Tannic Acid-Loaded Gellan Gum Hydrogels Reduce In Vitro Chemokine Expression in Oral Cells
Abstract
1. Introduction
2. Results
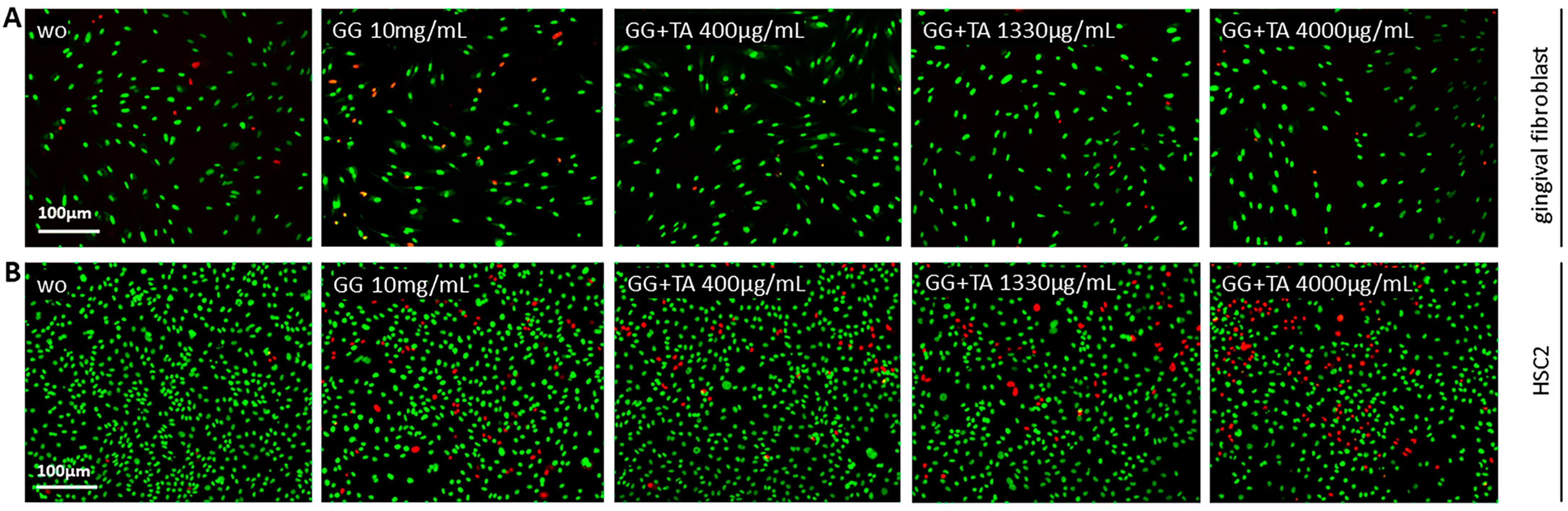
2.1. Screening of TA Concentrations
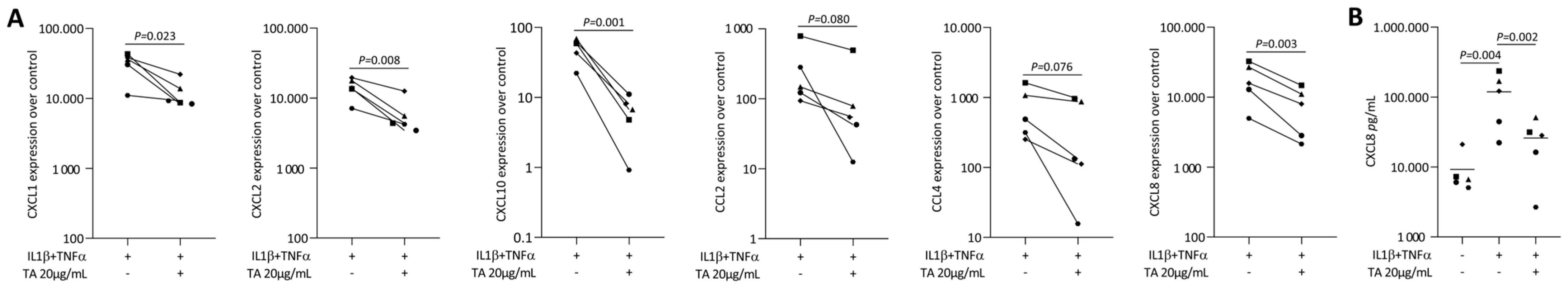
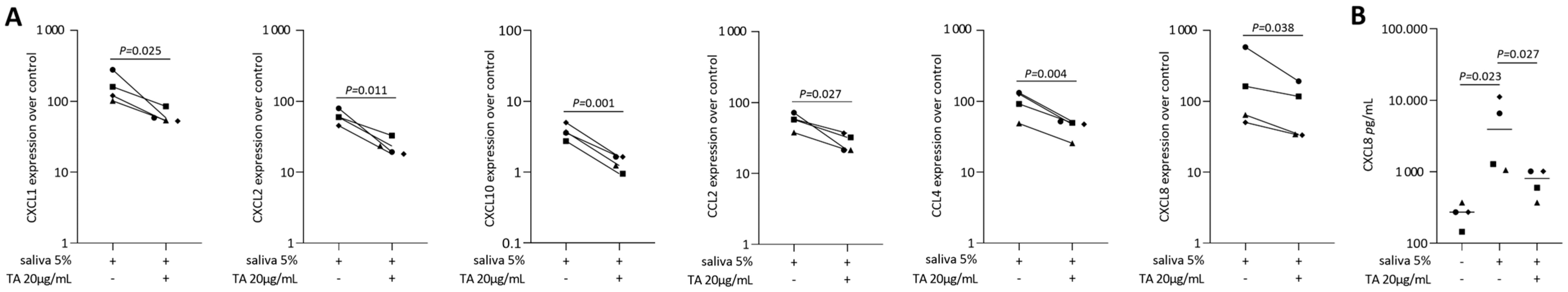
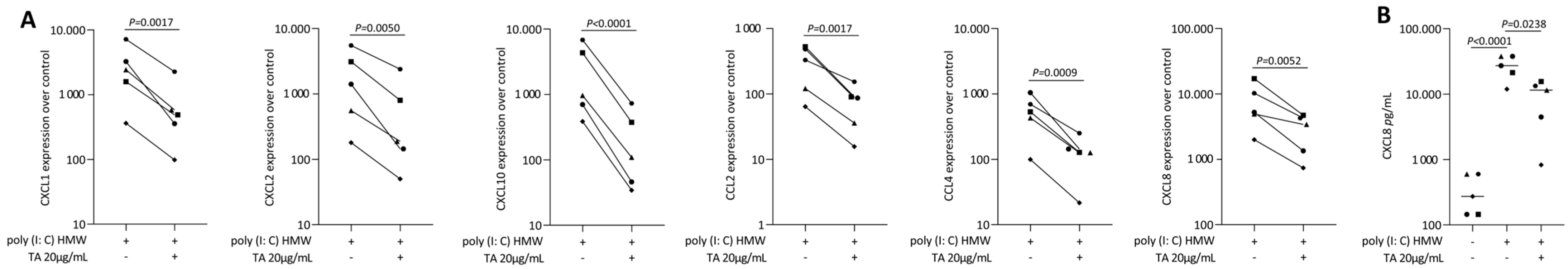
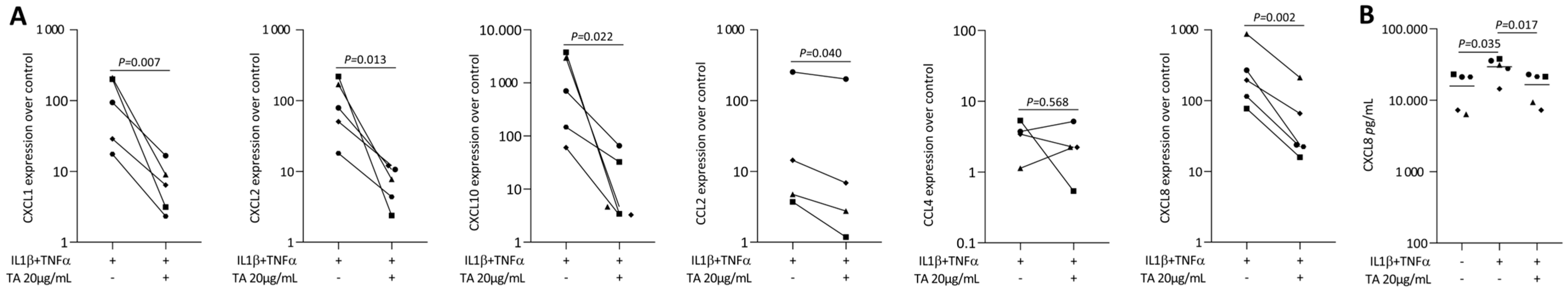
2.2. TA Reduced IL1β and TNFα, SLV or Poly I: C HMW-Induced Chemokines in Gingival Fibroblasts
2.3. TA Reduced IL1β and TNFα-Induced Chemokines in HSC2 Cells
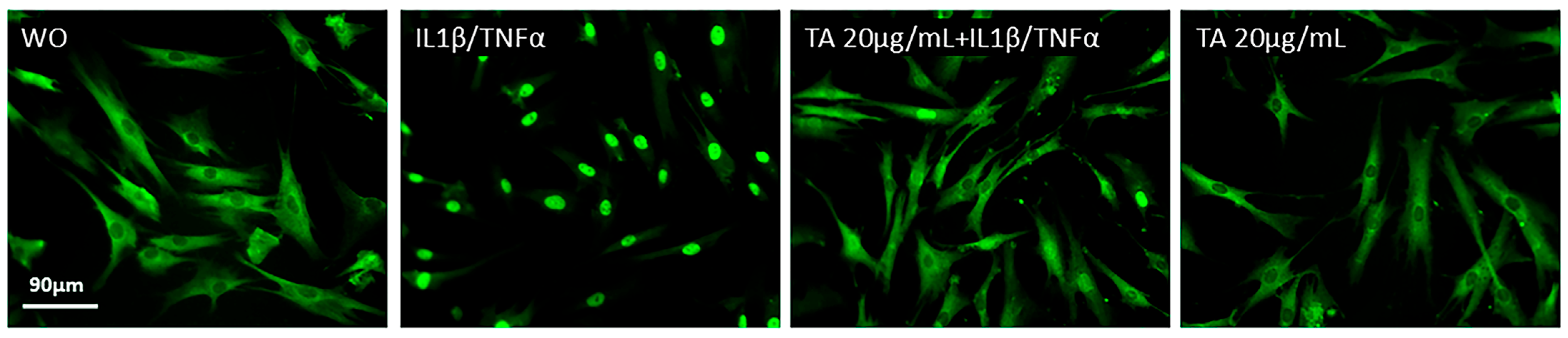
2.4. TA Inhibits the Phosphorylation of ERK, JNK, and p65 in IL1β and TNFα-Induced Gingival Fibroblasts
2.5. Sustained Release of Tannic Acid from Gellan Gum Hydrogel (GGTA)
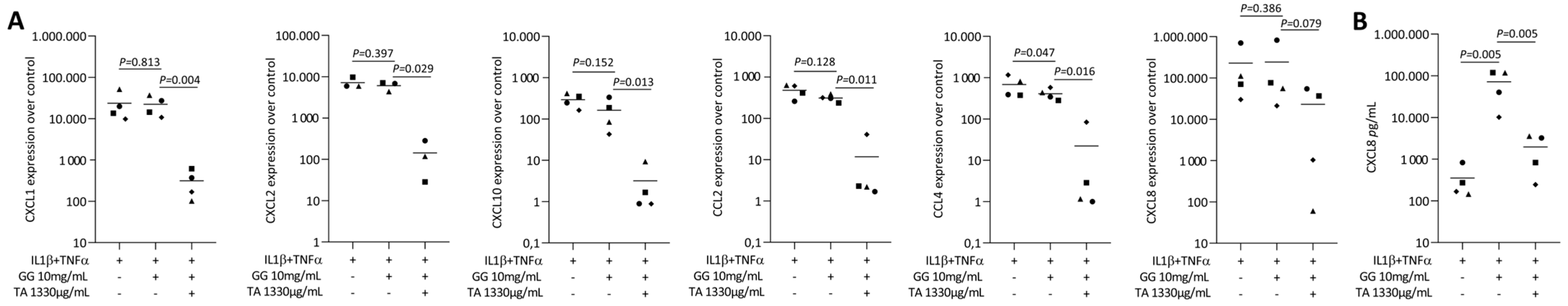
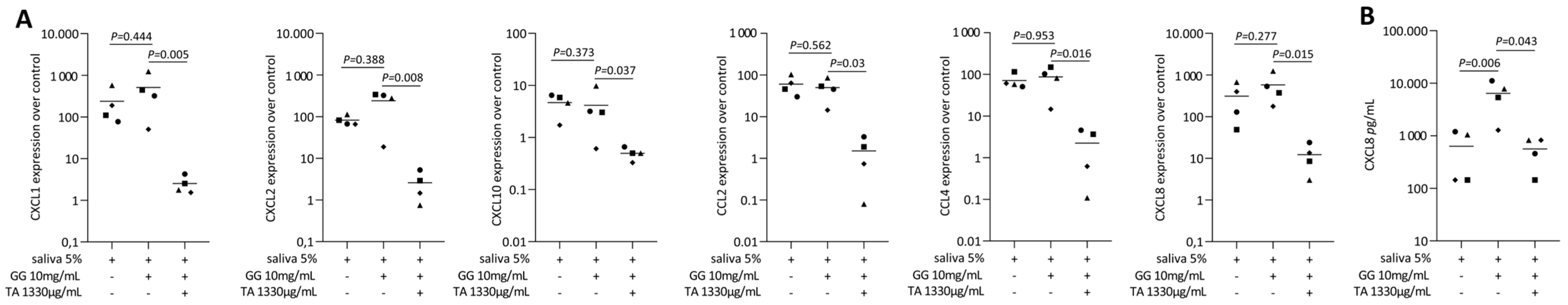
2.6. Tannic Acid-Loaded Gellan Gum Reduced IL1β and TNFα or SLV-Induced Chemokines in Gingival Fibroblasts
3. Discussion
4. Materials and Methods
4.1. Gingival Fibroblasts and Oral Squamous Carcinoma Cells
4.2. Tannic Acid-Loaded Gellan Gum Hydrogel
4.3. Release Kinetics of Tannic Acid from Gellan Gum Hydrogel
4.4. Viability Assay
4.5. Reverse Transcription-Quantitative Real-Time PCR and Immunoassay
4.6. Immunofluorescence Analysis
4.7. Western Blot
4.8. Mitochondrial Reactive Oxygen Species Release
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scannapieco, F.A.; Cantos, A. Oral Inflammation and Infection, and Chronic Medical Diseases: Implications for the Elderly. Periodontol. 2000 2016, 72, 153–175. [Google Scholar] [CrossRef]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal Diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef]
- Shen, L.; Hu, J.; Yuan, Y.; Wang, X.; Jiang, Q. Photothermal-Promoted Multi-Functional Gallic Acid Grafted Chitosan Hydrogel Containing Tannic Acid Miniaturized Particles for Peri-Implantitis. Int. J. Biol. Macromol. 2023, 253, 127366. [Google Scholar] [CrossRef]
- Shang, J.; Liu, H.; Zheng, Y.; Zhang, Z. Role of Oxidative Stress in the Relationship between Periodontitis and Systemic Diseases. Front. Physiol. 2023, 14, 1210449. [Google Scholar] [CrossRef]
- Renvert, S.; Persson, G.R.; Pirih, F.Q.; Camargo, P.M. Peri-Implant Health, Peri-Implant Mucositis, and Peri-Implantitis: Case Definitions and Diagnostic Considerations. J. Periodontol. 2018, 89 (Suppl. S1), S304–S312. [Google Scholar] [CrossRef]
