Photobiomodulation in Promoting Cartilage Regeneration
Abstract
1. Introduction
2. Photobiomodulation
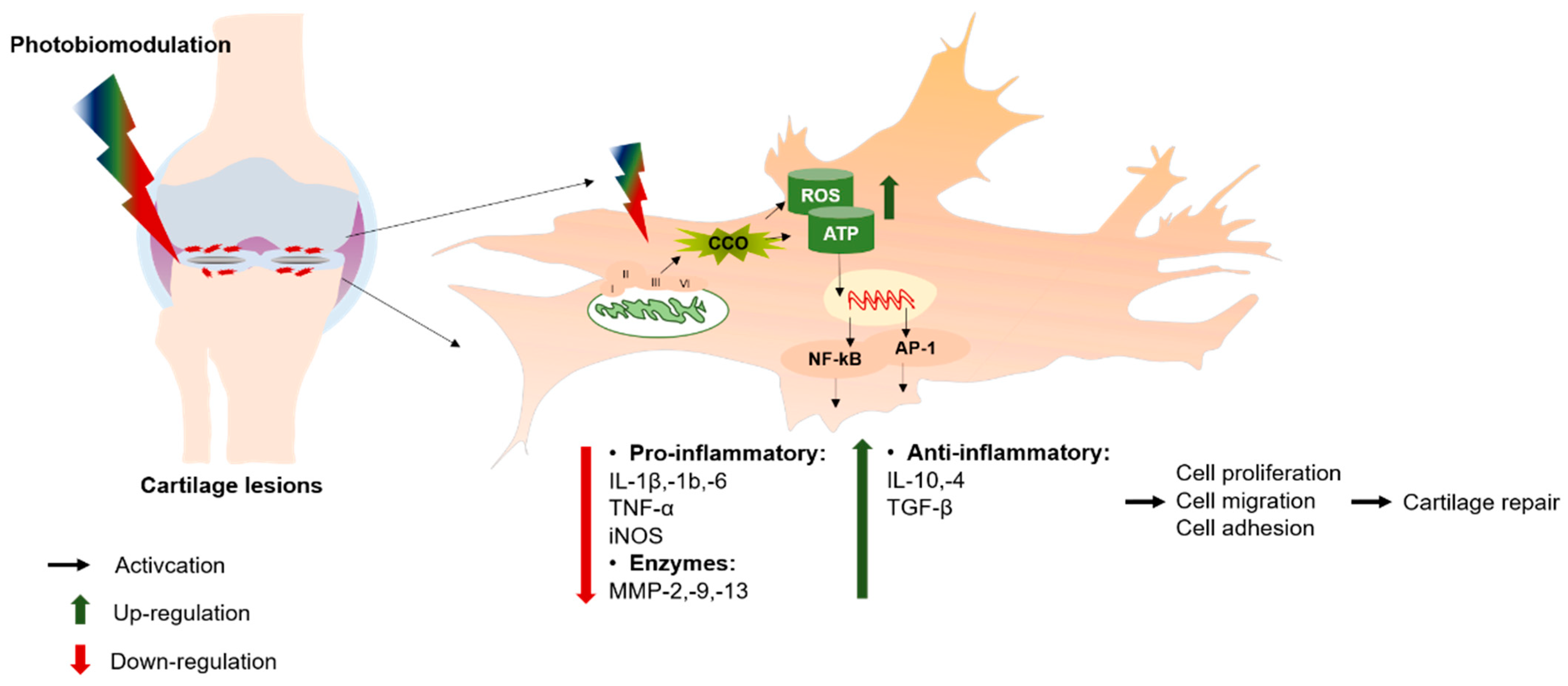
2.1. Mechanism of PBM in Promoting Cartilage Repair
2.2. In Vitro Studies of PBM Effects in Cartilage Regeneration
2.3. In Vivo Studies of PBM Effects in Cartilage Regeneration
| Objective | Laser Parameters | Outcome | References | ||||
|---|---|---|---|---|---|---|---|
| Light Sources | Wavelength | Power Output/Power Intensity | Irradiation Time | Fluency (J/cm2) | |||
| rMSCs and hMSCs | GaAlAs | 660 nm | 30 mW | 25 s 50 s 100 s 300 s | 0.75 J/cm2 1.5 J/cm2 3 J/cm2 9 J/cm2 | PBM (3 J/cm2) triggered statistically significant VEGF and VEGFR2 expression on hMSCs and rMSCs under low nutrient supplement (5% FBS) compared other groups. | de Oliveria., 2014 [29] |
| hUCMSCs | Laser | 635 nm 808 nm 1443 nm | 200 mW | Twice/day for 3 days | 12 J/cm2 | PBM (635 nm) showed a greater impact on proliferation rate and ROS production compared to PBM of 808 and 635 + 808 nm. PBM (808 nm) stimulated the expression of IL-1, IL-6, and NF-kB, which was not in VEGF. | Chen et al., 2016 [30] |
| hADSCs | Red laser | 660 nm ± 20 | 3-4.5 Mw | 1-3 h | 11-16 J/cm2 | PBM (11 J/cm2) had a greater impact on cell proliferation compared to other fluences. | Kan Yin et al., 2017 [31] |
| hADSCs | InGaAIP | 660 nm | 40 mW | 14 s 46 s 126 s | 20 J/cm2 70 J/cm2 180 J/cm2 | PBM (20 and 70 J/cm2) stimulated significantly cell proliferation after 3 days. Increase of mitochondrial activity was observed after 72 h. receiving PBM (20 and 70 J/cm2), where 180 J/cm2 inhibited mitochondrial functions. | de Abdrade et al., 2018 [9] |
| hBMMSCs and hADSCs | He-Ne red NIR | 632.8 nm 630 nm 810 nm | 0.5 mW 5 mW 5 mW | 3 times at day 1, 3 and 5 | 0.6, 1.2, 2.4 J/cm2 | Increase of cell viability (hADSCs/hBMMSCs) and decreased population doubling time (PDT) of hBMMSCs exhibited after irradiating at 630 nm. Combination of 630 and 810 nm promoted cell viability, decreased DPT and apoptosis of both cells. | Zare et al., 2019 [10] |
| hADSCs | Blue, green, red, NIR | 415 nm 540 nm 660 nm 810 nm | NA | 188 s | 3 J/cm2 | PBM (3 J/cm2, red/NIR) promoted ATP production and proliferation rate, while using blue/green wavelength downregulated ATP and inhibited cell proliferation. | Wang et al., 2017 [12] |
| rADSCs | GaAlAs | 660 nm | 30 mW | 9 s 16 s 25 s | 10 J/cm2 18 J/cm2 27 J/cm2 | PBM (10 J/cm2) reduced DOX-induced toxicity effects on cell viability, apoptosis, and inhibited oxidative stress in rADSCs. | Lima et al., 2019 [13] |
| Primary Chondrocyte in Rat | HN-8318 He-Ne | 632.8 nm | 12 mW | 8 min, daily treatment | 5.74 J/cm2 | PBM-treated chondrocytes significantly increased after 1 and 3 days, where GAGs level positively rose. Stimulation of PBM and IL-1β reduced TNF-α expression PBM upregulated protein (COL-II) and gene expression (ACAN, COLII, SOX-9). | Yang et al., 2020 [32] |
| rBMMSCs | He-Ne laser (helium-neon) | 632.8 nm | 1.7 mWt | 15 min with 40-min pause | NA | An increase in chondrogenesis gene expression (TGF-β3, COL2A1, and SOX-9) was shown in two groups: laser and recombinant protein tgfβ3-based treatment. The laser-treated group exhibited chondrogenic differentiation capacity that was less significant than tgfβ3-based treatment. | Bozhokin et al., 2021 [33] |
| hADSCs | LED (blue, green, red) | 475 nm 516 nm 635 nm | NA | 10 min | 40 J/cm2 | PBM (red, 635 nm) triggered the increase in the size of pellet (3D) and gene expression (COL-2A1, IL-1β), while PBM (green and blue) reduced the pellet size (3D), GAG/DNA content, and collagen type II. | Schneider et al., 2021 [34] |
| Normal human articular chondrocyte (NHAC-Kn) | GaAs | 910 nm | 300 mW | 256 s | 8 J/cm2 | PBM reduced inflammatory cytokine (IL-1β and IL-6) and DNA-binding activity of NF-κB in IL-1β-treated chondrocytes, where no impact on NF-kB responses in PBM-treated chondrocytes | Sakata et al., 2022 [35] |
2.4. Clinical Studies of PBM Effects in Cartilage Regeneration
| Objective | Laser Parameters | Disease Model (In Vivo) | Outcome | References | ||||
|---|---|---|---|---|---|---|---|---|
| Light Sources | Wavelength (Nm) | Power Output/Power Intensity | Irradiation Time | Fluency (J/cm2) | ||||
| Chondrocytes (Rat) | GaAlAs | 810 nm | 20 mW | 60 s/session 7 sessions within 2 weeks | 51.02 J/cm2 | TMJ in rats | Reduced inflammation after receiving PBM and steroid treatments statistically compared to control group. | Khozeimed et al., 2014 [36] |
| Chondrocytes (Rat) | Diode laser device (Azor-2k, Russia) | 880 nm | 100 mW | 10 min/day within 7 days | NA | TMJ arthritis in rats | Improvement of damaged cartilage and non-impact in angiogenesis observed after 7 days’ PBM. | Peimani et al., 2014 [37] |
| Chondrocytes (Rat) | GaAlAs | 830 nm | 30 mW | 12 s/session 7 sessions/48 h within 13 days | 3 J/cm2 | TMJ arthritis in rats | The PBM-treated group exhibited reduced thickness and higher collagen fibers in the articular region compared to non-PBM and simultaneously showed a decrease in MMP-2 and MMP-9 activities. | Lemos et al., 2016 [38] |
| Chondrocytes (Rat) | GaAIAs | 808 nm | 50 mW | 28 s, 3 days/ weeks for 8 weeks | 50 J/cm2 | KOA in rats | Reduction of IL-1b and MMP-13 shown in groups undergoing irradiation, aerobic exercise, and both, whereas caspase-3 indicated a reduction in groups combining aerobic exercise and irradiation. | Assis et al., 2016 [39] |
