High PD-L1 Expression in HRS Cells and Macrophages in Tumor Immune Microenvironment Is Associated with Adverse Outcome and EBV Positivity in Classical Hodgkin Lymphoma
Abstract
1. Introduction
2. Results
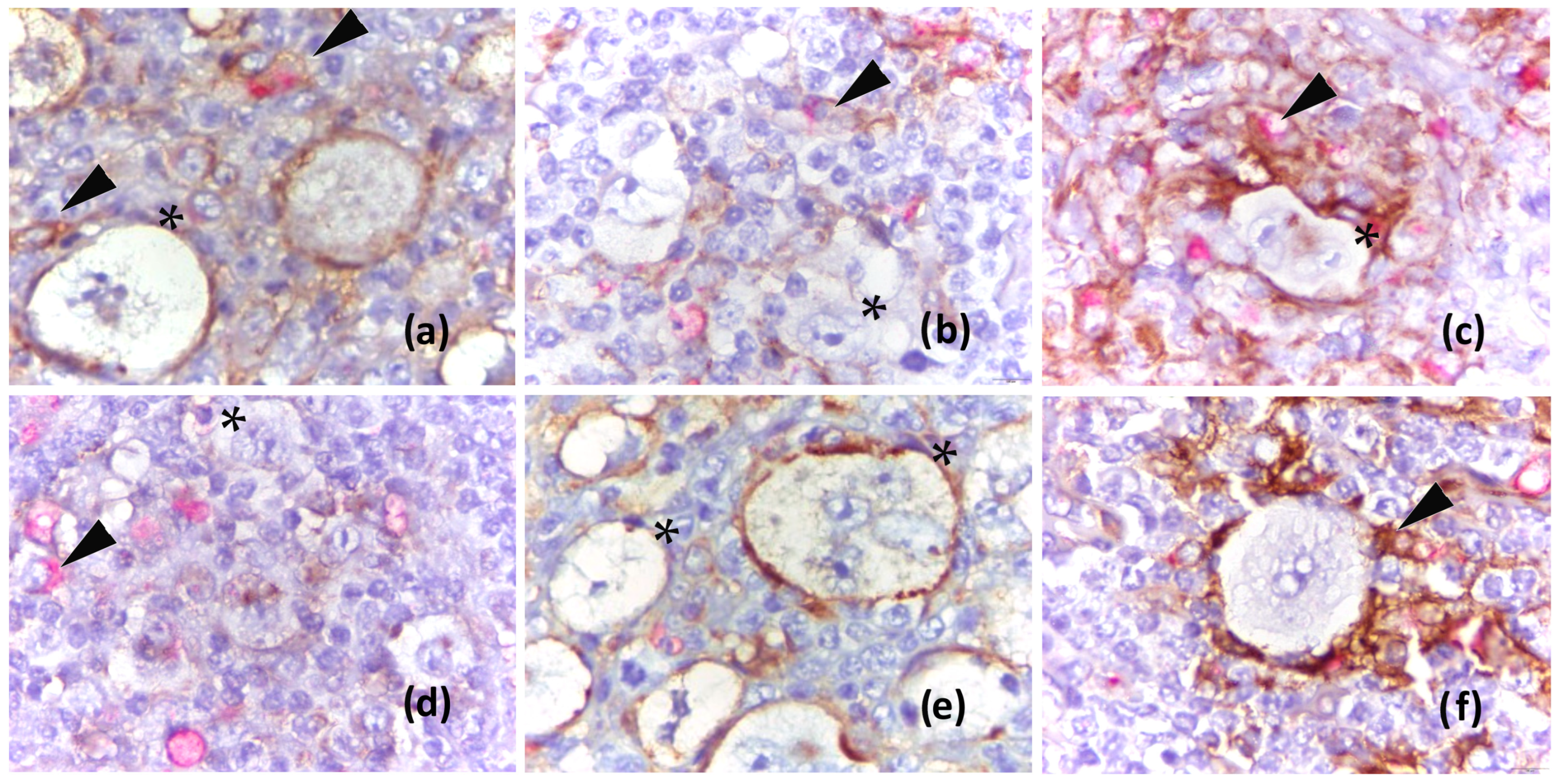
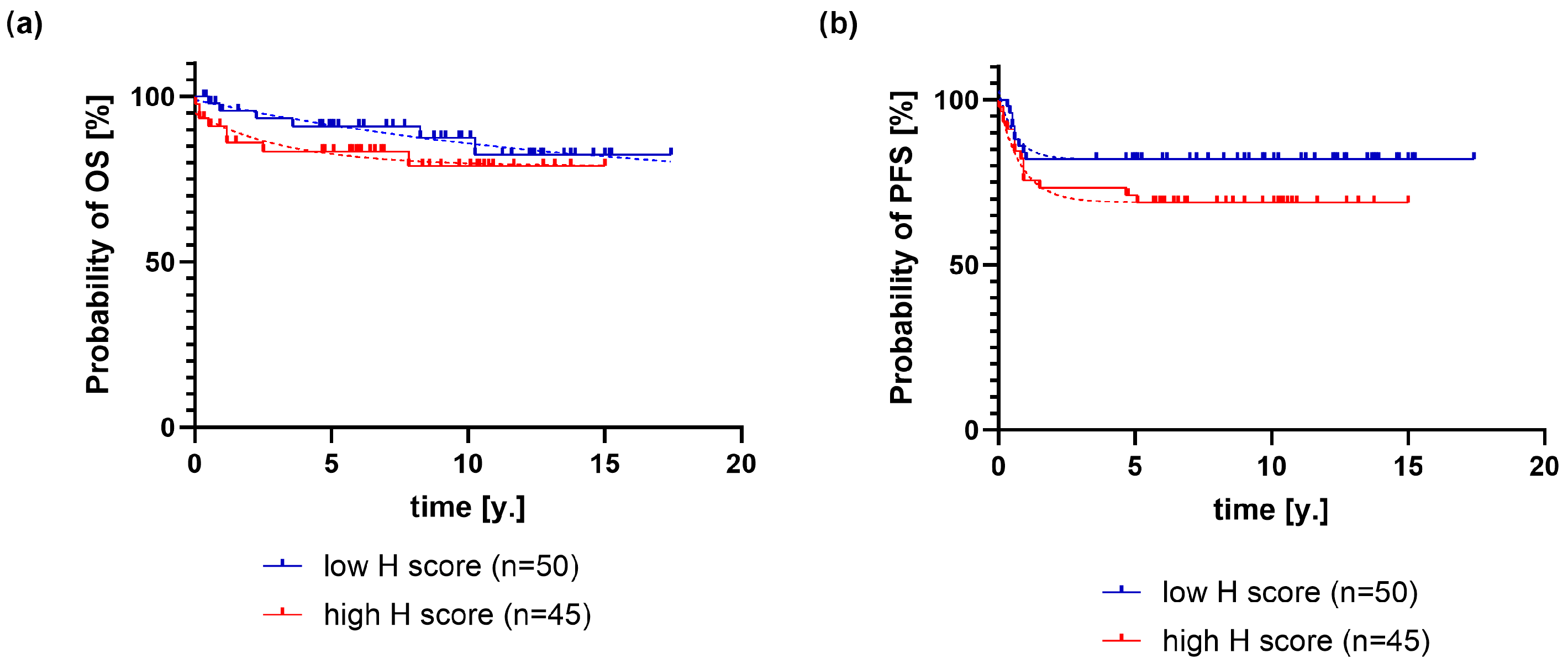
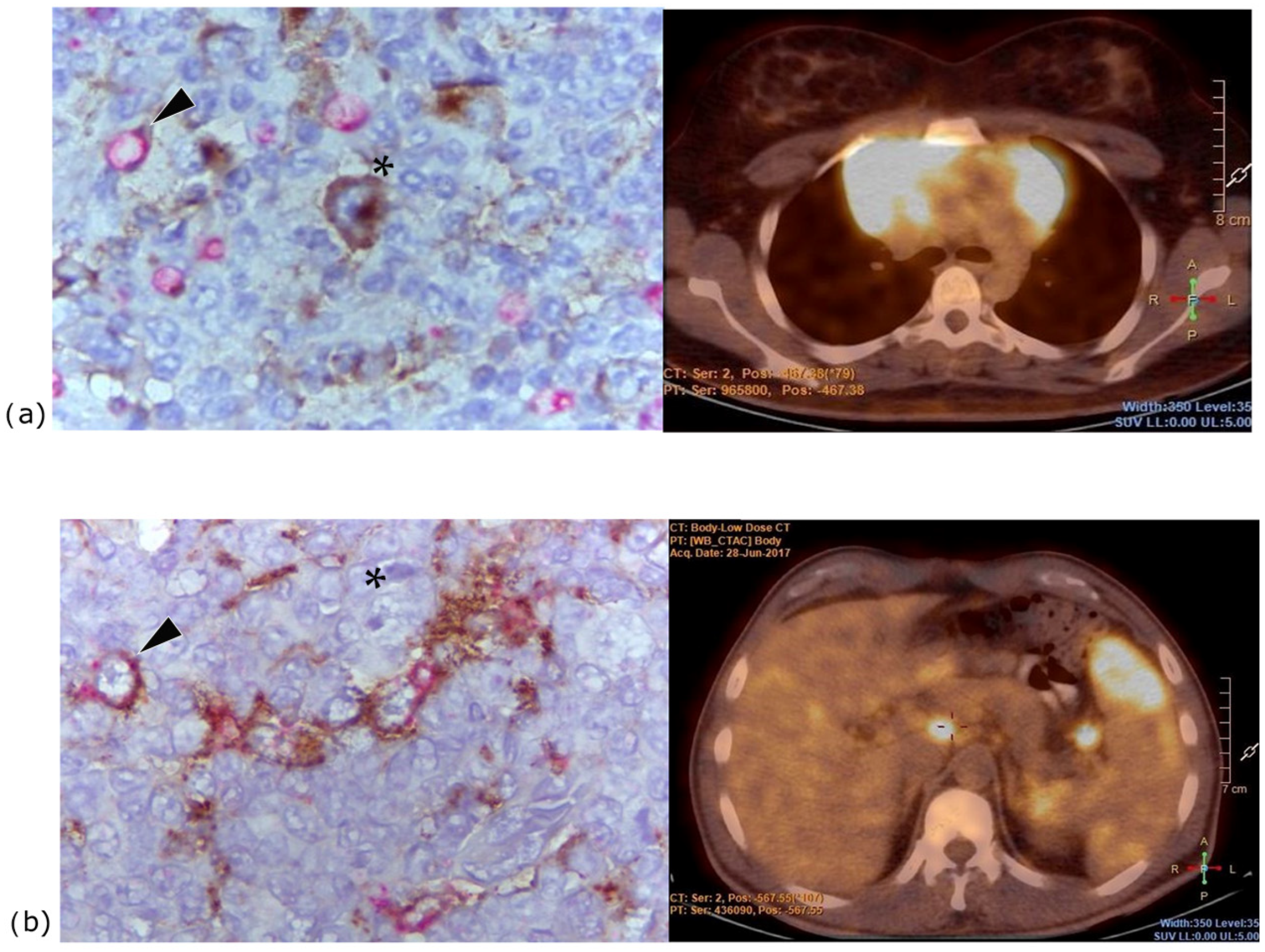
2.1. Association of PD-L1 Expression on HRS Cells with Clinical and Pathological Variables and Outcome of Classical Hodgkin Lymphoma
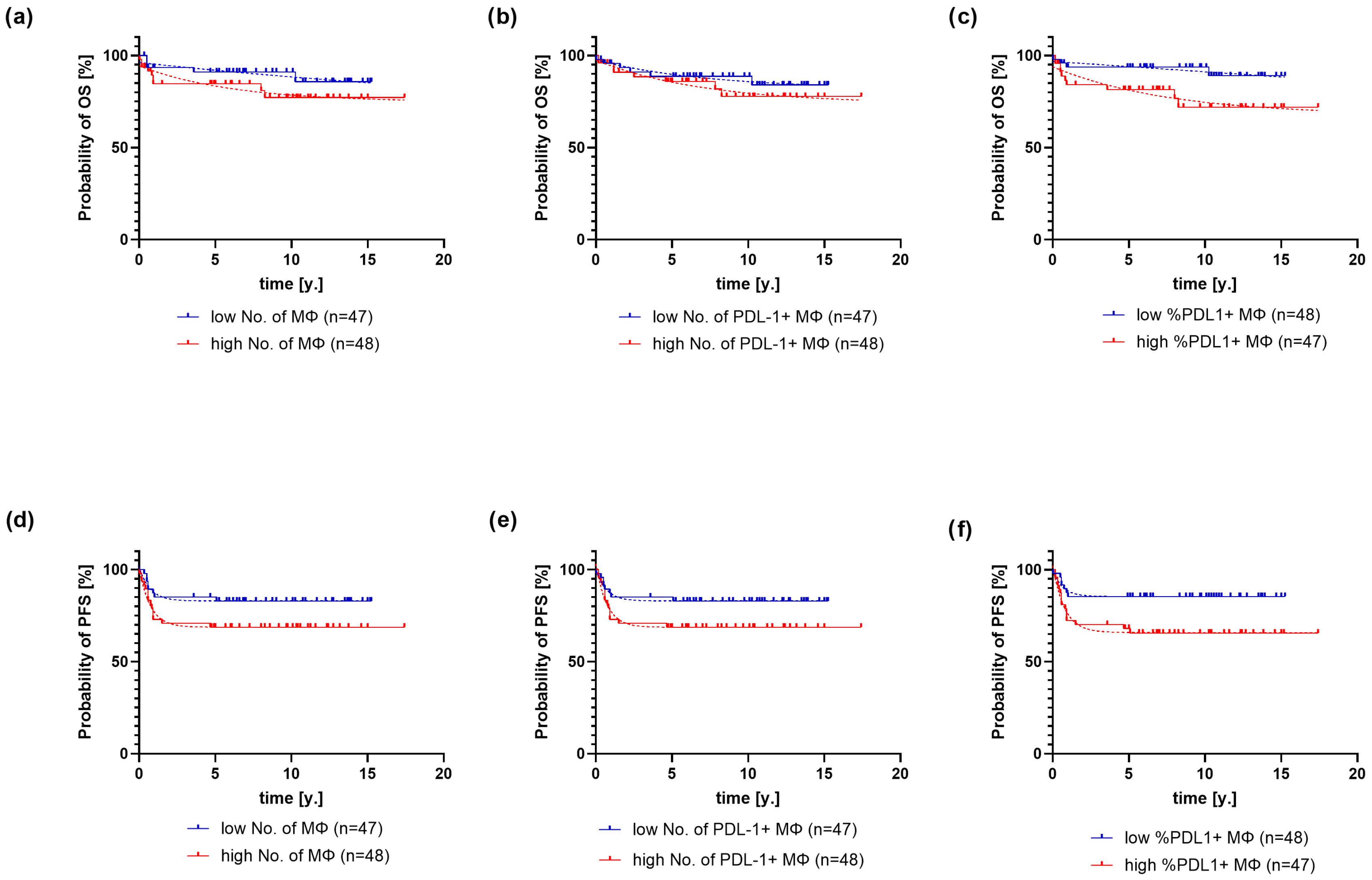
2.2. Association of PD-L1 Expression on Macrophages with Clinical and Pathological Variables and Outcome of Classical Hodgkin Lymphoma
3. Discussion
4. Materials and Methods
4.1. Patient Characteristics
4.2. Methods
4.2.1. Analysis of PD-L1 in HRS Cells
4.2.2. Analysis of PD-L1 in Macrophages
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PD-L1 | Programmed Death-Ligand 1 |
| HRS | Hodgkin and Reed–Sternberg |
| cHL | Classical Hodgkin Lymphoma |
| TME | Tumor Immune Microenvironment |
| OS | Overall Survival |
| PFS | Progression-Free Survival |
| EBV | Epstein–Barr Virus |
| H-score | Histologic Score |
| CR | Complete Remission |
| IFN-γ | Interferon Gamma |
| PD-1 | Programmed Cell Death 1 |
| ICIs | Checkpoint Inhibitors |
| R/R | Relapsed/Refractory |
| IQR | Interquartile Range |
| MΦ | Macrophages |
| EBER | Epstein–Barr Virus-Encoded RNA |
| CI | Confidence Interval |
| ANOVA | Analysis of Variance |
| PET-CT | Positron Emission Tomography–Computed Tomography |
| ER | Evidence Ratio |
| CROHEM | Croatian Cooperative Group for Hematologic Diseases |
| NCCN | National Comprehensive Cancer Network |
| ABVD | Chemotherapy protocol: Doxorubicin, Bleomycin, Vinblastine, and Dacarbazine |
| BEACOPP | Bleomycin, Etoposide, Doxorubicin, Cyclophosphamide, Vincristine, Procarbazine, and Prednisone |
| OEPA | Vincristine, Etoposide, Prednisone, and doxorubicin |
| DHAP | Cisplatin, Cytarabine, and Dexamethasone |