- Fürst, M.M.; Salvi, G.E.; Lang, N.P.; Persson, G.R. Bacterial Colonization Immediately after Installation on Oral Titanium Implants. Clin. Oral. Implant. Res. 2007, 18, 501–508. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Manoil, D. Microbial Community-Driven Etiopathogenesis of Peri-Implantitis. J. Dent. Res. 2021, 100, 21–28. [Google Scholar] [CrossRef]
- Renvert, S.; Roos-Jansåker, A.-M.; Claffey, N. Non-Surgical Treatment of Peri-Implant Mucositis and Peri-Implantitis: A Literature Review. J. Clin. Periodontol. 2008, 35, 305–315. [Google Scholar] [CrossRef]
- Li, D.; Tan, X.; Zheng, L.; Tang, H.; Hu, S.; Zhai, Q.; Jing, X.; Liang, P.; Zhang, Y.; He, Q.; et al. A Dual-Antioxidative Coating on Transmucosal Component of Implant to Repair Connective Tissue Barrier for Treatment of Peri-Implantitis. Adv. Healthc. Mater. 2023, 12, e2301733. [Google Scholar] [CrossRef]
- Williams, D.W.; Greenwell-Wild, T.; Brenchley, L.; Dutzan, N.; Overmiller, A.; Sawaya, A.P.; Webb, S.; Martin, D.; NIDCD/NIDCR Genomics and Computational Biology Core; Hajishengallis, G.; et al. Human Oral Mucosa Cell Atlas Reveals a Stromal-Neutrophil Axis Regulating Tissue Immunity. Cell 2021, 184, 4090–4104.e15. [Google Scholar] [CrossRef]
- Li, J.; Ye, L.J.; Dai, Y.W.; Wang, H.W.; Gao, J.; Shen, Y.H.; Wang, F.; Dai, Q.G.; Wu, Y.Q. Single-Cell Analysis Reveals a Unique Microenvironment in Peri-Implantitis. J. Clin. Periodontol. 2024, 51, 1665–1676. [Google Scholar] [CrossRef]
- Easter, Q.T.; Fernandes Matuck, B.; Beldorati Stark, G.; Worth, C.L.; Predeus, A.V.; Fremin, B.; Huynh, K.; Ranganathan, V.; Ren, Z.; Pereira, D.; et al. Single-Cell and Spatially Resolved Interactomics of Tooth-Associated Keratinocytes in Periodontitis. Nat. Commun. 2024, 15, 5016. [Google Scholar] [CrossRef]
- Jiang, W.; Xu, T.; Song, Z.; Wang, X.; Yuan, S.; Li, Q.; Wei, Y.; Wang, C.; Yang, G.; Cao, J.; et al. CCL2 Is a Key Regulator and Therapeutic Target for Periodontitis. J. Clin. Periodontol. 2023, 50, 1644–1657. [Google Scholar] [CrossRef]
- Alassy, H.; Parachuru, P.; Wolff, L. Peri-Implantitis Diagnosis and Prognosis Using Biomarkers in Peri-Implant Crevicular Fluid: A Narrative Review. Diagnostics 2019, 9, 214. [Google Scholar] [CrossRef]