| Chondrocytes (Rat) | GaAIAs | 808 nm | 50 mW | 28 s, 3 days/ weeks for 8 weeks | 50 J/cm2 | KOA in rats | A decrease of IL-1b and caspase-3 was exhibited in groups receiving aquatic exercise coupled with/without PBM, whereas MMP-3 was only increasingly expressed after aquatic exercise and PBM. | Milares et al., 2016 [40] |
| Chondrocytes (Rat) | GaAIAs | 808 nm | 50 mW | 28 s, 3 days/ weeks for 8 weeks | 50 J/cm2 | KOA in rats | PBM/non-PBM coupled with aerobic and exercise training performed increasingly in IL-10 and COL-II expression, where TGF-β levels were promoted with and without exposing PBM combined with aerobic exercise. | Assis et al., 2018 [41] |
| Chondrocytes (Rat) | Gallium arsenidephosphide (InGaAsP) | 940 nm | 20 mW | 60 s/session, 7 sessions, within 2 weeks | NA | OA in TMJ of rats | The moderate increase of GAGs and reduction of caspase-3 were illustrated in the PBM-treated group. | AbuBakr et al., 2018 [42] |
| Chondrocytes (Rat) | GaAIAs | 940 nm | 500 mW | 14 sessions/48 h, then 28 sessions/24 h | 15 J/cm2 | TMJ arthritis in Rat | No significant difference in improvement of cartilage, osteochondral junction, chondrocyte appearance, or subchondral ossification in the groups with and without PBM treatment. | Memis et al., 2018 [43] |
| Chondrocytes (Rat) | GaAIAs | 808 nm | 50 mW | 28 s, 3 days/ weeks | 50 J/cm2 | KOA in rats | Improvement in chondrocyte density of the treated-groups (PBM, chondroitin and glucosamine sulfate (CS/GL), PBM/CS/GL) statistically compared to control group. Group of PBM/CS+GL upregulated significantly inflammatory cytokine of COL-II, and downregulated IL-1β, also exhibited a decrease in OARS score compared to control group. | Shanches et al., 2018 [44] |
| Chondrocytes (Rat) | GaAIAs | 830 nm | 30 mW | 5–20 J/cm2 (20–80 s) 10 sessions/ 48 h, within 3 weeks | 5, 10, 20 J/cm2 | TMJ arthritis in rats | PBM (5 J/cm2) reduced significantly the thickness of articular cartilage at the middle region and increased the GAGs concentration compared other fluences. Both PBM-treated groups showed a greater reduction in MMP-9 and MMP-2 activities; and IL-1β concentration compared to the group of TMJ arthritis. | Lemos et al., 2020 [45] |
| Chondrocytes (Rat) | Laser Moxibustion Device | 10.6 µm | NA | 10 min/day, total of 7 times irradiation | 1500 J/cm2 | MIA model of OA in rat | Reduction of immunoexpression (MMP-3), protein expression (TNF-α, IL-1β, IL-6), and OARSI score detected in MIA/laser-based treatment compared to other treated-groups (MIA and MIA/sham laser). | Li et al., 2020 [46] |
| Chondrocytes (Rat) | GaAIAs | 850 nm | 200 mW | 30 s | NA | KOA in rats | PBM stimulated the increase of COL-II and TGF-β expression in rat OA model. Abnormal chondrocyte organization was observed in the PBM-treated group after histological analysis. | Trevisan et al., 2020 [47] |
| Chondrocytes (Rat) | GaAIAs | 850 nm | 100 mW | 40 s/site, 4 sites | 57.14 J/cm2 | MIA model of OA in rat | Non-PBM efficacy in movable activities, but regeneration of damaged cartilage was proved in superficial layers after PBM. | Balbinot et al., 2021 [48] |
| Chondrocytes (Rat) | LED | 630 nm | 300 mW | 30 s 3 times/ week for 8 weeks | 9 J/cm2 | KOA in rats | Cartilage thickness of LED-treated group (LEDG) exhibited significantly higher values compared to that in the OA group (OAG), but no difference compared to the control group (CG). The enzymatic activity of superoxide dismutase (SOD) and catalase (CAT) of LEDG was higher than that of CG, and thiobarbituric acid-reactive substances (TBARS) lower than that of CG and OAG. | Lorena et al., 2021 [49] |
| Chondrocytes (Rat) | GaAIAs | 808 nm | 50 mW | 16 s (in vitro) 28 s (in vivo), 3 days/ week for 4 and 8 weeks | 28 J/cm2 (in vitro) 50 J/cm2 (in vivo) | KOA in rats (in vitro and in vivo) | PBM enhanced chondrocyte proliferation significantly after 3 days in vitro. An increase of IL-4, IL-10, and COL-II expression was proved after 8 weeks of PBM, whereas a decrease of inflammatory cytokine (IL1-β) was detected after PBM-treated cartilage in vivo. PBM stimulated an increase in TGF-β, COL-2, and aggrecan expression after 4 weeks, whereas that was not observed after 8 weeks in vivo. | Tim et al., 2022 [50] |
| Chondrocytes (Mice) | GaAlAs | 830 nm | 10 mW | 15 s and 150 s 4 treatment sessions | 3 and 30 J/cm2 | Zymosan-induced arthritis in mice | A decrease in MMP-9 and an increase in TIMP-2 were indicated after exposing PBM. PBM of 3 J/cm2 expressed a level of MMP-2, and -9 significantly lower than that of 30 J/cm2; there was no significance in the MMP-13 level and a higher level in MMP-14. | Lucia et al., 2022 [51] |
| Chondrocyte (Rabbit) | YAG laser | 1064 nm | NA | once/day on day 0, 2 and 4 | 80 J/cm2 | Rabbit with the folded ears (in vitro and in vivo) | PBM upregulated aromatase (Cyp19) expression and increased collagen synthesis in chondrocytes in vivo. The estrogen rate in the cartilage tissue was significantly increased after PBM via the activation of Cyp19, which promoted chondrocyte proliferation and collagen synthesis. | Zhu et al., 2023 [52] |
| Chondrocytes (Rat) | NA | NA | NA | 48 h for 7 days (4 sessions) | 38 J/cm2 | TMJ arthritis in rats | Articular disc thickness, condylar cartilage thickness, and TMJ structure were improved after three weeks’ PBM compared to the arthritic group, with improvement in cartilage tissue and less osteochondral detachment. | Rana et al., 2023 [53] |