| ICE | Ifosfamide, Carboplatin, and Etoposide |
| ISH | In Situ Hybridization |
| DAB | 3,3’-diaminobenzidine |
| IHC | Immunohistochemistry |
References
- Press, O.W.; Li, H.; Schöder, H.; Straus, D.J.; Moskowitz, C.H.; LeBlanc, M.; Rimsza, L.M.; Bartlett, N.L.; Evens, A.M.; Mittra, E.S.; et al. US Intergroup Trial of Response-Adapted Therapy for Stage III to IV Hodgkin Lymphoma Using Early Interim Fluorodeoxyglucose-Positron Emission Tomography Imaging: Southwest Oncology Group S0816. J. Clin. Oncol. 2016, 34, 2020–2027. [Google Scholar] [CrossRef] [PubMed]
- Epperla, N.; Herrera, A.F. How I Incorporate Novel Agents into the Treatment of Classical Hodgkin Lymphoma. Blood 2021, 138, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Jang, B.-C.; Lee, S.-W.; Yang, Y.-I.; Suh, S.-I.; Park, Y.-M.; Oh, S.; Shin, J.-G.; Yao, S.; Chen, L.; et al. Interferon Regulatory Factor-1 Is Prerequisite to the Constitutive Expression and IFN-γ-Induced Upregulation of B7-H1 (CD274). FEBS Lett. 2006, 580, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Abiko, K.; Matsumura, N.; Hamanishi, J.; Horikawa, N.; Murakami, R.; Yamaguchi, K.; Yoshioka, Y.; Baba, T.; Konishi, I.; Mandai, M. IFN-γ from Lymphocytes Induces PD-L1 Expression and Promotes Progression of Ovarian Cancer. Br. J. Cancer 2015, 112, 1501–1509. [Google Scholar] [CrossRef]
- Green, M.R.; Monti, S.; Rodig, S.J.; Juszczynski, P.; Currie, T.; O’Donnell, E.; Chapuy, B.; Takeyama, K.; Neuberg, D.; Golub, T.R.; et al. Integrative Analysis Reveals Selective 9p24.1 Amplification, Increased PD-1 Ligand Expression, and Further Induction via JAK2 in Nodular Sclerosing Hodgkin Lymphoma and Primary Mediastinal Large B-Cell Lymphoma. Blood 2010, 116, 3268–3277. [Google Scholar] [CrossRef]