- Aleksandrowicz, P.; Brzezińska-Błaszczyk, E.; Kozłowska, E.; Żelechowska, P.; Borgonovo, A.E.; Agier, J. Analysis of IL-1β, CXCL8, and TNF-α Levels in the Crevicular Fluid of Patients with Periodontitis or Healthy Implants. BMC Oral. Health 2021, 21, 120. [Google Scholar] [CrossRef]
- Gonzáles, J.R.; Herrmann, J.M.; Boedeker, R.H.; Francz, P.I.; Biesalski, H.; Meyle, J. Concentration of Interleukin-1beta and Neutrophil Elastase Activity in Gingival Crevicular Fluid during Experimental Gingivitis. J. Clin. Periodontol. 2001, 28, 544–549. [Google Scholar] [CrossRef]
- Jing, W.; Xiaolan, C.; Yu, C.; Feng, Q.; Haifeng, Y. Pharmacological Effects and Mechanisms of Tannic Acid. Biomed. Pharmacother. 2022, 154, 113561. [Google Scholar] [CrossRef]
- Mohammadzadeh, V.; Mahmoudi, E.; Ramezani, S.; Navaeian, M.; Taheri, R.A.; Ghorbani, M. Design of a Novel Tannic Acid Enriched Hemostatic Wound Dressing Based on Electrospun Polyamide-6/Hydroxyethyl Cellulose Nanofibers. J. Drug Deliv. Sci. Technol. 2023, 86, 104625. [Google Scholar] [CrossRef]
- Wekwejt, M.; Małek, M.; Ronowska, A.; Michno, A.; Pałubicka, A.; Zasada, L.; Klimek, A.; Kaczmarek-Szczepańska, B. Hyaluronic Acid/Tannic Acid Films for Wound Healing Application. Int. J. Biol. Macromol. 2024, 254, 128101. [Google Scholar] [CrossRef]
- Wu, Y.; Zhong, L.; Yu, Z.; Qi, J. Anti-Neuroinflammatory Effects of Tannic Acid against Lipopolysaccharide-Induced BV2 Microglial Cells via Inhibition of NF-κB Activation. Drug Dev. Res. 2019, 80, 262–268. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Chen, Y.; Wang, L.; Liao, M. Preparation and Characterization of a Novel Polysialic Acid/Gelatin Composite Hydrogels Cross-Linked by Tannic Acid to Improve Wound Healing after Cesarean Section Dressing. J. Biomater. Sci. Polym. Ed. 2021, 32, 1927–1943. [Google Scholar] [CrossRef]
- de Veras, B.O.; da Silva, M.V.; Cabral Ribeiro, P.P. Tannic Acid Is a Gastroprotective That Regulates Inflammation and Oxidative Stress. Food Chem. Toxicol. 2021, 156, 112482. [Google Scholar] [CrossRef]
- Song, D.; Zhao, J.; Deng, W.; Liao, Y.; Hong, X.; Hou, J. Tannic Acid Inhibits NLRP3 Inflammasome-Mediated IL-1β Production via Blocking NF-κB Signaling in Macrophages. Biochem. Biophys. Res. Commun. 2018, 503, 3078–3085. [Google Scholar] [CrossRef]