| rADSC/ ADC-derived secretome | InGaAs | 980 nm | 500 mW | 60 s | 38 J/cm2 | TMJ arthritis in rats | Groups of ADSCs or secretome-based treatments exhibited an improvement in joint structure, articular disc, and condylar cartilage thickness; simultaneously, a reduction of TNF-α was detected after three weeks’ PBM compared to the arthritic group. No significance between using ADSCs and ADSC-derived secretome for improving TMJ arthritis in rats. | Rana et al., 2023 [54] |
| Fibroblast-like synoviocytes from RA patients (RA-FLSs) | LED | 610 nm | 5 and 10 mW/cm2 | 20 min | 12 J/cm2 | Collagen-induced arthritis in mouse (CIA) | TNF-α expression was decreased in proliferation, migration, and invasion in RA-FLSs, whereas NF-kB and NLRP3 inflammasomes were downregulated after receiving PBM. Integration of PBM and methotrexate, an anti-rheumatic drug, contributed to inhibiting CIA development. | Ryu et al., 2023 [55] |
2.5. PBM Strategies on Cartilage Regeneration-Related Studies
| Objective | Laser Parameters | Disease Model (In Vivo) | Outcome | References | ||||
|---|---|---|---|---|---|---|---|---|
| Light Sources | Wavelength (nm) | Power Output/Power Intensity | Irradiation Time | Fluency (J/cm2) | ||||
| Human | Semiconductive neodymium laser IV | 1064 nm | 12,000 mW | Single application/day, first three sessions, 2 min (medial and lateral area) Next 4 sessions, 10 min (medial area) | 12 J/cm2 120 J/cm2 | 72 patients (Pain in KOA) | Reduction in pain level (visual analogue scale (VAS) and dolorimetry) significantly improved in the laser-based treatment after 7 days and was maintained over 3 months. Laser-treated group showed a decrease in contact surface area (pedobarometric analysis), resulting in a balance between two legs in static and dynamic positions. | Angelova et al., 2016 [56] |
| Human | Pulsed Nd:YAG laser | 1064 nm | 10,500 mW | Twice/ week for 6 weeks | 5100–17,800 J/cm2 | 67 patients (KOA) | Pain levels were measured by VAS and Western Ontario and McMaster Universities Osteoarthritis (WOMAC) questionnaire, which were significantly reduced in all groups after 6 weeks. The treated group of HILT (laser) + glucosamine/chondroitin sulfate + exercise downregulated synovial thickness (ST), while there was no significance in femoral cartilage thickness (FCT). | Alayat et al., 2017 [57] |
| Human | Pulsed Nd:YAG laser | 1064 nm | 5000 mW | 8 min, 12 sessions 3 times/ week for 12 weeks | 60 J/cm2 | 93 patients (KOA) | HILT (laser) and exercise-based treatment increased knee flexion range of motion (FROM), improved WOMAC scores, and reduced the VAS after both treatment and 12 weeks. There was no difference in Timed Up and Go test (TUG), 6 min walk test (6MWT), or WOMAC pain subscale between HILT and conventional (TENS/US) treatment, but greater improvement compared to alone exercise therapy (ET). | Nazari et al., 2019 [58] |
| Human | 1 superpulsed laser 4 IR LEDs 4 red LEDs | 905 nm 875 nm 640 nm | 2.25 mW/cm2 77.76 mW/cm2 66.64 mW/cm2 | Twice a week/5 weeks, plus exercise | 0.12 J/cm2 4.46 J/cm2 4 J/cm2 | 60 patients (KOA) | The treated group of PBM plus exercise reduced the numerical rating pain scale (NRPS) compared to other groups (exercise and exercise/placebo PBM), whereas other responses including pressure pain threshold (PPT) and muscle strength or balance (functional reach test (FRT)) had a non-clinical significant difference. | Gomes et al., 2018 [59] |
| Human | GaAlAs | 860 nm | 100 mW | 3 sessions/week for 4 weeks | 7.5 J/cm2 | 40 patients (RA) | In the laser-treated group, a statistical difference in elbow extension ROM (range of motion) between pre- and post-treatment was detected, whereas there was no difference in elbow flexion ROM. | Afnan et al., 2018 [60] |
| Human | Pulsed Nd:YAG laser | 1064 nm | 12,000 mW | 5 sessions/ week, for 2 weeks 3 first sessions with 2 min 7 following sessions, 10 min | 12 J/cm2 120 J/cm2 | 54 patients (KOA) | HILT + ET integration indicated an greater improvement in VAS, WOMAC scores, FCT, and FROM after 2 weeks of irradiation; however, VAS and WOMAC scores were detected decreasingly in the 6th week compared to placebo laser (PL) + ET group. A significant improvement in cartilage thickness was obtained after 6 weeks receiving HILT plus ET. | Akaltun et al., 2020 [61] |
| Human | Laser acupoints | 808 nm | 100 mW/cm2 | 60 s | 7.5 J/cm2 | 60 patients (RA) | Treated group (laser+methotrexate+ET) showed significantly enhanced C-reactive protein (CRP), IL-6, malondialdehyde (MDA) oxidative marker, ATP compared to control group (methotrexate+ET). | Afnan et al., 2022 [62] |
| Human | Pulsed Nd:YAG laser | 1064 nm | 10,000 mW 5000 mW | 3 sessions/first week, 2 min 6 sessions within following 2 weeks, 10 min | 12 J/cm2 120 J/cm2 | 60 patients (KOA) | An improvement in reducing pain level and increasing FROM, muscle strength, WOMAC, and FCT in both HILT/ET-, and sham laser/ET-based treatment. | Burak et al., 2023 [63] |
3. PBM in Cartilage Tissue Engineering
3.1. Cartilage Tissue Engineering
3.1.1. Cell Sources
Chondrocytes
Mesenchymal Stem Cells
3.2. PBM-Induced Biological Effects Supporting Effective Cartilage Tissue Engineering
4. Conclusions and Prospects
| Objective | Laser Parameters | Biomaterials/Tissue Engineering | Cell Sources/Animal Model | Main Outcome | References | ||||
