.C.T.; Harada, H. HIF-1-Dependent Reprogramming of Glucose Metabolic Pathway of Cancer Cells and Its Therapeutic Significance. Int. J. Mol. Sci. 2019, 20, 238. [Google Scholar] [CrossRef] [PubMed]
- Lyles, R.H.; Poindexter, C.; Evans, A.; Brown, M.; Cooper, C.R. Nonlinear model-based estimates of IC(50) for studies involving continuous therapeutic dose-response data. Contemp. Clin. Trials 2008, 29, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, B.; Abasi, M.; Abbasi, M.M.; Jahanban-Esfahlan, R. Anti-Proliferative Properties of Cornus mass Fruit in Different Human Cancer Cells. Asian Pac. J. Cancer Prev. 2015, 16, 5727–5731. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zichri, S.B.; Kolusheva, S.; Shames, A.I.; Schneiderman, E.A.; Poggio, J.L.; Stein, D.E.; Doubijensky, E.; Levy, D.; Orynbayeva, Z.; Jelinek, R. Mitochondria membrane transformations in colon and prostate cancer and their biological implications. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183471. [Google Scholar] [CrossRef]
- Xu, H.; Ju, L.; Xiong, Y.; Yu, M.; Zhou, F.; Qian, K.; Wang, G.; Xiao, Y.; Wang, X. E3 ubiquitin ligase RNF126 affects bladder cancer progression through regulation of PTEN stability. Cell Death Dis. 2021, 12, 239. [Google Scholar] [CrossRef]
- Fulda, S.; Scaffidi, C.; Susin, S.A.; Krammer, P.H.; Kroemer, G.; Peter, M.E.; Debatin, K.M. Activation of mitochondria and release of mitochondrial apoptogenic factors by betulinic acid. J. Biol. Chem. 1998, 273, 33942–33948. [Google Scholar] [CrossRef]
- Nedopekina, D.A.; Gubaidullin, R.R.; Odinokov, V.N.; Maximchik, P.V.; Zhivotovsky, B.; Bel’skii, Y.P.; Khazanov, V.A.; Manuylova, A.V.; Gogvadze, V.; Spivak, A.Y. Mitochondria-targeted betulinic and ursolic acid derivatives: Synthesis and anticancer activity. Medchemcomm 2017, 8, 1934–1945. [Google Scholar] [CrossRef]
- Feron, O. Pyruvate into lactate and back: From the Warburg effect to symbiotic energy fuel exchange in cancer cells. Radiother. Oncol. 2009, 92, 329–333. [Google Scholar] [CrossRef]
- Faubert, B.; Solmonson, A.; DeBerardinis, R.J. Metabolic reprogramming and cancer progression. Science 2020, 368, eaaw5473. [Google Scholar] [CrossRef]
- Zois, C.E.; Harris, A.L. Glycogen metabolism has a key role in the cancer microenvironment and provides new targets for cancer therapy. J. Mol. Med. 2016, 94, 137–154. [Google Scholar] [CrossRef]
- McKenna, E.; Traganos, F.; Zhao, H.; Darzynkiewicz, Z. Persistent DNA damage caused by low levels of mitomycin C induces irreversible cell senescence. Cell Cycle 2012, 11, 3132–3140. [Google Scholar] [CrossRef] [PubMed]
- Nowak, D.; Kloskowski, T.; Goslinski, M.; Buhl, M.; Wojtowicz, E.; Poplawski, C.; Drewa, T.; Pokrywczynska, M. Antioxidant Properties of Aronia melanocarpa and Morinda citrifolia Juices and their Impact on Bladder Cancer Cell Lines. Med. Sci. Monit. 2025, 31, e945120. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Min, K.J. Betulinic Acid Increases the Lifespan of Drosophila melanogaster via Sir2 and FoxO Activation. Nutrients 2024, 16, 441. [Google Scholar] [CrossRef]
- Park, C.; Jeong, J.W.; Han, M.H.; Lee, H.; Kim, G.Y.; Jin, S.; Park, J.H.; Kwon, H.J.; Kim, B.W.; Choi, Y.H. The anti-cancer effect of betulinic acid in u937 human leukemia cells is mediated through ROS-dependent cell cycle arrest and apoptosis. Anim. Cells Syst. 2021, 25, 119–127. [Google Scholar] [CrossRef]
- Zhang, Y.; He, N.; Zhou, X.; Wang, F.; Cai, H.; Huang, S.H.; Chen, X.; Hu, Z.; Jin, X. Betulinic acid induces autophagy-dependent apoptosis via Bmi-1/ROS/AMPK-mTOR-ULK1 axis in human bladder cancer cells. Aging 2021, 13, 21251–21267. [Google Scholar] [CrossRef]
- Heiss, E.H.; Kramer, M.P.; Atanasov, A.G.; Beres, H.; Schachner, D.; Dirsch, V.M. Glycolytic switch in response to betulinic acid in non-cancer cells. PLoS ONE 2014, 9, e115683. [Google Scholar] [CrossRef]
- Dumas, J.F.; Brisson, L.; Chevalier, S.; Maheo, K.; Fromont, G.; Moussata, D.; Besson, P.; Roger, S. Metabolic reprogramming in cancer cells, consequences on pH and tumour progression: Integrated therapeutic perspectives with dietary lipids as adjuvant to anticancer treatment. Semin. Cancer Biol. 2017, 43, 90–110. [Google Scholar] [CrossRef]
- Zhu, X.; Jin, C.; Pan, Q.; Hu, X. Determining the quantitative relationship between glycolysis and GAPDH in cancer cells exhibiting the Warburg effect. J. Biol. Chem. 2021, 296, 100369. [Google Scholar] [CrossRef]