- Lee, H.-R.; Jeong, Y.-J.; Lee, J.-W.; Jhun, J.; Na, H.S.; Cho, K.-H.; Kim, S.J.; Cho, M.-L.; Heo, T.-H. Tannic Acid, an IL-1β-Direct Binding Compound, Ameliorates IL-1β-Induced Inflammation and Cartilage Degradation by Hindering IL-1β-IL-1R1 Interaction. PLoS ONE 2023, 18, e0281834. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, L.; Yang, F.; Tong, W.; Jia, R.; Zou, Y.; Yin, L.; Li, L.; He, C.; Liang, X.; et al. Tannic Acid Accelerates Cutaneous Wound Healing in Rats Via Activation of the ERK 1/2 Signaling Pathways. Adv. Wound Care 2019, 8, 341–354. [Google Scholar] [CrossRef]
- Sivanantham, A.; Pattarayan, D.; Rajasekar, N.; Kannan, A.; Loganathan, L.; Bethunaickan, R.; Mahapatra, S.K.; Palanichamy, R.; Muthusamy, K.; Rajasekaran, S. Tannic Acid Prevents Macrophage-Induced pro-Fibrotic Response in Lung Epithelial Cells via Suppressing TLR4-Mediated Macrophage Polarization. Inflamm. Res. 2019, 68, 1011–1024. [Google Scholar] [CrossRef]
- Tsai, K.-F.; Shen, C.-J.; Cheung, C.-W.; Wang, T.-L.; Chow, L.W.C.; Leung, Y.-M.; Wong, K.-L. Lipotoxicity in Human Lung Alveolar Type 2 A549 Cells: Mechanisms and Protection by Tannic Acid. Chin. J. Physiol. 2021, 64, 289–297. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, M.; Ou, J.; Li, K.; Ju, X.; Tian, Y.; Niu, Z. Multi-Responsive Sodium Hyaluronate/Tannic Acid Hydrogels with ROS Scavenging Ability Promote the Healing of Diabetic Wounds. Int. J. Biol. Macromol. 2024, 278, 134896. [Google Scholar] [CrossRef]
- Qin, J.; Li, Z.; Feng, Y.; Guo, Y.; Zhao, Z.; Sun, S.; Zheng, J.; Zhang, M.; Zhang, J.; Zhang, Y.; et al. Reactive Oxygen Species-Scavenging Mesoporous Poly(Tannic Acid) Nanospheres Alleviate Acute Kidney Injury by Inhibiting Ferroptosis. ACS Biomater. Sci. Eng. 2024, 10, 5856–5868. [Google Scholar] [CrossRef]
- Yeo, J.; Lee, J.; Yoon, S.; Kim, W.J. Tannic Acid-Based Nanogel as an Efficient Anti-Inflammatory Agent. Biomater. Sci. 2020, 8, 1148–1159. [Google Scholar] [CrossRef]
- Lengert, E.V.; Savkina, A.A.; Ermakov, A.V.; Saveleva, M.S.; Lagutina, D.D.; Stepanova, T.V.; Ivanov, A.N. Influence of the New Formulation Based on Silver Alginate Microcapsules Loaded with Tannic Acid on the Microcirculation of the Experimental Periodontitis in Rats. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 126, 112144. [Google Scholar] [CrossRef]
- Chen, C.; Yang, H.; Yang, X.; Ma, Q. Tannic Acid: A Crosslinker Leading to Versatile Functional Polymeric Networks: A Review. RSC Adv. 2022, 12, 7689–7711. [Google Scholar] [CrossRef]