|---|---|---|---|---|---|---|---|---|---|
| Light | Wavelength (Nm) | Power Output/Power Intensity | Time | Fluency | |||||
| To determine the Erbium:glass laser effect on costal and articular cartilage impregnated with SSNPs. | laser septochondrocorrection | 1.56 µm | 0.7 W | 5 s | NA | SSNPs | Porcine articular and costal cartilage disks | SSNPs can be safely used for the laser diagnostics and therapy.A slight decrease in acid proteoglycan content occurs after laser treatment, but the collagen network remains unchanged. | Soshnikova et al., 2015 [84] |
| Evaluate an application of PBM and BMSCs-seeded type I collagen-scaffold on cartilage repair in the rabbits | Ga-Al-As | 810 | NA | Every other day for three weeks | 4 J/cm2 | Type I collagen scaffold | AC defect in rabbits | No significant difference in new cartilage formation and inflammation among the groups, but new bone formation was significantly found. | Fekrazad et al., 2016 [81] |
| Investigating the modification of dECM scaffold’s surface using CO2 and Er:YAG lasers to improve chondrocyte migration within scaffolds. | CO2 laser (AcuPulse) Er:YAG laser (SupErb XL) | 10.600 nm 2.940 nm | 8 W NA | 0.03–0.06 s 0.1–1.0 ms | 1.2–4.8 J 0.5 J | DECM scaffold | Chondrocyte | Both laser types result in increased scaffold surface area and cell growth.Chondrocytes were inhibited to migrate in the CO2 laser-treated scaffold compared to Er:YAG laser-treated scaffold due to the present of thermal denaturation. | Eva et al., 2018 [77] |
| Evaluate PBM impacts on the differentiated potential of hDPSCs in 3D agarose gel culture | InGaAlP diode laser | 660 | NA | 10 s | 3.3 J/cm2 | 3D Agarose gel culture | hDPSCs | PBM/5% FBS and PBM/10% FBS significantly accelerated chondrogenic differentiation in 3D agarose gel culture compared to the control group (10% FBS) at day 14, but there was no significant difference between the two PBM groups on days 7 and 14. | Zaccara et al., 2018 [78] |
| Evaluate wavelength dependence on promoting chondrogenic differentiation potential of rMSCs. | IR laser, red laser, green laser, blue laser | 810 660 532 475 | 200 mW 30 mW 30 mW 30 mW | 3 s 24 s 15 s 15 s | 4 J/cm2 | micro mass culture system (Pellet) | rMSCs | IR laser had highest stimulatory effect on chondrogenesis-related gene expression (Col 10)IR + blue laser increased the expression of SOX-9, Col 2, aggrecan. | Fekrazad et al., 2019 [82] |
| Investigating PBM effects associated with chitosan viscosupplementation for osteoarthritis | GaAlAs diode | 808 | 50 mW | 28 s | 50 mJ/cm2 | Chitosan hydrogel | OA in Wistar rats | PBM combined with chondrocyte-seeded hydrogel inhibited articular degeneration by boosting TGF-β, decreasing TNF-α, and enhancing Col 2 expression in vivo. | Tim et al., 2020 [83] |
| Assessment the effects of extrinsic PBM conjungated with cell adhesion pattern in inducing adipogenic differentiation of WJ-MSCs | NA | 690 | 5 mW/cm2 | 10 s, every 2–3 days | 50 mJ/cm2 | Collagen microislands and TCPS | WJ-MSCs | The integration between EPM and collagen microislands exhibited the higher rate of adipogenic differentiation (~28%) than that of EPM and TCPS. A greater efficacy in inducing adipogenic differentiation of EPM and collagen microislands than using chemical factors was revealed. | Muneekaew et al., 2022 [80] |
| Evaluation of PBM therapy for chondrocyte-derived cellular responses in alginate scaffold | Infrared diode laser ((MLL-III-830 nm 2 W-EK10935) | 830 | 0.1 W | 32 s, every 2 days | 5 J/cm2 | Alginate scaffold | Chondrocyte | PBM (5 J/cm2) significantly upregulated cell proliferation, GAG synthesis, EV release, and SG formation in chondrocytes-seeded alginate scaffold.Multiple PBM sessions (one every 4 days) provided a greater improvement in chondrocyte-derived biological responses. | N.L.T. Hang et al., 2024 [18] |
| Determine GAG generation in differentiated MeSCs after PBM | LED (LH-SDT5W) | 660 | 35.398 W/cm2 | 113 s 508 s | 4 J/cm2 18 J/cm2 | Hydrogel | MeSCs | GAG production of PBM groups was significantly higher than in control group, but it was not between PBM groups. | Tong et al., 2024 [79] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Liu, Y.; Shah, K.M.; Luo, J. Strategies for articular cartilage repair and regeneration. Front. Bioeng. Biotechnol. 2021, 9, 770655. [Google Scholar] [CrossRef] [PubMed]
- Gahunia, H.K.; Pritzker, K.P. Structure and function of articular cartilage. In Articular Cartilage of the Knee: Health, Disease and Therapy; Springer: New York, NY, USA, 2020; pp. 3–70. [Google Scholar] [CrossRef]
- Medvedeva, E.V.; Grebenik, E.A.; Gornostaeva, S.N.; Telpuhov, V.I.; Lychagin, A.V.; Timashev, P.S.; Chagin, A.S. Repair of Damaged Articular Cartilage: Current Approaches and Future Directions. Int. J. Mol. Sci. 2018, 19, 2366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Qu, J. The mechanisms and efficacy of photobiomodulation therapy for arthritis: A comprehensive review. Int. J. Mol. Sci. 2023, 24, 14293. [Google Scholar] [CrossRef]
- Kilmer, C.E.; Battistoni, C.M.; Cox, A.; Breur, G.J.; Panitch, A.; Liu, J.C. Collagen type I and II blend hydrogel with autologous mesenchymal stem cells as a scaffold for articular cartilage defect repair. ACS Biomater. Sci. Eng. 2020, 6, 3464–3476. [Google Scholar] [CrossRef]