- Ansell, S.M.; Bröckelmann, P.J.; von Keudell, G.; Lee, H.J.; Santoro, A.; Zinzani, P.L.; Collins, G.P.; Cohen, J.B.; de Boer, J.P.; Kuruvilla, J. Nivolumab for Relapsed/Refractory Classical Hodgkin Lymphoma: 5-Year Survival from the Pivotal Phase 2 CheckMate 205 Study. Blood Adv. 2023, 7, 6266–6274. [Google Scholar] [CrossRef]
- Younes, A.; Santoro, A.; Shipp, M.; Zinzani, P.L.; Timmerman, J.M.; Ansell, S.; Armand, P.; Fanale, M.; Ratanatharathorn, V.; Kuruvilla, J. Nivolumab for Classical Hodgkin’s Lymphoma after Failure of Both Autologous Stem-Cell Transplantation and Brentuximab Vedotin: A Multi-Centre, Multicohort, Single-Arm Phase 2 Trial. Lancet Oncol. 2016, 17, 1283–1294. [Google Scholar] [CrossRef]
- Chen, R.; Zinzani, P.L.; Lee, H.J.; Armand, P.; Johnson, N.A.; Brice, P.; Radford, J.; Ribrag, V.; Molin, D.; Vassilakopoulos, T.P.; et al. Pembrolizumab in Relapsed or Refractory Hodgkin Lymphoma: 2-Year Follow-Up of KEYNOTE-087. Blood 2019, 134, 1144–1153. [Google Scholar] [CrossRef]
- De Re, V.; Caggiari, L.; Repetto, O.; Mussolin, L.; Mascarin, M. Classical Hodgkin’s Lymphoma in the Era of Immune Checkpoint Inhibition. J. Clin. Med. 2019, 8, 1596. [Google Scholar] [CrossRef]
- Pavlović, A.; Miljak, A.; Brzica, K.; Glavina Durdov, M. The Abundance of FOXP3, FOXP3/CD4 and CD8 Cells in the Microenvironment of Nodular Sclerosis and Mixed Cellularity Subtypes Is Associated with the Epstein–Barr Virus Status of Classic Hodgkin Lymphoma. Biomedicines 2024, 12, 1680. [Google Scholar] [CrossRef]
- Paydas, S.; Bağır, E.; Seydaoglu, G.; Ercolak, V.; Ergin, M. Programmed Death-1 (PD-1), Programmed Death-Ligand 1 (PD-L1), and EBV-Encoded RNA (EBER) Expression in Hodgkin Lymphoma. Ann. Hematol. 2015, 94, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Antel, K.; Chetty, D.; Oosthuizen, J.; Mohamed, Z.; Van der Vyver, L.; Verburgh, E. CD68-Positive Tumour Associated Macrophages, PD-L1 Expression, and EBV Latent Infection in a High HIV-Prevalent South African Cohort of Hodgkin Lymphoma Patients. Pathology 2021, 53, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, O.; Colli, S.; Garcia Lombardi, M.; Preciado, M.V.; De Matteo, E.; Chabay, P. Epstein–Barr Virus Recruits PDL1-Positive Cells at the Microenvironment in Pediatric Hodgkin Lymphoma. Cancer Immunol. Immunother. 