- Baldwin, A.; Booth, B.W. Biomedical Applications of Tannic Acid. J. Biomater. Appl. 2022, 36, 1503–1523. [Google Scholar] [CrossRef]
- Jafari, H.; Ghaffari-Bohlouli, P.; Niknezhad, S.V.; Abedi, A.; Izadifar, Z.; Mohammadinejad, R.; Varma, R.S.; Shavandi, A. Tannic Acid: A Versatile Polyphenol for Design of Biomedical Hydrogels. J. Mater. Chem. B 2022, 10, 5873–5912. [Google Scholar] [CrossRef]
- Yue, J.; Liu, Z.; Wang, L.; Wang, M.; Pan, G. Recent Advances in Bioactive Hydrogel Microspheres: Material Engineering Strategies and Biomedical Prospects. Mater. Today Bio 2025, 31, 101614. [Google Scholar] [CrossRef]
- Mahdi, M.H.; Conway, B.R.; Mills, T.; Smith, A.M. Gellan Gum Fluid Gels for Topical Administration of Diclofenac. Int. J. Pharm. 2016, 515, 535–542. [Google Scholar] [CrossRef]
- Osmalek, T.; Froelich, A.; Milanowski, B.; Bialas, M.; Hyla, K.; Szybowicz, M. pH-Dependent Behavior of Novel Gellan Beads Loaded with Naproxen. Curr. Drug Deliv. 2018, 15, 52–63. [Google Scholar] [CrossRef]
- Wang, L.; Dos Santos Sanches, N.; Panahipour, L.; Imani, A.; Yao, Y.; Zhang, Y.; Li, L.; Gruber, R. Dimethyl Fumarate-Loaded Gellan Gum Hydrogels Can Reduce In Vitro Chemokine Expression in Oral Cells. Int. J. Mol. Sci. 2024, 25, 9485. [Google Scholar] [CrossRef]
- Musazzi, U.M.; Cencetti, C.; Franzé, S.; Zoratto, N.; Di Meo, C.; Procacci, P.; Matricardi, P.; Cilurzo, F. Gellan Nanohydrogels: Novel Nanodelivery Systems for Cutaneous Administration of Piroxicam. Mol. Pharm. 2018, 15, 1028–1036. [Google Scholar] [CrossRef]
- Ng, J.Y.; Zhu, X.; Mukherjee, D.; Zhang, C.; Hong, S.; Kumar, Y.; Gokhale, R.; Ee, P.L.R. Pristine Gellan Gum–Collagen Interpenetrating Network Hydrogels as Mechanically Enhanced Anti-Inflammatory Biologic Wound Dressings for Burn Wound Therapy. ACS Appl. Bio Mater. 2021, 4, 1470–1482. [Google Scholar] [CrossRef]
- Spera, R. Gellan Gum for Tissue Engineering Applications: A Mini Review. Biomed. J. Sci. Tech. Res. 2018, 7, 1241–2574. [Google Scholar] [CrossRef]
- Wang, F.; Li, S.; Wang, X.; Yang, Q.; Duan, J.; Yang, Y.; Mu, H. Gellan Gum-Based Multifunctional Hydrogel with Enduring Sterilization and ROS Scavenging for Infected Wound Healing. Int. J. Biol. Macromol. 2024, 282, 136888. [Google Scholar] [CrossRef]