- Masoumi, M.; Bashiri, H.; Khorramdelazad, H.; Barzaman, K.; Hashemi, N.; Sereshki, H.A.; Sahebkar, A.; Karami, J. Destructive roles of fibroblast-like synoviocytes in chronic inflammation and joint damage in rheumatoid arthritis. Inflammation 2021, 44, 466–479. [Google Scholar] [CrossRef]
- Sumathi, L.; Pichaivel, M.; Kandasamy, T. Areview on Rheumatoid Arthiritis. Asian J. Pharm. Res. Dev. 2022, 10, 104–109. [Google Scholar] [CrossRef]
- Khan, A.; Pooja, V.; Chaudhury, S.; Bhatt, V.; Saldanha, D. Assessment of depression, anxiety, stress, and quality of life in rheumatoid arthritis patients and comparison with healthy individuals. Ind. Psychiatry J. 2021, 30, S195. [Google Scholar] [CrossRef]
- de Andrade, A.L.M.; Luna, G.F.; Brassolatti, P.; Leite, M.N.; Parisi, J.R.; de Oliveira Leal, Â.M.; Frade, M.A.C.; de Freitas Anibal, F.; Parizotto, N.A. Photobiomodulation effect on the proliferation of adipose tissue mesenchymal stem cells. Lasers Med. Sci. 2019, 34, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Zare, F.; Moradi, A.; Fallahnezhad, S.; Ghoreishi, S.K.; Amini, A.; Chien, S.; Bayat, M. Photobiomodulation with 630 plus 810 nm wavelengths induce more in vitro cell viability of human adipose stem cells than human bone marrow-derived stem cells. Photochem. Photobiol. B Biol. 2019, 201, 111658. [Google Scholar] [CrossRef]
- Yamada, E.F.; Bobinski, F.; Martins, D.F.; Palandi, J.; Folmer, V.; da Silva, M.D. Photobiomodulation therapy in knee osteoarthritis reduces oxidative stress and inflammatory cytokines in rats. J. Biophotonics 2020, 13, e201900204. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.-Y.; Wang, Y.; Lyu, P.; Hamblin, M.R. Red (660 nm) or near-infrared (810 nm) photobiomodulation stimulates, while blue (415 nm), green (540 nm) light inhibits proliferation in human adipose-derived stem cells. Sci. Rep. 2017, 7, 7781. [Google Scholar] [CrossRef] [PubMed]
- de Lima, R.d.N.; Vieira, S.S.; Antonio, E.L.; de Carvalho, P.d.T.C.; de Paula Vieira, R.; Mansano, B.S.D.M.; de Arruda, D.F., Jr.; Girardi, A.C.C.; Tucci, P.J.F.; Serra, A.J. Low-level laser therapy alleviates the deleterious effect of doxorubicin on rat adipose tissue-derived mesenchymal stem cells. Photochem. Photobiol. B Biol. 2019, 196, 111512. [Google Scholar] [CrossRef] [PubMed]
- Tuli, R.; Li, W.J.; Tuan, R.S. Current state of cartilage tissue engineering. Arthritis Res. Ther. 2003, 5, 235–238. [Google Scholar] [CrossRef]
- Chimutengwende-Gordon, M.; Donaldson, J.; Bentley, G. Current solutions for the treatment of chronic articular cartilage defects in the knee. EFORT Open Rev. 2020, 5, 156–163. [Google Scholar] [CrossRef]
- Huang, B.; Li, P.; Chen, M.; Peng, L.; Luo, X.; Tian, G.; Wang, H.; Wu, L.; Tian, Q.; Li, H. Hydrogel composite scaffolds achieve recruitment and chondrogenesis in cartilage tissue engineering applications. J. Nanobiotechnology 2022, 20, 25. [Google Scholar] [CrossRef]
- Bikmulina, P.; Kosheleva, N.; Shpichka, A.; Yusupov, V.; Gogvadze, V.; Rochev, Y.; Timashev, P. Photobiomodulation in 3D tissue engineering. J. Biomed. Opt. 2022, 27, 090901. [Google Scholar] [CrossRef]
- Hang, N.L.T.; Chuang, A.E.-Y.; Chang, C.-J.; Yen, Y.; Wong, C.-C.; Yang, T.-S. Photobiomodulation associated with alginate-based engineered tissue on promoting chondrocytes-derived biological responses for cartilage regeneration. Int. J. Biol. Macromol. 2024, 280, 135982. [Google Scholar] [CrossRef]
- Dompe, C.; Moncrieff, L.; Matys, J.; Grzech-Leśniak, K.; Kocherova, I.; Bryja, A.; Bruska, M.; Dominiak, M.; Mozdziak, P.; Skiba, T.H.I. Photobiomodulation—Underlying mechanism and clinical applications. J. Clin. Med. 2020, 9, 1724. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.-C.; Hang, N.-L.-T.; Colley, M.M.; Chang, J.; Hsiao, Y.-C.; Lu, L.-S.; Li, B.-S.; Chang, C.-J.; Yang, T.-S. Single cell effects of photobiomodulation on mitochondrial membrane potential and reactive oxygen species production in human adipose mesenchymal stem cells. Cells 2022, 11, 972. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and mitochondrial redox signaling in photobiomodulation. Photochem. Photobiol. 2018, 94, 199–212. [Google Scholar] [CrossRef]
- Chang, S.-Y.; Lee, M.Y.; Chung, P.-S.; Kim, S.; Choi, B.; Suh, M.-W.; Rhee, C.-K.; Jung, J.Y. Enhanced mitochondrial membrane potential and ATP synthesis by photobiomodulation increases viability of the auditory cell line after gentamicin-induced intrinsic apoptosis. Sci. Rep. 2019, 9, 19248. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.G.; Felix, F.N.; Woodley, D.T.; Shim, E.K. The role of oxygen in wound healing: A review of the literature. Dermatol. Surg. 2008, 34, 1159–1169. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, T.; Liu, L.; Ohulchanskyy, T.Y.; Qu, J. Dose–effect relationships for PBM in the treatment of Alzheimer’s disease. J. Phys. D Appl. Phys. 2021, 54, 353001. [Google Scholar] [CrossRef]
- Chen, A.C.; Arany, P.R.; Huang, Y.Y.; Tomkinson, E.M.; Sharma, S.K.; Kharkwal, G.B.; Saleem, T.; Mooney, D.; Yull, F.E.; Blackwell, T.S.; et al. Low-level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts. PLoS ONE 2011, 6, e22453. [Google Scholar] [CrossRef]