2021, 70, 1519–1526. [Google Scholar] [CrossRef]
- Hollander, P.; Kamper, P.; Smedby, K.E.; Enblad, G.; Ludvigsen, M.; Mortensen, J.; Amini, R.M.; Hamilton-Dutoit, S.; d’Amore, F.; Molin, D.; et al. High Proportions of PD-1+ and PD-L1+ Leukocytes in Classical Hodgkin Lymphoma Microenvironment Are Associated with Inferior Outcome. Blood Adv. 2017, 1, 1427–1439. [Google Scholar] [CrossRef] [PubMed]
- Roemer, M.G.M.; Advani, R.H.; Ligon, A.H.; Natkunam, Y.; Redd, R.A.; Homer, H.; Connelly, C.F.; Sun, H.H.; Daadi, S.E.; Freeman, G.J.; et al. PD-L1 and PD-L2 Genetic Alterations Define Classical Hodgkin Lymphoma and Predict Outcome. J. Clin. Oncol. 2016, 34, 2690–2697. [Google Scholar] [CrossRef]
- Koh, Y.W.; Jeon, Y.K.; Yoon, D.H.; Suh, C.; Huh, J. Programmed Death 1 Expression in the Peritumoral Microenvironment Is Associated with a Poorer Prognosis in Classical Hodgkin Lymphoma. Tumour Biol. 2016, 37, 7507–7514. [Google Scholar] [CrossRef]
- Steidl, C.; Lee, T.; Shah, S.P.; Farinha, P.; Han, G.; Nayar, T.; Delaney, A.; Jones, S.J.; Iqbal, J.; Weisenburger, D.D.; et al. Tumor-Associated Macrophages and Survival in Classic Hodgkin’s Lymphoma. N. Engl. J. Med. 2010, 362, 875–885. [Google Scholar] [CrossRef]
- Guo, B.; Cen, H.; Tan, X.; Ke, Q. Meta-Analysis of the Prognostic and Clinical Value of Tumor-Associated Macrophages in Adult Classical Hodgkin Lymphoma. BMC Med. 2016, 14, 159. [Google Scholar] [CrossRef]
- Karihtala, K.; Leivonen, S.-K.; Brück, O.; Karjalainen-Lindsberg, M.-L.; Mustjoki, S.; Pellinen, T.; Leppä, S. Prognostic Impact of Tumor-Associated Macrophages on Survival Is Checkpoint Dependent in Classical Hodgkin Lymphoma. Cancers 2020, 12, 877. [Google Scholar] [CrossRef]