- Scalia, F.; Vitale, A.M.; Picone, D.; De Cesare, N.; Swiontek Brzezinska, M.; Kaczmarek-Szczepanska, B.; Ronca, A.; Zavan, B.; Bucchieri, F.; Szychlinska, M.A.; et al. Exploring Methacrylated Gellan Gum 3D Bioprinted Patches Loaded with Tannic Acid or L-Ascorbic Acid as Potential Platform for Wound Dressing Application. Gels 2025, 11, 40. [Google Scholar] [CrossRef]
- Panahipour, L.; Abbasabadi, A.O.; Gruber, R. Oral Cell Lysates Reduce the Inflammatory Response of Activated Macrophages. J. Clin. Med. 2023, 12, 1701. [Google Scholar] [CrossRef]
- Liu, F.; Sheng, S.; Shao, D.; Xiao, Y.; Zhong, Y.; Zhou, J.; Quek, C.H.; Wang, Y.; Dawulieti, J.; Yang, C.; et al. Targeting Multiple Mediators of Sepsis Using Multifunctional Tannic Acid-Zn2+-Gentamicin Nanoparticles. Matter 2021, 4, 3677–3695. [Google Scholar] [CrossRef]
- Frasheri, I.; Heym, R.; Ern, C.; Summer, B.; Hennessen, T.G.; Högg, C.; Reichl, F.-X.; Folwaczny, M. Salivary and Gingival CXCL8 Correlation with Periodontal Status, Periodontal Pathogens, and Smoking. Oral Dis. 2022, 28, 2267–2276. [Google Scholar] [CrossRef]
- Imani, A.; Panahipour, L.; Dos Santos Sanches, N.; Wang, L.; Gruber, R. Platelet-Rich Fibrin Increases CXCL8 Expression in Gingival Fibroblasts. Biomedicines 2024, 12, 1326. [Google Scholar] [CrossRef]
- Bond, M.; Chase, A.J.; Baker, A.H.; Newby, A.C. Inhibition of Transcription Factor NF-kappaB Reduces Matrix Metalloproteinase-1, -3 and -9 Production by Vascular Smooth Muscle Cells. Cardiovasc. Res. 2001, 50, 556–565. [Google Scholar] [CrossRef]
- Daré, R.G.; Nakamura, C.V.; Ximenes, V.F.; Lautenschlager, S.O.S. Tannic Acid, a Promising Anti-Photoaging Agent: Evidences of Its Antioxidant and Anti-Wrinkle Potentials, and Its Ability to Prevent Photodamage and MMP-1 Expression in L929 Fibroblasts Exposed to UVB. Free Radic. Biol. Med. 2020, 160, 342–355. [Google Scholar] [CrossRef]
- Chiang, Y.-T.; Xiao, Y.-B.; Hsu, S.-H.; Chang, S.-W.; Chou, C.-C. Molecular Interactions of Tannic Acid and Matrix Metalloproteinases 2 and 9. Comput. Struct. Biotechnol. J. 2023, 21, 2792–2800. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, H.J.; Kwon, H.J.; Lee, J.Y.; Cho, B.K.; Lee, W.J.; Yang, Y.; Cho, D.H. UVB-Induced Interleukin-18 Production is Downregulated by Tannic Acids in Human HaCaT Keratinocytes. Exp. Dermatol. 2006, 15, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Azimullah, S.; Meeran, M.F.N.; Ayoob, K.; Arunachalam, S.; Ojha, S.; Beiram, R. Tannic Acid Mitigates Rotenone-Induced Dopaminergic Neurodegeneration by Inhibiting Inflammation, Oxidative Stress, Apoptosis, and Glutamate Toxicity in Rats. Int. J. Mol. Sci. 2023, 24, 9876. [Google Scholar] [CrossRef] [PubMed]