- Ryu, H.; Lee, S.; Song, S.H.; Lee, H.; Oh, J.J.; Hong, S.K.; Byun, S.S.; Song, B. Acidic urine as a prognostic factor after intravesical Bacillus Calmette-Guerin induction therapy for nonmuscle-invasive bladder cancer. World J. Urol. 2025, 43, 247. [Google Scholar] [CrossRef]
- McClure, M.B.; Kogure, Y.; Ansari-Pour, N.; Saito, Y.; Chao, H.H.; Shepherd, J.; Tabata, M.; Olopade, O.I.; Wedge, D.C.; Hoadley, K.A.; et al. Landscape of Genetic Alterations Underlying Hallmark Signature Changes in Cancer Reveals TP53 Aneuploidy-driven Metabolic Reprogramming. Cancer Res. Commun. 2023, 3, 281–296. [Google Scholar] [CrossRef]
- Cao, B.; Li, Q.; Xu, P.; Zhang, Y.; Cai, S.; Rao, S.; Zeng, M.; Dai, Y.; Jiang, S.; Zhou, J. Vesical Imaging-Reporting and Data System (VI-RADS) as a grouping imaging biomarker combined with a decision-tree mode to preoperatively predict the pathological grade of bladder cancer. Clin. Radiol. 2024, 79, e725–e735. [Google Scholar] [CrossRef] [PubMed]
- Golijanin, J.; Amin, A.; Moshnikova, A.; Brito, J.M.; Tran, T.Y.; Adochite, R.C.; Andreev, G.O.; Crawford, T.; Engelman, D.M.; Andreev, O.A.; et al. Targeted imaging of urothelium carcinoma in human bladders by an ICG pHLIP peptide ex vivo. Proc. Natl. Acad. Sci. USA 2016, 113, 11829–11834. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Lin, J.; Chen, X.; Zhou, X.; Zhang, Y.; Fan, M.; Xiang, J.; He, N.; Hu, Z.; Wang, F. CircXRN2 suppresses tumor progression driven by histone lactylation through activating the Hippo pathway in human bladder cancer. Mol. Cancer 2023, 22, 151. [Google Scholar] [CrossRef] [PubMed]
- Woolcott, C.G.; Maskarinec, G.; Haiman, C.A.; Henderson, B.E.; Kolonel, L.N. Diabetes and urothelial cancer risk: The Multiethnic Cohort study. Cancer Epidemiol. 2011, 35, 551–554. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; Ibrahim, R.S.; Arabi, B.; Brockmueller, A.; Shakibaei, M.; Busselberg, D. The effect of GLP-1R agonists on the medical triad of obesity, diabetes, and cancer. Cancer Metastasis Rev. 2024, 43, 1297–1314. [Google Scholar] [CrossRef]
- Mahato, A.; Jain, A.; Prakash, V.S.; Nair, R.; Joshi, R.; Paliwal, D.; Tiwari, A.; Khandpur, S.; Singh, H. Use of Oral Empagliflozin to Obtain Optimal Blood Sugar Levels for Conducting (18) F-FDG PET-CT in Patients with Hyperglycemia-A Pilot Study. World J. Nucl. Med. 2023, 22, 191–195. [Google Scholar] [CrossRef]
- Bouchelouche, K. PET/CT in Bladder Cancer: An Update. Semin. Nucl. Med. 2022, 52, 475–485. [Google Scholar] [CrossRef]
- Roth, B.J.; Gill, B.C.; Khooblall, P.; Vallabhaneni, S.; Bole, R.; Bajic, P. Associations Between Sodium-Glucose Co-transporter 2 Inhibitors and Urologic Diseases: Implications for Lower Urinary Tract Symptoms from a Multi-State Health System Analysis. Urology 2024, 192, 119–125. [Google Scholar] [CrossRef]


| Doubling Time (h) | T24 | TRT-HU1 |
|---|---|---|
| Basal Glucose | 18.3 ± 0.048 | 40.7 ± 0.024 |
| 10% Increased Glucose | 15.5 ± 0.126 | 39.4 ± 0.072 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganguly, A.; Halder, A.; Healy, K.; Daugherty, S.; Kimura, S.; Banerjee, R.; Beckel, J.M.; Tyagi, P. Metabolic Reprogramming of Urothelial Carcinoma—A Theragnostic Target for Betulinic Acid. Int. J. Mol. Sci. 2025, 26, 5598. https://doi.org/10.3390/ijms26125598
Ganguly A, Halder A, Healy K, Daugherty S, Kimura S, Banerjee R, Beckel JM, Tyagi P. Metabolic Reprogramming of Urothelial Carcinoma—A Theragnostic Target for Betulinic Acid. International Journal of Molecular Sciences. 2025; 26(12):5598. https://doi.org/10.3390/ijms26125598
Chicago/Turabian StyleGanguly, Anirban, Aratrika Halder, Keara Healy, Stephanie Daugherty, Shingo Kimura, Rajkumar Banerjee, Jonathan M. Beckel, and Pradeep Tyagi. 2025. "Metabolic Reprogramming of Urothelial Carcinoma—A Theragnostic Target for Betulinic Acid" International Journal of Molecular Sciences 26, no. 12: 5598. https://doi.org/10.3390/ijms26125598
APA StyleGanguly, A., Halder, A., Healy, K., Daugherty, S., Kimura, S., Banerjee, R., Beckel, J. M., & Tyagi, P. (2025). Metabolic Reprogramming of Urothelial Carcinoma—A Theragnostic Target for Betulinic Acid. International Journal of Molecular Sciences, 26(12), 5598. https://doi.org/10.3390/ijms26125598