- Carey, C.D.; Gusenleitner, D.; Lipschitz, M.; Roemer, M.G.M.; Stack, E.C.; Gjini, E.; Hu, X.; Redd, R.; Freeman, G.J.; Neuberg, D.; et al. Topological Analysis Reveals a PD-L1-Associated Microenvironmental Niche for Reed-Sternberg Cells in Hodgkin Lymphoma. Blood 2017, 130, 2420–2430. [Google Scholar] [CrossRef]
- Kawashima, M.; Carreras, J.; Higuchi, H.; Kotaki, R.; Hoshina, T.; Okuyama, K.; Suzuki, N.; Kakizaki, M.; Miyatake, Y.; Ando, K.; et al. PD-L1/L2 Protein Levels Rapidly Increase in Monocytes via Trogocytosis from Tumor Cells in Classical Hodgkin Lymphoma. Leukemia 2020, 34, 2405–2417. [Google Scholar] [CrossRef] [PubMed]
- Bettadapur, A.; Miller, H.W.; Ralston, K.S. Biting Off What Can Be Chewed: Trogocytosis in Health, Infection, and Disease. Infect. Immun. 2020, 88, e00930-19. [Google Scholar] [CrossRef]
- Zeng, Q.; Schwarz, H. The Role of Trogocytosis in Immune Surveillance of Hodgkin Lymphoma. OncoImmunology 2020, 9, 1781334. [Google Scholar] [CrossRef] [PubMed]
- Stewart, B.J.; Fergie, M.; Young, M.D.; Jones, C.; Sachdeva, A.; Blain, A.; Bacon, C.M.; Rand, V.; Ferdinand, J.R.; James, K.R.; et al. Spatial and Molecular Profiling of the Mononuclear Phagocyte Network in Classic Hodgkin Lymphoma. Blood 2023, 141, 2343–2358. [Google Scholar] [CrossRef]
- Jarrett, A.F.; Armstrong, A.A.; Alexander, E. Epidemiology of EBV and Hodgkin’s Lymphoma. Ann. Oncol. 1996, 7 (Suppl. S4), S5–S10. [Google Scholar] [CrossRef]
- Moyano, A.; Ferressini, N.; De Matteo, E.; Preciado, M.V.; Chabay, P. PD-L1 Is Upregulated in CD163+ Tonsillar Macrophages from Children Undergoing EBV Primary Infection. Front. Immunol. 2022, 13, 940910. [Google Scholar] [CrossRef]
- Yu, J.; Jin, S.; Yin, X.; Du, H. Expression of the Immune Checkpoint Molecules PDL1 and PD1 in EBV Associated Lymphoproliferative Disorders: A Meta-Analysis. Exp. Ther. Med. 2023, 7, 12294. [Google Scholar] [CrossRef]