- Turpaev, K. Reactive oxygen species and regulation of gene expression. Biochemistry 2002, 67, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Karin, M. NF-kappaB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- de Oliveira, T.S.; Serra, A.J.; Manchini, M.T.; Bassaneze, V.; Krieger, J.E.; de Tarso Camillo de Carvalho, P.; Antunes, D.E.; Bocalini, D.S.; Ferreira Tucci, P.J.; Silva, J.A. Effects of low level laser therapy on attachment, proliferation, and gene expression of VEGF and VEGF receptor 2 of adipocyte-derived mesenchymal stem cells cultivated under nutritional deficiency. Lasers Med. Sci. 2015, 30, 217–223. [Google Scholar] [CrossRef]
- Chen, H.; Wang, H.; Li, Y.; Liu, W.; Wang, C.; Chen, Z. Biological effects of low-level laser irradiation on umbilical cord mesenchymal stem cells. Aip Adv. 2016, 6, 045018. [Google Scholar] [CrossRef]
- Yin, K.; Zhu, R.; Wang, S.; Zhao, R.C. Low-level laser effect on proliferation, migration, and antiapoptosis of mesenchymal stem cells. Stem Cells Dev. 2017, 26, 762–775. [Google Scholar] [CrossRef]
- Yang, X.; Liu, T.C.; Liu, S.; Zhu, W.; Li, H.; Liang, P.; Ye, S.; Cui, S. Promoted viability and differentiated phenotype of cultured chondrocytes with low level laser irradiation potentiate efficacious cells for therapeutics. Front. Bioeng. Biotechnol. 2020, 8, 468. [Google Scholar] [CrossRef] [PubMed]
- Bozhokin, M.; Vcherashnii, D.; Yastrebov, S.; Beilinson, L.; Zherebtsova, J.V.; Khotin, M. Low-intensity photobiomodulation at 632.8 nm increases tgfβ3, col2a1, and sox9 gene expression in rat bone marrow mesenchymal stem cells in vitro. Lasers Med. Sci. 2021, 37, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Dungel, P.; Priglinger, E.; Danzer, M.; Schädl, B.; Nürnberger, S. The impact of photobiomodulation on the chondrogenic potential of adipose-derived stromal/stem cells. Photochem. Photobiol. B Biol. 2021, 221, 112243. [Google Scholar] [CrossRef]
- Sakata, S.; Kunimatsu, R.; Tsuka, Y.; Nakatani, A.; Gunji, H.; Yanoshita, M.; Kado, I.; Ito, S.; Putranti, N.A.R.; Prasetya, R.C. High-frequency near-infrared diode laser irradiation suppresses IL-1β-induced inflammatory cytokine expression and NF-κB signaling pathways in human primary chondrocytes. Lasers Med. Sci. 2022, 37, 1193–1201. [Google Scholar] [CrossRef]
- Khozeimeh, F.; Moghareabed, A.; Allameh, M.; Baradaran, S. Comparative evaluation of low-level laser and systemic steroid therapy in adjuvant-enhanced arthritis of rat temporomandibular joint: A histological study. Dent. Res. J. 2015, 12, 215–223. [Google Scholar]
- Peimani, A.; Sardary, F. Effect of low-level laser on healing of temporomandibular joint osteoarthritis in rats. J. Dent. 2014, 11, 319. [Google Scholar]
- Lemos, G.A.; Rissi, R.; de Souza Pires, I.L.; de Oliveira, L.P.; de Aro, A.A.; Pimentel, E.R.; Palomari, E.T. Low-level laser therapy stimulates tissue repair and reduces the extracellular matrix degradation in rats with induced arthritis in the temporomandibular joint. Lasers Med. Sci. 2016, 31, 1051–1059. [Google Scholar] [CrossRef]
- Assis, L.; Milares, L.P.; Almeida, T.; Tim, C.; Magri, A.; Fernandes, K.R.; Medalha, C.; Renno, A.M. Aerobic exercise training and low-level laser therapy modulate inflammatory response and degenerative process in an experimental model of knee osteoarthritis in rats. Osteoarthr. Cartil. 2016, 24, 169–177. [Google Scholar] [CrossRef]
- Milares, L.P.; Assis, L.; Siqueira, A.; Claudino, V.; Domingos, H.; Almeida, T.; Tim, C.; Renno, A.C. Effectiveness of an aquatic exercise program and low-level laser therapy on articular cartilage in an experimental model of osteoarthritis in rats. Connect. Tissue Res. 2016, 57, 398–407. [Google Scholar] [CrossRef]
- Assis, L.; Tim, C.; Magri, A.; Fernandes, K.R.; Vassão, P.G.; Renno, A.C.M. Interleukin-10 and collagen type II immunoexpression are modulated by photobiomodulation associated to aerobic and aquatic exercises in an experimental model of osteoarthritis. Lasers Med. Sci. 2018, 33, 1875–1882. [Google Scholar] [CrossRef]
- Abubakr, N.; Salem, Z.; Ali, Z.; Assaly, M.E. Comparative evaluation of the early effects of the low-level laser therapy versus intra-articular steroids on temporomandibular joint acute osteoarthritis in rats: A histochemical, molecular and imaging evaluation. Dent. Med. Probl. 2018, 55, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Memis, S.; Candirli, C.; Kerimoglu, G. Short term histopathological effects of GaAlAs laser on experimentally induced TMJ osteoarthritis in rabbits. Braz. Oral Res. 2018, 32, e90. [Google Scholar] [CrossRef] [PubMed]
- Sanches, M.; Assis, L.; Criniti, C.; Fernandes, D.; Tim, C.; Renno, A.C.M. Chondroitin sulfate and glucosamine sulfate associated to photobiomodulation prevents degenerative morphological changes in an experimental model of osteoarthritis in rats. Lasers Med. Sci. 2018, 33, 549–557. [Google Scholar] [CrossRef]
- Lemos, G.A.; Batista, A.U.D.; da Silva, P.L.P.; Araújo, D.N.; Sarmento, W.E.A.; Palomari, E.T. Photobiostimulation activity of different low-level laser dosage on masticatory muscles and temporomandibular joint in an induced arthritis rat model. Lasers Med. Sci. 2020, 35, 1129–1139. [Google Scholar] [CrossRef]
- Li, Y.; Wu, F.; Wei, J.; Lao, L.; Shen, X. The effects of laser moxibustion on knee osteoarthritis pain in rats. Photobiomodulation Photomed. Laser Surg. 2020, 38, 43–50. [Google Scholar] [CrossRef]
- Trevisan, E.S.; Martignago, C.C.S.; Assis, L.; Tarocco, J.C.; Salman, S.; Dos Santos, L.; Liebano, R.; Tim, C.R. Effectiveness of led photobiomodulation therapy on treatment with knee osteoarthritis: A rat study. Am. J. Phys. Med. Rehabil. 2020, 99, 725–732. [Google Scholar] [CrossRef]