- Meier, B.; Radeke, H.H.; Selle, S.; Younes, M.; Sies, H.; Resch, K.; Habermehl, G.G. Human Fibroblasts Release Reactive Oxygen Species in Response to Interleukin-1 or Tumour Necrosis Factor-Alpha. Biochem. J. 1989, 263, 539–545. [Google Scholar] [CrossRef]
- Son, Y.; Kim, S.; Chung, H.-T.; Pae, H.-O. Reactive Oxygen Species in the Activation of MAP Kinases. Methods Enzymol. 2013, 528, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Xiao, J.; Guan, S.; Geng, Z.; Zhao, R.; Gao, B. A Hydrogen-Bonded Antibacterial Curdlan-Tannic Acid Hydrogel with an Antioxidant and Hemostatic Function for Wound Healing. Carbohydr. Polym. 2022, 285, 119235. [Google Scholar] [CrossRef]
- Guo, Z.; Xie, W.; Lu, J.; Guo, X.; Xu, J.; Xu, W.; Chi, Y.; Takuya, N.; Wu, H.; Zhao, L. Tannic Acid-Based Metal Phenolic Networks for Bio-Applications: A Review. J. Mater. Chem. B 2021, 9, 4098–4110. [Google Scholar] [CrossRef]
- Tikoo, K.; Bhatt, D.K.; Gaikwad, A.B.; Sharma, V.; Kabra, D.G. Differential Effects of Tannic Acid on Cisplatin Induced Nephrotoxicity in Rats. FEBS Lett. 2007, 581, 2027–2035. [Google Scholar] [CrossRef]
- Jin, W.; Xue, Y.; Xue, Y.; Han, X.; Song, Q.; Zhang, J.; Li, Z.; Cheng, J.; Guan, S.; Sun, S.; et al. Tannic Acid Ameliorates Arsenic Trioxide-Induced Nephrotoxicity, Contribution of NF-κB and Nrf2 Pathways. Biomed. Pharmacother. 2020, 126, 110047. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, K.; Qin, M.; Lan, W.; Wang, L.; Liang, Z.; Li, X.; Wei, Y.; Hu, Y.; Zhao, L.; et al. Abundant Tannic Acid Modified Gelatin/Sodium Alginate Biocomposite Hydrogels with High Toughness, Antifreezing, Antioxidant and Antibacterial Properties. Carbohydr. Polym. 2023, 309, 120702. [Google Scholar] [CrossRef]
- Cvikl, B.; Lussi, A.; Moritz, A.; Sculean, A.; Gruber, R. Sterile-Filtered Saliva Is a Strong Inducer of IL-6 and IL-8 in Oral Fibroblasts. Clin. Oral Investig. 2015, 19, 385–399. [Google Scholar] [CrossRef]