- Green, M.R.; Rodig, S.; Juszczynski, P.; Ouyang, J.; Sinha, P.; O’Donnell, E.; Neuberg, D.; Shipp, M.A. Constitutive AP-1 Activity and EBV Infection Induce PD-L1 in Hodgkin Lymphomas and Post-Transplant Lymphoproliferative Disorders: Implications for Targeted Therapy. Clin. Cancer Res. 2012, 18, 1611–1618. [Google Scholar] [CrossRef]
- Moskowitz, A.J.; Perales, M.-A.; Kewalramani, T.; Yahalom, J.; Castro-Malaspina, H.; Zhang, Z.; Vanak, J.; Zelenetz, A.D.; Moskowitz, C.H. Outcomes for Patients Who Fail High-Dose Chemoradiotherapy and Autologous Stem Cell Rescue for Relapsed and Primary Refractory Hodgkin Lymphoma. Br. J. Haematol. 2009, 146, 158–163. [Google Scholar] [CrossRef]
- Roemer, M.G.M.; Redd, R.A.; Cader, F.Z.; Pak, C.J.; Abdelrahman, S.; Ouyang, J.; Sasse, S.; Younes, A.; Fanale, M.; Santoro, A.; et al. Major Histocompatibility Complex Class II and Programmed Death Ligand 1 Expression Predict Outcome After Programmed Death 1 Blockade in Classic Hodgkin Lymphoma. J. Clin. Oncol. 2018, 36, 942–950. [Google Scholar] [CrossRef]
- Bröckelmann, P.J.; Goergen, H.; Keller, U.; Meissner, J.; Ordemann, R.; Halbsguth, T.V.; Sasse, S.; Sökler, M.; Kerkhoff, A.; Mathas, S.; et al. Efficacy of Nivolumab and AVD in Early-Stage Unfavorable Classic Hodgkin Lymphoma: The Randomized Phase 2 German Hodgkin Study Group NIVAHL Trial. JAMA Oncol. 2020, 6, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.N.; Nahar, A.; Kim, E.; Marinello, P. Sequential Pembrolizumab (Pembro) and Chemotherapy (Chemo) in Newly Diagnosed, Early Unfavorable, or Advanced-Stage Classical Hodgkin Lymphoma (cHL): The Phase 2 KEYNOTE-C11 Study. J. Clin. Oncol. 2022, 40 (Suppl. S16), TPS7584. [Google Scholar] [CrossRef]
- Reinke, S.; Bröckelmann, P.J.; Iaccarino, I.; Garcia-Marquez, M.; Borchmann, S.; Jochims, F.; Kotrova, M.; Pal, K.; Brüggemann, M.; Hartmann, E. Tumor and Microenvironment Response but No Cytotoxic T-Cell Activation in Classic Hodgkin Lymphoma Treated with Anti-PD1. Blood 2020, 136, 2851–2863. [Google Scholar] [CrossRef] [PubMed]