- Balbinot, G.; Schuch, C.P.; Nascimento, P.S.d.; Lanferdini, F.J.; Casanova, M.; Baroni, B.M.; Vaz, M.A. Photobiomodulation therapy partially restores cartilage integrity and reduces chronic pain behavior in a rat model of osteoarthritis: Involvement of spinal glial modulation. Cartilage 2021, 13, 1309S–1321S. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.P.d.O.; Santos, F.F.d.; Costa, T.E.D.; Lacerda, A.C.R.; Santos, J.M.d.; Costa, K.B.; Santos, A.P.; Gaiad, T.P.; Pinfildi, C.E.; Rocha-Vieira, E. Photobiomodulation Therapy (Light-Emitting Diode 630 Nm) Favored the Oxidative Stress and the Preservation of Articular Cartilage in an Induced Knee Osteoarthritis Model. Photobiomodulation Photomed. Laser Surg. 2021, 39, 272–279. [Google Scholar] [CrossRef]
- Tim, C.R.; Martignago, C.C.S.; Assis, L.; Neves, L.M.; Andrade, A.L.; Silva, N.C.; Parizotto, N.; Pinto, K.Z.; Rennó, A.C. Effects of photobiomodulation therapy in chondrocyte response by in vitro experiments and experimental model of osteoarthritis in the knee of rats. Lasers Med. Sci. 2022, 37, 1677–1686. [Google Scholar] [CrossRef]
- Dos Anjos, L.M.J.; Quirino-Teixeira, A.C.; Hottz, E.D.; da Fonseca, A.d.S.; Gameiro, J.; de Paoli, F. Photobiomodulation effects in metalloproteinases expression in zymosan-induced arthritis. Lasers Med. Sci. 2022, 37, 3661–3670. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, X.; Peng, X.; Li, H.; Wang, H.; Guo, Z.; Xiong, Y.; Xu, J.; Ni, X.; Qi, X. 1064nm Nd: YAG laser promotes chondrocytes regeneration and cartilage reshaping by upregulating local estrogen levels. J. Biophotonics 2023, 17, e202300443. [Google Scholar] [CrossRef]
- El-Qashty, R.; Elkashty, O.A.; Hany, E. Therapeutic potential of low-level laser photobiomodulation on adjuvant induced temporomandibular joint arthritis in a rat model. Mansoura J. Dent. 2023, 10, 1–8. [Google Scholar] [CrossRef]
- El-Qashty, R.; Elkashty, O.A.; Hany, E. Photobiostimulation conjugated with stem cells or their secretome for temporomandibular joint arthritis in a rat model. BMC Oral Health 2023, 23, 720. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Park, J.; Kim, B.-Y.; Kim, Y.; Kim, N.G.; Shin, Y.-I. Photobiomodulation ameliorates inflammatory parameters in fibroblast-like synoviocytes and experimental animal models of rheumatoid arthritis. Front. Immunol. 2023, 14, 1122581. [Google Scholar] [CrossRef] [PubMed]
- Angelova, A.; Ilieva, E.M. Effectiveness of high intensity laser therapy for reduction of pain in knee osteoarthritis. Pain Res. Manag. 2016, 2016, 9163618. [Google Scholar] [CrossRef]
- Alayat, M.S.M.; Aly, T.H.A.; Elsayed, A.E.M.; Fadil, A.S.M. Efficacy of pulsed Nd: YAG laser in the treatment of patients with knee osteoarthritis: A randomized controlled trial. Lasers Med. Sci. 2017, 32, 503–511. [Google Scholar] [CrossRef]
- Nazari, A.; Moezy, A.; Nejati, P.; Mazaherinezhad, A. Efficacy of high-intensity laser therapy in comparison with conventional physiotherapy and exercise therapy on pain and function of patients with knee osteoarthritis: A randomized controlled trial with 12-week follow up. Lasers Med. Sci. 2019, 34, 505–516. [Google Scholar] [CrossRef]
- de Paula Gomes, C.A.; Leal-Junior, E.C.; Dibai-Filho, A.V.; de Oliveira, A.R.; Bley, A.S.; Biasotto-Gonzalez, D.A.; de Tarso Camillo de Carvalho, P. Incorporation of photobiomodulation therapy into a therapeutic exercise program for knee osteoarthritis: A placebo-controlled, randomized, clinical trial. Lasers Surg. Med. 2018, 50, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Adly, A.S.; Adly, A.S.; Adly, M.S.; Mostafa, Y.M. Effectiveness of laser acupuncture as adjunctive therapy for limited range of motion in rheumatoid arthritis. Laser Phys. 2018, 29, 025601. [Google Scholar] [CrossRef]
- Akaltun, M.S.; Altindag, O.; Turan, N.; Gursoy, S.; Gur, A. Efficacy of high intensity laser therapy in knee osteoarthritis: A double-blind controlled randomized study. Clin. Rheumatol. 2021, 40, 1989–1995. [Google Scholar] [CrossRef]
- Adly, A.S.; Adly, A.S.; Adly, M.S.; Abdeen, H.A.A. Effects of laser acupuncture tele-therapy for rheumatoid arthritis elderly patients. Lasers Med. Sci. 2022, 37, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Ekici, B.; Ordahan, B. Evaluation of the effect of high-intensity laser therapy (HILT) on function, muscle strength, range of motion, pain level, and femoral cartilage thickness in knee osteoarthritis: Randomized controlled study. Lasers Med. Sci. 2023, 38, 218. [Google Scholar] [CrossRef] [PubMed]
- Penberthy, W.T.; Vorwaller, C.E. Utilization of the 1064 nm wavelength in photobiomodulation: A systematic review and meta-analysis. J. Lasers Med. Sci. 2021, 12, e86. [Google Scholar] [CrossRef] [PubMed]
- Urlic, I.; Ivkovic, A. Cell Sources for Cartilage Repair-Biological and Clinical Perspective. Cells 2021, 10, 2496. [Google Scholar] [CrossRef]
- Akkiraju, H.; Nohe, A. Role of Chondrocytes in Cartilage Formation, Progression of Osteoarthritis and Cartilage Regeneration. J. Dev. Biol. 2015, 3, 177–192. [Google Scholar] [CrossRef]
- Demoor, M.; Ollitrault, D.; Gomez-Leduc, T.; Bouyoucef, M.; Hervieu, M.; Fabre, H.; Lafont, J.; Denoix, J.M.; Audigie, F.; Mallein-Gerin, F.; et al. Cartilage tissue engineering: Molecular control of chondrocyte differentiation for proper cartilage matrix reconstruction. Biochim. Biophys. Acta 2014, 1840, 2414–2440. [Google Scholar] [CrossRef]