- Ransy, C.; Vaz, C.; Lombès, A.; Bouillaud, F. Use of H2O2 to Cause Oxidative Stress, the Catalase Issue. Int. J. Mol. Sci. 2020, 21, 9149. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Elner, S.G.; Bian, Z.-M.; Till, G.O.; Petty, H.R.; Elner, V.M. Pro-Inflammatory Cytokines Increase Reactive Oxygen Species through Mitochondria and NADPH Oxidase in Cultured RPE Cells. Exp. Eye Res. 2007, 85, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Pan, Y. Reactive Oxygen Species Mediate TNF-α-Induced Inflammatory Response in Bone Marrow Mesenchymal Cells. Iran. J. Basic Med. Sci. 2019, 22, 1296–1301. [Google Scholar] [CrossRef] [PubMed]



| Gene | Forward (5′ → 3′) | Reverse (3′ → 5′) |
|---|---|---|
| hCXCL1 | TCCTGCATCCCCCATAGTTA | CTTCAGGAACAGCCACCAGT |
| hCXCL2 | CCCATGGTTAAGAAAATCATCG | CTTCAGGAACAGCCACCAAT |
| hCXCL8 | AACTTCTCCACAACCCTCTG | CTTCAGGAACAGCCACCAAT |
| hCXCL10 | TGCCATTCTGATTTGCTGCC | TTGGCAGCCTTCCTGATTTC |
| hCCL2 | AGAATCACCAGCAGCAAGTGTC | TCCTGAACCCACTTCTGCTTG |
| hCCL4 | AATCACCAGCAGCAAGTGTC | TTGGGTTGTGGAGTGAGTGT |
| hGAPDH | TGCACCACCAACTGCTTAGC | GGCATGGACTGTGGTCATGAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos Sanches, N.; Imani, A.; Wang, L.; Pacheco Vitória, O.A.; Reinert, H.; Panahipour, L.; Souza, F.Á.; Garcia Júnior, I.R.; Gruber, R. Tannic Acid-Loaded Gellan Gum Hydrogels Reduce In Vitro Chemokine Expression in Oral Cells. Int. J. Mol. Sci. 2025, 26, 5578. https://doi.org/10.3390/ijms26125578
dos Santos Sanches N, Imani A, Wang L, Pacheco Vitória OA, Reinert H, Panahipour L, Souza FÁ, Garcia Júnior IR, Gruber R. Tannic Acid-Loaded Gellan Gum Hydrogels Reduce In Vitro Chemokine Expression in Oral Cells. International Journal of Molecular Sciences. 2025; 26(12):5578. https://doi.org/10.3390/ijms26125578
Chicago/Turabian Styledos Santos Sanches, Natália, Atefe Imani, Lei Wang, Otávio Augusto Pacheco Vitória, Hannah Reinert, Layla Panahipour, Francisley Ávila Souza, Idelmo Rangel Garcia Júnior, and Reinhard Gruber. 2025. "Tannic Acid-Loaded Gellan Gum Hydrogels Reduce In Vitro Chemokine Expression in Oral Cells" International Journal of Molecular Sciences 26, no. 12: 5578. https://doi.org/10.3390/ijms26125578
APA Styledos Santos Sanches, N., Imani, A., Wang, L., Pacheco Vitória, O. A., Reinert, H., Panahipour, L., Souza, F. Á., Garcia Júnior, I. R., & Gruber, R. (2025). Tannic Acid-Loaded Gellan Gum Hydrogels Reduce In Vitro Chemokine Expression in Oral Cells. International Journal of Molecular Sciences, 26(12), 5578. https://doi.org/10.3390/ijms26125578