| Variables | Median, IQR |
|---|---|
| PD-L1+ HRS H-score | 80 (36.5–142.8) |
| Macrophages | 6.8 (5.3–8.9) |
| PD-L1+ macrophages | 5.4 (3.6–7.1) |
| Proportion of PD-L1+ macrophages (%) | 85.9 (65.4–91.9) |
| Variable | PD-L1 H-Score | IQR of H-Score | p |
|---|---|---|---|
| Histological subtype | 0.10 | ||
| Nodular sclerosis (n = 73) | 83 | 40–50 | |
| Mixed cellularity (n = 21) | 75 | 31–121 | |
| Lymphocyte-rich (n = 3) | 20 | 3–80 | |
| Lymphocyte-depleted (n = 1) | 216 | 216–216 | |
| Clinical stage | 0.28 | ||
| Early favorable (n = 23) | 81 | 25–145 | |
| Early unfavorable (n = 29) | 67 | 35–118 | |
| Advanced (n = 45) | 85 | 40–190 | |
| Extranodal disease | 0.35 | ||
| no (n = 66) | 84 | 40–154 | |
| yes (n = 30) | 77 | 33–142 | |
| Bulky disease | 0.69 | ||
| no (n = 72) | 82 | 28–133 | |
| yes (n = 26) | 80 | 38–145 | |
| B symptoms | 0.42 | ||
| no | 75 | 36–143.5 | |
| yes | 85 | 37.5–143.5 | |
| EBV status | 0.005 | ||
| no (n = 75) | 70 | 34–104 | |
| yes (n = 22) | 136 | 82.5–182.3 |
| Variable | Macrophages | PD-L1+ Macrophages | Proportion of PD-L1+ Macrophages | |||||
|---|---|---|---|---|---|---|---|---|
| Subtype | N | IQR | N | IQR | % | IQR | ||
| Nodular sclerosis | 6.3 | 4.65–7.73 | 4.9 | 3.21–6.65 | 85.3 | 63.3–91.2 | ||
| Mixed cellularity | 9.6 | 7.62–10.65 | 8.0 | 6.4–10.31 | 89.8 | 83.9–93.9 | ||
| Lymphocyte-rich | 5.2 | 4.3–6.7 | 4.2 | 1.4–4.6 | 68.7 | 32.6–80.8 | ||
| Lymphocyte-depleted | 8.7 | 8.7–8.7 | 4.6 | 4.6–4.6 | 52.9 | 52.9–52.9 | ||
| p | 0.0002 | <0.0001 | 0.02 | |||||
| Extranodal | ||||||||
| no | 6.65 | 4.92–7.92 | 5.0 | 3.21–6.8 | 85.1 | 63.1–90.2 | ||
| yes | 7.48 | 5.3–9.8 | 6.3 | 4.0–8.42 | 88.7 | 76.7–94.9 | ||
| p | 0.18 | 0.05 | 0.06 | |||||
| Bulky | ||||||||