- Gikas, P.D.; Bayliss, L.; Bentley, G.; Briggs, T.W. An overview of autologous chondrocyte implantation. J. Bone Jt. Surg. Br. 2009, 91, 997–1006. [Google Scholar] [CrossRef]
- Nawaz, S.Z.; Bentley, G.; Briggs, T.W.; Carrington, R.W.; Skinner, J.A.; Gallagher, K.R.; Dhinsa, B.S. Autologous chondrocyte implantation in the knee: Mid-term to long-term results. J. Bone Jt. Surg. Am. 2014, 96, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Guo, S.; Wang, Y.; Yu, Y. Adipose-derived stem cells and hyaluronic acid based gel compatibility, studied in vitro. Mol. Med. Rep. 2017, 16, 4095–4100. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.; Li, C.; Ji, W.; Wang, L.; Chen, X.; Zhao, S.; Xu, Z.; Ge, R.; Guo, X. Differentiation of human adipose derived stem cells into Leydig-like cells with molecular compounds. J. Cell. Mol. Med. 2019, 23, 5956–5969. [Google Scholar] [CrossRef]
- Hoang, D.M.; Pham, P.T.; Bach, T.Q.; Ngo, A.T.; Nguyen, Q.T.; Phan, T.T.; Nguyen, G.H.; Le, P.T.; Hoang, V.T.; Forsyth, N.R. Stem cell-based therapy for human diseases. Signal Transduct. Target. Ther. 2022, 7, 272. [Google Scholar] [CrossRef]
- Le, H.; Xu, W.; Zhuang, X.; Chang, F.; Wang, Y.; Ding, J. Mesenchymal stem cells for cartilage regeneration. J. Tissue Eng. 2020, 11, 2041731420943839. [Google Scholar] [CrossRef]
- Dai, R.; Wang, Z.; Samanipour, R.; Koo, K.-i.; Kim, K. Adipose-derived stem cells for tissue engineering and regenerative medicine applications. Stem Cells Int. 2016, 2016, 6737345. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.-H.; Kuo, T.-L.; Kuo, K.-K.; Hsiao, C.-C. Human adipose-derived stem cells: Isolation, characterization and current application in regeneration medicine. Genom. Med. Biomark. Health Sci. 2011, 3, 53–62. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Liu, H.-Y.; Chang, Y.-T.; Cheng, Y.-H.; Mersmann, H.J.; Kuo, W.-H.; Ding, S.-T. Isolation and differentiation of adipose-derived stem cells from porcine subcutaneous adipose tissues. J. Vis. Exp. 2016, 109, e53886. [Google Scholar] [CrossRef]
- Goldberg-Bockhorn, E.; Schwarz, S.; Subedi, R.; Elsässer, A.; Riepl, R.; Walther, P.; Körber, L.; Breiter, R.; Stock, K.; Rotter, N. Laser surface modification of decellularized extracellular cartilage matrix for cartilage tissue engineering. Lasers Med. Sci. 2018, 33, 375–384. [Google Scholar] [CrossRef]
- Zaccara, I.M.; Mestieri, L.B.; Moreira, M.S.; Grecca, F.S.; Martins, M.D.; Kopper, P.M.P. Photobiomodulation therapy improves multilineage differentiation of dental pulp stem cells in three-dimensional culture model. J. Biomed. Opt. 2018, 23, 095001. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Subbiah, S.K.; Rampal, S.; Ramasamy, R.; Wu, X.; You, Y.; Wang, J.; Mok, P.L. Effect of 660-nm LED photobiomodulation on the proliferation and chondrogenesis of meniscus-derived stem cells (MeSCs). Sci. Rep. 2024, 14, 19735. [Google Scholar] [CrossRef]
- Muneekaew, S.; Wang, M.-J.; Chen, S.-y. Control of stem cell differentiation by using extrinsic photobiomodulation in conjunction with cell adhesion pattern. Sci. Rep. 2022, 12, 1812. [Google Scholar] [CrossRef]
- Fekrazad, R.; Eslaminejad, M.B.; Shayan, A.M.; Kalhori, K.A.; Abbas, F.M.; Taghiyar, L.; Sepehr Pedram, M.; Ghuchani, M.S. Effects of photobiomodulation and mesenchymal stem cells on articular cartilage defects in a rabbit model. Photomed Laser Surg. 2016, 34, 543–549. [Google Scholar] [CrossRef]
- Fekrazad, R.; Asefi, S.; Eslaminejad, M.B.; Taghiar, L.; Bordbar, S.; Hamblin, M.R. Photobiomodulation with single and combination laser wavelengths on bone marrow mesenchymal stem cells: Proliferation and differentiation to bone or cartilage. Lasers Med. Sci. 2019, 34, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Tim, C.R.; Martignago, C.C.S.; Assis, L.; Andrade, A.L.; Neves, L.M.; Castro, C.; Parizotto, N.; Tarocco, J.C.; Liebano, R.; Cominetti, M. Effects of photobiomodulation associated with chitosan viscosupplementation for osteoarthritis: An in vitro and in vivo study. Res. Biomed. Eng. 2021, 37, 65–77. [Google Scholar] [CrossRef]
- Soshnikova, Y.M.; Shekhter, A.B.; Baum, O.I.; Shcherbakov, E.M.; Omelchenko, A.I.; Lunin, V.V.; Sobol, E.N. Laser radiation effect on chondrocytes and intercellular matrix of costal and articular cartilage impregnated with magnetite nanoparticles. Lasers Surg. Med. 2015, 47, 243–251. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hang, N.L.T.; Aviña, A.E.; Chang, C.-J.; Yang, T.-S. Photobiomodulation in Promoting Cartilage Regeneration. Int. J. Mol. Sci. 2025, 26, 5580. https://doi.org/10.3390/ijms26125580
Hang NLT, Aviña AE, Chang C-J, Yang T-S. Photobiomodulation in Promoting Cartilage Regeneration. International Journal of Molecular Sciences. 2025; 26(12):5580. https://doi.org/10.3390/ijms26125580
Chicago/Turabian StyleHang, Nguyen Le Thanh, Ana Elena Aviña, Cheng-Jen Chang, and Tzu-Sen Yang. 2025. "Photobiomodulation in Promoting Cartilage Regeneration" International Journal of Molecular Sciences 26, no. 12: 5580. https://doi.org/10.3390/ijms26125580
APA StyleHang, N. L. T., Aviña, A. E., Chang, C.-J., & Yang, T.-S. (2025). Photobiomodulation in Promoting Cartilage Regeneration. International Journal of Molecular Sciences, 26(12), 5580. https://doi.org/10.3390/ijms26125580