| no | 7.35 | 5.3–9.2 | 5.75 | 4.0–8.0 | 87.1 | 74.4–92.0 | ||
| yes | 6.2 | 4.1–7.78 | 3.9 | 2.87–6.4 | 82.3 | 56.2–91.7 | ||
| p | 0.07 | 0.04 | 0.04 | |||||
| EBV status | ||||||||
| negative | 6.3 | 4.6–7.8 | 4.8 | 3.3–6.8 | 84 | 64.1–90.9 | ||
| positive | 8.3 | 6.9–9.8 | 6.8 | 5.6–8.9 | 90.7 | 82.2 –94.6 | ||
| p | 0.008 | 0.005 | 0.11 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miljak, A.; Pavlović, A.; Benzon, B.; Čutura, L.V.; Galušić, D.; Vujčić, M.; Blaslov, V.; Durdov, M.G. High PD-L1 Expression in HRS Cells and Macrophages in Tumor Immune Microenvironment Is Associated with Adverse Outcome and EBV Positivity in Classical Hodgkin Lymphoma. Int. J. Mol. Sci. 2025, 26, 5592. https://doi.org/10.3390/ijms26125592
Miljak A, Pavlović A, Benzon B, Čutura LV, Galušić D, Vujčić M, Blaslov V, Durdov MG. High PD-L1 Expression in HRS Cells and Macrophages in Tumor Immune Microenvironment Is Associated with Adverse Outcome and EBV Positivity in Classical Hodgkin Lymphoma. International Journal of Molecular Sciences. 2025; 26(12):5592. https://doi.org/10.3390/ijms26125592
Chicago/Turabian StyleMiljak, Antonija, Antonia Pavlović, Benjamin Benzon, Lučana Vicelić Čutura, Davor Galušić, Milan Vujčić, Viktor Blaslov, and Merica Glavina Durdov. 2025. "High PD-L1 Expression in HRS Cells and Macrophages in Tumor Immune Microenvironment Is Associated with Adverse Outcome and EBV Positivity in Classical Hodgkin Lymphoma" International Journal of Molecular Sciences 26, no. 12: 5592. https://doi.org/10.3390/ijms26125592
APA StyleMiljak, A., Pavlović, A., Benzon, B., Čutura, L. V., Galušić, D., Vujčić, M., Blaslov, V., & Durdov, M. G. (2025). High PD-L1 Expression in HRS Cells and Macrophages in Tumor Immune Microenvironment Is Associated with Adverse Outcome and EBV Positivity in Classical Hodgkin Lymphoma. International Journal of Molecular Sciences, 26(12), 5592. https://doi.org/10.3390/ijms26125592






